Abstract
Background
We evaluated immunogenicity of SCB-2019, a subunit vaccine candidate containing a pre-fusion trimeric form of the SARS-CoV-2 spike (S)-protein adjuvanted with CpG-1018/alum.
Methods
The phase 2/3, double-blind, randomized SPECTRA trial was conducted in five countries in participants aged ≥ 18 years, either SARS-CoV-2-naïve or previously exposed. Participants were randomly assigned to receive two doses of SCB-2019 or placebo administered intramuscularly 21 days apart. In the phase 2 part of the study, on days 1, 22, and 36, neutralizing antibodies were measured by pseudovirus and wild-type virus neutralization assays to SARS-CoV-2 prototype and variants, and ACE2-receptor-binding antibodies and SCB-2019–binding antibodies were measured by ELISA. Cell-mediated immunity was measured by intracellular cytokine staining via flow cytometry.
Results
1601 individuals were enrolled between 24 March and 13 September 2021 and received at least one vaccine dose. Immunogenicity analysis was conducted in a phase 2 subset of 691 participants, including 428 SARS-CoV-2-naïve (381 vaccine and 47 placebo recipients) and 263 SARS-CoV-2-exposed (235 vaccine and 28 placebo recipients). In SARS-CoV-2-naïve participants, GMTs of neutralizing antibodies against prototype virus increased 2 weeks post-second dose (day 36) compared to baseline (224 vs 12.7 IU/mL). Seroconversion rate was 82.5 %. In SARS-CoV-2-exposed participants, one SCB-2019 dose increased GMT of neutralizing antibodies by 48.3-fold (1276.1 IU/mL on day 22) compared to baseline. Seroconversion rate was 92.4 %. Increase was marginal post-second dose. SCB-2019 also showed cross-neutralization capability against nine variants, including Omicron, in SARS-CoV-2-exposed participants at day 36. SCB-2019 stimulated Th1-biased cell-mediated immunity to the S-protein in both naïve and exposed participants. The vaccine was well tolerated, no safety concerns were raised from the study.
Conclusions
A single dose of SCB-2019 was immunogenic in SARS-CoV-2-exposed individuals, whereas two doses were required to induce immune response in SARS-CoV-2-naïve individuals. SCB-2019 elicited a cross-neutralizing response against emergent SARS-CoV-2 variants at antibody levels associated with clinical protection, underlining its potential as a booster.
Clinicaltrials.gov: NCT04672395; EudraCT: 2020-004272-17.
Keywords: COVID-19, SARS-CoV-2, SCB-2019, Vaccine, Immunogenicity, Cell-mediated immunity
1. Introduction
The assessment of efficacy and immunogenicity of COVID-19 vaccines was initially focused on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naïve population. However, during the pandemic, the overall seroprevalence increased rapidly due to asymptomatic SARS-CoV-2 infection and COVID-19 disease, as well as vaccination [1]. Therefore, evaluating the immunogenicity and efficacy of COVID-19 vaccines in both SARS-CoV-2-naïve and SARS-CoV-2-exposed individuals is essential for understanding the magnitude of the immune response and differences in the cross-neutralization of new emergent SARS-CoV-2 variants.
Clover Biopharmaceuticals has developed a subunit vaccine candidate, SCB-2019, using its innovative Trimer-Tag™ technology [2]. SCB-2019 contains the SARS-CoV-2 spike (S) glycoprotein (Wuhan-Hu-1 strain), in a native-like prefusion trimeric conformation, adjuvanted with the Toll-like receptor agonist CpG-1018 and alum [3]. The efficacy, immunogenicity, and safety of the SCB-2019 vaccine were assessed in the global phase 2/3 clinical trial (SPECTRA) with more than 30,000 study participants from five countries. In SARS-CoV-2-naïve subjects, the vaccine demonstrated 100 % efficacy against severe COVID-19 and COVID-19-associated hospitalizations, 67 % efficacy against COVID-19 of any severity for all strains, and 79 % efficacy against the predominant Delta variant [4]. As approximately half of the study participants had a history of previous exposure to SARS-CoV-2, the study has also assessed the efficacy, immunogenicity, and safety of SCB-2019 in this population [5]. On top of the 83.2 % protection afforded by previous exposure to SARS-CoV-2, a single SCB-2019 vaccine dose had an efficacy of 49.9 % against COVID-19 of any severity, whereas two doses had an additional efficacy of 64.2 % [5]. The cumulative effect of vaccinating participants with evidence of previous exposure to SARS-CoV-2 was 89.7 % after one dose and 93.8 % after two doses [5].
The vaccine was generally well tolerated in SARS-CoV-2-naïve and previously exposed individuals. Rates of solicited adverse events were similar in vaccine and placebo groups, except for a higher rate of short-lived, mild-to-moderate injection site pain reported in the vaccine group. Few severe or serious adverse events were reported, and no safety concerns were raised [4], [5].
Here, we describe the immunogenicity results of the SCB-2019 vaccine in both SARS-CoV-2-naïve and exposed individuals from the SPECTRA trial, including levels of cross-neutralizing antibodies against multiple SARS-CoV-2 variants that circulated during and after the study period, as well as vaccine-induced cell-mediated immune response.
2. Materials and methods
2.1. Study design
Clover’s SPECTRA is a randomized, placebo-controlled, phase 2/3 trial conducted in participants aged ≥ 12 years in five countries (Belgium, Brazil, Colombia, the Philippines, and South Africa) [4]. Here, we present the analysis of immunogenicity data obtained between 24 March 2021 and 13 September 2021 (data lock point) in participants ≥ 18 years enrolled in the phase 2 part of SPECTRA in Belgium, Colombia, and the Philippines. The trial was double-blinded; study participants, study center staff involved in the study conduct and monitoring, and laboratory staff conducting immunogenicity testing were blinded.
Individuals eligible to participate in this study were healthy individuals or had a stable pre-existing medical condition, were ≥ 12 years of age, and were willing to comply with the study requirements and procedures. Full inclusion and exclusion criteria have been previously described [4]. The main exclusion criteria were pregnancy; breastfeeding; a history of severe adverse reaction or anaphylaxis to any vaccine component; any chronic condition or therapy likely to impact immune responses; and prior receipt of any coronavirus vaccine. Individuals with a history of SARS-CoV-2 infection were allowed for enrollment (except RT-PCR-confirmed COVID-19 at screening or within 14 days before enrollment).
