Abstract
Introduction
Postoperative antibiotic treatment is indicated for 3–5 days following appendectomy for complex appendicitis. However, meeting discharge criteria may allow for safe discontinuation of antibiotics and discharge. This study assessed the association between time to reach discharge criteria and duration of postoperative antibiotic use and length of stay.
Methods
This is a multicenter retrospective cohort study including patients who underwent appendectomy for complex appendicitis and received postoperative antibiotics for >24 h. Main outcome measures were time to reach discharge criteria, duration of postoperative antibiotic use, length of hospital stay, and postoperative infectious complications. Discharge criteria were defined as absence of fever (temperature ≤38°C) for 24 h, ability to tolerate oral intake, and pain controlled by oral analgesics.
Results
Between May 2014 and January 2015, 124 patients were included. Time to reach discharge criteria was 2 days (interquartile range [IQR] 1–3). Patients received postoperative antibiotics and were in hospital for a median of 5 (IQR 3–5) and 5 (IQR 4–6) days, respectively. Infectious complications occurred in 12% and did not differ between patients reaching discharge criteria before or after 2 postoperative days.
Discussion
Discharge criteria were met by a median of 2 days after appendectomy for complex appendicitis. This suggests that postoperative antibiotics duration and thereby hospital stay can be reduced. In daily practice, prescribed antibiotics are not reduced in total days given. Prospective studies that evaluate limited postoperative antibiotic use, based on these criteria, are necessary.
Keywords: Appendectomy, Antibiotics, Discharge, Postoperative day
Introduction
Acute appendicitis is a common disease. Appendicitis can be classified as simple (nonperforated, phlegmonous inflammation) or complex (necrosis, perforation, and/or abscess) [1]. Complex appendicitis is associated with a higher rate of infectious complications compared to simple appendicitis [2, 3, 4]. To treat secondary peritonitis and reduce infectious complications, guidelines recommend postoperative intravenous (IV) antibiotics (ABs) in patients with complex appendicitis [5, 6, 7, 8, 9]. The optimal duration of AB treatment remains a topic of debate [2, 3, 10, 11, 12, 13, 14, 15]. Prolonged and ineffective use of antimicrobials is associated with the development of bacterial resistance [16]. Therefore, it is important to define the optimal postoperative AB regimen for complex appendicitis. Furthermore, improving treatment efficiency by reducing postoperative length of stay may reduce healthcare costs and improve treatment satisfaction. Reducing length of hospital stay has become all the more relevant in light of the COVID pandemic.
The recently updated Dutch guideline on acute appendicitis recommends a minimum of 3 days of postoperative ABs for complex appendicitis, starting with IV administration. In daily practice, ABs are often given for 5 postoperative days (PODs) [6, 15]. The guideline does not specifically address the duration of IV treatment. Recent studies reported on criteria that need to be met for a patient before discharge from the hospital [12, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27]. These discharge criteria include absence of fever (for 12 or 24 h), ability to tolerate oral intake, adequate pain control with oral analgesics, and a normal white blood cell (WBC) count. Some studies validated these discharge criteria [12, 20, 21, 23]. Meeting these discharge criteria may also allow for discontinuation of (IV) ABs. In most studies, only children were included and ABs were continued with an oral prescription. The complication rates for children discharged from hospital after meeting discharge criteria were similar to a historical control group receiving a pre-defined duration of postoperative ABs [20, 21, 22, 23]. Only 2 studies included adult patients, and one of them used discharge criteria but only after 3 days of postoperative AB treatment [12, 25].
The aim of this study was to assess the association between time to reach discharge criteria and length of administration of ABs in pediatric and adult patients with a complex appendicitis. We hypothesized that the use of discharge criteria may reduce the duration of postoperative ABs and length of stay.
Methods
All consecutive patients who underwent an appendectomy in 6 teaching hospitals in the Southwest of the Netherlands (1 academic center, 5 teaching hospitals) from June 2014 to January 2015 were selected from the hospital administration databases. This patient population was previously described in a study on risk factors for surgical site infections (SSIs) [4]. For the present study, patients were included if they had a complex appendicitis and received postoperative ABs for at least 24 h. Complex appendicitis was defined as a gangrenous or perforated appendicitis (including iatrogenic perforation) or appendicitis in the presence of purulent peritonitis or abscess (based on the operative notes of the surgeon). Exclusion criteria were interval appendectomy (appendectomy after resolution of the acute inflammation) or appendectomy for an indication other than acute appendicitis (e.g., chronic appendicitis, suspected malignancy).
