Abstract
Background:
Traumatic vertebral artery dissections (tVADs) occur in up to 20% of patients with head trauma, yet data on their presentation and associated sequelae are limited.
Aims and Objectives:
To characterize the tVAD population and identify factors associated with clinical outcomes.
Materials and Methods:
We retrospectively analyzed all cases of tVAD at our institution from January 2004 to December 2018 with respect to mechanism of injury, clinical presentation, anatomic factors, associated pathologies, and relevant outcomes.
Results:
Of the 123 patients with tVAD, the most common presenting symptoms were neck pain (n=76, 67.3%), headache (57.5%), and visual changes (29.6%). 101 cases (82.1%) were unilateral, and 22 cases (17.9%) were bilateral. V2 was the most involved anatomic segment (83 cases, 70.3). 30 cases (25.4%) led to stroke, and 39 cases (31.7%) had a concomitant cervical fracture. The anatomic segment and number of segments involved, and baseline clinical and demographic characteristics were not associated with risk of stroke. Patients with associated fractures were older (50.3 years v. 36.4 years, p=0.0233), had a higher comorbid disease burden (CCI 1 vs. CCI 1, p<0.0007), were more likely to smoke (OR 3.0 [1.2178, 7.4028], p=0.0202), be male (OR 7.125 [3.0181, 16.8236], p<0.0001), and have mRS≥3 at discharge (OR 3.0545 [1.0937, 8.5752], p=0.0449). On multivariable regression, only fracture independently predicted mRS≥3 at discharge (OR 5.6898 [1.5067, 21.4876], p=0.010).
Conclusion:
tVADs may be associated with stroke and/or cervical fracture. Presenting symptoms predict stroke, but baseline demographic and clinical characteristics do not. Comorbid cervical fractures, not stroke, drive negative outcomes
Keywords: Cervical fracture, cervical trauma, stroke, traumatic dissection, vertebral artery dissection
INTRODUCTION
Traumatic vertebral artery dissections (tVADs) are intimal tears of the vertebral artery that occur in 0.5%–2.0% of all trauma patients, and in up to 20% of patients with head-and-neck injuries.[1] tVADs are the leading cause of stroke in the young.[2,3,4,5] However, there is a paucity of literature discussing presentation, risk factors, and associated sequelae. In addition, some tVADs can have concomitant cervical spine fractures, however, the morbidity is not well characterized.[6,7] Therefore, the aim of our study was to characterize the tVAD patient population and identify factors associated with clinically relevant outcomes, including stroke and functional disability.
MATERIALS AND METHODS
Study population
Our case series consists of all cases of tVAD at the author's affiliated institution, an urban, tertiary academic center, and level 1 trauma center, between January 2004 and December 2018 were retrospectively analyzed. All patients were identified using the electronic data warehouse, an institution-specific clinical data repository. The Institutional Review Board approved this study, and patient consent was waived given the study's design. Patients were identified using International Classification of Diseases (ICD) 9 and ICD-10 codes and text queries of patient notes and confirmed with a review of radiology interpretations of angiographic data confirming the diagnosis of tVAD. Analysis was conducted using both single- and multivariable methods.
Variables analyzed included baseline demographic and clinical characteristics, presenting clinical symptoms, anatomic factors, associated pathologies, and functional outcomes at discharge. Baseline demographic and clinical characteristics included age, sex, smoking history (ever smoker vs. never smoker), and comorbid disease burden as measured by the Charlson Comorbidity Index (CCI). Anatomic factors that were considered included which anatomic segments of the vertebral arteries were involved in the dissection, the number of segments involved in the dissection, whether the dissection was unilateral or bilateral, and the laterality of the dissection, as determined by final radiographic interpretations by attending neuroradiologists. Given their possible association with vertebral artery dissection, stroke and concomitant cervical fracture, and their sequelae, were assessed. Functional outcome at discharge was measured by the modified Rankin Scale (mRS). MRS ≥3 at discharge, a score that corresponds to functional disability, was an outcome assessed.
