Abstract
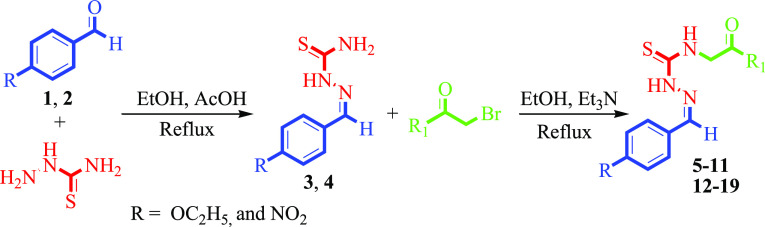
The current research reports the synthesis of 14 para-substituted thiosemicarbazone derivatives in good to excellent yields using standard procedures. Initially, 4-ethoxybenzaldehyde (1) and 4-nitrobenzaldehyde (2) were refluxed with thiosemicarbazide in the presence of acetic acid in ethanol for 4–5 h. Then, various substituted phenacyl bromides were treated with the desired thiosemicarbazones (3 and 4) in the presence of triethylamine in ethanol with constant stirring for 5–6 h. The resulting derivatives were confirmed through electron impact mass spectrometry and 1H NMR spectroscopy and evaluated for anticholinesterase inhibitory activity. Among the series, four compounds, 19, 17, 7, and 6, showed potent inhibitory activity against the acetylcholinesterase (AChE) enzyme, having IC50 values of 110.19 ± 2.32, 114.57 ± 0.15, 140.52 ± 0.11, and 160.04 ± 0.02 μM, respectively, compared with standard galantamine (IC50 = 104.5 ± 1.20 μM). Similarly, compounds 19 (IC50 = 145.11 ± 1.03 μM), 9 (IC50 = 147.20 ± 0.09 μM), 17 (IC50 = 150.36 ± 0.18 μM), and 6 (IC50 = 190.21 ± 0.13 μM) were the most excellent inhibitors of butyrylcholinesterase (BChE) when compared with the standard drug galantamine (IC50 = 156.8 ± 1.50 μM). In silico studies were accomplished on the produced derivatives in order to explain the binding interface of compounds with the active sites of AChE and BChE enzymes.
Introduction
Compounds possessing at least one carbon–nitrogen double bond are named Schiff’s bases.1 Schiff’s bases have been known since 1864; they were first discovered by Hugo Schiff through the condensation of primary amines with carbonyl compounds (aldehydes and ketones).2,3 The fundamental feature of these analogues is the azomethine group, which has a general formula of RHC = N–R1, (R and R1 may be alkyl, aryl, or heterocyclic groups). These compounds are also known as azomethine, imines, or anils.4−6 Schiff’s bases are the most important organic compounds with a variety of applications; these compounds are extensively used as catalysts in different organic reactions,7 as stabilizers,8 in the polymer industry,9 as pesticides,10 and also as dyes.11 Likewise, these compounds also possess various biological or pharmacological activities, like anti-inflammatory,12 anti-HIV,13 antiurease,14 anticholinesterase,15 antifungal,16 antioxidant,17 and α-glucosidase activities.18 One more application of imine linkage is in vision chemistry, where the link is formed between the active vitamin B6 (pyridoxal phosphate) and the amino acids for the purpose of transamination.19−21 Imine-containing compounds have the potential to form a defensive coating, which makes the surface safe from corrosion.22
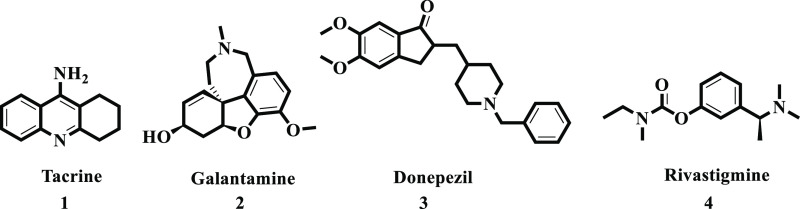
Alzheimer’s disease (AD) is an enlightened neurodegeneration leading to a decline of mental and physical health.23−25 Basically, it is found in elders frequently and is considered the most common form of dementia.26 The typical reason for AD is uncertain, though the cholinergic hypothesis characterizes it as the loss or damage of neurotransmitters in the brain which causes advanced mental impairment.27 Thus, drugs to treat AD are based on their ability to inhibit cholinesterase enzymes, that is, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE).28,29 Nowadays, various drugs are used for the treatment of AD, including rivastigmine, donepezil, galantamine, and tacrine (Figure 1). Synthetic organic chemists are working on new, chief, effective, and safe drugs for AD.30,31 In the near past, our research group has reported imine-containing derivatives with promising biological activities.32−35 The present work is mainly focused on the synthesis of various thiosemicarbazone derivatives with anticholinesterase inhibitory potential.
Figure 1.
Currently available marketed drugs as cholinesterase inhibitors.
Experimental Section
Materials and Methods
The chemicals, solvents, and reagents (4-ethoxybenzaldehyde CAS no 173606 and 4-nitrobenzaldehyde CAS no 130176) utilized in the current study were of analytical grade (98–99% pure) and purchased from Sigma-Aldrich (St Louis, MO, USA). The analytical grade chemicals, solvents, and reagents utilized in the current study were purchased from Sigma-Aldrich, MO, USA. Precoated aluminum sheets (silica gel 60F-254, Merck, Germany) were used for thin layer chromatography (TLC), and they were visualized with the help of UV light at 254 nm. A MAT312 spectrophotometer was used to obtain electron impact mass spectra, while an AVANCE AV 400 MHz was used to obtain NMR spectra of the synthesized compounds.
General Procedure for the Synthesis of Para-Substituted Carbothioamides (3 and 4)
In a 100 mL round-bottomed flask, 3 g of para-substituted benzaldehyde (4-nitrobenzaldehyde and 4-ethoxybenzaldehyde) was dissolved in 15 mL absolute ethanol containing a catalytic amount of glacial acetic acid. The reaction was stirred for 20–30 min; then, 1.8 g of thiosemicarbazide was added to it and refluxed for 3–4 h with constant stirring. The progress of the reaction was checked with the help of TLC in a 30% polar system of ethyl acetate and n-hexane. After completion, the reaction mixture was cooled to room temperature and poured into a beaker containing crushed ice; formed precipitates were filtered, washed with excess of water, dried under vacuum, and collected for further reactions. The synthesized analogues were confirmed with the help of electron impact mass spectrometry (EI-MS) and 1H NMR spectroscopy.
