Summary
Background
Indonesia had the second-highest number of COVID-19 cases and deaths in South-East Asia. We aimed to determine the factors associated with this mortality and the effect of the recommended COVID-19 treatment regimen during the first 10 months of the epidemic.
Methods
This was a retrospective cohort study using secondary data from medical records. In total, 689 adult COVID-19 inpatients hospitalized between March and December 2020 were enrolled. Clinical characteristics, laboratory parameters, and treatments were analyzed by survival outcome. Kaplan–Meier statistics were used to estimate survival.
Findings
Of the 689 patients enrolled, 103 (14.9%) died. Disease severity was highly associated with mortality (hazard ratio [HR]: 7.69, p < 0.001). Other clinical factors associated with mortality were older age and comorbidities. Based on laboratory parameters, higher procalcitonin and C-reactive protein contents and a neutrophil-to-lymphocyte ratio >3.53 were also linked to mortality. Favipiravir was associated with lower mortality, with adjusted HRs of 0.24 (0.11–0.54) and 0.40 (0.17–0.98) among the mild/moderate and severe cases, respectively. Among patients with severe disease, steroids showed some beneficial effects in the early days of hospitalization.
Interpretation
Older age and comorbidities were associated with disease severity and, consequently, higher mortality. Higher mortality after the second week of hospitalization may be related to secondary bacterial infection. Favipiravir showed significant benefit for COVID-19 survival, while steroids showed benefit only in the early days of admission among patients with severe disease.
Funding
This research did not receive a specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Keywords: SARS-Cov-2, Cohort, Survival, Comorbidity, Severity, Antiviral
Research in context.
Evidence before this study
Before this study, we searched PubMed for articles that assessed factors associated with mortality in COVID-19 inpatients, using search terms (“COVID-19” OR “coronavirus”) AND (“mortality” OR “death”), from March until April 2022. We found the mortality studies were mainly from China, America, and Europe that associated mortality with older age, male, and pre-existing comorbidities besides the severity of the disease itself. Indonesia had the second-highest total number of cases and deaths in South-East Asia. However, there were still limited studies and inadequate reliable data about COVID-19 mortality in Indonesia. Previously published studies in Indonesia reported a CFR of around 9.4%–18%, associated with older age, male, pre-existing comorbidity, immediate ICU admission, and intubation. Laboratory parameters such as NLR, D-dimer, and lymphocyte count were also associated with a greater risk of overall mortality.
Added value of this study
This study is a retrospective cohort study of hospitalized COVID-19 patients in a top referral hospital in Bandung of West Java, the largest province in Indonesia, during the first ten months of the epidemic. Our report is the most comprehensive mortality study in our region that analyzed the clinical characteristics, laboratory parameters, and treatments associated with survival outcomes. The case fatality rate of 14.9% was similar to previous studies in Indonesia. Clinical factors such as older age and comorbidities were associated with severe presentation and higher mortality. Severe cases can be identified based on clinical findings and several laboratory markers like the Leukocyte count, NLCR, CRP and PCT. Our analysis of treatment modalities remarkably confirmed that Favipiravir provided significant benefit among the mild/moderate cases, while steroids were associated with poorer survival in this group. Among the severe group, remdesivir did not significantly reduce mortality, while steroids and the use of mechanical ventilation only prevented death in the early days of hospitalization. We observed a high mortality rate after the second week of admission, which might be related to secondary bacterial infection. This study only reflects the situation in the first ten months of the COVID-19 pandemic; therefore, the generalizability may be limited.
Implications of all the available evidence
This study confirmed the higher mortality risk of covid-19 patients with older age and the presence of comorbidities. We recommend providing more attention to preventive means and early treatment modalities like the use of the vaccine, monoclonal antibodies and novel drugs for the elderly and those with comorbidities. Additional study is greatly needed to understand various clinical and management factors which affect poor outcomes and hospital-acquired infections among severe cases.
Introduction
COVID-19 caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has become a global pandemic. Up to October 2022, more than 600 million confirmed cases of COVID-19 had been reported worldwide.1 Since the beginning of this outbreak, Indonesia has reported 6,467,189 confirmed cases of COVID-19, with a case fatality rate of 2.45% (158,398 deaths up to October 2022). Indonesia had the second highest number of COVID-19 cases and deaths in South-East Asia and ranks 20th worldwide for disease incidence.1
SARS-CoV-2 has caused respiratory illnesses ranging from asymptomatic to severe pneumonia. Symptomatic cases occurred in 69%–72% of the infected patients, with a mortality rate of 1.83%.2 Our current knowledge of COVID-19 mortality is derived from clinical epidemiological studies undertaken in the early stages of the pandemic in China3,4 as well as in Western countries.5 In these studies, COVID-19 mortality has been associated with older age, being male, and pre-existing comorbidities such as hypertension, diabetes mellitus (DM), cardiovascular disease, chronic kidney disease (CKD), chronic pulmonary disease, and cancer, especially hematologic malignancy, in addition to the severity of the disease itself.3,4,6,7 Several laboratory markers have also been associated with death, such as lymphopenia, leukocytosis, elevated C-reactive protein (CRP), D-dimer, procalcitonin, and troponin I.4,8 The use of mechanical ventilation and intensive care unit (ICU) admission were also associated with higher mortality.4,9
Challenges in COVID-19 management differ between developed and developing countries. Because population, comorbidities, age distribution, and access to health services are unique to each developed country, guidelines and policies should be tailored to meet local needs.10 Despite the immensity of the problem, limited data are available regarding the characteristics and mortality of hospitalized patients with COVID-19 in Indonesia. In the first published cohort study in Jakarta, of 4052 patients with confirmed COVID-19, 381 (9.4%) died.11 In another cohort study of hospitalized patients in Jakarta, the overall mortality was 12% (497 out of 4265), and the identified risk factors were older age, being male, pre-existing comorbidities, immediate ICU admission, and intubation.10
We analyzed the clinical characteristics, laboratory parameters, and treatment data relating to the first wave of COVID-19 in Hasan Sadikin Hospital, a major referral hospital in West Java Province. We sought to determine the clinical characteristics and treatment factors associated with mortality during the first 10 months of the pandemic (from March to December 2020).
Methods
Study design and participants
This retrospective cohort study was based on medical records of adult (≥18 years old) inpatients from Hasan Sadikin Hospital who were diagnosed with COVID-19 according to World Health Organization (WHO) interim guidance between March 1, 2020, and December 31, 2020. Patients were diagnosed using real-time polymerase chain reaction detection of SARS-CoV-2 in nasopharyngeal swabs. The criteria for discharge were improvement in clinical condition and/or two throat-swab samples testing negative for SARS-CoV-2 RNA at least 24 h apart.
According to the national health protocol for the care of patients with mild/moderate and severe COVID-19, patients with mild disease were hospitalized if they either had comorbidities or were more than 59 years old and did not have a place for self-isolation. Choosing and evaluating therapeutic regimens for COVID-19 patients remains challenging because of the varied symptoms and severity. Supportive treatment was provided according to clinical symptoms. For the period March to August 2020, no antivirals or steroids were given. In August 2020, antivirals such as favipiravir (loading dose: 1600 mg two times daily on the first day and then 600 mg twice daily for 5 days) and remdesivir (loading dose: 200 mg on day 1, followed by 100 mg once daily for a total of 10 days), and low-dose corticosteroids (6 mg/day for 10 days) were introduced and became the standard treatment. Since then, remdesivir and steroids have been given to patients with severe disease or in critical condition, while favipiravir has been given to patients with mild/moderate disease.
