Abstract

Quantum chemical calculations have for some time predicted that perfluorinated polyhedral organic molecules should exhibit a low-energy LUMO consisting of the overlapping inward-pointing lobes of the C–F σ* orbitals. Accordingly, these molecules should be able to encapsulate an electron within the interior of their cavities. Inspired by the recent confirmation of this prediction for perfluorocubane, we have sought to identify additional perfluorinated cage molecules capable of this remarkable behavior, which we refer to as the perfluoro cage effect (PCE). Using DFT calculations with multiple well-tested exchange-correlation functionals and large STO-QZ4P basis sets, we have identified several systems including [n]prismanes (n = 3–6), [n]asteranes (n = 3–5), twistane, and two norbornadiene dimer cages that clearly exhibit the PCE. In other words, they exhibit a low-energy LUMO belonging to the total symmetric irreducible representation of the point group in question and adiabatic electron affinities ranging from somewhat under 1 eV to over 2 eV. A pronounced size effect appears to hold, with larger cages exhibiting higher electron affinities (EAs). The largest adiabatic EAs, well over 3 eV, are predicted for perfluorinated dodecahedrane and C60. In contrast, the PCE is barely discernible for perfluorinated tetrahedrane and bicyclo[1.1.1]pentane.
Introduction
Polyfluorination and perfluorination typically affect organic molecules in a profound manner.1−3 One such influence is the so-called perfluoro effect, observed for planar conjugated molecules, in which the fluorines exert a much stronger stabilizing influence on the σ molecular orbitals than on the π molecular orbitals.4−7 Perfluorinated polyhedral organic molecules have been theoretically examined and a key prediction is a low-energy, totally symmetric LUMO derived from the overlapping inward-pointing lobes of the C–F σ* orbitals.8,9 The molecules thus exhibit a significant electron affinity (EA), accommodating the electron largely within central cavity of the polyhedra. This prediction has now been experimentally realized in the form of the perfluorocubane anion radical with Oh symmetry.10 Herein, we have used density functional theory (DFT) to explore both the generality and limitations of the electron-encapsulating effect across a wide range of organofluorine cages. The effect, hereafter referred to as the perfluoro cage effect (PCE), indeed appears to be general, with only a handful of exceptions. Several new examples of the PCE are predicted.
Results and Discussion
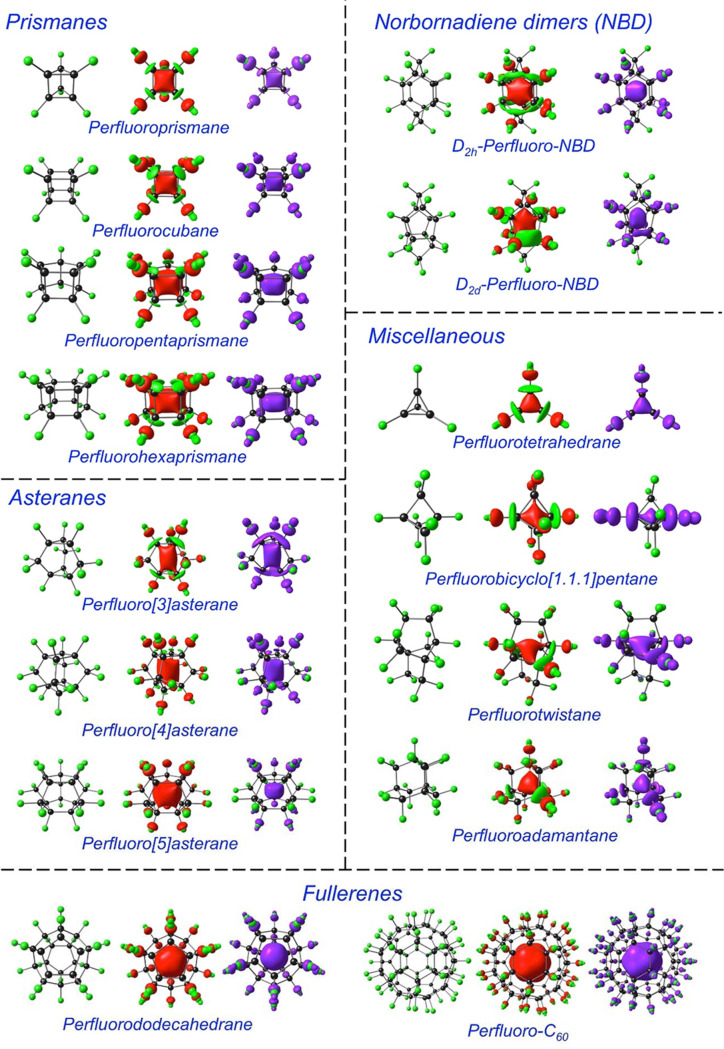
Sixteen perfluorinated polyhedral and/or cage molecules were examined with three well-tested11−15 exchange-correlation functionals, OLYP,16,17 B3LYP,18,19 and B3LYP*,20,21 augmented with D322 dispersion corrections, and large STO-QZ4P basis sets. Table 1 presents their calculated adiabatic EAs, while Figure 1 presents graphical representations of their LUMOs and the spin densities of their anion-radicals. In pretty much every case examined, the LUMO belongs to the totally symmetric irreducible representation of the point group in question. The vast majority of the molecules also exhibit a significant electron-encapsulating ability, as measured by the adiabatic EAs, which, by and large, are mutually consistent across the three functionals.
