Abstract
The transgender community has expressed concerns regarding drug-drug interactions between HIV-pre-exposure prophylaxis (PrEP) and gender-affirming hormones. In this study, we evaluated emtricitabine (F, FTC)/tenofovir (TFV) disoporoxil fumarate (TDF) pharmacokinetics (PK) among adolescent and young adult transgender persons receiving gender-affirming hormone therapy (GAHT). This was a prospective, observational study among transgender women (TW) and men (TM) without HIV, 15–24 years of age, receiving GAHT (estradiol with/without spironolactone, or testosterone). Participants received 1 month of directly observed daily F/TDF. Weekly convenience blood samples were collected for plasma TFV and FTC, and intracellular TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) in peripheral blood mononuclear cells (PBMC) and dried blood spots (DBS). After 2–3 weeks of F/TDF dosing, intensive PK sampling was conducted. PK parameters were estimated using noncompartmental methods. Data were log-transformed and compared between TM and TW, and to historical data among cisgender adults. Plasma TFV exposures were similar between TM and TW [geometric mean ratio (GMR); confidence interval (95% CI): 1.06 (0.89–1.28)], whereas FTC plasma exposures were 21% higher in TM versus TW (95% CI: 1.07–1.38). TFV-DP in PBMC and DBS and FTC-TP in DBS did not differ between TM versus TW after controlling for creatinine clearance (CrCl), but FTC-TP in PBMC remained 46% (95% CI: 1.15–1.86) higher in TM versus TW. All PK exposures were within expected ranges based on historical studies. TM had higher FTC exposures compared with TW, but overall plasma and intracellular exposures for both drugs were within the range of historical studies, suggesting high PrEP efficacy will be retained in adolescent and young adult transgender persons. Registered at ClinicalTrials.gov (NCT03652623)
Keywords: transgender women, transgender men, PrEP, pharmacology, F/TDF
Background
Transgender men (TM) and transgender women (TW) are at a higher risk of HIV infection compared to the general U.S. population,1 emphasizing the potential positive impact pre-exposure prophylaxis (PrEP) could have on this population. Despite the knowledge of their increased vulnerability, transgender individuals have previously been underrepresented in clinical trials for PrEP.2,3 Historically, TM are often excluded from these trials and TW are typically grouped with cisgender men who have sex with men, making up only a small percentage of the study population.
The lack of data in TM and TW from clinical trials has led to concerns about the potential for drug-drug interactions between PrEP and exogenous hormones (i.e., estradiol in TW and testosterone in TM). Recently, there have been a few pharmacokinetic (PK) studies that were conducted mostly in adult TW to assess whether such interactions exist.4–7 The results of these studies have varied, with some showing significantly lower plasma exposures of tenofovir (TFV) and/or emtricitabine (F,FTC),4,6,7 whereas others have shown no difference in TW versus cisgender controls.5 It is important to understand the effects of gender-affirming hormone therapy (GAHT) not only on plasma but also on intracellular concentrations, as peripheral blood mononuclear cells (PBMC) include cells where HIV establishes infection, and thus are the target cells for these drugs.
Furthermore, the extended half-life of TFV-diphosphate (DP) in red cells, measured by dried blood spots (DBS), allows us to understand the long-term exposure and adherence to PrEP. Among studies that also compared TFV-DP and FTC-triphosphate (FTC-TP) in PBMC, there was no significant difference in concentrations of either anabolite between TW and cisgender controls, but the studies generally lacked statistical power.5,6 Thus, the potential drug-drug interaction between exogenous estradiol use among adult TW and F/tenofovir disoporoxil fumarate (TDF) remains unresolved.4–7 Furthermore, fewer data exist for TM receiving exogenous testosterone and among adolescent transgender individuals.
The objectives of this study (i.e., the TransPrEP study) were to (1) compare plasma TFV and FTC PK in TM versus TW, (2) compare TFV-diphosphate (TFV-DP) and FTC-TP in PBMC and DBS in TM versus TW, and (3) evaluate these concentrations relative to historical concentrations in cisgender adults without HIV. Serum hormone concentrations were also evaluated in the TransPrEP study, and are reported separately.
