Abstract
The preparation of novel sunlight active photocatalysts for complete removal of pollutants from aqueous solutions is a vital research topic in environmental protection. The present work reports the synthesis of a ZnO/BiVO4 S-scheme heterojunction photocatalyst for degradation of the reactive red dye and oxytetracycline antibiotic in wastewater. ZnO and BiVO4 were first fabricated by a hydrothermal technique, and then, the ZnO/BiVO4 heterostructure was synthesized using an ultrasonic route. An increase of the surface area, compared to that of ZnO, was found in ZnO/BiVO4. The enhancement of charge separation efficiency at the interface was obtained so that a remarkable enhancement of the photocatalytic performance was detected in the prepared heterojunction photocatalyst. Complete detoxification of harmful pollutants was achieved by using the economical solar energy. The removal of the pollutants follows the first-order reaction with the highest rate constant of 0.107 min–1. The stability of the prepared photocatalyst was detected after five cycles of use. The ZnO/BiVO4 S-scheme heterostructure photocatalyst still provides high photoactivity even after five times of use. Hydroxyl radicals play an important role in the removal of the pollutant. This work demonstrates a new route to create the step-scheme heterojunction with high photoactivity for complete removal of the toxic dye and antibiotic in the environment.
1. Introduction
A large variety of harmful dyes have been found in toxic wastewater effluents from various industries including textile and pharmaceutical sectors.1−5 Reactive dyes are basically classified by the presence of azo chromophores, which are considered toxic and mutagenic to living organisms.6−9 A very small amount of the reactive red azo dye in water is visible and undesirable.10 Thus, it is a matter of high concern to find out a cost-effective technology for the treatment of water containing this toxic dye.10,11 The oxytetracycline (OTC) antibiotic is extensively used in humans for the treatment of bacterial infectious diseases.12−15 However, this drug causes a serious problem to the environment. After OTC is metabolized in the body, there is a chance that the toxic byproducts could enter the natural water.16,17 This causes damage to the ecosystem and human health. Therefore, complete removal of the toxic organic contaminants from the aqueous phase is urgently needed.18−22
Several techniques including adsorption were used to treat the pollutants in wastewater. However, the incomplete removal of the contaminants and the generation of secondary pollutants are the main drawbacks of these conventional methods.23−25 Interestingly, a semiconducting photocatalytic route is a promising technique for complete removal of the toxic organic pollutants.5,16,17 Basically, the commercially available TiO2 is a UV-active photocatalyst with low photoactivity under natural sunlight.26−31 The ZnO photocatalyst has gained much attention because it shows excellent transport property, is inexpensive, and has a versatile morphology.32−34 In addition, based on our previous studies, controlled synthesis can be performed very easily for the creation of ZnO with high performance and also with a low cost of production.1,3,5−7 Therefore, in comparison to TiO2, ZnO is much cheaper and very easy to synthesize controllably. Nevertheless, low sunlight performance and occurrence of the photocorrosion are the main drawbacks influencing the practical application of ZnO.35 Alternatively, preparation of the visible-light-responsive photocatalysts is one of the most promising research topics in the field of photocatalysis due to the advantage of complete degradation of the toxic pollutants by the utilization of abundant solar energy.36−39
Sunlight active photocatalysts including bismuth vanadate (BiVO4) have gained much attention. Monoclinic BiVO4 comprising an Aurivillius structure shows the advantages of non-toxicity, excellent structural stability, and good visible light response.40−45 However, this pristine photocatalyst faces the problem of a high electron–hole recombination rate and severe photocorrosion after photodegradation study.46−48 In theory, doping of noble metals or fabrication of the semiconducting heterojunctions are the two main strategies for the improvement of the resultant photocatalytic performance.49−54 However, utilization of gold or silver metals is quite expensive. Therefore, generation of a heterojunction is a promising alternative method to create the new photocatalyst with high performance. According to the literature, some heterojunctions based on BiVO4 have been reported.55−57
After the coupling of ZnO and BiVO4, an improvement of electron–hole separation efficiency is expected. This will end up with an enhancement of the resultant photocatalytic performance. On examining the ZnO/BiVO4 heterojunction, it is noted that many techniques have been used for the preparation of the ZnO/BiVO4 binary composite.36−38,41,43,45−47 Among these various methods, ultrasonication is one of the most popular techniques for the creation of the photocatalyst heterojunction. This is due to the advantages of low cost, low energy consumption, and easy control.58−64
To study a heterojunction semiconductor, fabrication of a new heterostructure photocatalyst by coupling of two photocatalysts was introduced. It is well-known that both type II and Z-scheme heterojunctions have previously been proposed.65,66 However, the electron transfer mechanism of a conventional Z-scheme photocatalyst faces some drawbacks so that the new model based on a step scheme or S-scheme photocatalyst with excellent charge separation and high redox ability was introduced. In principle, the advantages of extended photoabsorption, excellent charge separation, and strong redox potential are obtained.65−68
From the above discussion, the present work aims to create a ZnO/BiVO4 S-scheme heterostructure photocatalyst for the degradation of the dye and antibiotic by the utilization of abundant solar energy. Bare ZnO and pristine BiVO4 were prepared first by a facile hydrothermal method. Subsequently, the ZnO/BiVO4 S-scheme photocatalyst was then synthesized using an ultrasonic route. An improvement of the surface area, compared to that of bare ZnO, and a dramatic lowering of the electron–hole recombination rate at the interface are the two key factors concerning the remarkable enhancement of the photocatalytic performance detected in the prepared ZnO/BiVO4 heterojunction photocatalyst. Complete removal of toxic pollutants under sunlight was obtained. The excellent stability of the photocatalyst, with high photoactivity, was confirmed after five times of use. Hydroxyl radicals play a major role in the pollutant removal, while photogenerated holes and electrons play a minor role. Charge transfer at the interface belongs to the S-scheme heterojunction. The novelty of the work is based on the use of an ultrasonic route to create the S-scheme ZnO/BiVO4 heterostructure photocatalyst for the removal of the OTC antibiotic. This type of heterojunction thermodynamically favors photocatalytic reactions due to the huge driving force provided by its strong redox abilities. This work offers a novel avenue to fabricate the heterostructure photocatalyst with high photoactivity for complete detoxification of the harmful dye and antibiotic in natural water.
2. Experiment
2.1. Chemicals and Reagents
All chemicals were used as received. Ultrapure water [deionized (DI) water, 18.2 MΩ·cm–1] was used.
2.2. Synthesis of BiVO4
The bismuth vanadate (BiVO4) catalyst was prepared by a hydrothermal method.16 First, solution A was prepared by the dispersion of 2.4238 g of Bi(NO3)3·5H2O in 30 cm3 of 1.5 M HNO3. Second, solution B was obtained by the addition of 0.5849 g of NH4VO3 and 1.2656 g of ethylenediaminetetraacetic acid (EDTA) in 30 cm3 of DI water. Third, solution B was added to solution A, and the pH of the mixture was adjusted to reach about 7 by the addition of NaOH solution. It was then transferred into a 100 cm3 Teflon-lined autoclave, which maintains the temperature of 100 °C for 24 h. After that, the precipitate was collected, washed with water and ethanol, and finally dried at 60 °C for 15 h.
2.3. Synthesis of ZnO
The zinc oxide (ZnO) photocatalyst was also fabricated via a hydrothermal route as well.5 The Zn2+ solution was prepared by dissolving 3.6100 g of Zn(CH3COO)2·2H2O in 30 cm3 of DI water. Meanwhile, the OH– solution was separately prepared by dissolving 3.2900 g of NaOH in 30 cm3 of DI water. The OH– solution was then added dropwise to the Zn2+ solution with continuous stirring for 30 min. The reaction mixture was transferred into an autoclave that maintains the temperature of 150 °C for 24 h. The resultant precipitate was collected, washed with water and ethanol, and finally dried at 80 °C for 15 h.
2.4. Synthesis of ZnO/BiVO4
ZnO/BiVO4 was fabricated by an ultrasonic route. In a typical procedure, about 0.2 g of ZnO was dispersed in 10 cm3 of DI water for 60 min in an ultrasonic bath (dispersion A). Separately, ultrasonication of BiVO4 (0.2 g of the sample in 10 cm3 of DI water) was also performed for 60 min (dispersion B). Dispersion A was then added into dispersion B, and the mixture was further ultrasonicated for 120 min. Afterward, about 15 cm3 of acetone was added to the mixture, which was then left for 24 h with continuous stirring. After the centrifugation, the product was collected, washed with water and ethanol, and finally dried at 60 °C for 15 h.
2.5. Characterization
All sample were characterized using the same method described previously.1,3,5−7 Basically, the structure of the prepared catalyst was recorded by X-ray diffraction (XRD) using a PANalytical EMPYREAN X-ray diffractometer comprising monochromatic Cu Kα radiation. The morphological structure and the element constitution of the samples were examined by field emission scanning electron microscopy (FESEM, FEI Helios NanoLab G3 CX). The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images of the photocatalyst were obtained on an FEI TECNAI G2 20. The Fourier transform infrared (FT-IR) spectra were recorded on a PerkinElmer Spectrum One FT-IR spectrophotometer. The sample preparation was performed by a KBr pellet method. The Brunauer–Emmett–Teller (BET) specific surface area and porosity were elucidated by a Micromeritics ASAP 2460 surface area and porosity analyzer. Optical properties were determined through ultraviolet–visible (UV–vis) diffused reflectance spectroscopy (Shimazu UV–VISNIR-3101PC scanning spectrophotometer) and photoluminescence (PL) spectroscopy (Shimazu RF-5301PC).
2.6. Photocatalytic Degradation of the Pollutants
The photocatalytic performance of all prepared photocatalysts was determined by monitoring the degradation of the OTC antibiotic and RR141 dye under UV light (a mercury lamp, 125 W) and natural sunlight. The details of the photocatalytic study can be found elsewhere.5,16,17
Typically, the blank experiment was carried out by irradiating the pollutant solution without the incorporation of the photocatalyst.16,17 The photocatalytic study was performed in an aqueous solution (200 cm3) of either the OTC antibiotic or the RR141 dye (concentration = 10 ppm) in the presence of the photocatalyst (50 mg). The solution of 5 cm3 was sampled after light irradiation. The exact concentration of either RR141 or OTC was determined at the λmax values of 543 and 354 nm, respectively, by a spectrophotometric method.
The photoactivity toward the degradation of the pollutant was calculated as follows.
| 1 |
where C0 and C represent the initial concentration and the concentration of the pollutant solution after different times of photo illumination, respectively.
The performance of the prepared photocatalyst can also be determined from the photocatalytic degradation rate as shown below.
| 2 |
| 3 |
where k1 is the rate constant of the first-order degradation reaction.
To study the crucial species involved in the removal of the pollutant, each scavenger (5 mM), namely, isopropyl alcohol (IPA), NaN3, EDTA-2Na, or K2Cr2O7, was separately added for the quenching of hydroxyl radicals, superoxide anion radicals, holes, and electrons, respectively.3 In addition, KI was also incorporated as a scavenger of surface hydroxyl radicals and holes. Each scavenger was separately incorporated in the presence of the prepared photocatalyst.5,16,17
To confirm the generation of the hydroxyl radicals, the photocatalyst was dispersed in terephthalic acid (TA) solution.17 The formation of the radicals was examined by using a spectrofluorometer (excitation wavelength of 315 nm).
To investigate the reusability of the photocatalyst, the used photocatalyst was filtered, washed with ethanol and water, and then dried before use in the second run.3,5,17 The reuse of the sample was carried out for five consecutive runs.
3. Results and Discussion
3.1. Characterization of the Photocatalyst
The XRD patterns of all prepared photocatalyst are shown in Figure 1a. The monoclinic BiVO4 photocatalyst displayed diffraction at 2θ values of 18.67, 19.00, 28.90, 30.50, 34.50, 35.22 39.80, 42.50, 45.84, 46.74, 47.30, 50.30, and 53.20°, assigned to the reflection planes of (110), (011), (121), (040), (200), (002), (211), (051), (213), (240), (042), (202), and (161), respectively. This agrees well the JCPDS no. 14-0688 file.16,17 The pattern from the XRD diffractogram of bare ZnO belongs to the hexagonal wurtzite structure (JCPDS no. 36-1451).5 The diffraction located at 2θ values of 31.76, 34.41, 36.25, 47.53, 56.5962.85, 66.37, 67.94, and 69.08° corresponded to the reflection from crystal planes of (100), (002), (101), (102), (110), (103), (200), (112), and (201), respectively. It can be seen that the ZnO/BiVO4 photocatalyst showed the diffraction peaks contributing from both ZnO and BiVO4. The result confirms the successful creation of the heterojunction.
Figure 1.
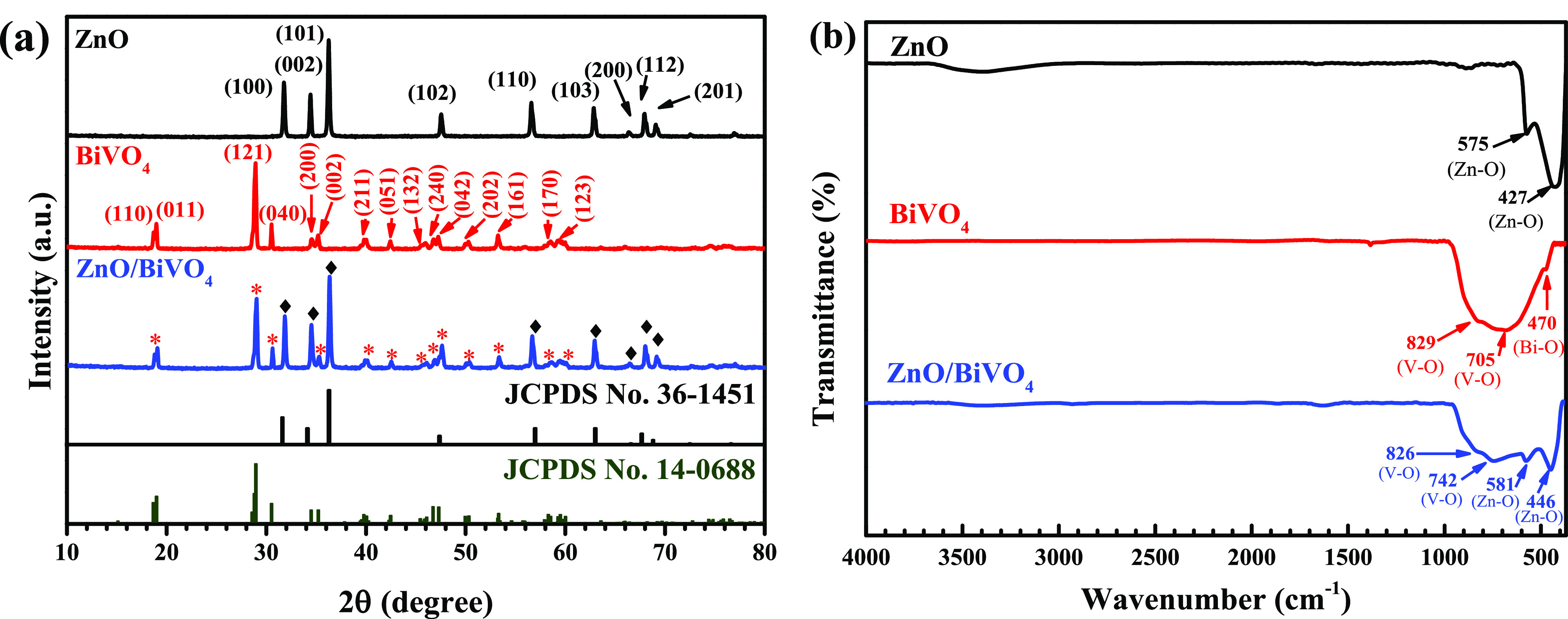
XRD patterns (a) and FT-IR spectra (b) of ZnO, BiVO4, and ZnO/BiVO4 photocatalysts.