Table 1. Adiabatic Electron Affinities (eV) of the Compounds Studied.
| adiabatic EA |
||||||
|---|---|---|---|---|---|---|
| compound | point group | LUMO irrep | OLYP | OLYP (ZPE) | B3LYP* | B3LYP |
| Prismanes | ||||||
| Perfluoroprismane | D3h | a′1 | 0.97 | 1.04 | 0.87 | 0.80 |
| Octafluorocubane | Oh | a1g | 1.55 | 1.67 | 1.43 | 1.35 |
| Perfluoropentaprismane | D5h | a′1 | 1.98 | 2.12 | 1.85 | 1.77 |
| Perfluorohexaprismane | D6h | a1g | 2.15 | 2.28 | 2.04 | 1.96 |
| Asteranes | ||||||
| Perfluoro[3]asterane | C3h | a′ | 1.36 | 1.54 | 1.18 | 1.08 |
| Perfluoro[4]asterane | D4h | a1g | 2.03 | 2.19 | 1.91 | 1.82 |
| Perfluoro[5]asterane | D5h | a′1 | 2.43 | 2.59 | 2.39 | 2.33 |
| Perfluoronorbornadiene dimers | ||||||
| D2d-Perfluoro-NBD | D2d | a1 | 2.02 | 2.18 | 1.90 | 1.81 |
| D2h-Perfluoro-NBD | D2h | ag | 2.05 | 2.26 | 1.96 | 1.87 |
| Miscellaneous | ||||||
| Perfluorotetrahedrane | Td | a1 | 0.02 | 0.10 | 0.07 | 0.07 |
| Perfluorobicyclo[1.1.1]pentane | D3h | a | –0.03 | –0.05 | –0.13 | |
| Perfluorotwistane | D2 | a | 0.73 | 0.99 | 0.59 | 0.48 |
| Perfluoroadamantane | Td | a1 | 1.09 | 1.29 | 0.93 | 0.81 |
| Fullerenes | ||||||
| Perfluorododecahedrane | Ih | a1g | 3.44 | 3.54 | 3.52 | 3.46 |
| Perfluoro-C60 | Ih | a1g | 3.87 | 4.53 | 4.50 | |
Figure 1.
Ball-and-stick structures, neutral LUMOs, anion spin densities of the perfluorinated cage molecules studied.
All the perfluorinated prismanes exhibit sizable electron affinities, with the following order across all three functionals (the values in eV shown within parentheses are for OLYP): hexaprismane23,24 (2.15) > pentaprismane25,26 (1.98) > cubane27−29 (1.55) > prismane30,31 (0.97). The ordering suggests a pronounced size effect on the PCE: larger fluorinated cages result in greater stabilization of the encapsulated electron.
The three asteranes32−36 examined also exhibit relatively large electron affinities, with that of perfluoro[5]asterane (OLYP: 2.43 eV) and perfluoro[4]asterane (2.03 eV) greatly exceeding that of perfluoro[3]asterane (1.36 eV). Again, there appears to be a pronounced size effect.
The two Td systems examined, tetrafluorotetrahedrane37,38 and perfluoroadamantane,39−43 exhibit dramatically different electron affinities. For the former, the value is near zero, suggesting that the relatively tiny tetrahedral cage cannot effectively encapsulate an electron. The same also holds for perfluorinated bicyclo[1.1.1]pentane.44,45 In contrast, perfluorinated twistane46 (OLYP: 0.73) and adamantane (1.09 eV) exhibit moderate, positive EAs.