Methods
Study design
TransPrEP was a prospective, PK study conducted at the University of Colorado Anschutz Medical Campus and the Stroger Hospital of Cook County. All research was conducted in accordance with the Declaration of Helsinki, national and institutional standards. The study was approved by both the Colorado Multiple Institution Review Board (COMIRB) and the Cook County Health Institutional Review Board and is registered at ClinicalTrials.gov (NCT03652623). Written informed consent was provided by all participants.
Study participants
Study participants were TM and TW between 15 and 24 years of age without HIV. They had to be receiving a stable hormone (estradiol ± spironolactone or testosterone) dose for at least 1 month or three consecutive doses, whichever was longer, before enrollment and be willing to continue this same dose throughout the study period. To minimize variability, only those receiving oral/sublingual or intramuscular estradiol, and only subcutaneous or intramuscular testosterone were included, as these were the most common formulations at each study site. Exclusion criteria were as follows: recent hospitalization, a condition that precludes their ability to complete study procedures, previous participation in an HIV vaccine study, use of F/TDF in the previous 3 months, or a Grade 3 or higher lab abnormality.
Procedures
Participants continued their current hormone regimen (i.e., estradiol ± spironolactone in TW or testosterone in TM) and dosing schedule for the entirety of the study. They received 1 month of directly observed (DOT) daily F/TDF. Observation of dosing was conducted either in person or by timestamped recorded video using a smart phone application. Dates and times of each dose were then recorded by study staff.
Weekly visits were conducted throughout the 1-month study, where convenience blood samples were collected for TFV, FTC, TFV-DP, and FTC-TP quantification. After 2 to 3 weeks of DOT F/TDF dosing (steady state for plasma and PBMC), intensive PK sampling was conducted. Blood samples were collected at 0 (pre-dose), 1, 2, 4, 6, 8, and 24 h post-dose for measurement of TFV and FTC in plasma. TFV-DP and FTC-TP were also measured in PBMC at 0 (pre-dose), 4, and 24 h post-dose. All participants were asked to fast for ∼10 h before the intensive PK visit, and to continue fasting ∼4 h post-dose.
For DBS, 25 μL of whole blood was spotted onto a Whatman 903 protein saver card five times. The cards were then air dried at room temperature for at least 3 h (up to overnight) before being stored at −80°C until analysis. For PBMC, blood from an EDTA tube was centrifuged with lymphocyte separation medium and the PBMC buffy layer was removed. This was followed by washes, red cell lysis, and counting of PBMC by an automated hemocytometer. PBMC were then lysed and suspended in 500 μL of cold 70/30 methanol/water and stored at −80°C until analysis.
TFV, FTC, TFV-DP, and FTC-TP were quantified using previously validated LC-MS/MS methods.8,9 The lower limit of quantitation for TFV and FTC was 10 ng/mL.9 For PBMC concentrations, values are reported as fmol/106 cells (TFV-DP) or pmol/106 cells (FTC-TP). For DBS, concentrations are quantified from one 3 mm punch and are reported as fmol/punch (TFV-DP) or pmol/punch (FTC-TP). The quantifiable linear range of the assay is 25–6,000 fmol/sample for TFV-DP and 0.1–200 pmol/sample for FTC-TP.
Sample size
This study was powered based on a comparison of plasma TFV area under the curve (AUC) to a historical control group in the Cell-PrEP Study.9 A sample size of 48 participants (24 TW and 24 TM) would provide at least 80% power to detect a difference of ∼17% in TFV AUC versus historical controls, assuming the standard deviation remained the same.
Analyses
Baseline clinical and demographic variables were summarized and compared between TW and TM using an analysis of variance. Continuous variables were reported as mean ± SD and categorical as N (%). Baseline variables included age, weight, creatinine clearance (CrCl), race/ethnicity, route of exogenous hormone use, and spironolactone use. CrCl was calculated using actual body weight and sex at birth. Schwartz equation was used if participants were <18 years of age and Cockcroft-Gault equation was used if they were 18 years of age or older.10,11
Plasma TFV and FTC PK parameters were determined from concentrations at intensive PK sampling time points, including AUCtau, maximum concentration (Cmax), time to maximum concentration (Tmax), trough concentration (Ctau), and half-life (T1/2) and PBMC TFV-DP and FTC-TP AUCs were calculated using noncompartmental methods (Phoenix WinNonlin version 8.2). PBMC average concentrations (Cavg) were calculated as AUClast/Tlast. PBMC PK parameters used to calculate Cavg were determined from concentrations at intensive PK sampling time points using noncompartmental methods. Analysis of TFV-DP and FTC-TP concentrations in DBS focused on week 4 results alone owing to the long half-life of TFV-DP in red blood cells (RBCs). All PK results were log-transformed before analysis.