FT-IR spectra of all samples were recorded and are shown in Figure 1b. BiVO4 showed bands at 705 and 829 cm–1, respectively, indicating the ν3 asymmetric and ν1 symmetric stretching of VO43. The presence of the peak at 470 cm–1 was attributed to the stretching of the Bi–O bond.16,17 The vibrational peaks at 427 and 575 cm–1 were due to stretching vibration from the Zn–O bond.5 In the case of the ZnO/BiVO4 photocatalyst, the vibrational bands of both the Bi–O bond and Zn–O bond were clearly observed. This confirms the co-existence of both ZnO and BiVO4. The result correlates well with those obtained by XRD.
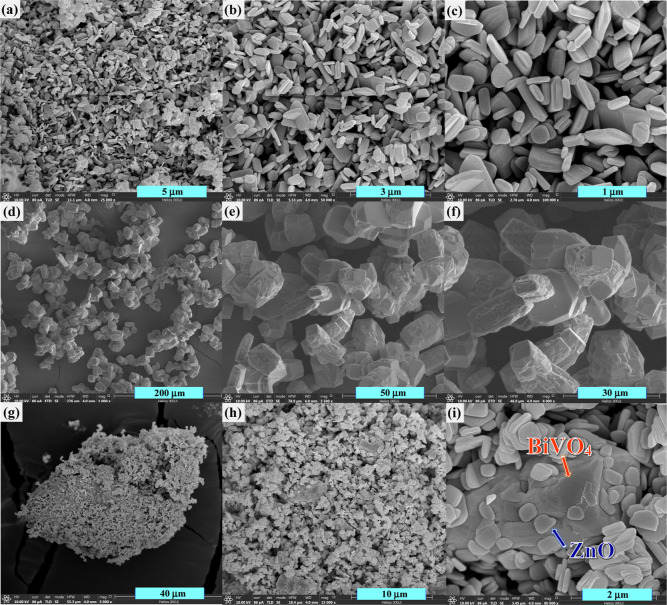
The morphological structures of ZnO, BiVO4, and ZnO/BiVO4 were elucidated by the scanning electron microscopy (SEM) method. The SEM images of pristine ZnO (Figure 2a–c) showed a thin disk-like morphological structure of about 0.30 μm × 0.50 μm × 0.12 μm dimensions. Meanwhile, the SEM images of the bare BiVO4 photocatalyst (Figure 2d–f) showed an irregular polygon morphology of about 8.84 μm × 8.50 μm dimensions. As seen in Figure 2g–i, the final morphology of ZnO/BiVO4 showed the co-existence of both ZnO and BiVO4 without altering the morphology of the individual photocatalyst.
Figure 2.
FESEM micrographs of ZnO (a–c), BiVO4 (d–f), and ZnO/BiVO4 photocatalysts (g–i).
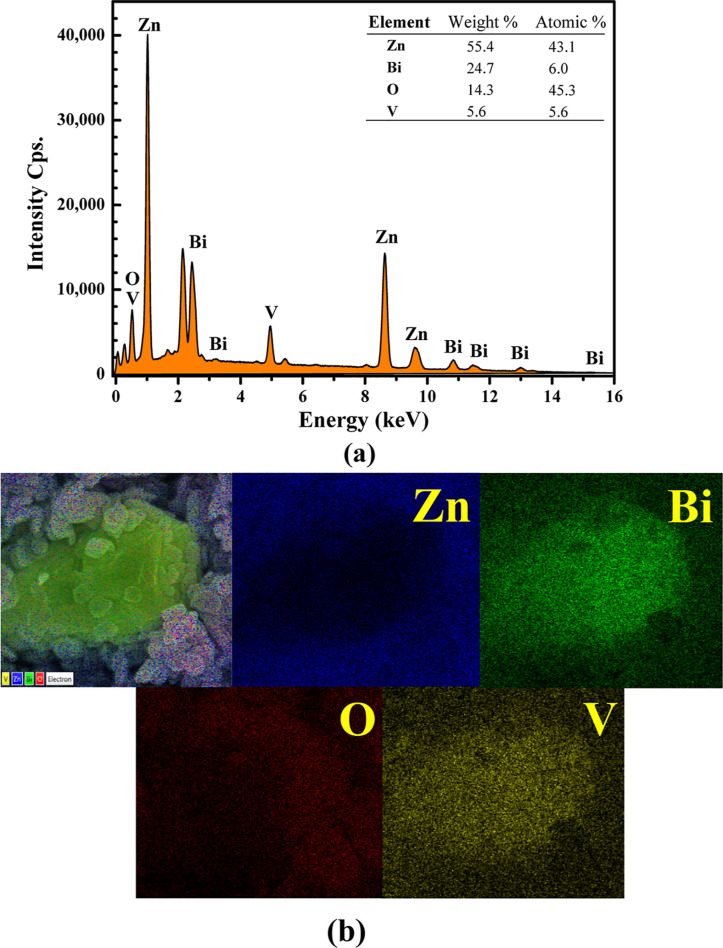
Energy-dispersive X-ray spectroscopy (EDS) was used to determine the elemental compositions in the sample. Four elements, including Zn, O, Bi, and V, were detected in the synthesized photocatalyst (Figure 3a). The atomic percentages of 43.1, 6.0, 45.3, and 5.6%, were detected for Zn, O, Bi, and V, respectively. The result confirms the element composition in the prepared photocatalyst. Furthermore, the dispersion of all elements was elucidated by EDS elemental mapping (Figure 3b). The uniform distribution of Zn, O, Bi, and V elements was confirmed.
Figure 3.
EDS spectrum and SEM image of the mapping area (a) and EDS elementary mapping of Zn O, Bi, and V obtained from the ZnO/BiVO4 photocatalyst (b).
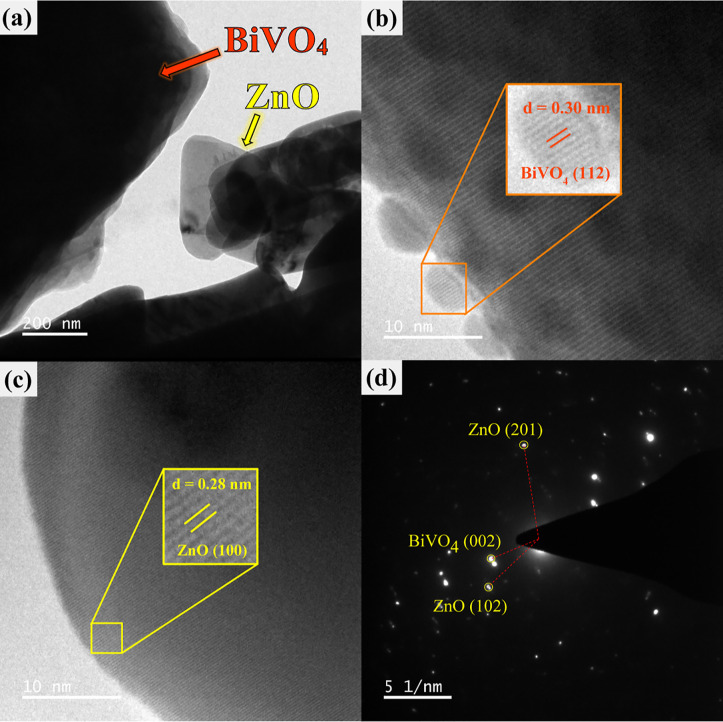
The successful creation of ZnO/BiVO4 was also examined by the TEM method. The TEM image of the heterojunction is shown in Figure 4a. The HR-TEM micrographs in Figure 4b,c showed the interplanar spacings of 0.30 and 0.28 nm, assigned to the existence of the (112) crystal plane of BiVO4 and the (100) plane of ZnO, respectively. The selected area electron diffraction (SAED) pattern (Figure 4d) revealed the monocrystalline nature of the prepared photocatalyst comprising those of ZnO and BiVO4.5 The results agree well with those from XRD, FT-IR spectroscopy, and EDS–FESEM presented previously. Thus, the co-existence of both ZnO and BiVO4 was confirmed after the fabrication of the ZnO/BiVO4 photocatalyst via a facile ultrasonication method.
Figure 4.
TEM image (a), HR-TEM image (b,c), and SAED pattern (d) obtained from the ZnO/BiVO4 photocatalyst.
The textural properties of the samples were examined from a nitrogen (N2) adsorption–desorption isotherm. According to the IUPAC classification, the samples showed a type IV isotherm, as seen in Figure 5a.5 The ZnO/BiVO4 catalyst showed a specific surface area of 4.2 m2/g, which is greater than that of bare ZnO. In addition, a Barett–Joyner–Halenda pore volume of about 0.32 cm3/g and an average pore diameter of about 310 nm were reported. The size distribution is shown in Figure 5b.
Figure 5.
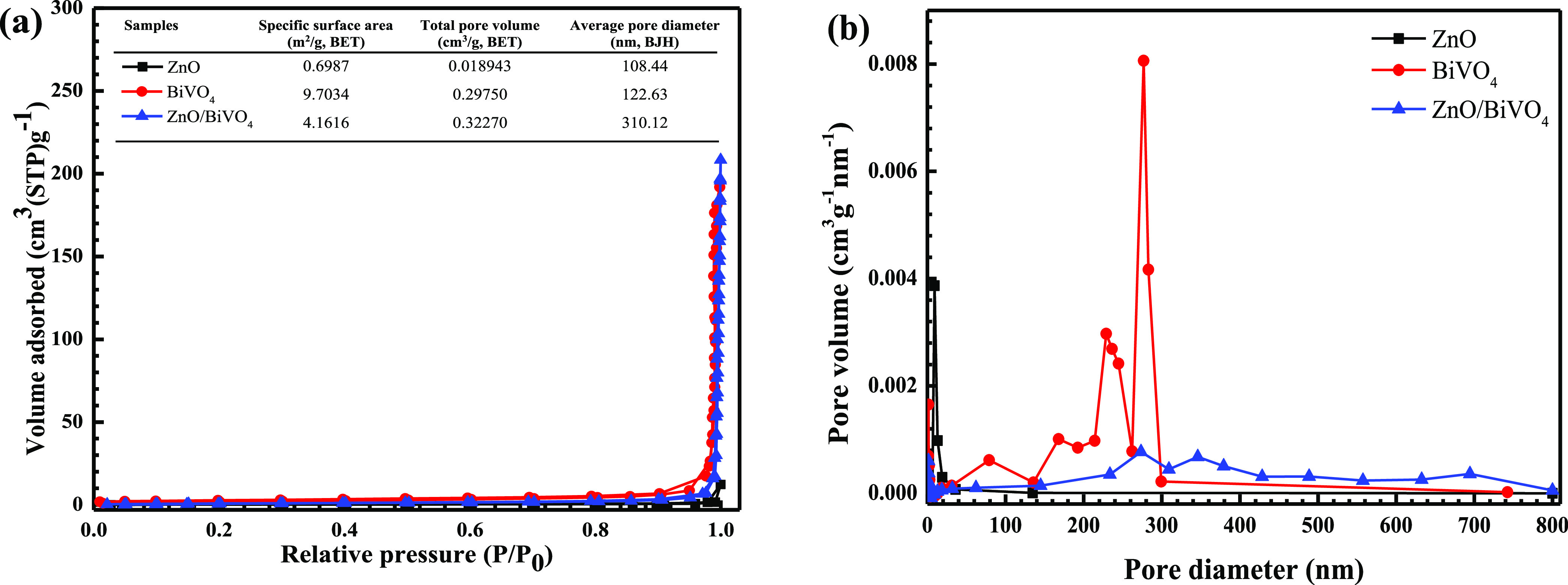
N2 adsorption–desorption isotherms (a) and pore size distributions (b) of ZnO, BiVO4, and ZnO/BiVO4 photocatalysts.
Figure 6a shows the UV–vis diffuse reflectance spectra of the samples. The band gap of each sample was determined by the Kubelka–Munk formula, as seen in Figure 6b.5,16,17 Accordingly, the absorption edge values of 387 and 585 nm were detected from pristine ZnO and bare BiVO4, respectively. After the creation of the binary composites, two absorption edge values at 397 and 585 nm were found. The former might be due to the contribution from the generated heterojunction, with a slight shift of the absorption edge, while the latter is mainly due to the contribution from the BiVO4 photocatalyst. The corresponding band gap values of bare ZnO and pristine BiVO4 were 3.20 and 2.12 eV, respectively. It should be noted that a slight shift of the band gap toward visible light was obtained after the creation of the ZnO/BiVO4 heterojunction. This will result in an increase in the resultant photoactivity under natural sunlight.
Figure 6.

Diffused reflectance spectra (a), Tauc plot for the determination of the band gap (b), and PL spectra of the samples using λexcitation values of 320 (c) and 360 nm (d).
