Abstract
Background
Netherton syndrome (NS) is a rare potential life-threatening disorder that causes severe defects to the skin barrier. No effective treatment options are available for patients with NS and current therapy is mostly supportive. The effects of intravenous immunoglobulins (IVIGs), ixekizumab, and dupilumab have scarcely been reported. Additionally, the role of anakinra in patients with NS has never been investigated.
Objectives
The objective was to report our experiences of treatment with IVIG, ixekizumab, dupilumab, and anakinra in patients with NS.
Methods
A retrospective case series, including 5 patients with NS, was performed in a tertiary referral hospital between 2016 and 2021. Patients were treated with IVIG, ixekizumab, dupilumab, and/or anakinra. Long-term experiences with treatment regimens and adverse events requiring medical attention were reported.
Results
IVIG, ixekizumab, dupilumab, and anakinra were well tolerated with no severe adverse events. The 2 patients that received IVIG showed clinical response for 6 months and 2.5 years. Ixekizumab was effective in 1 of our patients for 3.5 years, while in another patient ixekizumab lost its effect after 1.5 years. Dupilumab treatment did not result in persistent improvement of NS-related skin symptoms in 1 patient. Anakinra showed physician-assessed clinical response during the first months of treatment in 4 patients with NS. During anakinra treatment, no changes in blood levels of IL-1β, IL-6, and TNF-α levels were measured at routine blood examinations.
Conclusions
This case series suggests that the use of IVIG, ixekizumab, dupilumab, and anakinra in NS is safe and moderately effective on the short term. On the long term, a decline in effect was observed. Our experiences may help clinicians and researchers to provide adequate care and develop treatment for these severely affected patients. More international research, especially on the long term, is needed to determine if and which patients benefit most from the emerging therapies for NS.
Keywords: Netherton syndrome, Intravenous immunoglobulins, Ixekizumab, Dupilumab, Anakinra
Introduction
Netherton syndrome (OMIM #256500) (NS) is a rare genetic disorder caused by an autosomal recessive mutation in the serine peptidase inhibitor Kazal type 5 (SPINK5) gene [1, 2]. Pathogenic variants in SPINK 5 are associated with a defective lymphoepithelial Kazal-type-related inhibitor (LEKTI). The loss of LEKTI results in uninhibited activity of epidermal proteases causing severe skin barrier impairment in NS patients [2, 3]. Typical features associated with NS include congenital potential life-threatening erythroderma evolving in ichthyosis linearis circumflexa alternated with erythroderma, hair shaft abnormalities, and the atopic syndrome. Secondary skin inflammation is associated with overexpression of the T helper type 17 (Th17) pathway and IL-1β [4, 5, 6]. Moreover, patients with NS suffer from immunodeficiency secondary to a skin barrier defect [4], resulting in increased susceptibility to skin infections.
Currently, there are no registered effective treatment options available for patients with NS and treatment is mainly supportive. Current treatment options include topical corticosteroids, topical calcineurin inhibitors [7], retinoids [8], and narrowband ultraviolet B phototherapy [9]. Recent reports have described treatment of severe NS cases with biologicals targeting specific pro-inflammatory cytokines or immunoglobulins such as IL-4/IL-13, IL-17, TNF-α, IL-12/IL-23, IgE (dupilumab, infliximab, ustekinumab, secukinumab, and omalizumab) [10, 11, 12, 13, 14, 15, 16, 17]. These treatments have been shown to be effective in small case numbers [18]. However, most studies only describe the effects of these treatments on the short term. More evidence on the efficacy of these therapies, also on the long term, is required to develop treatment guidelines for NS.
In addition, the effects of other therapies should be further explored. Based on their mechanisms of action, intravenous immunoglobulins (IVIGs) and anakinra, specifically targeting the IL-1 pathway, could be of potential interest.
Immunoglobulins derived from human plasma donors have been used for treatment of a range of inborn errors of immunity and chronic inflammatory disorders [19, 20]. The role of IVIG extends to more than the mere replacement of antibodies [21]. More evidence emerges that IVIG may have an important role in immune modulation. In mice, IVIG induces expansion of FoxP3+ regulatory T cells, while downregulating the Th17 pathway [22]. In NS, treatment with IVIG may reduce inflammation by downregulating type 17 inflammation and restore immune homeostasis.
Anakinra is a human IL-1 receptor antagonist, used for treatment of auto-inflammatory conditions. In the skin of Cdsniep−/− mice (in which disruption of the stratum corneum can be induced to cause epidermal barrier defects), anakinra caused blockage of IL-1 and suppression of the Th2 and Th17 cytokines [23]. In addition, blockage of IL-1β prevents secretion of thymic stromal lymphopoietin, an IL-7-like cytokine, via NF-kB. This prevents reduction of filaggrin expression, an important protein for epidermal homeostasis [24]. Furthermore, IL-1β levels are elevated in skin biopsies from patients with NS when compared to healthy individuals [5]. Based on the mode of action and its safety profile, anakinra could also be of interest in treatment of NS. In this case series, we describe our experiences with IVIGs, ixekizumab, dupilumab, and, to our knowledge, for the first-time treatment with anakinra in the treatment of 5 patients with severe NS symptoms.
