Abstract

The performance of organic solar cells (OSCs) has been improving steadily over the last few years, owing to the optimization of device fabrication, fine-tuning of morphology, and thin-film processing. Thiophene core containing fused ring-type non-fullerene acceptors (NFAs) achieved significant proficiency for highly efficient OSCs. Quantum chemical computations are utilized herein with the motive of suggesting new NIR sensitive, highly efficient low-band gap materials for OSCs. A series of extended conjugated A−π–D−π–A architectured novel fused-ring NFAs (FUIC-1-FUIC-6) containing thieno[2,3-b]thiophene-based donor core are proposed by substituting the end-capped units of synthesized molecule F10IC. Different properties including frontier molecular orbital analysis, density of states analysis, transition density matrix analysis, excitation energy, reorganizational energies of both holes (λh) and electrons (λe), and open-circuit voltage (Voc) were performed employing the density functional theory approach. Charge transfer analysis of the best-designed molecule with the donor complex was analyzed to comprehend the efficiency of novel constructed molecules (FUIC-1–FUIC-6) and compared with the reference. End-caped acceptor alteration induces the reduction of the energy gap between HOMO–LUMO (1.88 eV), tunes the energy levels, longer absorption in the visible and near-infrared regions, larger Voc, smaller reorganizational energies, and binding energy values in designed structures (FUIC-1–FUIC-6) in comparison to reference (FUIC). The designed molecules show the best agreement with the PTBT-T donor polymer blend and cause the highest charge from the HOMO to the LUMO orbital. Our findings predicted that thieno[2,3-b] thiophene-based newly designed molecules would be efficient NFAs with outstanding photovoltaic characteristics and can be used in future applications of OSCs.
1. Introduction
Energy is essential for financial progression and human survival, but its resources are decreasing daily.1 The most acceptable source of renewable energy is solar cells since they are clean, non-polluting, have a low cost, are distributed worldwide, and are non-exhaustive.2 A solar cell is a device that converts sunlight directly into electrical energy via the photovoltaic effect.3−5 Organic solar cells, also known as OSCs, have become fascinating because of their benefits of being inexpensive, having a vast selection of organic materials, being solution processable, and having the potential to be used in large-area devices.6−8 To increase the OSC power conversion efficiencies (PCEs), light-absorbing materials have been widely studied.9 In fact, the device physic also plays a vital role in improving the performance of OSCs.10−13 As an alternative to fullerenes in organic photovoltaics, non-fullerene acceptors (NFAs) enable the rapid progress of OSCs with higher PCEs.14−16 Recently reported fused-ring acceptor–donor–acceptor (A–D–A)-type organic compounds containing effective two-photon absorption have resulted in a significant increase in photovoltaic competence OSCs.17−19 Since they are electronically coupled in-plane to acceptor groups at the molecule’s edge, electron-rich cores, planar, stiff, and highly delocalized π-systems, these materials’ structural motifs are also intriguing for two-photon absorption (2PA).20,21 Examples with strong, long wavelength 1PA are likely candidates for strong 2PA that extend into the near-infrared (NIR). The extensive material design efforts that were made to maximize the competency and spectrum range of a 2PA material are substantially responsible for the success of these endeavors. The many synthetic chemistries that can be used to modify the structures of conjugated molecules and polymers to produce strong 2PA responses is one of the many benefits of organic materials.22,23
Due to the significant 2PA increases that can be obtained through careful adjustments in their structural makeup, highly delocalized π-systems containing quadrupolar molecules, particularly, have attracted much attention. For their two-photon absorption (2PA) capabilities in organic photovoltaics, Allen et al. created quadrupolar A–D–A architecture based on fused-ring-type organic chromophores (F10IC).24 The F10IC has thieno[3,2-b]thiophene at its core as a donor, and indacene (IND)-centered analogues serve as the electron acceptors in the F10IC. In the scientific literature, modifications to the size, planarity, functionalization, and geometry of compounds containing π-conjugated fused rings have been shown to substantially influence the compounds’ optoelectronic competencies.20,21 The addition of spacers among terminal moieties, conjugated rings of donors, and additional electron-withdrawing and conjugated acceptors can improve the photovoltaic characteristics of molecular frameworks.25,26 The amount of quadrupolar ground-to-excited-state charge transfer (CT) is also controllable by adjusting the strength of the acceptor (A). Using electron-deficient and rich structural motifs for building OSCs is one of the molecular engineering techniques for designing non-fullerene acceptors (NFAs). Through the flexible structural adjustability, such a combination of electron-donating and electron-withdrawing moiety could create an electron donor–acceptor (D–A) architecture that is advantageous for adjusting the frontier orbital levels, band gap, light-absorption capacity, intramolecular CT, and intermolecular charge separation and recombination.27−29
Under these assumptions, it is intended to change the IND-centered analogues of the F10IC with strong reported end-capped acceptors with efficient electron-withdrawing features for developing innovative non-fullerene acceptors while maintaining the donor core of the thieno[3,2-b]thiophene. Researchers are increasingly using density functional theory (DFT) and time-depended density functional theory (TDDFT) approaches to explore the photoelectric characteristics of organic semiconductors and identify potential candidates for solar cell applications. First-principles computational molecular design could provide an effective method to forecast the qualities we are interested in, avoiding the need for experimental trial-and-error searches for new materials. In this work, we aim to inaugurate structure–property connections of thieno[3,2-b]thiophene-based molecules to evaluate the optoelectronic viability of these cutting-edge materials and conduct a computational investigation on the effect of the enhanced electron-pulling capacity of the terminal IND on their photovoltaic performance. We calculated various parameters, including the frontier molecular orbital (FMO), the density of state (DOS) transition density matrix (TDM), absorption maxima, reorganizational energies, excitation energy, and open-circuit voltage. Charge-transfer analysis was also carried out on the optimally designed molecules in conjunction with the suitable donor complexes, and the results were compared to those of the reference molecule (FUIC). We are optimistic that these freshly developed chemicals will make promising high-performance OSC candidates.
2. Results and Discussion
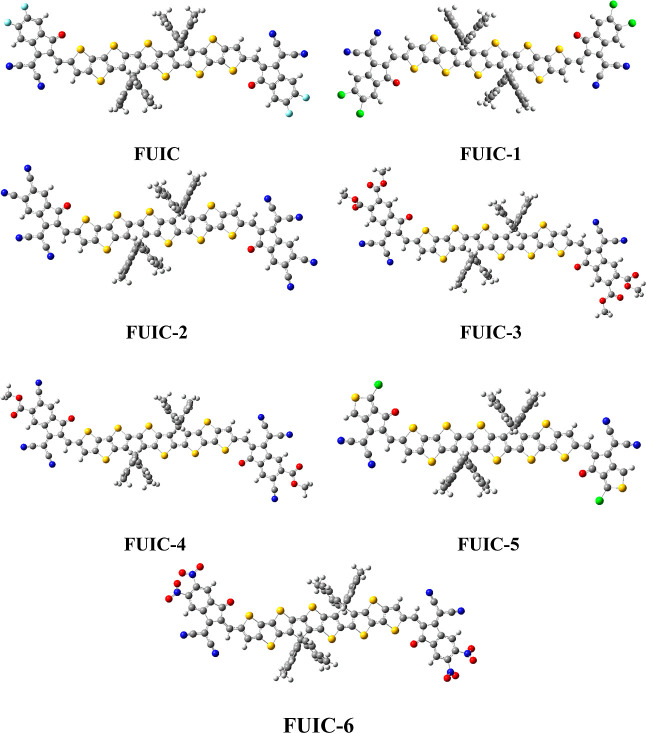
The focus of this study is to construct and investigate novel A−π–D−π–A quadrupolar-type fused-ring, highly conjugated acceptors with improved optoelectronic assets for their usage in solar cells. For this, F10IC,24 which is a novel fused-ring, highly conjugated, A−π–D−π–A backbone having thieno[2,3-b] thiophene-based core as the donor moiety and 2-(5,6-difluoro-2-methylene-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile as end-capped acceptor groups with significant electron-withdrawing properties,24 is used as a reference molecule (F10IC) in this report. In structural tailoring of the reference F10IC, the acceptor unit of F10IC is switched using six dissimilar designed end-capped acceptors (1–6), as represented in Scheme 1, and six novel A−π–D−π–A acceptor molecules (FUIC-1–FUIC-6) are being proposed for promising photovoltaic and optoelectronic applications. The central donor core was kept fixed while end-capped units were changed to design the similar core-based efficient new molecules. The names of the end-capped acceptors (1–6) are mentioned in Scheme 1.
