Abstract
The production of extracellular vesicles (EVs) has emerged as an important process in bacterial biology and host-pathogen interactions. Like many other bacteria, mycobacteria, including Mycobacterium tuberculosis (Mtb), the causative agent of human tuberculosis (TB), produces EVs in vitro and in vivo. These membrane-enclosed nanoparticles enable Mtb to secrete hydrophobic molecules, proteins, lipids and glycolipids in a concentrated and protected manner and engage in remote interactions with the host. The nature of the material secreted in mycobacterial EVs, the functional attributes of these vesicles and their potential as protective antigens have stimulated great interest in the mycobacterial field. Although the field of EVs in mycobacterial infections is developing, it has already uncovered a whole new dimension for Mtb-host interactions potentially relevant to TB pathogenesis.
In this mini-review, we discuss the current evidence supporting an important role of mycobacterial EVs in modulating cellular immune response, the challenges and recent advances in understanding the mechanisms of vesicle biogenesis and the implications for development of new preventive and therapeutic tools against TB.
Keywords: Extracellular vesicles, Mycobacterium tuberculosis, Immunomodulation, Hostpathogen interactions, Cell-to-cell communication
1. Introduction
Bacteria depend on specialized protein secretion systems to interact with other cells and modulate their environment (Ligon et al., 2012). Like most organisms from other domains of life (Eukarya and Archaea), bacteria actively secrete extracellular vesicles (EVs) (Gill et al., 2018). EVs are membrane enclosed spherical nanostructures, 20-500 nm in diameter, naturally released by intact bacteria in vitro and in vivo. While secretion systems allow the export of individual proteins or small complexes, EVs can deliver, into the extracellular milieu, a diverse cargo of proteins, lipids and nucleic acids (Kulp and Kuehn, 2010). EVs released by pathogenic bacteria frequently contain toxins and other virulence factors. EVs originated at the outer membrane of the Gram-negative bacterium Escherichia coli were first detected more than 60 years ago. In contrast, EV production in Gram-positive bacteria and mycolic-acid containing microorganisms like mycobacteria was recognized only in the last decade.
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), the most lethal infectious disease globally, produces EVs in vitro and in vivo. It is well known that Mtb has evolved multiple and sophisticated mechanisms to manipulate host cellular physiology and evade the immune system. These interactions with the host are mediated in large part by mycobacterial cell envelope components that are now known to be exported in EVs (Dulberger et al., 2020). Indeed, mycobacterial EVs (MEVs) have strong immunomodulatory properties in vitro and when administered to mice.
In this minireview we provide an overview of the current knowledge about EVs and host-pathogen interactions in TB. We discuss MEVs biogenesis, immunological activity and potential for medical applications including vaccine and diagnostics.
2. MEVs biogenesis.
MEVs were first observed embedded in the extracellular matrix of Mycobacterium ulcerans biofilms and recovered from biopsies of Buruli ulcer-like lesions in infected mice (Marsollier et al., 2007). These EVs serve as vehicles for the lipidic toxin mycolactone, the main M. ulcerans virulence factor. Subsequently, a comprehensive study of EV production in multiple mycobacterial species confirmed that live mycobacteria, including virulent and non-virulent species, actively released EVs in vitro and in vivo (Prados-Rosales et al., 2011). Analysis of MEV’s lipid components showed enrichment of phospholipids and lipoproteins consistent with their origin from the cytoplasmic membrane. Polyacylated trehalose and phenolic glycolipids, which are mycomembrane components, were also extracted from MEV preparations, albeit in low abundance. It is possible that these lipids associate with MEVs formed at the plasma membrane as they traffic out of the cell, or they may be derived of a small sub population of vesicles originated at the mycomembrane. Indeed, in addition to the most abundant outer membrane vesicles (OMV), Gram-negative bacteria release outer-inner vesicles containing periplasmic and cytoplasmic components (Pérez-Cruz et al., 2013).
MEVs production is an active process regulated by iron (Prados-Rosales et al., 2014b), VirR (vesiculogenesis and immune response regulator) (Rath et al., 2013) and the two-component system SenX3-RegX3 (White et al., 2018). While iron limitation, a stressful condition encountered in the host, and activation of SenX3-RegX3 signaling induce vesiculation, VirR expression restricts vesicle release; deletion of virR leads to hypervesiculation. The way these regulators control MEVs release is not understood. However, in E. coli, in which OMV release is also regulated by iron, derepression of a phospholipid translocator repressed by the Ferric uptake repressor (Fur), and the consequent accumulation of phospholipids in the outer leaflet of the outer membrane lead to hypervesiculation (Roier et al., 2016). A comprehensive analysis of structural changes in the mycobacterial cell envelope in response to iron availability, VirR and SenX3-RegX3 activation may provide insights on the mechanisms underpinning the action of these regulators.