To roughly compare the charge carrier separation efficiency in the prepared photocatalysts, the PL spectroscopic method was performed.65 According to the PL spectra in Figure 6c,d, the ZnO/BiVO4 photocatalyst provided the lowest intensity in the PL spectra, compared to pristine components, implying the lowest charge carrier recombination rate found in this sample. Therefore, the highest photocatalytic performance, compared to that of ZnO and BiVO4, would be observed.
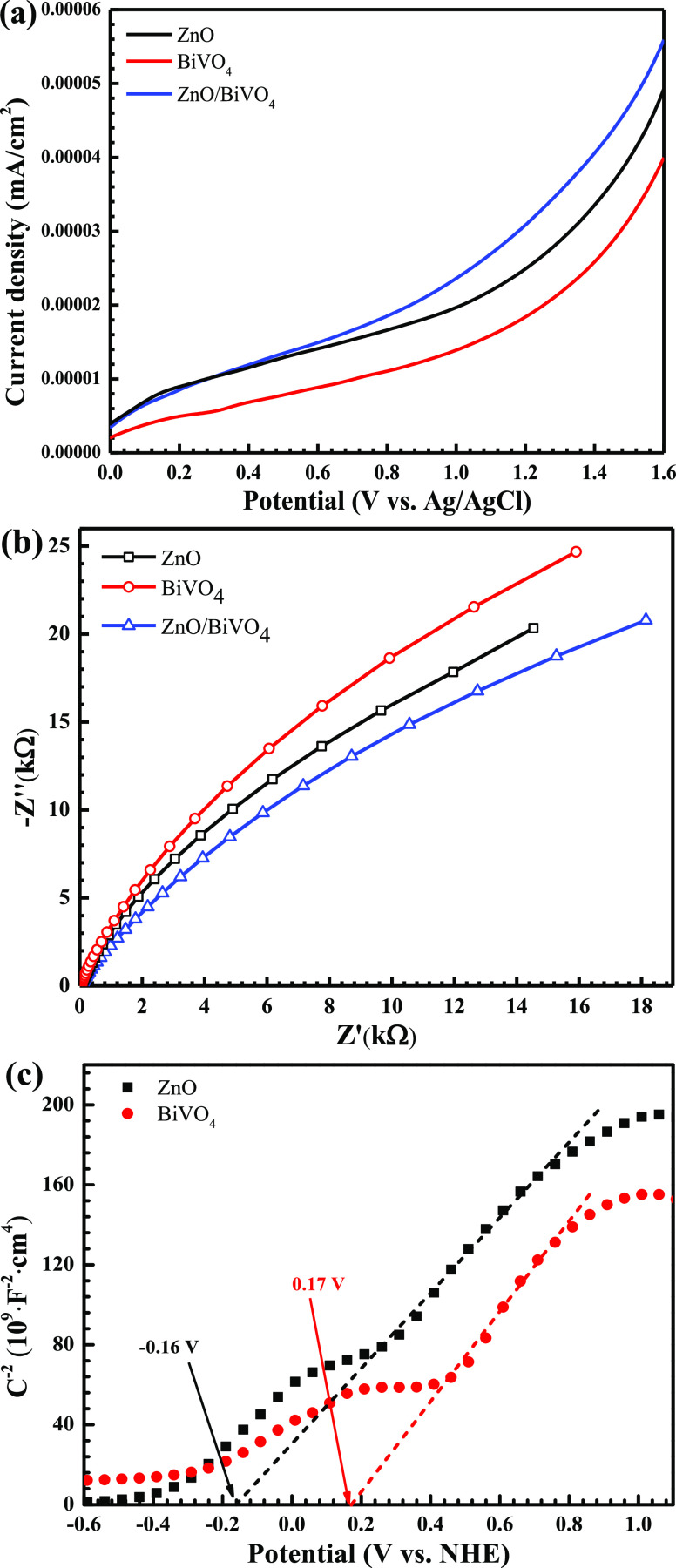
The electrochemical technique was performed to determine the spatial transfer and separation of electron–hole pairs using the linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS) methods.16,17 The highest current density was obtained from ZnO/BiVO4, compared to the bare photocatalysts (Figure 7a), suggesting that the highest photoactivity could be found from this sample. The successful generation of the heterojunction photocatalyst provides an improvement of charge separation efficiency, causing an increase in the resultant photoactivity.5,6,19 The result agrees well with the trend of relative intensity found in the PL spectra.
Figure 7.
LSV scans plots (a), EIS Nyquist plots (b), and Mott–Schottky plots (c) of the samples using a frequency of 30 Hz in 0.1 M Na2SO4.
The charge recombination rate was estimated by performing EIS. In principle, the arc radius provides the information concerning the charge transfer process at the electrode/electrolyte interface. The lowest radius being obtained from the ZnO/BiVO4 photocatalyst (Figure 7b) implies the lowest charge-transfer resistance, indicating that the greatest photocatalytic performance is expected to be detected from the ZnO/BiVO4 heterojunction, compared to that of bare ZnO and pristine BiVO4. It can be concluded that the results from the PL spectra, the LSV plots, and the EIS plots confirm the enhanced photoactivity of the prepared heterojunction, in comparison to those obtained from the individual photocatalysts.
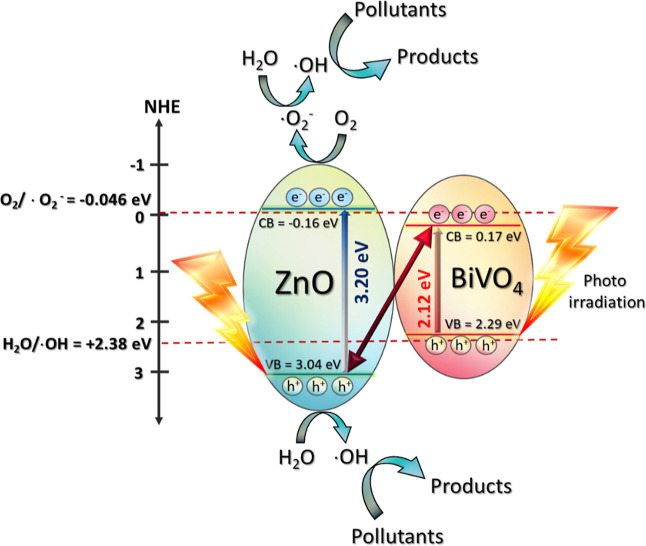
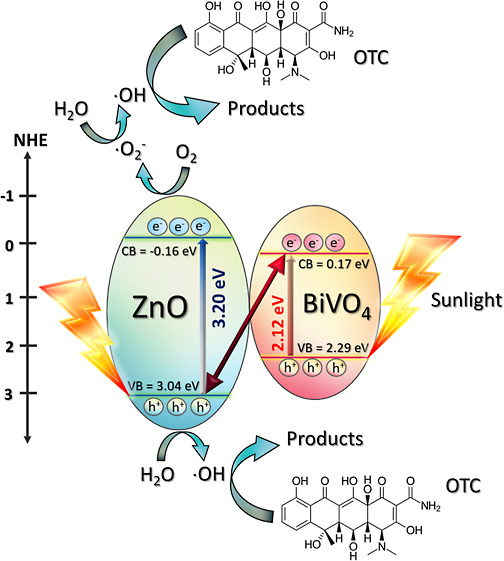
To understand the mechanism regarding the enhanced efficiency of the photocatalyst, the Mott–Schottky plots were elucidated for the determination of the band structures of the heterojunction (Figure 7c). The details have been shown previously.5,17 The flat band (VFB) level of bare ZnO was found to be −0.16 eV, while that of pristine BiVO4 was about 0.17 eV. The values are nearly the same as the conduction band (VCB) levels of the photocatalysts5,6 such that the VCB values of ZnO and BiVO4 were found to be −0.16 and 0.17 eV, respectively (see Figure 7c). The prepared ZnO (Eg of 3.20 eV) showed the VVB level of 3.04 eV. BiVO4 (Eg of 2.12 eV) displayed the VVB value of 2.29 eV. In summary, the values of VCB and VVB levels obtained from both ZnO and BiVO4 are depicted later in Figure 13. The charge transfer between ZnO and BiVO4 was also proposed.
Figure 13.
Photocatalytic mechanism schemes based on degradation of the pollutants after photoirradiation of the ZnO/BiVO4 photocatalyst.
3.2. Photodegradation Study
The photocatalytic degradation of the RR141 dye and OTC antibiotic was investigated under both simulated UV light and natural solar light.
3.2.1. Photodegradation of the Pollutants under UV Light
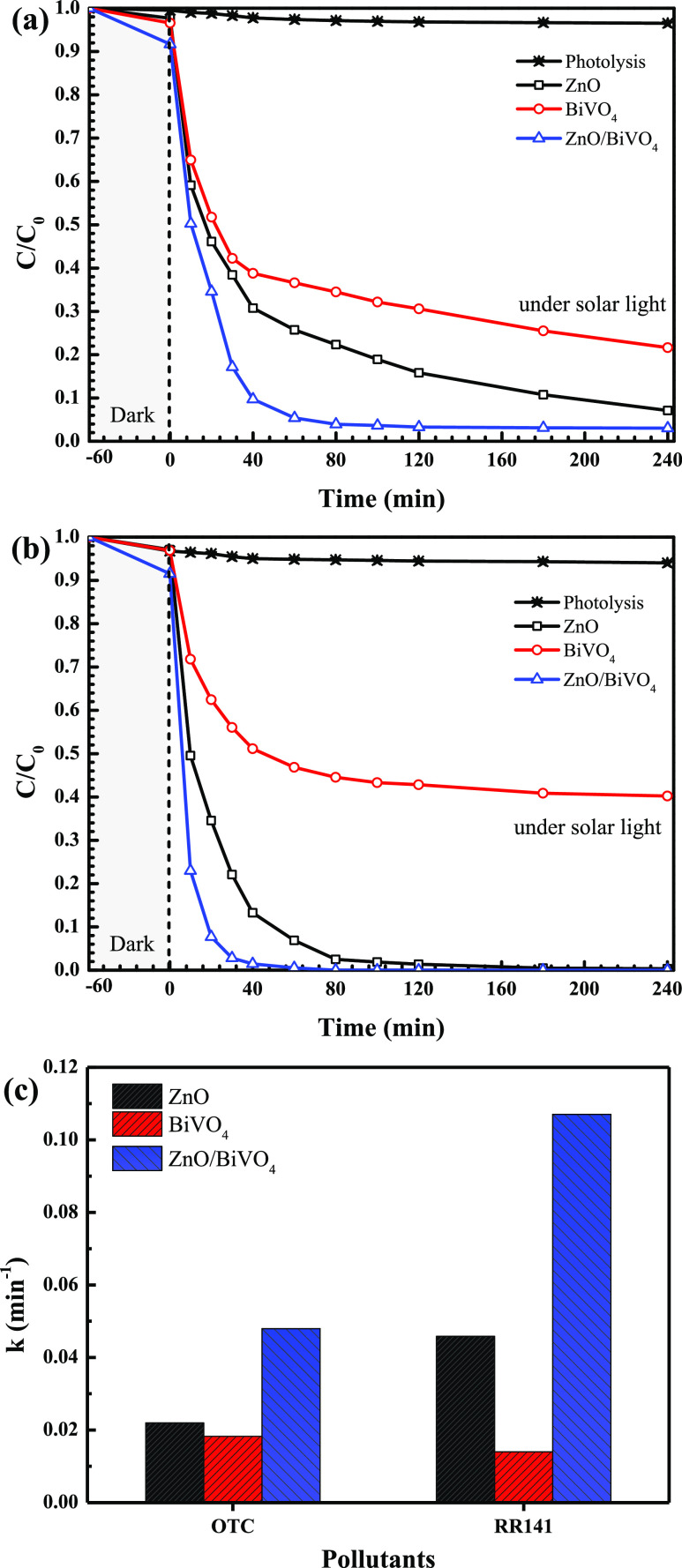
Photodegradation of the OTC antibiotic and RR141 dye after UV light irradiation (a mercury lamp, 125 W) was studied. As seen in Figure 8a, lowering of OTC content with time indicates the removal of this antibiotic. The photolysis of OTC is negligible. Less than 5% removal of OTC via the adsorption process was detected in the presence of ZnO/BiVO4. Interestingly, enhanced detoxification of OTC was detected under light illumination. The photoactivity of about 88% was reported after 240 min (Figure 8c). The photoactivity of the binary heterojunction is about 1.5 and 1.9 times higher than those obtained from bare ZnO and pristine BiVO4, respectively.
Figure 8.
