Abstract
Background:
Limited literature is available to define the impact of the longus colli muscle, a deep flexor of the spine, on cervical spine stability despite its close proximity to the vertebrae.
Aims and Objectives:
The purpose of this study was to determine if longus colli cross-sectional area (CSA) is associated with the severity preoperative cervical degenerative spondylolisthesis.
Materials and Methods:
Patients undergoing elective anterior cervical discectomy and fusion (ACDF) for cervical spondylolisthesis between 2010-2021 were retrospectively identified. Longus colli cross-sectional areas (CSA) were measured from preoperative MRI images at the C5 level. Preoperative spondylolisthesis measurements were recorded with cervical radiographs. Patients were grouped by quartiles respectively according to longus colli CSAs. Statistical tests compared patient demographics, surgical characteristics, and surgical outcomes between groups. Multiple linear regression analysis was utilized to assess if longus colli CSA predicted cervical spondylolisthesis.
Results:
A total of 157 patients met inclusion criteria. Group 1 (first quartile) was the oldest (60.4 ± 12.0 years, P = 0.024) and was predominantly female (59.0%, P = 0.001). Group 1 also had the highest maximum spondylolisthesis (0.19 mm, P = 0.031) and highest proportion of grade 2 spondylolisthesis (23.1%, P = 0.003). On regression analysis, lowest quartile of longus colli CSA was an independent predictor of larger measured maximum spondylolisthesis (β: 0.04, P = 0.012).
Conclusion:
Smaller longus colli CSA is independently associated with a higher grade and degree of preoperative cervical spondylolisthesis, but this finding does not result in adverse postsurgical outcomes.
Keywords: Cervical, longus colli, spine, spondylolisthesis
INTRODUCTION
Spinal biomechanics has predominantly focused on the unique relationship that exists between bony and soft tissue architecture. One of the first widely accepted theories of spinal biomechanics was proposed by Panjabi in the early 1990s, which described three essential components of the spinal column: The bony architecture (passive support), the soft tissue and muscular architecture (active support), and the neural coordination that balances the two components based on a person's position in 3-dimensional space.[1,2] In the lumbar spine, Bergmark's biomechanical model of stability further subclassified the muscular component of the above system into either global (providing truncal) or local (providing segmental) support.[3,4] Disease in one or more of the above spinal components may alter the function of the other, especially since many spine abnormalities are associated with segmental spine instability.
Evidence in the lumbar spine literature highlights an association between disease within the small lumbar stabilizers and spinal instability.[3] It is believed that either atrophy or fatty infiltration within these muscles diminish their ability to properly function. Thus, their ability to provide segmental stability to the lumbar spine is compromised resulting in increased intersegmental micromotion. This may ultimately overwhelm the spinal column support provided by the bony architecture leading to instability and ultimately spondylolisthesis.[3] Previous literature has evaluated the role of ultrasound and magnetic resonance imaging (MRI) to measure cross-sectional areas (CSAs) of the paraspinal muscles, thus allowing physicians to assess their morphology.[3] Utilizing these modalities, research has suggested that smaller multifidus and transversus abdominis CSAs result in greater spondylolisthesis compared to patients with more robust abdominal and spinal muscles.[3,5] Additionally, it has been demonstrated that women experiencing chronic neck pain have smaller cervical multifidus CSA compared to women without neck pain, suggesting the same principles of segmental instability observed in the lumbar spine may apply to the cervical spine.[6]
However, limited literature is available to define the impact of the longus colli muscle, a deep flexor of the spine, on cervical spine stability despite its close proximity to the vertebrae.[7] Additionally, it has been shown that patients with bilateral chronic mechanical neck pain have smaller longus colli CSA compared with healthy controls.[8] To our knowledge, no studies have investigated longus colli CSA in patients with cervical spondylolisthesis. Therefore, the primary objective of this study aims at elucidating the relationship between longus colli CSA and the extent and severity of spondylolisthesis in a cohort of patients undergoing anterior cervical discectomy and fusion (ACDF) for radiculopathy and/or myelopathy. Secondarily, we will attempt to identify if longus colli CSA is associated with greater readmissions, complications, or revision procedures in patients undergoing ACDF.
METHODS
Patient selection and cohort generation
After approval from the Institutional Review Board, a retrospective cohort study was conducted on patients who underwent one-to five-level ACDF between 2010 and 2021 at a single, academic center for clinical symptoms of radiculopathy, myelopathy, or mixed radiculomyelopathy in the setting of cervical spondylolisthesis. Patient data was obtained using a Structured Query Language search and manual chart review. Patients who lacked a preoperative cervical spine MRI or radiograph or had surgical intervention to address infection, trauma, tumor, or revision of a prior instrumented cervical fusion surgery were excluded from analysis.