The study of the molecular mechanisms of vesicle biogenesis is still in its infancy. Nonetheless, in a recent preprint we reported the identification of the mycobacterial dynamin-like proteins IniA and IniC as critical factors in MEVs production thereby advancing knowledge in this area (Gupta et al., 2020). Dynamin and related proteins are large GTPases that function in membrane remodeling in eukaryotic and prokaryotic cells. Inactivation of iniAC in Mtb reduced vesicle production, and M. smegmatis IniA has been shown to mediate liposome membrane fission in vitro in a GTP dependent manner (Wang et al., 2019). We, therefore, have proposed a model in which IniA and IniC cooperate to restructure the mycobacterial membrane and allow scission of membrane vesicles. The iniBAC operon is induced in iron limited and VirR lacking mycobacterial cells, suggesting that, at least in part, iron and VirR regulate vesiculation by controlling iniAC expression. The iniBAC operon is also induced by isoniazid (INH). Accordingly, it was shown that subinhibitory concentrations of INH lead to increased MEV production in wild type Mtb, but not in an iniAC mutant (Gupta et al., 2020). This observation has important implications since INH is universally used to treat TB. Suboptimal exposure of Mtb to INH in the host environment might augment vesicle production and exacerbate MEVs effects on the immune system.
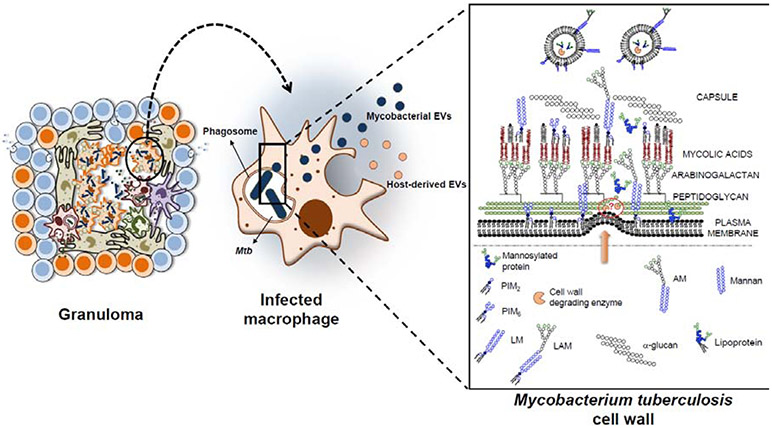
How EVs formed at the plasma membrane reach the extracellular environment is still a challenging question. Studies showing that antibiotics that weaken the cell wall, such as β-lactams, stimulate membrane vesicle formation in Gram-positive bacteria (Biagini et al., 2015; Toyofuku et al., 2017) and the fact that cell wall modifying enzymes are included in fungi and Staphylococcus aureus EVs (Brown et al., 2015; Lee et al., 2009) support the idea that cell envelope remodeling facilitate MEVs release (Fig. 1). In addition, highlighting an intricate connection between cell envelope remodeling and MEVs release, vesicle biogenesis regulators are closely linked to cell surface responses. Iron controls the expression of many cell envelope synthesis genes, SenX3-RegX3 regulates envelope stress responses, and VirR belongs to the LytR-CpsA-Psr (LCP) protein family, which include proteins involved in maintaining cell envelope integrity.
Figure 1. Mechanism of EVs secretion during a mycobacterial infection.
Mtb resides within phagocytic cell-phagosomes, restrained in the granuloma. Infected cells secrete both host-derived EVs and mycobacterial EVs (MEVs), the latter originated at the plasma membrane of the bacteria and released from the APC. MEVs reach the extracellular space through a mycobacterial envelope remodeling mechanisms yet to be identified.
3. MEVs cargo
The protein content of EVs produced by Mtb, M. bovis BCG, M. smegmatis and M. avium was studied by mass spectrometry, and some proteins validated based on recognition by specific antibodies. The proteomic profiles of MEVs encompassed over 200 proteins, mainly cell surface and secreted proteins, but also some cytoplasmic proteins. Vesicular proteins are functionally diverse (Chiplunkar et al., 2019; Layre, 2020; Prados-Rosales et al., 2011) (Table 1). Global proteomics highlighted differences in MEVs composition in various mycobacterial species and culture conditions. For instance, MEVs produced by virulent Mtb and M. bovis BCG, but not M. smegmatis are enriched in lipoproteins (Prados-Rosales et al., 2011). Additionally, the set of proteins in vesicles produced by M. avium cultured in medium with a metal composition and pH that seeks to reproduce the phagosome environment was distinct to that of EVs produced in minimal medium (Chiplunkar et al., 2019). This suggests that MEVs composition and therefore, biological properties may differ in various host microenvironments, according to the nutritional status of the parental bacterium and its response to environmental cues.
Table 1.
Summary of proteins identified in mycobacterial Extracellular Vesicles.
| Functional category | In-vitro and in-vivo detected vesicular proteins |
|---|---|
| Virulence associated proteins | ClpB, Cfp29, Eis, EphG, HspX, HtpG, GroEL1, GroES, GrpE, GlgX, KatG, MymT, Tpx, TreS, SodB, SodC, VapC10, VapC11 |
| Membrane remodeling proteins | Cut2, CrgA, CFP21, CwsA, DacB2, EmbC, FtsE, FtsH, FtsK, FtsY, PonA1, PonA2, ParA-related protein, ParB-like proteins, SppA, Tig, Wag31 |
| Lipoproteins | FecB, LpqD, LpqE, LpqH, LpqI, LpqJ, LpqL, LpqN, LpqT, LprA, LprC, LprF, LprG, LprQ, LppI, LppO, LppX, LppZ, SubI, ModA, PstS1, PstS2, PstS3 |
| Adhesins | Apa, DnaK, GroEL2, HBHA |
| Secretion associated proteins | EsxA, EsxB, EsxM, EsxN, EsxO, EspA, EspC, EspK |
| Transport system associated proteins | CtpC, DppA, DppD, MMPL3, Mkl, MscL, Rv1410c, Rv1747 ABC transporter, SecA, SecD, TatA, TrkB |
| Immunogenic proteins | Atg85a, Atg85b, Mpt53, Mpt63, Mpt64, Mtc28 |
MEVs lipids consist mainly of phospholipids (PG, PE, PI and cardiolipin), acylated phosphotidylinositol dimannosides (PIM2 and PIM6) and, in lower amounts, polyacylated trehalose and phenolic glycolipids (Prados-Rosales et al., 2011). MEVs produced by iron limited Mtb carry the lipidic siderophore mycobactin, which enables iron acquisition and growth of iron-deprived bacteria (Prados-Rosales et al., 2014b).
