Abstract
Patient: Male, 41-year-old
Final Diagnosis: Cerebral infarction • Libman-Sacks endocarditis • primary antiphospholipid syndrome
Symptoms: Dizziness
Clinical Procedure: —
Specialty: Neurology
Objective:
Rare coexistence of disease or pathology
Background:
Anticardiolipin antibodies in patients with Libman-Sacks endocarditis (LS) are indicative of comorbid antiphospholipid syndrome (APS) and can result in cerebral infarctions. We describe a case of LS and primary APS with recurrent cerebral infarctions despite anticoagulation treatment. The patient underwent surgery for enlarged LS vegetation with high titers of antiphospholipid antibodies.
Case Report:
A 41-year-old Japanese man was admitted to hospital for small cerebral infarction recurrence in a left parietal lesion. At age 35, the patient had suffered multiple cerebral infarctions. He was found to have high serum titers of all 3 antiphospholipid antibodies. Transesophageal echocardiography (TEE) findings were normal. Differential diagnosis ruled out other autoimmune diseases and a clinical diagnosis of primary APS was made. Warfarin anticoagulation was started. When cerebral infarction recurred 6 years after the first episode, serum titers of antiphospholipid antibodies remained high, and TEE showed a 7×8 mm area of mitral vegetation. A TEE results from his first admission revealed a 5×6 mm area of mitral vegetation, which was believed to be related to the current vegetation. As anticoagulation produced no improvement, the mitral valve was replaced with a mechanical valve. Examination of the excised vegetation found it to be consistent with LS. The patient made good progress within 3 years after surgery.
Conclusions:
LS size can increase despite anticoagulation in cases with high titers of all 3 antiphospholipid antibodies and cerebral infarction. Such patients require ongoing TEE follow-up and surgical treatment should be considered.
Keywords: Antiphospholipid Syndrome; Case Reports; Embolic Stroke; Lupus Erythematosus, Systemic; Mitral Valve Insufficiency
Background
Libman-Sacks endocarditis (LS) is related to anticardiolipin antibodies and other manifestations of primary antiphospholipid syndrome (APS) [1], and can be the source of cerebral infarctions [2]. Previous research on the presence of valvular abnormalities on transesophageal echocardiography (TEE) assessments in patients with primary APS found anticoagulant and/or antiplatelet treatment to be ineffective means of producing valvular lesion regression. The same study also demonstrated a relationship between the appearance of cardiac involvement and high immunoglobulin G (IgG) anticardiolipin antibody (aCL) titers [3]. In this report, we describe a case of enlarged LS vegetation with high antiphospholipid antibody titers, primary APS, and recurrent cerebral infarction. In line with previous studies, we found anticoagulation treatment ineffective, so the patient underwent mitral valve replacement surgery. Anticoagulation treatment was continued following surgery and, in the subsequent 3 years, there have been no further cerebral infarctions.
