Abstract
Emerging in November 2021, the SARS-CoV-2 Omicron variant of concern exhibited marked immune evasion resulting in reduced vaccine effectiveness against SARS-CoV-2 infection and symptomatic disease. Most vaccine effectiveness data on Omicron are derived from the first Omicron subvariant, BA.1, which caused large waves of infection in many parts of the world within a short period of time. BA.1, however, was replaced by BA.2 within months, and later by BA.4 and BA.5 (BA.4/5). These later Omicron subvariants exhibited additional mutations in the spike protein of the virus, leading to speculation that they might result in even lower vaccine effectiveness. To address this question, the World Health Organization hosted a virtual meeting on December 6, 2022, to review available evidence for vaccine effectiveness against the major Omicron subvariants up to that date. Data were presented from South Africa, the United Kingdom, the United States, and Canada, as well as the results of a review and meta-regression of studies that evaluated the duration of the vaccine effectiveness for multiple Omicron subvariants. Despite heterogeneity of results and wide confidence intervals in some studies, the majority of studies showed vaccine effectiveness tended to be lower against BA.2 and especially against BA.4/5, compared to BA.1, with perhaps faster waning against severe disease caused by BA.4/5 after a booster dose. The interpretation of these results was discussed and both immunological factors (i.e., more immune escape with BA.4/5) and methodological issues (e.g., biases related to differences in the timing of subvariant circulation) were possible explanations for the findings. COVID-19 vaccines still provide some protection against infection and symptomatic disease from all Omicron subvariants for at least several months, with greater and more durable protection against severe disease.
Keywords: Covid-19 vaccines, Vaccine effectiveness, Omicron variant
1. Background and meeting objectives
The World Health Organization (WHO) declared the Omicron variant, first detected in South Africa, as a variant of concern on November 26, 2021 due to its high transmissibility and immune evasion potential [1]. These early cases were mostly the BA.1 lineage. Within one month, studies from the United Kingdom, Denmark, Canada, and South Africa showed that the vaccine effectiveness (VE) in real-world settings of the primary series of COVID-19 vaccines was lower against BA.1 infection and symptomatic disease compared to previous circulating variants [2], [3], [4], [5], [6]. Data showed that primary series VE against hospitalization was also lower compared to previous circulating variants of concern, though higher than the VE against infection and symptomatic disease [6]. A booster dose improved VE over the primary series against all BA.1-related outcomes, but was still lower when compared to booster dose VE for previous circulating variants. Lower VE for Omicron was confirmed by many subsequent studies; moreover, further evidence suggested that there was rapid waning of protection against infection and symptomatic disease due to Omicron [7], [8].
Other Omicron subvariant lineages, which had additional mutations in the spike protein compared with BA.1, started cocirculating in early 2022 and soon replaced BA.1 as the predominant Omicron lineage [9], [10]. Subvariant BA.2 dominated in South Africa by late January and globally by March [11], [12]. After BA.2 had become predominant, BA.4 and BA.5 began to increase in prevalence [11]. BA.4 and BA.5 were first detected in South Africa in January and February 2022, respectively; by early July, BA.4 made up 11 % of global cases and BA.5 made up 52 % of global cases [9], [10]. BA.5 descendent lineages remained predominant globally through the end of November 2022, with a global prevalence of 74 % [13].
Given the different spike protein mutations that defined subsequent Omicron subvariants, there was concern that vaccines might have lower VE to these subvariants compared to BA.1. However, measuring such decreases in VE can be challenging for several reasons. First, if VE is only slightly decreased for these subvariants, it might not be detected from inadequately powered studies. Second, due to the speed with which Omicron subvariants have replaced each other, contemporaneous measurements of different subvariants during the same period in one geographic setting have rarely been possible. Moreover, because genomic sequencing has declined, few studies have had enough molecular characterization of infections to calculate VE in settings of co-circulation of subvariants. Consequently, most studies that have assessed VE for multiple subvariants have done so after each subvariant has become predominant in the study setting. This leads to VE assessments in different periods of time for different subvariants, which leads to the potential for time-varying confounders to bias results.
To address whether VE differs by Omicron subvariant, and to define which biases might affect the results, WHO held a virtual meeting of the COVID-19 Vaccine Effectiveness Methods Forum on December 6, 2022, to review the evidence from several studies that assessed COVID-19 VE against Omicron subvariants. This report summarizes the results of these studies, as well as other relevant studies in the pre-print and published literature and discusses potential biases that should be considered when interpreting VE results for Omicron subvariants. Of note, this report covers the Omicron subvariants BA.1, BA.2 and BA.4/5, but does not address VE for more recently emerging Omicron subvariants (e.g., BQ.1.1, XBB).
2. Neutralization by Omicron subvariant
Multiple studies have clearly shown that there is significant reduction in neutralization by vaccine-induced antibodies against the Omicron variant of concern, compared to the ancestral SARS-CoV-2 strain as well as prior variants of concern (i.e., Alpha, Delta) [14], [15], [16], [17]. Differences in neutralization among Omicron BA subvariants, however, are minimal. A recent review of 178 studies that summarized post-vaccination neutralization against multiple Omicron subvariants showed that in general BA.2 subvariants had similar neutralization capacity after booster dose vaccination compared to BA.1, across all vaccines tested (i.e., mRNA, vector-based, inactivated, protein subunit); the relative fold reduction in neutralization of BA.2 compared to BA.1 was 0.9 [Inter-quartile range (IQR) 0.7–1.2] [18]. However, neutralization titers against BA.4/5 subvariants were lower compared to BA.1 (median 2.1 fold reduction, IQR 1.5–3.1). Based on vaccine-induced neutralizing antibodies, this study suggests that VE against symptomatic infection could be lower for BA.4/5 compared to the original BA.1 Omicron subvariant. Of note, this difference in neutralization between BA.1 and BA.4/5 is substantially less than the difference in neutralization that was observed between BA.1 and previous variants of concern (e.g., Alpha and Delta) [19], [20]. Also of note, this study did not assess more recent Omicron subvariants, such as BQ.1.1 and XBB, nor did it assess cellular immunity.