Comparisons were performed between TM and TW using linear regression. Covariates, including CrCl, age, weight, and race, were univariately assessed as predictors of each of the PK outcomes. Exploratory, post hoc subgroup analyses were conducted to compare PK outcomes between TW receiving oral (PO)/sublingual (SL) estradiol versus intramuscular (IM) estradiol, between TW not taking versus taking spironolactone, and between TM taking subcutaneous versus intramuscular testosterone. All results were back-transformed and reported on the original scale.
In addition to the primary comparisons between TM and TW, PK results were also qualitatively compared against previous studies to further assess findings between different studies for clinical relevance. Historical data from cisgender controls were previously published values from past studies. Studies to be used as reference in this comparison were selected because they were another transgender PK study (plasma), and/or an intensive PK study (PBMC or DBS concentrations).
Studies included Cell-PrEP [NCT01040091; N = 34 (22 male, 12 female), median (range) age = 31 (20–52) years], A PK Evaluation of TFV/FTC as HIV PrEP in TW [Cirrincione et al.; NCT03270969; N = 19 (15 male, 4 female), mean (range) age = 26 (19–41) years], Finding the Right TFV/FTC Regimen for PrEP in TW [Shieh et al.6; NCT03060785; N = 8 cisgender men, median (IQR) age = 46 (28–52) years], iFACT [Hiransuthikul et al.7; NCT03620734; N = 20 TW not receiving GAHT, median (IQR) age = 21.5 (21–26) years], HPTN066 [Hendrix et al.12; NCT01276600; N = 15 (5 male, 10 female), median (IQR) age = 31 (24–37) years], and DOT-DBS [NCT02022657; N = 32 (15 cisgender men, 17 cisgender women), median (IQR) age = 28 (26–39) years for cisgender men and 30 (27–32) years for cisgender women].
Results
Available data were included in analyses up until the time in which participants withdrew/were lost to follow-up. Fifty (26 TW and 24 TM) participants were enrolled and included in analyses. Baseline clinical and demographic characteristics were similar between TM and TW (Table 1). Although not significant, TW had higher CrCl compared with TM.
Table 1.
Baseline Clinical and Demographic Characteristics of Study Population
| Transgender women (N = 26) | Transgender men (N = 24) | p | |
|---|---|---|---|
| Age | 19.9 ± 2.5 | 20.3 ± 2.3 | .551 |
| Weight (kg) | 69.1 ± 11.9 | 68.5 ± 21.4 | .893 |
| CrCl (mL/min) | 136.2 ± 34.4 | 116.9 ± 48.4 | .108 |
| Race/ethnicitya | |||
| White | 19 (73.1%) | 20 (83.3%) | .462 |
| Black | 4 (15.4%) | 2 (8.3%) | |
| Asian | 0 (0%) | 2 (8.3%) | |
| Hispanic | 9 (39.1%) | 2 (8.7%) | |
| Other | 1 (3.9%) | 2 (8.3%) | |
| Route of estradiol administration | |||
| Oral/sublingual | 14 (53.9%) | — | |
| Intramuscular | 12 (46.2%) | ||
| Route of testosterone administration | |||
| Subcutaneous | — | 12 (50%) | |
| Intramuscular | 12 (50%) | ||
| Spironolactone useb | 18 (69.2%) | — | |
All values presented as mean ± SD or n (%).
Participants may have self-reported as multiple races or ethnicities.
There were eight transgender women receiving progesterone, one receiving finasteride, and one receiving leuprolide as part of their gender affirming hormone therapy.
CrCl, Creatinine clearance.