The primary and secondary efficacy and safety results of the SPECTRA study have been previously described [4], [5]. The immunogenicity of SCB-2019 was to be evaluated in a subset of phase 2 participants. The exploratory immunogenicity objectives were to assess the cell-mediated immunity (CMI) elicited by SCB-2019 in a subset of adult participants and to explore the cross-neutralization of new emergent SARS-CoV-2 variants of concern (VOCs) by antibodies elicited from two doses of SCB-2019 vaccination.
The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulations. The protocol was approved by all site institutional review boards and applicable national authorities. All participants provided written informed consent before enrollment.
2.2. Vaccine
The SCB-2019 vaccine was supplied as 720 μg of SCB-2019 (Clover Biopharmaceuticals) in 1 mL (20 doses) of solution for injection. The CpG-1018 adjuvant (Dynavax Technologies) was provided in a 2-mL vial containing 12 mg/mL of a 22-mer phosphothioate oligodeoxynucleotide in Tris-buffered saline (24 mg per vial) and alum was provided as a vial containing 10 mg/mL of aluminum hydroxide (Alhydrogel®, Croda Health Care). All vaccine components, except aluminum hydroxide, were stored at 2–8 °C and mixed such that each vaccine dose contained 30 μg SCB-2019, 1.5 mg CpG-1018, and 0.75 mg alum. The vaccine or placebo (0.9 % sodium chloride) was then administered by intramuscular injection (0.5 mL per dose) in the deltoid region of the non-dominant arm. The vaccine syringes were opacified to avoid unblinding, and vaccine administration was carried out by an unblinded dosing team who were not involved in any study evaluation.
All adults were to receive two doses of the vaccine or placebo 21 days apart (on days 1 and 22) according to the randomization stratified by site, age group (≥18 to < 65 years of age vs ≥ 65 years of age), absence/presence of comorbidities associated with a high risk of severe COVID-19, and a known history of COVID-19.
2.3. Assessments of humoral immune responses
Previous exposure to the SARS-CoV-2 virus was assessed in all participants at baseline (day 1) using Roche Elecsys anti-SARS-CoV-2 S kit to measure the anti-S immunoglobulins (Ig). All participants were also tested for antibodies against the nucleocapsid (N)-protein using Roche Elecsys anti-SARS-CoV-2 kit (anti-N test), a component not included in the SCB-2019 vaccine. Individuals having a positive result with the anti-S ELISA test or the anti-N test at baseline and individuals with a documented history of SARS-CoV-2 were considered SARS-CoV-2-exposed.
Humoral immune responses induced by SCB-2019 in SARS-CoV-2-naïve and exposed subjects were compared to the placebo using four different assays: SCB-2019-binding antibody ELISA to analyze SCB-2019-specific antibodies; two virus neutralization assays (wild-type virus and pseudovirus using a biosafety level 2-level recombinant VSV backbone expressing S protein from SARS-CoV-2 and green fluorescent protein) to assess neutralizing antibodies to the prototype SARS-CoV-2 (Wuhan-Hu-1), as well as to SARS-CoV-2 Alpha (B.1.1.7), Beta (B1.351), Gamma (P.1), Delta (B.1.617), Mu (B.1.621), and Omicron BA.1, BA.2, BA.4, and BA.5 (B.1.1.529) variants; and ACE2-competitive ELISA to analyze serum ACE2 receptor-binding IgG antibodies. Details on the assays are provided in the Supplementary materials. Geometric mean titers (GMTs) for the virus neutralization assays with the prototype SARS-CoV-2 and the SCB-2019-binding antibody ELISA were expressed in IU/mL, whereas GMTs for neutralization assays against variants were expressed in 1/dilution.
2.4. Assessments of cell-mediated immune responses
CMI was evaluated in 150 adult participants (75 in the SCB-2019 arm and 75 in the placebo arm) randomly enrolled at selected sites (two in Belgium and one in Colombia) able to collect and process the peripheral blood mononuclear cells (PBMC) samples before shipping to testing laboratory. Whole blood (32 mL) was collected at baseline (day 1) and 14 days after the second vaccination (day 36). The number of lymphocytes and intracellular cytokine staining (ICS) of PBMCs were analyzed using flow cytometry to evaluate CD4+/CD3+ T cells expressing markers of T-helper type 1 cells (interferon γ [IFNγ], interleukin [IL]-2, and tumor necrosis factor α [TNF α]), T-helper type 2 cells (IL-4, IL-5), and T-helper type 17 cells (IL-17) after in vitro stimulation with 15-mer (with 11 overlapping amino acids) peptide pools spanning across S1 and S2 subunits of SARS-CoV-2 spike protein.
2.5. Assessment of safety
The detailed reactogenicity data were presented earlier by Bravo et al. [4]. Unsolicited adverse events, medically attended adverse events, adverse events of special interest, and serious adverse events were collected during the entire study period. The detailed safety data obtained in all adult participants who received at least one dose of the SCB-2019 vaccine or placebo are reported separately (Husain et al., manuscript in revision).
2.6. Statistical analysis
A total of 1600 participants were planned to be recruited in the phase 2 part of the SPECTRA trial. The immunogenicity subset was to include all 800 subjects in the SCB-2019 arm and 100 randomly selected participants in the placebo arm who received at least one dose of the study vaccine and had available blood sample at day 36 for immunogenicity assessment. Only a subset of participants was selected from the placebo arm for immunogenicity testing because no increase of antibody titers was expected in this group. SARS-CoV-2 naïve participants who received two doses and had no major protocol deviations that could affect the immunogenicity of the vaccine were included in the Immunogenicity-per-protocol set (PPS). Immunogenicity analysis of SARS-CoV-2-exposed individuals was performed on the Immunogenicity-full analysis set (FAS) Exposed cohort, which included individuals who received two doses and had been exposed to SARS-CoV-2 or had a history of COVID-19 before vaccination.
Immunogenicity endpoints included GMTs, geometric mean fold rise (GMFR post-vaccination to baseline), the proportion of participants with seroconversion, and the proportion of participants with antibody titer above the lower limit of quantification (LLoQ).
