Abstract
Background
Stress hyperglycemia ratio (SHR) is significantly related to adverse cardiovascular clinical outcomes and increased in-hospital mortality. However, the relationship between SHR and coronary artery disease (CAD) severity has hitherto not been reported. This study sought to clarify the relationship between the SHR and CAD severity of individuals with different glucose metabolic statuses.
Methods
A retrospective analysis was performed on 987 patients who underwent coronary angiography (CAG) from October 2020 to May 2022. Based on CAG results, patients were divided into single-vessel CAD and multi-vessel CAD groups. All subjects were stratified into three groups according to the tertiles of the SHR (T1 group: SHR < 0.930; T2 group: 0.930 ≤ SHR < 1.154; T3 group: 1.154 ≤ SHR). Moreover, according to glucose metabolism status, study subjects were divided into normal glucose regulation (NGR), pre-diabetes mellitus (pre-DM) and diabetes mellitus (DM) groups. Finally, the correlation between SHR and CAD severity was analyzed by logistic regression analysis and receiver operating characteristic (ROC) curve.
Results
The results showed significantly higher SHR in the multi-vessel CAD group than in the single-vessel group. Logistic regression analysis showed that SHR was an independent risk factor for multi-vessel CAD when used as a continuous variable (OR, 4.047; 95% CI 2.137–7.663; P < 0.001). After adjusting for risk factors, the risk of multi-vessel CAD in the T2 and T3 groups was 1.939-fold (95% CI 1.341–2.804; P < 0.001) and 1.860-fold (95% CI 1.272–2.719; P = 0.001) higher than in the T1 group, respectively. The area under the curve (AUC) of ROC plots was 0.613 for SHR. In addition, SHR was significantly correlated with an increased risk of multi-vessel CAD in the pre-DM and DM groups.
Conclusions
Our study indicated that SHR was significantly correlated with the risk of multi-vessel CAD and predicted CAD severity, especially in pre-DM and DM patients.
Keywords: Stress hyperglycemia ratio, Coronary artery disease, Normal glucose regulation, Pre-diabetes mellitus, Diabetes mellitus
Introduction
The poor dietary habits and lifestyle of the general population, and global population aging account for the significant increase in morbidity and mortality attributed to coronary artery disease (CAD) in recent years [1], bringing a serious economic and health burden on our public health systems. Indeed, it is well-established that coronary heart disease (CHD) patients with abnormal glucose metabolism have a higher risk of cardiovascular adverse events [2, 3]. It is widely acknowledged that the number of stenotic vessels determines the severity and prognosis of CAD [3, 4]. Therefore, it is very important for identify high-risk populations with multi-vessel CAD, especially those with abnormal glucose metabolism, to more accurately and efficiently judge the condition of patients and provide optimal treatment.
Stress hyperglycemia represents a transient physiologic response to acute diseases [5]. There is a growing consensus that stress hyperglycemia is associated with adverse cardiovascular clinical outcomes and increased in-hospital mortality [5–10]. Chronic hyperglycemia in diabetic patients is a well-defined risk factor for adverse cardiovascular events [11], but those with stress hyperglycemia are at higher risk of adverse outcomes than those with pre-existing diabetes [5, 6]. Stress hyperglycemia is greatly affected by the glycemia background. To better evaluate the actual blood glucose status of patients, researchers have proposed to use the stress hyperglycemia ratio (SHR) to estimate relative hyperglycemia in patients with or without diabetes to identify and quantify stress hyperglycemia [12]. Current evidence suggests that the glycemia background does not affect the relationship between SHR and critical illness. Studies have also confirmed that relative hyperglycemia measured by SHR was more associated with critical illness than absolute hyperglycemia and was a better biomarker for critical illness than absolute hyperglycemia [12]. Moreover, overwhelming evidence substantiates that SHR is significantly related to adverse cardiovascular outcomes and related mortality [11, 13–15].
However, the relationship between SHR and CAD severity remains largely unclear, warranting further research. This study sought to clarify the correlation between SHR and CAD severity and the strength of the correlation under different glucose metabolic conditions.
Methods
Study design and population
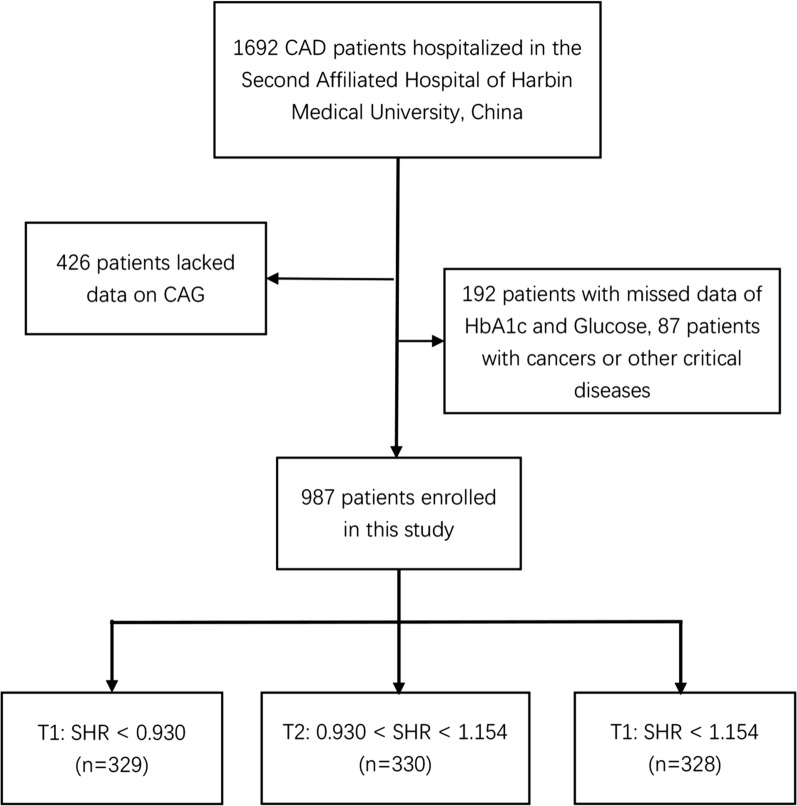
This retrospective study conformed to the Declaration of Helsinki and was approved by the Ethics Committee in the Second Affiliated Hospital of Harbin Medical University. 1692 CAD patients hospitalized in the Second Affiliated Hospital of Harbin Medical University from October 2020 to May 2022 were selected. The exclusion criteria were as follows: (1) patients under 18 years of age; (2) patients with missing data on glycated hemoglobin A1c (HbA1c), admission glucose and coronary angiography (CAG); (3) patients with life-threatening diseases such as tumors, infectious diseases or severe liver or kidney diseases. Ultimately, 987 eligible participants were enrolled in the study. Study subjects were grouped based on tertiles as follows: T1 group, SHR < 0.930 (n = 329); T2 group, SHR ≥ 0.930 to < 1.154 (n = 330); and T3 group, SHR ≥ 1.154 (n = 328). The study flow chart is shown in Fig. 1.