3. Results on Omicron sub-variant VE from four sites presented at meeting
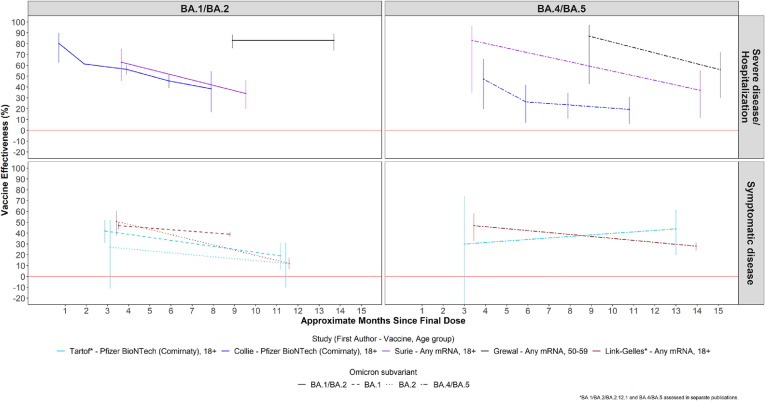
During the meeting four studies that compared VE for multiple Omicron subvariants were presented (Table 1, Table 2 ). Using data from Discovery Health, a large medical care organization in South Africa, adult cases of Omicron hospitalized during periods of combined BA.1 and BA.2 (BA.1/2) and BA.4/5 predominance were compared with test-negative controls from within the organization’s hospitalized population [6], [21]. Comparing absolute VE within two-month time intervals since the most recent vaccine dose, VE of the primary series of BNT162b2 (monovalent Pfizer-BioNTech) tended to be 10–20 percentage points lower during the BA.4/5 period than the BA.1/2 period from 3 to 8 months after vaccination, although with overlapping confidence intervals; no difference was observed in VE after the first booster dose of BNT162b2, with up to 8 months follow-up, and also no difference was observed following a second booster dose with up to 4 months follow-up.
Table 1.
Primary series absolute VE by Omicron subvariant from studies that include subvariant-specific estimates over time since final dose.
| Study (Country, Vaccine) | Outcome | Age group (years) | Time interval since last dose used for VE estimate | Median of time interval since last dose (days) |
2 dose VE (95 % CI) |
|||
|---|---|---|---|---|---|---|---|---|
| BA.1 | BA.2* |
BA.1/BA.2* |
BA.4/BA.5 | |||||
| Chemaitelly et al (Qatar, BNT162b2) [29] |
Symptomatic disease | All ages | 1–3 m | 46.6 (33.4, 57.2) | 51.7 (43.2, 58.9) | |||
| 4–6 m | 8.8 (-4.1, 20.1) | 12.4 (3.8, 20.3) | ||||||
| ≥7 m | −17.8 (-28.2, −8.2) | −12.1 (−19.1, −5.5) | ||||||
| Chemaitelly et al (Qatar, mRNA-1273) [29] |
1–3 m | 71.0 (24.0, 89.0) | 35.9 (-5.9, 61.2) | |||||
| 4–6 m | 31.3 (19.1, 41.7) | 9.9 (-0.3, 19.0) | ||||||
| ≥7 m | −10.2 (–23.1, 1.3) | −20.4 (-30.2, −11.2) | ||||||
| Collie et al (South, Africa, BNT162b2) [21] |
Hospitalization (excluding admissions unlikely related to COVID-19) | ≥18 | 14–27 d | 80.3 (62.8, 89.5) | ||||
| 1–2 m | 61.3 (54.7, 66.9) | |||||||
| 3–4 m | 56.3 (51.6, 60.5) | 47.4 (19.9, 65.5) | ||||||
| 5–6 m | 45.6 (39.3, 51.3) | 26.3 (7.1, 41.6) | ||||||
| 7–8 m | 38.4 (16.9, 54.4) | 23.6 (11.1, 34.3) | ||||||
| ≥9 m | 19.3 (6.3, 30.5) | |||||||
| Link-Gelles et al (USA, any mRNA) [25], [26] |
Emergency department or urgent care encounters for COVID-like illness | ≥18 | 14–149 d | 107 (BA.1), 104 (BA.2), 105 (BA.4/BA.5) |
47 (44, 50) | 51 (38, 60) | 47 (33, 58) | |
| ≥150 d | 267 (BA.1), 352 (BA.2), 424 (BA.4/BA.5) |
39 (37, 41) | 12 (7, 17) | 28 (24, 31) | ||||
| Hospitalization with COVID-like illness | 14–149 d | 105 (BA.1), 102 (BA.2) |
68 (63, 73) | 57 (19, 77) | ||||
| ≥150 d | 289 (BA.1), 371 (BA.2), 450 (BA.4/BA.5) |
61 (58, 63) | 24 (12, 35) | 25 (17, 32) | ||||
| Surie et al (USA, any mRNA) [30] |
Hospitalization with COVID-like illness | ≥18 | 14–150 d | 111 (BA.1/BA.2), 102 (BA.4/BA.5) | 63 (46, 75) | 83 (35, 96) | ||
| ≥150 d | 290 (BA.1/BA.2), 430 (BA.4/BA.5) | 34 (20, 46) | 37 (12, 55) | |||||
| Tartof et al (USA, BNT162b2) [31], [32] |
Emergency department admissions for acute respiratory infection | ≥18 | <6 m | 42 (31, 52) | 27 (-11, 52) | 30 (-86, 74) | ||
| ≥6 m | 19 (6, 31) | 12 (-10, 31) | 44 (20, 61) | |||||
| Hospitalization for acute respiratory infection | <6 m | 54 (38, 65) | 56 (-2, 81) | |||||
| ≥6 m | 32 (16, 45) | 56 (28, 73) | −4 (-118, 50) | |||||
| Grewal et al (Canada, any mRNA) [28] |
Hospitalization due to or partially due to COVID-19 | 50–59 | 240–299 d | 83 (76, 88) | 87 (43, 97) | |||
| ≥300 d | 83 (74, 89) | 56 (30, 72) | ||||||
| 60–69 | ≥300 d | 79 (71, 86) | 43 (16, 61) | |||||
| 70–79 | ≥300 d | 80 (72, 86) | 48 (25, 64) | |||||
| ≥ 80 | ≥300 d | 72 (62, 79) | 40 (18, 56) | |||||
Abbreviations: VE = vaccine effectiveness, CI = confidence interval, d = days, m = months. *BA.2.12.1 was not distinguished from BA.2 for all included studies.