General Procedure for the Synthesis of Carbothioamide Derivatives (5–19)
The resultant para-substituted thiosemicarbazones (3 and 4) were further treated with various substituted phenacyl bromides in a basic medium using ethanol as a solvent. In a typical reaction, thiosemicarbazones were dissolved in absolute ethanol containing a catalytic amount of triethylamine, and the reaction was stirred for 30 min. Then, various substituted phenacyl bromides were added to it and further refluxed for 4–5 h with constant stirring. The progress of the reaction was monitored with the help of TLC using a solvent system of ethyl acetate and n-hexane (3:7). The mixture was poured into a beaker containing ice-cold water after completion. Precipitates formed were filtered, washed with excess of water, dried under air, and recrystallized with ethanol to obtain pure products. All the synthesized analogues were structurally deduced with the help of EI-MS and 1H NMR spectroscopy.
Anti-cholinesterase Inhibition Assay
The anti-cholinesterase activity of the obtained products was accomplished according to the described procedure.36 Electric eel AChE (type-VI-S), potassium phosphate buffer (pH 8.0), equine BChE, butyryl thiocholine iodide, 5,5-dithio-bis-nitrobenzoic acid (DTNB), galantamine from Lycoris sp., and acetyl thiocholine iodide, were used in the present study.
Spectrophotometric Ellman’s technique was used to carry out the AChE and BChE assays using butyryl thiocholine iodide and acetyl thiocholine iodide as substrates. Two mixtures having 5 μL of AChE (0.03 μg/mL) and 5 μL of BChE (0.01 μg/mL) particularly and each one added with 205 μL of samples (125–1000 μg/mL) and DTNB (5 μL) were incubated for 15 min at 30 °C. After 15 min, 5 μL of substrates was added to the reaction mixture, and the reaction was detected for 4 min at 412 nm by using a UV–visible spectrophotometer. The presence of yellow color in the mixture showed the creation of the 5-thio-2-nitrobenzoate anion by reaction of thiocholines and DTNB. In the absence of samples and enzymes, nonenzymatic hydrolysis of substrate was also examined. The reaction mixture was considered as a control in the absence of samples. Percent inhibition and enzyme activities were calculated through the following formulas
where V is the rate of reaction in the presence of an inhibitor and Vmax is the rate of reaction without the inhibitor.
Molecular Docking Assay
Molecular docking studies are mainly used to study the intermolecular interactions of a chemical/molecule with a protein/enzyme or biological target to find its drug-like properties. In the current work, we have studied the intermolecular interactions of synthesized compounds with AChE and BchE via molecular docking.37 The crystal structures of human AChE and BChE with PDB codes 4EY6 and 6QAE were downloaded from the protein data bank by visiting www.rcsb.com. These proteins were prepared and minimized/stabilized in MOE 2016. The compounds 6, 7, 9, 17, and 19 having effective IC50 values against these targets, along with the reference molecule galantamine, were docked into the active site of these minimized proteins using the default docking protocol implemented in MOE 2016. Pymol was used for ligand interaction and visualization.
Spectral Interpretation of the Synthesized Compounds (5–19)
2-(4-Ethoxybenzylidene)-N-(2-oxo-2-(m-tolyl)ethyl)hydrazine-1-carbothioamide (5)
White powder; yield: 89%; 1H NMR (400 MHz, DMSO-d6): δ 12.05 (s, 1H, −NH), 8.01 (s, 1H, −CH=N−), 7.85 (d, 1H, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.64–7.62 (m, 4H, Ar-H, H-2′, H-4′, H-5′, H-6′), 7.41 (s, 1H, −NH), 7.03 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-4), 4.13 (q, J = 8.0 Hz, 2H, −OCH2CH3), 1.39 (t, J = 8.0 Hz, 3H, −OCH2CH3), 1.30 (s, 3H, −CH3); EI-MS m/z (rel. abund, %): 355 (M+, 100), 313.9 (15), 163.9 (22), 149.9 (50), 120.0 (41), 104.0 (22).
N-(2-(4-Bromophenyl)-2-oxoethyl)-2-(4-ethoxybenzylidene)hydrazine-1-carbothioamide (6)
White powder; yield: 90%; 1H NMR (400 MHz, CDCl3): δ 7.78 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.31 (d, J = 8.0 Hz, 2H, Ar-H, H-2′, H-6′), 7.26 (d, J = 8.0 Hz, 2H, Ar-H, H-3′, H-5′), 6.89 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-5), 6.82 (s, 1H, −NH), 4.12 (q, J = 8.0 Hz, 2H, −OCH2CH3), 2.38 (s, 2H, −CH2), 1.47 (t, J = 8.0 Hz, 3H, −OCH2CH3); EI-MS m/z (rel. abund, %): 419 (M+, 2), 327.2 (2), 235.3 (8), 219.4 (5), 185.2 (21), 171.2 (35).
N-(2-(4-Chlorophenyl)-2-oxoethyl)-2-(4-ethoxybenzylidene)hydrazine-1-carbothioamide (7)
Off white powder; yield: 84%; 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 8.0 Hz, 2H, Ar-H, H-2′, H-6′), 7.78 (s, 1H, −NH), 7.44–7.41 (m, 4H, Ar-H, H-2, H-6, H-3′, H-5′), 6.93 (d, J = 7.2 Hz, 2H, Ar-H, H-3, H-5), 6.87 (s, 1H, −NH), 4.13 (q, J = 8.0 Hz, 2H, −OCH2CH3), 1.51 (t, J = 8.0 Hz, 3H, −OCH2CH3); EI-MS m/z (rel. abund, %): 376 (M+, 99), 285.3 (4), 193.1 (53), 182.1 (49), 155.4 (8), 103.4 (3), 91.1 (33), 65.1 (3).
2-(4-Ethoxybenzylidene)-N-(2-oxo-2-phenylethyl)hydrazine-1-carbothioamide (8)
Yellow powder; yield: 82%; 1H NMR (400 MHz, DMSO-d6): δ 11.17 (s, 1H, −CH=N−), 7.88 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.46–7.35 (m, 4H, Ar-H, H-2′, H-3′, H-5′, H-6′), 6.90–6.87 (m, 3H, Ar-H, H-4′, H-3, H-5), 4.12 (q, J = 8.0 Hz, 2H, −OCH2CH3), 2.67 (s, 2H, −CH2), 1.51 (t, J = 8.0 Hz, 3H, −OCH2CH3); EI-MS m/z (rel. abund, %): 341 (M+,100), 312 (57), 163 (72), 133 (69).