As it has been reported for EVs produced by other bacteria (Bitto et al., 2017; Dauros-Singorenko et al., 2018), it is likely that MEVs contain nucleic acids such as DNA and different kind of RNAs. One study reported that no RNA was detected within Mtb EVs after RNAseA treatment (Singh et al., 2015), although further research is needed in order to ascertain this finding. In the case of M. avium-derived EVs, dsDNA was detected both within the vesicles and externally associated with them (Chiplunkar et al., 2019).
4. MEVs mediate release of Mtb molecules from infected macrophages
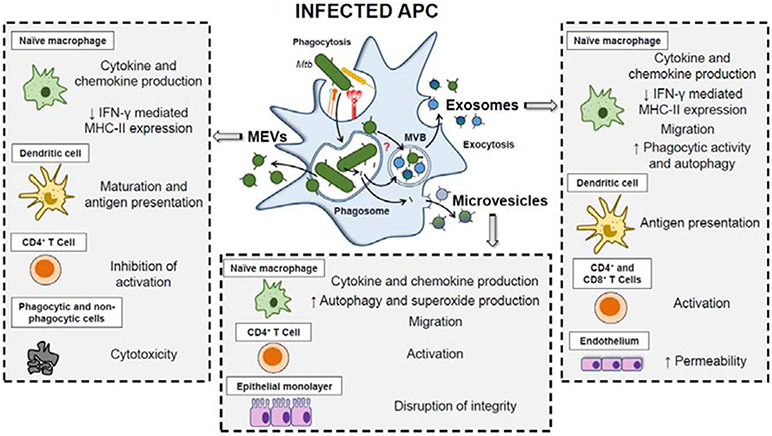
Although in macrophages, Mtb resides mostly within the phagosome, as early as few hours post infection, mycobacterial cell wall glycolipids and lipoproteins traffic out of the phagosome and disseminate throughout endocytic compartments of infected macrophages (Pethe et al., 2004). Subsequently, bacterial cell envelope components are detected in EVs released by infected macrophages. The size and surface markers present in these EVs are consistent with exosomes, a type of EVs that originate at the multivesicular endosomal compartment (MVE), and are released following fusion of the MVE with the plasma membrane. Today it is clear that EVs released by Mtb infected macrophages are a heterogeneous mix, including vesicles containing bacterial material such as lipoarabinomannan (LAM), lipomannan (LM) and lipoproteins but devoid of host markers, consistent with a bacterial origin. When separated from exosomes these MEVs have potent immunomodulatory properties as described below (Fig. 2).
Figure 2. Immunomodulatory properties of MEVs and host-derived vesicles.
Different types of mycobacterial-antigen containing EVs are released from Mtb-infected APCs, including MEVs, host-derived exosomes and microvesicles. These EVs have been shown to modulate both innate and adaptive immune responses. The mechanisms by which MEVs and mycobacterial antigens traffic outside the phagosome and the cell remain to be established. APC (Antigen-presenting cell); MVB (Multivesicular body); MEVs (Mycobacterial extracellular vesicles).
5. Modulation of the immune response by EVs released during mycobacterial infection.
5.1. MEVs
The first report of MEVs demonstrated the cytotoxic activity of M. ulcerans-derived EVs on both phagocytic and non-phagocytic cells due to the fact that these EVs contained the toxin mycolactone (Marsollier et al., 2007). Notably, the toxicity of M. ulcerans EVs was superior to that of pure mycolactone, suggesting that packing into EVs potentiate mycolactone’s activity. In addition, encapsulation of mycolactone in MEVs also induce IL-1β production in human macrophages and a strong inflammatory response in mice (Foulon et al., 2020). Concentration of virulence factors and immune effectors in EVs is a common theme in bacteria. Mtb and M. bovis BCG EVs were shown to be enriched in lipoproteins and other TLR2 agonists (Prados-Rosales et al., 2011). Accordingly, stimulation of murine bone marrow-derived macrophages (BMMs) with MEVs triggered a TLR2-dependent proinflammatory response characterized by enhanced release of IL-1β, IL-6, IL-12, TNF, CXCL1, MIP-1α/CCL3 (macrophage inflammatory protein-1α), and the anti-inflammatory cytokine IL-10 (Prados-Rosales et al., 2011). Such response was observed in vivo when BCG EVs were administered intratracheally to WT but not TLR2−/− naïve mice. Subsequent infection of MEV-primed mice resulted in increased granulomatous inflammation and higher bacterial burden in lungs and spleens (Prados-Rosales et al., 2011). Importantly, M. smegmatis-derived EVs, which were not enriched in lipoproteins, failed to induce such magnified proinflammatory responses in vitro, or in vivo. In another study, MEVs released by Mtb-infected macrophages, separated from exosomes, activated TLR2 in uninfected murine macrophages, promoting the release of TNF, IL-12, p40 and IL-10 (Athman et al., 2015). Although these studies suggest that MEVs promote continued inflammation that may be detrimental to the host, it is likely that MEVs-mediated Mtb-host interactions are complex and multifactorial. Indeed, Jurkoshek et al showed that MEVs purified from Mtb axenic cultures induced the expression of MHC-I, MCH-II and CD86 in murine dendritic cells (DCs), prompting their maturation and antigen presentation to Ag85b-specific CD4+ T cells (Jurkoshek et al., 2016). In contrast, they were found to diminish antigen presentation in naïve macrophages and CD11bhiCD11c− monocytes by impairing IFN-γ mediated MHC-II expression (Jurkoshek et al., 2016; Kania et al., 2013). Furthermore, LAM and other lipoglycans in MEVs inhibited IL-2 production and reduced T cell proliferation following TCR stimulation (Athman et al., 2017). Notably, MEVs given systemically prior to a virulent mycobacterial challenge induced both humoral and IFN-γ-mediated splenic Th1 cellular responses that protected mice against Mtb infection similarly to standard BCG vaccination (Prados-Rosales et al., 2014a), suggesting that developing immunity against MEVs will increase host resistance to Mtb infection.