Statistical methodology
Microsoft Excel version 16.4 (Microsoft, Redmond, WA, USA) was used to manage data. Statistical analysis was performed using Stata 12.0 (StataCorp, College Station, TX, USA) and Prism 9.0 (GraphPad Software, Inc., La Jolla, CA, USA). The decision to use parametric or nonparametric assumptions was based on assessing the kurtosis and skewness of the variables in question. Normally distributed data were reported as mean ± standard deviation and compared using analysis of variance or t-tests. Nonnormally distributed data were reported as median (25th percentile and 75th percentile) for nonbinary variables and compared using Mann–Whitney U or Fisher's exact tests, as appropriate.
Stepwise, forward-and-backward multivariable logistic regression was performed with candidate variables consisting of the aforementioned demographic and baseline clinical data. Candidate variables with P < 0.20 on univariable analysis were included in multivariable analysis. A value of P < 0.05 was considered statistically significant on multivariable analyses. This case series has been reported in line with the PROCESS Guideline.[8]
RESULTS
Patient demographics
We identified 123 patients with tVAD who met the criteria for inclusion. The median age of the population was 40.5 (32.3, 52.7), of whom 47 (38.2%) were male. Of the 104 patients for whom data were available, 34 patients (32.7%) had a history of smoking, and the median comorbid disease burden was a CCI of 0 (0 and 1).
Clinical presentation
Of the 123 patients, 101 cases (82.1%) were unilateral and 22 cases (17.9%) were bilateral, with 67 cases of left tVAD and 68 cases of right tVAD. The most common presenting symptoms were neck pain (n = 76, 67.3%), followed by headache (57.5%) and visual symptoms (29.6%). A full list of presenting symptoms is found in Table 1. V2 was the most involved segment (83 cases, 70.3%), with 54 cases (43.9%) involved multiple anatomic segments [Figure 1]. Thirty cases (25.4%) led to stroke, and 39 cases (31.7%) were associated with a cervical fracture, of which 8 (20.5%) were C1 fractures, 14 (35.9%) were C2 fractures, and the remainder were subaxial cervical spine fractures. The median follow-up time was 356 days.
Table 1.
Presenting symptoms
| Symptom | Percentage of patients |
|---|---|
| Headache | 57.5 |
| Neck pain | 67.3 |
| Facial pain | 2.6 |
| Visual symptoms | 29.6 |
| HTN | 2.6 |
| Sensory symptoms | 27.8 |
| Weakness | 20.9 |
| Ataxia/dysequilibrium | 11.3 |
| Paresis plegia | 6.1 |
| Syncope | 8.6 |
| Dizziness/vertigo | 27.0 |
| Nausea | 17.4 |
| Vomiting | 7.8 |
| Nystagmus | 1.7 |
| Auditory symptoms | 4.3 |
| Clumsiness | 1.7 |
| Lightheadedness | 7.8 |
| Aphasia | 2.6 |
| Dysmetria | 0.9 |
| Chest pain | 1.7 |
| Unresponsive | 0.9 |
| Other neurologic symptoms | 4.3 |
| Dysarthria | 2.6 |
HTN - Hypertension
Figure 1.
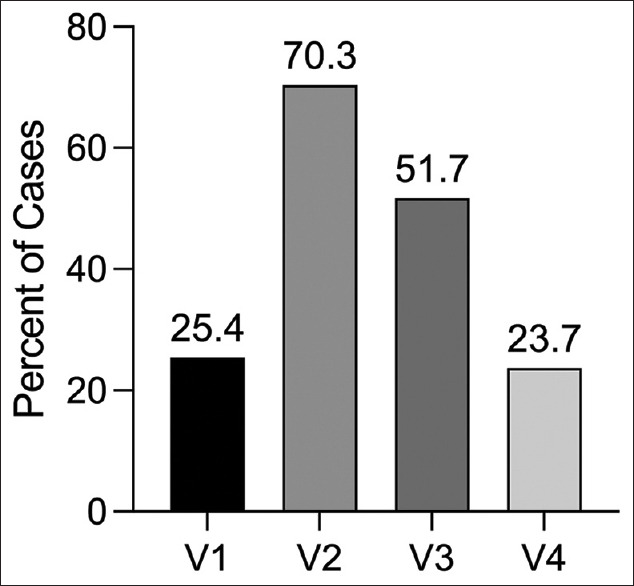
The percent of tVAD patients with a given vertebral artery segment involved. V2 was the most commonly involved segment (70.9%), and 43.9% involved multiple segments. tVAD - Traumatic vertebral artery dissection
Patients with concomitant fractures
Patients with fractures were older (50.3 years vs. 36.4 years, P = 0.0233), had a higher comorbid disease burden (CCI 1 vs. CCI 1, P < 0.0007), were more likely to smoke (odds ratio [OR] 3.0 [1.2178, 7.4028], P = 0.0202), be male (OR 7.125 [3.0181, 16.8236], P < 0.0001), and have associated intracranial bleeding (OR 6.455 (1.493, 32.21), P = 0.0205). They were less likely to develop a pseudoaneurysm (OR 0.0 [0, 0.3601], P = 0.0011), and their rate of stroke was no different (OR 0.9138 [0.3834, 2.1858], P = 1.000) and the proportion whose mRS improved at 3-month follow-up was no different (0.9023 [0.3831, 2.1265], P = 0.8292).