2-(4-Ethoxybenzylidene)-N-(2-(2-hydroxyphenyl)-2-oxoethyl)hydrazine-1-carbothioamide (9)
Pale yellow powder; yield: 78%; 1H NMR (400 MHz, CDCl3): δ 11.23 (s, 1H, −CH=N−), 9.66 (s, 1H, −OH), 7.91 (s, 1H, −NH), 7.63 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.47 (m, 1H, Ar-H, H-4′), 7.24 (m, 1H, Ar-H, H-5′), 6.96 (d, J = 8.0 Hz, 2H, Ar-H, H-3′, H-6′), 6.40 (s, 1H, −NH), 4.14 (q, J = 8.0 Hz, 2H, −OCH2CH3), 1.49 (t, J = 8.0 Hz, 3H, −OCH2CH3); EI-MS m/z (rel. abund, %): 357 (M+, 12), 327.3 (3), 311.4 (3), 297.4 (4), 277.3 (5), 263.3 (4), 249.3 (4), 235.5 (8), 219.3 (10), 185.2 (66), 171.2 (58).
N-(2-(2,4-Dichlorophenyl)-2-oxoethyl)-2-(4-ethoxybenzylidene)hydrazine-1-carbothioamide (10)
Reddish powder; yield: 88%; 1H NMR (400 MHz, CDCl3): δ 8.03 (s, 1H, −CH=N−), 8.0 (s, 1H, −NH), 7.89 (d, J = 7.2 Hz, 2H, Ar-H, H-2, H-6), 7.78 (d, J = 8.0 Hz, 1H, Ar-H, H-6), 7.64 (s, 1H, Ar-H, H-3′), 7.36 (d, J = 8.0 Hz, 2H, Ar-H, H-5′), 7.03 (d, J = 7.2 Hz, 2H, Ar-H, H-3, H-5), 7.0 (s, 1H, −NH), 4.05 (q, J = 8.0 Hz, 2H, −OCH2CH3), 2.24 (s, 2H, −CH2), 1.34 (t, J = 8.0 Hz, 3H, −OCH2CH3); EI-MS m/z (rel. abund, %): 411 (M+2, 66), 409.9 (M+, 100), 374 (58), 340 (81), 163 (68).
N-(2-([1,1′-Biphenyl]-4-yl)-2-oxoethyl)-2-(4-ethoxybenzylidene)hydrazine-1-carbothioamide (11)
Brown powder; yield: 82%; 1H NMR (400 MHz, CDCl3): δ 7.96 (d, J = 8.0 Hz, 2H, Ar-H, H-2′, H-6′), 7.69 (t, J = 8.0 Hz, 3H, Ar-H, H-3″, H-4″, H-5″), 7.62 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.50 (s, 1H, −NH), 7.46 (d, J = 8.0 Hz, 2H, Ar-H, H-3′, H-5′), 7.41 (d, J = 8.0 Hz, 2H, Ar-H, H-2″, H-6″), 6.94 (s, 1H, −NH), 6.86 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-5), 4.08 (q, J = 8.0 Hz, 2H, −OCH2CH3), 1.48 (t, J = 8.0 Hz, 3H, −OCH2CH3); EI-MS m/z (rel. abund, %): 417 (M+,100), 222 (85), 195 (63), 163 (80).
2-(4-Nitrobenzylidene)-N-(2-oxo-2-(p-tolyl)ethyl)hydrazine-1-carbothioamide (12)
White powder; yield: 86%; 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 7.2 Hz, 2H, Ar-H, H-3, H-5), 8.03 (s, 1H, −CH=N−), 8.01 (s, 1H, −NH), 7.97 (d, J = 7.2 Hz, 2H, Ar-H, H-2, H-6), 7.44 (d, J = 8.0 Hz, 2H, Ar-H, H-3′, H-5′), 6.72 (d, J = 8.0 Hz, 2H, Ar-H, H-2′, H-6′), 4.85 (s, 2H, −CH2), 2.41 (s, 3H, −CH3); EI-MS m/z (rel. abund, %): 356 (M+, 100), 310 (68), 221 (85), 163 (85).
2-(4-Nitrobenzylidene)-N-(2-(4-nitrophenyl)-2-oxoethyl)hydrazine-1-carbothioamide (13)
Orange powder; yield: 77%; 1H NMR (400 MHz, CDCl3): δ 8.40 (d, J = 8.0 Hz, 2H, Ar-H, H-3′, H-5′), 8.31 (d, J = 8.0 Hz, 2H, Ar-H, H-2′, H-6′), 8.27 (d, J = 7.2 Hz, 2H, Ar-H, H-3, H-5), 8.03 (s, 1H, −CH=N−), 8.01 (s, 1H, −NH), 7.97 (d, J = 7.2 Hz, 2H, Ar-H, H-2, H-6), 4.85 (s, 2H, −CH2); EI-MS: m/z (rel. abund, %): 387 (M+, 100), 341 (58), 252 (84), 163 (97).
N-(2-(4-Chlorophenyl)-2-oxoethyl)-2-(4-nitrobenzylidene)hydrazine-1-carbothioamide (14)
Off white powder; yield: 79%; 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-5), 8.06 (d, J = 7.2 Hz, 2H, Ar-H, H-2′, H-6′), 8.03 (s, IH, −CH=N−), 8.00 (s, 1H, −NH), 7.97 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.68 (d, J = 7.2 Hz, 2H, Ar-H, H-3′, H-5′), 7.00 (s, 1H, −NH), 4.85 (s, 2H, −CH2); EI-MS m/z (rel. abund, %): 422 (M2+, 98), 420 (M+, 100), 375 (77), 341 (60) 223 (77).
N-(2-(2-Hydroxyphenyl)-2-ozoethyl)-2-(4-nitrobenzylidene)hydrazine-1-carbothioamide (15)
Yellowish powder; yield: 80%; 1H NMR (400 MHz, DMSO-d6): δ 11.03 (s, 1H, −CH=N−), 9.56 (s, 1H, −OH), 7.93 (s, 1H, −NH), 7.64 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.45 (m, 1H, Ar-H, H-4′), 7.34 (m, 1H, Ar-H, H-5′), 6.96 (d, J = 8.0 Hz, 2H, Ar-H, H-3′, H-6′), 6.40 (s, 1H, −NH), 4.14 (q, J = 8.0 Hz, 2H, −OCH2CH3), 1.49 (t, J = 8.0 Hz, 3H, −OCH2CH3); EI-MS m/z (rel. abund, %): 358 (M+, 100), 341 (58), 223 (100).
N-(2-(2,4-Dichlorophenyl)-2-ozoethyl)-2-(4-nitrobenzylidene)hydrazine-1-carbothioamide (16)
White powder; yield: 82%; 1H NMR (400 MHz, DMSO-d6): δ 12.07 (s, 1H, −CH=N−), 7.83 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-5), 7.80 (m, 2H, Ar-H, H-2, H-6), 7.71 (m, 2H, Ar-H, H-5′, H-6′), 7.70 (s, 1H, −NH), 7.69 (s, 1H, −NH), 7.24 (s, 1H, Ar-H, H-3′), 3.17 (s, 2H, −CH2); EI-MS m/z (rel. abund, %): 410 (M+2,66), 410 (M+,100), 375 (62), 341 (86), 223 (46).