All patients were treated according to the Dutch guideline on acute appendicitis, published in 2010 [5]. This includes administration of preoperative AB prophylaxis within 30 min before skin incision followed by 3–7 days of postoperative ABs in patients with a complex appendicitis. Most patients received cefuroxime and metronidazole as postoperative regime. In the preoperative setting, cefazolin and metronidazole were given. Operative approach depended on the preference of the surgeon. This study has been approved by the Institutional Review Board (Medical Ethics Committee) of the Erasmus Medical Center.
Data were extracted from electronic patient charts, including operation and pathology reports. Pre- and intraoperative data included sex, age, American Society of Anesthesiologist (ASA) classification, and operative approach (laparoscopic or open appendectomy). Laparoscopic operations that were converted to open were classified as open appendectomy. Collected postoperative data included daily body temperature, type of pain medication, ability to tolerate oral intake, duration and route of administration of postoperative ABs, length of hospital stay, and postoperative complications (including superficial SSI, intra-abdominal abscess [IAA], pneumonia, urinary tract infection, cardiovascular, ileus, bleeding, and mortality). Data on WBC count and C-reactive protein were not retrieved. Routine blood tests for C-reactive protein and WBC are not recommended in the Dutch guidelines. The severity of the complications was assessed using the Clavien-Dindo classification (grade I-V) [28].
Outcome Measures
The two main outcome measures were time to reach discharge criteria (in PODs) and postoperative infectious complications. In this study, we defined discharge criteria as (1) absence of fever (temperature ≤38°C) for 24 h, (2) ability to tolerate oral intake, and (3) pain controlled by oral analgesics. Since body temperature is often measured more than once a day, the highest temperature reported on that day was used. Patients were considered able to tolerate oral intake when explicitly reported in the patient's notes or when the patient did not report feeling nausea and the patient did not vomit and when there was no nasogastric tube in situ. Infectious complications included IAA and SSI, defined according to the Center for Disease Control (CDC) criteria (29). An IAA is an infection that involves the abdominal part of the body deeper than the fascial/muscle layers that is opened or manipulated during the operative procedure. IAA can be diagnosed through imaging or during reintervention, through purulent drainage from a drain placed into the IAA, or isolation of organisms from a culture of the IAA [29]. SSI can be either deep or superficial, involving the skin, subcutaneous tissue, and/or deep soft tissues at the site of the incision. Infectious complications were defined as IAA and SSI together.
Statistical Analysis
Infectious complication rates were compared between patients that met discharge criteria within 2 days after appendectomy and patients that did not. The cut-off at 2 days was chosen based on the recommendation by the Dutch Association of Surgeons Guideline Committee (which applied during the study period) being 48 h of postoperative IV AB treatment [5]. For assessing the relationship between variables and study outcomes, the independent Student's T test or the Mann-Whitney test was used in case of continuous outcome variables and the χ2 or Fisher's exact test in case of categorical outcome variables, as appropriate. A value of p < 0.05 was considered statistically significant. All analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp).
Results
Some 637 patients with acute appendicitis were identified from the multi-institutional database [4]. After exclusion of patients without complex appendicitis (n = 453), administration of <24 h of AB (n = 31), and excluding patients from one hospital where registration was no longer possible (n = 29), a total of 124 patients were included. Baseline characteristics are shown in Table 1. The median (interquartile range [IQR]) time to reach discharge criteria was 2 (1–3) days. Sixty-seven percent of all patients met discharge criteria within 2 days after surgery (Table 2). Infectious complications occurred in 15 patients (12%). Patients were given postoperative ABs for a median (IQR) duration of 5 (3–5) days.
Table 1.