Scheme 1. Schematic Diagram Representing Different End-Capped Acceptors (1–6) for Designing Thieno[2,3-b]thiophene-Based NFAs.
Long alkyl chains in the reference and developed compounds (FUIC and FUIC-1–FUIC-6) were swapped with methyl groups to save computational expenses. According to earlier publications, these simplifications do not affect the relative features of the acquired values or the essential features of the studied molecules.30 Numerous functionals are examined in this quantum chemistry inquiry to determine the best functional for estimating the photovoltaic and optoelectronic characteristics of designed structures.
Figure 1 depicts a bar chart comparing experimental λmax values of the reference (FUIC) molecule to DFT-estimated values in chloroform at different functionals. The calculated values of λmax at B3LYP, CAM-B3LYP, B3PW91, MPW1PW91, and ωB97XD/6-31G(d,p) functionals are 845, 580, 595, 780, and 601 nm correspondingly. The maximum wavelength measured experimentally for the standard molecule is 799 nm.24 Therefore, MPW1PW91/6-31G (d,p) functional demonstrates the most satisfactory compatibility with the reported highest absorption, and it can be employed for further analysis of FUIC and designed (FUIC-1-FUIC-9) molecule.
Figure 1.

Bar chart representation for selection of DFT functional.
The optimized structures of all the designed molecules at the MPW1PW91/6-31G(d,p) functional are displayed in Figure 2.
Figure 2.
Optimized structure of FUIC and constructed (FUIC-1–FUIC-6).
2.1. Frontier Molecular Orbital (FMO) Analysis
FMO is critical in understanding charge conduction and conducting electricity. The sequence of HOMO and LUMO is considered necessary in OSCs to transfer charge.31,32 In OSCs, the conduction band can be regarded as HOMO, and the valence band is considered LUMO according to the Band theory. Meanwhile, the difference between the conduction and valence bands is called a band gap.33,34 The photovoltaic character has considerable dependence on the band gap value. The band gap value is not only crucial for the FMOs but is also employed for the determination of Voc and binding energy (Eb). The good efficiency of OSCs is related to the lower band gap value and vice versa.35 With the help of FMO analysis, we can predict the conduction behavior of FUIC and FUIC-1 to FUIC-6. The HOMO and LUMO energies, together with the band gaps for FUIC and FUIC-1-FUIC-6, are displayed in electron Volts in Table 1.
Table 1. FMOs and Their Energy Gap Values of FUIC and FUIC-1 to FUIC-6.
| molecules | EHOMO | ELUMO | Egap = ELUMO – EHOMO |
|---|---|---|---|
| FUIC | –5.38 | –3.38 | 1.99 |
| FUIC-1 | –5.52 | –3.51 | 2.01 |
| FUIC-2 | –5.79 | –3.89 | 1.90 |
| FUIC-3 | –5.48 | –3.47 | 2.01 |
| FUIC-4 | –5.60 | –3.64 | 1.96 |
| FUIC-5 | –5.46 | –3.45 | 2.01 |
| FUIC-6 | –5.81 | –3.93 | 1.88 |
Table 1 represents the HOMO (−5.38 eV) and LUMO (−3.38 eV) for reference FUIC, and the energy gap is 1.99 eV. For designed molecules, we used different end-capped to enhance the efficiency by decreasing the energy gap. The HOMO energy values in electron Volts are calculated as −5.52, −5.79, −5.48, −5.60, −5.46, and 5.81 eV for FUIC-1, FUIC-2, FUIC-3, FUIC-4, FUIC-5, and FUIC-6, respectively. On the other hand, LUMO energy values calculated for FUIC-1, FUIC-2, FUIC-3, FUIC-4, FUIC-5, and FUIC-6 are −3.51, −3.89, −3.47, −3.64, −3.45, and −3.93 eV, respectively.
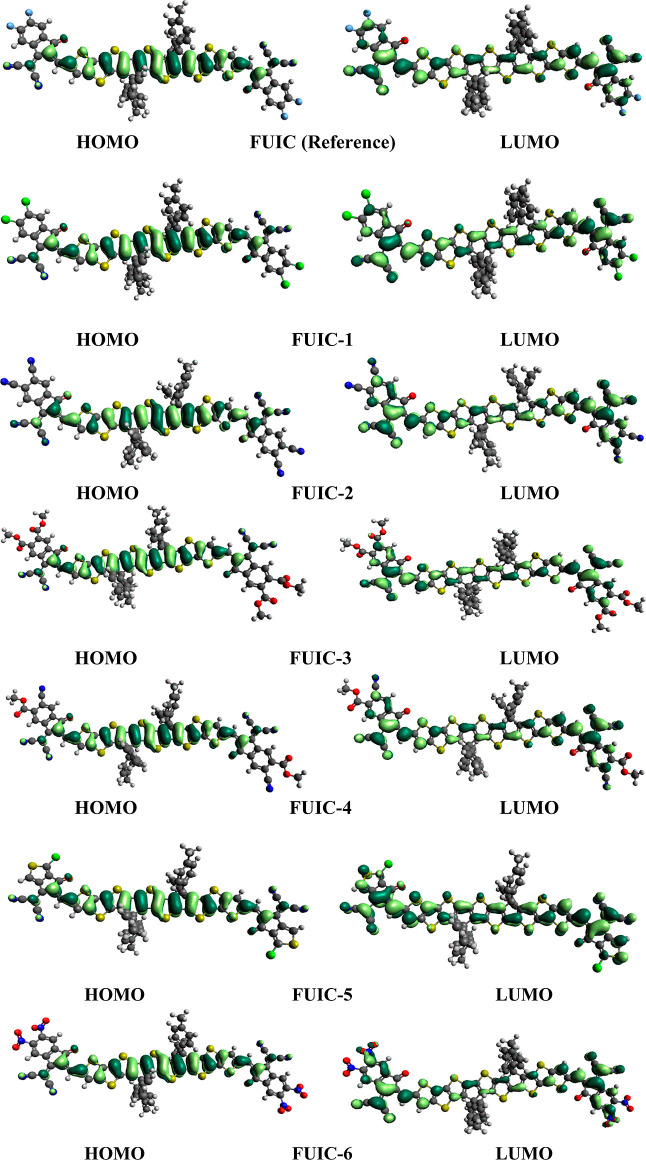
From the HOMO and LUMO values, the band gap was predicted as 2.01, 1.90, 2.01, 1.96, 2.01, and 1.88 eV for FUIC-1, FUIC-2, FUIC-3, FUIC-4, FUIC-5, and FUIC-6, respectively. The lowest energy gap is observed for FUIC-6 (1.88 eV). This is because of two efficient electron-withdrawing groups (CN and NO2) in this structure that extended the conjugation. Overall, CN, COOCH3, and NO2 containing units in end-capped acceptors 2, 4, and 6 lowered the energy gap than the reference. From the data, it can be predicted that the descending sequence of the HOMO and LUMO band gap of the designed structures (FUIC-1 to FUIC-6) and reference molecule (FUIC) is FUIC-1 = FUIC-3 = FUIC-5 > FUIC > FUIC-4 > FUIC-2 > FUIC-6. It is concluded that among all, the FUIC-6 molecule has the lowest band gap value. Lower energy gap values imply an efficient design of molecules and their propensity to transform more considerable charges, eventually aiding in the expansion of photovoltaic features. To have further insights into charge delocalization and shifting of density in different parts of investigated molecules, HOMO LUMO diagrams are generated and portrayed in Figure 3.