N-(2-(2-Bromophenyl)-2-oxoethyl)-2-(4-nitrobenzylidene)hydrazine-1-carbothioamide (17)
Yellow powder; yield: 76%; 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-5), 8.03 (s, 1H, −CH=N−), 8.00 (s, 1H, −NH), 7.97 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.79 (d, J = 7.2 Hz, 1H, Ar-H, H-6′), 7.39–7.38 (m, 2H, Ar-H, H-4′, H-5′), 7.65 (d, J = 8.0 Hz, 1H, Ar-H, H-3′), 4.85 (s, 2H, −CH2); EI-MS m/z (rel. abund, %): 422 (M+2, 98), 420 (M+, 100), 341 (77), 223 (61).
N-(2-([1,1′-Biphenyl]-4-yl)-2-ozoethyl)-2-(4-nitrobenzylidene)hydrazine-1-carbothioamide (18)
Off white powder; yield: 68%; 1H NMR (400 MHz, CDCl3): δ 7.98 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-5), 7.83 (s, 1H, −NH), 7.66 (d, J = 8.0 Hz, 2H, Ar-H, H-2′, H-6′), 7.59 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.43 (m, 4H, Ar-H, H-3′, H-5′, H-2″, H-6″), 7.19 (t, J = 8.0 Hz, 3H, Ar-H, H-3″, H-4″, H-5″), 4.85 (s, 2H, −CH2); EI-MS m/z (rel. abund, %): 418 (M+, 36), 376.2 (10), 284.1 (9), 199.1 (58), 178.0 (12), 104.9 (100), 77.0 (30), 51.0 (8).
N-(2-(Chlorophenyl)-2-ozoethyl)-2-(4-nitrobenzylidene)hydrazine-1-carbothioamide (19)
Yellow powder; yield: 73%; 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 8.0 Hz, 2H, Ar-H, H-3, H-5), 8.03 (s, 1H, −NH), 8.00 (s, 1H, −NH), 7.97 (d, J = 8.0 Hz, 2H, Ar-H, H-2, H-6), 7.84 (d, J = 7.2 Hz, 1H, Ar-H, H-6′), 7.44 (t, J = 8.0 Hz, 1H, Ar-H, H-4′), 7.38–7.32 (m, 2H, Ar-H, H-3′, H-5′), 7.00 (s, 1H, −NH), 4.85 (s, 2H, −CH2); EI-MS m/z (rel. abund, %): 378 (M+2, 37), 376 (100), 341 (70), 223 (51).
Results and Discussion
Chemistry
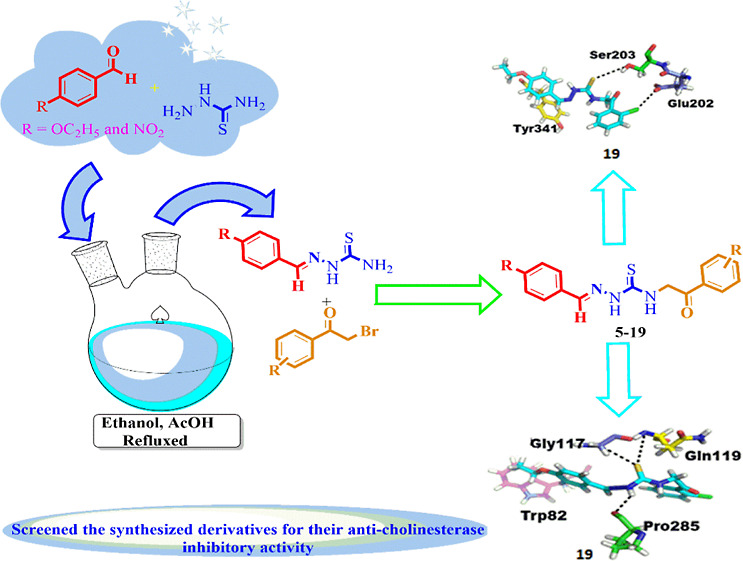
To discover various pharmacologically/biologically potent cholinesterase inhibitors, efforts were made to synthesize various novel derivatives of para-substituted benzaldehydes in good to excellent yields through multistep reactions. Initially, 4-ethoxybenzaldehyde (1) and 4-nitrobenzaldehyde (2) were refluxed with thiosemicarbazide in the presence of acetic acid in ethanol for 4–5 h. Then, various substituted phenacyl bromides were treated with the desired thiosemicarbazones (3 and 4) in the presence of triethylamine in ethanol with constant stirring for 5–6 h (Scheme 1). The reaction progress was monitored with the help of TLC using a 30% polar solvent system of ethyl acetate and n-hexane (3:7). After completion, the reaction mixtures were cooled to room temperature and poured into a beaker containing ice-cold distilled water. Precipitates were formed, which were filtered, washed with excess of water, dried under air, recrystallized with absolute ethanol, and collected.
Scheme 1. Thiosemicarbazone Derivatives of Para-Substituted Benzaldehydes (5–19).