Patients who developed stroke
There was no difference between patients who suffered strokes and those who did not with respect to age (40.4 vs. 40.1, P = 0.7904), CCI (0 vs. 0, P = 0.5973), smoking history (P = 1.0), sex (P = 0.5202), or associated intracranial hemorrhage (P = 1.00). The proportion of patients who developed a stroke was not different depending on which vertebral segment (P > 0.10). Comparing patients with tVAD involving only one anatomic segment to those involving ≥2 anatomic segments demonstrates no difference in the proportion of patients who developed a stroke (31.8% stroke for multiple segments vs. 20.0% for one segment, P > 0.10).
On multivariable analysis, presenting with dizziness/vertigo (OR 4.5017 [1.5594, 12.9954, P = 0.005]) or ataxia/dysequilibrium (OR 5.8154 (1.3318, 25.3936), P = 0.019) was associated with having a stroke, with an area under the receiver operating characteristic curve of 0.7472 [Figure 2 and Table 2]. No demographic risk factors were independently associated with stroke.
Figure 2.
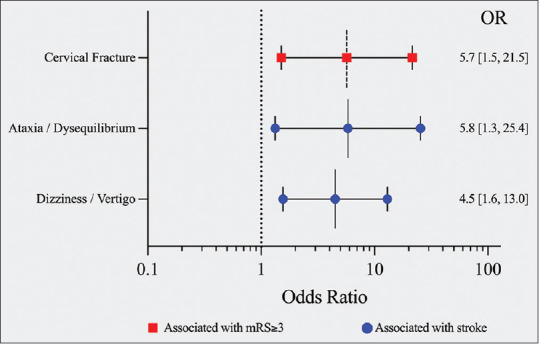
Forest plot demonstrating the odds ratios from multivariable analysis to identify factors independently associated with mRS ≥3 at discharge (red), and factors independently associated with stroke (blue). mRS - Modified Rankin Scale
Table 2.
Factors independently associated with stroke or disability
| OR | 95% CI | P | |
|---|---|---|---|
| Stroke | |||
| Ataxia/dysequilibrium | 5.815 | 1.332-25.394 | 0.019 |
| Dizziness/vertigo | 4.502 | 1.559-12.995 | 0.005 |
| mRS ≥3 at discharge | |||
| Fracture | 5.690 | 1.507-21.488 | 0.01 |
OR - Odds ratio; CI - Confidence interval; mRS - Modified Rankin Scale
Factors associated with poor modified Rankin Scale at discharge
Of 99 patients for whom mRS was available at the time of discharge, 27 (27.3%) had mRS ≥3 at discharge. Patients who suffered a stroke had a higher mRS at discharge than patients who did not (P = 0.0143) and were more likely to have mRS ≥3 at discharge on single-variable analysis (OR 2.9333 [1.1258, 7.6717], P = 0.0368). Similarly, patients who had a fracture had a higher mRS at discharge (2.3 vs. 1.4, P = 0.0128) and were more likely to have mRS ≥3 at discharge on single-variable analysis (OR 3.0545 [1.0937, 8.5752], P = 0.0449). The proportion of patients with mRS ≥3 at discharge was equivalent between patients with strokes, patients with fractures, and patients with both (0.7988). On multivariable regression, only fracture independently predicted mRS ≥3 at discharge (OR 5.6898 [1.5067, 21.4876), P = 0.010) [Table 2].