Demographics and outcomes
Demographic parameters including age, sex, diabetes status, body mass index (BMI) (kg/m2), Charlson Comorbidity Index (CCI), smoking status (never, current, former smoker), primary preoperative diagnosis, number of levels fused, cervical vertebral levels involved, hospital length of stay, and disposition status were recorded. Subsequently, baseline and postoperative (minimum 1-year postoperative) radiographic measurements and postoperative surgical outcomes, such as readmissions, revisions, and perioperative complications, were collected for everyone.
T1-weighted axial MRI images were evaluated independently by two reviewers. Using our institution's Picture Archiving and Communication System (PACS; Sectra AB, Linköping, Sweden) the CSA of the longus colli muscles were measured. The measurements were recorded using the axial T1 MRI at the midpoint of the C5 vertebral body for statistical comparison and consistency among patients, and the average of the two reviewers' measurements were recorded [Figure 1].[9] The Meyerding classification was utilized to determine the degree of spondylolisthesis using standing flexion and extension lateral radiographs of the cervical spine.[10] Grades I to IV represent translations of 0%–25%, 26%–50%, 51%–75%, 75%–99% and >100%, respectively. For each patient, the highest degree of spondylolisthesis measured among levels fused was determined as the “maximum spondylolisthesis measured.”
Figure 1.
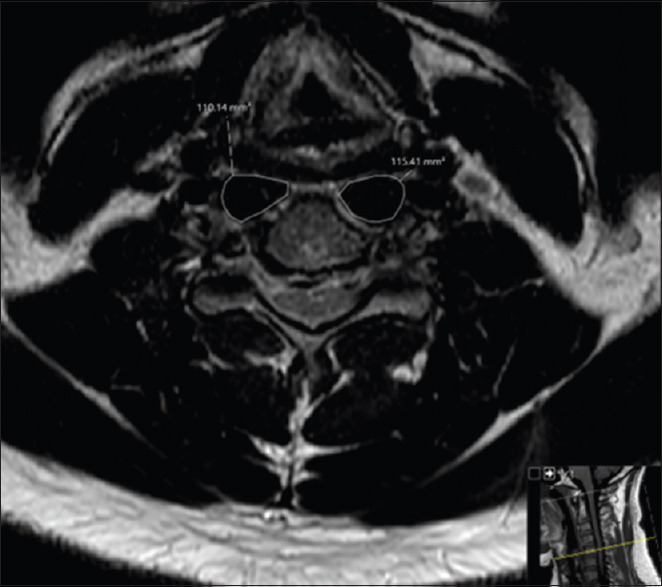
Representative axial T1 magnetic resonance imaging obtained at the C5 vertebral body. The image is representative of measurements obtained for the CSA of the longus colli muscle. CSA - Cross-sectional area
Statistical analysis
Based on the measured longus colli muscle CSA distribution, patients were grouped evenly by quartiles. Each group was compared for differences in demographic characteristics using Pearson Chi-square (χ2) test or Fisher's exact test for categorical variables. Sample means between the two groups were compared using a parametric analysis of variance test or a nonparametric Kruskal–Wallis test. Multiple linear regression analysis was performed to determine whether longus colli CSA significantly predicted the maximum spondylolisthesis after controlling for age and sex. All statistical analyses were conducted using R Studio (Version 3.6.3, Vienna, Austria). The threshold for statistical significance was set at P < 0.05.
RESULTS
Patients were grouped by quartiles according to longus colli CSAs and were comprised of 39, 39, 39 and 40 patients, respectively. The mean area (mm2) for each group was 49.9 ± 6.53 mm2, 64.6 ± 3.19 mm2, 78.8 ± 6.21 mm2, and 106 ± 13.6 mm2, respectively. Patients in Group 1 (first quartile) were the oldest (60.4 ± 12.0 vs. 56.7 ± 12.9 vs. 52.8 ± 11.5 vs. 53.4 ± 12.1 years, P = 0.024) and had the greatest proportion of female patients (59.0% vs. 51.3% vs. 38.5% vs. 17.5%, P = 0.001). There were no statistically significant differences in patients' diabetes status (14.3% vs. 28.0% vs. 32.0% vs. 19.2%, P = 0.408), BMI (28.8 ± 4.69 vs. 30.0 ± 5.30 vs. 29.8 ± 6.52 vs. 29.1 ± 4.63, P = 0.751), CCI (0.41 ± 0.71 vs. 0.53 ± 0.86 vs. 0.38 ± 0.85 vs. 0.29 ± 0.52, P = 0.580), length of stay (1.61 ± 1.06 vs. 1.84 ± 1.75 vs. 1.57 ± 1.89 vs. 1.62 ± 1.28 days, P = 0.447), smoking status (14.8% vs. 23.1% vs. 27.3% vs. 11.5% current smokers, P = 0.479), or discharge to home (97.2% vs. 100% vs. 97.1% vs. 92.1%, P = 0.669) between the groups [Table 1].