Table 2.
First booster dose absolute VE by Omicron subvariant from studies that include subvariant-specific estimates over time since final dose.
|
Study (Country, Vaccine) |
Outcome |
Age group (years) | Time interval since last dose used for VE estimate | Median of time interval since last dose (days) |
3 dose VE (95 % CI) |
|||
|---|---|---|---|---|---|---|---|---|
| BA.1 | BA.2* | BA.1/BA.2* | BA.4/BA.5 | |||||
| Chemaitelly et al (Qatar, BNT162b2) [29] |
Symptomatic disease | All ages | <1 m | 59.9 (51.2, 67.0) | 43.7 (36.5, 50.0) | |||
| ≥1 m | 40.5 (30.8, 48.8) | 40.2 (34.2, 45.7) | ||||||
| Collie et al (South, Africa, BNT162b2) [21] | Hospitalization (excluding admissions unlikely related to COVID-19) | ≥18 | 14–27 d | – | – | 81.6 (68.1, 89.4) | ||
| 1–2 m | 66.4 (53.7, 75.6) | 68.8 (59.5, 76.0) | ||||||
| 3–4 m | – | – | 50.0 (4.4, 73.9) | 46.8 (35.3, 56.2) | ||||
| UKHSA (United Kingdom, ChAdOx1-S + any mRNA) [24] |
Hospitalization for ≥ 2 days and respiratory primary diagnosis | ≥65 | 14 d − 2 m | 87 (85, 88) | 80 (77, 84) | 76 (67, 83) | ||
| 3–5 m | 83 (81, 85) | 68 (64, 72) | 66 (59, 72) | |||||
| 6–8 m | 59 (53, 64) | 58 (52, 62) | ||||||
| 9–11 m | 55 (48, 60) | |||||||
| 12–14 m | 60 (41, 72) | |||||||
| Link-Gelles et al (USA, any mRNA) [25], [26] |
Emergency department/ Urgent care visit for COVID-like illness | ≥18 | 7–119 d |
66 (BA.1), 94 (BA.2), 77 (BA.4/BA.5) |
84 (83, 85) | 56 (51, 61) | – | 62 (54, 68) |
| ≥120 d |
132 (BA.1), 166 (BA.2), 228 (BA.4/BA.5) |
73 (68, 77) | 26 (21, 30) | 32 (29, 36) | ||||
| Hospitalization with COVID-like illness | 7–119 d | 72 (BA.1), 94 (BA.2), 76 (BA.4/BA.5) |
92 (91, 93) | 69 (58, 76) | 68 (50, 80) | |||
| ≥120 d | 132 (BA.1), 168 (BA.2), 235 (BA.4/BA.5) |
85 (81, 89) | 52 (44, 59) | 36 (29, 42) | ||||
| Surie et al (USA, any mRNA [30] |
Hospitalization with COVID-like illness | ≥18 | 7–120 d | 80 (BA.1/BA.2), 74 (BA.4/BA.5) | 79 (74, 84) | 60 (12, 81) | ||
| ≥120 d | 180 (BA.1/BA.2), 237 (BA.4/BA.5) | 41 (23, 55) | 29 (3, 48) | |||||
| Tartof et al (USA, BNT162b2) [31], [32] |
Emergency department admissions for acute respiratory infection |
≥18 | <3 m | 74 (69, 78) | 59 (40, 72) | 71 (18, 90) | ||
| 3–5 m | 36 (-3, 60) | |||||||
| ≥3 m | 65 (56, 73) | 5 (-21, 25) | ||||||
| ≥6 m | 37 (8, 57) | |||||||
| Hospitalization for acute respiratory infection | <3 m | 80 (74, 84) | 74 (47, 87) | |||||
| 3–5 m | 72 (13, 91) | |||||||
| ≥3 m | 76 (69, 82) | 70 (53, 81) | ||||||
| ≥6 m | 38 (-31, 71) | |||||||
| Tseng et al (USA, any mRNA) [33] | Infection | ≥18 | 14–30 d | 85.8 (82.7, 88.3) |
BA.2: 61.0 (27.6, 79.0) BA.2.12.1: 82.7 (44.2, 94.7) |
BA.4: 72.6 (-54.7, 96.6) BA.5: 90.6 (30.6, 98.7) |
||
| 31–90 d | 76.3 (73.9, 78.6) |
BA.2: 41.2 (28.3, 51.8) BA.2.12.1: 37.2 (14.1, 54.0) |
BA.4: 0.7 (-53.6, 54.2) BA.5: 57.0 (26.2, 75.0) |
|||||
| 91–150 d | 67.3 (62.0, 71.9) |
BA.2: 10.8 (0.8, 19.8) BA.2.12.1: 9.8 (-3.1, 21.2) |
BA.4: 23.2 (-12.3, 48.3) BA.5: 20.7 (-1.6, 38.2) |
|||||
| >150 d | 54.9 (35.6, 68.4) |
BA.2: −24.9 (–32.3, −16.7) BA.2.12.1: −26.8 (-34.6, −18.0) |
BA.4: −16.4 (-35.8, 8.2) BA.5: −17.9 (-29.6, −4.2) |
|||||
| Grewal et al (Canada, any mRNA†) [28] |
Hospitalization due to or partially due to COVID-19 | 50–59 | 120–179 d | 96 (93, 97) | 77 (50, 89) | |||
| 180–239 d | 98 (93, 99) | 75 (60, 84) | ||||||
| 60–69 | 60–119 d | 95 (93, 96) | 94 (53, 99) | |||||
| 120–179 d | 92 (90, 94) | 74 (48, 87) | ||||||
| 180–239 d | 90 (79, 95) | 69 (55, 78) | ||||||
| 70–79 | 7–59 d | 96 (96, 97) | 86 (37, 97) | |||||
| 60–119 d | 93 (92, 95) | 86 (57, 95) | ||||||
| 120–179 d | 92 (90, 94) | 58 (30, 75) | ||||||
| 180–239 d | 91 (85, 95) | 59 (44, 70) | ||||||
| ≥ 80 | 7–59 d | 92 (90, 93) | 76 (27, 92) | |||||
| 60–119 d | 88 (85, 90) | 83 (61, 93) | ||||||
| 120–179 d | 85 (82, 88) | 64 (44, 77) | ||||||
| 180–239 d | 87 (80, 91) | 52 (36, 64) | ||||||
Abbreviations: VE = vaccine effectiveness, CI = confidence interval, d = days, m = months.