Baseline characteristics of the study group (n = 124)
| Male sex, n (%) | 75 (61) |
| Age, median (IQR), years | 36 (22; 55.75) |
| ASA score, n (%) | |
| ASA I | 85 (69) |
| ASA II | 33 (27) |
| ASA III | 6 (5) |
| Surgical approach, n (%) | |
| Laparoscopic | 112 (90) |
| Open | 12 (10) |
| Type of appendicitis, n (%) | |
| Gangrenous (nonperforated) | 35 (28) |
| Perforated | 75 (60) |
| Iatrogenic perforation | 14 (11) |
| Prescribed AB duration, median (IQR)*, days | 5 (3; 5) |
| Prescribed AB duration, n (%)* | |
| <3 days | 5 (4) |
| 3 days | 53 (43) |
| 5 days | 60 (49) |
| >5 days | 5 (4) |
IQR, interquartile range; ASA, American Society of Anesthesiologists; AB, antibiotic(s). * 1 missing.
Table 2.
Postoperative outcomes (n = 124)
| Time to reach discharge criteria, median (IQR), days | 2 (1–3) |
| Time between discharge criteria and length of ABs treatment, median (IQR)*, days | 2 (1–4) |
| Time between discharge criteria and length of stay, median (IQR)*, days | 3 (2–4) |
| Cumulative no. of patients having met discharge criteria, n (%)* | |
| POD 1 | 56 (46) |
| POD 2 | 81 (67) |
| POD 3 | 98 (81) |
| POD 4 | 111 (92) |
| POD 5 | 117 (97) |
| >POD 5 | 121 (100) |
| Received AB duration, median (IQR), days | 5 (3; 5) |
| Received AB duration in days, n (%) | |
| 2 days | 2 (2) |
| 3 days | 34 (27) |
| 4 days | 2 (2) |
| 5 days | 64 (52) |
| >5 days | 22 (17) |
| Deviation from prescribed AB duration, n (%)** | 44 (33) |
| Reduced | 3 (2) |
| Prolonged | 40 (33) |
| Route of administration, n (%)*** | |
| IV only | 80 (65) |
| IV/oral combination (IV/PO) | 36 (29) |
| Length of stay, median (IQR), days | 5 (4; 6) |
| Any postoperative complication, n (%) | 26 (21) |
| Infectious complications, n (%) | 15 (12) |
| IAA, n (%) | 14 (11) |
| SSI, n (%) | 1 (1) |
POD, postoperative day; AB, antibiotic(s); IQR, interquartile range; IAA, intra-abdominal abscess; SSI, surgical site infection; IV, intravenous.
3 missing,
1 missing,
8 missing.
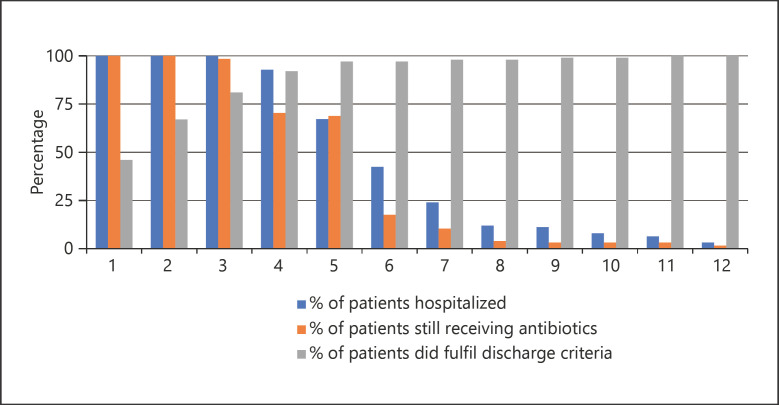
Continuation of ABs after meeting discharge criteria occurred in 103 of 124 patients (83%). Figure 1 shows the percentage of patients hospitalized, patients receiving ABs and patients having met discharge criteria for each POD over time. On POD 3, 81% of all patients had met all discharge criteria, yet on POD 4 70% of patients still received ABs and 92% were still hospitalized.
Fig. 1.
Percentage of patients hospitalized (blue), patients receiving antibiotics (orange), and patients having met discharge criteria (gray) for each POD over time.
Table 3 shows characteristics and outcomes for patients that met the discharge criteria on POD 2 versus POD 3 or later. The percentage of infectious complications was 12% in patients that met discharge criteria before or on POD 2 versus 15% for patients that did not (p = 0.433).
Table 3.