Among the molecules examined here, the largest adiabatic EAs have been calculated for perfluorinated dodecahedrane, C20F20,47−49 and buckministerfullerene, C60F60,50−52 the OLYP values being 3.44 and 3.87 eV, respectively. In each case, the spin density of the anion radical has the shape of a spheroidal shell within the interior of the polyhedral skeletons.
Concluding Remarks
Given the large number of polyhedral or cage-shaped molecules that have been synthesized and the vastly greater number that are theoretically possible, a clear conclusion from the present study is that electron encapsulation by their perfluorinated counterparts should be widely prevalent – indeed more the rule than the exception – and limited only by the accessibility of the compounds in question. The only exceptions appear to be the smallest cages such as tetrahedrane and bicyclo[1.1.1]pentane.
Acknowledgments
This work was supported by grant no. 324139 of the Research Council of Norway (A.G.) and grant nos. 129270 and 132504 of South African National Research Foundation (J.C.).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07374.
Optimized Cartesian coordinates (10 pages) (PDF)
The authors declare no competing financial interest.
Notes
The data underlying this study are available in the published article and its online Supplementary Material.
Supplementary Material
References
- Biffinger J. C.; Kim H. W.; DiMagno S. G. The polar hydrophobicity of fluorinated compounds. ChemBioChem 2004, 5, 622–627. 10.1002/cbic.200300910. [DOI] [PubMed] [Google Scholar]
- Johnson B. M.; Shu Y. Z.; Zhuo X.; Meanwell N. A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 2020, 63, 6315–6386. 10.1021/acs.jmedchem.9b01877. [DOI] [PubMed] [Google Scholar]
- Berger R.; Resnati G.; Metrangolo P.; Weber E.; Hulliger J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. 10.1039/c0cs00221f. [DOI] [PubMed] [Google Scholar]
- Brundle C. R.; Robin M. B.; Kuebler N. A.; Basch H. Perfluoro effect in photoelectron spectroscopy. I. Nonaromatic molecules. J. Am. Chem. Soc. 1972, 94, 1451–1465. 10.1021/ja00760a007. [DOI] [Google Scholar]
- Brundle C. R.; Robin M. B.; Kuebler N. A. Perfluoro effect in photoelectron spectroscopy. II. Aromatic molecules. J. Am. Chem. Soc. 1972, 94, 1466–1475. 10.1021/ja00760a008. [DOI] [Google Scholar]
- Van den Ham D. M. W.; Van der Meer D. Perfluoro effect in the photoelectron spectra of quinoline and isoquinoline. Chem. Phys. Lett. 1972, 15, 549–552. 10.1016/0009-2614(72)80368-3. [DOI] [Google Scholar]
- Decleva P.; Stener M.; Holland D. M. P.; Potts A. W.; Karlsson L. Perfluoro effects in the occupied and virtual valence orbitals of hexafluorobenzene. J. Phys. B: At., Mol. Opt. Phys. 2007, 40, 2939. 10.1088/0953-4075/40/14/012. [DOI] [Google Scholar]
- Irikura K. K. Sigma stellation: A design strategy for electron boxes. J. Phys. Chem. A 2008, 112, 983–988. 10.1021/jp710372p. [DOI] [PubMed] [Google Scholar]
- Wang Y. F.; Li Y.; Li Z. R.; Ma F.; Wu D.; Sun C. C. Perfluorinated exohedral potassium-metallofullerene K···CnFn (n = 20 or 60): partial interior and surface excess electron state. Theor. Chem. Acc. 2010, 127, 641–650. 10.1007/s00214-010-0763-1. [DOI] [Google Scholar]
- Sugiyama M.; Akiyama M.; Yonezawa Y.; Komaguchi K.; Higashi M.; Nozaki K.; Okazoe T. Electron in a cube: Synthesis and characterization of perfluorocubane as an electron acceptor. Science 2022, 377, 756–759. 10.1126/science.abq0516. [DOI] [PubMed] [Google Scholar]
- Jacobsen H.; Cavallo L. Re-evaluation of the Mn(salen) mediated epoxidation of alkenes by means of the B3LYP* density functional. Phys. Chem. Chem. Phys. 2004, 6, 3747–3753. 10.1039/b402188f. [DOI] [Google Scholar]