*BA.2.12.1 was not distinguished from BA.2 for all included studies, unless otherwise specified.
†In Canada, persons 70 years and older receiving a booster dose of Moderna-mRNA-1273 received a 100 μg dose.
In the United Kingdom, a test-negative case-control study was undertaken among persons ≥ 65 years old admitted to hospital for two or more days with a respiratory code as the primary diagnosis during periods of BA.1, BA.2 and BA.4/5 predominance (updated analysis based on published methods) [22], [23], [24]. Absolute VE was calculated comparing persons receiving the first booster dose with those unvaccinated. Primary series included ChAdOx1-S (monovalent AstraZeneca), BNT162b2, or mRNA-1273 (monovalent Moderna), whereas the first booster dose was only the mRNA vaccines; vaccines were combined in the analysis. VE was significantly higher for BA.1 than both BA.2 and BA.4/5 at 2 weeks-to-2 months and 3–5 months after the first booster dose. Moreover, waning was substantially greater for BA.2 and BA.4/5 than BA.1 through 5 months. No difference was observed in VE between BA.2 and BA.4/5 through 8 months after the first booster dose.
Data were presented from two surveillance platforms in the United States of America. In the VISION multi-state network of electronic health records, a test-negative design compared vaccination status among test-positive cases and test-negative control patients seeking care in an emergency department or urgent care clinic (ED/UC), and separately among hospitalized patients. Among immunocompetent adults ≥ 18 years old, the absolute VE for either BNT162b2 or mRNA-1273 was lower for ED/UC visits for BA.2 and BA.4/5 compared to BA.1 after the primary series and the first booster dose; the differences in VE by subvariant were larger when more time had elapsed since the last dose suggestive of faster waning of VE against BA.2 and BA.4/5 [25], [26]. For hospitalization, similar results were observed, with lower VE and faster waning of VE for BA.2 and BA.4/5 compared to BA.1, although with wider confidence intervals than for ED/UC visits. Of note, although the analysis was stratified into two periods post-vaccination, the median follow-up time within each period was longer during the BA.2 and BA.4/5 periods, compared to the BA.1 period; for example, for the hospitalization outcome in the period post-vaccination indicated as “120 + days”, the median follow-up times since the booster dose for BA.1, BA.2 and BA.4/5 were 135, 168, and 235 days, respectively. These differences in median follow-up time could translate into greater waning of VE having occurred by the time the later subvariants were circulating. In the Increasing Community Access to Testing (ICATT) program for community-testing at pharmacies, a test-negative design undertaken during periods of BA.1, BA.2 and BA.4/5 predominance, relative VE of BNT162b2 or mRNA-1273 against symptomatic infection was calculated comparing those receiving the first booster dose with those receiving the primary series at least five months ago [27]. Among 16–49 year old persons, relative VEs for the three Omicron subvariants were similar within the first two months following the last dose, but by two-to-four months after the last dose waning VE was markedly more pronounced for BA.2 and BA.4/5 compared to BA.1; similar trends in VE were found for older age groups, although with wider confidence intervals.
In Ontario, Canada, a study evaluated the absolute VE of BNT162b2 or mRNA-1273 against severe outcomes (hospitalization and deaths) using a test-negative design among immunocompetent, non-institutionalized adults ≥ 50 years old [28]. Stratified by BA.1/2 and BA.4/5 predominant periods, the absolute VE was significantly lower in the BA.4/5 period > 300 days after the primary series and 120–239 days after the first booster dose. Of note, the VE estimates between the BA.1/2 and BA.4/5 predominant periods were not significantly different in the first 120 days after the booster dose. Results were presented for 70–79-year-olds, although similar trends were observed among the other age groups.
4. Summary of studies that assess VE of multiple Omicron subvariants
In addition to the four individual studies presented at the meeting, a summary was presented of all VE studies that compared Omicron subvariants, as of December 6, 2022. Based on an ongoing systematic review of COVID-19 VE studies, data from those that evaluated primary series and/or first booster dose VE against more than one Omicron subvariant and over more than one discrete time interval since receipt of final dose were extracted. Only studies that compared vaccinated persons to unvaccinated persons (i.e., absolute VE) were included. Full inclusion/exclusion criteria have been described elsewhere and are available online at https://www.VIEW-hub.org. Eight studies assessed VE of primary series or first booster dose vaccination separately for at least two different Omicron subvariants (six assessed VE of both primary series and booster dose vaccination and two assessed VE of a booster dose only, Table 1, Table 2) [21], [24], [25], [26], [28], [29], [30], [31], [32], [33]. Results presented from the United Kingdom Health Security Agency (UKHSA) are unpublished data of an updated analysis using methods that have been described previously [22], [23]. All studies assessed VE of two or three doses of monovalent mRNA vaccines except for one study from the United Kingdom which assessed the VE of a booster dose of primarily monovalent mRNA vaccines (a small percentage of participants received ChAdOx1-S for the booster dose) given after receipt of a primary series of mRNA or ChAdOx1-S vaccines. In summarizing the VE studies below, we report VE estimates by Omicron subvariants from individual studies as lower or higher if VE estimates for different subvariants have non-overlapping confidence intervals during at least one time interval of follow-up.