Comparison of patients meeting discharge criteria on POD 2 and after POD 2 (n= 121)*
| Variable | ≤POD2 (n = 81) | >POD2 (n = 40) | p value |
|---|---|---|---|
| Male sex, n (%) | 53 (65) | 20 (50) | 0.103 |
| Age, median (IQR), years | 36 (21; 55) | 36 (22.25; 54.75) | 0.798 |
| Laparoscopic approach, n (%) | 71 (88) | 38 (95) | 0.203 |
| Length of stay, median (IQR), days | 5 (4; 6) | 6 (5; 8.5) | 0.000 |
| Received AB duration, median (IQR), days | 5 (3; 5) | 5 (4.25; 5) | 0.311 |
| Any postoperative complication, n (%) | 12 (15) | 13 (33) | 0.024 |
| Infectious complication, n (%) | 9 (11) | 6 (15) | 0.541 |
| IAA, n (%) | 8 (10) | 6 (15) | 0.407 |
| SSI, n (%) | 1 (1) | 0 | 0.480 |
POD, postoperative day; IQR, interquartile range; AB, antibiotic(s); IAA, intra-abdominal abscess; SSI, surgical site infection; Ns, nonsignificant.
3 missing.
Table 4 shows characteristics and outcomes for patients according to duration of AB treatment. There was a discrepancy between the duration of ABs prescribed and administered in 43 of 123 patients (35%). On the one hand, patients received ABs for a median (IQR) of 5.5 (5–7) days, while the prescribed duration was for a median (IQR) of 3 (3–5) days. On the other hand, 3 of 123 patients (2.4%) were prescribed ABs for a median of 5 days but received ABs for a median (IQR) of 3 (3 to −) days. The patients that received a shorter course of ABs did not have more complications. In 14 patients, an IAA occurred, and no differences in duration of achieving the several discharge criteria, total duration of postoperative AB treatment, and other risk factors like age, sex, and operative approach (laparoscopic or open appendectomy) were found in this group.
Table 4.
Comparison of patients receiving ABs for prolonged, per protocol or reduced duration (n = 123)*
| Variable | Prolonged (n = 40) | Per protocol (n = 80) | Reduced (n = 3) | p value |
|---|---|---|---|---|
| Prescribed AB duration, median (IQR), days | 3 (3; 5) | 5 (3; 5) | 5 (5;–) | 0.000 |
| Received AB duration, median (IQR), days | 5.5 (5; 7) | 5 (3; 5) | 3 (3;–) | 0.000 |
| Discharge reached, median (IQR) | 2 (1; 3) | 2 (1; 3)** | 2 (1;–) | 0.889 |
| Length of stay, median (IQR), days | 5 (4; 7) | 5 (4; 6) | 4 (3;–) | 0.831 |
| Any postoperative complication, n (%) | 7 (18) | 18 (23) | 1 (33) | 0.714 |
| Infectious complication, n (%) | 4 (10) | 11 (14) | 0 | 0.678 |
| IAA, n (%) | 3 (8) | 11 (14) | 0 | 0.490 |
| SSI, n (%) | 1 (3) | 0 | 0 | 0.351 |
IQR, interquartile range; AB, antibiotic(s); IAA, intra-abdominal abscess; SSI, surgical site infection; Ns, nonsignificant.
1 missing,
3 missing.
Discussion
Previous studies have shown that criteria can be used to safely discharge children after appendectomy for (complex) appendicitis and that stopping ABs is safe when these criteria are met [12, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27]. The present study shows that discharge criteria are reached after a median of 2 days in patients who underwent appendectomy for complex appendicitis. This is much earlier than the actual discharge after a median of 5 days likely explained by continuation of (IV) ABs in line with the Dutch guideline [5, 6, 15]. The Dutch guideline recommends 3–5 days of postoperative AB treatment irrespective of clinical status of the patient. Hence, applying discharge criteria in daily practice as a way to reduce postoperative AB use may be feasible as postoperative infectious complications were not different between the groups. This policy could result in a substantial reduction of AB use and earlier discharge. In a time where AB overuse and resistance is a well-recognized issue, restriction of AB treatment is warranted. Furthermore, use of these discharge criteria may lead to reduction of hospital stay and savings in hospital costs in the future.