Fig. 1.
Flow chart of patient recruitment. CAD Coronary artery disease, CAG coronary angiography, HbA1c glycated hemoglobin A1c, SHR stress hyperglycemia ratio
Data collection and definitions
Sociodemographic characteristics (age, sex, height, weight, smoking status, and alcohol consumption), medical history (hypertension, diabetes, cancer and other previous medical conditions), and laboratory results were derived from hospital medical records. Admission glucose was measured for the first time within 24 h after admission. Moreover, we recorded the patients' clinical data, including CAG results, blood-related indicators and echocardiography-related parameters. Blood-related indicators included admission glucose, HbA1c, C-reactive protein (CRP), B-type natriuretic peptide (BNP), creatinine (Cr), uric acid (UA), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Echocardiographic data included left atrial diameter (LAD), left ventricular end-diastolic diameter (LVDd), left ventricular systolic diameter (LVDs), interventricular septal thickness (IVS), left posterior wall thickness (LVPW), and left ventricular ejection fraction (LVEF) [12].
According to glucose metabolism status, study subjects were divided into normal glucose regulation (NGR), pre-diabetes mellitus (pre-DM) and diabetes mellitus (DM) groups. NGR was defined as HbA1c < 5.7% and no previous history of diabetes; 5.7% ≤ HbA1c < 6.5% and without a history of diabetes were classified as pre-DM; patients with a history of diabetes or HbA1c ≥ 6.5% were classified as DM [16].
It is well-established that the three main coronary arteries include the left anterior descending, left circumflex, and right coronary arteries, and ≥ 50% stenosis in at least one main coronary artery is considered CAD. In our study, based on the CAG results, patients with one lesion were defined as single-vessel CAD, and the involvement of two or more coronary arteries was defined as multi-vessel CAD. However, multi-vessel CAD was also defined with left main coronary artery stenosis ≥ 50% [3].
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables are expressed as numbers and percentages. χ2 test was used for comparing categorical variables, and the t-test, analysis of variance, Mann–Whitney U or Kruskal–Wallis H test were used for continuous variable. To analyze the association between SHR and CAD severity, odds ratios (OR) and 95% confidence intervals (CI) were calculated using logistic regression. The receiver operating characteristic (ROC) and the area under the curve (AUC) were used to determine the sensitivity and specificity of SHR in predicting CAD severity. All data were statistically analyzed by SPSS 26.0 (IBM Corp, New York, NY, USA). A P-value < 0.05 was statistically significant.
Results
Baseline characteristics according to single-vessel or multi-vessel CAD
A total of 987 patients were included in this study, with a median age of 62 (IQR, 58–68 years), exhibiting male predominance (71.5%). Based on the CAG results, single- and multi-vessel lesions were found in 27.36% and 72.64% of patients, respectively. Based on the glucose metabolism status, the patients were divided into the NGR group (26.6%), the pre-DM group (34%), and the DM group (39.4%). Table 1 shows the baseline characteristics of the single-vessel and multi-vessel CAD groups. Compared with the single-vessel CAD group, patients in the multi-vessel CAD group were significantly older, had a higher prevalence of hypertension, and were more prone to glucose metabolism disorders (P < 0.05). In addition, patients in the multi-vessel CAD group had significantly higher HbA1c, glucose, BNP, D-dimer, LAD and SHR, and lower LVEF than their counterparts in the single-vessel group (P < 0.05) (Table 1).
Table 1.
