Abstract
Introduction
Electronic cigarettes (ECIGs) heat a nicotine-containing liquid to produce an inhalable aerosol. ECIG power (wattage) and liquid nicotine concentration are two factors that predict nicotine emission rate (“flux”). These factors can vary greatly across devices and users.
Aims and Methods
The purpose of this study was to examine ECIG device and liquid heterogeneity in “real world” settings and the association with predicted nicotine flux, nicotine yield, and total particulate matter (TPM) emissions. Past 30-day ECIG users (n = 84; mean age = 23.8 years [SD = 9.6]) reported device and liquid characteristics. Device power was measured via multimeter, device display screens, or obtained via labeling. Liquid nicotine concentration was obtained via labeling or through chemical analysis. Predicted nicotine flux, nicotine yield, and TPM associated with 10 4-second puffs were calculated for participants’ primary devices.
Results
Participants’ primary devices were box mods (42.9%), disposable vapes (20.2%), and pod mods (36.9%). Most participants (65.5%) reported not knowing their primary device wattage. Rebuildable/box mods had the greatest power range (11.1–120.0 W); pod mod power also varied considerably (4.1–21.7 W). Unlike device wattage, most participants (95.2%) reported knowing their liquid nicotine concentration, which ranged from 3.0 to 86.9 mg/ml (M = 36.0, SD = 29.3). Predicted nicotine flux varied greatly across products (range =12.0–160.1 μg/s, M = 85.6 μg/s, SD = 34.3). Box mods had the greatest variability in wattage and predicted nicotine flux, nicotine yield, and TPM yield.
Conclusions
ECIG device and liquid heterogeneity influence nicotine and other toxicant emissions. Better measurement of ECIG device and liquid characteristics is needed to understand nicotine and toxicant emissions and to inform regulatory policy.
Implications
ECIG device and liquid heterogeneity cause great variability in nicotine flux and toxicants emitted. These data demonstrate the need to examine device and liquid characteristics to develop empirically informed, health-promoting regulatory policies. Policies may include setting product standards such that ECIG products cannot (1) have nicotine fluxes much greater than that of a cigarette to decrease the risk of dependence, (2) have nicotine fluxes that are very low and thus would have minimal appeal to cigarette smokers and may serve as starter products for youth or nontobacco users, and (3) emit large amounts of particulate matter and other toxicants.
Introduction
Electronic cigarettes (ECIGs) heat a liquid containing propylene glycol, vegetable glycerin, chemical flavorants, and nicotine to create an inhalable aerosol.1 While ECIG use among US adults remains relatively low, with 2.8% reporting current ECIG use in 2017 and 3.2% in 2018,2 use has increased more rapidly among youth. In 2011, current ECIG use among US middle and high school students was 1.5% and increased to 11.7% in 2017.3 Since 2014, ECIGs have been the most common tobacco product used by youth in the United States4 and in 2020 13.1% of middle and high school students reported current (past 30-day) ECIG use.5 ECIG use among youth decreased in 2021, with 11.3% of high school and 2.8% of middle school students reporting ECIG use, but still remained the most commonly used tobacco product among youth.6 During this time, ECIG products have evolved with the recent proliferation of “pod-mod” products7 and disposable ECIG devices.8
The continued use of ECIGs by youth and adults as well as the diversification of products on the market has presented challenges for health professionals and regulators given that the potential public health benefits or harms associated with long-term ECIG use remain unknown. Specifically, ECIGs may play a role in aiding some cigarette smokers in cigarette smoking cessation, but they may also be appealing to youth or nontobacco users. One factor that likely affects public health impact is an ECIG device’s ability to emit nicotine. Several reviews9–14 conclude that there is limited evidence that ECIGs are effective for smoking cessation and that there is “moderate evidence” that ECIGs containing nicotine are more effective than those without nicotine. ECIG devices that can deliver more nicotine in the same number of puffs to users can have greater withdrawal suppression and positive subjective effects.15 Thus, the rate at which an ECIG device emits nicotine may impact other outcomes, such as the likelihood of youth initiation, the probability of continued ECIG use, dependence, user puffing behaviors, and toxicant exposure.
One challenge in understanding ECIG nicotine emissions is that ECIGs are a heterogenous product class that includes numerous different devices and liquids. Some researchers have attempted to identify product subgroups to characterize ECIGs with greater specificity, such as cigalikes, vape pens, box mods, pod mods, or disposables. These categories are limited, as they provide little information regarding the amount of nicotine emitted from a user’s device because they do not focus on characteristics that impact nicotine emissions. Holding puffing behavior constant, devices that operate at higher electrical power (ie, wattage) and use a liquid with a higher nicotine concentration are associated with higher nicotine emissions relative to lower wattage devices or lower nicotine concentration liquids.16 Higher wattage devices also produce and expose users to higher amounts of particulate matter (PM; ie, suspended liquid particles generated from heating the ECIG liquid) and other chemicals.16
Because many “open system” ECIG devices allow users to change device wattage and liquid nicotine concentration easily, some researchers have proposed the use of nicotine flux, or the amount of nicotine emitted from an ECIG per second, as a measure for nicotine emissions.17,18 Using nicotine flux as a standardized measurement of nicotine emissions has many benefits for research, specifically: (1) it can be calculated for any ECIG device with a known power and liquid nicotine concentration19 and (2) it can be used to compare nicotine emission between ECIG device and liquid combinations and other non-ECIG tobacco products, such as cigarettes. Preliminary findings also demonstrate that nicotine flux is a strong predictor of nicotine delivery. In a recent study20 participants were asked to use a single ECIG product with various nicotine flux conditions by varying ECIG liquid nicotine concentration and device power. After using the ECIG device with various nicotine flux set ups, blood was drawn from participants and plasma nicotine concentrations were recorded. Data from this study demonstrate that nicotine flux is correlated strongly with plasma nicotine concentration (R2 = 0.96) with higher nicotine flux being associated with greater plasma nicotine concentration. Therefore, because preliminary evidence suggests that greater nicotine flux is associated with greater nicotine delivery,21 greater knowledge of the nicotine flux associated with ECIG users’ device and liquid combinations could be informative for identifying which ECIG products are likely to deliver high amounts of nicotine to users or those that are likely to deliver minimal amounts of nicotine.