Plasma TFV and FTC PKs
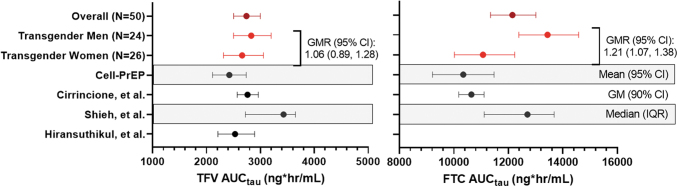
Plasma TFV PK parameters in TM and TW, as well as those previously reported from past studies among cisgender participants,4,6,7 are shown in Figure 1 and Supplementary Table S1. There was no significant difference in geometric mean [GM; confidence interval (95% CI)] TFV AUCtau between TW [2,660 (95% CI: 2,314–3,057) ng·h/mL] and TM [2,829 (2,502–3,199) ng·h/mL]; geometric mean ratio, GMR (95% CI): 1.06 (0.89–1.28). Overall exposures were similar to those seen among historical cisgender controls.4,6,7 Within TW and within TM, there was no difference in TFV PK parameters by route of exogenous hormone administration (oral/sublingual vs. intramuscular estradiol for TW and subcutaneous vs. intramuscular testosterone for TM). In addition, there was no significant association between spironolactone use and TFV PK among TW. These results are shown in Supplementary Table S2.
FIG. 1.
Plasma TFV and FTC Exposures. Plasma exposures (AUCtau) of TFV and FTC are shown [as geometric mean, GM (95% CI)] for the overall study population, and among TW and TM. Shown in black are previously published AUC values among cisgender participants from separate studies. Unless otherwise labeled, they are expressed as GM (95% CI).4,6,7,9 Hiransuthikul et al.7 reported TFV GM AUC (CV%); this was converted to GM (95% CI); FTC exposures were not reported for this study. The Cell-PrEP study9 reported mean (95% CI) Css, which was calculated as AUC/24 h; mean (95% CI) AUC was then determined for comparison here as reported Css × 24 h. AUC, area under the curve; CI, confidence interval; FTC, emtricitabine; GM, geometric mean; GMR, geometric mean ratio; PrEP, pre-exposure prophylaxis; TFV, tenofovir; TM, transgender men; TW, transgender women.
As shown in Figure 1 and Supplementary Table S3, TM had a significantly higher plasma FTC Cmax [GMR (95% CI): 1.25 (1.04–1.50), p = .019] and AUCtau [GMR (95% CI): 1.21 (1.07–1.38), p = .004] versus TW. Exposures in TW were similar to previously reported values from historical cisgender controls, whereas TM had higher exposures than those of historical controls.4,6 After adjusting for CrCl, the difference in Cmax was no longer statistically significant [GMR (95% CI): 1.20 (1.00–1.44), p = .052], but remained significant for AUCtau [GMR (95% CI): 1.16 (1.03–1.30), p = .016]. Similar to TFV, there was no significant difference in FTC PK within TW and within TM by route of exogenous hormone administration, or within TW by spironolactone use (Supplementary Table S4).
TFV-DP and FTC-TP in PBMC
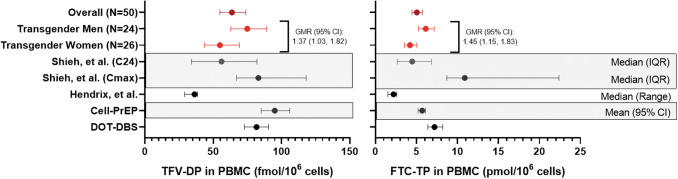
TFV-DP and FTC-TP Cavg in PBMC, as well as previously reported concentrations among cisgender participants,6,9,12 are presented in Figure 2 and Supplementary Table S5. Overall GM (95% CI) TFV-DP Cavg in PBMC was 63.7 (54.9–73.9) fmol/106 cells, with TM having 37% higher TFV-DP [GMR (95% CI): 1.37 (1.03–1.82), p = .034] and 45% higher FTC-TP [GMR (95% CI): 1.45 (1.15–1.83), p = .002]. After controlling for CrCl, the difference in Cavg PBMC TFV-DP was no longer statistically significant [GMR (95% CI): 1.23 (0.95–1.66), p = .104]. However, controlling for renal function (CrCl) did not account for differences in Cavg FTC-TP between groups [GMR (95% CI): 1.46 (1.15–1.86)].
