Abstract
Background
Primary cardiac lymphoma (PCL) is a rare cardiac tumour with various presentations, which might cause a complete atrioventricular (AV) block, which can, in turn, cause heart failure symptoms.
Case summary
We report a case of a 55-year-old woman with a chief complaint of exertional dyspnoea. Her vital signs showed bradycardia, and electrocardiography revealed a complete AV block. Transthoracic echocardiography revealed a large intra-cardiac mass in the right atrium. Full-body positron emission tomography showed an elevated fluorodeoxyglucose uptake in the right atrial mass, interatrial septum, and wall of the left atrium. Since the tumour could obstruct the tricuspid valve, urgent tumour debulking surgery and epicardial lead implantation were performed. Histopathological examination results were consistent with diffuse large B-cell lymphoma. After several courses of chemotherapy, we kept her in complete remission of the tumour for 2 years.
Discussion
Primary cardiac lymphoma was complicated by a complete AV block and diagnosed by using the samples that we obtained in the surgery. A surgical resection of the tumour and epicardial lead implantation, combined with chemotherapy, can be an option, especially in patients who require cardiac surgery.
Keywords: Cardiac lymphoma, Atrioventricular block, Surgery, Epicardial lead implantation, Heart failure, Case report
Learning points.
Primary cardiac lymphoma often involves the right heart and possibly causes obstruction.
Primary cardiac lymphoma complicated with complete atrioventricular block can be treated with epicardial lead implantation when the tumour impairs transvenous lead delivery.
Debulking tumours using surgery with epicardial lead implantation can be considered as a method of management.
Introduction
Primary cardiac lymphoma (PCL) is a rare malignant tumour accounting for ∼1% of primary cardiac tumours and 0.5% of extranodal lymphomas.1 In contrast, secondary cardiac involvement due to lymphoma can occur in up to 20% of patients with lymphoma. Almost all cardiac lymphomas are of B-cell lineage, and ∼80% of PCL is diffuse large B-cell lymphoma.1,2 Primary cardiac lymphoma has various presentations, of which atrioventricular (AV) block occurs in 22% of patients, and 61% of AV blocks are complete.3 Pacemaker implantation is indicated for patients with PCL complicated by a complete AV block. Some PCL patients with a complete AV block can regain their AV conduction function with chemotherapy.4–6 However, patients with a haemodynamically unstable status or heart failure require urgent pacemaker implantation. The transvenous approach is usually selected, but some patients require the epicardial approach due to the obstruction of the superior vena cava or the tricuspid valve.7–9
We present a case of PCL with a complete AV that was treated with chemotherapy combined with a surgical resection of the tumour and epicardial lead implantation.
Timeline
| Time | Events |
|---|---|
| Day 1 | A complete atrioventricular block was detected on electrocardiography and a mass in the right atrium was detected on transthoracic echocardiography |
| Day 3 | A surgical resection of tumour and epicardial lead implantation were performed |
| The diagnosis of malignant lymphoma was confirmed with a histopathological analysis | |
| Day 4 | Chemotherapy was initiated |
| Month 2 | Whole body positron emission tomography-computed tomography (PET-CT) showed no clinically significant accumulation of fluorodeoxyglucose |
| Month 29 | PET-CT showed no sign of recurrence |
Case summary
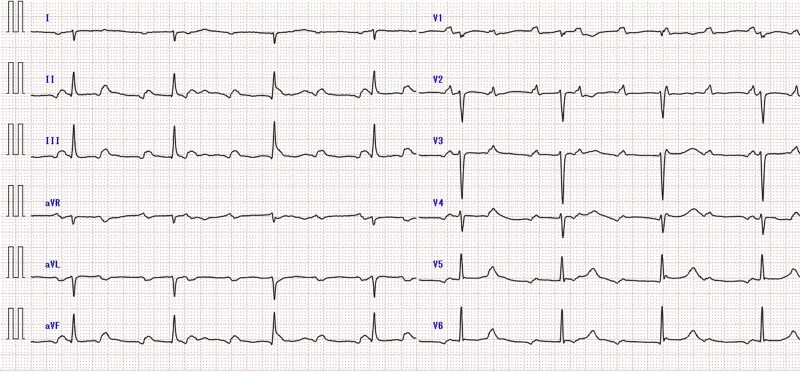
A 55-year-old woman with a history of total hysterectomy for uterine cancer and rheumatoid arthritis presented with a 2-week history of exertional dyspnoea and was admitted with bradycardia. On admission, her blood pressure was 127/67 mmHg, the pulse rate was 50 b.p.m., respiratory rate was 12 breaths/min, body temperature was 36.7°C, and SpO2 was 100% (room air). Her heart and pulmonary sounds were normal, and her extremities showed no oedema. The chest radiograph was normal. Electrocardiography (ECG) showed a complete AV block without ST-T segment changes and a heart rate of 46 b.p.m. (Figure 1). A laboratory test revealed normal complete blood test results and renal and hepatic function, although N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels were remarkably elevated to 1583 pg/mL and the soluble interleukin-2 receptor level (sIL-2R) was also elevated to 984 U/mL.