Case Report
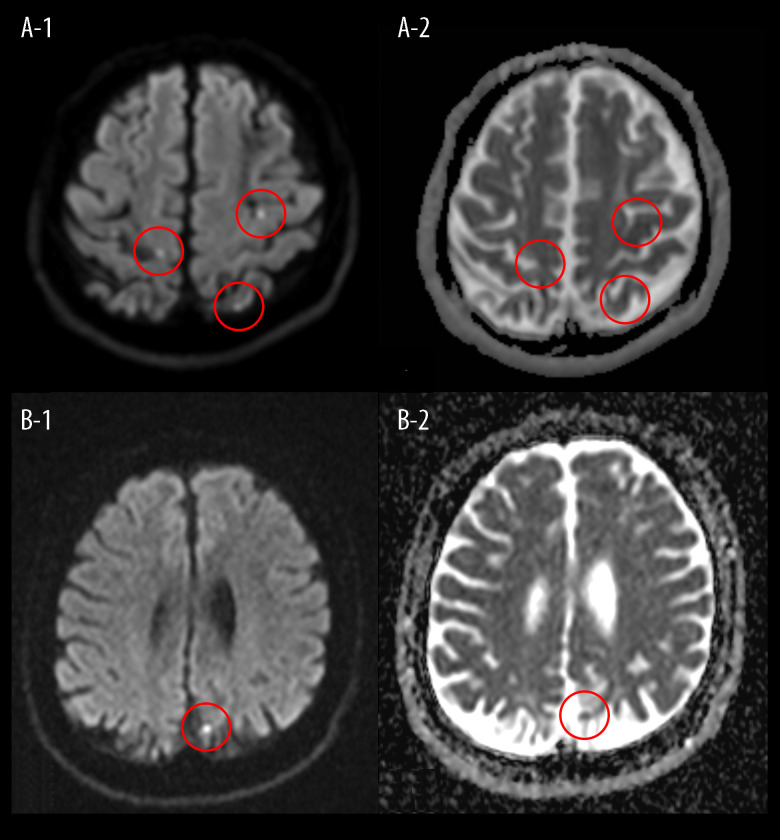
A 41-year-old Japanese man presented at our hospital with persistent dizziness and occipital pain. When he was 35 years old, he experienced left lower-limb weakness and hypoesthesia and was diagnosed with multiple cerebral infarctions, which were found by his previous doctor in bilateral anterior and posterior circulation lesions on magnetic resonance imaging (MRI) (Figure 1A–1, 1A–2). One month after symptom onset, the patient was admitted to our hospital for further examination of his cerebral infarctions. He had received no previous anticoagulation therapy. Apart from a history of smoking, he had no risk factors for cardiovascular diseases such as high blood pressure, diabetes, or hypercholesterolemia, and no family history of stroke or cardiovascular events, nor did he have any significant or relevant medical history. At that time, neither vascular stenosis nor occlusion were detected by magnetic resonance angiography and carotid ultrasonography, and no arrhythmias that can cause stroke were detected by 12-lead electrocardiography and cardiac monitoring. There was mild mitral regurgitation preserved ejection fraction in transthoracic echocardiography, and normal TEE findings. Laboratory findings showed high serum titers of lupus anticoagulant (LA) (2.05) (normal <1.2), IgG aCL (≥120 U/mL) (normal ≤12.3 U/mL), and IgG anti-β 2 glycoprotein-I antibody (anti-β 2GPI) (≥125 U/mL) (normal <3.5 U/mL) at measurement intervals of over 12 weeks (1.44, ≥120 U/mL and ≥125 U/mL). Anti-nuclear antibodies were <1: 40 (normal levels <1: 160), anti-Sm antibodies were ≤7 U/mL (normal <10 U/mL), anti-double-stranded deoxyribonucleic acid (anti-dsDNA) IgG antibodies were <10 U/mL (normal <12 U/mL), C3 was 98 mg/dL (normal=73–138 mg/dL), and C4 was 27 mg/dL (normal=11–31 mg/dL). Based on the diagnostic criteria for primary APS [4] and the absence of signs or symptoms of other autoimmune diseases, including systemic lupus erythematosus (SLE), the patient was clinically diagnosed with primary APS. This was considered to be the etiology of his cerebral infarctions, as all the other possible causes had been ruled out. As the acute phase had passed, heparin anticoagulation was postponed, and warfarin anticoagulation for primary APS and cerebral infarctions was initiated, with a target prothrombin time/international normalized ratio (PT/INR) therapeutic range of 2.0–3.0. The PT/INR was maintained within this range via outpatient treatment. The patient experienced ongoing left lower-limb weakness (grade 4 on the manual muscle test) and mild hypoesthesia as sequelae, although these neurological symptoms gradually improved.
Figure 1.