Data extraction and outcome
Data were extracted from the clinical records of laboratory-confirmed COVID-19 patients admitted to Hasan Sadikin Hospital. The information extracted included age, sex, date of admission, date of onset of symptoms, reported signs and symptoms, clinical condition (heart rate, respiratory rate, oxygen saturation, temperature), the presence of comorbidities as reported by the patients (chronic heart disease, hypertension, DM, CKD, and others), laboratory results, treatments given (oxygen treatment, antibiotics, antivirals, anticoagulants, and steroids), final outcome, and date of the final outcome. The primary outcome was survival status (discharged alive/died) and time to outcome from the date of hospitalization (in days). Data that did not have an outcome were excluded.
Definitions
The presence of comorbidities was determined based on clinical examination, including history taking. Hypertension was defined as blood pressure ≥140/90 mmHg.12 DM was defined as fasting plasma glucose ≥126 mg/dL, or HbA1c ≥ 6.5%, or 2-h plasma glucose ≥200 mg/dL during an oral glucose tolerance test, or random plasma glucose ≥200 mg/dL in a patient with classic symptoms.13 Patients with CKD included those with a glomerular filtration rate of less than 60 mL/min/1.73 m2 for at least 3 months.14 Patients with chronic heart disease included those with a history of several types of heart conditions, such as coronary artery disease, cardiomyopathy, and rheumatic heart diseases, among others. Fever was defined as an axillary temperature of at least 37.8 °C. Severe COVID-19 was defined according to WHO guidelines, as follows: oxygen saturation below 90% at room air, or respiratory rate >30 breaths/min, or signs of severe respiratory distress (use of accessory muscles, inability to complete full sentences), or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen (PaO2: FiO2) <300, and/or lung infiltrates >50% within 24–48 h.15 Patients with mild disease were defined as those with signs and symptoms of COVID-19 but without shortness of breath, dyspnea, or abnormal chest imaging, while patients with moderate disease were defined as those with lower respiratory tract disease (clinical assessment/abnormal chest imaging) with oxygen saturation ≥90% at room air.15 Anemia was defined according to WHO guidelines, namely, hemoglobin levels below 13 g/dL for men and below 12 g/dL for women.16 The neutrophil-to-lymphocyte count ratio (NLCR) cut-off was defined as >3.5 according to a previous study showing that the normal range of NLCR in healthy adults was 0.78–3.53 and the optimal cut-off value for identifying patients with severe COVID was 3.3.17
Statistical analysis
Descriptive analysis was undertaken for age, sex, clinical characteristics, laboratory parameters, and treatments based on survival outcome. The normality of the data was tested using the Kolmogorov–Smirnov or the Shapiro–Wilk test. For comparison of the variables of the two groups, normally distributed numeric variables were tested by the Student's t-test while non-normally distributed variables were tested by the Mann–Whitney U test. Categorical variables were tested using the chi-square statistic.
Univariate and multivariate Cox regression analysis with computed hazard ratio (HR) and 95% CIs with survival day as a time scale was used to estimate the effect of age, sex, and comorbidity on survival. Variables chosen for multivariable analysis were based on the univariate analysis and clinical relevance. All statistical tests were two-sided, and p-values less than 0.05 were considered significant. In sensitivity analysis, we examined the effect of age, sex, and comorbidity in all cases, including the probable cases. All analyses were performed with STATA software version 14.1 (StataCorp, College Station, TX, USA). Kaplan–Meier statistics were used to estimate survival probabilities and the log-rank test was employed to assess the significance of differences in survival among the groups.
To determine the effect of a drug regimen during hospitalization, we used Kaplan–Meier statistics and Cox regression analysis for sex, age, comorbidity, and each drug provided in the model. These analyses were conducted separately in the severe COVID-19 group and the mild/moderate group because of the different protocols provided for each category.
Ethical considerations
This was a retrospective cohort study using secondary data extracted from the medical records of patients. The study was approved by the Ethics Committee of Dr. Hasan Sadikin General Hospital, ethics number LB.02.01/X.6.5/100/2020, 6 May 2020, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was waived by the Ethics Committee because of the secondary use of medical data. All data were kept anonymous.
Role of funding source
None.
Results
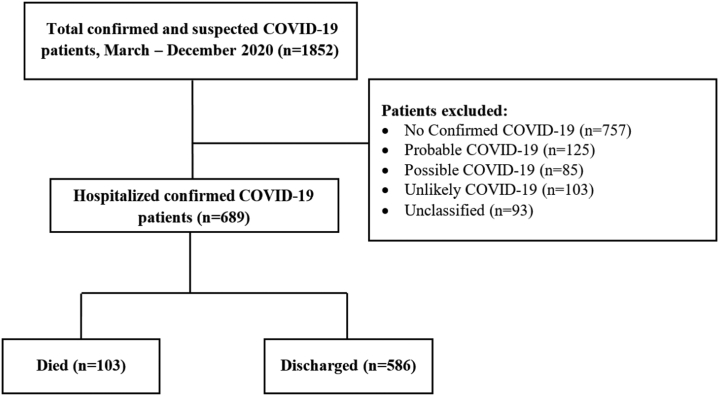
From March 2020 until December 2020, we admitted 1852 patients with suspected and confirmed COVID-19 to Hasan Sadikin Hospital. A total of 689 patients with confirmed COVID-19 were included in this study (Fig. 1). Of the patients, 586 (84.4%) were discharged when they were well or showed improvement, while 103 (14.9%) died. The demographic and baseline characteristics of the patients are presented in Table 1.
Fig. 1.
Flow diagram of data and patient outcomes.
Table 1.
Demographic and clinical characteristics of COVID-19 patients.