The geometric mean was calculated as the mean of the antibody titers after the data were log-transformed, followed by exponentiating the log mean to present the results on the original scale. A two-sided 95 % confidence interval (CI) was obtained by taking the log-transform of the antibody results and calculated based on t-distribution. GMFR was calculated as the mean of the difference after log-transformed results (post-vaccination minus baseline) and exponentiating the mean; a two-sided 95 % CI for GMFR was obtained using paired t-distribution. Seroconversion was defined as four times the LLoQ for seronegative participants at baseline and as a 4-fold increase in the titer from the pre-vaccination value for seropositive participants. The Clopper-Pearson method was used to calculate the CIs for seroconversion rates and the proportion of participants with antibody titer above LLoQ. Missing data were not imputed. Statistical analyses were done using SAS software version 9.4 (SAS Institute).
3. Results
3.1. Study population
A total of 1660 individuals were screened for enrolment in the phase 2 part of the SPECTRA study, of whom 1601 were enrolled (Fig. 1 ). Among them, 808 participants were randomized to the SCB-2019 arm and 793 to the placebo arm. All participants received at least one vaccine dose. The immunogenicity subset included 759 participants in the SCB-2019 arm and 95 participants in the placebo arm. Of those, 248 participants in the vaccine arm and 32 participants in the placebo arm were seropositive for SARS-CoV-2. In total, 143 participants in vaccine arm and 20 participants in placebo arm had protocol deviations (mainly missing blood samples post-vaccination, deviations from vaccination, or blood sample schedule) or reported RT-PCR-confirmed COVID-19 before day 36.
Fig. 1.
Participant flow diagram. Flow diagram of participants enrolled in the phase 2 part of the phase 2/3 SPECTRA trial. Participants included in the immunogenicity analysis are highlighted in grey. BL - baseline; Ab - antibodies.
Analysis of SARS-CoV-2-naïve participants was conducted in the Immunogenicity-PPS, which included 381 participants in the SCB-2019 arm and 47 participants in the placebo arm. Analysis of SARs-CoV-2-exposed participants was conducted in the Immunogenicity-FAS, which included 235 participants in the SCB-2019 arm and 28 participants in the placebo arm.
The demographic characteristics were similar between the SCB-2019 and placebo arms in both the SARS-CoV-2-naïve and exposed populations analyzed (Table 1 ). More SARS-CoV-2-naïve participants were females , but more participants were males in the placebo arm of the SARS-CoV-2-exposed cohort. The median age of participants per group ranged between 34.9 and 38.5 years. The SARS-CoV-2-naïve cohort included proportionally more participants from Belgium and who identified themselves as White than the exposed cohort, which included mostly participants from the Philippines. This difference was due to the higher number of SARS-CoV-2-naïve participants recruited from Belgium than from the Philippines [5]. In the SARS-CoV-2-exposed cohort, 7.2 % of the participants had a known history of COVID-19, and 99.6 % of the participants were positive for SARS-CoV-2 serostatus at baseline.
Table 1.
Baseline characteristics of the SARS-CoV-2-naïve and exposed populations.
|
SARS-CoV-2-naïve population (Immunogenicity-PPS) |
SARS-CoV-2-exposed population (Immunogenicity-FAS Exposed) |
|||||
|---|---|---|---|---|---|---|
|
SCB-2019 N = 381 |
Placebo N = 47 |
Total N = 428 |
SCB-2019 N = 235 |
Placebo N = 28 |
Total N = 263 |
|
| Sex, n (%) | ||||||
| Male | 160 (42.0) | 19 (40.4) | 179 (41.8) | 114 (48.5) | 17 (60.7) | 131 (49.8) |
| Female | 221 (58.0) | 28 (59.6) | 249 (58.2) | 121 (51.5) | 11 (39.3) | 132 (50.2) |
| Age (years) | ||||||
| Mean (SD) | 36.0 (12.5) | 38.5 (13.8) | 36.3 (12.7) | 36.1 (13.2) | 34.9 (14.1) | 36.0 (13.3) |
| Median (range) | 35.0 (18–77) | 39.0 (18–68) | 35.0 (18–77) | 34.0 (18–72) | 31.5 (19–81) | 34.0 (18–81) |
| Age group, n (%) | ||||||
| ≥18 to ≤ 64 years | 374 (98.2) | 45 (95.7) | 419 (97.9) | 224 (95.3) | 27 (96.4) | 251 (95.4) |
| ≥65 years | 7 (1.8) | 2 (4.3) | 9 (2.1) | 11 (4.7) | 1 (3.6) | 12 (4.6) |
| ≥65 to ≤ 74 years | 6 (1.6) | 2 (4.3) | 8 (1.9) | 11 (4.7) | 0 | 11 (4.2) |
| ≥75 years | 1 (0.3) | 0 | 1 (0.2) | 0 | 1 (3.6) | 1 (0.4) |
| ≥18 to ≤ 59 years | 357 (93.7) | 43 (91.5) | 400 (93.5) | 220 (93.6) | 27 (96.4) | 247 (93.9) |