- Conradie M. M.; Conradie J.; Ghosh A. Capturing the spin state diversity of iron(III)-aryl porphyrins OLYP is better than TPSSh. J. Inorg. Biochem. 2011, 105, 84–91. 10.1016/j.jinorgbio.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Hirao H. Which DFT Functional Performs Well in the Calculation of Methylcobalamin? Comparison of the B3LYP and BP86 Functionals and Evaluation of the Impact of Empirical Dispersion Correction. J. Phys. Chem. A 2011, 115, 9308–9313. 10.1021/jp2052807. [DOI] [PubMed] [Google Scholar]
- Conradie J.; Ghosh A. DFT Calculations on the Spin-Crossover Complex Fe(salen)(NO): A Quest for the Best Functional. J. Phys. Chem. B 2007, 111, 12621–12624. 10.1021/jp074480t. [DOI] [PubMed] [Google Scholar]
- Siegbahn P. E. M.; Blomberg M. R. A. A Systematic DFT Approach for Studying Mechanisms of Redox Active Enzymes. Front. Chem. 2018, 6, 644. 10.3389/fchem.2018.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy N. C.; Cohen A. J. Left-right correlation energy. Mol. Phys. 2001, 99, 403–412. 10.1080/00268970010018431. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098–3100. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Miehlich B.; Savin A.; Stoll H.; Preuss H. Results Obtained with the Correlation Energy Density Functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. 10.1016/0009-2614(89)87234-3. [DOI] [Google Scholar]
- Reiher M.; Salomon O.; Hess B. A. Reparameterization of hybrid functionals based on energy differences of states of different multiplicity. Theor. Chem. Acc. 2001, 107, 48–55. 10.1007/s00214-001-0300-3. [DOI] [Google Scholar]
- Salomon O.; Reiher M.; Hess B. A. Assertion and validation of the performance of the B3LYP* functional for the first transition metal row and the G2 test set. J. Chem. Phys. 2002, 117, 4729–4737. 10.1063/1.1493179. [DOI] [Google Scholar]
- Grimme S.; Anthony J.; Ehrlich S.; Krieg H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Allinger N. L.; Eaton P. E. The geometries of pentaprismane and hexaprismane insights from molecular mechanics. Tetrahedron Lett. 1983, 24, 3697–3700. 10.1016/S0040-4039(00)94512-X. [DOI] [Google Scholar]
- Chou T. C.; Lin G. H.; Yeh Y. L.; Lin K. J. Synthetic Approach Towards Hexaprismane. A Novel Entry to Homosecohexaprismane Skeleton by Cage Enlargement. J. Chin. Chem. Soc. 1997, 44, 477–493. 10.1002/jccs.199700073. [DOI] [Google Scholar]
- Eaton P. E.; Or Y. S.; Branca S. J. Pentaprismane. J. Am. Chem. Soc. 1981, 103, 2134–2136. 10.1021/ja00398a062. [DOI] [Google Scholar]
- Eaton P. E.; Or Y. S.; Branca S. J.; Shankar B. R. The synthesis of pentaprismane. Tetrahedron 1986, 42, 1621–1631. 10.1016/S0040-4020(01)87579-7. [DOI] [Google Scholar]
- Eaton P. E.; Cole T. W. Cubane. J. Am. Chem. Soc. 1964, 86, 3157–3158. 10.1021/ja01069a041. [DOI] [Google Scholar]
- Eaton P. E.; Cole T. W. The cubane system. J. Am. Chem. Soc. 1964, 86, 962–964. 10.1021/ja01059a072. [DOI] [Google Scholar]
- Biegasiewicz K. F.; Griffiths J. R.; Savage G. P.; Tsanaktsidis J.; Priefer R. Cubane: 50 years later. Chem. Rev. 2015, 115, 6719–6745. 10.1021/cr500523x. [DOI] [PubMed] [Google Scholar]
- Wilzbach K. E.; Kaplan L. Photoisomerization of Tri-t-butylbenzenes. Prismane and Benzvalene Isomers. J. Am. Chem. Soc. 1965, 87, 4004–4006. 10.1021/ja01095a055. [DOI] [Google Scholar]
- Katz T. J.; Acton N. Synthesis of prismane. J. Am. Chem. Soc. 1973, 95, 2738–2739. 10.1021/ja00789a084. [DOI] [Google Scholar]