| Variable | Total (n = 6 89) | Died (n = 1 03) | Discharged alive (n = 5 86) | p-value |
|---|---|---|---|---|
| Age categories, years (%) | ||||
| 18–39 | 199 (100) | 6 (3.0) | 193 (97.0) | <0.001∗ |
| 40–49 | 136 (100) | 15 (11.0) | 121 (89.0) | |
| 50–59 | 157 (100) | 30 (19.1) | 127 (80.9) | |
| 60–69 | 126 (100) | 26 (20.6) | 100 (79.4) | |
| ≥70 | 71 (100) | 26 (36.6) | 45 (63.4) | |
| Sex (%)∗ | ||||
| Male | 358 (100) | 62 (17.3) | 296 (82.7) | 0.070 |
| Female | 331 (100) | 41 (12.4) | 290 (87.6) | |
| Comorbidities (%)+ | ||||
| Any | 396 (100) | 77 (19.4) | 319 (80.6) | <0.001∗ |
| Coronary heart disease | 71 (100) | 17 (23.9) | 54 (76.1) | <0.001∗ |
| Hypertension | 187 (100) | 42 (22.5) | 145 (77.5) | <0.001∗ |
| Diabetes mellitus | 121 (100) | 33 (27.3) | 88 (72.7) | <0.001∗ |
| Chronic kidney disease | 42 (100) | 12 (28.6) | 30 (71.4) | <0.001∗ |
| No comorbidities | 293 (100) | 26 (8.9) | 267 (91.1) | |
| Signs and symptoms on presentation (%) | ||||
| Cough | ||||
| Yes | 484 (100) | 85 (17.6) | 399 (82.4) | 0.003∗ |
| No | 204 (100) | 18 (8.8) | 186 (91.2) | |
| Fever | ||||
| Yes | 469 (100) | 81 (17.3) | 388 (82.7) | 0.013∗ |
| No | 219 (100) | 22 (10.0) | 197 (90.0) | |
| Dyspnea | ||||
| Yes | 366 (100) | 91 (24.9) | 275 (75.1) | <0.001∗ |
| No | 322 (100) | 12 (3.7) | 310 (96.3) | |
| Headache | ||||
| Yes | 86 (100) | 10 (11.6) | 76 (88.4) | 0.353 |
| No | 602 (100) | 93 (15.4) | 509 (84.6) | |
| Nausea or vomiting | ||||
| Yes | 78 (100) | 9 (11.5) | 69 (88.5) | 0.367 |
| No | 610 (100) | 94 (15.4) | 516 (84.6) | |
| Diarrhea | ||||
| Yes | 34 (100) | 6 (17.6) | 28 (82.4) | 0.654 |
| No | 654 (100) | 97 (14.8) | 557 (85.2) | |
| Time from illness onset to hospital admission, (IQR), days∗ | 6 (3–8) | 5 (3–7) | 6 (4–9) | 0.009∗ |
| ≤7 | 392 (100) | 77 (19.6) | 315 (80.4) | 0.047∗ |
| 8–14 | 140 (100) | 16 (11.4) | 124 (88.6) | |
| >14 | 45 (100) | 5 (11.1) | 40 (88.9) | |
| Respiratory rate, median (IQR), breaths/min | 22 (20–26) | 28 (24–32) | 22 (20–24) | <0.001∗ |
| >24 | 216 (100) | 73 (33.8) | 143 (66.2) | <0.001∗ |
| ≤24 | 472 (100) | 30 (6.4) | 442 (93.6) | |
| Pulse, median (IQR), beats/min | 92 (84–104) | 102 (92–112) | 92 (84–100) | <0.001∗ |
| ≥100 | 190 (100) | 52 (27.4) | 138 (72.6) | <0.001∗ |
| <100 | 498 (100) | 51 (10.2) | 447 (89.8) | |
| Oxygen saturation, median (IQR), % | 96 (94–98) | 92 (80–96) | 97 (95–98) | <0.001∗ |
| <90 | 106 (100) | 46 (43.4) | 60 (56.6) | <0.001∗ |
| ≥90 | 575 (100) | 55 (9.6) | 520 (90.4) | |
| Temperature, median (range) | 36.8 (36.5–37.2) | 37.0 (36.5–37.8) | 36.8 (36.5–37.0) | 0.010∗ |
| ≥37.5 | 139 (100) | 35 (25.2) | 104 (74.8) | <0.001∗ |
| <37.5 | 550 (100) | 68 (12.4) | 482 (87.6) | |
| Disease severity (%) | ||||
| Mild/moderate | 538 (100) | 39 (7.2) | 499 (92.8) | <0.001∗ |
| Severe | 151 (100) | 64 (42.4) | 87 (57.6) |
COVID-19, coronavirus disease 2019; IQR, Interquartile Range; ∗, statistically significant (p-value <0.05); + comorbidity categories are not mutually exclusive.
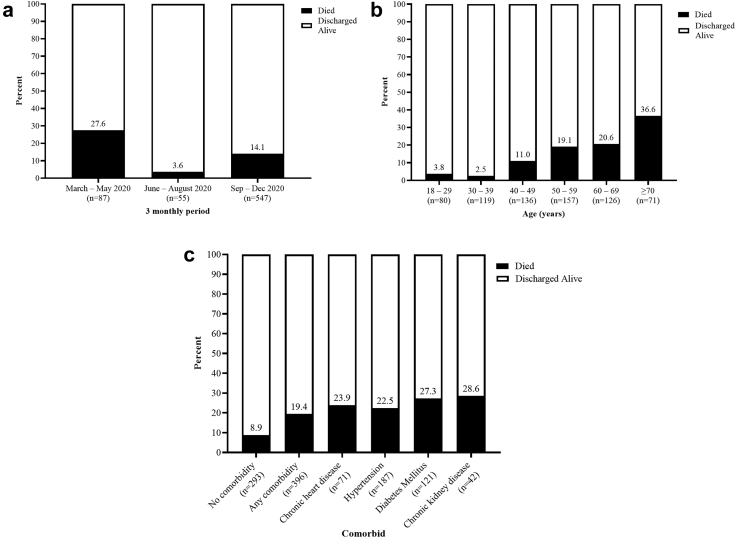
The records of the patients spanned from the beginning of the pandemic in March up to December 2020. The highest mortality was recorded between March and May 2020, with a death rate of 27.6% (24/87), but decreased in the next period. The greatest number of patients (547/689) were admitted between September and December 2020, with a 14.1% mortality rate (Fig. 2a).
Fig. 2.
a) Proportion of deaths during different periods of management. b) Proportion of mortality by age group. c) Proportion of deaths among patients with and without comorbidity.
Our cohort had a median age of 50 years (IQR 36–62). The median age of those who died was significantly higher (60 years; IQR 53–70) than that of those who survived (48 years; IQR 35–59). Mortality increased with age, ranging from 3.8% to 2.5% among patients aged 18 to 29 and 30–39 years, respectively, to 36.6% among those aged 70 years and older (Fig. 2b).
Three hundred and fifty-eight (51.9%) patients admitted were male and 396 (57.5%) had at least one comorbidity (Table 1). The most commonly reported comorbidities were hypertension (187, 27.1%), DM (121, 17.5%), and coronary heart disease (71, 10.3%). All comorbidities were associated with higher mortality, the main one being CKD, followed by DM (Fig. 2c).
The median time from illness onset to admission was 6 days (IQR 3–8). Patients who died had a shorter median duration of illness. Among the symptoms, cough (70.3%) was the most common presenting symptom, followed by fever (68%) and dyspnea (53.1%). These three symptoms were related to mortality. Mortality was significant among patients who presented with a respiratory rate >24 breaths/min, a pulse rate ≥100 beats/min, oxygen saturation <90%, and a temperature ≥37.5 °C. Severe COVID-19 was also associated with higher mortality (42.4% vs. 7.2%).