| ≥60 years | 24 (6.3) | 4 (8.5) | 28 (6.5) | 15 (6.4) | 1 (3.6) | 16 (6.1) |
| Race, n (%) | ||||||
| American Indian or Alaska Native | 30 (7.9) | 2 (4.3) | 32 (7.5) | 24 (10.2) | 0 | 24 (9.1) |
| Asian | 199 (52.2) | 20 (42.6) | 219 (51.2) | 174 (74.0) | 22 (78.6) | 196 (74.5) |
| Black or African American | 4 (1.0) | 0 | 4 (0.9) | 0 | 0 | 0 |
| White | 124 (32.5) | 23 (48.9) | 147 (34.3) | 20 (8.5) | 6 (21.4) | 26 (9.9) |
| Other | 24 (6.3) | 2 (4.3) | 26 (6.1) | 17 (7.2) | 0 | 17 (6.5) |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 64 (16.8) | 9 (19.1) | 73 (17.1) | 59 (25.1) | 1 (3.6) | 60 (22.8) |
| Not Hispanic or Latino | 317 (83.2) | 38 (80.9) | 355 (82.9) | 176 (74.9) | 27 (96.4) | 201 (77.2) |
| Country, n (%) | ||||||
| Belgium | 131 (34.4) | 23 (48.9) | 154 (36.0) | 20 (8.5) | 6 (21.4) | 26 (22.8) |
| Colombia | 52 (13.6) | 4 (8.5) | 56 (13.1) | 41 (17.5) | 0 | 41 (15.6) |
| Philippines | 198 (52.0) | 20 (42.6) | 218 (50.9) | 174 (70.0) | 22 (78.6) | 196 (74.5) |
| High risk of severe COVID-19, n (%) | ||||||
| No | 307 (80.6) | 36 (76.6) | 343 (80.1) | 187 (79.6) | 24 (85.7) | 211 (80.2) |
| Yes | 74 (19.4) | 11 (23.4) | 85 (19.9) | 48 (20.4) | 4 (14.3) | 52 (19.8) |
| Obesity (BMI ≥ 30 kg/m2) | 46 (12.1) | 2 (4.3) | 48 (11.2) | 26 (11.1) | 1 (3.6) | 27 (10.3) |
| Hypertension | 24 (6.3) | 6 (12.8) | 30 (7.0) | 24 (10.2) | 2 (7.1) | 26 (9.9) |
| Type 2 diabetes mellitus | 7 (1.8) | 1 (2.1) | 8 (1.9) | 4 (1.7) | 0 | 4 (1.5) |
| Asthma | 7 (1.8) | 2 (4.3) | 9 (2.1) | 2 (0.9) | 0 | 2 (0.8) |
| Baseline SARS-Cov-2 serostatus, n (%) | ||||||
| Negative | 381 (100.0) | 47 (100.0) | 428 (100.0) | 1 (0.4)1 | 0 | 1 (0.4) |
| Positive | 0 | 0 | 0 | 234 (99.6) | 28 (1 0 0) | 262 (99.6) |
| Known history of COVID-19 at baseline, n (%) | ||||||
| No | 381 (100.0) | 47 (100.0) | 428 (100.0) | 217 (92.3) | 27 (96.4) | 244 (92.8) |
| Yes | 0 | 0 | 0 | 18 (7.7) | 1 (3.6) | 19 (7.2) |
| Baseline evidence of prior SARS-CoV-2 infection, n (%) | ||||||
| No | 381 (100.0) | 47 (100.0) | 428 (100.0) | 0 | 0 | 0 |
| Yes | 0 | 0 | 0 | 235 (1 0 0) | 28 (1 0 0) | 163 (1 0 0) |
Abbreviations: BMI, body mass index; PPS, per protocol set; FAS, full analysis set; SD, standard deviation.
N is the number of participants in each arm; n is the number of participants with available data.
This participant had a history of confirmed COVID-19.
3.2. Humoral immune responses induced by SCB-2019
In SARS-CoV-2-naïve participants (381 in the SCB-2019 arm and 47 in the placebo arm), a robust increase of the GMTs as measured by the wild-type virus neutralization assay was observed 2 weeks after the second dose of SCB-2019 (day 36) compared to baseline (224 vs 12.7 IU/mL, respectively) (Table 2 ). The GMT increased by 17.5-fold (95 % CI: 15.1–20.2) between baseline and day 36 in SCB-2019 recipients. On day 36, the percentage of SCB-2019 recipients who seroconverted was 82.5 % (179/217) and 96.8 % (213/220) of SCB-2019 recipients had titers above the LLoQ. No increase was detected in GMTs, seroconversion rates, or proportions of participants with titers above the LLoQ on day 36 over baseline in placebo recipients.
Table 2.
Neutralizing antibodies induced by SCB-2019.
|
SARS-CoV-2-naïve population (Immunogenicity-PPS) |
SARS-CoV-2-exposed population (Immunogenicity-FAS Exposed) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
SCB-2019 N = 381 |
Placebo N = 47 |
SCB-2019 N = 235 |
Placebo N = 28 |
|||||
| Endpoint | n | Value (95 % CI) | n | Value (95 % CI) | n | Value (95 % CI) | n | Value (95 % CI) |
| Wild-type SARS-CoV-2 neutralizing antibodies (IU/mL) | ||||||||
| Day 1 | ||||||||
| Mean GMT | 219 | 12.7 (12.6–12.9) | 27 | 12.5 (–) | 118 | 26.4 (22.3–31.3) | 18 | 32.7 (16.3–65.8) |
| >LLoQ, % | 219 | 0.9 (0.1–3.3) | 27 | 0.0 (0.0–12.8) | 118 | 44.1 (34.9–53.5) | 18 | 38.9 (17.3–64.3) |
| Day 22 | ||||||||
| Mean GMT | 215 | 15.7 (14.1–17.5) | 28 | 12.8 (12.4–13.3) | 118 | 1276.1 (999.3–1629.7) | 18 | 42.1 (20.6–86.6) |
| GMFR | 212 | 1.2 (1.1–1.4) | 27 | 1.0 (1.0–1.1) | 118 | 48.3 (36.9–63.2) | 18 | 1.3 (0.7–2.3) |
| SCR, % | 212 | 4.2 (2.0–7.9) | 27 | 0.0 (0.0–12.8) | 118 | 92.4 (86.0–96.5) | 18 | 5.6 (0.1–27.3) |
| >LLoQ, % | 215 | 9.3 (5.8–14.0) | 28 | 0.0 (0.0–12.3) | 118 | 97.5 (92.7–99.5) | 18 | 61.1 (35.7–82.7) |
| Day 36 | ||||||||
| Mean GMT | 220 | 224 (194.0–258.7) | 28 | 12.8 (12.4–13.3) | 118 | 1831.4 (1545.9–2169.8) | 18 | 58.3 (26.6–128.1) |
| GMFR | 217 | 17.5 (15.1–20.2) | 27 | 1.0 (1.0–1.1) | 118 | 69.3 (55.9–85.8) | 18 | 1.8 (0.8–3.8) |