Overall, current evidence suggests that Mtb generate and release MEVs as a means of transporting immunomodulatory molecules and virulence factors to host cells beyond the phagosome in which they reside. Concurrently, the host immune system has evolved to recognize antigens enclosed within MEVs, triggering both innate and adaptive immune responses that can aid control of Mtb infection. The apparent contradictory outcomes reported for MEVs could also be explained by the temporal dependence of TLR2 stimulation. It has been reported that TLR2 does not seem to play an essential role during acute Mtb infection. However, an initial TLR2-triggered proinflammatory cytokine release contributes to the developing of immune responses to control bacterial replication (Gopalakrishnan and Salgame, 2016). In addition, continuous TLR2 stimulation reduces MHC-II expression in macrophages and the block of responsiveness to IFN-γ (Harding and Boom, 2010). These effects, which have been recapitulated by TLR2 agonists-containing MEVs, play an important role in immunomodulation during chronic Mtb infection, becoming beneficial for the pathogen as its persistence increases, in part, by diminished antigen presentation. The recent isolation of a mutant deficient in vesicle production will enable direct testing of the relevance of MEVs in pathological Mtb-host interactions (Gupta et al., 2020).
5.2. Host-derived EVs
As previously stated, most mammalian cells release EVs. Based on their origin, and according to the surface markers they display, these vesicles are classified into i) exosomes, generated through the endocytic pathway and released by the exocytosis of multivesicular bodies; ii) microvesicles, also referred to as microparticles or ectosomes, originated by budding from the plasma membrane and iii) apoptotic bodies, derived from the plasma membrane during cell apoptosis (Gill et al., 2018).
There are many examples in the literature suggesting that exosomes containing pathogen associated molecular patterns (PAMPs) may have distal effects and contribute to microbial pathogenesis (Schwab et al., 2015). For example, during viral infections exosomes can spread viral miRNAs, undetectable to the immune system (Laganà et al., 2013). Similarly, macrophages infected with Salmonella typhimurium (Bhatnagar et al., 2007) or Mycoplasma (Yang et al., 2012) can shed exosomes loaded with PAMPs and remotely induce a proinflammatory response.
In fact, this is not different for mycobacteria. Phagocytic cells harboring Mtb (mainly macrophages, dendritic cells and neutrophils) release vesicles that contain mycobacterial components capable of modulating the immune response. Exosomes isolated from BCG-, Mtb- or M. avium-infected macrophages were shown to promote inducible nitric oxide synthase (iNOS) expression and the release of proinflammatory cytokine and chemokines including TNF, Regulated on Activation, Normal T cell Expressed and Secreted (RANTES), MCP-5, MIP-1α, MIP-1β, G-CSF, sICAM-1, MIP-2, and IL-1RA in uninfected macrophages (Bhatnagar et al., 2007; Bhatnagar and Schorey, 2007; Singh et al., 2012; Wang et al., 2015). Moreover, the study of Bhatnagar et al showed that TNF production induced by exosomes correlated with the content of LAM and the 19-kDa lipoprotein. However, other PAMPs are likely to be involved since the response was completely dependent on the adapter protein MyD88, but only partially dependent on TLR2 and TLR4 (Bhatnagar et al., 2007; Bhatnagar and Schorey, 2007). Treatment of naïve macrophages with exosomes released by Mtb-infected macrophages also enhanced expression of signalling molecules such as CD40, CD80, CD81, CD86, HLA-DR, and CD195 (Wang et al., 2015). Likewise, exosomes produced during BCG-infection isolated from bronchoalveolar lavage fluid (BALF), or mice serum recapitulated naive macrophage production of proinflammatory cytokines and chemokines (Bhatnagar et al., 2007; Singh et al., 2012). Exosomes isolated from Mtb-infected macrophages or from sera from Mtb-infected mice promoted endothelial cell activation (Li et al., 2018). Through activation of NF-κB and the Type 1 interferon pathways, exosomes enhanced cell monolayer permeability and upregulated expression of genes involved in adhesion and inflammation, like Vascular Cell Adhesion-1 (VCAM1), TLR2, SVEC4-10 and CCL2. Besides macrophage’s exosomes, microvesicles derived from neutrophils and macrophages similarly promoted a proinflammatory response in macrophages through TLR2/6 ligands, disrupted epithelial monolayers and induced immune cell recruitment to the injection site in mice (Alvarez-Jiménez et al., 2018; Walters et al., 2013).