4.1. Primary series VE by Omicron subvariant
VE of primary series vaccination is shown in Table 1 and Fig. 1 . Of three studies that compared VE against SARS-CoV-2 infection and/or symptomatic COVID-19 disease between BA.1 and BA.2 periods, two showed lower VE against BA.2 at 4–6 months or ≥ 5 months post final dose [26], [29], [31]. Of the two studies that assessed VE against infection/symptomatic disease during BA.1 and BA.4/5 periods, one reported lower VE against A.4/5 at ≥ 5 months post final dose [25], [26], [31], [32]. Of the two studies that assessed VE against infection/symptomatic disease during BA.2 and BA.4/5 periods, one reported lower VE against BA.2 compared to BA.4/5 at ≥ 5 months post final dose, although both VE estimates were very low (12 % and 28 %, respectively), while the second study showed no differences in VE [25], [26], [31], [32]. Of two studies reporting primary series VE against hospitalization/severe disease against BA.1, BA.2, and BA.4/5, one reported lower VE against BA.2 and BA.4/5 than BA.1, while the other showed no statistically significant differences [25], [26], [31], [32]. Among three studies that compared VE of BA.1/2 combined to VE of BA.4/5, one showed lower VE against BA.4/5 than BA.1/2 at about ≥ 10 months post final dose, while the other two showed no statistically significant difference [21], [28], [30].
Fig. 1.
COVID-19 Primary Series Vaccine Effectiveness by Omicron Subvariant.
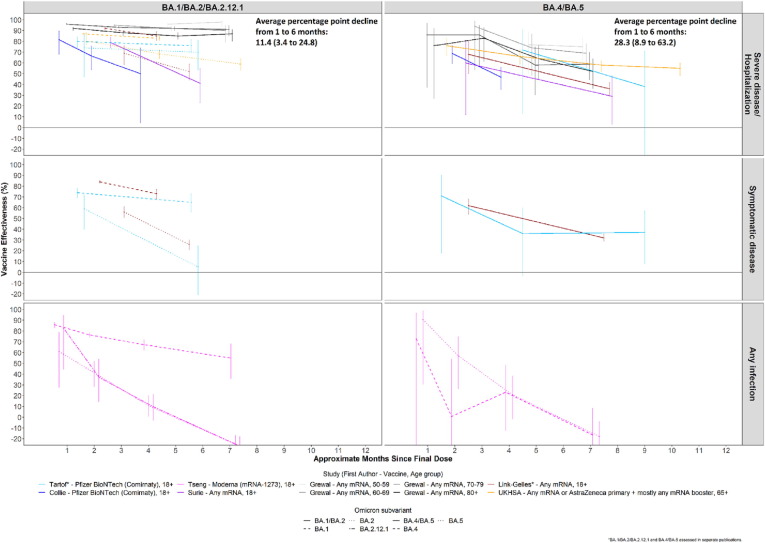
4.2. First booster dose VE by subvariant
The VE of a first booster dose by Omicron subvariant is shown in Table 2 and Fig. 2 . Among 4 studies that compared VE against outcomes of SARS-CoV-2 infection and/or symptomatic COVID-19 disease between BA.1 and BA.2, all showed lower VE against BA.2 [26], [29], [31], [33]. Of three studies assessing VE against infection/symptomatic disease during both BA.1 and BA.4/BA.5 periods, two showed lower VE against BA.4/5 [25], [26], [31], [32], [33]. Of three studies assessing VE against infection/symptomatic disease during both BA.2 and BA.4/BA.5 periods, all showed similar VE estimates or estimates with overlapping 95 % confidence intervals against both subvariants [25], [26], [31], [32], [33].
Fig. 2.
COVID-19 First Booster Dose Vaccine Effectiveness by Omicron Subvariant.
For hospitalization/severe disease outcomes, among three studies comparing booster VE against BA.1 and BA.2, the VE was lower against BA.2 than BA.1 in two studies and similar in one study [24], [26], [31]. Among three studies comparing VE against BA.1 and BA.4/5, two showed a lower VE against BA.4/5 [24], [25], [26], [31], [32]. Among three studies comparing VE of BA.2 and BA.4/5, one showed a lower VE against BA.4/5 and two showed similar VE [24], [25], [26], [31], [32]. Among three studies that compared VE against BA.1/2 with BA.4/5, one showed a similar VE within the first four months following a booster dose, but lower VE against BA.4/5 beyond 4 months; one study showed similar VE at all periods; the third showed lower VE against BA.4/5 than BA.1/2 at all periods, though confidence intervals overlapped [21], [28], [30].
5. Duration of VE against Omicron subvariants
To assess differences in waning of VE over time due to BA.1 and/or BA.2 compared to BA.4/BA.5, a meta-regression was conducted; meta-regression methods used were reported previously; p-values for comparisons between subvariants accounted for within-study effects [7], [34]. Only those studies assessing VE against multiple subvariants were included in the meta-regression to minimize the likelihood that any observed difference between subvariants might be due to inter-study differences in methodology. Where available for a study, the median time since vaccination among all individuals contributing to a VE estimate for a particular time interval since final dose was used in the meta-regression. Where median time since vaccination were not available, the median time point of the interval was calculated and used in the meta-regression (e.g., 52 days would be inputted as the time since final dose for an interval of 14–90 days; for open-ended time intervals, such as ≥ 120 days, the median time point between 120 days and the maximum length of time participants could have been vaccinated based on vaccine introduction date and the end date of the study period was inputted). For the single study that evaluated VE separately for four different age groups, all age groups were included in the meta-regression; a sensitivity analysis was conducted including only a single age group (70–79 years) [28]. The meta-regression was only done for the booster dose VE against severe disease outcomes; due to a limited number of studies, the meta-regression was not run for any primary series vaccination nor for infection and symptomatic disease outcomes for the booster dose.
Results of the meta-regression show that on average VE of first booster vaccination for severe disease/hospitalization was lower against BA.4/5 than BA.1/2 combined (p = 0.007); the average VE of a first booster dose of mRNA vaccine decreased more quickly over time against BA.4/5 (average decline from one to six months of 28.3 percentage points, 95 % CI: 8.9–63.2) than against BA.1, BA.2 and BA.1/2 (average decline of 11.4 percentage points, 95 % CI: 3.4–24.8), but the difference in waning over six months was not statistically significant (p = 0.17 for difference in waning over six months) (Fig. 2). Although few (n = 3) studies had data enabling comparison of VE against BA.1 to BA.2, these studies suggest VE against BA.2 is lower (p = 0.014), but there was no significant difference in waning between BA.1 and BA.2 over 6 months (p = 0.21).