| SCR, % | 217 | 82.5 (76.8–87.3) | 27 | 0.0 (0.0–12.8) | 118 | 98.3 (94.0–99.8) | 18 | 16.7 (3.6–41.4) |
| >LLoQ, % | 220 | 96.8 (93.6–98.7) | 28 | 0.0 (0.0–12.3) | 118 | 100.0 (96.9–100.0) | 18 | 61.1 (35.7–82.7) |
| Pseudovirus neutralizing antibodies (IU/mL) | ||||||||
| Day 1 | ||||||||
| Mean GMT | 219 | 15.8 (15.5–16.1) | 27 | 15.5 (–) | 119 | 47.6 (39.2–57.9) | 18 | 56.7 (27.5–117.0) |
| >LLoQ, % | 219 | 1.4 (0.3–4.0) | 27 | 0.0 (0.0–12.8) | 119 | 63.9 (54.6–72.5) | 18 | 61.1 (35.7–82.7) |
| Day 22 | ||||||||
| Mean GMT | 214 | 22.9 (20.1–26.1) | 28 | 15.9 (15.0–16.9) | 118 | 1559.9 (1266.3–1921.5) | 18 | 70.8 (32.5–154.3) |
| GMFR | 212 | 1.5 (1.3–1.6) | 27 | 1.0 (1.0–1.1) | 118 | 32.4 (25.0–42.2) | 18 | 1.2 (0.6–2.4) |
| SCR, % | 212 | 7.5 (4.4–12.0) | 27 | 0.0 (0.0–12.8) | 118 | 90.7 (83.9–95.3) | 18 | 5.6 (0.1–27.3) |
| >LLoQ, % | 214 | 19.6 (14.5–25.6) | 28 | 3.6 (0.1–18.3) | 118 | 97.5 (92.7–99.5) | 18 | 66.7 (41.0–86.7) |
| Day 36 | ||||||||
| Mean GMT | 220 | 540.3 (472.8–617.5) | 28 | 20.3 (14.8–27.8) | 119 | 2192.1 (1931.7–2487.6) | 18 | 74.0 (35.3–155.0) |
| GMFR | 218 | 34.1 (29.8–39.0) | 27 | 1.3 (1.0–1.8) | 119 | 46.0 (37.1–57.0) | 18 | 1.3 (0.7–2.5) |
| SCR, % | 218 | 93.6 (89.5–96.4) | 27 | 3.7 (0.1–19.0) | 119 | 95.8 (90.5–98.6) | 18 | 11.1 (1.4–34.7) |
| >LLoQ, % | 220 | 98.2 (95.4–99.5) | 28 | 14.3 (4.0–32.7) | 119 | 100.0 (96.9–100.0) | 18 | 66.7 (41.0 –86.7) |
Abbreviations: CI, confidence interval; FAS, full analysis set; GMFR, geometric mean fold rise; GMT, geometric mean titer; LLoQ, lower limit of quantification; PPS, per protocol set; SCR, seroconversion rate.
N is the number of participants in each arm; n is the number of participants with available data.
Titer value measured as below LLoQ of the assay was set to LLoQ/2.
In previously SARS-CoV-2-exposed participants (235 in the SCB-2019 arm and 28 in the placebo arm), neutralizing antibodies increased by 48.3 fold in SCB-2019 recipients on day 22 (from 26.4 to 1276.1 IU/mL), 3 weeks after the first vaccine dose. On day 36, 2 weeks after the second dose, the GMT reached 1831.4 IU/mL and increased by 69.3-fold compared to baseline (95 % CI: 55.9–85.8). In contrast, the GMTs on days 1, 22, and 36 remained within a range from 32.7 to 58.3 IU/mL in placebo recipients. In addition, 92.4 % (95 % CI: 86.0–96.5) of the SARS-CoV-2-exposed participants seroconverted in the SCB-2019 arm, compared to 5.6 % (95 % CI: 0.1–27.3) in the placebo arm. Most SARS-CoV-2-exposed participants had titers above the LLoQ after vaccination with SCB-2019 (44.1 % on day 1, 97.5 % on day 22, and 100 % on day 36), compared to 38.9 % (day 1) and 61.1 % (days 22 and 36) in the placebo arm. Similar kinetics of SARS-CoV-2-specific neutralizing antibodies in SCB-2019 recipients were revealed by testing with a pseudovirus neutralization assay. In addition to neutralizing antibodies, we also measured overall SCB-2019 binding and ACE2-receptor binding antibodies using SCB-2019 binding and ACE2-competitive binding ELISAs, respectively. The results of these assays were also consistent with those of the wild-type SARS-CoV-2 neutralization assays (Supplementary results and Table S1). Two doses of SCB-2019 were immunogenic in SARS-CoV-2-naïve participants, and a single dose of SCB-2019 showed a significant immune response in SARS-CoV-2-exposed participants.
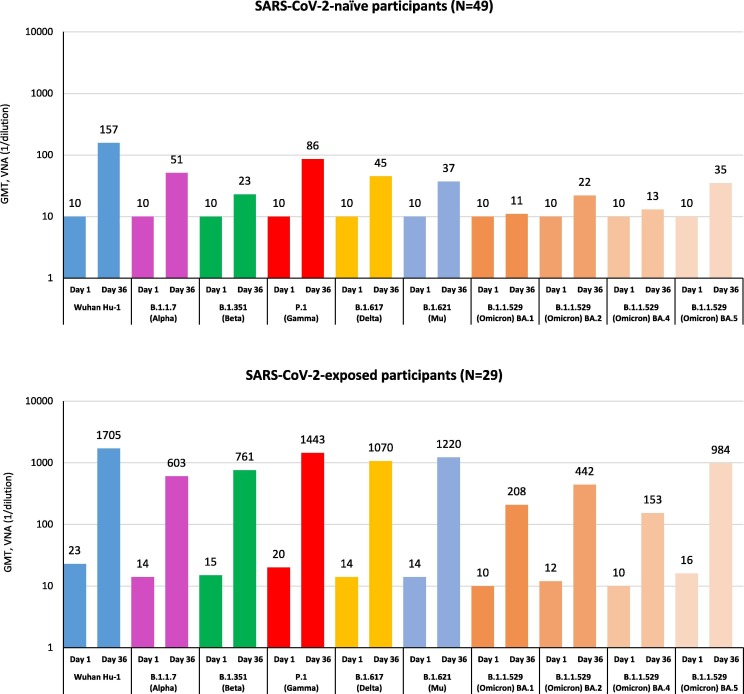
Cross-neutralizing immune responses against nine variants of SARS-CoV-2 (Alpha, Beta, Gamma, Delta, Mu, and Omicron BA.1, BA.2, BA.4, and BA.5) were evaluated in SARS-CoV-2-naïve and SARS-CoV-2-exposed participants after two doses of SCB-2019 vaccine (Fig. 2 ). The cross-neutralization capability of SCB-2019 was shown against Alpha, Beta, Gamma, Delta, and Mu variants in SARS-CoV-2-naïve participants after the second vaccine dose and to a greater extent in SARS-CoV-2-exposed participants. Whereas no antibodies against the Omicron BA.1 and BA.4 variants and low cross-neutralizing antibodies against the Omicron BA.2 and BA.5 variants were detected in SARS-CoV-2-naïve participants at day 36, high titers of cross-neutralizing antibodies against all these Omicron variants were noted in SARS-CoV-2-exposed participants 2 weeks after the second vaccine dose (MN50 [1/dilution], 208 for BA.1, 442 for BA.2, 153 for BA.4, and 984 for BA.5). The GMT of neutralizing antibodies against all Omicron variants in this cohort was comparable to GMTs against the original Wuhan-Hu-1 strain observed in SARS-CoV-2-naïve participants on day 36 (MN50, 157).