Baseline laboratory findings for the confirmed COVID-19 patients are presented in Table 2. There was no difference in hemoglobin, hematocrit, or proportion of anemia between those who died and those who survived. The platelet count was slightly lower among the patients who died (242 vs. 267 × 109 per L). The lymphocyte percentage and absolute count were significantly lower in non-survivors (10.5%, IQR 7.0%–16.3%) than in survivors (19%, IQR 12%–29%). The leucocyte count and NLCR were higher in non-survivors than in non-survivors (7.7 vs. 3.8, respectively). Inflammatory markers such as CRP (12.3 mg/dL vs. 2.8 mg/dL) and procalcitonin (0.38 ng/mL vs. 0.05 ng/mL) were also higher in non-survivors. Compared with survivors (3.1 g/L), the coagulation marker D-dimer was higher among non-survivors, whereas albumin was lower (2.3 g/L). Based on these laboratory parameters, mortality was associated with a leucocyte count >10 × 109 per L (21.8%), NLCR >3.53 (22.4%), higher CRP (26.2%), hypoalbuminemia (17.4%), D-dimer >1000 mcg/L (18.5%), and procalcitonin ≥0.5 (34.3%). Higher mortality was inversely correlated with lymphocyte count.
Table 2.
Laboratory findings in COVID-19 patients.
| Variable | Total (n = 689) | Died (n = 103) | Discharged alive (n = 586) | p-value |
|---|---|---|---|---|
| Hemoglobin, g/dL (n = 668) | 13.8 (12.5–15.0) | 13.9 (12.7–15.3) | 13.7 (12.5–14.9) | 0.203 |
| Anemia | ||||
| Yes | 432 (100) | 69 (16.0) | 363 (84.0) | 0.257 |
| No | 236 (100) | 30 (12.7) | 206 (87.3) | |
| Hematocrit, % (n = 667) | 40.0 (36.4–43.2) | 40.1 (36.3–44.1) | 40.0 (36.4–43.2) | 0.509 |
| WBC count, × 109 per L (n = 667) | 7.9 (5.9–10.8) | 9.4 (6.6–14.0) | 7.7 (5.8–10.4) | 0.001∗ |
| <4 | 34 (100) | 4 (11.8) | 30 (88.2) | 0.004∗ |
| 4–10 | 431 (100) | 53 (11.8) | 378 (88.2) | |
| >10 | 202 (100) | 46 (21.8) | 156 (78.2) | |
| Neutrophil, % (n = 659) | 74.0 (63.0–82.0) | 83.0 (76.0–87.0) | 72.0 (61.0–81.0) | <0.001∗ |
| Lymphocyte, % (n = 659) | 18.0 (11.0–27.0) | 10.5 (7.0–16.3) | 19.0 (12.0–29.0) | <0.001∗ |
| Absolute lymphocyte count (n = 659) | 1360 (948–1890) | 996 (690–1424) | 1440 (1010–1931) | <0.001∗ |
| Neutrophil lymphocyte count ratio (n = 659) | 4.2 (2.3–7.4) | 7.7 (4.7–12.8) | 3.8 (2.2–6.5) | <0.001∗ |
| >3.53 | 384 (100) | 86 (22.4) | 298 (77.6) | <0.001∗ |
| ≤3.53 | 275 (100) | 12 (4.4) | 263 (95.5) | |
| Platelet count, × 109 per L (n = 6 63) | 265 (208–326) | 242 (170–324) | 267 (216–327) | 0.028∗ |
| C-reactive protein, mg/L (n = 506) | 3.5 (0.6–9.8) | 12.3 (6.9–18.2) | 2.8 (0.5–8.8) | <0.001∗ |
| ≥100 | 122 (100) | 32 (26.2) | 90 (73.8) | <0.001∗ |
| <100 | 384 (100) | 23 (6.0) | 361 (94.0) | |
| Albumin, g/L (n = 436) | 2.9 (2.4–3.7) | 2.3 (2.2–2.6) | 3.1 (2.5–3.7) | <0.001∗ |
| <3.5 | 303 (100) | 53 (17.4) | 251 (82.8) | <0.001∗ |
| ≥3.5 | 133 (100) | 1 (1.9) | 131 (98.5) | |
| D-dimer, mcg/L (n = 492) | 0.8 (0.4–2.0) | 1.9 (1.0–4.8) | 0.8 (0.4–1.8) | <0.001∗ |
| >1000 | 211 (100) | 39 (18.5) | 172 (81.5) | <0.001∗ |
| >500–1000 | 126 (100) | 7 (5.6) | 119 (94.4) | |
| ≤500 | 156 (100) | 3 (1.9) | 153 (98.1) | |
| Procalcitonin, ng/mL (n = 4 66) | 0.05 (0.01–0.20) | 0.38 (0.16–1.21) | 0.05 (0.01–0.15) | <0.001∗ |
| ≥0.5 | 67 (100) | 23 (34.3) | 44 (65.7) | <0.001∗ |
| <0.5 | 399 (100) | 27 (6.8) | 372 (93.2) |
COVID-19, coronavirus disease 2019; WBC, white blood cell count; ∗, statistically significant (p-value <0.05).
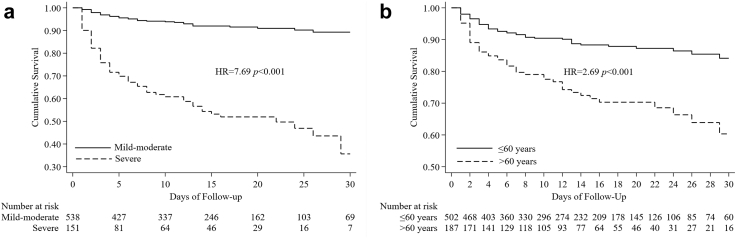
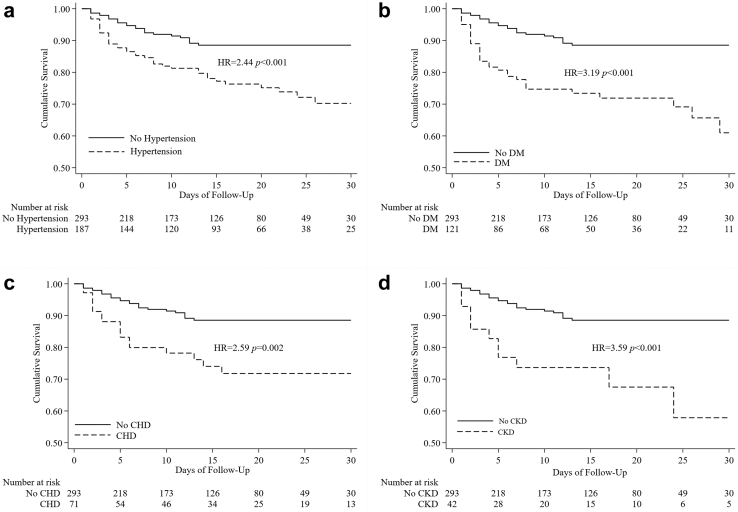
Multivariate analysis showed that older age was related to a fatal outcome, increasing from an adjusted HR of 2.66 (1.02–6.97) for the 40–49 age group to an HR of 8.96 (3.60–22.32) for those aged ≥70 years. Having any comorbidity was also associated with higher mortality, with a HR of 2.23 (CI: 1.43–3.49) and an adjusted HR of 1.56 (CI: 0.97–2.51). The time of illness onset to hospital admission was significantly lower in patients with a fatal outcome (Table 3). All of these factors showed similar effects when analyzed with respect to disease severity. Increasing age and the presence of comorbidity increased disease severity. More than 7 days after illness onset was also related to severe illness (Table 4). As shown in the Kaplan–Meier survival curve in Fig. 3a, the survival probability of severely ill patients was 65.4% on day 7, declined to 54.4% on day 14, and was further reduced to 35.6% on day 28. This was significantly lower than that recorded in the mild/moderate group, in which the survival probability until day 28 was 99.4% (Fig. 3a). Kaplan–Meier curve analysis also showed a significantly lower survival rate in older patients (Fig. 3b) and patients with comorbidity (Fig. 4a–d). The results of sensitivity analyses including the probable cases were consistent with those of the primary analyses (see Supplementary Tables S1 and S2).