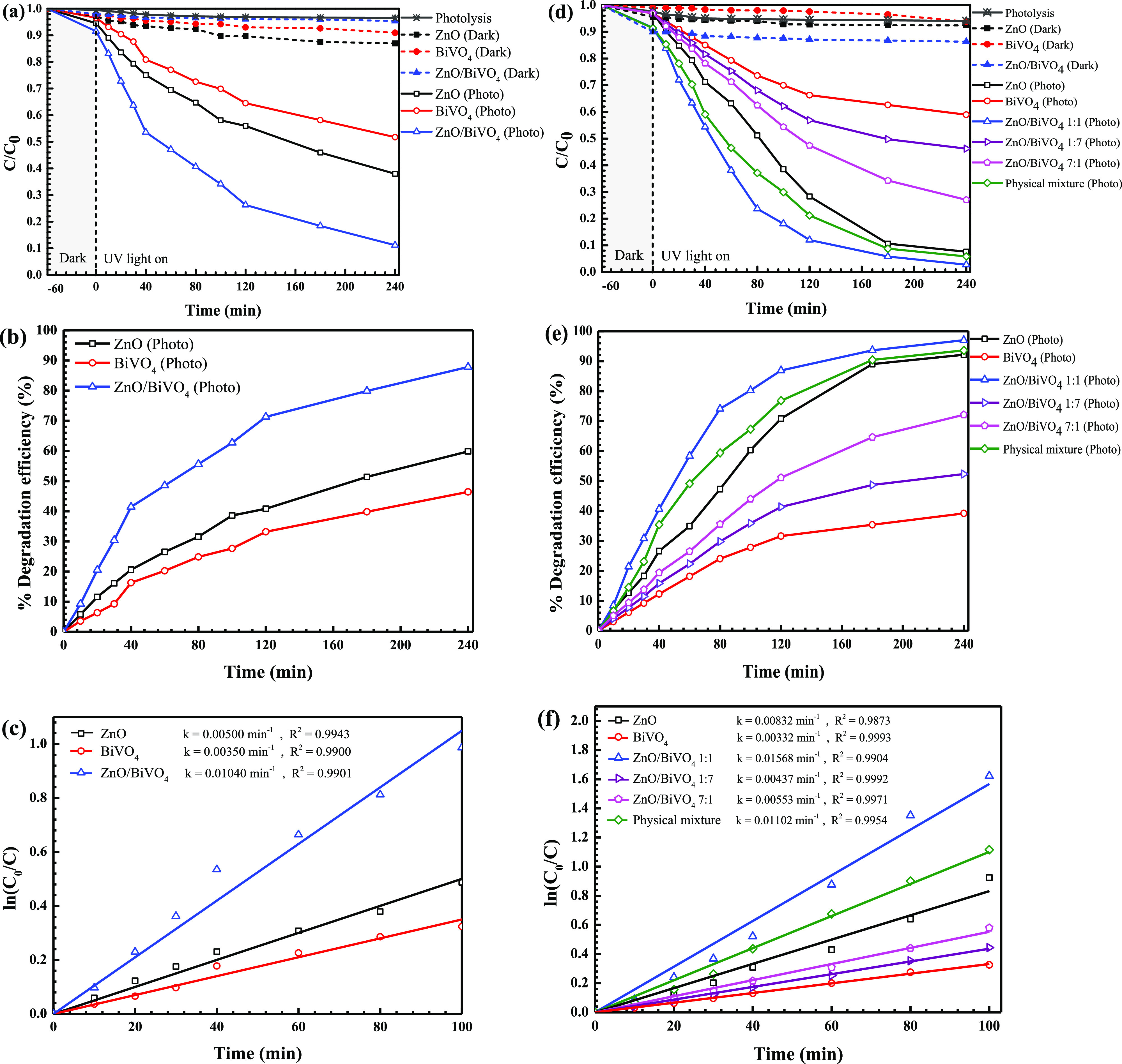
Plots of OTC antibiotic concentration (a) and RR141 dye (b) vs time, the photodegradation efficiency of OTC (c) and RR141 (d) degradation, and ln(C0/C) vs time obtained from the photodegradation of the OTC antibiotic (e) and RR141 (f).
On examining the RR141 degradation, Figure 8b shows a decrease of RR141 concentration with time. The photolysis of the RR141 dye can be neglected. In addition, in the presence of ZnO/BiVO4, lower than 13% of RR141 was removed via the adsorption process. Interestingly, enhanced photocatalytic degradation of RR141 under UV light was detected. The photoactivity reaches about 97% after 240 min (Figure 8d). For comparison, the physical mixture of ZnO and BiVO4 was also used as a photocatalyst. However, this sample still shows lower performance than the ZnO/BiVO4 photocatalyst. The result strongly indicates that the most important role is of the heterojunction photocatalyst for the improvement of the resultant photoactivity. As seen from Figure 8d, the heterojunction provides an enhanced performance, which is about 1.04, 2.66, and 1.03 times greater than those reported from bare ZnO, pristine BiVO4, and the physical mixture, respectively, after 180 min.
The photodegradation reaction of both the OTC antibiotic and RR141 dye follows the first-order reaction (Figure 8e,f). The rate constants (k) of 0.0104 and 0.0157 min–1 were observed after UV light irradiation. ZnO/BiVO4 shows the highest rate constants among all prepared samples. This correlates well with the greatest efficiency found in the heterojunction presented previously. On examining OTC removal (Figure 8e), ZnO/BiVO4 shows a rate constant that is about 2.08 and 2.97 times greater than those of ZnO and BiVO4, respectively. On examining RR141 degradation (Figure 8f), it is noted that the ZnO/BiVO4 heterojunction provides a rate constant that is about 1.89, 4.76, and 1.43 times greater than those obtained from bare ZnO, pristine BiVO4, and the physical mixture, respectively. All in all, the greatest photodegradation efficiency together with the highest rate constant found in the ZnO/BiVO4 heterojunction photocatalyst is mainly due to the enhancement of the surface area, compared to that of bare ZnO, and the suppression of the charge carrier recombination rate after successful construction of the binary heterostructure.
3.2.2. Photodegradation of the Pollutants under Natural Sunlight
The photocatalytic degradation of OTC and RR141 was also studied under sunlight, as seen in Figure 9. By determining OTC degradation, an efficiency of about 95% was achieved after 80 min, in the presence of the ZnO/BiVO4 photocatalyst (Figure 9a). Accordingly, a rate constant of about 0.0479 min–1 was obtained (Figure 9c). It can be clearly seen that the ZnO/BiVO4 heterojunction shows the highest efficiency and the greatest rate constant in comparison to those of bare ZnO and pristine BVO4. In the case of RR141 degradation, the same trend was also observed. With the addition of ZnO/BiVO4, the highest performance reached 100% after only 80 of solar light irradiation (Figure 9b). The rate constant of 0.1071 min–1 was obtained. All in all, the results from both UV light and solar light support the enhanced photocatalytic performance of the binary heterojunction, compared to that of the individual photocatalysts. This is mainly due to the improvement of the surface area, compared to that of bare ZnO, and the remarkable enhancement of charge separation at the interface.
Figure 9.
Lowering of OTC antibiotic (a) and RR141 dye (b) concentrations with time under natural sunlight and the rate constants of the photocatalysts toward the degradation of the OTC antibiotic and RR141 dye (c).
3.2.3. Influence of the Experimental Parameters on Photoactivity
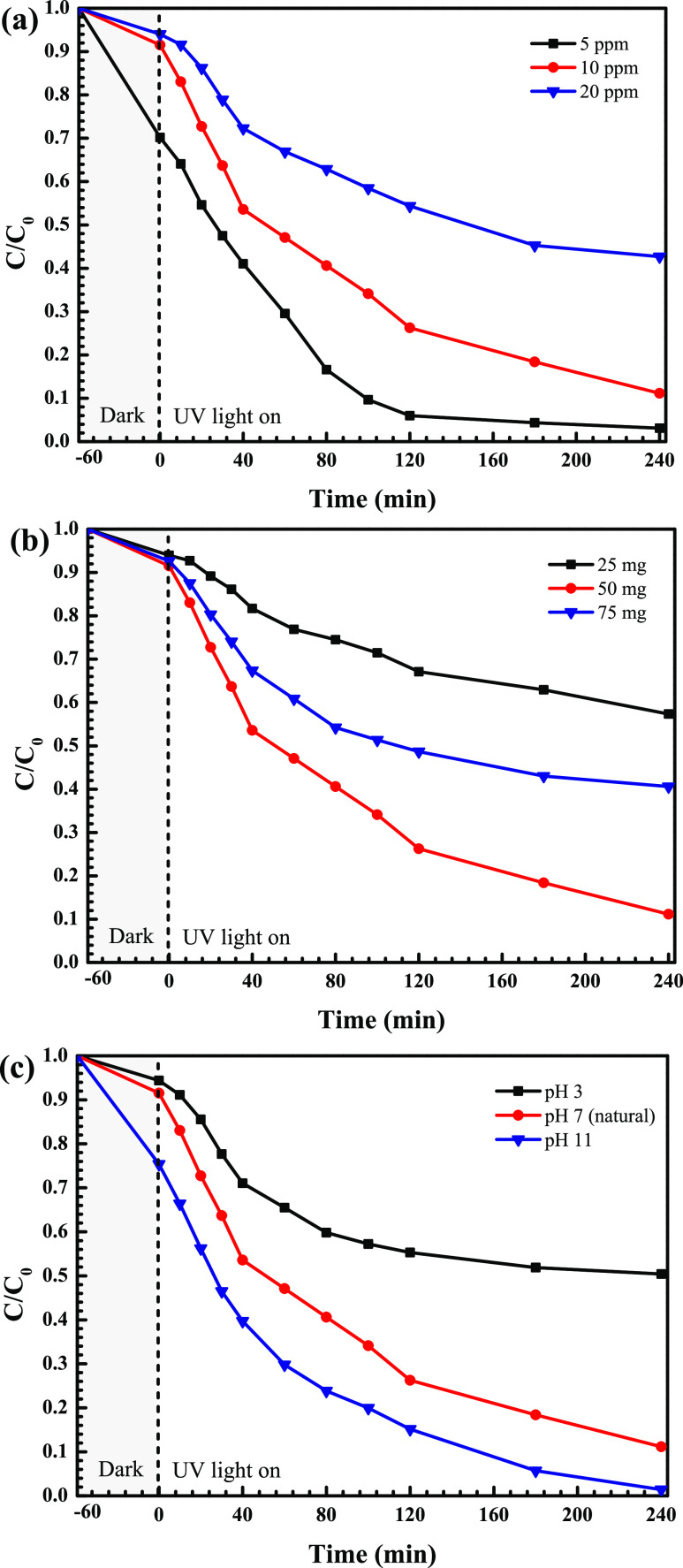
The influence of some experimental parameters on OTC degradation was investigated. It can be seen from Figure 10a that increasing OTC concentration results in lowering of degradation efficiency. The enhancement of OTC concentration results in much more absorption of light by the OTC molecules instead of the catalyst. Therefore, a decrease of photon flux reaching the photocatalyst causes the lowering of the resultant photocatalytic performance. The addition of 20 ppm OTC showed the lowest photoactivity. It should be noted that after 240 min, the efficiency reached about 90% with an initial OTC concentration of 10 ppm. Thus, this concentration was used for further study.
Figure 10.
Effect of initial concentration (a), catalyst content (b), and initial solution pH (c) on photodegradation of the OTC antibiotic.
The effect of photocatalyst content on photocatalytic performance was also elucidated. As seen in Figure 10b, the addition of a catalyst content of up to 50 mg causes an increase in photoactivity, resulting from the enhancement of OTC adsorption on the photocatalyst surface together with the increment of the photocatalyst density over one unit area of photo irradiation.5,17 However, a drop of the performance was found after 75 mg of the catalyst was added. Therefore, the catalyst loading was fixed at 50 mg for further investigation.
The effect of the initial solution pH (3–11) on photocatalytic activity was also elucidated (Figure 10c). The OTC aqueous solution showed the natural solution pH of 7. A drastic decrease of the photocatalytic performance at an extremely acidic condition (pH = 3) is mainly assigned to the dissolution of the photocatalyst. On examining the extreme basic condition of pH = 11, in principle, a negative charge on the photocatalyst surface and a negative charge of OTC molecules (pKa = 3.57, 7.79, and 9.88) co-existed.5,17 This causes the repulsion between the photocatalyst and the OTC antibiotic so that the lowering of the performance could be expected. However, the pH of about 11 provides the highest photoactivity compared to the natural pH of about 7. This can be explained in terms of the much higher production of active species such as hydroxyl radicals under the basic condition.16,17 The higher the number of active radicals, the greater the resultant photoactivity.
Furthermore, the influence of some parameters on RR141 degradation was also elucidated (see Figure 11). First, the lowest dye concentration of 5 ppm showed the greatest performance (Figure 11a). A decrease of the efficiency with an increment of dye concentration was observed. However, 10 ppm of RR141 is the optimal concentration for further investigation. Second, the effect of the catalyst content on the efficiency is shown in Figure 11b. An increase in the catalyst loading of up to 50 mg provided the maximum photoactivity within 240 min. Third, the influence of pH on the photocatalytic activity was studied over the pH range of 3–11 (Figure 11c). The natural pH of the dye solution is about 7. A dramatic drop of the performance at a pH of 3 was mainly due to the dissolution of the prepared photocatalyst under the acidic condition. The performance obtained from the natural pH of 7 showed the efficiency to be as high as that obtained from the basic condition (pH of 11). Therefore, the result confirms no need to adjust the initial solution pH of the RR141 dye.
Figure 11.
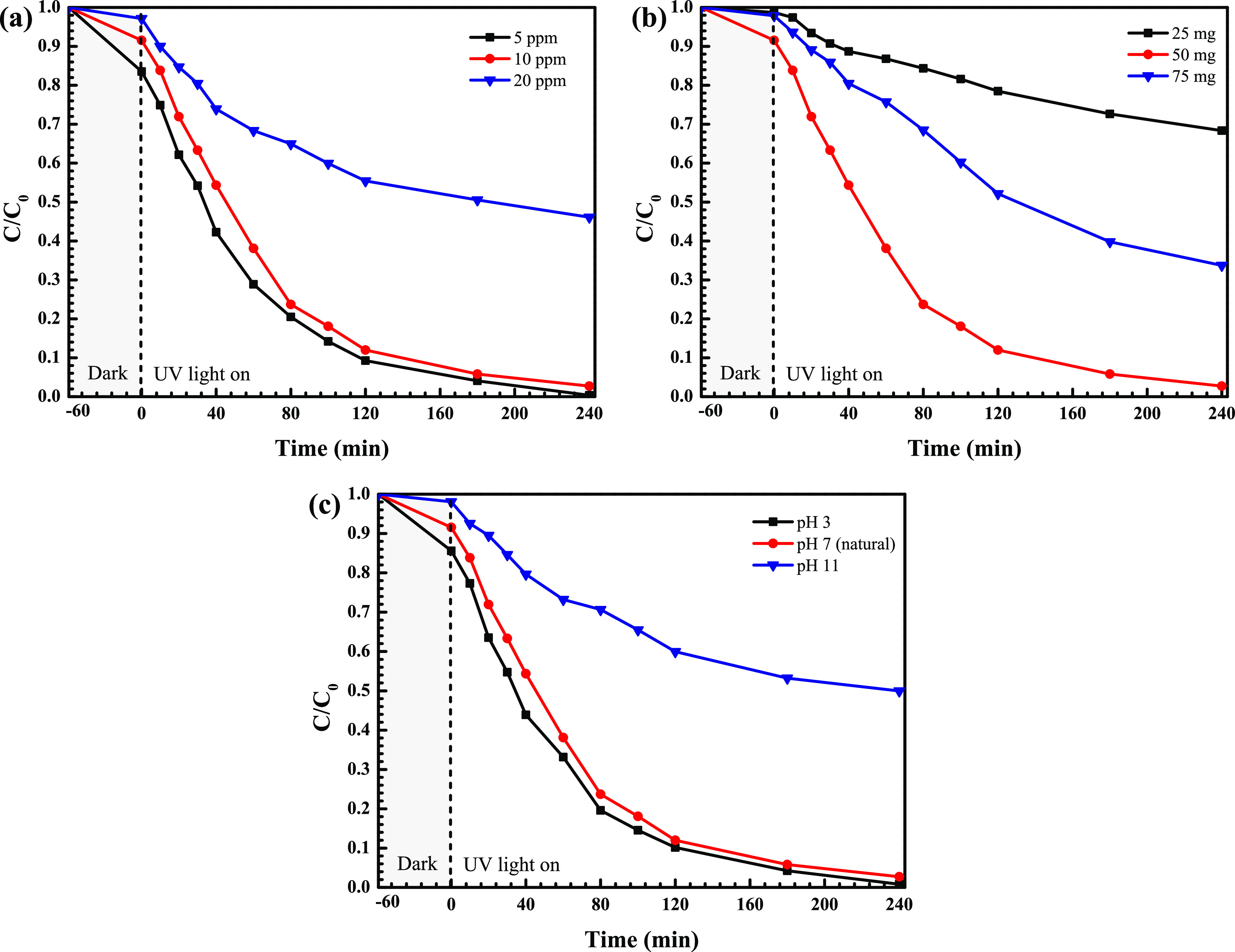
Effect of initial concentration (a), catalyst content (b), and initial solution pH (c) on photodegradation of the RR141 dye.