Fig. 2.
Cross-neutralization of new emergent variants of SARS-CoV-2 by SCB-2019 vaccine-elicited sera. Geometric mean titers (GMTs) of cross-neutralizing antibodies against different SARS-CoV-2 variants are shown in serum samples collected at days 1 and 36 from SARS-CoV-2-naïve (top) and exposed (bottom) participants vaccinated with SCB-2019, as measured by virus microneutralization assay (VNA).
3.3. Cell-mediated immune responses induced by SCB-2019
Cell-mediated immune responses were measured by ICS in a subset of SARS-CoV-2-naïve and exposed participants (Fig. 3 ). Overall, 137 subjects were included in the CMI analysis, 70 from the SCB-2019 arm and 67 from the placebo arm. Increases of Th1-type cytokine-expressing cells (i.e., CD4+/CD3+ T cells producing IFNγ, IL-2, or TNFα) were observed between the baseline and 2 weeks after the second vaccine dose (day 36) in CD4+/CD3+ T lymphocytes from both SARS-CoV-2-naïve and exposed participants vaccinated with SCB-2019, after in vitro stimulation with peptide pools from S1 and S2 subunits of SARS-CoV-2 spike protein. The magnitude of immune response was higher in previously SARS-CoV-2-exposed individuals. No increases in Th2 (CD4+/CD3+ T cells producing IL-4 or IL-5) or Th17 (CD4+/CD3+ T cells producing IL-17) cellular immune responses were observed (data not shown), suggesting that a Th1-biased cell-mediated immune response specific to the SARS-CoV-2 S-protein was induced after a two-dose vaccination with SCB-2019. In the placebo recipients, neither a Th1 nor a Th2 response could be detected, as expected.
Fig. 3.
Cell-mediated immunity of SCB-2019 vaccine. Cell-mediated immunity was measured by intracellular cytokine staining and flow cytometry in serum samples from a subset of participants vaccinated with SCB-2019 (N = 70) or placebo (N = 67). Results are geometric mean (GM) of cytokine expressed in CD4+/CD3+ T cells (i.e., IFNγ+/CD4+/CD3+, IL-2+/CD4+/CD3+, and TNF α +/CD4+/CD3+) with 95 % confidence intervals (indicated by black lines on top of the bars). Top panel, GMs in samples collected at days 1 and 36 from SARS-CoV-2-naïve and exposed participants vaccinated with SCB-2019 or placebo. Bottom panel, GMs in samples collected at days 1 and 36 in participants vaccinated with SCB-2019 or placebo (regardless of SARS-CoV-2 exposure history), after in vitro stimulation with peptide pools spanning across S1 or S2 subunit of SARS-CoV-2 spike protein.
3.4. Overall SCB-2019 safety profile
The detailed safety data obtained in all 30,137 adult participants who received at least one dose of SCB-2019 (N = 15,070) or placebo (N = 15,067) between 24 March 2021 and 01 December 2021 are reported separately (Husain et al., manuscript in revision). SCB-2019 has an acceptable safety profile. Overall, unsolicited adverse events, medically attended adverse events, adverse events of special interest, and serious adverse events were reported in similar frequencies in vaccine and placebo arms over the 6-month follow-up period.
4. Discussion
All approved COVID-19 vaccines were designed to elicit neutralizing immune responses against the SARS-CoV-2 spike protein of the Wuhan strain and were initially tested in SARS-CoV-2-naïve individuals. The primary population for evaluating the protective efficacy of all approved COVID-19 vaccines were individuals without evidence of previous SARS-CoV-2 infection. However, during the pandemic, a significant proportion of any study population was naturally primed due to asymptomatic or symptomatic SARS-CoV-2 infection. A systematic review and meta-analysis of the global seroprevalence of SARS-CoV-2 antibodies, conducted for a period from 01 January 2020 to 31 December 2020, estimated a global population-wide seroprevalence of 4.5 % [6]. In April 2021, WHO assessed SARS-CoV-2 seroprevalence as 26 % overall, ranging between 0.3 % in WHO’s Western Pacific Region to 57 % in high-income countries in the Americas [7]. Based on the recent U.S. CDC data, the seroprevalence of infection-induced SARS-CoV-2 antibodies increased from 33.5 % (95 % CI: 33.1–34.0) to 57.7 % (95 % CI: 57.1–58.3) for the period from December 2021 to February 2022 [1]. In our study, approximately half of the participants had been exposed to SARS-CoV-2 at the time of enrollment [5]. This proportion varied between countries, from 12.6 % in Belgium to 64.9 % in the Philippines [5].
As such, the assessment of vaccine-induced immune response and protection against COVID-19 in SARS-CoV-2-naïve and previously exposed individuals is now essential for understanding the benefit of vaccination campaigns and optimal booster strategy. The results reported here were obtained in the phase 2 part of the Clover SPECTRA phase 2/3 clinical trial, which was conducted to evaluate the humoral and cell-mediated immune responses induced by SCB-2019 in both SARS-CoV-2-naïve and exposed participants. As measured by SARS-CoV-2 neutralization assays, SCB-2019-binding ELISA, and ACE2-competitive ELISA, we showed that two doses of SCB-2019 were immunogenic in-SARS-CoV-2-naïve participants. Furthermore, in SARS-CoV-2-exposed participants, a single dose of SCB-2019 elicits a rapid and significant immune response, which is consistent with prior observations that a single dose of SARS-CoV-2 vaccine (mRNA or adenovirus-vectored) elicits a robust immune response in individuals previously infected with SARS-CoV-2 [8], [9], [10]. These results support the efficacy data obtained in the trial. From 2 weeks after the second vaccination, for all the VOCs evaluated, SCB-2019 demonstrated 100 % efficacy against severe COVID-19, 83.7 % efficacy against moderate-to-severe disease, and 67.2 % efficacy against COVID-19 of any severity in SARS-CoV-2-naïve individuals [4]. After only one vaccination, in SARS-CoV-2-exposed participants, the incremental vaccine efficacy over that afforded by natural infection against all VOCs was 49.9 % and it increased to 64.2 % after the second vaccination. Immune response against new emergent VOCs appeared lower compared to those against homologous Wuhan-Hu-1 prototype virus. However, despite being low, the titers of neutralizing antibodies induced by SCB-2019 against Delta (45 IU/mL), Gamma (86 IU/mL), and Mu (37 IU/mL) variants in naïve subjects were associated with clinical protection against COVID-19 of any severity (78.7 %, 91.8 %, and 58.6 %, respectively) [4].