In Vitro Anti-cholinesterase Inhibitory Activity
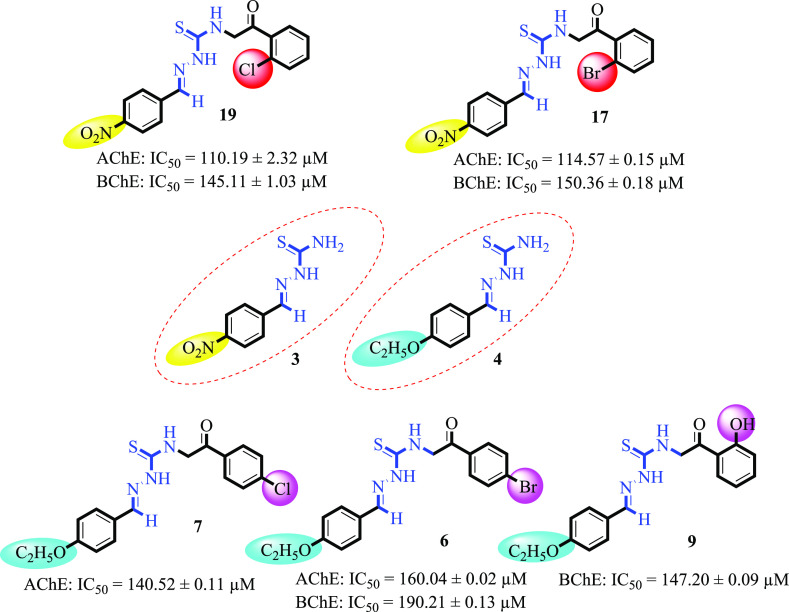
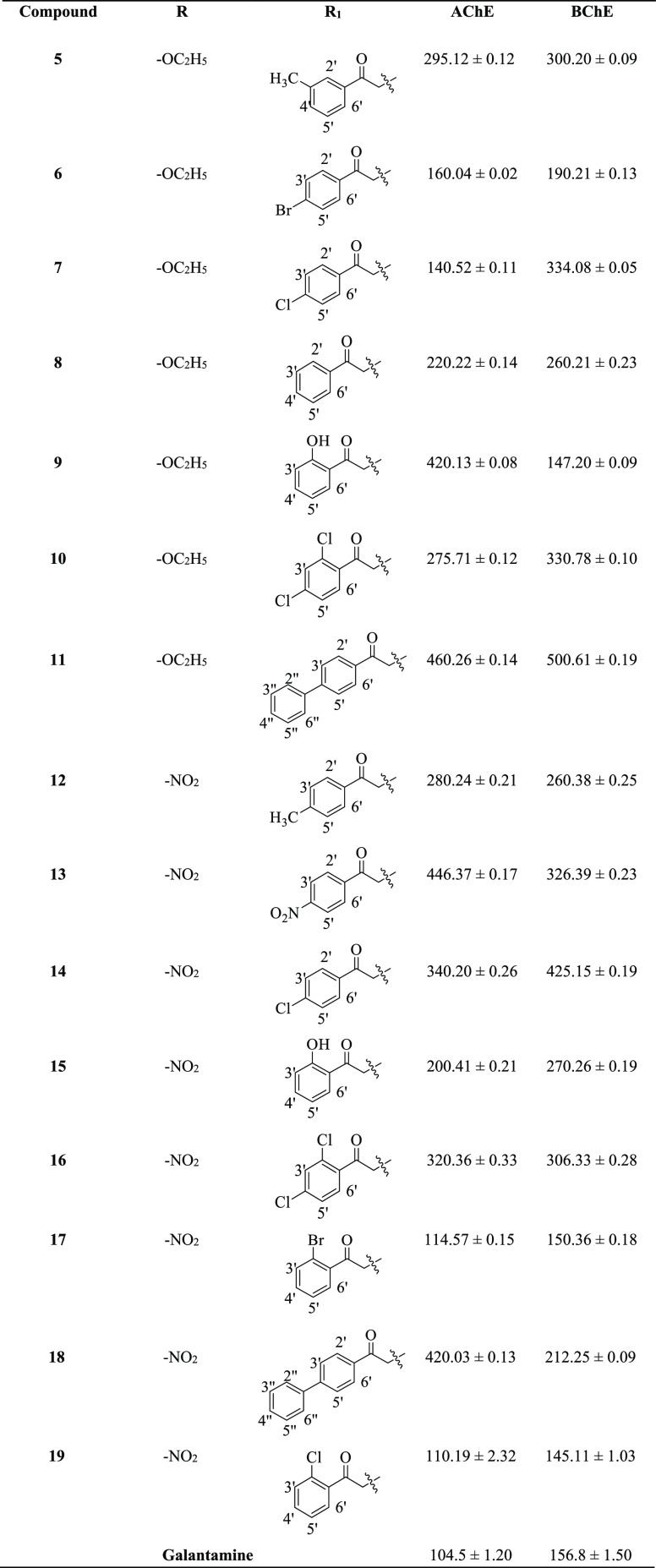
All the synthesized derivatives (5–19) were screened for their anti-cholinesterase inhibitory potential and compared with the standard drug galantamine. All the compounds showed good to moderate inhibitory activity. Among the synthesized series, four compounds 19, 17, 7, and 9 showed potent inhibitory activity against the AChE enzyme, having IC50 values of 110.19 ± 2.32, 114.57 ± 0.15, 140.52 ± 0.11, and 160.04 ± 0.02 μM, respectively, compared with standard galantamine (IC50 = 104.5 ± 1.20 μM). Similarly, compounds 19 (IC50 = 145.11 ± 1.03 μM), 9 (IC50 = 147.20 ± 0.09 μM), 17 (IC50 = 150.36 ± 0.18 μM), and 6 (IC50 = 190.21 ± 0.13 μM) (Figure 2) were the most excellent inhibitors of BChE when compared with standard drug galantamine (IC50 = 156.8 ± 1.50 μM). However, compounds 15 (IC50 = 200.41 ± 0.21 μM), 8 (IC50 = 220.22 ± 0.14 μM), 10 (IC50 = 275.71 ± 0.12 μM), 12 (IC50 = 280.24 ± 0.21 μM), and 5 (IC50 = 295.12 ± 0.12 μM) showed good inhibitory potential against the AChE enzyme. Furthermore, four compounds 18 (IC50 = 212.25 ± 0.09 μM), 8 (IC50 = 260.21 ± 0.23 μM), 12 (IC50 = 260.38 ± 0.25 μM), and 15 (IC50 = 270.26 ± 0.19 μM) displayed good inhibitory potential toward the BChE enzyme, while the remaining compounds demonstrated less enzyme inhibitory activity (Table 1).
Figure 2.
Most active compounds of the series.
Table 1. AChE and BChE Inhibitory Activities of Compounds (5–19)a.
Standard positive control galanthamine was used, and data are mean ± SEM.
The most active compound in the series against AChE and BChE is compound 19 having IC50 values of 110.19 ± 2.32 μM and 145.11 ± 1.03 μM, respectively. The potency of this compound might be due to the attachment of a chlorine atom in the ortho position; the activity of compound 19 against BChE is near that of standard galantamine. Similarly, compound 17 was found to be the second most active compound against the desired enzymes, with IC50 values of 114.57 ± 0.15 and 150.36 ± 0.18 μM. The highest activity of the compound may be due to the presence of a bromine atom in the ortho position. Compound 3 was the third active compound against the AChE enzyme with an IC50 value of 140.52 ± 0.11 μM, while being less active against BChE. The activity of this compound could be due to the presence of a chlorine atom at the para position of the benzene ring. Galantamine was used as a standard drug for AChE and BChE enzymes, with IC50 values of 104.5 ± 1.20 and 156.8 ± 1.50 μM, respectively.
Molecular Docking Analysis
Molecular docking was performed in order to explore in vitro the intermolecular interactions of para-substituted thiosemicarbazone derivatives with human AChE and BchE enzymes. Among the series, the most active derivatives were docked into the active site of these targets, which had effective IC50 values in an in vitro assay along with the reference substrate galantamine. All these compounds had stronger and comparable interactions with target proteins, which means that they may have the ability to alter the chemistries of these proteins and might act as an anticholinesterase.