Figure 3.
FMOs diagrams of FUIC and constructed molecules FUIC-1–FUIC-6.
The green color indicates the positive sites, while yellow pointing to the negative ends. The repetitive pattern of FUIC and FUIC-1 to FUIC-6 demonstrates the right strategy to architect the molecules. Charge resides on the donor core in HOMO and on the acceptors in case of LUMO. This promotes efficient CT. Through prolonged conjugation bridges, the charge was transferred from the donor HOMO to the LUMO end cap, creating a charge separation state that should improve the photocatalytic activity of these newly created near-infrared sensitive NFAs.
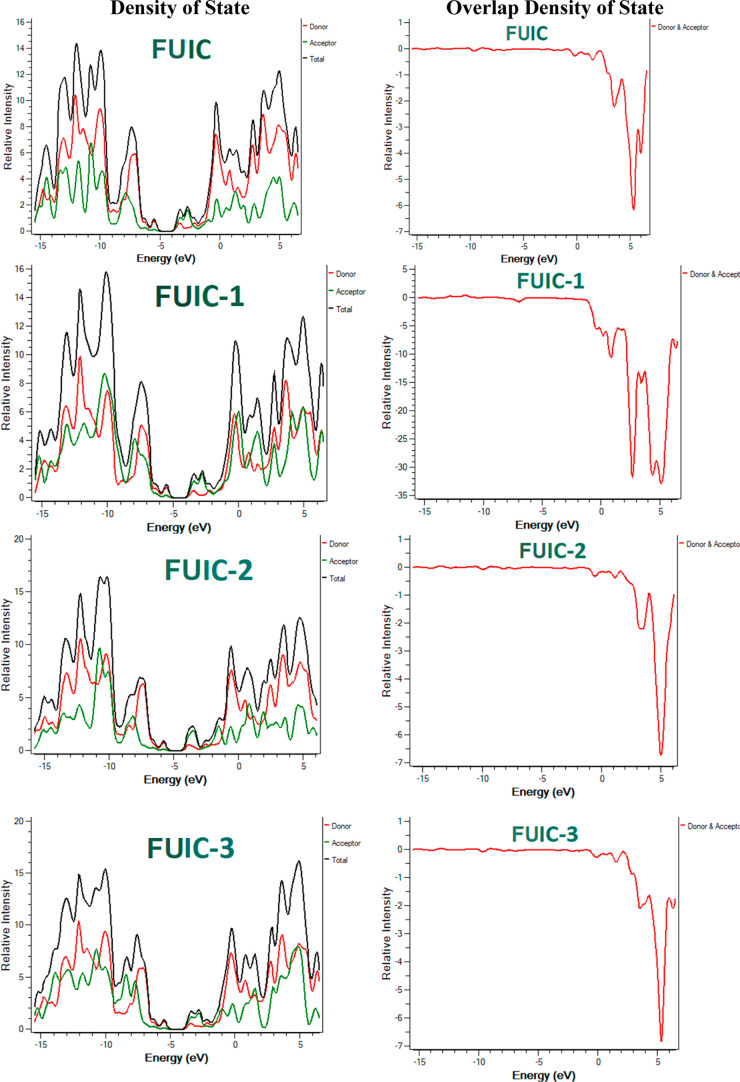
2.2. Density of States and Optical Density of States
The density of states (DOS) and optical density of states (ODOS) analyses are also carried out to strengthen the FMO at DFT/MP1PW91/6-31G(d,p). It can forecast the percentage contribution value for all the fragments present in the molecule.36,37 It also gives important information about charge distribution.38Figure 4 represents the percentage impact of the donor and acceptor sections in each molecule. Regarding FUIC, 84.7% of the contribution was from donors and 15.3% from acceptors in the case of HOMO. In contrast, the trend was reversed in the case of LUMO, that is, 43.1% of donors and 56.9% of acceptors. Then, the improved trend was observed in designed molecules. For donors, the percentage contribution values of HOMO are 80.3, 83.2, 84.3, 84.3, 83.8, and 83.2 for FUIC-1, FUIC-2, FUIC-3, FUIC-4, FUIC-5, and FUIC-6, respectively. Consequently, for acceptors, percentage contribution of HOMO is 19.7, 16.8, 15.7, 15.7, and 16.8%, for FUIC-1, FUIC-2, FUIC-3, FUIC-4, FUIC-5, and FUIC-6, respectively. In donors, the percentage contribution values of LUMO are 33.1, 37.0, 39.8, 38.4, 43.8, and 34% for FUIC-1, FUIC-2, FUIC-3, FUIC-4, FUIC-5, and FUIC-6, respectively.
Figure 4.
DOS and ODOS around the HOMO LUMO for FUIC and FUIC-1–FUIC-6 graphs.
Ultimately, in acceptors, these values are 66.9, 63.0, 60.2, 61.6, 56.2, and 66.0%, for FUIC-1, FUIC-2, FUIC-3, FUIC-4, FUIC-5, and FUIC-6 correspondingly. All evidence points to the terminal acceptor part of designed compounds being more densely inhabited by LUMO states than the central core HOMO states. For an intramolecular CT to occur, there must be a movement of charge density from the donor via the bridge and finally to the end-capped acceptors. This demonstrates the efficacy of our end-capped modification technique in creating novel NFAs with desirable photovoltaic properties for OSCs.
2.3. Optical Properties
TDDFT calculations were employed to determine the optical features of the investigated molecules (FUIC and FUIC-1–FUIC-6) using UV–visible analysis in chloroform and gas.39,40 For the determination of the photophysical properties of FUIC and FUIC-1-FUIC-6, the conductor-like polarizable continuum model (CPCM) model is applied in chloroform. Tables 2 and 3 summarize the findings of E, fos, λmax, and transition natures in studied molecules (FUIC and FUIC-1–FUIC-6) measured in solvent and gas phases.
Table 2. Calculated E (Transition Energy), fos (Oscillator Strengths), λmax (Maximum Absorption Wavelengths), and Transition Natures of FUIC and FUIC-1–FUIC-6 in eV at DFT/MPW1PW91/6-31G(d,p) Functional in Chloroform (Solvent).
| molecules | calculated λmax (nm) | expected λmax (nm) | Ex (eV) | fos | major MO assignmentsa |
|---|---|---|---|---|---|
| FUIC | 780.01 | 79924 | 1.59 | 3.48 | H → L (97%) |
| FUIC-1 | 792.98 | 1.56 | 3.56 | H → L (96%) | |
| FUIC-2 | 846.12 | 1.46 | 3.31 | H → L (96%) | |
| FUIC-3 | 803.26 | 1.54 | 3.42 | H → L (96%) | |
| FUIC-4 | 817.72 | 1.51 | 3.38 | H → L (96%) | |
| FUIC-5 | 788.79 | 1.57 | 3.62 | H → L (97%) | |
| FUIC-6 | 862.31 | 1.43 | 2.88 | H → L (96%) |
H = HOMO; L = LUMO.
Table 3. Calculated E (Transition Energy), fos (Oscillator Strengths), λmax (Maximum Absorption Wavelengths), and Transition Natures of FUIC and FUIC-1–FUIC-6 in eV at DFT/MPW1PW91/6-31G(d,p) Functional in the Gas Phase.
| molecules | calculated λmax (nm) | expected λmax (nm) | Ex (eV) | fos | major MO assignmentsa |
|---|---|---|---|---|---|
| FUIC | 717.71 | 1.72 | 3.16 | H → L (98%) | |
| FUIC-1 | 729.87 | 1.69 | 3.26 | H → L (98%) | |
| FUIC-2 | 770.08 | 1.61 | 3.09 | H → L (98%) | |
| FUIC-3 | 732.41 | 1.69 | 3.18 | H → L (98%) | |
| FUIC-4 | 746.66 | 1.66 | 3.19 | H → L (98%) | |
| FUIC-5 | 728.24 | 1.71 | 3.25 | H → L (98%) | |
| FUIC-6 | 776.98 | 1.59 | 2.83 | H → L (98%) |
H = HOMO; L = LUMO.