Materials and Methods
A retrospective case series of 5 patients with severe NS receiving systemic therapy, part of our national NS cohort (total of 21 patients), was conducted between 2016 and 2021 at the Erasmus MC University Medical Center Rotterdam-Sophia Children's Hospital, the National Expert Center for NS acknowledged by the Ministry of Health and part of the European Reference Network SKIN (https://ern-skin.eu). The members of the NS expert team that have taken care of these patients consist of a dermatologist, clinical immunologist, pediatrician, and pediatric psychologist. This study was approved by the Medical Ethical Committee (METC) of the Erasmus MC University Medical Center (MEC-2020-0447), the Netherlands. Written informed consent was obtained from all patients and/or parents.
During regular care visits at our outpatient clinic effects, adverse events of IVIG, ixekizumab, dupilumab, and anakinra were assessed and recorded in the electronic patient record. Additionally, standardized photographs were taken by a medical photographer and blood tests were performed. The Index for Ichthyosis Severity (VIIS) was assessed on these photographs [25]. During anakinra treatment, blood levels of IL-1β, IL-6, and TNF-α levels were measured at routine blood examinations.
Results
Details of Study Population
A total of 5 patients (mean age 29.6 years [SD: 16.6]; 1 man and 4 women) were studied (Table 1). In all patients, NS was confirmed by genetic analysis and patients were treated at the Erasmus MC University Medical Center. All patients had previously undergone unsuccessful treatments with topical corticosteroids, antibiotics, and prednisone. All patients suffered from erythroderma, combined with ichthyosis linearis circumflexa, before start of treatment with IVIG, ixekizumab, dupilumab, and anakinra. Concomitant systemic and topical therapies were continued in all cases (Table 1). A summary of the given treatments can be found in Table 2.
Table 1.
Patient characteristics
| Patient | Age, years | Gender | Previous therapies | Concomitant therapies |
|---|---|---|---|---|
| 1 | 25 | Female | Glucocorticoids (topical and systemic), antibiotics (silver sulfadiazine, clarithromycin, amoxicillin, doxycycline, clindamycin), pimecrolimus, emollients | Glucocorticoids (topical), doxycycline, zincoxide, desloratadine, emollients |
|
| ||||
| 2 | 25 | Male | Glucocorticoids (topical and systemic), cyclosporine, mycophenolic acid, antibiotics (clarithromycin), emollients | Glucocorticoids (topical), desloratidine, ketotifen, emollients |
|
| ||||
| 3 | 41 | Female | Glucocorticoids (topical and systemic), antibiotics (cefuroxime), cyclosporine, emollients | Glucocorticoids (topical), zincoxide, emollients |
|
| ||||
| 4 | 7 | Female | Glucocorticoids (topical), antibiotics (erythromycin), topical coal tar, pimecrolimus, emollients | Glucocorticoids (topical), antibiotics (erythromycin), alimemazine, zincoxide, emollients |
|
| ||||
| 5 | 50 | Female | Glucocorticoids (topical and systemic), antibiotics (erythromycin, flucloxacillin), antihistamines (fexofenadine, hydroxyzine, dimetindene), emollients | Glucocorticoids (topical), antibiotics (amoxicillin), zincoxide, desloratidine, emollients |
Table 2.
Summary of the given treatments
| Treatment | Patient | Total treatment duration | Initial response | Effectiveness | Adverse events | Discontinuation |
|---|---|---|---|---|---|---|
| IVIG | Patient 1 | 4 years | 2 months | Less effective after 2.5 years | No | Yes |
|
|
||||||
| Patient 2 | 8 months | 2 months | Less effective after 6 months | No | Yes | |
|
| ||||||
| Ixekizumab | Patient 1 | 1 year and 9 months | 1.5 months | Less effective after 1.5 years | Regular common colds | Yes |
|
|
||||||
| Patient 2 | 3.5 years | 3 months | Not applicable | Nail infection | No | |
|
| ||||||
| Dupilumab | Patient 3 | 6 months | 2 months | Less effective after 5 months | Fatigue in the first month | Yes |
|
| ||||||
| Anakinra | Patient 1 | 5.5 months | 2 weeks | Less effective after 5 months | Increased itch | Yes |
|
|
||||||
| Patient 3 | 14 months | 1 month | Less effective after 10 months | Initial pain and itch at local injection site | Yes | |
|
|
||||||
| Patient 4 | 5 months | 1 month | Less effective after 3 months | Increased itch, struggle with injections | Yes | |
|
|
||||||
| Patient 5 | 9 months | 1 month | Less effective after 4 months | Burning and dry sensation of the eyes, upper respiratory tract infection | Yes | |
IVIG, intravenous immunoglobulin.