Figure 1.
Electrocardiogram at admission showed a complete atrioventricular block at a heart rate of 46 b.p.m. without ST-T segment changes. AV, atrioventricular.
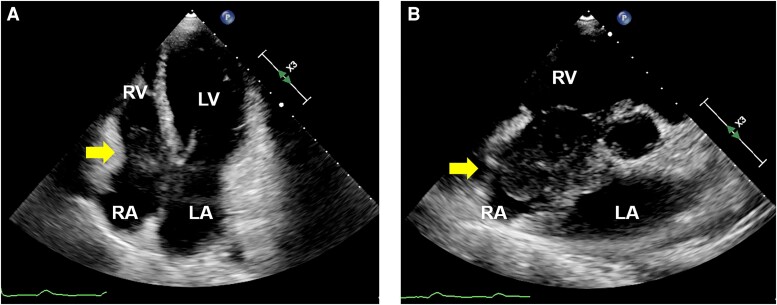
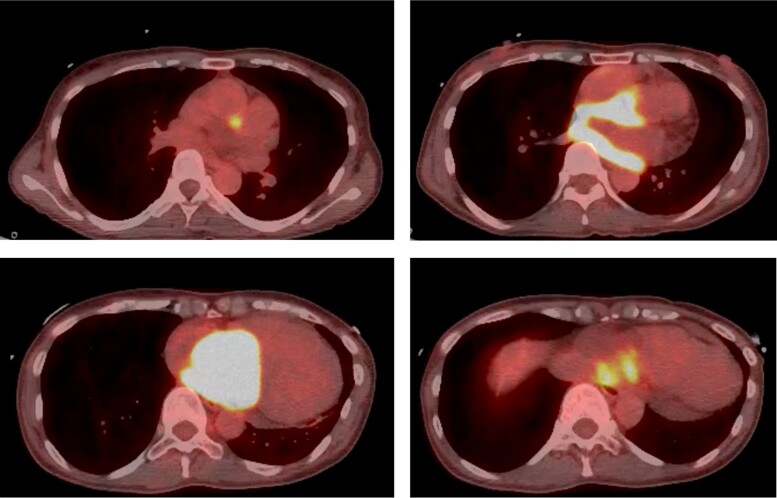
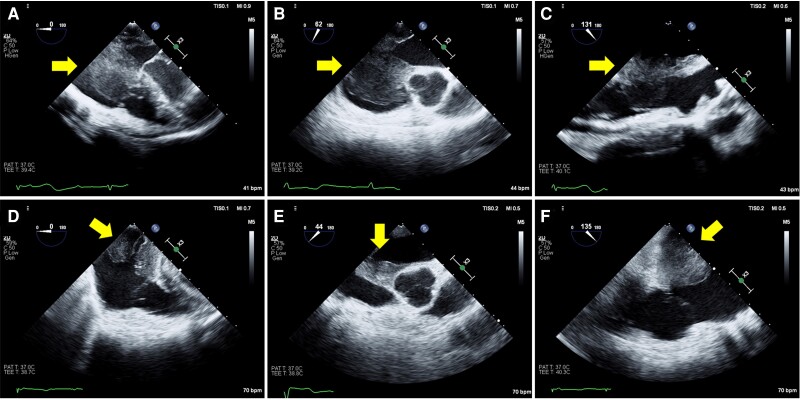
Transthoracic echocardiography (TTE) revealed a large intra-cardiac mass from the interatrial septum, which occupied a major portion of the right atrium (RA) (Figure 2). There was no sign of an elevation in the estimated pulmonary artery and central venous pressure, but the mass made contact with the broad area of the tricuspid valve, which was especially the posterior leaflet, during the diastolic phase. Computed tomography (CT), which was performed prior to the uterine surgery 9 months before, showed an endometrial thickening but no signs of metastasis (see Supplementary material online, Figure S1A). Conversely, at admission, CT showed a newly emerged intra-cardiac mass that was not rich in vascularity (see Supplementary material online, Figure S1B). Full-body positron emission tomography (PET) showed a clinically significant accumulation of fluorodeoxyglucose at the mass of the RA, parietal portion of the left atrium, interatrial septum, and minor mediastinal lymph nodes (Figure 3). Based on examinations revealing extensive cardiac involvement and elevated sIL-2R, malignant tumours such as lymphoma were suspected. Since a large tumour could obstruct the tricuspid valve, we resected the cardiac mass and implanted an epicardial pacemaker lead. During the surgery, the RA was longitudinally incised after a median sternotomy and careful observation. We removed as much of the tumour as possible, but the tumour involving the interatrial septum and coronary sinus remained, which was a significant extension as confirmed by intraoperative transoesophageal echocardiography (Figure 4, Supplementary material online, Videos S1 and S2). Histopathological examination results were consistent with diffuse large B-cell lymphoma. Bone marrow biopsy performed the day after the surgery showed no signs of lymphomatous infiltration. The pacemaker was programmed to perform VVI at 80 b.p.m.