(A–1) Head MRI of our patient’s first cerebral infarction showed hyperintensity (circle) in diffusion-weighted images (A–2) and low intensity (circle) on an apparent diffusion coefficient map, along bilateral anterior and posterior circulation lesions. (B–1) Head MRI of the recurrence of our patient’s cerebral infarction showed faint hyperintensity (circle) in diffusion-weighted images (B–2) and low intensity (circle) on an apparent diffusion coefficient map, along a left parietal lesion (TR/TE): (4000/95). MRI – magnetic resonance imaging; TR/TE – repetition time/echo time (for the control of image contrast in weighted MRI).
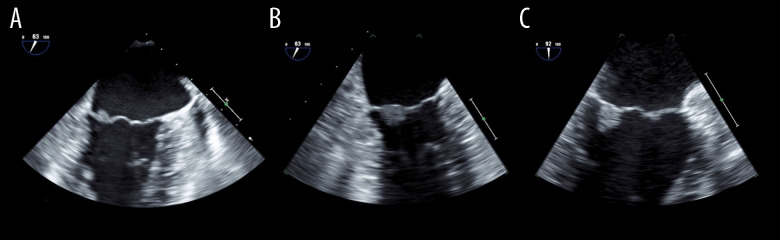
Figure 2.
(A) Transesophageal echocardiography image of the patient’s first cerebral infarction, showing a 5×6 mm image of the mitral vegetation on the posterior leaflet; (B) Enlarged (to 7×8 mm) image of the recurrent cerebral infarction site; (C) No change was observed 72 days after the last cerebral infarction.
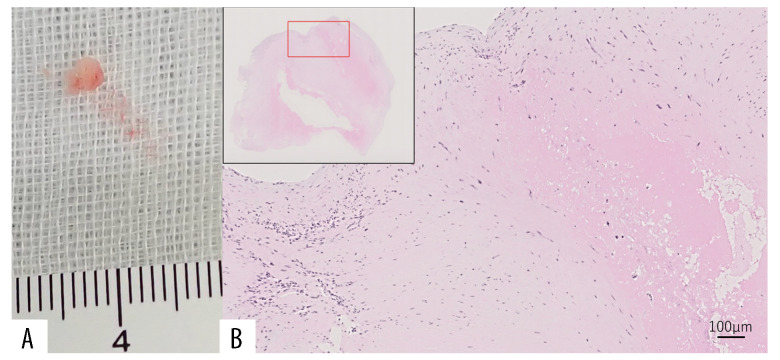
When his cerebral infarction recurred 6 years after the first episode (day 1), the patient presented with normal blood pressure, no fever, no heart murmurs, and no neurological signs other than the sequelae of his first cerebral infarctions. Diffusion-weighted MRI revealed a small cerebral infarction in a left parietal lesion (Figure 1B–1, 1B–2). Due to this finding, he was admitted to the hospital. Laboratory tests found warfarin to have achieved a PT/INR of 2.46, which was within the target therapeutic range. However, the high serum titers of LA (2.50), IgG aCL (≥120 U/mL), and IgG anti-β 2GPI (≥125 U/mL) remained present. Anti-nuclear antibodies were 1: 80, homogeneous+, nucleolar+, anti-Sm antibodies were ≤1 U/mL, anti-dsDNA IgG antibodies were <10 U/mL, C3 was 91 mg/dL, and C4 was 14 mg/dL. TEE on day 7 showed a 7×8 mm area of mitral vegetation on the posterior leaflet (Figure 2B). The TEE results from the patient’s first admission were interpreted as normal at that time. We performed a thorough retrospective reevaluation of these findings and identified a 5×6 mm area of mitral vegetation on the posterior leaflet (Figure 2A). This was considered to be the same vegetation as that diagnosed following the patient’s most recent admission, which had enlarged over the intervening 6 years. No periodic echocardio-graphic monitoring was performed in the years between infarction episodes due to the TEE findings on the first admission. As the cerebral infarction was cortical and there were no signs or symptoms of other potential causes, this enlarged vegetation was identified as the cause of the cerebral infarction recurrence and was also suspected to be among the causes of the first cerebral infarctions. Infectious endocarditis was ruled out based on the patient’s general condition and blood culture results. The persistence of the primary APS and the enlargement of the vegetation were considered indicative of LS. Upon admission, his anticoagulation treatment was switched to unfractionated heparin. However, we resumed warfarin treatment on day 7 with a target PT/INR of 3.0–4.0 on day 7 after finding the suspected LS vegetation on TEE. The PT/INR was >2.0 on day 15, and 3.4–4.6 on days 17–43. On day 43, we also initiated 35 mg/day of prednisolone since TEE on day 42 revealed no reduction in vegetation size. A previous study has shown that the inflammatory reaction in the affected valves can be suppressed by steroids due to the