Intravenous Immunoglobulins
Patient 1, a woman of 25 years old, and patient 2, a man of 25 years old, were treated with prednisone without improvement of skin symptoms. Therefore, IVIG treatment every 4 weeks was started (patient one 0.4 g/kg and patient two 0.8 g/kg). Both patients responded within 2 months of treatment and experienced less skin symptoms (less erythema and scaling) and skin infections (impetiginization). These effects persisted for 2.5 years and 6 months in patient 1 and patient 2, respectively. Thereafter, both patients experienced multiple exacerbations with more erythroderma and recurrent skin infections. Increasing the IVIG dose in patient 1 (0.9 g/kg) did not result in improvement of skin symptoms. Therefore, after 4 years and 8 months, respectively, treatment was stopped in both patients.
Ixekizumab
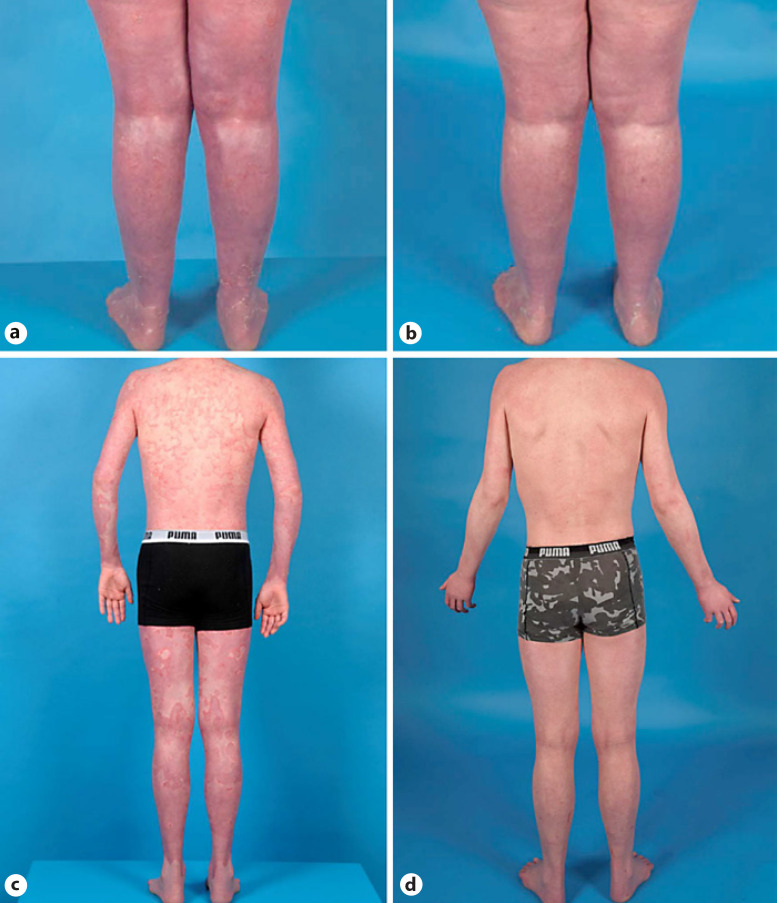
Patient 1 and 2 switched to ixekizumab after IVIG treatment, with an initial dose of 160 mg followed by a dosage of 80 mg every 2 weeks. After 3 months (patient 1) and 6 months (patient 2), the interval between injections was prolonged to 80 mg every 4 weeks. For 1.5 years, patient 1 responded well (Fig. 1a, b), with minimal exacerbations, less erythema, scaling, and skin infections. Thereafter, her skin condition declined and she suffered from more scaling and erythema. After a total duration of 1 year and 9 months, ixekizumab was discontinued due to reduced effectiveness. Patient 2 is currently still treated with ixekizumab, for over 3.5 years, with feasible results. He experiences less erythema, less exacerbations, and more energy overall compared to prior treatments (Fig. 1c, d).
Fig. 1.
Cases of NS treated with ixekizumab. a Patient 1 after 6 weeks of treatment with ixekizumab. b Improved skin condition after 1 year of treatment. c Widespread polycyclic, double-edged scaling and erythematous plaques before treatment with ixekizumab in patient 2. d Substantial improvement after 2 years and 4 months of treatment.
Dupilumab
Patient 3, a woman of 41 years old, was referred to our outpatient clinic from another tertiary hospital for treatment of severe NS. Due to exacerbation under topical corticosteroid treatment, and good experiences with dupilumab of a family member with NS, dupilumab was started abroad. After the initial dose of 600 mg, treatment was continued with 300 mg every 2 weeks. During treatment, her skin condition somewhat improved (less erythema and scaling); however, after 5 months of treatment skin symptoms began to deteriorate and the dosage was raised to 300 mg once a week. The erythema improved only temporally and after 6 months of treatment dupilumab was terminated due to lack of effectiveness and persistent skin symptoms and pain. Except for complaints of fatigue the first month of treatment, no adverse events during treatment with dupilumab were noted.