Baseline characteristics according to single-vessel or multi-vessel CAD
| Total (n = 987) | Single-vessel CAD (n = 270) | Multi-vessel CAD (n = 717) | P-value | |
|---|---|---|---|---|
| Age (years) | 62 (54, 69) | 58 (51, 67) | 63 (56, 70) | < 0.001 |
| Male (n, %) | 706 (71.50%) | 205 (75.90%) | 501 (69.90%) | 0.060 |
| NGR (n, %) | 262 (26.60%) | 85 (31.50%) | 177 (24.70%) | < 0.001 |
| Pre-DM (n, %) | 336 (34%) | 114 (42.20%) | 222 (31%) | |
| DM (n, %) | 389 (39.40%) | 71 (26.30%) | 318 (44.40%) | |
| Smoking (n, %) | 428 (43.40%) | 130 (48.10%) | 298 (41.60%) | 0.063 |
| Drinking (n, %) | 134 (13.60%) | 41 (15.20%) | 93 (13%) | 0.365 |
| Hypertension (n, %) | 475 (48.10%) | 114 (42.20%) | 361 (50.30%) | 0.023 |
| BMI (kg/m2) | 25.39 (23.03, 28.01) | 25.10 (23.12, 28.08) | 25.39 (23.03, 27.97) | 0.762 |
| HbA1c (%) | 6 (5.60, 7) | 5.80 (5.53, 6.30) | 6.10 (5.60, 7.30) | < 0.001 |
| Glucose (mmol/l) | 7.33 (5.99, 9.85) | 6.62 (5.48, 7.97) | 7.67 (6.21, 10.69) | < 0.001 |
| TC (mmol/l) | 4.71 (3.95, 5.51) | 4.75 (3.99, 5.42) | 4.68 (3.93, 5.52) | 0.924 |
| TG (mmol/l) | 1.29 (0.91, 1.81) | 1.28 (0.96, 1.77) | 1.30 (0.90, 1.85) | 0.959 |
| HDL-C(mmol/l) | 1.10 (0.92, 1.28) | 1.14 (0.94, 1.33) | 1.09 (0.91, 1.26) | 0.074 |
| LDL-C(mmol/l) | 3.07 (2.44, 3.73) | 3.10 (2.48, 3.66) | 3.05 (2.39, 3.77) | 0.957 |
| UA (umol/l) | 376.05 (307.08, 459.03) | 376 (313.45, 449.48) | 376.20 (304.95, 461.43) | 0.782 |
| Cr (umol/l) | 80 (67, 97.25) | 78 (66, 90) | 81 (67, 100) | 0.091 |
| BNP (pg/ml) | 732 (227.50, 2240) | 488 (175, 1477.25) | 867 (243, 2544.50) | < 0.001 |
| D-dimer (ug/ml) | 106 (58, 224.25) | 91.50 (50, 212.75) | 110.50 (61, 225.25) | 0.029 |
| CRP (mg/l) | 6.46 (2.45, 13.48) | 5.72 (2.05, 13.07) | 6.79 (2.64, 13.70) | 0.086 |
| LAD (mm) | 35.80 (32.58, 38.70) | 35.25 (32.13, 38.28) | 36 (32.80, 38.90) | 0.030 |
| LVDd (mm) | 46.90 (43.90, 50.20) | 47 (44, 49.70) | 46.85 (43.90, 50.50) | 0.480 |
| LVDs (mm) | 30 (26.10, 34.50) | 30 (25.73, 33.95) | 30 (26.20, 34.63) | 0.103 |
| IVS (mm) | 10.80 (10.10, 11.70) | 10.70 (10, 11.60) | 10.90 (10.10, 11.70) | 0.128 |
| LVPW (mm) | 10.50 (10, 11.20) | 10.50 (10, 11.08) | 10.50 (10, 11.20) | 0.624 |
| LVEF (%) | 58 (58.88, 62) | 58.40 (51.63, 62) | 58 (48, 62) | 0.028 |
| SHR | 1.05 (0.88, 1.23) | 0.99 (0.81, 1.17) | 1.07 (0.90, 1.27) | < 0.001 |
CAD Coronary artery disease, NGR normal glucose regulation, Pre-DM pre-diabetes mellitus, DM diabetes mellitus, BMI body mass index, HbA1c glycated hemoglobin A1c, TC total cholesterol, TG triglycerides, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, UA uric acid, Cr creatinine, BNP B-type natriuretic peptide, CRP C-reactive protein, LAD left atrial diameter, LVDd left ventricular end diastolic diameter, LVDs left ventricular systolic diameter, IVS interventricular septal thickness, LVPW left posterior wall thickness, LVEF left ventricular ejection fraction, SHR stress hyperglycemia ratio
Baseline characteristics according to the tertiles of SHR
Table 2 shows the baseline characteristics according to the tertiles of SHR. A significantly great number of males and patients with glucose metabolism disorders were found in the T3 group (P < 0.05). The levels of HbA1c, glucose, TC, HDL-C and LDL-C in the T3 group were significantly higher than in the T1 group (P < 0.05), and the proportion of multi-vessel CAD cases was higher in the T3 group than in the other groups (P < 0.05).
Table 2.