Table 1.
Demographics of Cohort
| Variable | 1st quartile (n=39), n (%) | 2nd quartile (n=39), n (%) | 3rd quartile (n=39), n (%) | 4th quartile (n=39), n (%) | P 1, 2 |
|---|---|---|---|---|---|
| Age | 60.4±12.0 | 56.7±12.9 | 52.8±11.5 | 53.4±12.1 | 0.024* |
| Sex | |||||
| Female | 23 (59.0) | 20 (51.3) | 15 (38.5) | 7 (17.5) | 0.001* |
| Male | 16 (41.0) | 19 (48.7) | 24 (61.5) | 33 (82.5) | |
| Diabetes | |||||
| No | 24 (85.7) | 18 (72.0) | 17 (68.0) | 21 (80.8) | 0.408 |
| Yes | 4 (14.3) | 7 (28.0) | 8 (32.0) | 5 (19.2) | |
| BMI | 28.8±4.69 | 30.0±5.30 | 29.8±6.52 | 29.1±4.63 | 0.751 |
| CCI | 0.41±0.71 | 0.53±0.86 | 0.38±0.85 | 0.29±0.52 | 0.580 |
| Smoking status | |||||
| Current | 4 (14.8) | 6 (23.1) | 6 (27.3) | 3 (11.5) | 0.479 |
| Former | 5 (18.5) | 8 (30.8) | 7 (31.8) | 6 (23.1) | |
| Never | 18 (66.7) | 12 (46.2) | 9 (40.9) | 17 (65.4) | |
| Hospital length of stay | 1.61±1.06 | 1.84±1.75 | 1.57±1.89 | 1.62±1.28 | 0.447 |
| Disposition | |||||
| Home | 35 (97.2) | 38 (100) | 33 (97.1) | 35 (92.1) | 0.669 |
| Skilled nursing facility | 1 (2.78) | 0 | 1 (2.94) | 2 (5.26) | |
| Inpatient rehab | 0 | 0 | 0 | 1 (2.63) | |
1ANOVA test or Kruskal–Wallis test for age, BMI, CCI, hospital length of stay, 2Pearson Chi-square test or Fisher’s exact test for sex, diabetes, smoking status, and disposition, *Significance established at P<0.05. ANOVA - Analysis of variance, BMI - Body mass index, CCI - Charlson Comorbidity Index
When evaluating for spondylolisthesis measurements, group 1 had the highest maximum spondylolisthesis measured (0.19 ± 0.07 mm vs. 0.16 ± 0.07 mm vs. 0.16 ± 0.06 mm vs. 0.14 ± 0.04 mm, P = 0.031) and the highest proportion of grade 2 spondylolisthesis (23.1% vs. 2.56% vs. 5.13% vs. 2.50%, P = 0.003). No patients within the cohort had grade 3 or higher spondylolisthesis. There were no differences for levels fused between groups (2.44 ± 0.88 vs. 2.10 ± 0.88 vs. 2.18 ± 0.91 vs. 2.33 ± 0.89, P = 0.356) [Table 2]. Revision procedures (0.00% vs. 2.56% vs. 12.8% vs. 7.50% P = 0.066), 90-day readmission (2.56% vs. 5.13% vs. 5.13% vs. 7.50%, P = 0.958), and surgical post-operative complications (0.00% vs. 0.00% vs. 2.56% vs. 2.50%, P = 1.000) were no different between groups [Table 3]. On regression analysis, longus colli CSA in the first quartile was an independent predictor of increased maximum spondylolisthesis (β: 0.03, P = 0.012) [Table 4].
Table 2.