Anakinra
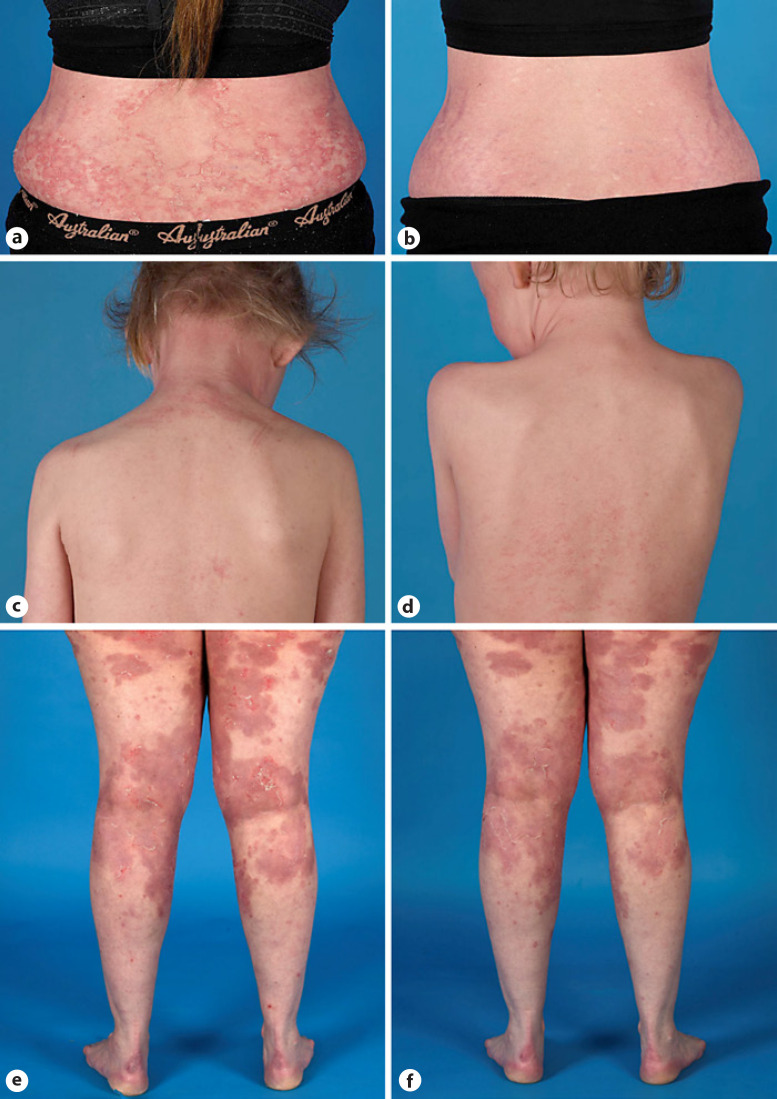
Patient 1, 3, 4, and 5 were all treated with daily anakinra (100 mg) after failing of abovementioned treatments or standard care. Skin condition of patient 1 was characterized by an increase of the affected skin surface with worsening of erythema, scaling, and pain prior to starting anakinra (Fig. 2a, b). Within 2 weeks of initiation of treatment with anakinra, her skin condition improved (VIIS 27 at start and 14 after 2 weeks). Due to increased itch, desloratadine (5 mg twice daily) was prescribed. After 2 months of treatment, she developed erythroderma. Anakinra was temporarily halted and doxycycline (100 mg daily) was started since a skin infection was suspected. Anakinra was restarted after 2 months and within 2 weeks her skin condition and energy level improved. However, after 3 months of successful treatment, she experienced two periods with skin exacerbations and increase of pain. After a total of 5.5 months of treatment, anakinra was stopped.
Fig. 2.
Cases of Netherton treated with anakinra. a Lower back of patient 1 before treatment (widespread polycyclic, double-edged scaling and erythematous plaques) with anakinra and b improvement of the skin condition after 6 weeks of treatment. Patient 4 before (c) and after (d) 3 months of anakinra treatment. Patient 5 before (e) and after (f) 2.5 months of anakinra treatment.
Patient 3 had still complaints of a scaly skin, pain, and itch after treatment with dupilumab. Treatment with anakinra was started and within a month the patient experienced an improved skin condition and less itching. Except for initial pain and itch at local injection site for which topical clobetasol propionate was prescribed, no adverse events were noted. After several months, improvement stabilized and after 10 months of treatment the patient experienced worsening of the skin condition with erythema. After 14 months, treatment with anakinra was stopped due to lack of effectiveness.
Patient 4, a girl of 7 years old, and patient 5, a woman of 50, were treated with standard care. Anakinra was started because of insufficient disease control with the use of long-term topical corticosteroids, calcineurin inhibitors, oral antibiotics, and anti-histamines. The symptoms of patient 4 were characterized by erythroderma and ichthyosis linearis circumflexa, most prominently on the upper back and face (VIIS 14) and slightly improved within 1 month (VIIS 13) mainly with less erythroderma and scaling on the face (Fig. 2c, d). In addition, her parents noted that she had more energy and became more active and went to school again. Similar effects were seen in patient 5. In addition to less scaling and erythroderma, most prominent on the upper legs, she experienced less painful calves and more energy (Fig. 2e, f). Patient 4 initially experienced more itch and patient 5 complained of a burning and dry sensation of the eyes, which was adequately treated with artificial tears. Treatment in patient 5 was temporarily halted due to an upper respiratory tract infection. Shortly after restart of anakinra, patient 5 developed a skin infection which was treated with oral antibiotics. In both patients, skin condition improved within a month with lower NRS scores for skin symptoms and itch (online suppl. Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000525987). However, no sustained improvement was noted. In patient 4, administration of the daily subcutaneous injections became a struggle due to increasing fear for the injection although our NS pediatric psychologist was consulted. After 5 months, therapy with anakinra was stopped (VIIS: 16). Her skin condition worsened rapidly and she developed painful calves.