immune-mediated mechanisms in due to the immune-mediated mechanisms in APS [5,6]. Other research has found a combination of low-dose prednisolone, aspirin, and heparin can be of benefit in women with APS refractory to the standard treatment [7]. The prednisolone was reduced to 30 mg/day on day 59, and to 25 mg/day on day 72. The PT/INR was maintained at 2.1–2.8 on days 45–57, and at 3.0–5.0 on days 59–73. Another TEE on day 73 found that there was still no reduction in vegetation size (Figure 2C). As the pharmaceutical approach had produced no improvement, our patient was offered surgical treatment and he consented to mitral valve replacement. His PT/INR was maintained to at 1.9–2.5 until day 130, during which anticoagulation was switched to preoperative unfractionated heparin. Prednisolone was reduced to 17.5 mg/day on day 85, to 10 mg/day on day 98, to 5 mg/day on day 112, to 2.5 mg/day on day 127, and was stopped on day 130. On day 134, mitral valve replacement surgery was performed, providing the patient with a mechanical replacement valve. Pathological examination of the excised vegetation revealed an organized fibrinous thrombus with no neutrophilic infiltration, consistent with LS (Figure 3). In the 3 years since the surgery, warfarin anticoagulation has been maintained, with a target PT/INR of 2.0–3.0, and there has been no stroke recurrence.
Figure 3.
(A) Macroscopy of the mitral vegetation in the tissue sample of the patient. (B) Hematoxylin & eosin stain of the histopathological mitral vegetation tissue showing an organized fibrin thrombus with no neutrophilic infiltration (×20 magnification), consistent with Libman-Sacks endocarditis.
Discussion
In this case, there was recurrence of cerebral infarction after 6 years. We found vegetation on TEE and a reevaluation of the TEE results judged as normal after the first infarction revealed a small mitral vegetation on the same location as the TEE finding at the second episode. We diagnosed the vegetation on the TEE following the second episode as an enlargement of that seen after the first. This was identified as the etiology of both cerebral infarction events due to the multiple vascular lesions in the first episode, the cortical location of the second, and the absence of alternative potential causes. Based on the clinical diagnosis of primary APS and pathological analysis of the mitral valve tissue obtained during the mitral valve replacement, the mitral vegetation was attributed to LS. This enlargement had occurred despite ongoing anticoagulation with warfarin and maintenance of the target PT/INR range, which was appropriate for primary APS. A previous prospective study has described the presence of valvular abnormalities on TEE assessments in patients with primary APS over a 5-year follow-up [3]. The present case report may be the first to describe TEE detection of enlarged LS vegetation, with pathological confirmation in a patient with primary APS and recurrent cerebral infarction who received anticoagulation for primary APS.
LS was first described by Libman and Sacks in 1924, who described this nonbacterial verrucous valvular disease in 4 patients [8,9]. In 1940, Gross went on to show a relationship between SLE and LS [10], and in 1985 D’Alton et al found a further relationship between APS and LS [11]. Subsequent support has been provided for this relationship between APS and LS [12–14]; however, conflicting results from other research have shown no relationship [15].