Aside from the proinflammatory cytokine release required to mount an efficient immune response against Mtb, mycobacterial antigen delivery by infected host cell-derived vesicles to bystander naïve cells has been suggested to mediate host protection by different mechanisms. In particular, increased phagocytic (Wang et al., 2015) and bacterial clearance capacity either by enhanced autophagy and the production of high amounts of superoxide (Alvarez-Jiménez et al., 2018), or by phagosome maturation through a non-canonical LC3-dependent pathway was reported in macrophages (Cheng and Schorey, 2019). In mice, intranasal administration of exosomes derived from Mtb, or BCG-infected macrophage induced neutrophils and macrophages recruitment to the lungs and BALF (Bhatnagar et al., 2007; Singh et al., 2012).
Regarding adaptive immunity modulation, exosomes derived from Mtb-infected macrophages have been shown to regulate antigen presentation and T cell activation. One of the mechanisms employed by Mtb to evade host defences consists on the inhibition of antigen presentation by infected antigen-presenting cells (APCs) (Harding and Boom, 2010). To partially overcome this, mycobacterial antigen transport within exosomes has been proposed to mediate cross-presentation of mycobacterial antigens in distal sites. Indeed, Mtb-infected macrophage-derived exosomes bearing MHC-I, MHC-II and costimulatory molecules activated BCG-sensitized CD4+ and CD8+ T cells in vitro and naïve CD4+ and CD8+ T cells in vivo. However, maximum activation of sensitized cells in vitro required prior processing of exosomes by APCs (Giri and Schorey, 2008; Ramachandra et al., 2010). Moreover, the activation and maturation of DCs as measured by intracellular IL-12p40 levels and the expression of CD83, CD86, MHC-II upon exosome treatment was described (Giri and Schorey, 2008). The study of Smith et al emphasized the relevance of mycobacterial cargo-containing macrophage-derived exosomes in T cell activation by leveraging mice deficient in Rab27a, a GTPase that regulates multivesicular body fusion with the plasma membrane during exosome biogenesis. Rab27a-deficient mice showed decreased exosomes in serum after infection with Mtb, associated with reduced mycobacterial antigen trafficking via exosomes and low lung and splenic T cell activation. This limited immune response correlated with increased bacterial load; thus, supporting a beneficial role of exosomes in the control of Mtb infection (Smith et al., 2017).
In a similar fashion to exosomes, MHC-II-containing macrophage microvesicles were shown to promote the proliferation of antigen-specific CD4+ T cells both in vitro and in vivo, in an APC-dependent (Walters et al., 2013) or independent-manner (Ramachandra et al., 2010), according to different vesicle preparation methods. Additionally, mycobacterial culture filtrate protein-treated macrophage exosomes were able of priming a mycobacterial antigen-specific T cell response in mice (Pramod et al., 2010. Altogether, exosome-mediated mycobacterial antigen transport appears to generate protective innate and adaptive responses against Mtb. However, exosome-mediated immunosuppressive effects have also been reported. Mtb-infected macrophage-exosomes partially repressed IFN-γ mediated macrophage activation by inhibiting the expression of MHC-II and CD64 in a TLR2 and MyD88-dependent manner (Singh et al.,2011).
Beside the demonstrated ability of both Mtb and its host of releasing MEVs and exosomes, respectively, it is not clear how trafficking of MEVs in the context of infection occurs. The fact that modulation of the immune system by Mtb-infected host-derived vesicles resembles that of the isolated MEVs (Fig. 2) could be explained if MEVs and host-derived EVs carry similar Mtb antigens, or MEVs are co-purified with host-derived EVs devoid of Mtb antigens. The most commonly used technique for EV isolation includes an ultracentrifugation at 100,000 x g, followed by a linear sucrose density gradient to get rid of co-precipitated contaminants. According to Athman et al, coexisting MEVs and host-derived EVs in macrophage culture supernatants can be separated as two distinct populations in a sucrose density gradient (Athman et al., 2015). In this study, each population harbored either MEV markers (LAM, LM, LpqH, LprG) or exosomal markers (CD9, CD63, MHCII) as determined by western blot, immunoelectron microscopy and immunofluorescence microscopy. Little or no colocalization was found between MEV and exosomal markers. However, mycobacterial EV production during cell infection might have been overlooked by researchers focused on host-derived EVs, and their contribution to the pool of vesicles has not been studied in detail. The vast majority of the studies involving EVs derived from infected cells or isolated from infected body fluids assume that purified EVs are exclusively host-derived. It is plausible that those studies were carried out with a mixture of co-purified vesicles of different origins, as western blot or flow cytometry marker characterization fail to discern different populations within the same sample. Besides, MEVs and host cell EVs show similar appearance under transmission electron microscopy (Athman et al., 2015). Further, additional MEV markers to LAM or LpqH are needed to properly discern between MEVs and host cells-derived vesicles from infected cell cultures and body fluids.