In patient 5, improvement of the skin condition remained for over 4 months. After this period, she increasingly suffered from exacerbations and needed to use topical steroids more intensively. After 9 months, anakinra was stopped.
During treatment with anakinra, no changes in laboratory values were observed (online suppl. Table S1). Patient 1, patient 3, and patient 4 switched to ixekizumab. Patient 5 continued treatment using topical corticosteroids.
Discussion/Conclusion
In this study, we share our long-term experiences with IVIG, ixekizumab, dupilumab, and anakinra for the treatment of patients with severe NS. All treatment modalities were well tolerated and no severe side effects occurred. At least temporary cutaneous improvement (less erythroderma, scaling, and less frequent exacerbations of NS-related skin symptoms) and less tiredness improving experienced quality of life was noted in all administered therapies.
Intravenous Immunoglobulin
IVIG has been used for treatment of inborn errors of immunity [19, 20]. In the past years, several studies reported the use of IVIG in children with NS [4, 26, 27, 28]. Most patients showed improvement while on IVIG therapy. However, 1 patient showed lack of benefits which led to discontinuation of the IVIG treatment [27]. In our 2 adult patients treated with IVIG, cutaneous response was observed within 2 months and lasted over the course of 2.5 years overall. Although knowledge of the effects of IVIG in adult patients is scarce, our results suggest that IVIG may a therapeutic option in adults with NS [29]. The precise mechanism of IVIG in NS still remains unclear and more research is needed to assess the role and effectiveness of IVIG in NS. However, it is thought that in addition to the replacement of immunoglobulins, high dosage of IVIG may modulate the immune system and reduce inflammation [21]. We hypothesize that IVIG may downregulate Th17 cytokines in NS and thereby reduces inflammation. Treatment with IVIG could be an option for patients with severe NS-related skin symptoms.
Biologicals
As a consequence of LEKTI deficiency and serine protease overexpression, inflammation and allergic pathways in NS are upregulated [4, 5, 6, 30, 31]. In patients with NS, increased activity of the Th17 pathway and elevated expression of TNF-α and IL-1β have been described [4, 5, 6, 31]. Also, Th2 skewing has been reported [31, 32, 33]. With the increase of biologic therapies targeting these specific inflammatory cytokines and pathways, it is hypothesized that some therapies could be effective for NS.
Ixekizumab
In this case series, we show the short- and long-term effects of ixekizumab. Similar to the results of Luchsinger et al. [15] and the findings of Paller et al. [5], anti-IL-17 treatment of NS with ixekizumab was effective in our 2 patients on the short term. In 1 patient, effectiveness declined after 1.5 years and in the other patient treatment with ixekizumab has been effective for over 3.5 years. These findings suggest a role for anti-IL-17 treatment in severe NS. More cases with treatment effects on the long term are needed to determine if and which patients benefit most from anti-IL-17 treatment.
Dupilumab
In our patient, dupilumab treatment demonstrated little effectiveness on NS-related skin symptoms (even after raising the dosage) and was terminated after 6 months. Our results are in contrast with two published single case series, who showed major improvement on the affected body surface area and patient reported itch [13, 16]. Dupilumab is increasingly used for inflammatory skin diseases including atopic dermatitis and its safety profile is well documented [34]. Dupilumab may be an interesting treatment option for NS as many patients suffer from atopic manifestations and elevated expression of IL-4 and IL-13 have been reported in Tg-KLK5 mice [1, 35]. However, another study found similar IL-13 expression in patients with NS as to controls [5]. Based on this contradictory, we conclude that more research is needed to assess the role of dupilumab in NS. Fortunately, a trial investigating dupilumab (NCT04244006) is currently ongoing [36].
Anakinra
Since previous studies showed elevated levels of IL-1β both in skin biopsies and serum of NS patients, we hypothesized that IL-1 blockade would improve the skin condition [4, 5]. In this case series, we describe the first experience with anakinra in NS. Anakinra showed a rapid decrease in erythema, scaling, itch, and tiredness during the first months of treatment in 4 patients with NS. On the long term, its effectiveness declined over time. Anakinra may be a good option for patients with severe symptoms or exacerbations. More research is warranted to explain the declining in long-term effect to establish the role of anakinra in the personalized treatment of NS.