Table 3.
Cox regression analysis evaluating pre-existing conditions associated with mortality.
| Clinical characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR | p-value | Adjusted HR | p-value | |
| Sex (male) | 1.32 (0.89–1.97) | 0.159 | 1.21 (0.81–1.82) | 0.356 |
| Age category (years) | ||||
| 18–39 | 1 (ref) | 1 (ref) | ||
| 40–49 | 3.67 (1.42–9.45) | 0.007∗ | 2.66 (1.02–6.97) | 0.046∗ |
| 50–59 | 5.99 (2.49–14.39) | <0.001∗ | 3.96 (1.61–9.76) | 0.003∗ |
| 60–69 | 6.95 (2.86–16.90) | <0.001∗ | 4.83 (1.95–11.97) | 0.001∗ |
| ≥70 | 12.88 (5.30–31.30) | <0.001∗ | 8.96 (3.60–22.32) | <0.001∗ |
| Comorbidity present (any) | 2.23 (1.43–3.49) | <0.001∗ | 1.56 (0.97–2.51) | 0.067 |
| Time from illness onset to hospital admission, days∗ | ||||
| ≤7 | 1 (ref) | 1 (ref) | ||
| 8–14 | 0.53 (0.31–0.90) | 0.019∗ | 0.54 (0.32–0.93) | 0.026∗ |
| >14 | 0.56 (0.23–1.39) | 0.212 | 0.52 (0.21–1.29) | 0.158 |
COVID-19, coronavirus disease 2019; HR, hazard ratio; ∗, statistically significant (p-value <0.05).
Table 4.
Cox regression analysis evaluating pre-existing conditions associated with disease severity.
| Clinical characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR | p-value | Adjusted HR | p-value | |
| Sex (male) | 1.23 (0.89–1.70) | 0.209 | 1.14 (0.82–1.59) | 0.440 |
| Age category (years) | ||||
| 18–39 | 1 (ref) | 1 (ref) | ||
| 40–49 | 2.96 (1.53–5.74) | 0.001∗ | 2.43 (1.25–4.74) | 0.009∗ |
| 50–59 | 3.20 (1.70–6.02) | <0.001∗ | 2.33 (1.21–4.48) | 0.011∗ |
| 60–69 | 4.82 (2.58–9.01) | <0.001∗ | 3.65 (1.91–6.96) | <0.001∗ |
| ≥70 | 8.10 (4.27–15.35) | <0.001∗ | 5.70 (2.91–11.14) | <0.001∗ |
| Comorbidity present (any) | 1.87 (1.31–2.66) | <0.001∗ | 1.44 (0.98–2.10) | 0.064 |
| Time from illness onset to hospital admission, days∗ | ||||
| ≤7 | 1 (ref) | 1 (ref) | ||
| 8–14 | 0.61 (0.40–0.93) | 0.022∗ | 0.61 (0.40–0.93) | 0.022∗ |
| >14 | 0.64 (0.31–1.32) | 0.228 | 0.59 (0.29–1.21) | 0.147 |
COVID-19, coronavirus disease 2019; HR, hazard ratio; ∗, statistically significant (p-value <0.05).
Fig. 3.
a) Disease severity as risk factor for mortality. HR = hazard ratio. b) Age as risk factor for mortality. HR = hazard ratio.
Fig. 4.
Comorbidities as risk factors for mortality. Kaplan-Meier curve of mortality related to a) hypertension b) diabetes mellitus (DM), c) coronary heart disease (CHD) and d) chronic kidney disease (CKD). HR = hazard ratio.
The use of specific therapies and their association with mortality is shown in Table 5. Overall, the use of remdesivir and corticosteroids was associated with higher mortality when all disease severity levels are considered. This was due to the protocol, where these treatments were mostly given to severely ill patients. Intubation/mechanical ventilation was also associated with mortality (30.5% vs. 13.5%) for similar reasons. Meanwhile, only favipiravir was shown to be significantly related to lower mortality (5.8 vs. 21.0). Antibiotics, mainly levofloxacin, ceftriaxone, and azithromycin, were given to almost half of the patients (Table 5).
Table 5.
Frequency and association of treatments provided.
| Variable | Total (n = 6 89) | Died (n = 103) | Discharged alive (n = 586) | p-value |
|---|---|---|---|---|
| Antibiotics (%)∗ | ||||
| Yes | 345 (100) | 58 (16.8) | 287 (83.2) | 0.170 |
| No | 344 (100) | 45 (13.1) | 299 (86.9) | |
| Antiviral treatment, n = 464 (%) | ||||
| Yes | 333 (100) | 31 (9.3) | 302 (90.7) | 0.487 |
| No | 131 (100) | 15 (11.5) | 116 (88.5) | |
| Remdesivir (%)∗ | ||||
| Yes | 92 (100) | 22 (23.9) | 70 (76.1) | 0.010∗ |
| No | 597 (100) | 81 (13.6) | 516 (86.4) | |
| Favipiravir (%)∗ | ||||
| Yes | 274 (100) | 16 (5.8) | 258 (94.2) | <0.001∗ |
| No | 415 (100) | 87 (21.0) | 328 (79.0) | |
| Corticosteroids (%)∗ | ||||
| Yes | 188 (100) | 41 (21.8) | 147 (78.1) | 0.002∗ |
| No | 501 (100) | 62 (12.4) | 439 (87.6) | |
| Anticoagulants, n = 466 (%)∗ | ||||
| Yes | 286 (100) | 43 (15.0) | 243 (85.0) | 0.958 |
| No | 403 (100) | 60 (14.9) | 343 (85.1) | |
| High-flow nasal cannula, n = 463 (%) | ||||
| Yes | 35 (100) | 7 (20.0) | 28 (80.0) | 0.045∗ |
| No | 428 (100) | 40 (9.3) | 388 (90.7) | |
| Invasive mechanical ventilation, n = 463 (%) | ||||
| Yes | 59 (100) | 18 (30.5) | 41 (69.5) | <0.001∗ |
| No | 630 (100) | 85 (13.5) | 545 (86.5) |
∗, Statistically significant (p-value <0.05).