Goldblatt et al. applied a population-based approach to define an immunologic correlate of protection for COVID-19 vaccines based on anti-spike IgG antibodies. The authors proposed a protective threshold of 60 binding antibody units (BAU)/mL (95 % CI: 35–102), suggesting that relatively low levels of binding antibodies might be sufficient to protect against COVID-19 [11]. They also demonstrated a high correlation between binding and neutralizing antibodies and clinical protection against symptomatic COVID-19 disease. Goldblatt et al. also assessed responses by ELISA in a subset of naïve recipients of the SCB-2019 vaccine and found comparable concentrations in binding antibodies to mRNA vaccines [12]. These results support our observation of significant efficacy against variants of concerns in SARS-CoV-2-naïve participants with relatively low titers of cross-neutralizing antibodies as measured in neutralization assays.
In SARS-CoV-2-exposed individuals, a two-dose primary vaccination with SCB-2019 was associated with a significant increase of antibody titers against all tested VOCs including Omicron BA.1, BA.2, BA.4, and BA.5. The Omicron BA.1 variant contains more than 59 mutations throughout its genome, with over 36 of them occurring within the S protein, including 15 mutations in the receptor-binding region [13]. BA.1 has then evolved into several sub-variants [14]. The presence of these mutations in regions accessible to antibodies makes Omicron variants markedly different from the prototype Wuhan-Hu-1 virus and may explain the low cross-neutralization titers in SARS-CoV-2-naïve participants. However, SARS-CoV-2-exposed participants achieved higher cross-neutralizing titers against Omicron variants after receiving two doses of SCB-2019 than SARS-CoV-2-naïve participants. Thus, SCB-2019 elicited a robust cross-neutralizing response against all nine VOCs at antibody levels associated with clinical protection in participants with evidence of prior SARS-CoV-2 infection, underlining the additional value of SCB-2019 as a potential booster vaccine.
Neutralizing antibodies can prevent virus entry into host cells and play a central role in protecting against infection [15]. In addition, virus-specific CD4+ T-cell immune responses and CD8+ cytotoxic T-cell responses have been proposed as reducing the severity of disease [16], [17], [18], [19], [20], [21], [22]. SCB-2019 induced not only high neutralizing antibody titers but also stimulated Th1-biased cell-mediated immune responses in both SARS-CoV-2-naïve and exposed individuals, suggesting that this recombinant SCB-2019 vaccine, when delivered with CpG-1018/alum adjuvant, can elicit both proper B and T cell immune responses to the S protein and, subsequently, confer protection from SARS-CoV-2 infection. Both Th1 and Th2 cellular immune responses were consistent with those reported in the SCB-2019 phase 1 study and aligned with the responses elicited by other COVID-19 vaccines [3], [16], [23], [24], [25].
This study has a few limitations. Due to the availability of authorized COVID-19 vaccines for the populations at high risk of severe COVID-19, few older participants were enrolled in this study. The study population is composed mainly of young adults. Another limitation is that immunogenicity was analyzed only up to 14 days after the second vaccine dose and thus does not evaluate long-term persistence of the responses induced by SCB-2019. However, a previous phase 1 study showed that 25–35 % of the observed peak value of neutralizing and binding antibodies persist up to 6 months following vaccination with SCB-2019 [26]. Assessing the long-term persistence of the responses induced by SCB-2019 would be of interest because the duration of protection provided by the approved COVID-19 vaccines against disease caused by the circulating variants appears to be limited [27], [28]. This study does not provide a comparative evaluation of the immunogenicity of SCB-2019 to other authorized COVID-19 vaccines. Furthermore, a recent study has shown that antibody titers elicited by SCB-2019 against the original prototype Wuhan-Hu-1 and Alpha variants are comparable or superior to those induced by four approved COVID-19 vaccines [12].
5. Conclusions
We have shown in this study that the adjuvanted trimeric S protein recombinant vaccine SCB-2019 elicits a robust immune response in both SARS-CoV-2-naïve and previously exposed individuals, which correlates with the vaccine efficacy demonstrated against SARS-CoV-2 previously described. A single dose of SCB-2019 was immunogenic in naturally primed SARS-CoV-2-exposed individuals and a higher humoral immune response was obtained in previously exposed individuals than in SARS-CoV-2-naïve ones. The SCB-2019 induced cross-reactive neutralizing antibodies to several emergent SARS-CoV-2 variants and a robust Th1-biased cell-mediated immune response specific to the SARS-CoV-2 S protein and consistent with other COVID-19 vaccines.
Funding
This study was supported by grants from The Coalition for Epidemic Preparedness Innovations (CEPI), Oslo, Norway (grants RRCL2001 and RRCL2002) and by Clover Biopharmaceuticals.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MH received financial support from Clover Biopharmaceuticals, grants from the European Union, the Belgian Health Care Knowledge Centre, and Université Libre de Bruxelles, travel fees from Gilead and Pfizer, financial support from INSMED, is involved in the Belgian Society of Infectious Diseases and Clinical Microbiology, the working group on Belgian COVID-19 therapeutic guidelines, and the Belgian Task Force on therapeutics for COVID-19. GS served as a Scientific Advisory Board Member and received consulting fees from Clover Biopharmaceuticals, AdVaccine, CanSino, Everest Medicines, Valneva, Vaxart, Affinivax, Genocea, and Vaxxess. DA received consulting fees from Clover Biopharmaceuticals, Vaxxinity, Everest Medicines, Senda, served as a Scientific Advisory Board Member for Clover Biopharmaceuticals, Vaxxinity, Senda, Everest Medicines, and Inventprise, and served as a board member for Clover Biopharmaceuticals and Inventprise. RC received funding from the Bill & Melinda Gates Foundation, consulting fees from Icosavax, Hillevax, honoraria from AstraZeneca, served as a board member for Clover Biopharmaceuticals, Curevac, IVI, Inventprise, and owns stocks in Icosavax, Hillevax, Curevac, Novartis, Roche, GSK, and Clover Biopharmaceuticals. EB had contracts with Clover Biopharmaceuticals, Astra-Zeneca, Janssen, GSK, Merck, and Moderna. ERA, JCC, LFBF, and MEBM declare that they have no competing financial interests. BH, HHH, HQ, and HLC are employees of Clover Biopharmaceuticals. CB is an employee of Clover Biopharmaceuticals owns stocks in Clover Biopharmaceuticals, GSK, and Sanofi. PL and IS are employees of Clover Biopharmaceuticals and own stocks in the company.