How MEVs exit infected macrophages, whether they interact with the endocytic network and if they are the source of mycobacterial components exported in exosomes remains to be resolved. Technical advances that enable efficient isolation, purification and characterization of MEVs and host derived EVs released during TB infection will aid understanding the combined influence of these various types of EVs on the immune system and the outcome of infection.
6. Mycobacterial EVs in medicine.
6.1. MEVs as vaccines
The potential of bacterial EVs-based vaccines was demonstrated with the approval of a vaccine against Neisseria (Acevedo et al., 2014). In the context of TB, the systemic administration of MEVs provided similar protection than standard BCG vaccination in a murine model of infection with virulent Mtb. Protection translated into moderated control of bacterial replication in lungs and spleens (Prados-Rosales et al., 2014a). Importantly, immunogenicity analysis of MEVs showed a mixed humoral and cellular response directed to Mtb cell surface antigens. However, further studies that characterize the vesicle components responsible for the demonstrated protection are needed. This study also highlighted the heterogeneity of independent batches of natural MEVs in terms of protective potential. Recent advances in methods to generate and load artificial and natural vesicles could be applied, once protective components are identified, to generate nanoparticle vesicles with consistent protection activity.
6.2. MEVs as biomarkers of TB infection
An EV is a biological particle containing information about the cell that released it. This notion is particularly important for a cryptic microorganism like Mtb, whose physiology cannot be separated from that of its only host, the human being. Most of Mtb’s biology occurs inside host cells and MEVs derived from this interaction can provide valuable information about the status of both the bacterium and the host. A study of sera from a small cohort of people with smear-positive, or negative TB infection, and BCG-vaccinated with and without latent TB, was used to investigate the potential of Mtb and BCG EVs as biomarkers to discern between disease status (Ziegenbalg et al., 2013). Detection of three MEV-associated antigens discriminated between sera from TB and non-TB patients. These results are encouraging and justify expanding this study to a larger cohort of patients and including multiple batches of MEVs. Of note, when MEVs are isolated from cultures submitted to host-related conditions such as iron starvation (Prados-Rosales et al., 2014b) MEV composition changes and potentially immunogenicity may change too. Whether such changes can expand the source of biomarkers for analysis of clinical samples awaits further investigation.
7. Concluding remarks
Investigations on the effects of EVs, produced during TB infection, on the immune system suggest MEVs constitute a whole new dimension for Mtb-host interactions. That virulent Mtb, like many other pathogens, incorporate virulence factors into vesicles and that such vesicles have potent immunomodulatory properties suggest that vesicle release is one more strategy that Mtb uses to survive within the host. Realization that MEVs can get out of the infected phagocytic cell together with host-related EVs, such as exosomes or microparticles, suggests a complex and heterogeneous scenario including multiple EVs with different composition. This consideration should be extended to other intracellular pathogens where the study of EVs in the context of infection has been mostly focused on host-derived EVs. In order to have a complete picture of the impact of vesiculogenesis during infection, comprehensive compositional analysis of such EV populations are needed.
MEV production is a regulated process and it may involve multiple pathways. It is likely that cell envelope remodeling is needed so that a newly formed MEV can reach the extracellular space. However, the specific changes and regulatory mechanisms involved should be the focus of future studies.
The discovery of MEVs has revolutionized our understanding of mycobacterial physiology. Many challenges remain. However, we anticipate that continued studies that characterize MEVs produced during infection and their impact on the host as well as efforts to decipher the mechanistic aspects of vesicle secretion will deepen our understanding of Mtb pathogenic strategies and the host defence response. The study of MEVs can also provide new drug targets, unveil host-response independent markers of infection and inform the development of new immunization strategies and predictive biomarkers of treatment and vaccine efficiency.
Highlights.
Mycobacterium tuberculosis actively releases extracellular vesicles in vitro and in vivo.
During an ongoing infection, Mycobacterium tuberculosis produces extracellular vesicles that travel beyond the infected phagocytic cells.
Extracellular vesicle production in mycobacteria is a regulated process.
Mycobacterial extracellular vesicles display strong immunomodulatory properties.