Baseline characteristics according to the tertiles of SHR
| Total (n = 987) | T1(n = 329) | T2(n = 330) | T3(n = 328) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 62 (54, 69) | 63 (55, 69) | 62(53, 69.25) | 63 (55, 69) | 0.465 |
| Male (n, %) | 706 (71.50%) | 250 (76%) | 242(73.30%) | 214 (65.20%) | 0.006 |
| NGR (n, %) | 262 (26.60%) | 79 (24%) | 112(33.9) | 71 (21.60%) | < 0.001 |
| Pre-DM (n, %) | 336 (34%) | 132 (40.10%) | 117 (35.50%) | 87 (26.50%) | |
| DM (n, %) | 389 (39.40%) | 118 (35.90%) | 101 (30.60%) | 170 (51.80%) | |
| Smoking (n, %) | 428 (43.40%) | 168 (51.10%) | 147 (44.50%) | 113 (34.50%) | < 0.001 |
| Drinking (n, %) | 134 (13.60%) | 48 (14.60%) | 46 (13.9%) | 40 (12.20%) | 0.651 |
| Hypertension (n, %) | 475 (48.10%) | 162 (49.20%) | 154 (46.70%) | 159 (48.50%) | 0.794 |
| BMI (kg/m2) | 25.39 (23.03, 28.01) | 25.09 (23.12, 27.78) | 25.75 (23.03, 28.41) | 25.39 (22.76, 27.55) | 0.544 |
| HbA1c (%) | 6 (5.60, 7) | 5.90 (5.60, 6.70) | 5.80 (5.50, 6.53) | 6.20 (5.70, 7.60) | < 0.001 |
| Glucose (mmol/l) | 7.33 (5.99, 9.85) | 5.74 (5.19, 6.49) | 7.05 (6.39, 8.34) | 10.22 (8.28, 13.82) | < 0.001 |
| TC (mmol/l) | 4.71 (3.95, 5.51) | 4.47 (3.81, 5.28) | 4.84 (4.07, 5.68) | 4.83 (4.01, 5.52) | 0.002 |
| TG (mmol/l) | 1.29 (0.91, 1.81) | 1.41 (1.04, 1.99) | 1.21 (0.88, 1.68) | 1.23 (0.86, 1.72) | < 0.001 |
| HDL-C(mmol/l) | 1.10 (0.92, 1.28) | 1 (0.86, 1.22) | 1.16(0.97, 1.30) | 1.13 (0.96, 1.29) | < 0.001 |
| LDL-C(mmol/l) | 3.07 (2.44, 3.73) | 2.87 (2.22, 3.52) | 3.20 (2.58, 3.90) | 3.21 (2.51, 3.72) | < 0.001 |
| UA (umol/l) | 376.05 (307.08, 459.03) | 371.40 (306.75, 462.60) | 384.05 (306.58, 463.85) | 371.80 (307.70, 448.10) | 0.694 |
| Cr (umol/l) | 80 (67, 97.25) | 79 (66.50, 97.50) | 81 (66, 97) | 82 (68, 99) | 0.446 |
| BNP (pg/ml) | 732 (227.50, 2240) | 633 (217, 1831.50) | 744 (242, 2007.25) | 904 (217, 2808) | 0.131 |
| D-dimer (ug/ml) | 106 (58, 224.25) | 105 (54.50, 202.50) | 109 (59.75, 217) | 101 (58, 241) | 0.945 |
| CRP (mg/l) | 6.46 (2.45, 13.48) | 6.79 (2.35, 13.28) | 6.49 (2.12, 13.61) | 6.30 (2.73, 13.70) | 0.951 |
| LAD (mm) | 35.80 (32.58, 38.70) | 35.60 (32.10, 38.60) | 35.85 (32.65, 38.80) | 36 (33, 38.70) | 0.415 |
| LVDd (mm) | 46.90 (43.90, 50.20) | 46.90 (43.80, 50.15) | 47 (44, 50.20) | 46.60 (44, 50.50) | 0.938 |
| LVDs (mm) | 30 (26.10, 34.50) | 29.70 (25.90, 34.75) | 30 (26.18, 34.75) | 30.20 (26.30, 34.90) | 0.868 |
| IVS (mm) | 10.80 (10.10, 11.70) | 10.80 (10.10, 11.60) | 10.80 (10.10, 11.70) | 10.80 (10.10, 11.80) | 0.984 |
| LVPW (mm) | 10.50 (10, 11.20) | 10.50 (10, 11.05) | 10.50(10, 11.20) | 10.50 (10, 11.20) | 0.945 |
| LVEF (%) | 58 (58.88, 62) | 58.50 (52, 62) | 58 (49, 61) | 57 (47, 62) | 0.069 |
| Multi-vessel CAD (n, %) | 717 (72.60%) | 210 (29.30%) | 249 (34.70%) | 258 (36%) | < 0.001 |
SHR stress hyperglycemia ratio, NGR normal glucose regulation, Pre-DM pre-diabetes mellitus, DM diabetes mellitus, BMI body mass index, HbA1c, glycated hemoglobin A1c, TC total cholesterol, TG triglycerides, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, UA uric acid, Cr creatinine, BNP B-type natriuretic peptide, CRP C-reactive protein, LAD left atrial diameter, LVDd left ventricular end diastolic diameter, LVDs left ventricular systolic diameter, IVS interventricular septal thickness, LVPW left posterior wall thickness, LVEF left ventricular ejection fraction, CAD Coronary artery disease
Relationship between CAD severity and various risk factors
The association between CAD severity and various risk factors was analyzed by univariate logistic regression, taking the multi-vessel CAD as the dependent variable. We found that age, glucose metabolism state, hypertension, HbA1c, BNP, LAD and SHR were positively correlated with multi-vessel CAD (P < 0.05), and HDL-C was negatively correlated with multi-vessel CAD (P < 0.05) (Table 3).
Table 3.