3.2.4. Photodegradation Mechanism and Recycling Ability
The photodegradation mechanism of the OTC antibiotic was examined by the trapping method.5,16,17 The photoactivity, in the presence of various quenchers, was elucidated. A dramatic decrease of photoactivity was observe after the addition of IPA (Figure 12a,b), implying the most important role of the hydroxyl radicals in the degradation of the antibiotic. The rate constant also decreases with the addition of IPA (Figure 12c). In addition, photogenerated holes play a subordinate role in the removal of the pollutants.
Figure 12.
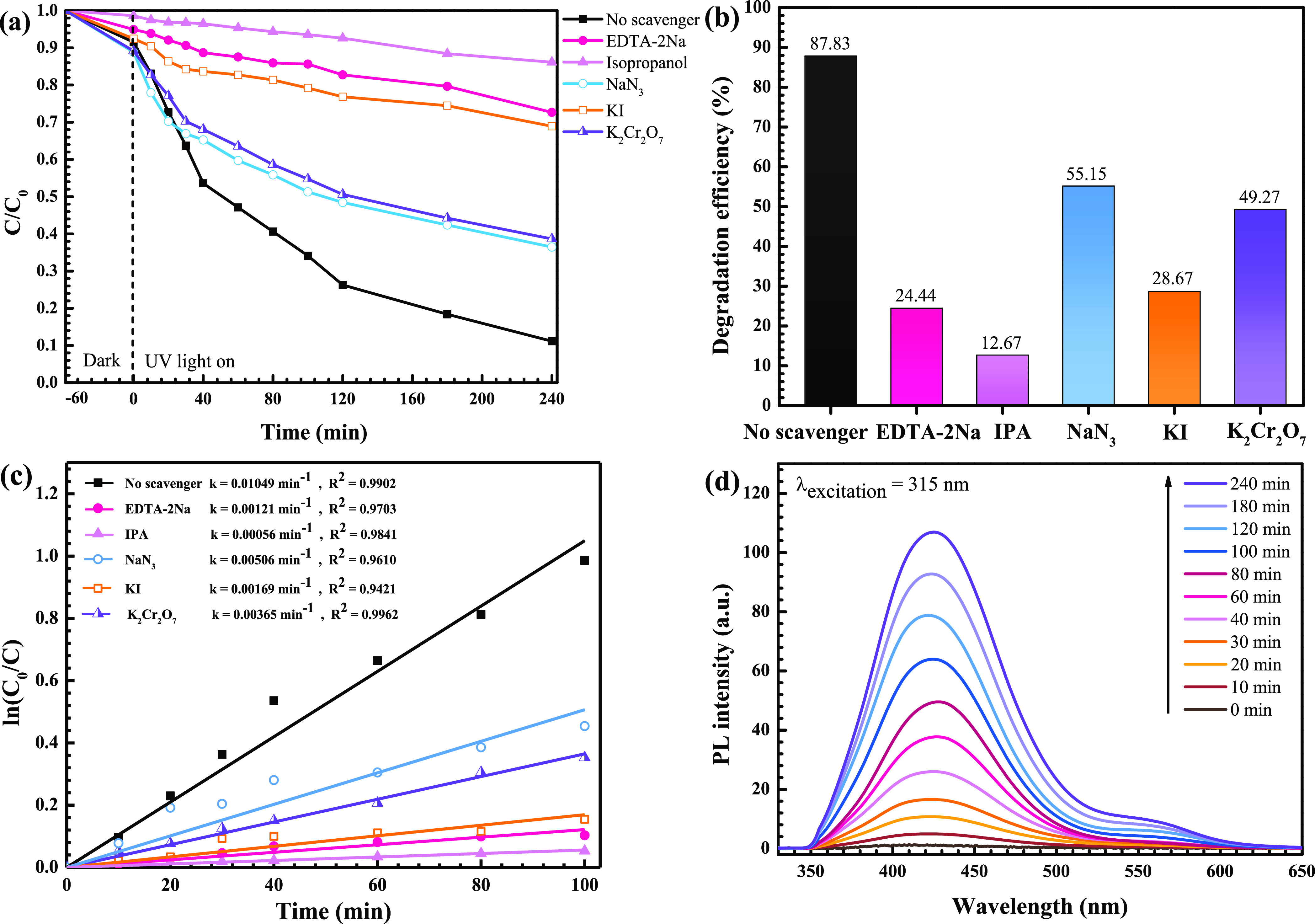
Lowering of C/C0 due to photodegradation (a), bar chart showing photodegradation efficiency (b), rate constants of the photocatalysts toward degradation of the OTC antibiotic after the addition of various scavengers (c), and hydroxyl radical trapping PL spectra of the solution after photo-illumination of the ZnO/BiVO4 photocatalyst (d).
Furthermore, monitoring of the hydroxyl radical (•OH) after light irradiation was carried out via the TA probe technique.17 The fluorescence product, 2-hydroxyterephthalic acid (TA-OH), was detected. The increasing of PL peak intensity at 425 nm with increasing irradiation time was clearly observed, as seen in Figure 12d. Again, the result strongly confirms the major role of the hydroxyl radicals in the degradation of the toxic pollutant.
Upon light irradiation, the photogeneration of the electrons and holes were occurred in the conduction band (CB) and the valence band (VB), respectively. After that, the formation of reactive species would be expected. In principle, the energy level of the CB and VB edges of both BiVO4 and ZnO photocatalysts can be determined by using the Mulliken electronegativity theory as follows
| 4 |
| 5 |
where EVB is the VB potential, ECB is the CB potential, EC is the standard hydrogen electrode potential (≈4.5 eV), and χ is the absolute value of the electronegativity of the semiconducting photocatalyst.3,6,16 The values of the electronegativity were 2.34 and 5.79 eV, respectively, for BiVO4 and ZnO.3,6,16 In theory, the VB and CB potentials of the BiVO4 photocatalyst were found to be 2.71 and 0.37 eV, respectively, while the corresponding values of the ZnO photocatalyst were found to be 2.90 and −0.31 eV, respectively. In practice, however, after the creation of the heterojunction, the CB potential levels of ZnO and BiVO4 are found to be −0.16 and 0.17 eV, respectively, as shown previously from the Mott–Schottky plots in Figure 7c. The band gap energy of ZnO and BiVO4 are 3.20 and 2.12 eV, respectively. Therefore, the VB potential levels of 3.04 and 2.29 eV can be obtained for ZnO and BiVO4, respectively.
To determine the heterojunction photocatalyst, the creation of a heterostructure based on the coupling of two photocatalysts has been demonstrated.39,47,69−71 The type II and Z-scheme heterojunctions were previously proposed. However, it is accepted that the electron transfer mechanism of the traditional Z-scheme photocatalyst and all-solid-state Z-scheme photocatalyst still exhibit some drawbacks. Alternatively, the novel model based on a S-scheme photocatalyst was proposed.65−68 In this case, the charge separation and high redox ability are obtained. In theory, extended photo-absorption, excellent charge separation, and a strong redox potential are collectively gained. This heterojunction mechanism can be applied nicely in the present research. Therefore, the removal of toxic pollutants by the ZnO/BiVO4 S-scheme heterojunction photocatalyst is proposed in Figure 13. The photocatalytic degradation of the toxic contaminant can be summarized as follows.
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
Reusability is a crucial factor influencing the practical use of the synthesized photocatalyst.5,6,16,17 Thus, the cycling ability of the photocatalyst after the removal of the RR141 dye and OTC antibiotic was studied. The ZnO/BiVO4 photocatalyst still provides high photocatalytic performance even after five cycles of use (Figure 14). The chemical structural of the photocatalyst after pollutant degradation was investigated. The stability of the structure was confirmed (Figure 15a). The identical FT-IR spectra (Figure 15b) and PL spectra (Figure 15c,d) suggested the chemical stability of the photocatalyst as well. Additionally, the morphology of the used photocatalyst (Figure 15e) is more or less the same as that received from the fresh photocatalyst (Figure 2), implying the stability of the sample morphology.
Figure 14.
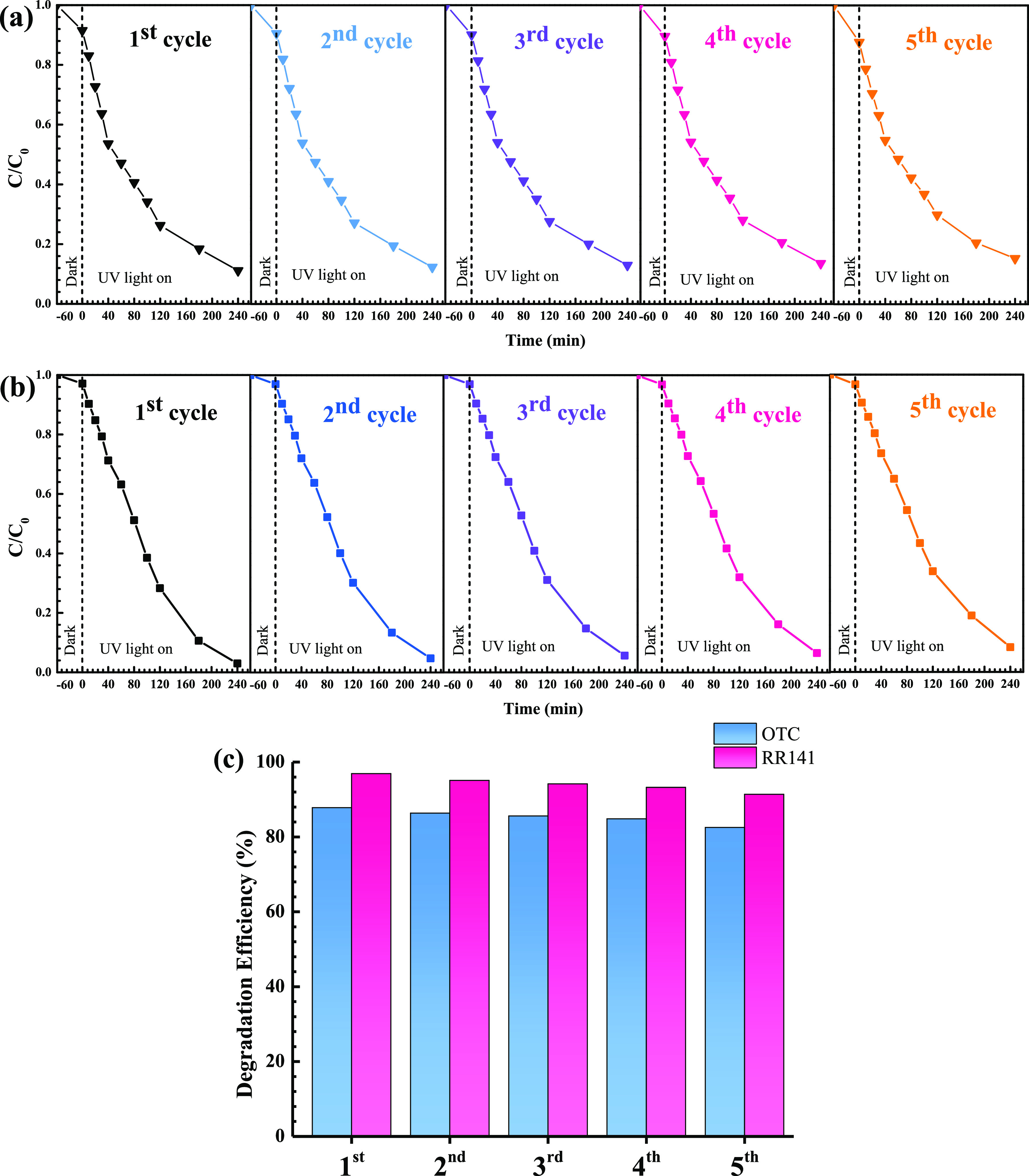
Reusability of the ZnO/BiVO4 photocatalyst for photodegradation of the OTC antibiotic (a) and RR141 dye (b) and the bar charts (c) showing the cycling ability of the photocatalyst.
Figure 15.