The results of this case series describe that some therapies were effective in NS on the short and medium long term; in some patients, this treatment effect declined on the long term. As far as we know, both the long-term effect and the decline over time have not been reported before in patients with NS. Although the precise mechanism behind this phenomenon remains unclear, this phenomenon can also be seen in other complex immune-mediated inflammatory diseases [37]. Immunogenicity might play a role, although no anti-drug antibodies were found [38]. Immunogenicity could be countered by adding an immunomodulator from the start of the therapy [39]. To address this issue, more research investigating the effects of a combination of therapies is needed. In addition to elongation of treatment response, a combination of treatment might be more effective. In rheumatoid arthritis, it has been demonstrated that monotherapy with biologics is inferior to a combination of therapies [40]. This might be the case for NS as well.
Currently, we cannot predict effectiveness of therapy in the individual NS patient. As we have observed in our case series, some patients might benefit more from a certain treatment and for a longer duration. Given this variability in treatment response, a more tailored approach is needed. Further research into personalized factors predicting and evaluating treatment response in NS is necessary, including genotype-phenotype and involved skin and serum biomarkers.
The scarcity of current literature on the role of treatment in NS, including IVIG and biologicals, further emphasizes the need for larger, controlled, long-term cohort studies and trials specially designed for rare skin diseases in an international setting based on standardized outcome measures, in cooperation with expert centers and patient associations. To establish this, we recently initiated the International Netherton Network for both patients and professionals (https://nethertonnetwork.com).
Limitations
This study is a case series of only one International Netherton Expert Center representing the more severe NS patients that needed a step up in their treatment of its national NS cohort. Some data were lost due to failure of patients to fill in the questionnaires and logistical problems caused by the COVID-19 pandemic.
Conclusion
Our experience suggests that IVIG, ixekizumab, dupilumab, and anakinra are safe and can improve NS with variability in treatment response on the long term. Of these treatments, IVIG and ixekizumab showed the longest treatment effects. Larger international studies in collaboration with expert centers internationally and patient associations are needed to determine if and which patients benefit most from the emerging therapies for NS.
Key Message
Intravenous immunoglobulins, ixekizumab, dupilumab, and anakinra are safe treatment options for Netherton syndrome.
Statement of Ethics
This study was approved by the Medical Ethical Committee (METC) of the Erasmus MC University Medical Center (MEC-2020-0447), the Netherlands. Written informed consent was obtained from all patients and of the parents of the participating child to participate in this study. Written informed consent was obtained from all patients and the parents/legal guardians of the minor patient for publication of the details of their medical cases and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received for this study.
Author Contributions
Aviël Ragamin, Minke M.F. van Mierlo, Virgil A.S.H. Dalm, and Suzanne G.M.A. Pasmans designed the study. Minke M.F. van Mierlo, Aviël Ragamin, and Anouk E.M. Nouwen were responsible for the data collection. Aviël Ragamin and Anouk E.M. Nouwen wrote the manuscript. Carsten R. Lincke provided pediatric care and support and delivered treatment to pediatric patients. Virgil A.S.H. Dalm and Suzanne G.M.A. Pasmans supervised the study. All authors critically commented on the manuscript.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy legislation but are available from Suzanne G.M.A. Pasmans (s.pasmans@erasmusmc.nl) upon reasonable request. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgments
We thank the patients and their families for their contributions. This study is part of the ERN-SKIN-subthematic group Ichthyosis (https://ern-skin.eu), the International Netherton Network (https://nethertonnetwork.com/thinc-2021), and the Center of Rare Skin diseases, Netherton Expert Center, Erasmus MC, acknowledged by the Ministry of Health. We thank Kira Stuvel, Rachel Stehouwer, Cornelus J.G. Sanders, and Karin Veldman for their contributions to the Netherton projects. We thank Errol P. Prens and H.B. Thio for their fruitful discussion.
Funding Statement
No funding was received for this study.