Relationship between CAD severity and various risk factors
| Variables | Multi-vessel coronary artery disease | ||
|---|---|---|---|
| OR(95%CI) | β | P-value | |
| Age | 1.033 (1.020–1.046) | 0.032 | < 0.001 |
| Sex | |||
| Male | Reference | ||
| Female | 1.360 (0.986–1.875) | 0.307 | 0.061 |
| Glucose metabolism state | |||
| NGR | Reference | ||
| Pre-DM | 0.935 (0.663–1.318) | − 0.067 | 0.702 |
| DM | 2.151 (1.493–3.098) | 0.766 | < 0.001 |
| Smoking | |||
| NO | Reference | ||
| YES | 0.766 (0.578–1.015) | − 0.267 | 0.063 |
| Drinking | |||
| NO | Reference | ||
| YES | 0.832 (0.559–1.239) | − 0.183 | 0.366 |
| Hypertension | |||
| NO | Reference | ||
| YES | 1.388 (1.046–1.840) | 0.328 | 0.023 |
| BMI | 1.015 (0.971–1.061) | 0.015 | 0.502 |
| HbA1c | 1.293 (1.148–1.456) | 0.257 | < 0.001 |
| Glucose | 1.180 (1.119–1.245) | 0.166 | < 0.001 |
| TC | 1.005 (0.897–1.126) | 0.005 | 0.936 |
| TG | 1.009 (0.884–1.151) | 0.009 | 0.896 |
| HDL-C | 0.568 (0.344–0.938) | − 0.566 | 0.027 |
| LDL-C | 0.996 (0.868–1.144) | − 0.004 | 0.960 |
| UA | 1.002 (0.999–1.006) | 0.002 | 0.117 |
| Cr | 0.999 (0.998–1.001) | < − 0.001 | 0.972 |
| BNP | 1.000 (1.000–1.001) | < 0.001 | 0.009 |
| D-dimer | 1.000 (0.999–1.001) | < 0.001 | 0.763 |
| CRP | 1.020 (0.994–1.047) | 0.020 | 0.135 |
| LAD | 1.032 (1.000–1.064) | 0.031 | 0.048 |
| LVDd | 1.013 (0.988–1.039) | 0.013 | 0.319 |
| LVDs | 1.021 (0.999–1.043) | 0.021 | 0.053 |
| IVS | 1.068 (0.962–1.187) | 0.066 | 0.216 |
| LVPW | 1.021 (0.909–1.146) | 0.020 | 0.731 |
| LVEF | 0.999 (0.999–1.000) | < − 0.001 | 0.053 |
| SHR | 4.590 (2.598–8.111) | 1.524 | < 0.001 |
OR odds ratios, CI confidence interval, β regression coefficient, NGR normal glucose regulation, Pre-DM pre-diabetes mellitus, DM diabetes mellitus, HbA1c glycated hemoglobin A1c, BMI body mass index, TC total cholesterol, TG triglycerides, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, UA uric acid, Cr creatinine, LAD left atrial diameter, LVDd left ventricular end diastolic diameter, LVDs left ventricular systolic diameter, IVS interventricular septal thickness, LVPW left posterior wall thickness, LVEF left ventricular ejection fraction, BNP B-type natriuretic peptide, CRP C-reactive protein, SHR stress hyperglycemia ratio
Association between SHR and CAD severity
As shown in Table 4, multivariate logistic regression analysis was constructed to analyze the correlation between SHR and CAD severity. Multi-vessel CAD was used as the dependent variable, and there was no multicollinearity between the independent variables. Confounding factors were not adjusted in model 1, age and sex were adjusted for model 2, and model 3 was adjusted for age, sex, glucose metabolism state, hypertension, smoking, HDL-C, BNP, LAD, LVDs, and LVEF. We found that SHR was an independent risk factor for multi-vessel CAD when used as a continuous variable (P < 0.05). When SHR was a categorical variable, the risk of multi-vessel CAD in the T2 and T3 groups was higher than T1. After adjusting for all relevant risk factors in model 3, the risk of multi-vessel CAD in the T2 and T3 groups was 1.939 (95% CI 1.341–2.804; P < 0.001) and 1.860 (95% CI 1.272–2.719; P = 0.001) fold higher than in the T1 group, respectively (Table 4).
Table 4.
Association between SHR and CAD severity
| Variables | Multi-vessel coronary artery disease | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| SHR | 4.590 (2.598–8.111) | < 0.001 | 4.567 (2.555–8.163) | < 0.001 | 4.047 (2.137–7.663) | < 0.001 |
| T1 | Reference | Reference | Reference | |||
| T2 | 1.742 (1.244–2.440) | 0.001 | 1.813 (1.287–2.554) | 0.001 | 1.939 (1.341–2.804) | < 0.001 |
| T3 | 2.089 (1.476–2.955) | < 0.001 | 2.061 (1.449–2.932) | < 0.001 | 1.860 (1.272–2.719) | 0.001 |
OR odds ratios, CI confidence interval, SHR stress hyperglycemia ratio
Model 1: unadjusted;
Model 2: adjusted for age and sex;
Model 3: adjusted for age, sex, glucose metabolism state, hypertension, smoking, HDL-C, BNP, LAD, LVDs, LVEF
T1: SHR < 0.930; T2: 0.930 ≤ SHR < 1.154; T3: 1.154 ≤ SHR
The predictive value of SHR for CAD severity
The ROC curve for multi-vessel CAD and SHR is shown in Fig. 2. The AUC of multi-vessel CAD evaluated by SHR was 0.613 (95% CI 0.572–0.653), yielding an optimal cut-off value of 0.840 at a sensitivity of 85.8% and a specificity of 33% (P < 0.05) (Table 5).
Fig. 2.