6. Interpretation of VE results by Omicron subvariant
Despite some heterogeneity in results, the majority of studies showed that VE tended to be lower against BA.2 and especially against BA.4/5 subvariants compared to BA.1, after both the primary series and the first booster dose. The results were more mixed when comparing BA.2 and BA.4/5, with some studies showing similar VE results and a few showing lower VE for BA.4/5. No study reported a higher booster dose VE estimate for BA.4/5 with non-overlapping confidence intervals compared to BA.1, BA.2, or BA.1/2 for either symptomatic or severe disease. Although the confidence intervals overlapped, there was a trend towards more rapid waning of VE after first booster vaccination against severe disease due to BA.4/5 compared with BA.1/2; more studies are needed to confirm this.
Possible explanations were discussed for the lower VE against BA.4/5 (and perhaps BA.2) compared to BA.1 (Table 3 ). First, the lower VE against BA.4/5 might reflect greater immune escape. This explanation is supported by neutralization data that show approximately a two-fold reduction in neutralization comparing BA.4/5 to BA.1. While neutralization correlates most closely with protection against infection and symptomatic disease [35], several studies of hospitalization/severe disease also showed lower VE for BA.4/5. It has been postulated that neutralizing antibodies can also protect against severe disease, albeit at lower concentrations than needed to protect against symptomatic disease [36], Alternatively, definitions of severe disease that rely solely on hospitalization might in fact include persons hospitalized with incidental Omicron infection or exacerbations of underlying illness triggered by SARS-CoV-2 infection, both of which would result in a lower VE against hospitalization if VE is truly lower against BA.4/5 infection [37].
Table 3.
Reasons for differences in vaccine effectiveness between Omicron subvariants.
| Reason | How to identify? | Potential way to correct bias? | Comment |
|---|---|---|---|
| Real difference | |||
| Greater immune escape (i.e., BA.4/5 vs. BA.1) | Neutralization supports VE results | Not applicable | Likely affects VE for infection/symptomatic disease more than severe disease (i.e., cellular immunity more important for severe disease) |
| Methodological differences | |||
| Differences in time elapsed since last vaccine dose between periods of subvariant circulation | Compare median time since last dose within periods | -Use shorter periods of comparison (e.g., 1–2 months) -Adjust for time since last dose in statistical comparisons of VE |
The steeper the waning of VE, the greater the bias since differences in VE could occur with relatively small differences in time since vaccination during different subvariant periods |
| Infection-induced immunity among unvaccinated and under-vaccinated persons differs by subvariant period (i.e., depletion of susceptibles bias) | Assess seroprevalence during periods of each subvariant circulation | Adjust for prior infection in analysis, if possible, and assess whether there is confounding or effect modification | -If initial subvariant causes a large wave of infection (e.g., BA.1), this is likely to affect VE for later subvariants --Infection rates higher for unvaccinated than vaccinated even with Omicron |
| Differences in testing between subvariant periods | Assess testing rates during different periods | -Adjust for testing rates, if possible -Assess within a sub-population with regular testing (e.g. health care workers) |
-Affects VE for infection/symptomatic disease more than severe disease -TND case-control studies probably less susceptible to this bias -Testing likelihood would need to be associated with vaccine status; direction of bias unclear |
| Differential force of infection during different subvariant periods | Assess incidence of infection during different periods | Use stricter case definitions for severe disease and restricted to respiratory diagnoses, to exclude incidental infections | -Would affect VE against hospitalization due to more incidental infections among hospitalized persons during different subvariant periods |
| Different vaccinated population during different subvariant periods (i.e., cohort effect) | Assess distribution of characteristics by subvariant periods | Stratify or restrict analysis by risk group (e.g., age, comorbidities) | -People vaccinated earlier, , have different risk; for severe disease (e.g., elderly, comorbidities), or for infection (e.g., less risky behaviors) |
| Combining subvariants in analysis that might have different VE | Assess proportion of subvariants in combined group (e.g., BA.1/2) | -Do separate VE estimates by subvariant, when feasible -Define subvariant predominant periods when high percentage of infections due to a single subvariant (e.g., >90 % rather than >51 %) |
-Real or methodological difference in individual subvariant VE can distort combined VE -Different proportions of subvariant by site can make comparisons of studies difficult |
Abbreviations: TND, Test-negative design.
Several methodological issues and potential biases that might explain the findings were discussed (Table 3). The fact that BA.1, BA.2 and BA.4/5 circulated during different periods, with the latter subvariants usually circulating several months later, creates several potential time-variable biases. For example, more time might have elapsed since the last vaccine dose during the BA.4/5 period, allowing more time for waning of VE against Omicron infection, which occurs within a matter of months [7]. For several studies that evaluated the median time since last dose, indeed the time since last dose was longer for BA.4/5 compared to BA.1 [25], [26], [30]. In contrast, in the study from Ontario where differences in VE between the BA.1/2 and BA.4/5 periods were large, differences in time since last dose were less than one month between subvariant periods [28]. Another time-variable bias could be that, due to the large BA.1 and/or BA.2 waves in many settings, there was likely high prevalence of recent infection by the time BA.4/5 circulated. This could confer infection-induced immunity, which would presumably be greater among unvaccinated or under-vaccinated (e.g., primary series only) persons, who would be more susceptible to infection in the initial BA.1 wave. This so-called “depletion of susceptibles” could lead to spuriously lower VE estimates during the BA.4/5 period [38]. In the one VE study that did provide data on prior infection, the percentage of people infected between the BA.1/2 and BA.4/5 periods increased more among unvaccinated persons (from 15 % to 39 %) than among vaccinated persons (from 14 % to 25 %) [31], [32]. However, individual-level data on prior infection are becoming increasingly difficult to ascertain in VE studies due to lack of testing, no systematic reporting of home-based test results, remote infections, and multiple infections. Some studies have shown higher seroprevalence in the general population during the BA.4/5 period than during periods of BA.1/BA.2 predominance [39], [40]. Several other potential biases to explain differences in VE by Omicron subvariant, along with suggestions for how to correct or reduce them, are described in Table 3. Because post-vaccination neutralization was similar for BA.1 and BA.2, it is possible that the lower VE against BA.2 reflects the contribution of methodological issues, whereas the lower VE against BA.4/5 is explained by a mixture of immunological and methodological reasons.