Mycobacterial extracellular vesicles are a potential source of infection biomarkers and have vaccinogenic properties.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- Acevedo R, Fernández S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, Rosenqvist E, Campa C, Cardoso D, Garcia L, Perez JL, 2014. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol 10.3389/fimmu.2014.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jiménez VD, Leyva-Paredes K, García-Martinez M, Vázquez-Flores L, García-Paredes VG, Campillo-Navarro M, Romo-Cruz I, Rosales-García VH, Castañeda-Casimiro J, González-Pozos S, Manuel Hernández J, Wong-Baeza C, García-Pérez BE, Ortiz-Navarrete V, Estrada-Parra S, Serafín-López J, Wong-Baeza I, Chacón-Salinas R, Estrada-García I, 2018. Extracellular vesicles released from Mycobacterium tuberculosis-Infected neutrophils promote macrophage autophagy and decrease intracellular mycobacterial survival. Front. Immunol 9, 1–12. 10.3389/fimmu.2018.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman JJ, Sande OJ, Groft SG, Reba SM, Nagy N, Wearsch PA, Richardson ET, Rojas R, Boom WH, Shukla S, Harding CV, 2017. Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J. Immunol 198, 2028–2037. 10.4049/jimmunol.1601199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman JJ, Wang Y, McDonald DJ, Boom WH, Harding CV, Wearsch PA, 2015. Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages . J. Immunol 195, 1044–1053. 10.4049/jimmunol.1402894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Schorey JS, 2007. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem 282, 25779–25789. 10.1074/jbc.M702277200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS,2007. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110, 3234–3244. 10.1182/blood-2007-03-079152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini M, Garibaldi M, Aprea S, Pezzicoli A, Doro F, Becherelli M, Taddei AR, Tani C, Tavarini S, Mora M, Teti G, D’Oro U, Nuti S, Soriani M, Margarit I, Rappuoli R, Grandi G, Norais N, 2015. The human pathogen Streptococcus pyogenes releases lipoproteins as lipoprotein-rich membrane vesicles. Mol. Cell. Proteomics 14, 2138–2149. 10.1074/mcp.M114.045880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, D’Cruze T, Reynolds EC, Dashper SG, Turnbull L, Whitchurch CB, Stinear TP, Stacey KJ, Ferrero RL, 2017. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep 7, 1–11. 10.1038/s41598-017-07288-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Wolf JM, Prados-Rosales R, Casadevall A, 2015. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol 10.1038/nrmicro3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Schorey JS, 2019. Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing . EMBO Rep. 20. 10.15252/embr.201846613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiplunkar SS, Silva CA, Bermudez LE, Danelishvili L, 2019. Characterization of membrane vesicles released by Mycobacterium avium in response to environment mimicking the macrophage phagosome. Future Microbiol. 14, 293–313. 10.2217/fmb-2018-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauros-Singorenko P, Blenkiron C, Phillips A, Swift S,2018. The functional RNA cargo of bacterial membrane vesicles. FEMS Microbiol. Lett 365, 1–9. 10.1093/femsle/fny023 [DOI] [PubMed] [Google Scholar]
- Dulberger CL, Rubin EJ, Boutte CC, 2020. The mycobacterial cell envelope — a moving target. Nat. Rev. Microbiol 10.1038/s41579-019-0273-7 [DOI] [PubMed] [Google Scholar]
- Foulon M, Robbe-Saule M, Manry J, Esnault L, Boucaud Y, Alcaïs A, Malloci M, Fanton d’Andon M, Beauvais T, Labarriere N, Jeannin P, Abel L, Saint-André JP, Croué A, Delneste Y, Boneca IG, Marsollier L, Marion E, 2020. Mycolactone toxin induces an inflammatory response by targeting the IL-1β pathway: Mechanistic insight into Buruli ulcer pathophysiology. PLOS Pathog. 16, e1009107. 10.1371/journal.ppat.1009107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Catchpole R, Forterre P,2018. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev 42. 10.1093/femsre/fuy042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri PK, Schorey JS, 2008. Exosomes derived from M. bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One 3. 10.1371/journal.pone.0002461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan A, Salgame P,2016. Toll-like receptor 2 in host defense against Mycobacterium tuberculosis: To be or not to be - that is the question. Curr. Opin. Immunol 42, 76–82. 10.1016/j.coi.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Palacios A, Khataokar A, Weinrick B, Lavin J, Sampedro L, Gil D, Anguita J, Menendez M, Garcia M, Dogra N, Neiditch B, Prados-Rosales R, Rodriguez G, 2020. Dynamin-like proteins are essential for vesicle biogenesis in Mycobacterium tuberculosis. bioRxiv. [Google Scholar]
- Harding CV, Boom WH, 2010. Regulation of antigen presentation by Mycobacterium tuberculosis: A role for Toll-like receptors. Nat. Rev. Microbiol 10.1038/nrmicro2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkoshek KS, Wang Y, Athman JJ, Barton MR, Wearsch PA, 2016. Interspecies Communication between Pathogens and Immune Cells via Bacterial Membrane Vesicles. Front. Cell Dev. Biol 4, 1–8. 10.3389/fcell.2016.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania G, Siegert S, Behnke S, Prados-Rosales R, Casadevall A, Lüscher TF, Luther SA, Kopf M, Eriksson U, Blyszczuk P, 2013. Innate signaling promotes formation of regulatory nitric oxide-producing dendritic cells limiting t-cell expansion in experimental autoimmune myocarditis. Circulation 127, 2285–2294. 10.1161/CIRCULATIONAHA.112.000434 [DOI] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ, 2010. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol 64, 163–184. 10.1146/annurev.micro.091208.073413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganà A, Russo F, Veneziano D, Di Bella S, Giugno R, Pulvirenti A, Croce CM, Ferro A, 2013. Extracellular circulating viral microRNAs : current knowledge and perspectives. Front. Genet 4, 1–8. 10.3389/fgene.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layre E, 2020. Trafficking of Mycobacterium tuberculosis Envelope Components and Release Within Extracellular Vesicles: Host-Pathogen Interactions Beyond the Wall. Front. Immunol 10.3389/fimmu.2020.01230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS, 2009. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9, 5425–5436. 10.1002/pmic.200900338 [DOI] [PubMed] [Google Scholar]