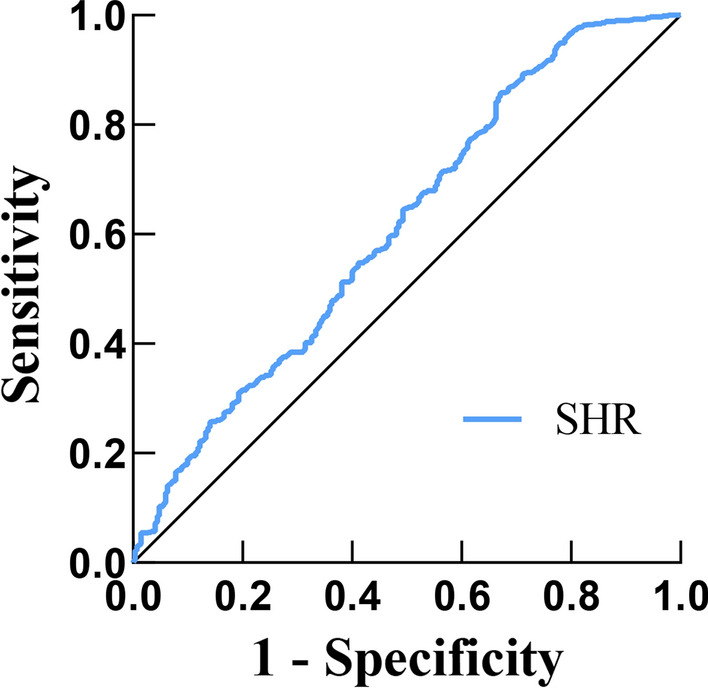
ROC curve for the use of SHR in the detection of multi-vessel CAD
Table 5.
The predictive value of SHR for CAD severity
| Variable | AUC | 95% CI | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| SHR | 0.613 | 0.572–0.653 | 0.840 | 85.8 | 33.0 |
AUC area under the curve, CI confidence interval, SHR stress hyperglycemia ratio
Correlations between SHR and CAD severity in different glucose metabolism states
These patients were divided into NGR, pre-DM and DM groups according to their glucose metabolic status. Logistic regression analysis was performed on the three groups with multi-vessel CAD as the dependent variable. In the NGR group, no significant association was found between SHR and the occurrence of multi-vessel CAD. In the pre-DM group, it was observed that SHR was an independent risk factor for multi-vessel CAD as a continuous variable (P < 0.05). And as a categorical variable, both T2 and T3 groups were correlated with an increased risk of multi-vessel CAD than the T1 group (P < 0.05). In the DM group, we also found that SHR was statistically significantly correlated with the increased risk of multi-vessel CAD (P < 0.05). Compared with the T1 group, T2 and T3 groups were more significantly correlated with the occurrence of multi-vessel CAD in models 4 and 5. However, after adjusting for all confounding risk factors, only the T3 group was associated with a significantly increased risk of multi-vessel CAD than the T1 group in model 6 (P < 0.05) (Table 6).
Table 6.
Correlations between SHR and CAD severity in different glucose metabolism states
| Glucose regulation state | Variables | Multi-vessel coronary artery disease | |||||
|---|---|---|---|---|---|---|---|
| Model 4 | Model 5 | Model 6 | |||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| NGR | SHR | 2.982 (0.911–9.759) | 0.071 | 2.903 (0.845–9.982) | 0.091 | 3.715 (0.957–14.412) | 0.058 |
| T1 | Reference | Reference | Reference | ||||
| T2 | 1.546 (0.842–2.838) | 0.16 | 1.534 (0.809–2.910) | 0.190 | 1.879 (0.944–3.740) | 0.073 | |
| T3 | 1.538 (0.778–3.038) | 0.216 | 1.526 (0.750–3.106) | 0.244 | 1.828 (0.854–3.914) | 0.120 | |
| Pre-DM | SHR | 5.655 (1.899–16.837) | 0.002 | 5.536 (1.838–16.674) | 0.002 | 5.833 (1.805–18.852) | 0.003 |
| T1 | Reference | Reference | Reference | ||||
| T2 | 1.799 (1.061–3.049) | 0.029 | 1.876 (1.092–3.223) | 0.023 | 1.905 (1.077–3.370) | 0.027 | |
| T3 | 1.934 (1.079–3.466) | 0.027 | 1.871 (1.034–3.386) | 0.038 | 1.890 (1.012–3.528) | 0.046 | |
| DM | SHR | 3.623 (1.527–8.595) | 0.003 | 3.722 (1.561–8.874) | 0.003 | 3.855 (1.494–9.947) | 0.005 |
| T1 | Reference | Reference | Reference | ||||
| T2 | 2.312 (1.154–4.634) | 0.018 | 2.309 (1.150–4.637) | 0.019 | 1.983 (0.935–4.201) | 0.074 | |
| T3 | 2.158 (1.200–3.883) | 0.010 | 2.190 (1.214–3.951) | 0.009 | 2.059 (1.063–3.988) | 0.032 | |
OR odds ratios, CI confidence interval, SHR stress hyperglycemia ratio, NGR normal glucose regulation, Pre-DM pre-diabetes mellitus, DM diabetes mellitus
Model 4: unadjusted;
Model 5: adjusted for age and sex;
Model 6: adjusted for age, sex, hypertension, smoking, HDL-C, BNP, LAD, LVDs, LVEF
Discussion
The present study showed that SHR was significantly associated with the severity of CAD, especially in the pre-DM and DM groups. Importantly, this is the first study to reveal the relationship between SHR and the risk of multi-vessel CAD to our best knowledge.
Herein, we observed that patients in the multi-vessel CAD group were older, had higher SHR, and were more prone to glucose metabolism disorders than the single-vessel group. Multivariate logistic regression analysis revealed that SHR was significantly associated with the risk of multi-vessel CAD. Next, based on tertiles, we divided the subjects into three groups. After adjusting for all relevant risk factors, we found that patients with the higher tertile of SHR were associated with an increased risk of multi-vessel CAD compared to those with the lowest tertile. Patients were stratified according to glucose metabolism status into NGR, Pre-DM and DM groups to observe the association between SHR and multi-vessel CAD. Unlike the NGR group, we found SHR was significantly correlated with the higher risk of multi-vessel CAD in the Pre-DM and DM groups, and the higher tertile of SHR was more significantly correlated with the occurrence of multi-vessel CAD than the lowest tertile.