FIG. 2.
Cavg of TFV-DP and FTC-TP in PBMC. PBMC concentrations of TFV-DP and FTC-TP are shown for the overall study population, and among TW and TM. Shown in black are previously published values among cisgender participants from three separate studies.6,9,12,13 Unless otherwise labeled, values are shown as geometric mean (95% CI). Cavg, average concentrations; DBS, dried blood spots; FTC-TP, emtricitabine triphosphate; PBMC, peripheral blood mononuclear cells; TFV-DP, TFV-diphosphate.
Among TW, those receiving oral/sublingual estradiol had significantly higher TFV-DP in PBMC versus those receiving intramuscular estradiol [GMR (95% CI): 1.67 (1.09–2.55), p = .019]. Similarly, TW receiving oral/sublingual estradiol had significantly higher FTC-TP in PBMC versus TW receiving intramuscular estradiol [GMR (95% CI): 1.68 (1.25–2.25), p = .001]. Adjusting for renal function did not account for differences seen in either TFV-DP or FTC-TP in PBMC among TW by route of estradiol administration (p = .035 and .003, respectively). TW using spironolactone had significantly higher TFV-DP in PBMC [GMR (95% CI): 1.61 (1.00–2.56), p = .0498], but not FTC-TP, vs. those not using spironolactone. However, this was no longer statistically significant after adjusting for CrCl [GMR (95% CI): 1.49 (0.94–2.38), p = .087]. There was no difference observed among TM receiving subcutaneous vs. intramuscular testosterone in TFV-DP or FTC-TP in PBMC (p = .589 and .157, respectively).
TFV-DP and FTC-TP in DBS
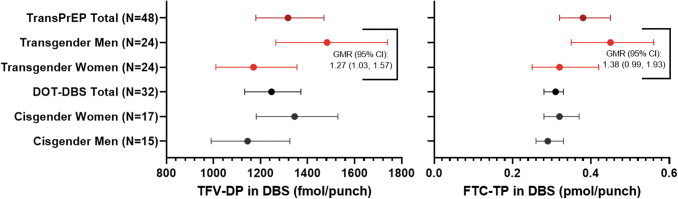
Concentrations of TFV-DP and FTC-TP in DBS at week 4 are shown in Figure 3 and Supplementary Table S6. Two TW had missing week 4 samples and were not included in this analysis. Overall GM (95% CI) TFV-DP concentrations were 1,317 (1.180–1.470) fmol/punch and FTC-TP concentrations were 0.38 (0.32–0.45) pmol/punch. TM had significantly higher TFV-DP, but not FTC-TP, in DBS at week 4 versus TW [GMR (95% CI): 1.27 (1.03–1.57), p = .028]. However, this difference was no longer statistically significant after controlling for renal function (p = .098). Compared to cisgender women and cisgender men receiving daily F/TDF in the DOT-DBS study,13 observed week 4 concentrations of both TFV-DP and FTC-TP in DBS were comparable to those seen in TW and TM (Fig. 3 and Supplementary Table S6).
FIG. 3.
Week 4 concentrations of TFV-DP and FTC-TP in DBS. All concentrations are shown as geometric mean (95% CI). Concentrations of TFV-DP and FTC-TP are shown for the overall study population, TW, and TM in red. The DOT-DBS study was a randomized crossover study in which participants received directly observed TDF/FTC for 12 weeks.13 They were assigned to two DOT treatment regimens of 33%, 67%, or 100% daily dosing, separated by a 12-week washout. Since all TransPrEP participants were instructed to take TDF/FTC daily, only those participants randomized to 100% daily dosing were included in this summary. DBS concentrations from the DOT-DBS study following 4 weeks of 100% dosing (i.e., week 4 or 28 for those randomized to 100% dosing for the first or second dosing regimen, respectively) are shown in black for the overall study population, cisgender women, and cisgender men. TDF, tenofovir disoporoxil fumarate.