The λmax of the FUIC is obtained at 780.01 nm, which is in good accordance with the experimentally recorded λmax of 799 nm24 for solvent, as illustrated in Table 2. The λmax values of the entire investigated systems (FUIC and FUIC-1–FUIC-6) are revealed in Tables 2 (for solvent) and 3 (for gas). In the solvent phase, the order of λmax values is FUIC-6 > FUIC-2 > FUIC-4 > FUIC-3 > FUIC-1 > FUIC-5 > FUIC while in the gas phase, it is FUIC-6 > FUIC-2 > FUIC-4 > FUIC-3 > FUIC-1 > FUIC-5 > FUIC. The λmax values of all six designed molecules FUIC-1–FUIC-6 are greater than the FUIC molecule. In both solvent and gas phases, the proposed FUIC-5 molecule has the smallest, and the FUIC-6 structure has the largest λmax among all the designed molecules FUIC-1–FUIC-6. End-capped acceptors affect the λmax values of all investigated molecules because of electron-withdrawing groups present in the end-capped units. The λmax of FUIC-1 is 780.01 nm (in the solvent) and 729.87 nm in the gas phase, which are slightly greater than the FUIC molecule due to the occurrence of chloro (Cl) on end-capped acceptors. The end-capped 2 in FUIC-2, having two cyano (CN) groups on both ends of the molecule, has a stronger withdrawing effect and shifts the absorption maximum toward a longer wavelength (846.12 nm in the solvent and 770.08 nm in the gas phase). The acceptor 3 contains two ester groups (COOCH3) in the FUIC-3 molecule that cause higher absorption values of 803.26 nm (in the solvent) and 732.41 nm (in the gas phase) than to FUIC, FUIC-1, and FUIC-5. The combined effect of (COOCH3) and (CN) groups on end-capped acceptor 4 are smaller than end-capped acceptor 3. FUIC-5 molecule shows λmax values of 788.79 nm in the solvent and 728.24 nm in the gas phase, which is smaller than all other designed molecules because of the lower effect of five terminal acceptors (end-capped). The λmax values of the FUIC-6 designed molecule are 862.31 nm in the solvent and 776.98 nm in the gas phase, which are the highest among all other reference and designed molecules. The reason is that the end-capped acceptors contain two nitro (NO2) groups with the highest pulling effect and extended conjugation in contrast to all other groups. The ascending order of end-capped acceptors’ capability is 6 > 2 > 4 > 3 > 1 > 5, which is identical to their performance in reducing energy band gaps. Moreover, the decreasing sequence of λmax values shows better accordance with the rising sequence of the energy gap, which is applicable for designing and developing high-performance NFAs molecules with improved optoelectronic properties.
The UV spectra of FUIC and FUIC-1–FUIC-6 computed in both gas and solvent phases are illustrated in Figure 5a,b, which show that end-capped acceptors had a remarkable impact on the values of λmax by promoting the absorption in near IR spectra with lower excitation energy and same significant MO contribution in all of the investigated molecules. Alteration of end-capped (acceptor moieties) is a powerful technique to fine-tune the optoelectronic characteristics by reducing the transition energy and increasing the absorption values, as shown in the previous discussion. As a result, novel created molecules offer greater optoelectronic capabilities than FUIC, which would increase their demand for solar energy.
Figure 5.
UV–visible absorption spectra (a) in the chloroform solvent and (b) in the gas phase.
Another parameter that improved the optoelectronic features of SCs is the excitation E. Compared to the reference (1.72 eV), all of our created molecules have smaller excitation energy values (1.59–1.71 eV) that are generally thought to be more favorable for efficient photovoltaic properties. Additionally, more outstanding CTs occur from lesser excitation energy, and reduced excitation energy results in the maximum power conversion efficiencies. The descending sequence of excitation energy for all examined structures is FUIC-6 < FUIC-2 < FUIC-4 < FUIC-3 = FUIC-1 < FUIC-5 < FUIC in both solvent and gas phases. This seems applicable for designing molecules that are good candidates for obtaining excellent optoelectronic features.
2.4. Reorganizational Energy
Another valuable tool used to measure the overall CT between donor and acceptor components is reorganizational energy.41 In most cases, two distinct forms of reorganizational energy exist. The first refers to the reorganizational energy (λint) generated within a system, while the second refers to the reorganizational energy (λext) generated outside a system. The λint explains variation in the internal structure, and λext gives information about variations in the external environment.42,43 Marcus’ equation calculates reorganization energy. The hole λh and electron λe reorganizational energy values of FUIC and FUIC1– FUIC6 are computed at MPW1PW91/6-31G(d,p) functional, and findings are mentioned in Table 4.
Table 4. Reorganizational Energy Values of the Hole and Electron for FUIC and FUIC1–FUIC6.
| molecules | λe (Eh) | λh (Eh) |
|---|---|---|
| FUIC | 0.0053 | 0.0082 |
| FUIC-1 | 0.0049 | 0.0082 |
| FUIC-2 | 0.0035 | 0.0079 |
| FUIC-3 | 0.0050 | 0.0083 |
| FUIC-4 | 0.0043 | 0.0081 |
| FUIC-5 | 0.0050 | 0.0080 |
| FUIC-6 | 0.0043 | 0.0081 |
It is clear from Table 4 that λe and λh statics reveal remarkable findings. The value of λe for FUIC is computed as 0.0053 eV, and λe value of designed molecules (FUIC-1–FUIC-6) is calculated as 0.0049, 0.0034, 0.0049, 0.0042, 0.0049, and 0.0043 eV correspondingly. The increasing order for λe values of reference FUIC and designed compounds (FUIC-1–FUIC-6) is FUIC-2 < FUIC-4 < FUIC-6 < FUIC-1 < FUIC-5 < FUIC3 < FUIC. The calculated λe values for all the engineered molecules are lower than those of the reference FUIC. The λh value of FUIC is calculated as 0.0082. In contrast, λh value of constructed molecules is computed as 0.0081, 0.0079, 0.0083, 0.0081, 0.0080, and 0.0080 eV, respectively. The increasing order for λh is FUIC-2 < FUIC-5 < FUIC-6 < FUIC-4 < FUIC-1 < FUIC < FUIC-3. According to this order, all examined structures exhibit a lower λh value than reference FUIC except FUIC-3. The tailored molecules FUIC-2 revealed the lowest λh value than all other structures, possibly due to the additional two terminal groups. Overall, FUIC-2 has minimum values of λe and λh, which is why it offers a maximum hole and electron transfer rate so that it would be a good structure for use in solar cell devices. The preceding discussion makes it evident that all designed molecules can act as excellent electron- and hole-transfer candidates with better potential reorganizational energy features than reference.
2.5. Open-Circuit Voltage
The Voc can also be used to assess the function of OSCs. The Voc can be defined as the highest quantity of voltage that can be obtained via any display device. It is the maximum voltage at the zero current stage, and this information was obtained via solar technology.44 Mainly, two types of current affect the Voc values, one is saturation voltage and the other is photo-generated current. Equation 1 is used to find Voc values of FUIC and FUIC-1–FUIC-6.
| 1 |
A larger Voc is a result of a faster rate of electron transport from the donor HOMO to the acceptor LUMO. PTBT-T is the most commonly used donor material in the literature. In this work, we took PTBT-T as a donor polymer. The results acquired from eq 1 conducted concerning HOMOPTBT-T–LUMOacceptorEg are shown in Figure 6.
Figure 6.
Voc of FUIC and designed structures FUIC-1–FUIC-6.