Although the pathophysiology of LS remains unclear, the deposition of immunoglobulins, including aCL, in valves is known to cause endothelial damage [16]. This can produce a hypercoagulative state conducive to the formation of fibrin clots. The presence of aCL may promote thrombus formation in the endothelia of already damaged valves through the deposition of immune complexes, which causes further valve dysfunction and inflammation [17]. LS is associated with the duration and disease activity of lupus, as well as aCL and APS manifestations [1], and can be the source of cerebral infarctions [2]. The histopathology of LS includes fibroblast hyperplasia, fibrin deposition, angiogenesis, hematoxylin bodies, and nonbacterial inflammation [9,17–19]. This is consistent with our findings in this case.
The EULAR (European League Against Rheumatism) has provided recommendations for the management of APS [20]. This includes treatment with vitamin K antagonists (VKA), and a target PT/INR of 2–3, or 3–4 in patients with definite APS and first arterial thrombosis, with considerations made for the individual’s bleeding risk and the occurrence of recurrent thrombosis. However, the guidelines include no mention of treatment for associated LS. Treatment for primary APS with warfarin and a target PT/INR of 2–3 at the first cerebral infarction was considered appropriate in the present case since the risk for warfarin-related intracerebral hemorrhage is significantly greater in Asians and patients with atrial fibrillation [21]. Additionally, EULAR recommendations agree that an increase of the target PT/INR to 3–4, a PT/INR of 2–3 with the addition of low-dose aspirin, or switching to low-molecular-weight heparin are acceptable options for arterial thrombosis that recurs despite VKA treatment, after allowing for other thrombosis risk factors and ensuring adherence to VKA treatment. In our case, the LS vegetation failed to regress even after implementing high-dose warfarin treatment on day 7 and increasing the PT/INR >3 for 42 days and then >2 for 15 day. Rivaroxaban is contraindicated in patients with triple aPL positivity and arterial events, and direct oral anticoagulants are not recommended in patients with definite APS and arterial events due to the high risk of recurrent thrombosis [20]. Randomized controlled trials are required to establish the effectiveness of hydroxychloroquine for APS thrombosis prevention in SLE [22,23]. Complement inhibition therapy using eculizumab is also a potential therapeutic option for treating recurrent APS thrombosis [24]. Thus, there is no established treatment for LS, and surgical intervention may be required in patients with large mobile vegetations, mitral regurgitation, significant valvular dysfunction, or recurrent embolic events [25,26]. However, cardiac surgery for valve diseases with APS has high morbidity and mortality rates [27]. In our patient, mitral valve replacement was performed due to the enlargement of the mitral vegetation despite appropriate medical treatment for primary APS, and he made good progress in the 3 years since the surgery. Tissue samples taken during surgery provided pathological confirmation of LS. Patients that are positive for all 3 antiphospholipid antibodies (LA, aCL, and anti-β 2GPI) are thought to be at the highest risk of adverse events [22], and both LA and aCL increase the cerebral infarction recurrence risk [28], likely due to the relationship between APS and LS [10,12,13,16]. Therefore, despite anticoagulation, the main cause of the LS enlargement that led to the cerebral infarction recurrence in our patient is likely to have been the persistent primary APS with high titers of all 3 antiphospholipid antibodies. Our case was consistent with the findings of previous prospective studies that have shown anticoagulant and/ or antiplatelet treatment to be ineffective against LS and that there is a correlation between cardiac involvement and high levels of antiphospholipid antibodies [3]. In patients such as ours, with enlarged LS and high titers of all 3 antiphospholipid antibodies despite anticoagulation, surgical intervention should be considered. As previously reported, TEE is valuable both in LS diagnosis and the monitoring of LS for enlargement [3,29].
A limitation of this report was that no antiplatelet drugs were administered, so we were unable to evaluate the effects of antiplatelet drugs on primary APS with LS. Additionally, LS may not always be associated with antiphospholipid antibodies. A previous study found that up to 60% of LS cases occur in the absence of APS [1] and patients with SLE have a similar prevalence and severity of valve disease, regardless of the presence or absence of antiphospholipid antibodies [15]. There has also been a case report of cerebral infarction with LS negative for antiphospholipid antibodies [30], meaning that the mechanisms and optimal medical treatment of LS remain undetermined.