Among TW receiving oral/sublingual versus intramuscular estradiol, there was no significant difference in TFV-DP in DBS [GM (95% CI): 1,251 (980–1,598) vs. 1,080 (907–1,288) fmol/punch; GMR (95% CI): 1.16 (0.86–1.56), p = .313] or FTC-TP in DBS [GM (95% CI): 0.28 (0.18–0.43) vs. 0.38 (0.28–0.52) pmol/punch; GMR (95% CI): 0.73 (0.44–1.22), p = .216]. In TW not taking versus those taking spironolactone, there was also no difference in TFV-DP in DBS [GM (95% CI): 1,086 (784–1,504) vs. 1,214 (1,018–1,449) fmol/punch; GMR (95% CI): 0.89 (0.65–1.23), p = .472] or FTC-TP in DBS [GM (95% CI): 0.33 (0.16–0.68) vs. 0.32 (0.25–0.41) pmol/punch; GMR (95% CI): 1.02 (0.58–1.78), p = .949].
Similarly, between TM receiving subcutaneous versus intramuscular testosterone, there was no difference in TFV-DP in DBS [GM (95% CI): 1,629 (1,278–2,077) vs. 1,351 (1,072–1,702); GMR (95% CI): 1.21 (0.88–1.65), p = .231] or FTC-TP in DBS [GM (95% CI): 0.46 (0.40–0.54) vs. 0.43 (0.26–0.68); GMR (95% CI): 1.09 (0.69–1.75), p = .693].
Discussion
This was a prospective, observational, PK study of F/TDF among adolescent and young adult TM and TW without HIV receiving GAHT. Plasma TFV PK was similar in TM and TW, but plasma FTC exposures were higher in TM versus TW. In PBMC, TM had higher concentrations of both TFV-DP and FTC-TP versus TW, and significantly higher TFV-DP in DBS versus TW.
In both PBMC and DBS, differences in TFV-DP concentrations appeared to be partially due to differences in renal function between groups. We did not observe significantly higher plasma TFV exposures with TM versus TW, as expected with differences attributed to renal function. It is possible that plasma TFV concentrations in TM were slightly, nonsignificantly higher than those in TW, which over time accumulated and manifested as significantly higher intracellular concentrations of TFV-DP, as was observed here. In addition, we calculated CrCl using identified gender and mean values were 133.6 and 120.5 mL/min for TM and TW, respectively. Differences in TFV-DP in PBMC and DBS remained significant after controlling for CrCl calculated using identified gender.
Importantly, when compared to historical cisgender controls in previously published studies,4–7,9,12,13 the plasma TFV/FTC PK parameters and intracellular anabolite concentrations observed in both TM and TW appeared to fall within or above the ranges of previously reported values. This provides reassurance that the use of exogenous hormone therapy does not affect F/TDF PK among adolescent and young adult TM and TW without HIV to a clinically meaningful extent and suggests that F/TDF should continue to be offered as PrEP for those at risk of HIV.
The higher FTC and FTC-TP exposures in TM compared with TW are unlikely important clinically, given the extensive safety history for FTC, but the potential mechanism(s) is unclear. A study in mice showed increased OCT2 and MATE1 expression with testosterone, which would theoretically increase FTC clearance, but this is not consistent with our findings.14 In general, interpretation is difficult because drug interaction studies with testosterone in humans are lacking, which highlights a need for future research.15
In a post hoc analysis, we found that within TW, those taking oral/sublingual estradiol had significantly higher TFV-DP and FTC-TP Cavg in PBMC versus participants taking intramuscular estradiol. Similarly higher TFV-DP was observed in TW receiving versus not receiving spironolactone, but this relationship was not significant after controlling for renal function. It is important to note that these findings are hypothesis-generating and should not influence changes in practice.
These relationships were not observed for TFV or FTC in plasma, or either anabolite in DBS, which is puzzling. It should be noted that all intramuscular participants were from the Chicago site and the PO participants from Denver. Although the same cell-processing procedures were used, PBMC analyses are exquisitely dependent on cell processing/counting and a potential site bias cannot be ruled out. Biologically, in vitro studies suggest higher TFV-DP in epithelial cells exposed to estradiol, with no effect on immunologic cells, which would not explain these findings.16 Taken together, these findings require confirmation and if confirmed, follow-up studies.