Spondylolisthesis among longus colli cross-sectional area groups
| Variable | 1st quartile (n=39) | 2nd quartile (n=39) | 3rd quartile (n=39) | 4th quartile (n=39) | P 1 |
|---|---|---|---|---|---|
| Longus colli area (mm2) | 49.9±6.53 | 64.6±3.19 | 78.8±6.21 | 106±13.6 | <0.001* |
| Maximum spondylolisthesis measured (mm) | 0.19±0.07 | 0.16±0.07 | 0.16±0.06 | 0.14±0.04 | 0.031* |
| Spondylolisthesis grade (1-4) | 1.23±0.43 | 1.03±0.16 | 1.05±0.22 | 1.02±0.16 | 0.003* |
| Levels fused | 2.44±0.88 | 2.10±0.88 | 2.18±0.91 | 2.33±0.89 | 0.445 |
1ANOVA test or Kruskal-Wallis test for comparing area groups, *Significance established at P<0.05. ANOVA - Analysis of variance
Table 3.
Complications, 90-day readmissions, and revisions
| Variable | 1st quartile (n=39) | 2nd quartile (n=39) | 3rd quartile (n=39) | 4th quartile (n=39) | P 1 |
|---|---|---|---|---|---|
| 90-day readmissions | |||||
| No | 38 (97.4) | 37 (94.9) | 37 (94.9) | 37 (92.5) | 0.958 |
| Yes | 1 (2.56) | 2 (5.13) | 2 (5.13) | 3 (7.50) | |
| Surgical complications | |||||
| No | 39 (100) | 39 (100) | 38 (97.4) | 39 (97.5) | 1.000 |
| Yes | 0 | 0 | 1 (2.56) | 1 (2.50) | |
| Revisions | |||||
| No | 39 (100) | 38 (97.4) | 34 (87.2) | 37 (92.5) | 0.066 |
| Yes | 0 | 1 (2.56) | 5 (12.8) | 3 (7.50) | |
1Pearson Chi-square test or Fisher’s exact test comparing groups
Table 4.
Multiple linear regression analysis for spondylolisthesis measured
| Variable | Maximum spondylolisthesis measured | P 1 |
|---|---|---|
| 1st CSA quartile | 0.03 (0.01-0.06) | 0.012* |
| Age | 0.00 (0.00-0.00) | <0.001* |
| Sex (male) | 0.01 (−0.01-0.03) | 0.456 |
1Multiple linear regression analysis performed with values reported as such: β, P value. Female sex and 4th quartile served as references, *Statistical significance threshold was set to P<0.05. CSA - Cross-sectional area
DISCUSSION
Spondylolisthesis is less commonly found in the cervical spine compared to the lumbar spine, but it is more prevalent than originally thought with reported rates ranging from 4% to 20%.[11,12] Degenerative cervical spondylolisthesis is one potential cause of myelopathy in the elderly population. One retrospective study found that 55 of 80 (69%) patients with cervical spondylotic myelopathy had evidence of degenerative spondylolisthesis with displacement greater than 2 mm.[13] A separate retrospective study observed the progression of degenerative cervical spondylolisthesis where neck pain was the initial symptom in all patients presenting with degeneration of the facet joints. At more advanced staging, wherein degeneration was observed in both the facet joints and vertebral bodies, the predominant presenting complaint was radiculomyelopathy or myelopathy. In the most severe spine deformities, severe myelopathy was the leading symptom.[14] Park et al. found that the natural history of cervical listhesis appeared to be stable in patients who did not develop myelopathy during an extensive 8-year follow up period in their study.[15] Therefore, at an early stage of listhesis with less slippage (1–2 mm), patients will likely remain relatively asymptomatic with management focused on conservative management. These findings highlight the importance of monitoring the natural progression of cervical spondylolisthesis in high-risk patients to prevent further development of symptoms.