This is the first study to demonstrate a potential reduction in median AB treatment duration and hospital stay of 2 and 3 days, respectively. Previous studies in children showed reduced hospital stay by 1.5 day at most [22, 23]. Complications occurred in 21% of patients after appendectomy for complex appendicitis, which is in line with another study [2]. IAA is the most common complication [3, 21, 30, 31, 32, 33, 34, 35, 36], seen in 11% of patients in the present study. Risk factors for these complications have been identified [2, 3, 4]. Duration of postoperative AB treatment was not an independent factor affecting complication rate in most studies. Hence, the present study suggests that AB use could be reduced and patients can indeed be discharged safely before POD 3. However, it may well be that continuation of postoperative ABs in patients that reached discharge criteria on POD 2 was indicated based on judgment by the surgeon. Prolongation of ABs beyond 2 days may have prevented infectious complications. However, a higher incidence of postoperative complications was seen in patients that did not meet discharge criteria within 2 days compared to patients that did. This implies that discharge criteria, if not met within 2 days, indicate a certain risk for developing of a postoperative complication. Yet, it remains unclear whether prolonging/continuing AB treatment until criteria are met can prevent complications. However, it seems reasonable to keep these patients admitted for continued observation and consider additional laboratory tests and/or imaging studies.
The main limitation of this study is the retrospective design. The true individual reasons for prolongation or reducing AB treatment (compared to the prescribed duration) are not clear, and the clinical judgment on patient's well-being could have played a role. In addition, it is unclear for what reasons patients remained hospitalized when their condition according to the criteria would allow discharge. Maybe discharge criteria were not used in clinical practice to guide termination of ABs. Thirty-five percent of all patients did not receive the prescribed duration of ABs. Nevertheless, for the patients that received ABs as per protocol, most met discharge criteria well prior to AB treatment cessation. And in only 3 patients AB treatment was stopped before the aforementioned fixed duration. Due to the small study population, no definite conclusions can be drawn from these results.
Conclusion
The present results suggest that AB use and length of hospital stay after appendectomy for complex appendicitis can be reduced. Prospective studies that evaluate limited postoperative AB use are necessary to confirm that the benefits outweigh a possible harm.
Statement of Ethics
This retrospective study was approved by the Local Medical Ethical Committee of the Erasmus MC (Registration No. MEC-2021-0013), and in this specific study, the Ethical Committee approved the lack of informed consent from the patients.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received for this study.
Author Contributions
Anne Loes van den Boom initiated this study. Anne Loes van den Boom, Louis Giesen, and Charles van Rossem collected data. Anne Loes van den Boom, Elisabeth de Wijkerslooth, and Louis Giesen performed data analysis and drafted the manuscript. Anne Loes van den Boom, Louis Giesen, Elisabeth de Wijkerslooth, Charles van Rossem, Boudewijn Toorenvliet, and Bas Wijnhoven read and revised the manuscript and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Funding Statement
No funding was received for this study.
References
- 1.Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT. Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet. 2015;386((10000)):1278–1287. doi: 10.1016/S0140-6736(15)00275-5. [DOI] [PubMed] [Google Scholar]
- 2.van Rossem CC, Schreinemacher MHF, van Geloven AAW, Bemelman WA, Snapshot Appendicitis Collaborative Study Group Antibiotic duration after laparoscopic appendectomy for acute complicated appendicitis. JAMA Surg. 2016;151((4)):323–329. doi: 10.1001/jamasurg.2015.4236. [DOI] [PubMed] [Google Scholar]
- 3.Van Rossem CC, Schreinemacher MHF, Treskes K, Van Hogezand RM, Van Geloven AAW. Duration of antibiotic treatment after appendicectomy for acute complicated appendicitis. Br J Surg. 2014;101((6)):715–719. doi: 10.1002/bjs.9481. [DOI] [PubMed] [Google Scholar]
- 4.Giesen LJX, van den Boom AL, van Rossem CC, den Hoed PT, Wijnhoven BPL. Retrospective multicenter study on risk factors for surgical site infections after appendectomy for acute appendicitis. Dig Surg. 2017;34((2)):103–107. doi: 10.1159/000447647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nederlandse Vereniging voor Heelkunde (NVvH; Dutch Surgical Association) Richtlijn voor diagnostiek en behandeling van acute appendicitis (Guideline on acute appendicitis diagnostics and treatment) 2010.
- 6.Nederlandse Vereniging voor Heelkunde (NVvH; Dutch Surgical Association) Richtlijn voor diagnostiek en behandeling van acute appendicitis (Guideline on acute appendicitis diagnostics and treatment) 2019.