References
- 1.Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013 Feb;351((2)):289–300. doi: 10.1007/s00441-013-1558-1. [DOI] [PubMed] [Google Scholar]
- 2.Sarri CA, Roussaki-Schulze A, Vasilopoulos Y, Zafiriou E, Patsatsi A, Stamatis C, et al. Netherton syndrome: a genotype-phenotype review. Mol Diagn Ther. 2017 Apr;21((2)):137–152. doi: 10.1007/s40291-016-0243-y. [DOI] [PubMed] [Google Scholar]
- 3.Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell. 2007 Sep;18((9)):3607–3619. doi: 10.1091/mbc.E07-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renner ED, Hartl D, Rylaarsdam S, Young ML, Monaco-Shawver L, Kleiner G, et al. Comel-Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol. 2009 Sep;124((3)):536–543. doi: 10.1016/j.jaci.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paller AS, Renert-Yuval Y, Suprun M, Esaki H, Oliva M, Huynh TN, et al. An IL-17-dominant immune profile is shared across the major orphan forms of ichthyosis. J Allergy Clin Immunol. 2017 Jan;139((1)):152–165. doi: 10.1016/j.jaci.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik K, He H, Huynh TN, Tran G, Mueller K, Doytcheva K, et al. Ichthyosis molecular fingerprinting shows profound TH17 skewing and a unique barrier genomic signature. J Allergy Clin Immunol. 2019 Feb;143((2)):604–618. doi: 10.1016/j.jaci.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan AC, Honig PJ, Ming ME, Weber J, Shah KN. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010 Jan;146((1)):57–62. doi: 10.1001/archdermatol.2009.326. [DOI] [PubMed] [Google Scholar]
- 8.Happle R, van de Kerkhof PC, Traupe H. Retinoids in disorders of keratinization: their use in adults. Dermatologica. 1987;175((Suppl 1)):107–124. doi: 10.1159/000248867. [DOI] [PubMed] [Google Scholar]
- 9.Maatouk I, Moutran R, Tomb R. Narrowband ultraviolet B phototherapy associated with improvement in Netherton syndrome. Clin Exp Dermatol. 2012 Jun;37((4)):364–366. doi: 10.1111/j.1365-2230.2011.04231.x. [DOI] [PubMed] [Google Scholar]
- 10.Fontao L, Laffitte E, Briot A, Kaya G, Roux-Lombard P, Fraitag S, et al. Infliximab infusions for Netherton syndrome: sustained clinical improvement correlates with a reduction of thymic stromal lymphopoietin levels in the skin. J Invest Dermatol. 2011 Sep;131((9)):1947–1950. doi: 10.1038/jid.2011.124. [DOI] [PubMed] [Google Scholar]
- 11.Yalcin AD. A case of netherton syndrome: successful treatment with omalizumab and pulse prednisolone and its effects on cytokines and immunoglobulin levels. Immunopharmacol Immunotoxicol. 2016;38((2)):162–166. doi: 10.3109/08923973.2015.1115518. [DOI] [PubMed] [Google Scholar]
- 12.Roda A, Mendonca-Sanches M, Travassos AR, Soares-de-Almeida L, Metze D. Infliximab therapy for Netherton syndrome: a case report. JAAD Case Rep. 2017 Nov;3((6)):550–552. doi: 10.1016/j.jdcr.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreasen TH, Karstensen HG, Duno M, Lei U, Zachariae C, Thyssen JP. Successful treatment with dupilumab of an adult with Netherton syndrome. Clin Exp Dermatol. 2020 Oct;45((7)):915–917. doi: 10.1111/ced.14317. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard SK, Prose NS. Successful use of secukinumab in Netherton syndrome. JAAD Case Rep. 2020 Jun;6((6)):577–578. doi: 10.1016/j.jdcr.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luchsinger I, Knopfel N, Theiler M, Bonnet des Claustres M, Barbieux C, Schwieger-Briel A, et al. Secukinumab therapy for Netherton syndrome. JAMA Dermatol. 2020 Aug 1;156((8)):907–911. doi: 10.1001/jamadermatol.2020.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steuer AB, Cohen DE. Treatment of Netherton syndrome with dupilumab. JAMA Dermatol. 2020 Mar 1;156((3)):350–351. doi: 10.1001/jamadermatol.2019.4608. [DOI] [PubMed] [Google Scholar]
- 17.Volc S, Maier L, Gritsch A, Aichelburg MC, Volc-Platzer B. Successful treatment of Netherton syndrome with ustekinumab in a 15-year-old girl. Br J Dermatol. 2020 Jul;183((1)):165–167. doi: 10.1111/bjd.18892. [DOI] [PubMed] [Google Scholar]
- 18.Nouwen AEM, Schappin R, Nguyen NT, Ragamin A, Bygum A, Bodemer C, et al. Outcomes of systemic treatment in children and adults with Netherton syndrome: a systematic review. Front Immunol. 2022;13:864449. doi: 10.3389/fimmu.2022.864449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurcan HM, Ahmed AR. Efficacy of various intravenous immunoglobulin therapy protocols in autoimmune and chronic inflammatory disorders. Ann Pharmacother. 2007 May;41((5)):812–823. doi: 10.1345/aph.1K037. [DOI] [PubMed] [Google Scholar]
- 20.De Ranieri D, Fenny NS. Intravenous immunoglobulin in the treatment of primary immunodeficiency diseases. Pediatr Ann. 2017 Jan 1;46((1)):e8–e12. doi: 10.3928/19382359-20161213-03. [DOI] [PubMed] [Google Scholar]