There is a rich literature available suggesting that stress hyperglycemia is transient hyperglycemia secondary to neurohormonal disorders and inflammatory responses [5, 17, 18]. Besides, stress hyperglycemia is very common in critical illness and is a sign of disease severity [19–21]. Roberts et al. first proposed that SHR was not affected by background glycemia and could better evaluate the true blood glucose status of patients [12]. Multi-vessel CAD is associated with a high risk of death and adverse events [3]. In this respect, compared with single-vessel CAD, multi-vessel CAD increased the difficulty of percutaneous coronary intervention and had a worse prognosis [22], and has attracted much interest in recent years. Ample literature suggests that SHR is associated with increased risk of adverse cardiovascular clinical outcomes and in-hospital mortality and is an independent predictor of poor prognosis [5–10, 23].
Chu et al. suggested that high SHR was independently related to large thrombus burden and had a better predictive value for adverse complications and events than admission blood glucose level [24]. Moreover, Kojima et al. found that high SHR was significantly associated with a poorer long-term prognosis in non-diabetic individuals [25]. Cui et al. also demonstrated a strong positive correlation between SHR and long-term mortality in acute myocardial infarction (AMI) patients with or without diabetes [26]. However, Schmitz et al. advocated that SHR was significantly associated with higher short-term mortality in patients with AMI, and was only found to be associated with higher long-term mortality in patients with diabetes [27]. Besides, Chen et al. reported that SHR was an effective predictor of in-hospital outcomes in patients > 75 years of age with AMI, especially in non-diabetic individuals [28]. Last but not least, results of a large observational study based on 274 centers in China by Xu et al., showed that SHR was independently associated with the risk of major adverse cardiovascular events (MACEs) and all-cause mortality in ST-segment elevation myocardial infarction (STEMI) patients [15].
Our study also corroborated the adverse effect of SHR on cardiovascular disease and provided hitherto undocumented evidence that SHR was an independent risk factor for CAD severity; high SHR was closely related to multi-vessel CAD, especially in pre-DM and DM populations. Previous studies suggested that pre-DM was an elevated risk state for cardiovascular events [29–32]. Besides, a meta-analysis of 53 prospective studies showed that pre-DM was associated with an increased risk of CAD [33]. Schlesinger et al. also summarized the results of 95 meta-analyses and found that pre-DM was positively associated with the risk of all-cause mortality and cardiovascular outcomes [34]. These results raise awareness of the need to dynamically monitor blood glucose changes in patients with and without diabetes in the future.
In future clinical practice, we can measure the admission glucose and calculate SHR for all CAD patients, preliminarily assess the severity of condition, and make more timely, accurate and appropriate treatment according to the results, which can also reduce the delay of the condition of critically ill patients with not obvious clinical symptoms. For high-risk groups of CAD, dynamic monitoring of SHR changes can also be used to understand the changes of the condition, so as to seek medical treatment in time and improve the prognosis.
Strengths and limitations
This is the first study to explore the relationship between SHR and CAD severity, providing the foothold for the prediction and diagnosis of multi-vessel CAD. However, this study still had some limitations. The study population came from a single center, the sample size was small, and information on past medication history was not collected. Accordingly, the generalizability of our results is limited. Further research is needed to validate our findings.
Conclusion
In summary, our study indicated that high SHR was significantly related to increased risk of multi-vessel CAD, and SHR is a predictor of CAD severity, especially for those with Pre-DM and DM. Risk management of such populations can be done through monitoring the SHR and better health management programs after admission.
Acknowledgements
We are grateful to all subjects who participated in the study.
Abbreviations
- CAD
Coronary artery disease
- CHD
Coronary heart disease
- CAG
Coronary angiography
- SHR
Stress hyperglycemia ratio
- BMI
Body mass index
- NGR
Normal glucose regulation
- Pre-DM
Pre-diabetes mellitus
- DM
Diabetes mellitus
- HbA1c
Glycated hemoglobin A1c
- TC
Total cholesterol
- TG
Triglycerides
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- UA
Uric acid
- BNP
B-type natriuretic peptide
- CRP
C-reactive protein
- Cr
Creatinine
- LAD
Left atrial diameter
- LVDd
Left ventricular end diastolic diameter
- LVDs
Left ventricular systolic diameter
- IVS
Interventricular septal thickness
- LVPW
Left posterior wall thickness
- LVEF
Left ventricular ejection fraction
- OR
Odds ratios
- CI
Confidence interval
- β
Regression coefficient
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- AMI
Acute myocardial infarction
- MACEs
Major adverse cardiovascular events
- STEMI
ST-segment elevation myocardial infarction
Author contributions
All authors contributed substantially to this work. YZ and HS designed the research, analysed the data and wrote the manuscript. YZ, JB, JX, GW, LZ, YW and YQ collected the data and performed the statistical analysis. HS and YZ critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
No specific fund supports the current analysis.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee of the Second Affiliated Hospital of Harbin Medical University. Informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Zhang, Email: zhangyuhhha@163.com.
Haiyan Song, Email: songhy6605@126.com.
Jing Bai, Email: 1003090359@qq.com.
Jiahui Xiu, Email: 3020028954@qq.com.
Ganggang Wu, Email: 1920875916@qq.com.
Liao Zhang, Email: Zhangl990129@163.com.
Yunhe Wu, Email: wuyunhe0331@163.com.