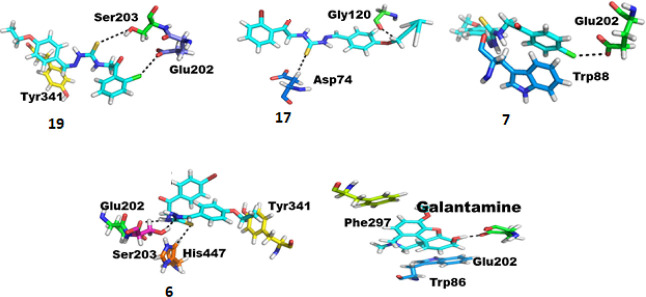
For AChE, among these derivatives, 19, 17, 7, and 6 have been found with much stronger and comparable interactions as compared to galantamine. The docking scores of all the compounds 5–19 against AChE are given in Table S2. Compound 19 has stronger interactions as it has a highly electronegative chlorine on the terminal aromatic ring, which forms a stronger hydrogen bond with the oxygen of residue Glu202. In addition, this compound forms two other stronger intermolecular interactions, that is, one hydrogen bond between sulfur of the ligand and the oxygen of residue Ser203, followed by a π bond between the delocalized electrons of the aromatic ring of the compound with alpha-carbon of Tyr341 as shown in Figure 3. These interactions made the compound a reasonable inhibitor of the enzyme. Similarly, compound 17 has also formed a stronger bond with AChE. Compound 17 is bonded to residues Gly120 and Asp74 of the active site of the enzyme. With the residues the compound has formed strong hydrogen bonds, Gly120 has an electronegative oxygen which interacts with the hydrogen of the main chain of the compound. In the same way, the other residue, Asp70, is linked with the sulfur of the compound via hydrogen bonding, as shown in Figure 3. These interactions may alter/inhibit the activity of the enzyme. Likewise, we have compound 7 with a considerable IC50 value and good docking interactions. This compound also forms stronger molecular interactions with the target but is considerably weaker than the former two, as in the former compounds there have been at least two hydrogen bonds found in each case, but here we have only one hydrogen with a considerably weaker π bond. Still, the compound has shown interactions and might have the ability to disturb the activity of the enzyme. Compound 7 forms a hydrogen bond by its chlorine with the oxygen of residue Glu202 and the delocalized electrons of the aromatic ring of the compound form a π–π interaction with a similar aromatic ring of Trp86 as shown in Figure 3. Except for these compounds, there is still another compound (6) with attractive docking results and effective interactions with the active site residues of the enzyme. The compound formed three hydrogen bonds and one H−π interaction, making this compound a possible inhibitor of the enzyme. Among the three hydrogen bonds, one hydrogen bond is formed between the nitrogen of the ligand and the oxygen of Glu202, another one is formed between the nitrogen of the ligand and the oxygen of Ser203, and the third hydrogen bond is formed between the sulfur of the ligand and the alpha carbon of His447. There is also an H−π interaction between the carbons of the ligand and the aromatic ring of Tyr341, as shown in Figure 3.
Figure 3.
Protein–ligand interaction of AChE with compounds 19, 17, 7, and 6 in comparison with galantamine.
Likewise, in AChE, the synthesized derivatives have also been found equally effective against BChE and showed considerable interactions with the enzymes. The docking scores of all the compounds 5–19 against BChE are given in Table S3. Compound 19 has been found to be very much effective against the enzyme; this compound might have formed the strongest interactions of this study. Compound 19 is bonded to four active site residues of the enzyme. Three bonds were strong intermolecular hydrogen bonds, and one was H−π interactions. Among the three hydrogen bonds, one was formed between the nitrogen of the ligand and the oxygen of Pro285, the second hydrogen bond formed by the sulfur of the compound with the hydrogen attached to the alpha carbon of Gly117, and the third hydrogen bond formed by the sulfur of the ligand with the nitrogen of Glu119. The H−π bond has been formed by the carbon of the ligand with an aromatic ring of Trp82 as shown in Figure 4. Such strong and stable linkages may be considered as the best inhibitory source. Along with this compound, there was still another compound which also showed much stronger and more stable interactions during the course of docking, compound 17. Compound 17 also formed four bonds similar to compound 15, but they were slightly weaker than the former as the latter has two hydrogen bonds and two π interactions, whereas the former has three hydrogen bonds. Anyhow, the latter is also of much importance as it has stronger interactions than galantamine. Compound 17 has two adjacent highly electronegative nitrogen atoms which both formed hydrogen bonds with the oxygen of Asp70, Along with the terminal aromatic ring of the compound formed two π interactions, one π–π interaction with 5-ring of Trp82 and one with 6-ring of Trp82, as shown in Figure 4. There was still another base with strong interactions with active site residues of the enzyme; compound 7 also formed three intermolecular interactions, indicating the ability of the inhibition. Among these three bonds, one was a hydrogen bond formed between the oxygen of the compound and His438; besides this, it has two π interactions, both formed by the aromatic ring of the compound. There was a π–H interaction formed by the aromatic ring of the compound with hydrogen attached to the alpha carbon of Phe329 and a π–π interaction between the same ring of the compound and the benzene ring of Tyr332, as shown in Figure 4.
Figure 4.
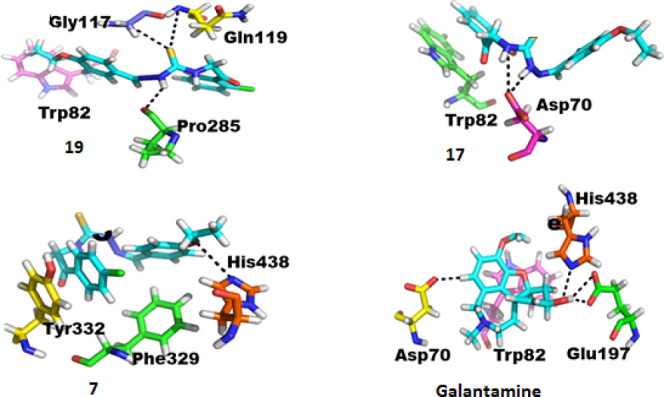
Protein–ligand interactions of BChE with compounds 19, 17, and 7 in comparison with galantamine.
Conclusions
A novel series of para-substituted thiosemicarbazone derivatives were synthesized in good to excellent yields using standard procedures. The resulting compounds were structurally elucidated with the help of EI-MS and 1H NMR spectroscopy and finally evaluated for their anticholinesterase inhibitory potential. Four compounds showed excellent AChE inhibitory activity in the range of 110.19 ± 2.32 to 160.04 ± 0.02 μM compared with the standard drug galantamine (IC50 = 104.5 ± 1.20 μM). Nevertheless, the other four compounds displayed potent BChE inhibitory activity in the range of 145.11 ± 1.03 to 190.21 ± 0.13 μM when compared with galantamine (156.8 ± 1.50 μM). In silico studies were accomplished on the synthesized analogues in order to explain the binding interface of compounds with the active sites of AChE and BChE enzymes.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c08108.