The Voc for FUIC and FUIC-1–FUIC-6 is computed as: 1.65, 1.52, 1.14, 1.56, 1.39, 1.58, and 1.1 V, respectively. The Voc values for investigated systems are in the following order: FUIC > FUIC-5 > FUIC-3 > FUIC-1 > FUIC-4 > FUIC-2 > FUIC-6. The newly designed molecules FUIC-5 and FUIC-3 exhibit Voc values that are almost equal to reference FUIC. While other molecules revealed Voc value lower than reference FUIC. Higher Voc values represent the larger voltage obtained from the solar cell, high conversion efficiency, and vice versa. All developed molecules of the studied family with sufficient Voc values can be considered better entrants for achieving maximum voltage from the solar cell and high conversion efficiency than the reference.
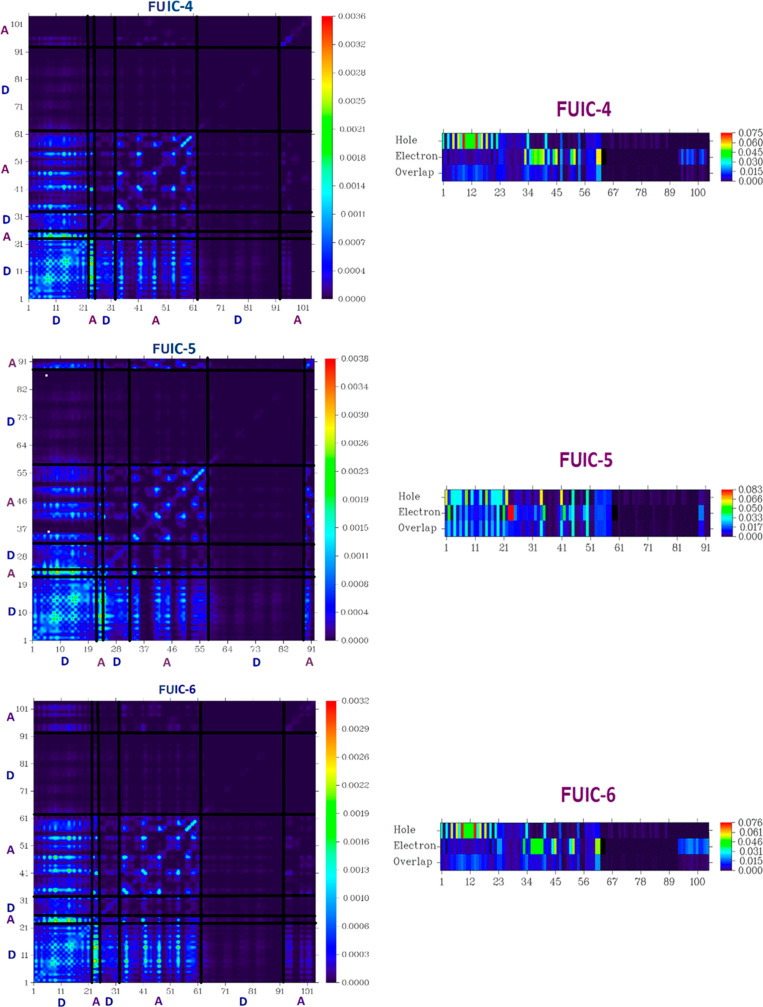
2.6. TDM Analysis and Exciton Binding Energy (Eb)
TDM is another valuable technique to estimate the transition between the donor and acceptor parts in the excited state.45 The hydrogen (H) atoms play no role in transition; basically, they are ignored. TDM analysis for reference FUIC and designed molecules (FUIC-1–FUIC-6) were computed with S1 state under vacuum at MPW1PW91/6-31G(d,p) level of theory. In the current investigation, the designed molecules are divided into two parts: core (D) and accepter. TDM results for all molecules, including reference, are shown in Figure 7. In FUIC and designed FUIC1–FUIC6 molecules, almost similar segment contributed in CT, as indicated with heat maps. Transfer of charge occurred from atom number 1–21 designated as D in the TDM surfaces of all investigated molecules. Charge is found to be shifted in the acceptor segment with atom numbers 31–61 in all studied molecules.
Figure 7.
TDM surfaces for all reference and designed molecules.
TDM pictures depicted that all investigated structures exhibit consistency of charges. A diagonal electron coherence transfer is envisioned for all investigated molecules FUIC-1–FUIC-6 and reference FUIC. The presence of electron coherence in investigated molecules FUIC and FUIC-1–FUIC-6 assures that charge consistency effectively transfers from the central donor part to the bridging unit, facilitating the shifting of electrons without tapping them. Finally, the charge density shifts to another end-capped unit. Figure 7 represents the TDM functionality and charge distribution, which is very important for developing the solar cell.
Binding energy is also significant for evaluating the OSC efficiency. Smaller Eb values boost exciton dissociation by reducing hole–electron Coulombic forces.46 Small Coulombic communication among hole and electron facilitates the separation of charge.47 The binding energy is equal to the difference between the initial excitation energy Eopt and the energy gap (Eg) calculated utilizing eq 2, and the results are given in Table 5.
| 2 |
Table 5. Exciton Binding Energies (Eb) of FUIC and FUIC-1–FUIC-6.
| molecules | EH–L (eV) | Eopt (eV) | Eb (eV) |
|---|---|---|---|
| FUIC | 2.00 | 1.60 | 0.40 |
| FUIC-1 | 2.01 | 1.56 | 0.45 |
| FUIC-2 | 1.90 | 1.47 | 0.43 |
| FUIC-3 | 2.01 | 1.54 | 0.47 |
| FUIC-4 | 1.96 | 1.52 | 0.44 |
| FUIC-5 | 2.01 | 1.57 | 0.44 |
| FUIC-6 | 1.88 | 1.44 | 0.44 |
The Eb energy of FUIC is computed as 0.40 eV. The Eb of FUIC-1–FUIC-6 is noted as 0.45, 0.43, 0.47, 0.44, 0.44, and 0.44 eV, correspondingly. The declining sequence of Eb for all structures, including reference, is FUIC < FUIC-2 < FUIC-4 = FUIC-5 = FUIC-6 < FUIC-1 < FUIC-3. These calculated values indicate that FUIC-4, FUIC-5, and FUIC-6 have Eb values that are almost similar to those of the FUIC molecule. It may be due to additional end-capped groups. As a result, these constructed compounds can be used as good acceptor molecules in OSCs.
2.7. Charge-Transfer Analysis between PBDT-T and FUIC-6
In order to verify that the created molecules may be used in solar cell materials, CT analysis is performed on them. For this, the acceptor molecules are modeled into a layer that contains a well-known and well-characterized donor. Among the investigated systems, the FUIC-6 molecule was chosen for charge-transfer analysis with an established PBDT-T polymer, and the PBDT-T/FUIC-6 complex has been optimized. This was due to the FUIC-6 molecule having the lowest energy gap, the lowest Ex, the lowest Eb, the lowest λh and λh values, and the highest λmax values. Figure 8 depicts the PBDT-T/FUIC-6 complex optimized shape at the MPW1PW91/6-31G(d,p) functional.
Figure 8.

Optimized structure of the PBDT-T and FUIC-6 complex.
Figure 8 depicts the relative positions of PBDT-T and FUIC-6. The orientation of the PBDT-T/FUIC-6 complex is also helpful for the electron-transfer process. To facilitate electron transport between donor and acceptor moieties, the core regions of FUIC-6 and PBDT-T are arranged in a parallel fashion. The aforesaid complex was then used to get the HOMO and LUMO. The HOMO charge is localized on the donor polymer, while the LUMO charge is dispersed across the FUIC-6 acceptor molecule. There is promising evidence that tailored molecules can be useful in real-world solar cell applications, thanks to the transmission of charge density from the donor (HOMO) to the acceptor (LUMO) layer in the PBDT-T/FUIC-6 blend (Figure 9).
Figure 9.