- Biethan U.; Gizycki U. V.; Musso H. Asterane. Tetrahedron Lett. 1965, 6, 477–1482. [Google Scholar]
- Musso H. Asterane. Angew. Chem., Int. Ed. 1965, 80, 290–291. [Google Scholar]
- Hoffmann V. T.; Musso H. Nonacyclo[10.8.0.02,11.04,9.04,19.06,17.07,16.09,14.014,19]icosan, ein doppeltes Tetraasteran. Angew. Chem., Int. Ed. 1987, 99, 1036–1037. 10.1002/ange.19870991008. [DOI] [Google Scholar]
- Ebel K.; Krüger H.; Musso H. Asterane, XX. Studien in der Pentaasteranreihe. Chem. Ber. 1988, 121, 323–326. 10.1002/cber.19881210219. [DOI] [Google Scholar]
- Bader A.; Ebel K.; Musso H.; Skuballa N. Asterane, XXI. Weitere Versuche zur Synthese des Pentaasterans. Chem. Ber. 1988, 121, 327–338. 10.1002/cber.19881210220. [DOI] [Google Scholar]
- Maier G. Tetrahedrane and cyclobutadiene. Angew. Chem., Int. Ed. 1988, 27, 309–332. 10.1002/anie.198803093. [DOI] [Google Scholar]
- Maier G.; Neudert J.; Wolf O.; Pappusch D.; Sekiguchi A.; Tanaka M.; Matsuo T. Tetrakis(trimethylsilyl)tetrahedrane. J. Am. Chem. Soc. 2002, 124, 13819–13826. 10.1021/ja020863n. [DOI] [PubMed] [Google Scholar]
- Schleyer P. v. R. A simple preparation of adamantane. J. Am. Chem. Soc. 1957, 79, 3292–3292. 10.1021/ja01569a086. [DOI] [Google Scholar]
- Fort R. C.; Schleyer P. v. R. Adamantane: consequences of the diamondoid structure. Chem. Rev. 1964, 64, 277–300. 10.1021/cr60229a004. [DOI] [Google Scholar]
- Schwertfeger H.; Fokin A. A.; Schreiner P. R. Diamonds are a chemist’s best friend: diamondoid chemistry beyond adamantane. Angew. Chem., Int. Ed. 2008, 47, 1022–1036. 10.1002/anie.200701684. [DOI] [PubMed] [Google Scholar]
- Robertson G.; Liu E. K. S.; Lagow R. J. Synthesis of perfluoroadamantane compounds by direct fluorination. J. Org. Chem. 1978, 43, 4981–4983. 10.1021/jo00420a020. [DOI] [Google Scholar]
- Li Q. S.; Feng X. J.; Xie Y.; Schaefer H. F. Perfluoroadamantane and its negative ion. J. Phys. Chem. A. 2005, 109, 1454–1457. 10.1021/jp040538h. [DOI] [PubMed] [Google Scholar]
- Wiberg K. B.; Williams V. Z. Jr. Bicyclo [1.1.1] pentane derivatives. J. Org. Chem. 1970, 35, 369–373. 10.1021/jo00827a018. [DOI] [Google Scholar]
- Kanazawa J.; Uchiyama M. Recent advances in the synthetic chemistry of bicyclo[1.1.1] pentane. Synlett 2019, 30, 1–11. 10.1055/s-0037-1610314. [DOI] [Google Scholar]
- Gauthier J.; Deslongchamps P. A new synthesis of twistane. Can. J. Chem. 1967, 45, 297–300. 10.1139/v67-052. [DOI] [Google Scholar]
- Ternansky R. J.; Balogh D. W.; Paquette L. A. Dodecahedrane. J. Am. Chem. Soc. 1982, 104, 4503–4504. 10.1021/ja00380a040. [DOI] [Google Scholar]
- Paquette L. A.; Ternansky R. J.; Balogh D. W.; Kentgen G. Total synthesis of dodecahedrane. J. Am. Chem. Soc. 1983, 105, 5446–5450. 10.1021/ja00354a043. [DOI] [Google Scholar]
- Schulman J. M.; Disch R. L. Theoretical studies of dodecahedrane. 2. Dodecahedrane, inclusion compounds, and fluorine derivatives. J. Am. Chem. Soc. 1978, 100, 5677–5681. 10.1021/ja00486a016. [DOI] [Google Scholar]
- Ol’ga V. B.; Galeva N. A. Direct fluorination of fullerenes. Russ. Chem. Rev. 2000, 69, 609–621. 10.1070/RC2000v069n07ABEH000579. [DOI] [Google Scholar]
- Holloway J. H.; Hope E. G.; Taylor R.; Langley G. J.; Avent A. G.; Dennis T. J.; Hare J. P.; Kroto H. W.; Walton D. R. Fluorination of buckminsterfullerene. J. Chem. Soc. Chem. Commun. 1991, 966–969. 10.1039/c39910000966. [DOI] [Google Scholar]
- Ravaine S.; Agricole B.; Mingotaud C.; Cousseau J.; Delhaes P. Langmuir and Langmuir-Blodgett films of a perfluoro C60 derivative. Chem. Phys. Lett. 1995, 242, 478–482. 10.1016/0009-2614(95)00756-T. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.