1H-NMR and EI-MS spectra of the synthesized compounds; currently available marketed drugs as cholinesterase inhibitors; most active compounds; protein ligand interactions of AChE and BChE with compounds 19, 17, 7, and 6 in comparison with galantamine; thiosemicarbazone derivatives of para-substituted benzaldehydes; AChE and BChE inhibitory activities of compounds; and docking scores of compounds 5–19 against AChE and BChE (PDF)
Funding was provided by the Dean of Scientific Research at Umm Al-Qura University under grant code: 22UQU4331128DSR25.
The authors declare no competing financial interest.
Supplementary Material
References
- Mohammed Khan K.; Shah Z.; Uddin Ahmad V.; Khan M.; Taha M.; Rahim F.; Ali S.; Ambreen N.; Perveen S.; Iqbal Choudhary M. 2,4,6-Trichlorophenylhydrazine Schiff Bases as DPPH Radical and Super Oxide Anion Scavengers. Med. Chem. 2012, 8, 452–461. 10.2174/1573406411208030452. [DOI] [PubMed] [Google Scholar]
- Scavenging D. R. Synthesis of benzophenone hydrazone analogs and their DPPH radical scavenging and urease inhibitory activities. J. Chem. Soc. Pak. 2015, 37, 479. [Google Scholar]
- Mohammed Khan K.; Rahim F.; Ambreen N.; Taha M.; Khan M.; Jahan H.; Shaikh A.; Iqbal S.; Perveen S.; Iqbal Choudhary M. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013, 9, 588–595. 10.2174/1573406411309040013. [DOI] [PubMed] [Google Scholar]
- Khan K. M.; Taha M.; Rahim F.; Fakhri M. I.; Jamil W.; Khan M.; Rasheed S.; Karim A.; Perveen S.; Iqbal M. Acylhydrazide Schiff bases: Synthesis and antiglycation activity. J. Chem. Soc. Pak. 2013, 34, 930–938. [Google Scholar]
- Mohammed Khan K.; Shah Z.; Uddin Ahmad V.; Khan M.; Taha M.; Rahim F.; Jahan H.; Perveen S.; Iqbal Choudhary M. Synthesis of 2,4,6-Trichlorophenyl Hydrazones and their Inhibitory Potential Against Glycation of Protein. Med. Chem. 2011, 7, 572–580. 10.2174/157340611797928415. [DOI] [PubMed] [Google Scholar]
- Ahad G.; Khan M.; Khan A.; Ibrahim M.; Salar U.; Khan K. M.; Perveen S. Synthesis, structural characterization, and antioxidant activities of 2, 4-dinitrophenyl-hydrazone derivatives. J. Chem. Soc. Pak. 2018, 40, 961. [Google Scholar]
- Shen Y. M.; Duan W. L.; Shi M. Chemical Fixation of Carbon Dioxide Co-Catalyzed by a Combination of Schiff Bases or Phenols and Organic Bases. Eur. J. Org. Chem. 2004, 2004, 3080–3089. 10.1002/ejoc.200400083. [DOI] [Google Scholar]
- Terenzi A.; Bonsignore R.; Spinello A.; Gentile C.; Martorana A.; Ducani C.; Högberg B.; Almerico A. M.; Lauria A.; Barone G. Selective G-quadruplex stabilizers: Schiff-base metal complexes with anticancer activity. RSC Adv. 2014, 4, 33245–33256. 10.1039/c4ra05355a. [DOI] [Google Scholar]
- Antony R.; Arun T.; Manickam S. T. D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. 10.1016/j.ijbiomac.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhang D.; Liu Z.; Xu Y.; Wang D. Synthesis, characterization of a ternary Cu(II) Schiff base complex with degradation activity of organophosphorus pesticides. Inorg. Chim. Acta 2018, 471, 280–289. 10.1016/j.ica.2017.11.024. [DOI] [Google Scholar]
- Abuamer K. M.; Maihub A. A.; El-Ajaily M. M.; Etorki A. M.; Abou-Krisha M. M.; Almagani M. A. The role of aromatic Schiff bases in the dyes techniques. Int. J. Org. Chem. 2014, 4, 7–15. 10.4236/ijoc.2014.41002. [DOI] [Google Scholar]
- Rakesh K.; Manukumar H.; Gowda D. C. Schiff’s bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg. Med. Chem. Lett. 2015, 25, 1072–1077. 10.1016/j.bmcl.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Al-Masoudi N. A.; Aziz N. M.; Mohammed A. T. Synthesis and In Vitro Anti-HIV Activity of Some New Schiff Base Ligands Derived from 5-Amino-4-phenyl-4H-1,2,4-triazole-3-thiol and Their Metal Complexes. Phosphorus, Sulfur Silicon Relat. Elem. 2009, 184, 2891–2901. 10.1080/10426500802591630. [DOI] [Google Scholar]
- Aslam M. A. S.; Mahmood S.-u.; Shahid M.; Saeed A.; Iqbal J. Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur. J. Med. Chem. 2011, 46, 5473–5479. 10.1016/j.ejmech.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Kamalı A.; Çakmak R.; Boğa M. Anticholinesterase and antioxidant activities of novel heterocyclic Schiff base derivatives containing an aryl sulfonate moiety. J. Chin. Chem. Soc. 2022, 69, 731–743. 10.1002/jccs.202100511. [DOI] [Google Scholar]
- Hamad A.; Chen Y.; Khan M. A.; Jamshidi S.; Saeed N.; Clifford M.; Hind C.; Sutton J. M.; Rahman K. M. Schiff bases of sulphonamides as a new class of antifungal agent against multidrug-resistant Candida auris. MicrobiologyOpen 2021, 10, e1218 10.1002/mbo3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K. M.; Khan M.; Ambreen N.; Rahim F.; Muhammad B.; Ali S.; Haider S. M.; Perveen S.; Choudhary M. Bis-Schiff bases of isatins: a new class of antioxidant. J. Pharmacol. Res. 2011, 4, 3402–3404. [Google Scholar]
- Alam A.; Ali M.; Rehman N. U.; Ullah S.; Halim S. A.; Latif A.; Zainab; Khan A.; Ullah O.; Ahmad S.; Al-Harrasi A. Bio-Oriented Synthesis of Novel (S)-Flurbiprofen Clubbed Hydrazone Schiff’s Bases for Diabetic Management: In Vitro and In Silico Studies. Pharmaceuticals 2022, 15, 672. 10.3390/ph15060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkop B.; Beiler T. W. Studies on Schiff bases in connection with the mechanism of transamination. J. Am. Chem. Soc. 1954, 76, 5589–5597. 10.1021/ja01651a002. [DOI] [Google Scholar]