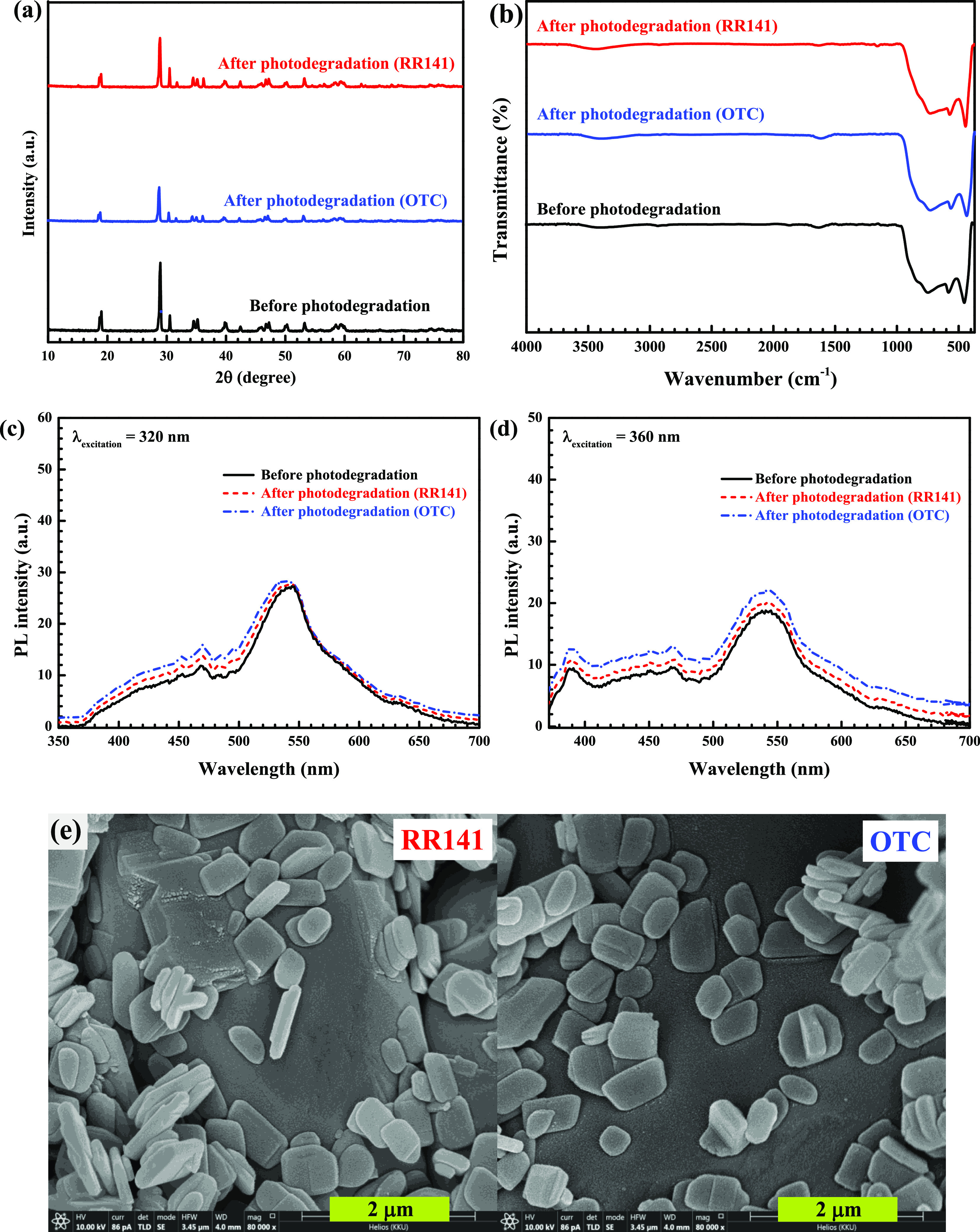
XRD patterns (a), vibrational spectra (b), and PL spectra using λexcitation values of 320 (c) and 360 nm (d) of the fresh and used photocatalyst and SEM images of the ZnO/BiVO4 photocatalyst after the removal of the RR141 dye and OTC antibiotic (e).
It is important to noted that leaching of Zn2+ after photodegradation was also one major concern. The measurement of Zn2+ in the solution was performed. It can be shown that bare ZnO showed 7 ppm Zn2+ in the pollutant solution, while ZnO/BiVO4 exhibited only 0.7 ppm Zn2+. The result strongly confirms the improvement of the photocatalyst stability after the creation of the heterojunction. The photoactivity of various catalysts toward degradation of the RR141 dye and OTC antibiotic has been studied.1,6,7,13,16,17,20,21,72−84 In this work, the ZnO/BiVO4 photocatalyst is used for the degradation of the toxic pollutants under UV light and natural sunlight. The performance of the synthesized photocatalyst together with those reported in the literature is shown in Table 1. By determining RR141 degradation, bare ZnO provided the photoactivity of 95–98%.6,72,76 Capped ZnO, by using sodium dodecyl sulfate (SDS) or polyvinylpyrrolidone (PVP), showed the performance of 60–100%.1,7,73 In the case of metal-doped ZnO, copper-doped ZnO and lead-doped ZnO displayed the performances of 89 and 96%, respectively.76,77 The ZnO/CdS composite exhibited the photoactivity of 80%.6 In addition, the pristine bismuth molybdate photocatalyst showed the efficiency of 37–70%.74,75 Interestingly, in the present work, the ZnO/BiVO4 heterojunction showed the performances of 97 and 100% under UV light and sunlight, respectively. In terms of OTC removal, bare ZnO showed the efficiency of 50% after 150 min of visible light irradiation.78 In the case of pristine BiVO4, the performance of 52–93% was detected.13,16,17,79 The binary composites based on BiVO4 showed a high efficiency of about 68% .20,80 The ZnO–TiO2 photocatalyst provided the performance of 90%.81 Enhancement of the efficiency to about 55–99.8% was reported in the ternary composites based on either ZnO or BiVO4.13,21,74,82−84 In the present work, the prepared ZnO/BiVO4 catalyst exhibited high photocatalytic performances of 88 and 95% under UV light and sunlight, respectively. To sum up, the prepared heterojunction catalyst displayed enhanced performance toward the degradation of the RR141 dye and OTC antibiotic under the economical natural sunlight. The synthesis of the photocatalyst can be performed perfectly without the doping of expensive noble metals such as gold or silver. The present research shows the novel route for detoxification of the harmful dye and antibiotic in aqueous solution by utilization of the abundant solar energy.
Table 1. Comparison of the RR141 Azo Dye and OTC Antibiotic Degradation by Using Various Photocatalysts.
| catalyst | concentration (mg L–1) | catalyst loading (mg) | light source | lamp (W) | time (min) | photodegradation (%) | refs |
|---|---|---|---|---|---|---|---|
| Photodegradation of RR141 Azo Dye | |||||||
| ZnO | 10 | 50 | UV | 125 | 240 | 95 | (72) |
| ZnO | 10 | 50 | UV | 125 | 240 | 98 | (6) |
| ZnO | 30 | 30 | UV | 120 | 96 | (76) | |
| SDS-capped ZnO | 10 | 50 | UV | 125 | 240 | 100 | (1) |
| SDS-capped ZnO | 10 | 50 | UV | 125 | 240 | 95 | (7) |
| SDS-capped ZnO | 10 | 50 | visible | 15 | 240 | 60 | (7) |
| SDS-capped ZnO | 10 | 50 | solar light | 240 | 88 | (7) | |
| PVP-capped ZnO | 10 | 50 | UV | 125 | 120 | 100 | (73) |
| Cu–ZnO | 50 | 100 | UV | 120 | 89 | (77) | |
| 3% Pb–ZnO | 30 | 30 | UV | 120 | 96 | (76) | |
| ZnO/CdS | 10 | 50 | visible | 15 | 120 | 80 | (6) |
| Bi2MoO6 | 10 | 50 | UV | 125 | 240 | 37 | (74) |
| Bi2MoO6 | 10 | 50 | visible | 15 | 240 | 45 | (74) |
| Bi4MoO9 | 10 | 50 | UV | 125 | 240 | 68 | (75) |
| Bi4MoO9 | 10 | 50 | solar light | 240 | 70 | (75) | |
| ZnO–BiVO4 | 10 | 50 | UV | 125 | 240 | 97 | this work |
| ZnO–BiVO4 | 10 | 50 | solar light | 60 | 100 | this work | |
| Photodegradation of OTC Antibiotic | |||||||
| ZnO | 10 | 100 | visible | 300 | 150 | 50 | (78) |
| BiVO4 | 10 | 50 | visible | 15 | 240 | 55.5 | (17) |
| BiVO4 | 10 | 50 | natural sunlight | 240 | 82.8 | (17) | |
| BiVO4 | 10 | 50 | visible | 15 | 240 | 62 | (16) |
| BiVO4 | 10 | 50 | solar light | 240 | 93 | (16) | |
| BiVO4 | 10 | 50 | visible | 1000 | 120 | 61.1 | (13) |
| BiVO4 | 10 | 20 | visible | 300 | 120 | 52.7 | (79) |
| BiVO4/TiO2 | 10 | visible | 1000 | 120 | 68.4 | (20) | |
| Bi2S3/BiVO4 | 200 | 100 | visible | 500 | 960 | 67 | (80) |
| ZnO–TiO2 | 60 | 5 | solar light | 8 | 90.3 | (81) | |
| Cu doped ZnO-MWCNT | 50 | 5 | visible | 400 | 240 | 55 | (82) |
| NiCo/ZnO/g-C3N4 | 10 | 20 | visible | 300 | 50 | 71.3 | (83) |
| Ag/AgCl/BiVO4 | 20 | 50 | UV | 1000 | 120 | 97.6 | (13) |
| Ag/Ag2S/BiVO4 | 20 | 20 | visible | 500 | 150 | 99.8 | (21) |
| Ag/BiVO4/GO | 20 | 100 | visible | 500 | 70 | 90 | (84) |
| ZnO/ZnFe2O4/diatomite | 10 | 100 | visible | 300 | 150 | 95 | (78) |
| ZnO–BiVO4 | 10 | 50 | UV | 125 | 240 | 88 | this work |
| ZnO–BiVO4 | 10 | 50 | solar light | 100 | 95 | this work | |
4. Conclusions
The ZnO/BiVO4 S-scheme photocatalyst has been generated very easily by a facile hydrothermal technique followed by an ultrasonic route. The prepared ZnO/BiVO4 heterojunction showed the improvement of the surface area, compared to that of bare ZnO. The heterojunction also exhibited the lowest PL intensity, compared to those of bare ZnO and BiVO4. Accordingly, the heterostructure photocatalyst showed the enhanced sunlight-active photocatalytic activities of 100 and 95% toward degradation of the RR141 dye and OTC antibiotic, respectively. The photodegradation of the pollutant follows the first-order kinetic model. Hydroxyl radicals play the most important role in the removal of the toxic pollutants. The synthesized ZnO/BiVO4 photocatalyst still maintains excellent performance even after five cycles of use, indicating its promising cycling ability. The present finding demonstrates a promising avenue to fabricate a novel sunlight active S-scheme heterostructure photocatalyst for environmental remediation.
Acknowledgments
We would like to thank the fund from Materials Chemistry Research Center (MCRC), Khon Kaen University, Thailand. S.N. would like to thank the fund supported by Research and Graduate Studies, Khon Kaen University (Research Program, grant number RP64-2-Canabis/001), fiscal year 2021. S.N. also wishes to acknowledge the financial support from the Center of Excellence for Innovation in Chemistry (PERCH-CIC) and the Center of Excellence in Petrochemical and Materials technology (PETROMAT), Ministry of Higher Education, Science, Research, and Innovation.
The authors declare no competing financial interest.