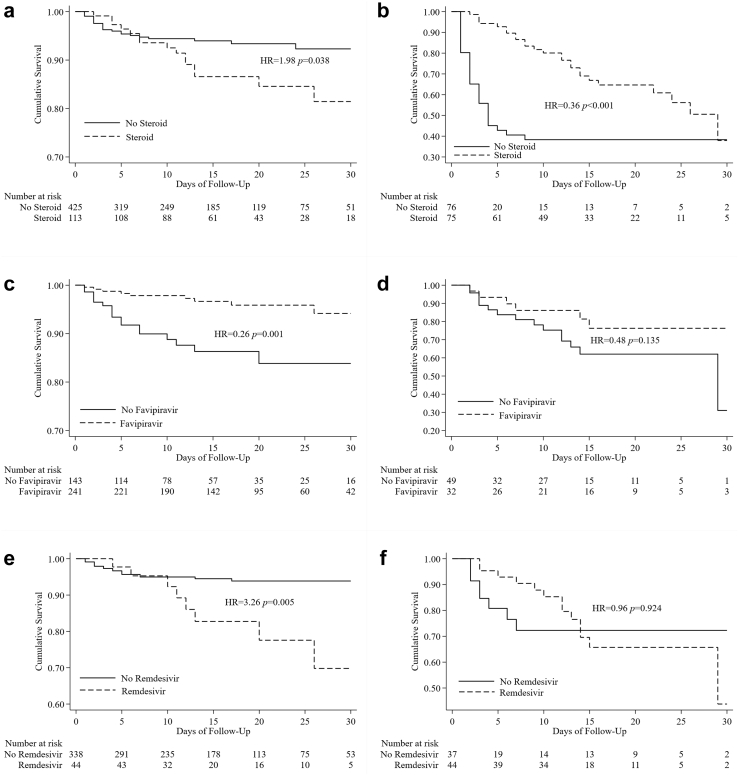
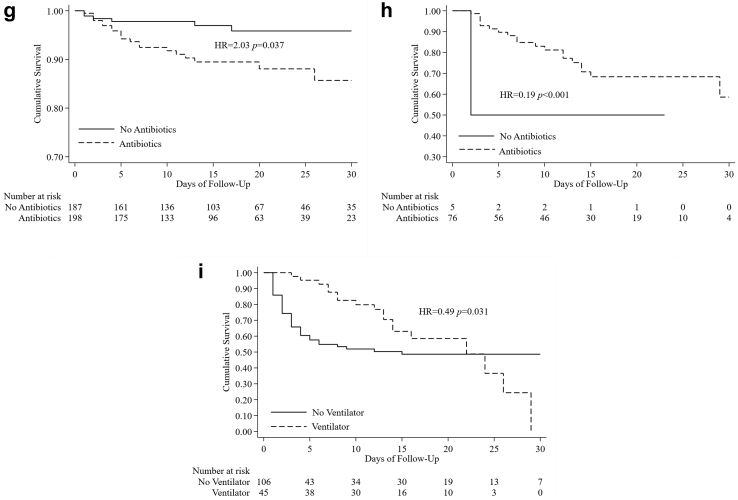
Because of the different protocols implemented for patients with severe disease versus those with mild/moderate illness, we stratified the subjects into these two case groups. Among the mild/moderate cases, Cox regression analysis (Table 6) showed that the use of steroids was associated with higher mortality, with an adjusted HR of 2.81 (1.09–7.25), while favipiravir was associated with lower mortality, with an adjusted HR of 0.24 (0.11–0.54). These associations were later confirmed via analysis of the respective Kaplan–Meier curves (Fig. 5a and c, respectively). Remdesivir was correlated with higher mortality, especially in the second week of hospital admission (Fig. 5e). Although not significant in the multivariate analysis, the Kaplan–Meier curve showed that the use of antibiotics was also related to increased mortality in this group (Fig. 5g).
Table 6.
Cox regression analysis evaluating the association of drugs with mortality among patients with mild and moderate disease.
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR | p-value | Adjusted HR | p-value | |
| Sex (male) | 1.07 (0.57–2.02) | 0.826 | 1.00 (0.52–1.92) | 1.000 |
| Age (>60 years) | 3.94 (2.10–7.39) | <0.001∗ | 3.17 (1.63–6.17) | 0.001∗ |
| Comorbidity present (any) | 2.91 (1.38–6.13) | 0.005∗ | 2.41 (1.12–5.18) | 0.024∗ |
| Drugs | ||||
| Steroids | 1.98 (1.04–3.78) | 0.038∗ | 2.81 (1.09–7.25) | 0.033∗ |
| Antibiotics | 2.03 (1.04–3.95) | 0.037∗ | 1.23 (0.59–2.59) | 0.579 |
| Anticoagulants | 0.93 (0.49–1.77) | 0.834 | 0.60 (0.23–1.55) | 0.291 |
| Favipiravir | 0.29 (0.14–0.60) | 0.001∗ | 0.24 (0.11–0.54) | 0.001∗ |
| Remdesivir | 2.32 (1.06–5.04) | 0.034∗ | 0.89 (0.31–2.53) | 0.827 |
COVID-19, coronavirus disease 2019; HR, hazard ratio; ∗, statistically significant (p-value <0.05).
Fig. 5.
Relationship between treatment provided and patient survival. Kaplan-Meier curve shows the survival difference of the mild/moderate patients receiving a) steroid, c) favipiravir, e) remdesivir and g) antibiotics. Similar comparison shows the survival difference of the severe patients receiving b) steroid, d) favipiravir, f) remdesivir and h) antibiotics and i) ventilator. HR = hazard ratio.
Among patients with severe COVID-19, antibiotics and favipiravir were significantly associated with lower mortality, with adjusted HRs of 0.29 (0.13–0.65) and 0.40 (0.17–0.98), respectively (Table 7). This association was also shown in the Kaplan–Meier curve (Fig. 5d) for the use of favipiravir. The Kaplan–Meier curve for the use of antibiotics could not be interpreted because the sample size was too small (Fig. 5h). Among patients with severe disease, we found that remdesivir was related to lower mortality in the first 10 days of admission, but not overall (Fig. 5f). A similar pattern was observed among the patients who were given steroids (Fig. 5b) and mechanical support (Fig. 5i). In these two groups, mortality was lower in the first 15 days but increased markedly after day 20, likely due to secondary bacterial infection.
Table 7.
Cox regression analysis evaluating the association of drugs with mortality among patients with severe disease.
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR | p-value | Adjusted HR | p-value | |
| Sex (male) | 1.17 (0.70–1.95) | 0.550 | 0.86 (0.51–1.48) | 0.592 |
| Age (≥60 years) | 0.94 (0.58–1.54) | 0.810 | 1.06 (0.63–1.78) | 0.818 |
| Comorbidity present (any) | 1.00 (0.57–1.74) | 0.994 | 0.94 (0.52–1.69) | 0.827 |
| Drugs | ||||
| Steroids | 0.36 (0.21–0.59) | <0.001∗ | 1.62 (0.62–4.28) | 0.328 |
| Antibiotics | 0.19 (0.12–0.33) | <0.001∗ | 0.29 (0.13–0.65) | 0.003∗ |
| Anticoagulants | 0.21 (0.12–0.36) | <0.001∗ | 0.43 (0.17–1.12) | 0.080 |
| Favipiravir | 0.29 (0.13–0.63) | 0.002∗ | 0.40 (0.17–0.98) | 0.044∗ |
| Remdesivir | 0.45 (0.25–0.82) | 0.009∗ | 0.76 (0.33–1.77) | 0.523 |
COVID-19, coronavirus disease 2019; HR, hazard ratio; ∗, statistically significant (p-value <0.05).