DISCUSSION
tVADs occur in 0.5%–2.0% of all trauma patients, and in up to 20% of patients with head trauma, yet modern data on their presentation and associated sequelae are limited.[1] In our study of 123 patients with tVADs, which to our knowledge is the largest single-institutional tVAD cohort, we analyzed characteristics of presentation, mechanism of injury, and factors predictive of disability and stroke. The median age of the patient population was 40.5 years, the majority of patients were female (62.8%), and presenting with dizziness/vertigo or ataxia/dysequilibrium was associated with stroke. However, the associated cervical fracture was the primary driver of disability in these patients, 31.7% of patients presented with concomitant fractures, and only fractures predicted functional disability at discharge.
In order, the most common presenting symptoms in our patients with tVAD were neck pain, headache, visual disturbances, sensory changes, and dizziness or vertigo. Similarly, Gottesman et al., in a systematic review of 1927 patients that experienced spontaneous or tVADs identified the three most common presenting symptoms as dizziness/vertigo, headache, and neck pain.[9] Notably, visual and sensory changes were not assessed in many of these series, and no distinction was made between traumatic and spontaneous VAD in this review. As such, our finding that visual and sensory disturbances are common following tVAD is novel. While there are numerous, nonspecific presenting symptoms of tVAD, a clearer characterization of them may allow for improved detection based on clinical suspicion.
Notably, we found that the v2 segment (foraminal segment) was involved in 70% of tVAD patients in our population, which is a departure from the literature on spontaneous VAD. Ginoza et al. report that the majority of spontaneous dissections occur in the v3 segment and may extend into the v4 segment.[10] While tVAD is known to be associated with upper cervical fractures that are in proximity to distal vertebral artery segments, our finding that proximal vertebral artery segments are more commonly involved is novel.[6] As v2 is the longest segment anatomic vertebral artery segment, it is reasonable for it to be the most likely to be involved in any given injury, but establishing the cause for this finding is out of the purview of this study. Nevertheless, proximal dissections may in fact be more common than distal dissections in the tVAD population.
Overall, approximately one-third of the patients in our study with tVAD were found to have a comorbid cervical fracture, and the fracture was the primary driver of disability at discharge. Moreover, while most of our tVAD patients were relatively young women, which is consistent with existing literature,[11] patients who were found to have a concomitant fracture on imaging, were more likely to be older, male, have a smoking history, and a greater CCI disease burden. Indeed, cervical spine fractures are more common in the elderly.[12,13] Older men may, therefore, represent a subgroup of tVAD patients at high risk for concomitant cervical fracture, and the increased morbidity associated with it.
Stroke, on the other hand, was less common than cervical fractures in our series, and was not a significant driver of functional disability at discharge. Moreover, we found that a patient's presenting symptoms, rather than their baseline demographic and clinical characteristics, were predictors of stroke following tVAD. The classic symptoms of limb paresis, facial paralysis, sensory disturbances, and speech deficits are well-described in literature as the presenting symptoms of hemispheric stroke.[14,15,16] However, the symptoms of stroke in patients following tVAD have not been well-studied. Given our findings, these symptoms should prompt further stroke workup in the tVAD population. Notably, vertebral artery strokes can lead to severe disability, particularly in the context of brainstem ischemia, though our analysis here suggests that less disabling strokes are more common in the tVAD population.[17,18,19]
Our study is not without limitations. The retrospective nature of this study required post hoc determination of mRS scores and presents a potential source of bias. Therefore, there is potential for additional neurologic injury independent of the VAD to contribute to a patient's neurologic status and our interpretation of the mRS score.[20] Moreover, while our study is the largest single-center analysis of patients with tVADs, the outcomes are inherently dependent on the patient selection, medical decision-making, and management preferences of our institution and providers. Furthermore, given our position as an urban, academic, tertiary referral center, and level 1 trauma center, our patient population may not be representative of the tVAD population more broadly, which may limit the generalizability of our findings. In addition, our patient population had differential losses to follow-up preventing assessment of prognosis past the point of discharge.
Despite these limitations, this series, to our knowledge, is the largest to date on tVADs, with data sufficiently rich to allow the use of multivariable analysis to identify factors independently associated with negative outcomes. Moreover, we have provided a detailed assessment of this population's clinical presentation, including presenting symptoms that predict stroke. Future, prospective series with longer follow-ups could further characterize this patient population, and the factors affecting the prognosis for these patients.