References
- Juabrum S.; Chankhanittha T.; Nanan S. Hydrothermally Grown SDS-Capped ZnO Photocatalyst for Degradation of RR141 Azo Dye. Mater. Lett. 2019, 245, 1–5. 10.1016/j.matlet.2019.02.080. [DOI] [Google Scholar]
- Mokhtari-Shourijeh Z.; Langari S.; Montazerghaem L.; Mahmoodi N. M. Synthesis of Porous Aminated PAN/PVDF Composite Nanofibers by Electrospinning: Characterization and Direct Red 23 Removal. J. Environ. Chem. Eng. 2020, 8, 103876. 10.1016/j.jece.2020.103876. [DOI] [Google Scholar]
- Chankhanittha T.; Yenjai C.; Nanan S. Utilization of Formononetin and Pinocembrin from Stem Extract of Dalbergia Parviflora as Capping Agents for Preparation of ZnO Photocatalysts for Degradation of RR141 Azo Dye and Ofloxacin Antibiotic. Catal. Today 2022, 384-386, 279–293. 10.1016/j.cattod.2021.03.002. [DOI] [Google Scholar]
- Mahmoodi N. M.; Keshavarzi S.; Oveisi M.; Rahimi S.; Hayati B. Metal-Organic Framework (ZIF-8)/Inorganic Nanofiber (Fe2O3) Nanocomposite: Green Synthesis and Photocatalytic Degradation Using LED Irradiation. J. Mol. Liq. 2019, 291, 111333. 10.1016/j.molliq.2019.111333. [DOI] [Google Scholar]
- Chankhanittha T.; Komchoo N.; Senasu T.; Piriyanon J.; Youngme S.; Hemavibool K.; Nanan S. Silver Decorated ZnO Photocatalyst for Effective Removal of Reactive Red Azo Dye and Ofloxacin Antibiotic under Solar Light Irradiation. Colloids Surf., A 2021, 626, 127034. 10.1016/j.colsurfa.2021.127034. [DOI] [Google Scholar]
- Senasu T.; Chankhanittha T.; Hemavibool K.; Nanan S. Visible-Light-Responsive Photocatalyst Based on ZnO/CdS Nanocomposite for Photodegradation of Reactive Red Azo Dye and Ofloxacin Antibiotic. Mater. Sci. Semicond. Process. 2021, 123, 105558. 10.1016/j.mssp.2020.105558. [DOI] [Google Scholar]
- Kakarndee S.; Nanan S. SDS Capped and PVA Capped ZnO Nanostructures with High Photocatalytic Performance toward Photodegradation of Reactive Red (RR141) Azo Dye. J. Environ. Chem. Eng. 2018, 6, 74–94. 10.1016/j.jece.2017.11.066. [DOI] [Google Scholar]
- Kumar A.; Subash B.; Krishnakumar B.; Sobral A. J. F. N.; Sankaran K. R. Synthesis Characterization and Excellent Catalytic Activity of Modified ZnO Photocatalyst for RR 120 Dye Degradation under UV-A and Solar Light Illumination. J. Water Proc. Eng. 2016, 13, 6–15. 10.1016/j.jwpe.2016.07.009. [DOI] [Google Scholar]
- Kalaiarasan S.; Uthirakumar P.; Shin D.; Lee I. H. The Degradation Profile of High Molecular Weight Textile Reactive Dyes: A Daylight Induced Photocatalytic Activity of ZnO/Carbon Quantum Dot Photocatalyst. Environ. Nanotechnol., Monit. Manage. 2021, 15, 100423. 10.1016/j.enmm.2020.100423. [DOI] [Google Scholar]
- Almasian A.; Mahmoodi N. M.; Olya M. E. Tectomer Grafted Nanofiber: Synthesis, Characterization and Dye Removal Ability from Multicomponent System. J. Ind. Eng. Chem. 2015, 32, 85–98. 10.1016/j.jiec.2015.08.002. [DOI] [Google Scholar]
- Mahmoodi N. M.; Karimi B.; Mazarji M.; Moghtaderi H. Cadmium Selenide Quantum Dot-Zinc Oxide Composite: Synthesis, Characterization, Dye Removal Ability with UV Irradiation, and Antibacterial Activity as a Safe and High-Performance Photocatalyst. J. Photochem. Photobiol., B 2018, 188, 19–27. 10.1016/j.jphotobiol.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Ye S.; Zhou X.; Xu Y.; Lai W.; Yan K.; Huang L.; Ling J.; Zheng L. Photocatalytic Performance of Multi-Walled Carbon Nanotube/BiVO4 Synthesized by Electro-Spinning Process and Its Degradation Mechanisms on Oxytetracycline. J. Chem. Eng. 2019, 373, 880–890. 10.1016/j.cej.2019.05.109. [DOI] [Google Scholar]
- Dai Y.; Liu Y.; Kong J.; Yuan J.; Sun C.; Xian Q.; Yang S.; He H. High Photocatalytic Degradation Efficiency of Oxytetracycline Hydrochloride over Ag/AgCl/BiVO4 Plasmonic Photocatalyst. Solid State Sci. 2019, 96, 105946. 10.1016/j.solidstatesciences.2019.105946. [DOI] [Google Scholar]
- Jia L.; Jin Y.; Li J.; Wei Z.; Chen M.; Ma J. Study on High-Efficiency Photocatalytic Degradation of Oxytetracycline Based on a Spiral Microchannel Reactor. Ind. Eng. Chem. Res. 2022, 61, 554–565. 10.1021/acs.iecr.1c03495. [DOI] [Google Scholar]
- Xu J.; Bian Z.; Xin X.; Chen A.; Wang H. Size Dependence of Nanosheet BiVO4 with Oxygen Vacancies and Exposed {0 0 1} Facets on the Photodegradation of Oxytetracycline. J. Chem. Eng. 2018, 337, 684–696. 10.1016/j.cej.2017.12.133. [DOI] [Google Scholar]
- Hemavibool K.; Sansenya T.; Nanan S. Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation. J. Antibiot. 2022, 11, 761. 10.3390/antibiotics11060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senasu T.; Youngme S.; Hemavibool K.; Nanan S. Sunlight-Driven Photodegradation of Oxytetracycline Antibiotic by BiVO4 Photocatalyst. J. Solid State Chem. 2021, 297, 122088. 10.1016/j.jssc.2021.122088. [DOI] [Google Scholar]
- Xu W.; Zhang Q.; Xu K.; Qiu L.; Song J.; Wang L. Study on Visible Light Photocatalytic Performance of BiVO4 Modified by Graphene Analogue Boron Nitride. Chemosphere 2022, 307, 135811. 10.1016/j.chemosphere.2022.135811. [DOI] [PubMed] [Google Scholar]
- Lakhera S. K.; Hafeez H. Y.; Venkataramana R.; Veluswamy P.; Choi H.; Neppolian B. Design of a Highly Efficient Ternary AgI/RGO/BiVO4 Nanocomposite and Its Direct Solar Light Induced Photocatalytic Activity. Appl. Surf. Sci. 2019, 487, 1289–1300. 10.1016/j.apsusc.2019.05.201. [DOI] [Google Scholar]
- Wang W.; Han Q.; Zhu Z.; Zhang L.; Zhong S.; Liu B. Enhanced Photocatalytic Degradation Performance of Organic Contaminants by Heterojunction Photocatalyst BiVO4/TiO2/RGO and Its Compatibility on Four Different Tetracycline Antibiotics. Adv. Powder Technol. 2019, 30, 1882–1896. 10.1016/j.apt.2019.06.006. [DOI] [Google Scholar]
- Wei Z.; Benlin D.; Fengxia Z.; Xinyue T.; Jiming X.; Lili Z.; Shiyin L.; Leung D. Y. C.; Sun C. A Novel 3D Plasmonic P-n Heterojunction Photocatalyst: Ag Nanoparticles on Flower-like p-Ag2S/n-BiVO4 and Its Excellent Photocatalytic Reduction and Oxidation Activities. Appl. Catal., B 2018, 229, 171–180. 10.1016/j.apcatb.2018.02.008. [DOI] [Google Scholar]
- Ali A. A.; El Fadaly E. A.; Deraz N. M. Auto-Combustion Fabrication, Structural, Morphological and Photocatalytic Activity of CuO/ZnO/MgO Nanocomposites. Mater. Chem. Phys. 2021, 270, 124762. 10.1016/j.matchemphys.2021.124762. [DOI] [Google Scholar]
- Chuenpratoom T.; Hemavibool K.; Rermthong K.; Nanan S. Removal of Lead by Merlinoite Prepared from Sugarcane Bagasse Ash and Kaolin: Synthesis, Isotherm, Kinetic, and Thermodynamic Studies. Molecules 2021, 26, 7550. 10.3390/molecules26247550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Li Z.; Yahyaoui S.; Hanafy H.; Seliem M. K.; Bonilla-Petriciolet A.; Luiz Dotto G.; Sellaoui L.; Li Q. Effective Adsorption of Dyes on an Activated Carbon Prepared from Carboxymethyl Cellulose: Experiments, Characterization and Advanced Modelling. J. Chem. Eng. 2021, 417, 128116. 10.1016/j.cej.2020.128116. [DOI] [Google Scholar]
- Du X.; Yang W.; Liu Y.; Zhang W.; Wang Z.; Nie J.; Li G.; Liang H. Removal of Manganese, Ferrous and Antibiotics from Groundwater Simultaneously Using Peroxymonosulfate-Assisted in-Situ Oxidation/Coagulation Integrated with Ceramic Membrane Process. Sep. Purif. Technol. 2020, 252, 117492. 10.1016/j.seppur.2020.117492. [DOI] [Google Scholar]
- Rajput R. B.; Shaikh R.; Sawant J.; Kale R. B. Recent Developments in ZnO-Based Heterostructures as Photoelectrocatalysts for Wastewater Treatment: A Review. Environ. Adv. 2022, 9, 100264. 10.1016/j.envadv.2022.100264. [DOI] [Google Scholar]
- Jiang C. H.; Yao C. B.; Wang Z. M.; Wang X.; Wang L. Y. Heterostructure MoS2@ZnO Nanowires: Preparation, Ultrafast Nonlinear Optical Behavior and Photoelectric Functional Application. Appl. Surf. Sci. 2022, 599, 153920. 10.1016/j.apsusc.2022.153920. [DOI] [Google Scholar]
- Wang J.; Feng J.; Wei C. Molecularly Imprinted Polyaniline Immobilized on Fe3O4/ZnO Composite for Selective Degradation of Amoxycillin under Visible Light Irradiation. Appl. Surf. Sci. 2023, 609, 155324. 10.1016/j.apsusc.2022.155324. [DOI] [Google Scholar]
- Choudhary S.; Sharma M.; Krishnan V.; Mohapatra S. Facile Synthesis, Structural, Morphological, Photocatalytic and Optical Properties of CoFe2O4/ZnO Hybrid Nanostructures. Ceram. Int. 2022, 48, 34033–34045. 10.1016/j.ceramint.2022.08.003. [DOI] [Google Scholar]
- Ouriques Brasileiro I. L.; Madeira V. S.; Lopes-Moriyama A. L.; Rodrigues de Almeida Ramalho M. L. Addition of G-C3N4 to ZnO and ZnFe2O4 to Improve Photocatalytic Degradation of Emerging Organic Pollutants. Ceram. Int. 2023, 49, 4449–4459. 10.1016/j.ceramint.2022.09.331. [DOI] [Google Scholar]
- Li D.; Zhang W.; Niu Z.; Zhang Y. Improvement of Photocatalytic Activity of BiOBr and BiOBr/ZnO under Visible-Light Irradiation by Short-Time Low Temperature Plasma Treatment. J. Alloys Compd. 2022, 924, 166608. 10.1016/j.jallcom.2022.166608. [DOI] [Google Scholar]
- Yang X.; Sun S.; Ye L.; Yun D.; Liu C.; Guo Y.; Yang B.; Yang M.; Yang Q.; Liang S.; Cui J. One-Pot Integration of S-Doped BiOCl and ZnO into Type-II Photocatalysts: Simultaneously Boosting Bulk and Surface Charge Separation for Enhanced Antibiotic Removal. Sep. Purif. Technol. 2022, 299, 121725. 10.1016/j.seppur.2022.121725. [DOI] [Google Scholar]
- uz Zaman F.; Nagamuthu S.; Cui K.; Hou L.; Yuan C. Microwave-Assisted Synthesis of Porous Heterojunction ZnO/ZnMn2O4 Microrods for Efficient Degradation of Organic Pollutants. Inorg. Chem. Commun. 2022, 144, 109845. 10.1016/j.inoche.2022.109845. [DOI] [Google Scholar]
- Ramesh Reddy N. R.; Mohan Reddy P. M.; Hak Jung J. H.; Woo Joo S. W. Construction of Various Morphological ZnO-NiO S-Scheme Nanocomposites for Photocatalytic Dye Degradation. Inorg. Chem. Commun. 2022, 146, 110107. 10.1016/j.inoche.2022.110107. [DOI] [Google Scholar]
- Chen Z.; Dedova T.; Spalatu N.; Maticiuc N.; Rusu M.; Katerski A.; Acik I. O.; Unold T.; Krunks M. ZnO/NiO Heterostructures with Enhanced Photocatalytic Activity Obtained by Ultrasonic Spraying of a NiO Shell onto ZnO Nanorods. Colloids Surf., A 2022, 648, 129366. 10.1016/j.colsurfa.2022.129366. [DOI] [Google Scholar]
- Yang J. S.; Wu J. J. Low-Potential Driven Fully-Depleted BiVO4/ZnO Heterojunction Nanodendrite Array Photoanodes for Photoelectrochemical Water Splitting. Nano Energy 2017, 32, 232–240. 10.1016/j.nanoen.2016.12.039. [DOI] [Google Scholar]
- Srinivasan N.; Anbuchezhiyan M.; Harish S.; Ponnusamy S. Hydrothermal Synthesis of C Doped ZnO Nanoparticles Coupled with BiVO4 and Their Photocatalytic Performance under the Visible Light Irradiation. Appl. Surf. Sci. 2019, 494, 771–782. 10.1016/j.apsusc.2019.07.093. [DOI] [Google Scholar]
- Singh S.; Sharma R.; Mehta B. R. Enhanced Surface Area, High Zn Interstitial Defects and Band Gap Reduction in N-Doped ZnO Nanosheets Coupled with BiVO4 Leads to Improved Photocatalytic Performance. Appl. Surf. Sci. 2017, 411, 321–330. 10.1016/j.apsusc.2017.03.189. [DOI] [Google Scholar]
- Raja A.; Rajasekaran P.; Vishnu B.; Selvakumar K.; Yeon Do J.; Swaminathan M.; Kang M. Fabrication of Effective Visible-Light-Driven Ternary Z-Scheme ZnO-Ag-BiVO4 Heterostructured Photocatalyst for Hexavalent Chromium Reduction. Sep. Purif. Technol. 2020, 252, 117446. 10.1016/j.seppur.2020.117446. [DOI] [Google Scholar]
- Zhang J.; Xie L. Synthesis and Sonophotocatalytic Activities of ZnO\BiVO4\Co3O4 Composites. Chem. Phys. Lett. 2021, 775, 138660. 10.1016/j.cplett.2021.138660. [DOI] [Google Scholar]
- Raja A.; Rajasekaran P.; Selvakumar K.; Arunpandian M.; Kaviyarasu K.; Asath Bahadur S.; Swaminathan M. Visible Active Reduced Graphene Oxide-BiVO4-ZnO Ternary Photocatalyst for Efficient Removal of Ciprofloxacin. Sep. Purif. Technol. 2020, 233, 115996. 10.1016/j.seppur.2019.115996. [DOI] [Google Scholar]
- Mohamed N. A.; Safaei J.; Ismail A. F.; Khalid M. N.; Mohd Jailani M. F. A.; Noh M. F. M.; Arzaee N. A.; Zhou D.; Sagu J. S.; Teridi M. A. M. Boosting Photocatalytic Activities of BiVO4 by Creation of G-C3N4/ZnO@BiVO4 Heterojunction. Mater. Res. Bull. 2020, 125, 110779. 10.1016/j.materresbull.2020.110779. [DOI] [Google Scholar]