Discussion
Here, we report a case fatality rate (CFR) of 14.9% in a cohort of patients admitted to our hospital in the first 10 months of the COVID-19 pandemic. Higher mortality was observed in the first 3 months of the pandemic and was associated with older age, comorbidities, and severe illness. Other studies reported higher or similar CFRs among hospitalized COVID patients in the early phase of the pandemic.3, 4, 5 Studies involving community-level hospitals, such as the one in Jakarta, Indonesia, reported lower mortality rates (9.4%11 and 12%10), as did one study from China (3.7%).3 The higher mortality rates reported in this study likely occurred because more severely ill patients were admitted to our hospital, which is a provincial, top referral hospital with a greater number of ICU beds.
Numerous reports have confirmed the high association of severe COVID-19 with mortality,3,4 and our observations are consistent with these findings. The parameters and factors that were associated with severity and mortality in our study are also in line with previous reports.3,4,6,8
Older age was related to higher mortality independently of comorbidities, with an overall CFR of 26.4% among patients >60 years of age. This value was slightly higher than that reported in a previous study in Jakarta, which recorded a CFR of 23% among a similar age group,18 but lower compared to a study from Iran19 which reported a CFR of 41.6%. In addition to having more comorbidities, age-related immune senescence was also a factor in the high mortality rate seen in elder patients. This condition affects the general immune system and is characterized by significant dysregulation of the adaptive immune response.3,20,21
Chronic kidney disease was associated with the highest mortality in our COVID-19 patients. Studies have shown that the ACE2 receptor is overexpressed in tubular cells in COVID-19 patients with kidney disease, which renders CKD patients more susceptible to COVID-19 infection.22 The proposed hypothesis is that elevated expression of the ACE2 receptor coupled with dysregulation of immune function increases the risk of mortality in COVID-19 patients with CKD.21 Patients with CKD often are also older, may have DM, may be malnourished, or may be on routine hemodialysis. All these factors contribute to an impaired inflammatory response, resulting in inadequate control of viral replication and prolonged proinflammatory responses.3,21
The presence of DM was also associated with high mortality in our study. We found that patients with DM had a 62.7% survival probability, which was lower than in other studies. Zhou et al.4 reported a 79% survival rate with an HR of 2.85 (95% CI: 1.35–6.05) while Wu et al.23 found that DM was associated with a survival rate of 75% (HR of 1.58 [95% CI, 0.80 to 3.13]) among COVID-19 patients. The association of DM with the severity of COVID-19 and mortality has been reported in other studies, while poor glycemic control was proposed to have value as a predictor of worse outcomes.4,23,24 Increased risk and severity of SARS-CoV-2 infection in diabetes are hypothesized to be related to the increased expression of ACE2, the receptor for SARS-CoV-2, which facilitates viral entry into host cells, impairs T-cell function, and increases interleukin (IL)-6 content.23,24 The overwhelming metabolic dysregulation in DM predisposes to infections in general, which further complicates the response to COVID-19.24
Aside from pre-existing conditions, we analyzed inflammatory markers and clinical conditions associated with mortality. Univariate analysis of laboratory markers showed that high NLCR, CRP, and procalcitonin were associated with higher mortality, with HRs of 5.36, 4.76, and 6.87, respectively. In agreement with our study, a previous meta-analysis also demonstrated that higher serum CRP was related to severe infection, with an optimal cut-off of 33.55 mg/L and with 89.5% sensitivity and specificity. Increased CRP may result from a robust inflammatory response to SARS-CoV-2 or bacterial co-infection, both of which are associated with severe COVID disease.25
The increase in procalcitonin levels among patients with severe or fatal disease has also been previously reported. One study demonstrated that increased procalcitonin values are related to nearly 5-fold higher risk of severe COVID infection (odds ratio [OR], 4.76; 95% CI, 2.74–8.29).26 In a cohort of inpatients with COVID-19 in Wuhan, China, the mean serum procalcitonin levels were over four to eight times higher in patients with severe and critical disease compared to patients with more moderate disease.27 The high procalcitonin value was associated with bacterial co-infection, which increasingly occurred with longer hospitalization time and the use of ventilators.26,27
We evaluated the association of drugs with mortality by separating the analysis into severe and mild/moderate groups. Oral favipiravir, remdesivir, and dexamethasone were introduced in June/July 2020. After adjustment for other factors, we first found that dexamethasone was not beneficial in mild and moderate cases but showed some beneficial effects in the first 3 weeks of admission in patients with severe illness. Our observation confirms the benefit of using steroids only for the severely ill.28
The use of remdesivir in our study was not related to decreased mortality. Other studies report conflicting results. Some studies support the association of remdesivir with reduced 30-day mortality in patients with moderate COVID-19 and that they hasten clinical recovery.29,30 In contrast, other studies have shown that the benefits of remdesivir are still not apparent in patients with severe disease.29 Meanwhile, we found that favipiravir was associated with decreased mortality in patients with mild/moderate disease. These findings are in line with a previous trial in Japan showing that favipiravir shortened the time to clinical improvement by approximately 3 days in COVID-19 patients with moderate disease.31
The use of antibiotics has been proposed for the treatment of COVID-19 patients; however, they were deemed unnecessary for mild/moderate or uncomplicated COVID-19 illness.32 Our study confirmed that the use of antibiotics in mild/moderate cases did not reduce the fatality rate. On the other hand, a secondary bacterial infection often occurred in COVID-19 patients with severe illness and undergoing mechanical ventilation, and substantial evidence supports the provision of antibiotics in this situation. However, we could not determine the effect of antibiotics among this group of patients because the number of subjects who did not take antibiotics was too small.
Similar to a previous report,4 mortality increased among patients with severe illness after 2 weeks, even after receiving a treatment regimen for severe COVID-19 (remdesivir and corticosteroids). The use of ventilators is also a significant risk factor for death. The high mortality observed may be associated with acquired secondary infection in patients hospitalized for more than 2 weeks and requiring mechanical ventilation, as also reported by other studies.4,9 The incidence of secondary lung infection reported in the literature is approximately 15%–20%,33, 34, 35 with higher incidence observed in patients with severe COVID-19 admitted to the ICU and requiring mechanical ventilation (∼30%).33,35
Our study had several limitations. First, our data were incomplete for some baseline variables and laboratory data, especially markers such as procalcitonin and CRP, which were not routinely screened. Comorbidities were self-reported, potentially resulting in underreporting. Additionally, details regarding the incidence of secondary infections were not analyzed, and the treatments received were not randomized; accordingly, the findings of this study may not reflect the association of treatments with mortality in the general population. Our study was not designed to analyze drug effects and was thus inferior to regimented clinical trials. Our protocol indicates the use of remdesivir in moderate and severe cases, steroids in severe cases, and favipiravir in mild and moderate cases. These selections affect the evaluation of the drugs. However, our analysis showed that remdesivir and steroids were beneficial in the earlier stages of COVID-19.