The possibility of faster waning against severe disease caused by BA.4/5 than BA.1 is somewhat surprising given that vaccine protection against severe disease has been shown to wane much less rapidly than against symptomatic infection [7], [34]. It is possible that the waning against severe disease caused by BA.4/5 reflects a larger impact of the methodological biases at more distant time intervals from vaccination. Additionally, a larger percentage of hospitalizations during the Omicron period likely had incidental, rather than causal, infections with SARS-CoV-2, and infections would be more affected by true waning of VE [37], [41].
7. Summary and conclusions
VE studies that evaluated several Omicron subvariants and disease outcomes showed a heterogeneity of findings. Moreover, many subvariant-specific VE estimates had wide and overlapping confidence intervals. Nonetheless, the majority of studies showed that VE tended to be lower against both BA.2 and especially against BA.4/5, than against BA.1. The results were more mixed when comparing BA.2 and BA.4/5, with some studies showing similar VE results and a few showing lower VE against BA.4/5. Given these findings, it is advisable to analyze VE of Omicron subvariants BA.1, BA.2 and BA.4/5 separately, when possible, defining subvariant-specific VE based on testing results (i.e., sequencing or S-gene target failure) or periods of clear subvariant predominance. The explanations for the lower VE against BA.2 and BA.4/5 include both immunological factors (i.e., more immune escape with BA.4/5) and methodological issues (e.g., biases related to differences in the timing of subvariant circulation). If possible, researchers should report the median time of each time interval since the last dose for which VE is estimated to assess the potential for differential amounts of waning by subvariant. Of note, despite the generally lower VE against BA.4/5, vaccines do provide some degree of protection against this subvariant, at least for a few months against infection/symptomatic disease, and for longer duration against severe disease. Newer subvariants of Omicron (e.g., BQ.1.1, XBB) are emerging and becoming predominant in some locations [13], [42]. Neutralization data suggests some of these new subvariants have even more immune escape than the original BA.5 subvariant [43], [44]. Moreover, the time since the last vaccine dose will likely be even greater for these newer Omicron subvariants than for BA.4/5. Results of future VE studies of these new subvariants are needed and should be interpreted considering both immunological and methodological factors. Moreover, vaccine effectiveness against Omicron subvariants of variant-containing bivalent vaccines might be different than that of the ancestral virus monovalent vaccines presented in this meeting; this question needs to be evaluated in future studies. While the VE data presented at this meeting concerned the original Omicron variant and its subvariants, the same interpretation of VE data would likely apply for a future new variant of concern that spawns subsequent waves of subvariants over time.
8. Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the World Health Organization or of the U.S. Centers for Disease Control and Prevention.
Declaration of Competing Interest
None
Acknowledgments
We acknowledge Anurima Baidya and Karoline Walter, International Vaccine Access Center, for their work in extracting data for studies for the ongoing systematic review.
Data availability
Data will be made available on request.
References
- 1.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. World Health Organization 2021; published online Nov 26. Available from: https://www.who.int/news/item/26-11-2021-classification-of-Omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed Dec. 26, 2022).
- 2.United Kingdom Health Security Agency. COVID-19 vaccine surveillance report - week 50. United Kingdom Health Security Agency 2021; published online Dec 12. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1041593/Vaccine-surveillance-report-week-50.pdf (accessed Jan. 23, 2023).
- 3.Sheikh A, Kerr S, Woolhouse M, et al. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. University of Edinburgh 2021; published online Dec 22. https://www.research.ed.ac.uk/en/publications/severity-of-Omicron-variant-of-concern-and-vaccine-effectiveness- (preprint) [DOI] [PMC free article] [PubMed]
- 4.Hansen CH, Schelde AB, Mousten-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. medRxiv 2021; published online Dec 23. doi: 10.1101/2021.12.20.21267966. (preprint).
- 5.Buchan S.A., Chung H., Brown K.A., et al. Estimated effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022 Sep 1;5(9):e2232760. doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collie S., Champion J., Moultrie H., et al. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022 Feb 3;386(5):494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higdon M.M., Baidya A., Walter K.K., et al. Duration of effectiveness of vaccination against COVID-19 caused by the Omicron variant. Lancet Infect Dis. 2022 Aug;22(8):1114–1116. doi: 10.1016/S1473-3099(22)00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Külper-Schiek W., Piechotta V., Pilic A., et al. Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review. Front Immunol. 2022 Aug;24(13) doi: 10.3389/fimmu.2022.940562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Weekly epidemiological update on COVID-19 - 17 August 2022. World Health Organization 2022; published online Aug 17. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---17-august-2022.
- 10.World Health Organization. Weekly epidemiological update on COVID-19 - 23 November 2022. World Health Organization 2022; published online Nov 23. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---23-november-2022.
- 11.Tegally H., Moir M., Everatt J., et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022 Sep;28(9):1785–1790. doi: 10.1038/s41591-022-01911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Weekly epidemiological update on COVID-19 - 22 March 2022. World Health Organization 2022; published online Mar 22. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---22-march-2022.
- 13.World Health Organization. Weekly epidemiological update on COVID-19 - 14 December 2022. World Health Organization 2022; published online Dec 14. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---14-december-2022.
- 14.Sitaras I., Jacobsen H., Higdon M.M., et al. Systematic review of primary and booster COVID-19 sera neutralizing ability against SARS-CoV-2 Omicron variant. npj Vaccines. 2022 Nov 15;7(1):147. doi: 10.1038/s41541-022-00565-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Ma Y., Xu Y., et al. Resistance of SARS-CoV-2 Omicron variant to convalescent and CoronaVac vaccine plasma. Emerg Microbes Infect. 2022 Dec;11(1):424–427. doi: 10.1080/22221751.2022.2027219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ai J., Zhang H., Zhang Y., et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022 Dec;11(1):337–343. doi: 10.1080/22221751.2021.2022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobsen H., Strengert M., Maaß H., et al. Diminished neutralization responses towards SARS-CoV-2 Omicron VoC after mRNA or vector-based COVID-19 vaccinations. Sci Rep. 2022 Nov 18;12(1):19858. doi: 10.1038/s41598-022-22552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen H., Katzmarzyk M., Higdon M.M., et al. Post-Vaccination Neutralization Responses to Omicron Sub-Variants. Vaccines (Basel) 2022 Oct 20;10(10):1757. doi: 10.3390/vaccines10101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Lusvarghi S, Subramanian R, et al. Post-vaccination Omicron infections induce broader immunity across antigenic space than prototype mRNA COVID-19 booster vaccination or primary infection. bioRxiv 2022; published online Jul 6. doi: 10.1101/2022.07.05.498883. (preprint).