- Kumar E. T. D.; Thirumalai K.; Balachandran S.; Aravindhan R.; Swaminathan M.; Raghava Rao J. Solar Light Driven Degradation of Post Tanning Water at Heterostructured BiVO4-ZnO Mixed Oxide Catalyst Interface. Surf. Interfaces 2017, 8, 147–153. 10.1016/j.surfin.2017.05.009. [DOI] [Google Scholar]
- Kim M. W.; Kim K.; Ohm T. Y.; Yoon H.; Joshi B.; Samuel E.; Swihart M. T.; Choi S. K.; Park H.; Yoon S. S. Electrosprayed BiVO4 Nanopillars Coated with Atomic-Layer-Deposited ZnO/TiO2 as Highly Efficient Photoanodes for Solar Water Splitting. J. Chem. Eng. 2018, 333, 721–729. 10.1016/j.cej.2017.09.130. [DOI] [Google Scholar]
- Hou H.; Liu H.; Gao F.; Shang M.; Wang L.; Xu L.; Wong W. Y.; Yang W. Packaging BiVO4 Nanoparticles in ZnO Microbelts for Efficient Photoelectrochemical Hydrogen Production. Electrochim. Acta 2018, 283, 497–508. 10.1016/j.electacta.2018.06.148. [DOI] [Google Scholar]
- Peng F.; Ni Y.; Zhou Q.; Kou J.; Lu C.; Xu Z. Construction of ZnO Nanosheet Arrays within BiVO4 particles on a Conductive Magnetically Driven Cilia Film with Enhanced Visible Photocatalytic Activity. J. Alloys Compd. 2017, 690, 953–960. 10.1016/j.jallcom.2016.08.189. [DOI] [Google Scholar]
- Chang J. S.; Phuan Y. W.; Chong M. N.; Ocon J. D. Exploration of a Novel Type II 1D-ZnO Nanorods/BiVO4 Heterojunction Photocatalyst for Water Depollution. J. Ind. Eng. Chem. 2020, 83, 303–314. 10.1016/j.jiec.2019.12.002. [DOI] [Google Scholar]
- Hu C.; Tian M.; Wu L.; Chen L. Enhanced Photocatalytic Degradation of Paraben Preservative over Designed G-C3N4/BiVO4 S-Scheme System and Toxicity Assessment. Ecotoxicol. Environ. Saf. 2022, 231, 113175. 10.1016/j.ecoenv.2022.113175. [DOI] [PubMed] [Google Scholar]
- Kansaard T.; Ishihara K. N.; Pecharapa W. Structural, Optical and Photo-Induced Catalytic Properties of Derived-Leucoxene /BiVO4 Composite Prepared by Sonochemical Process. Optik 2022, 267, 169665. 10.1016/j.ijleo.2022.169665. [DOI] [Google Scholar]
- Li Y.-l.; Liu Y.; Hao Y.-j.; Wang X.-j.; Liu R.-h.; Li F.-t. Fabrication of Core-Shell BiVO4@Fe2O3 Heterojunctions for Realizing Photocatalytic Hydrogen Evolution via Conduction Band Elevation. Mater. Des. 2020, 187, 108379. 10.1016/j.matdes.2019.108379. [DOI] [Google Scholar]
- Ullah S.; Fayeza; Khan A. A.; Jan A.; Aain S. Q.; Neto E. P. F.; Serge-Correales Y. E.; Parveen R.; Wender H.; Rodrigues-Filho U. P.; Ribeiro S. J. L. Enhanced Photoactivity of BiVO4/Ag/Ag2O Z-Scheme Photocatalyst for Efficient Environmental Remediation under Natural Sunlight and Low-Cost LED Illumination. Colloids Surf., A 2020, 600, 124946. 10.1016/j.colsurfa.2020.124946. [DOI] [Google Scholar]
- Lin Y.; Pan D.; Luo H. Hollow Direct Z–Scheme CdS/BiVO4 Composite with Boosted Photocatalytic Performance for RhB Degradation and Hydrogen Production. Mater. Sci. Semicond. Process. 2021, 121, 105453. 10.1016/j.mssp.2020.105453. [DOI] [Google Scholar]
- Tang Q. Y.; Luo X. L.; Yang S. Y.; Xu Y. H. Novel Z-Scheme In2S3/BiVO4 Composites with Improved Visible-Light Photocatalytic Performance and Stability for Glyphosate Degradation. Sep. Purif. Technol. 2020, 248, 117039. 10.1016/j.seppur.2020.117039. [DOI] [Google Scholar]
- Wang S.; Zhao L.; Gao L.; Yang D.; Wen S.; Huang W.; Sun Z.; Guo J.; Jiang X.; Lu C. Fabrication of Ternary Dual Z-Scheme AgI/ZnIn2S4/BiVO4 Heterojunction Photocatalyst with Enhanced Photocatalytic Degradation of Tetracycline under Visible Light. Arabian J. Chem. 2022, 15, 104159. 10.1016/j.arabjc.2022.104159. [DOI] [Google Scholar]
- Wang T.; Cai J.; Zheng J.; Fang K.; Hussain I.; Husein D. Z. Facile Synthesis of Activated Biochar/BiVO4 Heterojunction Photocatalyst to Enhance Visible Light Efficient Degradation for Dye and Antibiotics: Applications and Mechanisms. J. Mater. Res. Technol. 2022, 19, 5017–5036. 10.1016/j.jmrt.2022.06.177. [DOI] [Google Scholar]
- Zhu P.; Sun X.; Zhu K.; Wang J.; Bai J. Preparing Co-Pi/BiVO4 for Effective Degradation of Sulfur-Containing Organics in Wastewater. Environ. Technol. Innovation 2022, 28, 102928. 10.1016/j.eti.2022.102928. [DOI] [Google Scholar]
- Li Y.; Xiao X.; Ye Z. Facile Fabrication of Tetragonal Scheelite (t-s) BiVO4/g-C3N4 Composites with Enhanced Photocatalytic Performance. Ceram. Int. 2018, 44, 7067–7076. 10.1016/j.ceramint.2018.01.143. [DOI] [Google Scholar]
- Pirhashemi M.; Habibi-Yangjeh A. Ultrasonic-Assisted Preparation of Plasmonic ZnO/Ag/Ag2WO4 Nanocomposites with High Visible-Light Photocatalytic Performance for Degradation of Organic Pollutants. J. Colloid Interface Sci. 2017, 491, 216–229. 10.1016/j.jcis.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Liu W.; Liang B.; Ma Y.; Liu Y.; Zhu A.; Tan P.; Xiong X.; Pan J. Well-Organized Migration of Electrons for Enhanced Hydrogen Evolution: Integration of 2D MoS2 Nanosheets with Plasmonic Photocatalyst by a Facile Ultrasonic Chemical Method. J. Colloid Interface Sci. 2017, 508, 559–566. 10.1016/j.jcis.2017.08.084. [DOI] [PubMed] [Google Scholar]
- Aguilera-Ruiz E.; Zambrano-Robledo P.; Vazquez-Arenas J.; Cruz-Ortiz B.; Peral J.; García-Pérez U. M. Photoactivity of Nanostructured Spheres of BiVO4 Synthesized by Ultrasonic Spray Pyrolysis at Low Temperature. Mater. Res. Bull. 2021, 143, 111447. 10.1016/j.materresbull.2021.111447. [DOI] [Google Scholar]
- Yu Y.; Yao B.; He Y.; Cao B.; Ren Y.; Sun Q. Piezo-Enhanced Photodegradation of Organic Pollutants on Ag3PO4/ZnO Nanowires Using Visible Light and Ultrasonic. Appl. Surf. Sci. 2020, 528, 146819. 10.1016/j.apsusc.2020.146819. [DOI] [Google Scholar]
- Qin H.; Wang K.; Jiang L.; Li J.; Wu X.; Zhang G. Ultrasonic-Assisted Fabrication of a Direct Z-Scheme BiOI/Bi2O4 Heterojunction with Superior Visible Light-Responsive Photocatalytic Performance. J. Alloys Compd. 2020, 821, 153417. 10.1016/j.jallcom.2019.153417. [DOI] [Google Scholar]
- Phadtare V. D.; Parale V. G.; Kim T.; Lee K. Y.; Kadam A. N.; Choi H.; Kim Y.; Dhavale R. P.; Lee S. W.; Park H. H. Ultrasonically Dispersed Ultrathin G-C3N4 Nanosheet/BaBi2Nb2O9 Heterojunction Photocatalysts for Efficient Photocatalytic Degradation of Organic Pollutant. J. Alloys Compd. 2021, 884, 161037. 10.1016/j.jallcom.2021.161037. [DOI] [Google Scholar]
- Li Y.; Chen H.; Wang L.; Wu T.; Wu Y.; He Y. KNbO3/ZnO Heterojunction Harvesting Ultrasonic Mechanical Energy and Solar Energy to Efficiently Degrade Methyl Orange. Ultrason. Sonochem. 2021, 78, 105754. 10.1016/j.ultsonch.2021.105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Khosla A.; Kumar Sharma S.; Dhiman P.; Sharma G.; Gnanasekaran L.; Naushad M.; Stadler F. J. A Review on S-Scheme and Dual S-Scheme Heterojunctions for Photocatalytic Hydrogen Evolution, Water Detoxification and CO2 Reduction. Fuel 2023, 333, 126267. 10.1016/j.fuel.2022.126267. [DOI] [Google Scholar]
- Shawky A.; Mohamed R. M. S-Scheme Heterojunctions: Emerging Designed Photocatalysts toward Green Energy and Environmental Remediation Redox Reactions. J. Environ. Chem. Eng. 2022, 10, 108249. 10.1016/j.jece.2022.108249. [DOI] [Google Scholar]
- Xu Q.; Zhang L.; Cheng B.; Fan J.; Yu J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. 10.1016/j.chempr.2020.06.010. [DOI] [Google Scholar]
- Wang L.; Zhu B.; Zhang J.; Ghasemi J. B.; Mousavi M.; Yu J. S-Scheme Heterojunction Photocatalysts for CO2 Reduction. Matter 2022, 5, 4187–4211. 10.1016/j.matt.2022.09.009. [DOI] [Google Scholar]
- Ghazal N.; Mohamed S. A.; Hameed M. F. O.; Obayya S. S. A.; El Nazer H. A.; Madkour M. Surface and Optoelectronic Impacts of ZnO/BiVO4/MWCNT Nanoheterostructure toward Photodegradation of Water Contaminants. Surf. Interfaces 2022, 33, 102278. 10.1016/j.surfin.2022.102278. [DOI] [Google Scholar]
- Zhang L.; Zhang J.; Yu H.; Yu J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. 10.1002/adma.202107668. [DOI] [PubMed] [Google Scholar]
- Xu Q.; Wageh S.; Al-Ghamdi A. A.; Li X. Design Principle of S-Scheme Heterojunction Photocatalyst. J. Mater. Sci. Nanotechnol. 2022, 124, 171–173. 10.1016/j.jmst.2022.02.016. [DOI] [Google Scholar]
- Chankhanittha T.; Nanan S. Hydrothermal Synthesis, Characterization and Enhanced Photocatalytic Performance of ZnO toward Degradation of Organic Azo Dye. Mater. Lett. 2018, 226, 79–82. 10.1016/j.matlet.2018.05.032. [DOI] [Google Scholar]
- Chankhanittha T.; Watcharakitti J.; Nanan S. PVP-Assisted Synthesis of Rod-like ZnO Photocatalyst for Photodegradation of Reactive Red (RR141) and Congo Red (CR) Azo Dyes. J. Mater. Sci.: Mater. Electron. 2019, 30, 17804–17819. 10.1007/s10854-019-02132-z. [DOI] [Google Scholar]
- Chankhanittha T.; Somaudon V.; Watcharakitti J.; Piyavarakorn V.; Nanan S. Performance of Solvothermally Grown Bi2MoO6 Photocatalyst toward Degradation of Organic Azo Dyes and Fluoroquinolone Antibiotics. Mater. Lett. 2020, 258, 126764. 10.1016/j.matlet.2019.126764. [DOI] [Google Scholar]
- Chankhanittha T.; Somaudon V.; Watcharakitti J.; Nanan S. Solar Light-Driven Photocatalyst Based on Bismuth Molybdate (Bi4MoO9) for Detoxification of Anionic Azo Dyes in Wastewater. J. Mater. Sci.: Mater. Electron. 2021, 32, 1977–1991. 10.1007/s10854-020-04965-5. [DOI] [Google Scholar]
- Gnanamozhi P.; Rajivgandhi G.; Alharbi N. S.; Kadaikunnan S.; Khaled J. M.; Almanaa T. N.; Pandiyan V.; Li W. J. Enhanced Antibacterial and Photocatalytic Degradation of Reactive Red 120 Using Lead Substituted ZnO Nanoparticles Prepared by Ultrasonic-Assisted Co-Precipitation Method. Ceram. Int. 2020, 46, 19593–19599. 10.1016/j.ceramint.2020.05.020. [DOI] [Google Scholar]
- Khan H. R.; Murtaza G.; Choudhary M. A.; Ahmed Z.; Malik M. A. Photocatalytic Removal of Carcinogenic Reactive Red S3B Dye by Using ZnO and Cu Doped ZnO Nanoparticles Synthesized by Polyol Method: A Kinetic Study. J. Sol. Energy 2018, 173, 875–881. 10.1016/j.solener.2018.08.038. [DOI] [Google Scholar]
- Xue L.; Liang E.; Wang J. Fabrication of Magnetic ZnO/ZnFe2O4/Diatomite Composites: Improved Photocatalytic Efficiency under Visible Light Irradiation. J. Mater. Sci.: Mater. Electron. 2022, 33, 1405–1424. 10.1007/s10854-021-07568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral B.; Mansingh S.; Reddy K. H.; Bariki R.; Parida K. Architecting a Double Charge-Transfer Dynamics In2S3/BiVO4 n-n Isotype Heterojunction for Superior Photocatalytic Oxytetracycline Hydrochloride Degradation and Water Oxidation Reaction: Unveiling the Association of Physicochemical, Electrochemical, and Ph. ACS Omega 2020, 5, 5270–5284. 10.1021/acsomega.9b04323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D. K.; Guan M. L.; Liu S. S.; Zhang Y. Q.; Zhang C. W.; He Y. X.; Huang S. M. Controlled Synthesis of Olive-Shaped Bi2S3/BiVO4 Microspheres through a Limited Chemical Conversion Route and Enhanced Visible-Light-Responding Photocatalytic Activity. Dalton Trans. 2012, 41, 5581–5586. 10.1039/c2dt30099k. [DOI] [PubMed] [Google Scholar]
- Singh J.; Kumar S.; Rishikesh; Manna A. K.; Soni R. K. Fabrication of ZnO–TiO2 Nanohybrids for Rapid Sunlight Driven Photodegradation of Textile Dyes and Antibiotic Residue Molecules. Opt. Mater. 2020, 107, 110138. 10.1016/j.optmat.2020.110138. [DOI] [Google Scholar]
- Toloman D.; Popa A.; Stan M.; Stefan M.; Vlad G.; Ulinici S.; Baisan G.; Silipas T. D.; Macavei S.; Leostean C.; Pruneanu S.; Pogacean F.; Suciu R. C.; Barbu-Tudoran L.; Pana O. Visible-Light-Driven Photocatalytic Degradation of Different Organic Pollutants Using Cu Doped ZnO-MWCNT Nanocomposites. J. Alloys Compd. 2021, 866, 159010. 10.1016/j.jallcom.2021.159010. [DOI] [Google Scholar]
- Wu J.; Hu J.; Qian H.; Li J.; Yang R.; Qu L. NiCo/ZnO/g-C3N4 Z-Scheme Heterojunction Nanoparticles with Enhanced Photocatalytic Degradation Oxytetracycline. Diamond Relat. Mater. 2022, 121, 108738. 10.1016/j.diamond.2021.108738. [DOI] [Google Scholar]
- Ouyang K.; Yang C.; Xu B.; Wang H.; Xie S. Synthesis of Novel Ternary Ag/BiVO4/GO Photocatalyst for Degradation of Oxytetracycline Hydrochloride under Visible Light. Colloids Surf., A 2021, 625, 126978. 10.1016/j.colsurfa.2021.126978. [DOI] [Google Scholar]