CONCLUSION
Overall, our study represents a robust evaluation of patient presentation, characteristics, and prognosis associated with tVADs. Patients with tVAD can have an associated cervical fracture and/or develop an ischemic stroke. Presenting symptoms predict stroke, but baseline demographic and clinical characteristics do not. Regarding neurologic prognosis, a comorbid cervical fracture is associated with negative outcomes and functional disability, but in many cases, stroke alone is not.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Desouza RM, Crocker MJ, Haliasos N, Rennie A, Saxena A. Blunt traumatic vertebral artery injury: A clinical review. Eur Spine J. 2011;20:1405–16. doi: 10.1007/s00586-011-1862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold M, Bousser MG, Fahrni G, Fischer U, Georgiadis D, Gandjour J, et al. Vertebral artery dissection: Presenting findings and predictors of outcome. Stroke. 2006;37:2499–503. doi: 10.1161/01.STR.0000240493.88473.39. [DOI] [PubMed] [Google Scholar]
- 3.Park KW, Park JS, Hwang SC, Im SB, Shin WH, Kim BT. Vertebral artery dissection: Natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. 2008;44:109–15. doi: 10.3340/jkns.2008.44.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt TB, Agarwal S. Vertebral artery dissections. NIH Natl Libr Med. 2022;1:1–7. [Google Scholar]
- 5.Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383–8. doi: 10.1136/pgmj.2003.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloney M, Kim H, Riestenberg R, Dahdaleh NS. Risk factors for transverse ligament disruption and vertebral artery injury following an atlas fracture. World Neurosurg. 2021;146:e1345–50. doi: 10.1016/j.wneu.2020.11.172. [DOI] [PubMed] [Google Scholar]
- 7.Torretti JA, Sengupta DK. Cervical spine trauma. Indian J Orthop. 2007;41:255–67. doi: 10.4103/0019-5413.36985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ, Orgill DP, et al. The process 2018 statement: Updating consensus preferred reporting of case series in surgery (PROCESS) guidelines. Int J Surg. 2018;60:279–82. doi: 10.1016/j.ijsu.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman RF, Sharma P, Robinson KA, Arnan M, Tsui M, Ladha K, et al. Clinical characteristics of symptomatic vertebral artery dissection: A systematic review. Neurologist. 2012;18:245–54. doi: 10.1097/NRL.0b013e31826754e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginoza L, Lerner A, Sigman E. Vertebral artery dissection. J Orthop Sports Phys Ther. 2020;50:344. doi: 10.2519/jospt.2020.8858. [DOI] [PubMed] [Google Scholar]
- 11.Umasankar U, Carroll TJ, Famuboni A, Patel MD, Starke ID. Vertebral artery dissection: Not a rare cause of stroke in the young. Age Ageing. 2008;37:345–6. doi: 10.1093/ageing/afn004. [DOI] [PubMed] [Google Scholar]
- 12.Sander AL, El Saman A, Delfosse P, Wutzler S, Meier S, Marzi I, et al. Cervical spine fractures in the elderly: Morbidity and mortality after operative treatment. Eur J Trauma Emerg Surg. 2013;39:469–76. doi: 10.1007/s00068-013-0311-5. [DOI] [PubMed] [Google Scholar]
- 13.Bank M, Gibbs K, Sison C, Kutub N, Paptheodorou A, Lee S, et al. Age and other risk factors influencing long-term mortality in patients with traumatic cervical spine fracture. Geriatr Orthop Surg Rehabil. 2018;9:1–8. doi: 10.1177/2151459318770882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph SA. Ischemic stroke. Workplace Health Saf. 2016;64:444. doi: 10.1177/2165079916665400. [DOI] [PubMed] [Google Scholar]
- 15.Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528–36. [PubMed] [Google Scholar]
- 16.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: Findings from the atherosclerosis risk in communities study. Stroke. 2002;33:2718–21. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- 17.Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–14. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- 18.Prabhakar S, Bhatia R, Khandelwal N, Lal V, Das CP. Vertebral artery dissection due to indirect neck trauma: An underrecognised entity. Neurol India. 2001;49:384–90. [PubMed] [Google Scholar]
- 19.Gowda SN, De Jesus O. Brainstem infarction. NIH National Library of Medicine. 2022;1:1–15. [Google Scholar]
- 20.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]