- 7.Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, et al. The surgical infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt) 2017;18((1)):1–76. doi: 10.1089/sur.2016.261. [DOI] [PubMed] [Google Scholar]
- 8.Stichting Werkgroep Antibiotica Beleid (SWAB; Dutch Working Party on Antibiotic Policy) 2015 Peritonitis: secondary. Available from: https://adult.swabid.nl/nl/node/7369.
- 9.Di Saverio S, Birindelli A, Kelly MD, Catena F, Weber DG, Sartelli M, et al. WSES Jerusalem guidelines for diagnosis and treatment of acute appendicitis. World J Emerg Surg. 2016;11:34. doi: 10.1186/s13017-016-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Boom AL, de Wijkerslooth EML, van Rosmalen J, Beverdam FH, Boerma EJG, Boermeester MA, et al. Two versus five days of antibiotics after appendectomy for complex acute appendicitis (APPIC): study protocol for a randomized controlled trial. Trials. 2018;19((1)):263. doi: 10.1186/s13063-018-2629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llewelyn MJ, Fitzpatrick JM, Darwin E, SarahTonkin C, Gorton C, Paul J, et al. The antibiotic course has had its day. BMJ. 2017;358:j3418. doi: 10.1136/bmj.j3418. [DOI] [PubMed] [Google Scholar]
- 12.Taylor E, Dev V, Shah D, Festekjian J, Gaw F. Complicated appendicitis: is there a minimum intravenous antibiotic requirement? A prospective randomized trial. Am Surg. 2000;66((9)):887–890. [PubMed] [Google Scholar]
- 13.Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372((21)):1996–2005. doi: 10.1056/NEJMoa1411162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Boom AL, de Wijkerslooth EML, Wijnhoven BPL. Systematic review and meta-analysis of postoperative antibiotics for patients with a complex appendicitis. Dig Surg. 2020;37((2)):101–110. doi: 10.1159/000497482. [DOI] [PubMed] [Google Scholar]
- 15.van den Boom AL, de Wijkerslooth EML, Mauff KAL, Dawson I, van Rossem CC, Toorenvliet BR, et al. Interobserver variability in the classification of appendicitis during laparoscopy. Br J Surg. 2018;105((8)):1014–1019. doi: 10.1002/bjs.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Antimicrobial resistance: global report on surveillance. 2014. Available from: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf.
- 17.Emil S, Laberge JM, Mikhail P, Baican L, Flageole H, Nguyen L, et al. Appendicitis in children: a ten-year update of therapeutic recommendations. J Pediatr Surg. 2003;38((2)):236–242. doi: 10.1053/jpsu.2003.50052. [DOI] [PubMed] [Google Scholar]
- 18.Hoelzer DJ, Zabel DD, Zern JT. Determining duration of antibiotic use in children with complicated appendicitis. Pediatr Infect Dis J. 1999;18((11)):979–982. doi: 10.1097/00006454-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Keller MS, McBride WJ, Vane DW. Management of complicated appendicitis: a rational approach based on clinical course. Arch Surg. 1996;131((3)):261–264. doi: 10.1001/archsurg.1996.01430150039006. [DOI] [PubMed] [Google Scholar]
- 20.Fraser JD, Aguayo P, Leys CM, Keckler SJ, Newland JG, Sharp SW, et al. A complete course of intravenous antibiotics vs a combination of intravenous and oral antibiotics for perforated appendicitis in children: a prospective, randomized trial. J Pediatr Surg. 2010;45((6)):1198–1202. doi: 10.1016/j.jpedsurg.2010.02.090. [DOI] [PubMed] [Google Scholar]
- 21.Henry MCW, Walker A, Silverman BL, Gollin G, Islam S, Sylvester K, et al. Risk factors for the development of abdominal abscess following operation for perforated appendicitis in children: a multicenter case-control study. Arch Surg. 2007;142((3)):236–241. doi: 10.1001/archsurg.142.3.236. discussion 241. [DOI] [PubMed] [Google Scholar]