In conclusion, the risk factors associated with mortality in COVID-19 patients in a referral hospital in Bandung were older age and the presence of comorbidities, mainly CKD, DM, hypertension, and coronary heart disease. All these factors contributed to severe COVID-19 and, subsequently, to higher mortality. Among the administered drugs, favipiravir showed significant benefit for COVID-19 survival, while steroids showed benefit only in the earlier days of hospital admission among patients with severe disease. Because these findings are from the early stage of the COVID-19 pandemic, the results should be interpreted with caution. Mortality was high in the first few days of admission and after the second week of admission. The latter may be due to secondary bacterial infection, although more research is necessary to elucidate the causes of mortality.
Contributors
YH and BA designed and supervised the study and verified the data; they also took responsibility for the integrity and accuracy of the data analysis. YH, BA, and ES had full access to all the data and did the analysis. YH, JD, ES, EH, APAH, and BA collected and verified the data. All authors drafted and revised the manuscript and gave approval for the version to be published.
Data sharing statement
The datasets used for this study will be made available after publication to other researchers who provide a reasonable proposal to the corresponding author. The research proposal, statistical analysis plan, and de-identified participant data will be provided after written approval from the corresponding author and Universitas Padjajaran.
Declaration of interests
We declare that we have no competing interests.
Acknowledgements
We thank the Director of Hasan Sadikin Hospital, Bandung for managing COVID-19 patients and the Ministry of Health & the Government of Indonesia for financing COVID-19 care in Indonesia.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100167.
Contributor Information
Yovita Hartantri, Email: yhartantri@gmail.com.
Josephine Debora, Email: rabodedebora@gmail.com.
Leonardus Widyatmoko, Email: bpinsani@gmail.com.
Gezy Giwangkancana, Email: gezy.weita@unpad.ac.id.
Hendarsyah Suryadinata, Email: hendarsyahsuryadinata@gmail.com.
Evan Susandi, Email: evan.susandi@unpad.ac.id.
Elisabeth Hutajulu, Email: hutajuluelisabeth@gmail.com.
Assica Permata Amalya Hakiman, Email: Assicapermata@live.com.
Yesy Pusparini, Email: yesy.puspa@yahoo.com.
Bachti Alisjahbana, Email: b.alisjahbana@unpad.ac.id.
Appendix A. Supplementary data
References
- 1.World Health Organization . 2022. WHO Coronavirus (Covid-19) Dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data.https://COVID19.who.int/region/searo/country/id [Google Scholar]
- 2.Alene M., Yismaw L., Assemie M.A., et al. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: a systematic review and meta-analysis. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0249090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J., Wang X., Jia X., et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altonen B.L., Arreglado T.M., Leroux O., Murray-Ramcharan M., Engdahl R. Characteristics, comorbidities and survival analysis of young adults hospitalized with COVID-19 in New York City. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang J., Wang Q., Zhang H., et al. The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort study in Wuhan, China. Am J Med. 2021;134(1):e6–e14. doi: 10.1016/j.amjmed.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahu K.K., Cerny J. Taylor & Francis; 2020. Managing patients with hematological malignancies during COVID-19 pandemic; pp. 787–793. [DOI] [PubMed] [Google Scholar]
- 8.Lu W., Yu S., Liu H., et al. Survival analysis and risk factors in COVID-19 patients. Disaster Med Public Health Prep. 2021:1–6. doi: 10.1017/dmp.2021.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salinas-Escudero G., Carrillo-Vega M.F., Granados-García V., Martínez-Valverde S., Toledano-Toledano F., Garduño-Espinosa J. A survival analysis of COVID-19 in the Mexican population. BMC Publ Health. 2020;20(1):1–8. doi: 10.1186/s12889-020-09721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surendra H., Elyazar I.R., Djaafara B.A., et al. Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: a hospital-based retrospective cohort study. Lancet Reg Health West Pac. 2021;9 doi: 10.1016/j.lanwpc.2021.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozaliyani A., Savitri A.I., Setianingrum F., et al. Factors associated with death in COVID-19 patients in Jakarta, Indonesia: an epidemiological study. Acta Med Indones. 2020;52(3):246–254. [PubMed] [Google Scholar]
- 12.Whelton P.K., Williams B. The 2018 European Society of Cardiology/European Society of hypertension and 2017 American College of Cardiology/American heart association blood pressure guidelines: more similar than different. JAMA. 2018;320(17):1749–1750. doi: 10.1001/jama.2018.16755. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Supplement_1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A.S., Eckardt K.-U., Tsukamoto Y., et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . Vol. 25. 2021. COVID-19 Clinical Management: Living Guidance. [Google Scholar]
- 16.World Health Organization . 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Google Scholar]
- 17.Yang A.-P., Liu J.-P., Tao W.-Q., Li H.-M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharm. 2020;84 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azwar M.K., Setiati S., Rizka A., Fitriana I., Saldi S.R.F., Safitri E.D. Clinical profile of elderly patients with COVID-19 hospitalised in Indonesia's national general hospital. Acta Med Indones. 2020;52(3):199. [PubMed] [Google Scholar]
- 19.Jalili M., Payandemehr P., Saghaei A., Sari H.N., Safikhani H., Kolivand P. Characteristics and mortality of hospitalized patients with COVID-19 in Iran: a national retrospective cohort study. Ann Intern Med. 2021;174(1):125–127. doi: 10.7326/M20-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opal S.M., Girard T.D., Ely E.W. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Supplement_7):S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 21.Biswas M., Rahaman S., Biswas T.K., Haque Z., Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2021;64(1):36–47. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan C., Lu W., Li K., Ding Y., Wang J. ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 patients. Front Med. 2021:1045. doi: 10.3389/fmed.2020.563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu R., Wang L., Kuo H.-C.D., et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020;6(3):56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metabol Syndr: Clin Res Rev. 2020;14(4):303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariyanto T.I., Japar K.V., Kwenandar F., et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021;41:110–119. doi: 10.1016/j.ajem.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta Int J Clin Chem. 2020;505:190. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu R., Han C., Pei S., Yin M., Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Group R.C. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beigel J., Tomashek K., Dodd L., et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benfield T., Bodilsen J., Brieghel C., et al. Improved survival among hospitalized patients with coronavirus disease 2019 (COVID-19) treated with remdesivir and dexamethasone. A nationwide population-based cohort study. Clin Infect Dis. 2021;73(11):2031–2036. doi: 10.1093/cid/ciab536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinkai M., Tsushima K., Tanaka S., et al. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial. Infect Dis Therapy. 2021;10(4):2489–2509. doi: 10.1007/s40121-021-00517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinte L., Ceasovschih A., Niculae C.-M., et al. Antibiotic prescription and in-hospital mortality in COVID-19: a prospective multicentre cohort study. J Personalized Med. 2022;12(6):877. doi: 10.3390/jpm12060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox M.J., Loman N., Bogaert D., O'Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1(1):e11. doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chedid M., Waked R., Haddad E., Chetata N., Saliba G., Choucair J. Antibiotics in treatment of COVID-19 complications: a review of frequency, indications, and efficacy. J Infect Public Health. 2021;14(5):570. doi: 10.1016/j.jiph.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoso P., Sung M., Hartantri Y., et al. MDR pathogens organisms as risk factor of mortality in secondary pulmonary bacterial infections among COVID-19 patients: observational studies in two referral hospitals in West Java, Indonesia. Int J Gen Med. 2022;15:4741. doi: 10.2147/IJGM.S359959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.