- 21.Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin Exp Immunol. 2011 Jun;164((Suppl 2)):2–5. doi: 10.1111/j.1365-2249.2011.04387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Othy S, Hegde P, Topcu S, Sharma M, Maddur MS, Lacroix-Desmazes S, et al. Intravenous gammaglobulin inhibits encephalitogenic potential of pathogenic T cells and interferes with their trafficking to the central nervous system, implicating sphingosine-1 phosphate receptor 1-mammalian target of rapamycin axis. J Immunol. 2013 May 1;190((9)):4535–4541. doi: 10.4049/jimmunol.1201965. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Leyva-Castillo JM, Hener P, Eisenmann A, Zaafouri S, Jonca N, et al. Counterregulation between thymic stromal lymphopoietin- and IL-23-driven immune axes shapes skin inflammation in mice with epidermal barrier defects. J Allergy Clin Immunol. 2016 Jul;138((1)):150–61.e13. doi: 10.1016/j.jaci.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Bernard M, Carrasco C, Laoubi L, Guiraud B, Rozieres A, Goujon C, et al. IL-1β induces thymic stromal lymphopoietin and an atopic dermatitis-like phenotype in reconstructed healthy human epidermis. J Pathol. 2017 Jun;242((2)):234–245. doi: 10.1002/path.4887. [DOI] [PubMed] [Google Scholar]
- 25.Marukian NV, Deng Y, Gan G, Ren I, Thermidor W, Craiglow BG, et al. Establishing and validating an ichthyosis severity index. J Invest Dermatol. 2017 Sep;137((9)):1834–1841. doi: 10.1016/j.jid.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Small AM, Cordoro KM. Netherton syndrome mimicking pustular psoriasis: clinical implications and response to intravenous immunoglobulin. Pediatr Dermatol. 2016 May;33((3)):e222–3. doi: 10.1111/pde.12856. [DOI] [PubMed] [Google Scholar]
- 27.Eranko E, Ilander M, Tuomiranta M, Makitie A, Lassila T, Kreutzman A, et al. Immune cell phenotype and functional defects in Netherton syndrome. Orphanet J Rare Dis. 2018 Nov 26;13((1)):213. doi: 10.1186/s13023-018-0956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Pan C, Wei R, Li H, Yang Y, Chen J, et al. Netherton syndrome caused by compound heterozygous mutation, c.80A>G mutation in SPINK5 and large-sized genomic deletion mutation, and successful treatment of intravenous immunoglobulin. Mol Genet Genomic Med. 2021 Mar;9((3)):e1600. doi: 10.1002/mgg3.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aktas M, Salman A, Apti Sengun O, Comert Ozer E, Hosgoren Tekin S, Akin Cakici O, et al. Netherton syndrome: temporary response to dupilumab. Pediatr Dermatol. 2020 Nov;37((6)):1210–1211. doi: 10.1111/pde.14362. [DOI] [PubMed] [Google Scholar]
- 30.Hosomi N, Fukai K, Nakanishi T, Funaki S, Ishii M. Caspase-1 activity of stratum corneum and serum interleukin-18 level are increased in patients with Netherton syndrome. Br J Dermatol. 2008 Sep;159((3)):744–746. doi: 10.1111/j.1365-2133.2008.08706.x. [DOI] [PubMed] [Google Scholar]
- 31.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009 May 11;206((5)):1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Gysel D, Koning H, Baert MR, Savelkoul HF, Neijens HJ, Oranje AP. Clinico-immunological heterogeneity in Comel-Netherton syndrome. Dermatology. 2001;202((2)):99–107. doi: 10.1159/000051607. [DOI] [PubMed] [Google Scholar]
- 33.Konishi T, Tsuda T, Sakaguchi Y, Imai Y, Ito T, Hirota S, et al. Upregulation of interleukin-33 in the epidermis of two Japanese patients with Netherton syndrome. J Dermatol. 2014 Mar;41((3)):258–261. doi: 10.1111/1346-8138.12410. [DOI] [PubMed] [Google Scholar]
- 34.Thaci D, L Simpson E, Deleuran M, Kataoka Y, Chen Z, Gadkari A, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2) J Dermatol Sci. 2019 May;94((2)):266–275. doi: 10.1016/j.jdermsci.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Furio L, de Veer S, Jaillet M, Briot A, Robin A, Deraison C, et al. Transgenic kallikrein 5 mice reproduce major cutaneous and systemic hallmarks of Netherton syndrome. J Exp Med. 2014 Mar 10;211((3)):499–513. doi: 10.1084/jem.20131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazereeuw-Hautier J. A pilot study of the efficacy and safety of dupilumab versus placebo in patients with netherton syndrome (NS-DUPI) 2020 ClinicalTrials.gov . [Google Scholar]
- 37.Papamichael K, Vogelzang EH, Lambert J, Wolbink G, Cheifetz AS. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev Clin Immunol. 2019 Aug;15((8)):837–848. doi: 10.1080/1744666X.2019.1630273. [DOI] [PubMed] [Google Scholar]
- 38.de Vries MK, van der Horst-Bruinsma IE, Nurmohamed MT, Aarden LA, Stapel SO, Peters MJL, et al. Immunogenicity does not influence treatment with etanercept in patients with ankylosing spondylitis. Ann Rheum Dis. 2009 Apr;68((4)):531–535. doi: 10.1136/ard.2008.089979. [DOI] [PubMed] [Google Scholar]
- 39.Strober BE. Why biologic therapies sometimes lose efficacy. Semin Cutan Med Surg. 2016 Jun;35((4 Suppl 4)):S78–80. doi: 10.12788/j.sder.2016.022. [DOI] [PubMed] [Google Scholar]
- 40.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018 Oct 2;320((13)):1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy legislation but are available from Suzanne G.M.A. Pasmans (s.pasmans@erasmusmc.nl) upon reasonable request. Further inquiries can be directed to the corresponding author.