- 22.Skarda DE, Schall K, Rollins M, Andrews S, Olson J, Greene T, et al. Response-based therapy for ruptured appendicitis reduces resource utilization. J Pediatr Surg. 2014;49((12)):1726–1729. doi: 10.1016/j.jpedsurg.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Yu TC, Hamill JKM, Evans SM, Price NR, Morreau PN, Upadhyay VA, et al. Duration of postoperative intravenous antibiotics in childhood complicated appendicitis: a propensity score-matched comparison study. Eur J Pediatr Surg. 2014;24((4)):341–349. doi: 10.1055/s-0033-1349055. [DOI] [PubMed] [Google Scholar]
- 24.Fallon SC, Brandt ML, Hassan S, Wesson DE, Rodriguez JR, Lopez ME. Evaluating the effectiveness of a discharge protocol for advanced appendicitis at a children's hospital. J Surg Res. 2013;179((2)):221–222. doi: 10.1016/j.jss.2013.04.081. [DOI] [PubMed] [Google Scholar]
- 25.Basoli A, Chirletti P, Cirino E, D'Ovidio NG, Doglietto GB, Giglio D, et al. A prospective, double-blind, multicenter, randomized trial comparing ertapenem 3 vs >or=5 days in community-acquired intraabdominal infection. J Gastrointest Surg. 2008;12((3)):592–600. doi: 10.1007/s11605-007-0277-x. [DOI] [PubMed] [Google Scholar]
- 26.van Wijck K, de Jong JR, van Heurn LWE, van der Zee DC. Prolonged antibiotic treatment does not prevent intra-abdominal abscesses in perforated appendicitis. World J Surg. 2010;34((12)):3049–3053. doi: 10.1007/s00268-010-0767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunningham ME, Zhu H, Hoch CT, DeMello AS, Gusman ND, Fallon SC, et al. Effectiveness of a clinical pathway for pediatric complex appendicitis based on antibiotic stewardship principles. J Pediatr Surg. 2020;55((6)):1026–1031. doi: 10.1016/j.jpedsurg.2020.02.045. [DOI] [PubMed] [Google Scholar]
- 28.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250((2)):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 29.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36((5)):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Kelly KN, Fleming FJ, Aquina CT, Probst CP, Noyes K, Pegoli W, et al. Disease severity, not operative approach, drives organ space infection after pediatric appendectomy. Ann Surg. 2014;260((3)):466–471. doi: 10.1097/SLA.0000000000000874. discussion 472–3. [DOI] [PubMed] [Google Scholar]
- 31.Nataraja RM, Teague WJ, Galea J, Moore L, Haddad MJ, Tsang T, et al. Comparison of intraabdominal abscess formation after laparoscopic and open appendicectomies in children. J Pediatr Surg. 2012;47((2)):317–321. doi: 10.1016/j.jpedsurg.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 32.St Peter SD, Little DC, Calkins CM, Murphy JP, Andrews WS, Holcomb GW, 3rd, et al. A simple and more cost-effective antibiotic regimen for perforated appendicitis. J Pediatr Surg. 2006;41((5)):1020–1024. doi: 10.1016/j.jpedsurg.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 33.St Peter SD, Tsao K, Spilde TL, Holcomb GW, 3rd, Sharp SW, Murphy JP, et al. Single daily dosing ceftriaxone and metronidazole vs standard triple antibiotic regimen for perforated appendicitis in children: a prospective randomized trial. J Pediatr Surg. 2008;43((6)):981–985. doi: 10.1016/j.jpedsurg.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holcomb GW, 3rd, St Peter SD. Current management of complicated appendicitis in children. Eur J Pediatr Surg. 2012;22((3)):207–212. doi: 10.1055/s-0032-1320016. [DOI] [PubMed] [Google Scholar]
- 35.Vahdad MR, Troebs RB, Nissen M, Burkhardt LB, Hardwig S, Cernaianu G. Laparoscopic appendectomy for perforated appendicitis in children has complication rates comparable with those of open appendectomy. J Pediatr Surg. 2013;48((3)):555–561. doi: 10.1016/j.jpedsurg.2012.07.066. [DOI] [PubMed] [Google Scholar]
- 36.Emil S, Elkady S, Shbat L, Youssef F, Baird R, Laberge JM, et al. Determinants of postoperative abscess occurrence and percutaneous drainage in children with perforated appendicitis. Pediatr Surg Int. 2014;30((12)):1265–1271. doi: 10.1007/s00383-014-3617-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.