Acknowledgments
We thank all the participants in this clinical trial and team members from Clover Biopharmaceuticals, biostatisticians, contract research organizations, and clinical suppliers for their contributions to the study. VisMederi (Siena, Italy) conducted the wild-type virus neutralization assay and cross-neutralization assays for the different variants, SCB-2019 ELISA, and ACE2 competitive ELISA. Q2 Solutions (California, USA) conducted the pseudovirus neutralization assay. LabCorp conducted the intracellular cytokine assay. Dynavax Technologies provided the CpG-1018 adjuvant. We are particularly grateful to the Scientific Advisory Board members and The Coalition for Epidemic Preparedness Innovations (CEPI) team for their advice and guidance, and to the members of the data and safety monitoring board for their dedication to the trial. Medical writing was provided by Drs. Surayya Taranum and Julie Harriague (4Clinics, France) and funded by Clover Biopharmaceuticals.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.02.017.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Clarke KEN, M. JJ, Deng Y, Nycz E, Lee A, Iachan R, et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies — United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022;71:606-8. [DOI] [PMC free article] [PubMed]
- 2.Liu H., Su D., Zhang J., Ge S., Li Y., Wang F., et al. Improvement of Pharmacokinetic Profile of TRAIL via Trimer-Tag Enhances its Antitumor Activity in vivo. Sci Rep. 2017;7(1):8953. doi: 10.1038/s41598-017-09518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richmond P., Hatchuel L., Dong M., Ma B., Hu B., Smolenov I., et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10275):682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravo L., Smolenov I., Han H.H., Li P., Hosain R., Rockhold F., et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399(10323):461–472. doi: 10.1016/S0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolenov I., Han H.H., Li P., Baccarini C., Verhoeven C., Rockhold F., et al. Impact of previous exposure to SARS-CoV-2 and of S-Trimer (SCB-2019) COVID-19 vaccination on the risk of reinfection: a randomised, double-blinded, placebo-controlled, phase 2 and 3 trial. Lancet Infect Dis. 2022;22(7):990–1001. doi: 10.1016/S1473-3099(22)00144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobrovitz N., Arora R.K., Cao C., Boucher E., Liu M., Donnici C., et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLoS One. 2021;16(6):e0252617. doi: 10.1371/journal.pone.0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. True extent of SARS-CoV-2 Infection through seroprevalence studies. 2022; Available from: https://www.who.int/news/item/03-02-2022-true-extent-of-sars-cov-2-infection-through-seroprevalence-studies#:∼:text=Key%20findings%20from%20the%20meta,to%20infection%20at%20that%20time.
- 8.Havervall S., Marking U., Greilert-Norin N., Ng H., Gordon M., Salomonsson A.C., et al. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley T., Grundberg E., Selvarangan R., LeMaster C., Fraley E., Banerjee D., et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(20):1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldblatt D., Fiore-Gartland A., Johnson M., Hunt A., Bengt C., Zavadska D., et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine. 2022;40(2):306–315. doi: 10.1016/j.vaccine.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosino D., Han H.H., Hu B., Liang J., Clemens R., Johnson M., et al. Immunogenicity of SCB-2019 Coronavirus Disease 2019 Vaccine Compared With 4 Approved Vaccines. J Infect Dis. 2022;225(2):327–331. doi: 10.1093/infdis/jiab574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021; Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- 14.Shrestha L.B., Foster C., Rawlinson W., Tedla N., Bull R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev Med Virol. 2022:e2381. doi: 10.1002/rmv.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor P.C., Adams A.C., Hufford M.M., de la Torre I., Winthrop K., Gottlieb R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 17.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansari A., Arya R., Sachan S., Jha S.N., Kalia A., Lall A., et al. Immune Memory in Mild COVID-19 Patients and Unexposed Donors Reveals Persistent T Cell Responses After SARS-CoV-2 Infection. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.636768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L., Wang G., Wang Y., Zhang Q., Ren L., Gu X., et al. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: a longitudinal cohort study. Lancet. Microbe. 2022;3:348–356. doi: 10.1016/S2666-5247(22)00036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 22.Goldblatt D, Galit A, Crotty SH, Hane S, Plotkin S. Correlates of Protection Against SARS CoV-2 Infection and Covid 19 Disease. 2022. [DOI] [PMC free article] [PubMed]
- 23.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solforosi L., Kuipers H., Jongeneelen M., Rosendahl Huber S.K., van der Lubbe J.E.M., Dekking L., et al. Immunogenicity and efficacy of one and two doses of Ad26.COV2.S COVID vaccine in adult and aged NHP. J Exp Med. 2021;218(7) doi: 10.1084/jem.20202756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond P.C., Hatchuel L., Pacciarini F., Hu B., Smolenov I., Li P., Liang P., Han H.H., Liang J., Clemens R. Persistence of the Immune Responses and Cross-Neutralizing Activity With Variants of Concern Following 2 Doses of Adjuvanted SCB-2019 Coronavirus Disease 2019 Vaccine. J Infect Dis. 2021;224(10):1699–1706. doi: 10.1093/infdis/jiab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pegu A., O'Connell S.E., Schmidt S.D., O'Dell S., Talana C.A., Lai L., et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milne G., Hames T., Scotton C., Gent N., Johnsen A., Anderson R.M., et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med. 2021;9(12):1450–1466. doi: 10.1016/S2213-2600(21)00407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.