HOMO and LUMO calculated from the PBDT-T and FUIC-6 complex.
In silico engineering of thieno[2,3-b]thiophene core-based non-fullerene acceptors will lead to new developments in the next years, such as non-fullerene electron acceptors with larger solar spectrum absorption and fine optoelectronic characteristics. It raises hopes for high OSC PCE in the coming years.
3. Conclusions
In conclusion, advanced quantum chemical methods have been utilized to investigate the behavior of CT, structure–activity, photovoltaic and optoelectronic characteristics of six novel fused-ring, highly conjugated A−π–D−π–A designed structures FUIC-1-FUIC-6 designed by substituting the end-capped acceptors and compared with FUIC molecules. The UV–visible graphs showed that maximum absorption values of designed molecules shifted toward longer wavelengths in the range of 780.01–862.31 nm in the solvent phase while 728.24–776.98 nm in the gas phase in comparison to the reference molecule having 780.01 nm in the solvent and 717.71 nm in the gas phase. The HOMO–LUMO energy gap values of FUIC-1–FUIC-6 are reduced to 2.01–1.88 eV by end-capped acceptor substitution compared with FUIC 1.99 eV. The binding energy of the constructed structures is comparable to the reference FUIC, resulting in increased exciton dissociation in the excited state. The values of reorganizational energies of electron and hole of FUIC-1–FUIC-6 are observed to be lower than FUIC in the molecule. Consequently, the ascending sequence of open-circuit voltage is FUIC > FUIC-5 > FUIC-3 > FUIC-1 > FUIC-4 > FUIC-2 > FUIC-6 and found to be in better accordance with the ascending order of the excitation energy as follows FUIC-6 < FUIC-2 < FUIC-4 < FUIC-3 < FUIC-1 < FUIC-5 < FUIC. The increasing sequence of maximum absorption values is FUIC-6 > FUIC-2 > FUIC-4 > FUIC-3 > FUIC-1 > FUIC-5 > FUIC both in the solvent and gas phase. According to all analyses, FUIC-6 is the best and most efficient molecule among all investigated compounds because of its electron-withdrawing capacity and prolonged conjugation of the terminal acceptors (end-capped) of FUIC-6 due to the presence of NO2 groups. It possesses excellent photovoltaic features like less band gap (1.88 eV), lowest λe = 0.00431856 eV and λh = 0.00806781 eV, largest λmax values 862.31 nm (in solvent) and 776.98 nm (in gas), and Voc = 1.1 V concerning HOMOPTBT-T–LUMOacceptor. Our findings indicate that the designed structures, especially FUIC-6, are efficient acceptor moieties employed as hole- and electron-transporting material in OSCs with outstanding photovoltaic characteristics and can be used in future high-performance OSC systems.
4. Computational Procedures
The Gaussian 09 program was utilized to conduct all quantum chemistry studies.48 Using GaussView 5.0,49 all input files of reference and designed molecules (FUIC and FUIC-1-FUIC-6) are prepared. First, reference molecule is optimized using density DFT calculations at six different functionals, including B3LYP,50 CAM-B3LYP,51 M06-2X,52 MPW1PW91,53 and ωB97XD54 in combination with 6-31G(d,p) basis set. After frequency calculations of reference FUIC, the λmax (maximum absorption) was calculated. The λmax values calculated for the FUIC molecule at six different functionals were compared to the λmax values reported experimentally.24 The MPW1PW91/6-31G(d,p) functional was selected as the best functional among all six due to good harmony with the experimental value. The opted functional delivered strong evidence to undertake further all calculations of FUIC and constructed molecules (FUIC-1–FUIC-6). Therefore, optimizing designed molecules (FUIC-1–FUIC-6) was executed using selected functional. A vibrational study at the same opted functional was carried out to confirm the absence of negative eigenvalues and the appearance of optimized geometries in the lowest potential energy surfaces. The absorption spectrum of investigated molecules FUIC and FUIC-1–FUIC-6 was estimated by means of the CPCM model55 in the chloroform solvent at MPW1PW91/6-31G(d,p) functional. For the determination of λmax (in the solvent and gas), Swizard software was applied. Using Origin 8.0 software, the results of Gaussian calculations were shown. DFT/MPW1PW91/6-31G(d,p) functional was applied to measure different parameters, including FMOs, DOS calculations, reorganizational energies, TDM analysis, Voc, analysis of CT, and energy band gap of (FUIC and FUIC-1–FUIC-6) molecules. The DOS for all investigated molecules was calculated and displayed using PyMOlyze 2.056 software to determine the percentage impact of acceptor and donor fragments for radiation absorption. Electronic excitation transition density matrix (TDM) analysis is executed using Multiwfn 3.7 software.57 There are two different kinds of reorganization energies. Internal reorganization (λint) is first and is concerned with the internal structures of molecules, while the second is referred to as external reorganization (λext) and is concerned with the external environment, and its effect was ignored in this report because it is not very effective.58 For the CT study of all molecules, whether intermolecular or intramolecular, Marcus theory59 is very important. The following eq 3 can be used to understand electron mobility (λe) in terms of Marcus theory.
| 3 |
Furthermore, hole mobility (λh) is also essential for CT interactions in OSCs. Its value can be calculated using the formula given below
| 4 |
In these eqs 3, 4, the anion and cation single-point energies Eo– and Eo are found by optimizing neutral molecules. By their respective optimal geometries, cation and anion energies are denoted by E– and E+. The energies of the neutral molecule are represented by E–0 and E+0, which are obtained by optimizing the cation and anion energies, respectively. The energy of a neutral molecule in its ground state is denoted by the symbol Eo.60
Acknowledgments
S.S.A. extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Group Research Project under grant number 34/43, and S.S.A. also acknowledges the Research Center for Advance Materials (RCAMS) at King Khalid University, Saudi Arabia for their valuable technical support.
The authors declare no competing financial interest.
References
- Chaudhary S.; Mehra R.. Advances in Solar Cells as Renewable Energy. Proceedings of International Conference on Advancements in Computing & Management (ICACM) 2019; SSRN, 2019.