- 20.Zhou R., Liu N., Li X., et al. Three-dose vaccination-induced immune responses protect against SARS-CoV-2 Omicron BA.2: A population-based study in Hong Kong. Lancet Reg Health West Pac. 2022 Dec;23 doi: 10.1016/j.lanwpc.2022.100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collie S., Nayager J., Bamford L., et al. Effectiveness and Durability of the BNT162b2 Vaccine against Omicron Sublineages in South Africa. N Engl J Med. 2022 Oct 6;387(14):1332–1333. doi: 10.1056/NEJMc2210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsebom F.C.M., Andrews N., Stowe J., et al. COVID-19 vaccine effectiveness against the Omicron (BA.2) variant in England. Lancet Infect Dis. 2022 Jul;22(7):931–933. doi: 10.1016/S1473-3099(22)00309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirsebom F.C.M., Andrews N., Stowe J., et al. Effectiveness of the COVID-19 vaccines against hospitalisation with Omicron sub-lineages BA.4 and BA.5 in England. Lancet Reg Health Eur. 2022 Nov;11(23) doi: 10.1016/j.lanepe.2022.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United Kingdom Health Security Agency. Unpublished data on COVID-19 vaccine effectiveness against Omicron subvariants BA.1, BA.2, and BA.4/BA.5. United Kingdom Health Security Agency, 2022.
- 25.Link-Gelles R, Levy ME, Natarajan K, et al. Association between COVID-19 mRNA vaccination and COVID-19 illness and severity during Omicron BA.4 and BA.5 sublineage periods. medRxiv 2022; published online Oct 5. doi: 10.1101/2022.10.04.22280459. (preprint).
- 26.Link-Gelles R., Levy M.E., Gaglani M., et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA Vaccine Doses Among Immunocompetent Adults During Periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 Sublineages Predominated - VISION Network, 10 States, December 2021-June 2022. MMWR Morb Mortal Wkly Rep. 2022 Jul 22;71(29):931–939. doi: 10.15585/mmwr.mm7129e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link-Gelles R. Updates on COVID-19 Vaccine Effectiveness during Omicron. Presentation to Advisory Committee on Immunization Practices 2022 Sep; https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/04-COVID-Link-Gelles-508.pdf.
- 28.Grewal R, Nguyen L, Buchan SA, et al. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. medRxiv 2022; published online Nov 1. doi: 10.1101/2022.10.31.22281766. (preprint). [DOI] [PMC free article] [PubMed]
- 29.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022 Jun 2;13(1):3082. doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed]
- 30.Surie D., Bonnell L., Adams K., et al. Effectiveness of Monovalent mRNA Vaccines Against COVID-19-Associated Hospitalization Among Immunocompetent Adults During BA.1/BA.2 and BA.4/BA.5 Prepredominant Periods of SARS-CoV-2 Omicron Variant in the United States - IVY Network, 18 States, December 26, 2021-August 31, 2022. MMWR Morb Mortal Wkly Rep. 2022 Oct 21;71(42):1327–1334. doi: 10.15585/mmwr.mm7142a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tartof S.Y., Slezak J.M., Puzniak L., et al. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 Omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study. Lancet. Respir Med. 2022 Oct;7 doi: 10.1016/S2213-2600(22)00354-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tartof S.Y., Slezak J.M., Puzniak L., et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 Omicron BA.4 and BA.5. Lancet Infect Dis. 2022 Dec;22(12):1663–1665. doi: 10.1016/S1473-3099(22)00692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng HF, Ackerson BK, Bruxvoort KJ, et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 Omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat Commun. 2023 Jan 12;14(1):189. doi: 10.1038/s41467-023-35815-7. [DOI] [PMC free article] [PubMed]
- 34.Feikin D.R., Higdon M.M., Abu-Raddad L.J., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022 Mar 5;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 Jul;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 36.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibodies predict protection from severe COVID-19. medRxiv 2022; published online Jun 14. doi: 10.1101/2022.06.09.22275942. (preprint).
- 37.Feikin D.R., Abu-Raddad L.J., Andrews N., et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by Omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022 Jun 9;40(26):3516–3527. doi: 10.1016/j.vaccine.2022.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipsitch M., Goldstein E., Ray G.T., Fireman B. Depletion-of-susceptibles bias in influenza vaccine waning studies: how to ensure robust results. Epidemiol Infect. 2019 Nov;27(147):e306. doi: 10.1017/S0950268819001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kislaya I., Casaca P., Borges V. Comparative COVID-19 Vaccines Effectiveness in Preventing Infections, Hospitalizations, and Deaths with SARS-CoV-2 BA.5 and Ba.2 Omicron Lineages: A Case-Case and Cohort Study Using Electronic Health Records in Portugal. SSRN. 2022 doi: 10.2139/ssrn.4180482. published online Aug 3.(preprint. [DOI] [Google Scholar]
- 40.Lewnard JA, Hong V, Kim JS, et al. Association of SARS-CoV-2 BA.4/BA.5 Omicron lineages with immune escape and clinical outcome. medRxiv 2022; published online Nov 3. doi: 10.1101/2022.07.31.22278258. (preprint). [DOI] [PMC free article] [PubMed]
- 41.Stowe J, Andrews N, Kirsebom F, et al. Nat Commun. 2022 Sep 30;13(1):5736. doi: 10.1038/s41467-022-33378-7. [DOI] [PMC free article] [PubMed]
- 42.CDC, “COVID Data Tracker,” Centers for Disease Control and Prevention, Mar. 28, 2020. https://covid.cdc.gov/covid-data-tracker (accessed Dec. 26, 2022).
- 43.Hoffmann M., Behrens G.M.N., Arora P., et al. Effect of hybrid immunity and bivalent booster vaccination on Omicron sublineage neutralisation. Lancet Infect Dis. 2023 Jan;23(1):25–28. doi: 10.1016/S1473-3099(22)00792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q., Iketani S., Li Z., et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023 Jan 19;186(2):279–286.e8. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.