This study had many strengths, including the unique populations, its prospective design, the use of directly observed F/TDF to ensure adherence was accurately measured, and the measurement of TFV, FTC, and their active anabolites across multiple matrices. A limitation of this study is the lack of a within-person comparison. Because of this, we reported the values among cisgender controls in past PK studies,4–7,9,12,13 and formal statistical comparisons were not conducted due to differences in study designs and sampling. Another limitation is that concentrations at the potential sites of HIV infection were not assessed, such as genital tract concentrations. It is unclear to what extent concentrations at these sites translate to prevention efficacy of systemic PrEP agents, but this is something that should be evaluated further.
Furthermore, we did not assess differences in endogenous nucleotides, which could contribute to pharmacologic response.5 Importantly, this was a PK study that did not directly assess the effectiveness of PrEP in this population. Finally, the majority of participants in this study were white, and only TW receiving estradiol through oral/sublingual or intramuscular routes of administration were included in the study. This limited our ability to assess the influence of race, and alternate forms of hormone administration (e.g., transdermal estradiol) on F/TDF in adolescent transgender persons.
In conclusion, compared with TW, TM had significantly higher PBMC concentrations of TFV-DP and FTC-TP, higher TFV-DP in DBS, and higher FTC in plasma. However, some of these relationships were due to differences in renal function, and concentrations still fell within the range of previously reported values for cisgender controls in past studies.4,6,7,12 These findings should be reassuring to patients and providers with concerns regarding potential for drug-drug interactions between GAHT and PrEP. Daily F/TDF should continue to be recommended as PrEP for adolescent and young adult transgender persons at risk of HIV.
Supplementary Material
Author Disclosure Statement
J.Y. is an employee of Gilead Sciences and holds stock interest in the company. P.L.A. has received consulting fees from Gilead, ViiV, and Merck, and grants from Gilead Sciences, paid to his institution.
Funding Information
This study was funded by the National Institute of Mental Health (R01 MH114753). Study drug was provided by Gilead Sciences.
Supplementary Material
References
- 1. The Center for Disease Control and Prevention. HIV and Transgender Communities. [Issue Brief]. 2022. Available from: https://www.cdc.gov/hiv/pdf/policies/data/cdc-hiv-policy-issue-brief-transgender.pdf. [Last accessed: August 31, 2022].
- 2. Deutsch MB, Glidden DV, Sevelius J, et al. HIV pre-exposure prophylaxis in transgender women: A subgroup analysis of the iPrEx trial. Lancet HIV 2015;2(12):e512–e519; doi: 10.1016/S2352-3018(15)00206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020;396(10246):239–254; doi: 10.1016/S0140-6736(20)31065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cirrincione LR, Podany AT, Havens JP, et al. Plasma and intracellular pharmacokinetics of tenofovir disoproxil fumarate and emtricitabine in transgender women receiving feminizing hormone therapy. J Antimicrob Chemother 2020;75(5):1242–1249; doi: 10.1093/jac/dkaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cottrell ML, Prince HMA, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: Implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis 2019;69(12):2201–2204; doi: 10.1093/cid/ciz290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shieh E, Marzinke MA, Fuchs EJ, et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J Int AIDS Soc 2019;22(11):e25405; doi: 10.1002/jia2.25405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hiransuthikul A, Janamnuaysook R, Himmad K, et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: The iFACT study. J Int AIDS Soc 2019;22(7):e25338; doi: 10.1002/jia2.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng JH, Rower C, McAllister K, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016;122:16–20; doi: 10.1016/j.jpba.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seifert SM, Chen X, Meditz AL, et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses 2016;32(10–11):981–991; doi: 10.1089/AID.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20(3):629–637; doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16(1):31–41; doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12. Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016;32(1):32–43; doi: 10.1089/AID.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018;62(1); doi: 10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He R, Ai L, Zhang D, et al. Different effect of testosterone and oestrogen on urinary excretion of metformin via regulating OCTs and MATEs expression in the kidney of mice. J Cell Mol Med 2016;20(12):2309–2317; doi: 10.1111/jcmm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cirrincione LR, Huang KJ. Sex and gender differences in clinical pharmacology: implications for transgender medicine. Clin Pharmacol Ther 2021;110(4):897–908; doi: 10.1002/cpt.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen Z, Fahey JV, Bodwell JE, et al. Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture. PLoS One 2014;9(6):e100863; doi: 10.1371/journal.pone.0100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.