This is the first study to compare the longus colli CSA in patients with degenerative cervical spondylolisthesis. Our study suggests that decreased longus colli CSA is independently associated with a higher grade and degree of preoperative cervical spondylolisthesis. Smaller CSA of the longus colli may be attributed to muscle disuse as a result of axial and radicular pain causing the patient to limit activation of the muscles.[16,17] It has been theorized that smaller CSA of the cervical multifidus in females with chronic neck pain may be a result of disuse of the muscle secondary to pain.[6] One MRI study analyzing progressive degeneration of the cervical multifidus found a significant association of neck muscle fatty infiltrate and persistent neck disability, resulting in the inhibition of normal muscular activity and function.[18] In addition, avoidance behavior, which may ultimately lead to disuse based atrophy, is exhibited in patients with axial neck pain, further complicating this self-propagating cycle of pain and instability.[19] In a 2004 prospective study, custom electrodes were used to record electromyographic activity of deep cervical flexors of the cervical spine and found lower amplitude of deep cervical flexor Electromyography (EMG) activity in a patients with neck pain when compared to a control group.[16] Although not significant, their study also demonstrated greater sternocleidomastoid and anterior scalene muscle EMG activity in patients with neck pain, creating a theorized relationship between weakening of deep flexors and resultant compensatory hypertrophy of the superficial flexors. The role of targeted deep and superficial cervical flexor strengthening, in addition to targeted electrical muscle stimulation, as conservative management for this cohort, needs further study.[20,21]
The implications of our study have yet to be fully elucidated from both a conservative and operative treatment standpoint. The idea that disuse muscular atrophy of the cervical spine stabilizers and its association with instability represents both a challenge and opportunity for physicians. Recognizing that axial neck pain begets atrophy and ultimately instability allows for more targeted strengthening with physical therapy. A study by Tamai et al. observed this relationship using kinematic MRIs for patients with symptomatic neck pain or radiculopathy and found that muscle degeneration was correlated with disk degeneration and degenerative spondylolisthesis, suggesting that physical training should be employed to maintain or improve cervical alignment.[22] Although this may be one opportunity to help patients avoid surgery by preventing further progression of symptoms, prospective studies are needed to clarify the efficacy of deep cervical flexor strengthening and its benefits in reducing symptoms in the setting of cervical spondylolisthesis.
This retrospective study is not without its limitations. First and foremost, the retrospective nature of our study subjects our findings to the same inherent biases associated with all retrospective studies, which include, but are not limited to, differences in patient characteristics and demographics between study groups. Nonetheless, these variables were identified and controlled for in our regression analysis to minimize their impact on our study's conclusions. Another limitation of this study is the fact that we assessed longus colli CSA at the midpoint of the C5 vertebral body to standardize measurement across patients regardless of fusion level. There is undoubtedly anatomic variation that this standardized measurement may fail to appreciate; however, degenerative cervical spondylolisthesis has been shown to occur most often at the C4-C5 level.[23] Lastly, our study population only consisted of patients with Grade 1 and 2 spondylolistheses who were indicated for surgery which therefore limits the generalizability of our findings. Further studies observing both operative and nonoperative patients are needed to provide additional insight into the effect of longus colli CSA on the clinical presentation and progression of cervical spondylolisthesis.
CONCLUSION
Smaller longus colli CSA is independently associated with a higher grade and degree of preoperative cervical spondylolisthesis. Operative and nonoperative spine providers should be aware that patients with smaller longus colli CSA may be at increased risk of spondylolisthesis, but this finding does not significantly affect postsurgical outcomes. Further research targeted at improving our understanding of how longus colli strengthening affects cervical spondylolisthesis is indicated given our findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13:371–9. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 2.Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5:383–9. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Shadani A, Mohseni Bandpei MA, Rahmani N, Bassampour SA. A comparison of the abdominal and lumbar multifidus muscle size in patients with lumbar spondylolisthesis and healthy patients at rest and during contraction using ultrasonography. J Manipulative Physiol Ther. 2018;41:691–7. doi: 10.1016/j.jmpt.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl. 1989;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- 5.Lee ET, Lee SA, Soh Y, Yoo MC, Lee JH, Chon J. Association of lumbar paraspinal muscle morphometry with degenerative spondylolisthesis. Int J Environ Res Public Health. 2021;18:4037. doi: 10.3390/ijerph18084037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-de-las-Peñas C, Albert-Sanchís JC, Buil M, Benitez JC, Alburquerque-Sendín F. Cross-sectional area of cervical multifidus muscle in females with chronic bilateral neck pain compared to controls. J Orthop Sports Phys Ther. 2008;38:175–80. doi: 10.2519/jospt.2008.2598. [DOI] [PubMed] [Google Scholar]