- Hosenuzzaman M.; Rahim N. A.; Selvaraj J.; Hasanuzzaman M.; Malek A. A.; Nahar A. Global prospects, progress, policies, and environmental impact of solar photovoltaic power generation. Renew. Sustain. Energy Rev. 2015, 41, 284–297. 10.1016/j.rser.2014.08.046. [DOI] [Google Scholar]
- Khalid M.; Shafiq I.; Zhu M.; Khan M. U.; Shafiq Z.; Iqbal J.; Alam M. M.; Braga A. A. C.; Imran M. Efficient tuning of small acceptor chromophores with A1-π-A2-π-A1 configuration for high efficacy of organic solar cells via end group manipulation. J. Saudi Chem. Soc. 2021, 25, 101305. 10.1016/j.jscs.2021.101305. [DOI] [Google Scholar]
- Khan M. U.; Iqbal J.; Khalid M.; Hussain R.; Braga A. A. C.; Hussain M.; Muhammad S. Designing triazatruxene-based donor materials with promising photovoltaic parameters for organic solar cells. RSC Adv. 2019, 9, 26402–26418. 10.1039/c9ra03856f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M.; Momina; Imran M.; Rehman M. F. u.; Braga A. A. C.; Akram M. S. Molecular engineering of indenoindene-3-ethylrodanine acceptors with A2-A1-D-A1-A2 architecture for promising fullerene-free organic solar cells. Sci. Rep. 2021, 11, 20320. 10.1038/s41598-021-99308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.; Zheng T.; Wu Q.; Schneider A. M.; Zhao D.; Yu L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. 10.1021/acs.chemrev.5b00098. [DOI] [PubMed] [Google Scholar]
- Armin A.; Li W.; Sandberg O. J.; Xiao Z.; Ding L.; Nelson J.; Neher D.; Vandewal K.; Shoaee S.; Wang T.; Ade H.; Heumüller T.; Brabec C.; Meredith P. A History and Perspective of Non-Fullerene Electron Acceptors for Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2003570. 10.1002/aenm.202003570. [DOI] [Google Scholar]
- Riede M.; Spoltore D.; Leo K. Organic Solar Cells-The Path to Commercial Success. Adv. Energy Mater. 2021, 11, 2002653. 10.1002/aenm.202002653. [DOI] [Google Scholar]
- Cao D. H.; Stoumpos C. C.; Farha O. K.; Hupp J. T.; Kanatzidis M. G. 2D homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. 10.1021/jacs.5b03796. [DOI] [PubMed] [Google Scholar]
- Xu C.; Ma X.; Zhao Z.; Jiang M.; Hu Z.; Gao J.; Deng Z.; Zhou Z.; An Q.; Zhang J.; Zhang F. Over 17.6% efficiency organic photovoltaic devices with two compatible polymer donors. Sol. RRL 2021, 5, 2100175. 10.1002/solr.202100175. [DOI] [Google Scholar]
- Xu C.; Jin K.; Xiao Z.; Zhao Z.; Yan Y.; Zhu X.; Li X.; Zhou Z.; Jeong S. Y.; Ding L.; Woo H. Y.; Yuan G.; Zhang F. Efficient Semitransparent Layer-by-Layer Organic Photovoltaics via Optimizing Wide Bandgap and Narrow Absorption Polymer Layer Thickness. Sol. RRL 2022, 6, 2200308. 10.1002/solr.202200308. [DOI] [Google Scholar]
- Xu W.; Zhu X.; Ma X.; Zhou H.; Li X.; Jeong S. Y.; Woo H. Y.; Zhou Z.; Sun Q.; Zhang F. Achieving 15.81% and 15.29% efficiency of all-polymer solar cells based on layer-by-layer or bulk heterojunction structure. J. Mater. Chem. A 2022, 10, 13492. 10.1039/d2ta02914f. [DOI] [Google Scholar]
- Ma X.; Jiang Q.; Xu W.; Xu C.; Jeong S. Y.; Woo H. Y.; Wu Q.; Zhang X.; Yuan G.; Zhang F. Layered optimization strategy enables over 17.8% efficiency of layer-by-layer organic photovoltaics. Chem. Eng. J. 2022, 442, 136368. 10.1016/j.cej.2022.136368. [DOI] [Google Scholar]
- Zhang Y.; Ji Y.; Zhang Y.; Zhang W.; Bai H.; Du M.; Wu H.; Guo Q.; Zhou E. Recent Progress of Y6-Derived Asymmetric Fused Ring Electron Acceptors. Adv. Funct. Mater. 2022, 32, 2205115. 10.1002/adfm.202205115. [DOI] [Google Scholar]
- Nie Q.; Tang A.; Guo Q.; Zhou E. Benzothiadiazole-based non-fullerene acceptors. Nano Energy 2021, 87, 106174. 10.1016/j.nanoen.2021.106174. [DOI] [Google Scholar]
- Yang J.; Xiao B.; Tang A.; Li J.; Wang X.; Zhou E. Aromatic-Diimide-Based n-Type Conjugated Polymers for All-Polymer Solar Cell Applications. Adv. Mater. 2019, 31, 1804699. 10.1002/adma.201804699. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Wang J.; Zhang Z. G.; Bai H.; Li Y.; Zhu D.; Zhan X. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. 10.1002/adma.201404317. [DOI] [PubMed] [Google Scholar]
- Dai S.; Xiao Y.; Xue P.; James Rech J.; Liu K.; Li Z.; Lu X.; You W.; Zhan X. Effect of core size on performance of fused-ring electron acceptors. Chem. Mater. 2018, 30, 5390–5396. 10.1021/acs.chemmater.8b02222. [DOI] [Google Scholar]
- Li T.; Dai S.; Ke Z.; Yang L.; Wang J.; Yan C.; Ma W.; Zhan X. Fused Tris(thienothiophene)-Based Electron Acceptor with Strong Near-Infrared Absorption for High-Performance As-Cast Solar Cells. Adv. Mater. 2018, 30, 1705969. 10.1002/adma.201705969. [DOI] [PubMed] [Google Scholar]
- Katan C.; Tretiak S.; Werts M. H.; Bain A. J.; Marsh R. J.; Leonczek N.; Nicolaou N.; Badaeva E.; Mongin O.; Blanchard-Desce M. Two-photon transitions in quadrupolar and branched chromophores: experiment and theory. J. Phys. Chem. B 2007, 111, 9468–9483. 10.1021/jp071069x. [DOI] [PubMed] [Google Scholar]
- Delgado M. C. R.; Kim E.-G.; Filho D. t. A. d. S.; Bredas J.-L. Tuning the charge-transport parameters of perylene diimide single crystals via end and/or core functionalization: a density functional theory investigation. J. Am. Chem. Soc. 2010, 132, 3375–3387. 10.1021/ja908173x. [DOI] [PubMed] [Google Scholar]
- Terenziani F.; Katan C.; Badaeva E.; Tretiak S.; Blanchard-Desce M. Enhanced Two-Photon Absorption of Organic Chromophores: Theoretical and Experimental Assessments. Adv. Mater. 2008, 20, 4641–4678. 10.1002/adma.200800402. [DOI] [Google Scholar]
- Pawlicki M.; Collins H. A.; Denning R. G.; Anderson H. L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem., Int. Ed. 2009, 48, 3244–3266. 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]
- Allen T. G.; Benis S.; Munera N.; Zhang J.; Dai S.; Li T.; Jia B.; Wang W.; Barlow S.; Hagan D. J.; Van Stryland E. W.; Zhan X.; Perry J. W.; Marder S. R. Highly Conjugated, Fused-Ring, Quadrupolar Organic Chromophores with Large Two-Photon Absorption Cross-Sections in the Near-Infrared. J. Phys. Chem. A 2020, 124, 4367–4378. 10.1021/acs.jpca.0c02572. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Page Z. A.; Russell T. P.; Emrick T. Finely tuned polymer interlayers enhance solar cell efficiency. Angew. Chem. 2015, 127, 11647–11651. 10.1002/ange.201503933. [DOI] [PubMed] [Google Scholar]
- Spanggaard H.; Krebs F. C. A brief history of the development of organic and polymeric photovoltaics. Sol. Energy Mater. Sol. Cells 2004, 83, 125–146. 10.1016/j.solmat.2004.02.021. [DOI] [Google Scholar]
- Biswas S.; Pramanik A.; Sarkar P. Effect of additional donor group on the charge transfer/recombination dynamics of a photoactive organic dye: A quantum mechanical investigation. Comput. Theor. Chem. 2017, 1103, 38–47. 10.1016/j.comptc.2017.01.011. [DOI] [Google Scholar]
- Biswas S.; Pramanik A.; Sarkar P. Origin of different photovoltaic activities in regioisomeric small organic molecule solar cells: the intrinsic role of charge transfer processes. J. Phys. Chem. C 2018, 122, 14296–14303. 10.1021/acs.jpcc.8b02821. [DOI] [Google Scholar]
- Raheem A. A.; Kamaraj S.; Sannasi V.; Praveen C. New D-π-A push-pull chromophores as low band gap molecular semiconductors for organic small molecule solar cell applications. Org. Chem. Front. 2018, 5, 777–787. 10.1039/c7qo00920h. [DOI] [Google Scholar]