Figure 2.
Apical four-chamber view (A) and parasternal short-axis view (B) of transthoracic echocardiography showed a significantly large intra-cardiac mass (yellow arrows) that arose from the interatrial septum and occupied a major portion of the right atrium. LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium.
Figure 3.
Increased uptake of fluorodeoxyglucose on the right atrial mass, the parietal portion of the left atrium, the interatrial septum, and minor mediastinal lymph nodes on positron emission tomography.
Figure 4.
Preoperative transoesophageal echocardiography showed an intra-cardiac mass (yellow arrows) in the right atrium and a thickened interatrial septum (A and B). The mass made contact with the broad area of the tricuspid valve, which was especially the posterior leaflet, during the diastolic phase (C). Postoperative transoesophageal echocardiography showed a reduction of mass, but the tumour involving the interatrial septum remained (D–F). AV, aortic valve.
Two days after surgery, we initiated a first course of chemotherapy including 375 mg/m2 of rituximab, 750 mg/m2 of cyclophosphamide, 50 mg/m2 of doxorubicin, 2 mg of vincristine, and 80 mg of methylprednisolone for 5 days (R-CHOP). In the second course of therapy, 100 mg of prednisolone was administered instead of methylprednisolone, and intrathecal chemotherapy including 15 mg of methotrexate, 40 mg of cytarabine, and 10 mg of prednisolone was initiated. After two cycles of R-CHOP therapy, TTE showed a slight decrease in the left ventricular ejection fraction (LVEF) to 50.4%. Considering the cardiotoxicity, we started DA-EPOCH-R (50 mg/m2 of etoposide, 0.4 mg/m2 of vincristine, and 10 mg/m2 of doxorubicin for 4 days, 60 mg/m2 of prednisolone for 5 days, 750 mg/m2 of cyclophosphamide once, and 375 mg/m2 of rituximab once) instead of R-CHOP therapy and added 5 mg of carvedilol, 2.5 mg of enalapril, and 12.5 mg of spironolactone. Because of the palmar numbness, which was a side effect of vincristine, we administered a half of the standard dose of vincristine during the second course and discontinued vincristine administration on the fourth course. After two cycles of R-CHOP therapy and four cycles of R-EPOCH therapy, PET showed a complete remission of the cardiac lymphoma and no signs of metastasis to other organs. Despite the administration of cardioprotective medicine, LVEF did not improve after changes to the chemotherapy regimen, but it maintained around 50% without heart failure symptoms. Two years after diagnosis, PET showed no sign of tumour relapse, although ECG showed a pacemaker-dependent rhythm in the VVI mode.
Discussion
Chemotherapy is the main treatment for cardiac lymphoma. In contrast, the purpose of surgery is usually palliative and involves tumour debulking. We report a case of PCL with a complete AV block that was treated with chemotherapy combined with a partial surgical resection of the tumour and epicardial lead implantation.
Previous reports showed that cardiac lymphoma was mainly treated with chemotherapy.10 However, some patients in whom the tumour has extensive myocardial involvement could experience sudden cardiac arrest.11 Surgical resection is one method of management for patients with cardiac lymphoma and a high risk of sudden cardiac events due to cardiac involvement, as in this case. Furthermore, surgery is feasible for cases involving diagnostic difficulties. A previous report presented a similar case of PCL complicated with AV block, which was treated with epicardial lead implantation via the subxiphoid approach and chemotherapy, but axillary lymph nodes were obtained for diagnosis.8 In our case, because the tumour was located in the dorsal part of the heart, implantation of the epicardial lead was not enough, and we needed to perform a surgery for diagnosis as well.