- 7.Amiri-Arimi S, Mohseni Bandpei MA, Rezasoltani A, Javanshir K, Biglarian A. Measurement of cervical multifidus and longus colli muscle dimensions in patients with cervical radiculopathy and healthy controls using ultrasonography: A reliability study. PM R. 2019;11:236–42. doi: 10.1016/j.pmrj.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Javanshir K, Rezasoltani A, Mohseni-Bandpei MA, Amiri M, Ortega-Santiago R, Fernández-de-Las-Peñas C. Ultrasound assessment of bilateral longus colli muscles in subjects with chronic bilateral neck pain. Am J Phys Med Rehabil. 2011;90:293–301. doi: 10.1097/PHM.0b013e31820173e5. [DOI] [PubMed] [Google Scholar]
- 9.Cagnie B, Derese E, Vandamme L, Verstraete K, Cambier D, Danneels L. Validity and reliability of ultrasonography for the longus colli in asymptomatic subjects. Man Ther. 2009;14:421–6. doi: 10.1016/j.math.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Koslosky E, Gendelberg D. Classification in brief: The meyerding classification system of spondylolisthesis. Clin Orthop Relat Res. 2020;478:1125–30. doi: 10.1097/CORR.0000000000001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oichi T, Oshima Y, Taniguchi Y, Matsubayashi Y, Chikuda H, Takeshita K, et al. Cervical anterolisthesis: A predictor of poor neurological outcomes in cervical spondylotic myelopathy patients after cervical laminoplasty. Spine (Phila Pa 1976) 2016;41:E467–73. doi: 10.1097/BRS.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 12.Nouri A, Kato S, Badhiwala JH, Robinson M, Mejia Munne J, Yang G, et al. The influence of cervical spondylolisthesis on clinical presentation and surgical outcome in patients with DCM: Analysis of a multicenter global cohort of 458 patients. Global Spine J. 2020;10:448–55. doi: 10.1177/2192568219860827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tani T, Kawasaki M, Taniguchi S, Ushida T. Functional importance of degenerative spondylolisthesis in cervical spondylotic myelopathy in the elderly. Spine (Phila Pa 1976) 2003;28:1128–34. doi: 10.1097/01.BRS.0000067263.73474.97. [DOI] [PubMed] [Google Scholar]
- 14.Woiciechowsky C, Thomale UW, Kroppenstedt SN. Degenerative spondylolisthesis of the cervical spine - Symptoms and surgical strategies depending on disease progress. Eur Spine J. 2004;13:680–4. doi: 10.1007/s00586-004-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park MS, Moon SH, Oh JK, Lee HW, Riew KD. Natural history of cervical degenerative spondylolisthesis. Spine (Phila Pa 1976) 2019;44:E7–12. doi: 10.1097/BRS.0000000000002764. [DOI] [PubMed] [Google Scholar]
- 16.Falla DL, Jull GA, Hodges PW. Patients with neck pain demonstrate reduced electromyographic activity of the deep cervical flexor muscles during performance of the craniocervical flexion test. Spine (Phila Pa 1976) 2004;29:2108–14. doi: 10.1097/01.brs.0000141170.89317.0e. [DOI] [PubMed] [Google Scholar]
- 17.Chiu TT, Law EY, Chiu TH. Performance of the craniocervical flexion test in subjects with and without chronic neck pain. J Orthop Sports Phys Ther. 2005;35:567–71. doi: 10.2519/jospt.2005.35.9.567. [DOI] [PubMed] [Google Scholar]
- 18.Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish TB. The rapid and progressive degeneration of the cervical multifidus in Whiplash: An MRI study of fatty infiltration. Spine (Phila Pa 1976) 2015;40:E694–700. doi: 10.1097/BRS.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landers MR, Creger RV, Baker CV, Stutelberg KS. The use of fear-avoidance beliefs and nonorganic signs in predicting prolonged disability in patients with neck pain. Man Ther. 2008;13:239–48. doi: 10.1016/j.math.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Falla D. Unravelling the complexity of muscle impairment in chronic neck pain. Man Ther. 2004;9:125–33. doi: 10.1016/j.math.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Jull G, Kristjansson E, Dall'Alba P. Impairment in the cervical flexors: A comparison of whiplash and insidious onset neck pain patients. Man Ther. 2004;9:89–94. doi: 10.1016/S1356-689X(03)00086-9. [DOI] [PubMed] [Google Scholar]
- 22.Tamai K, Grisdela P, Jr, Romanu J, Paholpak P, Nakamura H, Wang JC, et al. The impact of cervical spinal muscle degeneration on cervical sagittal balance and spinal degenerative disorders. Clin Spine Surg. 2019;32:E206–13. doi: 10.1097/BSD.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 23.Jiang SD, Jiang LS, Dai LY. Degenerative cervical spondylolisthesis: A systematic review. Int Orthop. 2011;35:869–75. doi: 10.1007/s00264-010-1203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