- Ma W.; Jiao Y.; Meng S. Modeling charge recombination in dye-sensitized solar cells using first-principles electron dynamics: effects of structural modification. Phys. Chem. Chem. Phys. 2013, 15, 17187–17194. 10.1039/c3cp52458b. [DOI] [PubMed] [Google Scholar]
- Imran M.; Khalid M.; Jawaria R.; Ali A.; Asghar M. A.; Shafiq Z.; Assiri M. A.; Lodhi H. M.; Braga A. A. C. Exploration of Photophysical and Nonlinear Properties of Salicylaldehyde-Based Functionalized Materials: A Facile Synthetic and DFT Approach. ACS Omega 2021, 6, 33914–33922. 10.1021/acsomega.1c04984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M.; Khan M. U.; Hussain R.; Irshad S.; Ali B.; Braga A. A. C.; Imran M.; Hussain A. Exploration of second and third order nonlinear optical properties for theoretical framework of organic D−π–D−π–A type compounds. Opt. Quant. Electron. 2021, 53, 561. 10.1007/s11082-021-03212-3. [DOI] [Google Scholar]
- Khalid M.; Khan M. U.; Razia E.-t.; Shafiq Z.; Alam M. M.; Imran M.; Akram M. S. Exploration of efficient electron acceptors for organic solar cells: rational design of indacenodithiophene based non-fullerene compounds. Sci. Rep. 2021, 11, 19931. 10.1038/s41598-021-99254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq M.; Ali A.; Nawaz Tahir M. N.; Khalid M.; Assiri M. A.; Imran M.; Shahzad Munawar K. S.; Habiba U. Synthetic approach to achieve halo imine units: Solid-state assembly, DFT based electronic and non linear optical behavior. Chem. Phys. Lett. 2022, 803, 139843. 10.1016/j.cplett.2022.139843. [DOI] [Google Scholar]
- Mahmood A.; Abdullah M. I.; Nazar M. F. Quantum chemical designing of novel organic non-linear optical compounds. Bull. Korean Chem. Soc. 2014, 35, 1391–1396. 10.5012/bkcs.2014.35.5.1391. [DOI] [Google Scholar]
- Khalid M.; Zafar M.; Hussain S.; Asghar M. A.; Khera R. A.; Imran M.; Abookleesh F. L.; Akram M. Y.; Ullah A. Influence of End-Capped Modifications in the Nonlinear Optical Amplitude of Nonfullerene-Based Chromophores with a D−π-A Architecture: A DFT/TDDFT Study. ACS Omega 2022, 7, 23532–23548. 10.1021/acsomega.2c02052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan T.; Hussain R.; Khan M. U.; Habiba U.; Irshad Z.; Adnan M.; Lim J. Development of non-fused acceptor materials with 3D-Interpenetrated structure for stable and efficient organic solar cells. Mater. Sci. Semicond. Process. 2022, 151, 107010. 10.1016/j.mssp.2022.107010. [DOI] [Google Scholar]
- Rafiq A.; Hussain R.; Khan M. U.; Mehboob M. Y.; Khalid M.; Shehnaz M. M.; Alam M.; Imran K.; Ayub K. Novel Star-Shaped Benzotriindole-Based Nonfullerene Donor Materials: Toward the Development of Promising Photovoltaic Compounds for High-Performance Organic Solar Cells. Energy Technol. 2022, 10, 2100751. 10.1002/ente.202100751. [DOI] [Google Scholar]
- Mahmood A.; Khan S. U.-D.; Rana U. A.; Tahir M. H. Red shifting of absorption maxima of phenothiazine based dyes by incorporating electron-deficient thiadiazole derivatives as π-spacer. Arab. J. Chem. 2019, 12, 1447–1453. 10.1016/j.arabjc.2014.11.007. [DOI] [Google Scholar]
- Mahmood A.; HussainTahir M.; Irfan A.; Khalid B.; Al-Sehemi A. G. Computational Designing of Triphenylamine Dyes with Broad and Red-shifted Absorption Spectra for Dye-sensitized Solar Cells using Multi-Thiophene Rings in π-Spacer. Bull. Korean Chem. Soc. 2015, 36, 2615–2620. 10.1002/bkcs.10526. [DOI] [Google Scholar]
- Mahmood A.; Irfan A. Computational analysis to understand the performance difference between two small-molecule acceptors differing in their terminal electron-deficient group. J. Comput. Electron. 2020, 19, 931–939. 10.1007/s10825-020-01494-6. [DOI] [Google Scholar]
- Khalid M.; Khan M. U.; Ahmed S.; Shafiq Z.; Alam M. M.; Imran M.; Braga A. A. C.; Akram M. S. Exploration of promising optical and electronic properties of (non-polymer) small donor molecules for organic solar cells. Sci. Rep. 2021, 11, 21540. 10.1038/s41598-021-01070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. U.; Khalid M.; Hussain R.; Umar A.; Mehboob M. Y.; Shafiq Z.; Imran M.; Irfan A. Novel W-Shaped Oxygen Heterocycle-Fused Fluorene-Based Non-Fullerene Acceptors: First Theoretical Framework for Designing Environment-Friendly Organic Solar Cells. Energy Fuels 2021, 35, 12436–12450. 10.1021/acs.energyfuels.1c01582. [DOI] [Google Scholar]
- Janjua M. R. S. A.; Khan M. U.; Khalid M.; Ullah N.; Kalgaonkar R.; Alnoaimi K.; Baqader N.; Jamil S. Theoretical and conceptual framework to design efficient dye-sensitized solar cells (DSSCs): molecular engineering by DFT method. J. Cluster Sci. 2021, 32, 243–253. 10.1007/s10876-020-01783-x. [DOI] [Google Scholar]
- Khan M. U.; Khalid M.; Arshad M. N.; Khan M. N.; Usman M.; Ali A.; Saifullah B. Designing Star-Shaped Subphthalocyanine-Based Acceptor Materials with Promising Photovoltaic Parameters for Non-fullerene Solar Cells. ACS Omega 2020, 5, 23039–23052. 10.1021/acsomega.0c02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A.; Irfan A. Effect of fluorination on exciton binding energy and electronic coupling in small molecule acceptors for organic solar cells. Comput. Theor. Chem. 2020, 1179, 112797. 10.1016/j.comptc.2020.112797. [DOI] [Google Scholar]
- Mahmood A.; Khan S. U.-D.; Rana U. A. Theoretical designing of novel heterocyclic azo dyes for dye sensitized solar cells. J. Comput. Electron. 2014, 13, 1033–1041. 10.1007/s10825-014-0628-2. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G.; et al. Gaussian 09, Revision D. 01; Gaussian. Inc.: Wallingford, CT, 2009.
- Dennington R. D.; Keith T. A.; Millam J. M.. GaussView 5.0. 8; Gaussian Inc., 2008.
- Civalleri B.; Zicovich-Wilson C. M.; Valenzano L.; Ugliengo P. B3LYP augmented with an empirical dispersion term (B3LYP-D*) as applied to molecular crystals. CrystEngComm 2008, 10, 405–410. 10.1039/b715018k. [DOI] [Google Scholar]
- Yanai T.; Tew D. P.; Handy N. C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. 10.1016/j.cplett.2004.06.011. [DOI] [Google Scholar]
- Zhao Y.; Truhlar D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Adamo C.; Barone V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 1998, 108, 664–675. 10.1063/1.475428. [DOI] [Google Scholar]
- Chai J.-D.; Head-Gordon M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
- Barone V.; Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. 10.1021/jp9716997. [DOI] [Google Scholar]
- O’boyle N. M.; Tenderholt A. L.; Langner K. M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. 10.1002/jcc.20823. [DOI] [PubMed] [Google Scholar]
- Lu T.; Chen F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Ans M.; Ayub K.; Bhatti I. A.; Iqbal J. Designing indacenodithiophene based non-fullerene acceptors with a donor-acceptor combined bridge for organic solar cells. RSC Adv. 2019, 9, 3605–3617. 10.1039/c8ra09292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus R. A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599. 10.1103/revmodphys.65.599. [DOI] [Google Scholar]
- Tang S.; Zhang J. Design of donors with broad absorption regions and suitable frontier molecular orbitals to match typical acceptors via substitution on oligo(thienylenevinylene) toward solar cells. J. Comput. Chem. 2012, 33, 1353–1363. 10.1002/jcc.22966. [DOI] [PubMed] [Google Scholar]