- Li L, Cheng Y, Emrich S, Schorey J, 2018. Activation of endothelial cells by extracellular vesicles derived from Mycobacterium tuberculosis infected macrophages or mice. PLoS One 13. 10.1371/journal.pone.0198337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LS, Hayden JD, Braunstein M, 2012. The ins and outs of Mycobacterium tuberculosis protein export. Tuberculosis. 10.1016/j.tube.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsollier L, Brodin P, Jackson M, Korduláková J, Tafelmeyer P, Carbonnelle E, Aubry J, Milon G, Legras P, Saint André JP, Leroy C, Cottin J, Guillou MLJ, Reysset G, Cole ST, 2007. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 3, 0582–0594. 10.1371/journal.ppat.0030062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cruz C, Carrión O, Delgado L, Martinez G, López-Iglesias C, Mercade E, 2013. New type of outer membrane vesicle produced by the gram-negative bacterium Shewanella vesiculosa M7T: Implications for DNA content. Appl. Environ. Microbiol 10.1128/AEM.03657-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Russell DG, 2004. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.0401657101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR, Porcelli SA, Casadevall A, 2011. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest 121, 1471–1483. 10.1172/JCI44261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R, Carreño LJ, Batista-Gonzalez A, Baena A, Venkataswamy MM, Xu J, Yu X, Wallstrom G, Mitchell Magee D, LaBaer J, Achkar JM, Jacobs WR, Chan J, Porcelli SA, Casadevall A, 2014a. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of mycobacterium tuberculosis. MBio 5, e01921–14. 10.1128/mBio.01921-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R, Weinrick BC, Piqué DG, Jacobs WR, Casadevall A, Rodriguez GM, 2014b. Role for mycobacterium tuberculosis membrane vesicles in iron acquisition. J. Bacteriol 196, 1250–1256. 10.1128/JB.01090-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramod KG, Kruh NA, Dobos KM, Schorey JS, 2010. Proteomic analysis identifies highly antigenic proteins in exosomes from M . tuberculosis -infected and culture filtrate protein-treated macrophages. Proteomics. 10.1002/pmic.200900840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV, 2010. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect. Immun 78, 5116–5125. 10.1128/IAI.01089-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath P, Huang C, Wang T, Wang T, Li H, Prados-Rosales R, Elemento O, Casadevall A, Nathan CF, 2013. Genetic regulation of vesiculogenesis and immunomodulation in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci 10.1021/acsami.5b05228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, Klug L, Gadermaier B, Weinzerl K, Prassl R, Lass A, Daum G, Reidl J, Feldman MF, Schild S, 2016. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun 7, 1–13. 10.1038/ncomms10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A, Meyering SS, Lepene B, Iordanskiy S, van Hoek ML, Hakami RM, Kashanchi F, 2015. Extracellular vesicles from infected cells : potential for direct pathogenesis. Front. Microbiol 6, 1–18. 10.3389/fmicb.2015.01132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, LeMaire C, Tan JC, Zeng E, Schorey JS, 2011. Exosomes released from m.tuberculosis infected cells can suppress ifn-γ mediated activation of naïve macrophages. PLoS One 6. 10.1371/journal.pone.0018564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Li L, Jeffrey SS, 2015. Exosomal RNA from Mycobacterium tuberculosis infected cells is functional in recipient macrophages. Traffic 16, 555–571. 10.1111/tra.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Smith VL, Karakousis PC, Schorey JS, 2012. Exosomes Isolated from Mycobacteria-Infected Mice or Cultured Macrophages Can Recruit and Activate Immune Cells In Vitro and In Vivo. J. Immunol 189, 777–785. 10.4049/jimmunol.1103638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VL, Cheng Y, Bryant BR, Schorey JS, 2017. Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci. Rep 7, 1–12. 10.1038/srep43578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L, 2017. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun 8, 1–10. 10.1038/s41467-017-00492-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters SB, Kieckbusch J, Nagalingam G, Swain A, Latham SL, Grau GER, Britton WJ, Combes V, Saunders BM, 2013. Microparticles from Mycobacteria-Infected Macrophages Promote Inflammation and Cellular Migration. J. Immunol. 190, 669–677. 10.4049/jimmunol.1201856 [DOI] [PubMed] [Google Scholar]
- Wang J, Yao Y, Xiong J, Wu J, Tang X, Li G, 2015. Evaluation of the Inflammatory Response in Macrophages Stimulated with Exosomes Secreted by Mycobacterium avium -Infected Macrophages. Biomed Res. Int 2015. 10.1155/2015/658421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Guo X, Yang X, Zhang B, Ren J, Liu A, Ran Y, Yan B, Chen F, Guddat LW, Hu J, Li J, Rao Z, 2019. Mycobacterial dynamin-like protein IniA mediates membrane fission. Nat. Commun 10. 10.1038/s41467-019-11860-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Elliott SR, Odean E, Bemis LT, Tischler AD, 2018. Mycobacterium tuberculosis Pst/SenX3-RegX3 regulates membrane vesicle production independently of ESX-5 activity. MBio 9. 10.1128/mBio.00778-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chalasani G, Ng Y, Robbins PD, 2012. Exosomes Released from Mycoplasma Infected Tumor Cells Activate Inhibitory B Cells. PLoS One 7, e36138. 10.1371/journal.pone.0036138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenbalg A, Prados-Rosales R, Jenny-Avital ER, Kim RS, Casadevall A, Achkar JM, 2013. Immunogenicity of mycobacterial vesicles in humans: Identification of a new tuberculosis antibody biomarker. Tuberculosis 93, 448–455. 10.1016/j.tube.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]