Surgical epicardial lead implantation can be an alternative method for the treatment of AV block, especially in patients with cardiac lymphoma with broad cardiac involvement. Cardiac lymphoma can cause several arrhythmias, and a complete AV block is caused by an infiltration of the conduction system by lymphoma.11 In one study, 22% of cardiac lymphoma patients presented with AV block secondary to atrial arrhythmia.3 Although a complete AV block can be transient with a good response to chemotherapy,4–6 pacemaker lead implantation is frequently required because of heart failure symptoms caused by bradycardia. Several reports have documented successful transvenous lead implantation for AV block caused by cardiac lymphoma.4–6 In contrast, previous reports present patients with cardiac lymphoma with AV block or sick sinus syndrome that were treated with epicardial lead implantation.7–9 In our case, although we considered intravenous lead implantation, because of the concern that a large tumour in the RA could impair transvenous lead delivery, we chose surgical epicardial lead implantation with no complications.
Conclusion
A surgical resection of the tumour and epicardial lead implantation in combination with chemotherapy can be an option for the treatment of a complete AV block caused by PCL.
Supplementary Material
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report, including image(s) and associated text, has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Contributor Information
Ryo Shigeno, Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, 2-1-1 Minatojima-Minamimachi, Chuo-ku, Kobe 6500047, Japan.
Taiji Okada, Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, 2-1-1 Minatojima-Minamimachi, Chuo-ku, Kobe 6500047, Japan.
Tadaaki Koyama, Department of Cardiothoracic Surgery, Kobe City Medical Center General Hospital, Kobe, Japan.
Yutaka Furukawa, Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, 2-1-1 Minatojima-Minamimachi, Chuo-ku, Kobe 6500047, Japan.
Lead author biography
 Ryo Shigeno graduated from Shimane University in Japan. He is currently a senior resident of the Department of Cardiovascular Medicine at the Kobe City Medical Center General Hospital in Japan. He is interested in the areas of structural heart disease intervention and heart failure.
Ryo Shigeno graduated from Shimane University in Japan. He is currently a senior resident of the Department of Cardiovascular Medicine at the Kobe City Medical Center General Hospital in Japan. He is interested in the areas of structural heart disease intervention and heart failure.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
References
- 1. Gowda RM, Khan IA. Clinical perspectives of primary cardiac lymphoma. Angiology 2003;54:599–604. [DOI] [PubMed] [Google Scholar]
- 2. Jeudy J, Burke AP, Frazier AA. Cardiac lymphoma. Radiol Clin North Am 2016;54:689–710. [DOI] [PubMed] [Google Scholar]
- 3. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer 2011;117:581–589. [DOI] [PubMed] [Google Scholar]
- 4. Al-Darzi WK, Hana A, Lahiri MK, Dagher C, Greenberg JC, Alaswad K, et al. Diffuse B cell lymphoma leading to complete heart block: is this transient or permanent? Am J Case Rep 2020;21:e925760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tai CJ, Wang WS, Chung MT, Liu JH, Chiang CY, Yen CC, et al. Complete atrio-ventricular block as a major clinical presentation of the primary cardiac lymphoma: a case report. Jpn J Clin Oncol 2001;31:217–220. [DOI] [PubMed] [Google Scholar]
- 6. Montiel V, Maziers N, Dereme T. Primary cardiac lymphoma and complete atrio-ventricular block: case report and review of the literature. Acta Cardiol 2007;62:55–58. [DOI] [PubMed] [Google Scholar]
- 7. Jorge VC, Bernardino V, Araújo AC, Gomes S, Noronha C, Riso N, et al. Complete heart block as a complicating feature of a mediastinal lymphoma. BMJ Case Rep 2012;2012:bcr1020114890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al Mawed M, Brockmeier J, Haertel D, Ellermeier M, Hartmann F, Gielen S. From inoperable to back to life: a case report of successfully treated obstructive right ventricular primary cardiac lymphoma. Eur Heart J Case Rep 2022;6:ytac051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito K, Nishimura Y, Tanaka H, Tejima T. Epicardial pacemaker implantation for sick sinus syndrome in a patient with supra vena cava obstructed by a primary cardiac lymphoma. J Cardiol Cases 2020;21:234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sultan I, Aranda-Michel E, Habertheuer A, Kilic A, Arnaoutakis G, Bianco V, et al. Long-term outcomes of primary cardiac lymphoma. Circulation 2020;142:2194–2195. [DOI] [PubMed] [Google Scholar]
- 11. Ottaviani G, Matturri L, Rossi L, Jones D. Sudden death due to lymphomatous infiltration of the cardiac conduction system. Cardiovasc Pathol 2003;12:77–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.