Conclusions
LS, such as in the present patient, with high titers of all 3 antiphospholipid antibodies and cerebral infarction may increase in size despite anticoagulation for primary APS. Therefore, such patients require ongoing follow-up with TEE and head MRI. Surgical treatment should be considered. Further research is required to identify the optimal treatments for LS with high antiphospholipid antibodies antibody titers in patients with recurring thrombosis despite anticoagulation. This should include assessment of the efficacy of hydroxychloroquine and eculizumab as potential treatment options.
Acknowledgments
The authors would like to thank Enago for the English language review.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
Department of Neurology, Nara Medical University, Kashihara, Nara, Japan.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Moyssakis I, Tektonidou MG, Vasilliou VA, et al. Libman-Sacks endocarditis in systemic lupus erythematosus: Prevalence, associations, and evolution. Am J Med. 2007;120:636–42. doi: 10.1016/j.amjmed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Roldan CA, Sibbitt WL, Jr, Qualls CR, et al. Libman-Sacks endocarditis and embolic cerebrovascular disease. JACC Cardiovasc Imaging. 2013;6:973–83. doi: 10.1016/j.jcmg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turiel M, Sarzi-Puttini P, Peretti R, et al. Five-year follow-up by transesophageal echocardiographic studies in primary antiphospholipid syndrome. Am J Cardiol. 2005;96:574–79. doi: 10.1016/j.amjcard.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 5.Nesher G, Ilany J, Rosenmann D, Abraham AS. Valvular dysfunction in antiphospholipid syndrome: Prevalence, clinical features, and treatment. Semin Arthritis Rheum. 1997;27:27–35. doi: 10.1016/s0049-0172(97)80034-0. [DOI] [PubMed] [Google Scholar]
- 6.Atsumi T, Koike T. Cardiac valve diseases and antiphospholipid syndrome. Intern Med. 2000;39:446–47. doi: 10.2169/internalmedicine.39.446. [DOI] [PubMed] [Google Scholar]
- 7.Bramham K, Thomas M, Nelson-Piercy C, et al. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood. 2011;117(25):6948–51. doi: 10.1182/blood-2011-02-339234. [DOI] [PubMed] [Google Scholar]
- 8.Libman E, Sacks B. A hitherto undescribed form of valvular and mural endocarditis. Arch Intern Med. 1924;33:701–7. [Google Scholar]
- 9.Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Revisiting Libman-Sacks endocarditis: A historical review and update. Clin Rev Allergy Immunol. 2009;36:126–30. doi: 10.1007/s12016-008-8113-y. [DOI] [PubMed] [Google Scholar]
- 10.Gross L. The cardiac lesions in Libman-Sacks disease: With a consideration of its relationship to acute diffuse lupus erythematosus. Am J Pathol. 1940;16:375–408. [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alton JG, Preston DN, Bormanis J, et al. Multiple transient ischemic attacks, lupus anticoagulant and verrucous endocarditis. Stroke. 1985;16:512–14. doi: 10.1161/01.str.16.3.512. [DOI] [PubMed] [Google Scholar]
- 12.Khamashta MA, Cervera R, Asherson RA, et al. Association of antibodies against phospholipids with heart valve disease in systemic lupus erythematosus. Lancet. 1990;335:1541–44. doi: 10.1016/0140-6736(90)91373-i. [DOI] [PubMed] [Google Scholar]
- 13.Nihoyannopoulos P, Gomez PM, Joshi J, et al. Cardiac abnormalities in systemic lupus erythematosus. Association with raised anticardiolipin antibodies. Circulation. 1990;82:369–75. doi: 10.1161/01.cir.82.2.369. [DOI] [PubMed] [Google Scholar]