Ying Qu, Email: 664316692@qq.com.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the american heart association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the american heart association. Circulation. 2020;141(19):e779–e806. doi: 10.1161/CIR.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su J, Li Z, Huang M, Wang Y, Yang T, Ma M, et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):96. doi: 10.1186/s12933-022-01523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu MM, Peng J, Guo YL, Wu NQ, Zhu CG, Gao Y, et al. Impact of diabetes on coronary severity and cardiovascular outcomes in patients with heterozygous familial hypercholesterolaemia. Eur J Prev Cardiol. 2022;28(16):1807–1816. doi: 10.1093/eurjpc/zwab042. [DOI] [PubMed] [Google Scholar]
- 5.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 7.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara M, Kojima S, Sakamoto T, Asada Y, Tei C, Kimura K, et al. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J. 2005;150(4):814–820. doi: 10.1016/j.ahj.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Khalfallah M, Abdelmageed R, Elgendy E, Hafez YM. Incidence, predictors and outcomes of stress hyperglycemia in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Diab Vasc Dis Res. 2020;17(1):1479164119883983. doi: 10.1177/1479164119883983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. 2022;45(4):947–956. doi: 10.2337/dc21-1526. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, Song Q, Wang X, Zhao Z, Meng X, Xia C, et al. Association of stress hyperglycemia ratio and in-hospital mortality in patients with coronary artery disease: insights from a large cohort study. Cardiovasc Diabetol. 2022;21(1):217. doi: 10.1186/s12933-022-01645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100(12):4490–4497. doi: 10.1210/jc.2015-2660. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. 2017;241:57–63. doi: 10.1016/j.ijcard.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 14.Sia CH, Chan MH, Zheng H, Ko J, Ho AF, Chong J, et al. Optimal glucose, HbA1c, glucose-HbA1c ratio and stress-hyperglycaemia ratio cut-off values for predicting 1-year mortality in diabetic and non-diabetic acute myocardial infarction patients. Cardiovasc Diabetol. 2021;20(1):211. doi: 10.1186/s12933-021-01395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Yang YM, Zhu J, Wu S, Wang J, Zhang H, et al. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol. 2022;21(1):48. doi: 10.1186/s12933-022-01479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 17.Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, et al. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(3):304–310. doi: 10.1016/j.jacc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 18.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 19.Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305. doi: 10.1186/cc12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TF, Drake SM, Roberts GW, Bersten A, Stranks SN, Heilbronn LK, et al. Relative hyperglycemia is an independent determinant of in-hospital mortality in patients with critical illness. Crit Care Med. 2020;48(2):e115–e122. doi: 10.1097/CCM.0000000000004133. [DOI] [PubMed] [Google Scholar]
- 21.Bellaver P, Schaeffer AF, Dullius DP, Viana MV, Leitão CB, Rech TH. Association of multiple glycemic parameters at intensive care unit admission with mortality and clinical outcomes in critically ill patients. Sci Rep. 2019;9(1):18498. doi: 10.1038/s41598-019-55080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28(14):1709–1716. doi: 10.1093/eurheartj/ehm184. [DOI] [PubMed] [Google Scholar]
- 23.Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. 2019;42(7):1312–1318. doi: 10.2337/dc19-0274. [DOI] [PubMed] [Google Scholar]
- 24.Chu J, Tang J, Lai Y, Gao Y, Ye Z, Guan C, et al. Association of stress hyperglycemia ratio with intracoronary thrombus burden in diabetic patients with ST-segment elevation myocardial infarction. J Thorac Dis. 2020;12(11):6598–6608. doi: 10.21037/jtd-20-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima T, Hikoso S, Nakatani D, Suna S, Dohi T, Mizuno H, et al. Impact of hyperglycemia on long-term outcome in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2020;125(6):851–859. doi: 10.1016/j.amjcard.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Stress hyperglycemia ratio and long-term mortality after acute myocardial infarction in patients with and without diabetes: a prospective, nationwide, and multicentre registry. Diabetes Metab Res Rev. 2022;38(7):e3562. doi: 10.1002/dmrr.3562. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz T, Freuer D, Harmel E, Heier M, Peters A, Linseisen J, et al. Prognostic value of stress hyperglycemia ratio on short- and long-term mortality after acute myocardial infarction. Acta Diabetol. 2022;59(8):1019–1029. doi: 10.1007/s00592-022-01893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Li M, Wen X, Wang R, Zhou Y, Xue L, et al. Association between stress hyperglycemia ratio and in-hospital outcomes in elderly patients with acute myocardial infarction. Front Cardiovasc Med. 2021;8:698725. doi: 10.3389/fcvm.2021.698725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ertan C, Ozeke O, Gul M, Aras D, Topaloglu S, Kisacik HL, et al. Association of prediabetes with diffuse coronary narrowing and small-vessel disease. J Cardiol. 2014;63(1):29–34. doi: 10.1016/j.jjcc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Mutie PM, Pomares-Millan H, Atabaki-Pasdar N, Jordan N, Adams R, Daly NL, et al. An investigation of causal relationships between prediabetes and vascular complications. Nat Commun. 2020;11(1):4592. doi: 10.1038/s41467-020-18386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gujral UP, Jagannathan R, He S, Huang M, Staimez LR, Wei J, Singh N, Narayan KV, et al. Association between varying cut-points of intermediate hyperglycemia and risk of mortality, cardiovascular events and chronic kidney disease: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2021;9(1):e001776. doi: 10.1136/bmjdrc-2020-001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Guo M, Shi G. Prediabetes predicts adverse cardiovascular outcomes after percutaneous coronary intervention: a meta-analysis. Biosci Rep. 2020;40(1):1. doi: 10.1042/BSR20193130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W, et al. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65(2):275–285. doi: 10.1007/s00125-021-05592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.