- Mukherjee T.; Costa Pessoa J. C.; Kumar A.; Sarkar A. R. Synthesis, structure, magnetic properties and biological activity of supramolecular copper(ii) and nickel(ii) complexes with a Schiff base ligand derived from vitamin B6. Dalton Trans. 2013, 42, 2594–2607. 10.1039/c2dt31575k. [DOI] [PubMed] [Google Scholar]
- Li X.; Wen Q.; Gu J.; Liu W.; Wang Q.; Zhou G.; Gao J.; Zheng Y. Diverse reactivity to hypochlorite and copper ions based on a novel Schiff base derived from vitamin B6 cofactor. J. Mol. Liq. 2020, 319, 114124. 10.1016/j.molliq.2020.114124. [DOI] [Google Scholar]
- Elemike E. E.; Nwankwo H. U.; Onwudiwe D. C. Synthesis and comparative study on the anti-corrosion potentials of some Schiff base compounds bearing similar backbone. J. Mol. Liq. 2019, 276, 233–242. 10.1016/j.molliq.2018.11.161. [DOI] [Google Scholar]
- Elmaidomy A. H.; Abdelmohsen U. R.; Alsenani F.; Aly H. F.; Eldin Shams S. G. E.; Younis E. A.; Ahmed K. A.; Sayed A. M.; Owis A. I.; Afifi N.; El Amir D. The anti-Alzheimer potential of Tamarindus indica: an in vivo investigation supported by in vitro and in silico approaches. RSC Adv. 2022, 12, 11769–11785. 10.1039/d2ra01340a. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. 10.3892/mmr.2019.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.; Rehman M. U.; Qadir N.; Ashraf M.; Ismail T.; Kanwal A.; Salar U.; Khan K.; Parveen S. Acetyl-cholinesterase and butyrylcholinesterase inhibitory activities of 5-arylidene-N, N-diethylthiobarbiturates. J. Chem. Soc. Pak. 2020, 42, 134–148. [Google Scholar]
- Yoon Y. K.; Ali M. A.; Wei A. C.; Choon T. S.; Khaw K.-Y.; Murugaiyah V.; Osman H.; Masand V. H. Synthesis, characterization, and molecular docking analysis of novel benzimidazole derivatives as cholinesterase inhibitors. Bioorg. Chem. 2013, 49, 33–39. 10.1016/j.bioorg.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Yousaf M.; Khan M.; Ali M.; Wadood A.; Rehman A. U.; Jan M. S. 2-Mercaptobenzimidazole derivatives as novel butyrylcholinesterase inhibitors: biology-oriented drug synthesis (BIODS), in-vitro and in-silico evaluation. J. Chem. Soc. Pak. 2020, 42, 263. [Google Scholar]
- Hegazy M.-E. F.; Ibrahim A. Y.; Mohamed T. A.; Shahat A. A.; El Halawany A. M.; Abdel-Azim N. S.; Alsaid M. S.; Paré P. W. Sesquiterpene lactones from Cynara cornigera: acetyl cholinesterase inhibition and in silico ligand docking. Planta Med. 2016, 82, 138–146. 10.1055/s-0035-1558088. [DOI] [PubMed] [Google Scholar]
- Shadat A.; Ibrahim A. Y.; Ezzeldin E.; Alsaid M. S. Acetylcholinesterase inhibition and antioxidant activity of some medicinal plants for treating neuro degenarative disease. Afr. J. Tradit., Complementary Altern. Med. 2015, 12, 97–103. 10.4314/ajtcam.v12i3.12. [DOI] [Google Scholar]
- Agha K. A.; Abo-Dya N. E.; Issahaku A. R.; Agoni C.; Soliman M. E.; Abdel-Aal E. H.; Abdel-Samii Z. K.; Ibrahim T. S. Novel Sunifiram-carbamate hybrids as potential dual acetylcholinesterase inhibitor and NMDAR co-agonist: simulation-guided analogue design and pharmacological screening. J. Enzyme Inhib. Med. Chem. 2022, 37, 1241–1256. 10.1080/14756366.2022.2068147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. A.; Coats J. R. Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pestic. Biochem. Physiol. 2012, 102, 124–128. 10.1016/j.pestbp.2011.12.002. [DOI] [Google Scholar]
- Hayat M.; Khan K. M.; Saeed S.; Salar U.; Khan M.; Baig T.; Ahmad A.; Parveen S.; Taha M. Antimicrobial activities of synthetic arylidine nicotinic and isonicotinic hydrazones. Lett. Drug Des. Discovery 2018, 15, 1057–1067. 10.2174/1570180814666170914120337. [DOI] [Google Scholar]
- Shah S.; Khan M.; Ali M.; et al. Bis-1, 3, 4-Oxadiazole Derivatives as Novel and Potential Urease Inhibitors; Synthesis, In Vitro, and In Silico Studies. Med. Chem. 2022, 18 (7), 820–830. 10.2174/1573406418666220301161934. [DOI] [PubMed] [Google Scholar]
- Ahmad S.; Khan M.; Rehman N. U.; Ikram M.; Rehman S.; Ali M.; Uddin J.; Khan A.; Alam A.; Al-Harrasi A. Design, Synthesis, Crystal Structure, In Vitro and In Silico Evaluation of New N′-Benzylidene-4-tert-butylbenzohydrazide Derivatives as Potent Urease Inhibitors. Molecules 2022, 27, 6906. 10.3390/molecules27206906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S.; Khan M.; Shah M. I. A.; Ali M.; Alam A.; Riaz M.; Khan K. M. Synthetic Transformation of 2-{2-Fluoro[1,1′-biphenyl]-4-yl} Propanoic Acid into Hydrazide-Hydrazone Derivatives: In Vitro Urease Inhibition and In Silico Study. ACS Omega 2022, 7, 45077–45087. 10.1021/acsomega.2c05498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal E. U.; Sadiq A.; Murtaza S.; Rafique H.; Zafar M. N.; Riaz T.; Khan B. A.; Hameed A.; Khan K. M. Synthesis, structure-activity relationship and molecular docking of 3-oxoaurones and 3-thioaurones as acetylcholinesterase and butyrylcholinesterase inhibitors. Bioorg. Med. Chem. 2017, 25, 100–106. 10.1016/j.bmc.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Chemical Computing Group Inc. Molecular Operating Environment (MOE); Chemical Computing Group Inc: Montreal, QC, Canada, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.