- 14.Djokovic A, Stojanovich L, Stanisavljevic N, et al. Relationship between cerebrovascular and valvular manifestations in a Serbian cohort of patients with antiphospholipid syndrome. Clin Exp Rheumatol. 2018;36:850–55. [PubMed] [Google Scholar]
- 15.Roldan CA, Shively BK, Lau CC, et al. Systemic lupus erythematosus valve disease by transesophageal echocardiography and the role of antiphospholipid antibodies. J Am Coll Cardiol. 1992;20:1127–34. doi: 10.1016/0735-1097(92)90368-w. [DOI] [PubMed] [Google Scholar]
- 16.Ziporen L, Goldberg I, Arad M, et al. Libman-Sacks endocarditis in the antiphospholipid syndrome: Immunopathologic findings in deformed heart valves. Lupus. 1996;5:196–205. doi: 10.1177/096120339600500306. [DOI] [PubMed] [Google Scholar]
- 17.Ye T, Wang J, Liao S. Mitral valve repair for isolated Libman-Sacks endocarditis in a patient with primary antiphospholipid syndrome. Int Heart J. 2021;62:181–85. doi: 10.1536/ihj.20-260. [DOI] [PubMed] [Google Scholar]
- 18.Hojnik M, George J, Ziporen L, Shoenfeld Y. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation. 1996;93:1579–87. doi: 10.1161/01.cir.93.8.1579. [DOI] [PubMed] [Google Scholar]
- 19.Bouma W, Klinkenberg TJ, van der Horst IC, et al. Mitral valve surgery for mitral regurgitation caused by Libman-Sacks endocarditis: A report of four cases and a systematic review of the literature. J Cardiothorac Surg. 2010;5:13. doi: 10.1186/1749-8090-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296–304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–15. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 22.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378:2010–21. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 23.Panichpisal K, Rozner E, Levine SR. The management of stroke in antiphospholipid syndrome. Curr Rheumatol Rep. 2012;14:99–106. doi: 10.1007/s11926-011-0223-5. [DOI] [PubMed] [Google Scholar]
- 24.Hussain H, Tarantino MD, Chaturvedi S, et al. Eculizumab for refractory thrombosis in antiphospholipid syndrome. Blood Adv. 2022;6:1271–77. doi: 10.1182/bloodadvances.2021005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinstein AA, Giovanelli C, Romano JG, et al. Surgical treatment of nonbacterial thrombotic endocarditis presenting with stroke. J Neurol. 2005;252:352–55. doi: 10.1007/s00415-005-0660-z. [DOI] [PubMed] [Google Scholar]
- 26.Akhlaq A, Ali TA, Fatimi SH. Mitral valve replacement in systemic lupus erythematosus associated Libman-Sacks endocarditis. J Saudi Heart Assoc. 2016;28:124–26. doi: 10.1016/j.jsha.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colli A, Mestres CA, Espinosa G, et al. Heart valve surgery in patients with the antiphospholipid syndrome: Analysis of a series of nine cases. Eur J Cardiothorac Surg. 2010;37:154–58. doi: 10.1016/j.ejcts.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Brey RL, Chapman J, Levine SR, et al. Stroke and the antiphospholipid syndrome: Consensus meeting Taormina 2002. Lupus. 2003;12:508–13. doi: 10.1191/0961203303lu390oa. [DOI] [PubMed] [Google Scholar]
- 29.Turiel M, Muzzupappa S, Gottardi B, et al. Evaluation of cardiac abnormalities and embolic sources in primary antiphospholipid syndrome by transesophageal echocardiography. Lupus. 2000;9:406–12. doi: 10.1191/096120300678828532. [DOI] [PubMed] [Google Scholar]
- 30.Krawczyk M, Budhram A, Sposato LA. Ischemic stroke from Libman-Sacks endocarditis not associated with antiphospholipid antibodies: Good clinical outcome without anticoagulation. JACC Case Rep. 2019;1:297–300. doi: 10.1016/j.jaccas.2019.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]