Little is known about the extent to which ECIG devices and liquid heterogeneity among products used in real-world settings are associated with nicotine flux variability. One approach for examining nicotine flux is to use survey methods to ask ECIG users to report their device and liquid characteristics that are needed to calculate nicotine flux (ie, device wattage and nicotine concentration). However, obtaining the information needed to calculate nicotine flux may not be feasible using survey methods: a recent study found that while 90% of surveyed ECIG users reported knowing their liquid nicotine concentration, only 18.2% of users provided valid results associated with their ECIG device wattage.22 Despite these challenges, because of the known impact of ECIG device and liquid heterogeneity on nicotine and other toxicant emissions, there is a need to document ECIG device and liquid characteristics. Therefore, the purpose of this study was to examine ECIG device and liquid characteristics and estimate the nicotine flux, nicotine yield, and total particulate matter (TPM) emissions in “real-world” settings.
Methods
Overview
This study was approved by the Virginia Commonwealth University Institutional Review Board. In 2019 and 2020, current ECIG users were recruited to participate in an in-person study. Participants were recruited by posting advertisements on the local Craigslist website, public locations within the community, a large (29 000 students) public university, a community college, and seven tobacco or vape shops in the community. Advertisements included a link to a lab website where interested individuals could access a screening survey. Individuals were identified as eligible to participate in the study if they reported being over the age of 18 years, currently using an ECIG every day or some days, having used an ECIG at least 1 day in the past 30 days, and owning their own ECIG device.
Procedure
Eligible participants presented to the research lab for in-person visits and were instructed to bring all ECIGs and liquids that they owned. Because the purpose of this study was to predict product emissions from a variety of ECIG devices, efforts were made to limit duplicate devices. Therefore, participants were not invited to an in-person visit if they used a device that had been brought in by five or more previous participants. After providing consent, participants (n = 84) completed a brief questionnaire examining ECIG and other tobacco use, characteristics of their primary ECIG device (eg device type, brand name, model, voltage, resistance, and wattage), liquid characteristics (nicotine concentration, flavors, and other ingredients), ECIG dependence as measured by the E-Cigarette Dependence Scale,23 health effects, and demographic characteristics. Participants were allowed to examine their products while completing the questionnaire.
Trained research staff captured images of all devices and liquids that participants brought to the lab. Staff captured device and liquid images from multiple angles with a solid color background and placed a reference card next to devices and liquids when capturing images to be used as a size/scale reference. For devices, staff documented unique features, such as brand names/logos to aid in identifying devices from images. If devices included a display screen that included device specifications such as device wattage, voltage, resistance, or other settings, the staff asked participants to turn on the device to activate the display, and staff captured images of the illuminated display. For participants’ liquid bottles, packaging, or other storage containers, staff used multiple angles when capturing images to include text or images describing brand, flavor name, nicotine concentration, propylene glycol/vegetable glycerin ratio, ingredients, and other content displayed on the packaging.
After capturing device and liquid images, staff attempted to measure device electrical characteristics including voltage and resistance using a multimeter and recorded these values. When both values were able to be measured using the multimeter, device wattage was calculated using the formula Power = Voltage2/Resistance. For devices that included a display screen with electrical characteristic information, these data were also recorded. For devices that a multimeter could not be used to access electrical components and that did not include a display screen, research staff conducted an internet search of manufacturer websites (eg, www.smoktech.com) and third-party websites that display device specifications (eg, www.elementvape.com). If device wattages could not be determined using the multimeter, display screen, or internet searches, some devices were disassembled so that internal electrical characteristics could be measured and examined, including if devices were used to power or temperature control functions (as in24,25). This disassembly was most common for disposable devices. For devices for which wattage could not be determined, an estimate for wattage was used by assigning the device a wattage equivalent to the mean wattage value determined for other devices of the same device type (eg. box mod, pod mod, and disposable). Wattages were compared to product specifications from internet websites to validate that the wattages were feasible for the included devices.
For liquids, nicotine concentrations displayed on bottles were recorded. For liquids without packaging, research staff conducted an internet search of the manufacturer or third-party websites for nicotine concentration details. Several prefilled disposable devices that matched participants’ devices were purchased, disassembled, and analyzed for nicotine concentration as previous research has shown devices with prefilled liquid to have different liquid nicotine concentrations than labels display.24,25 Because there was less variability in ECIG liquid nicotine concentration, for liquids with unknown nicotine concentration, nicotine concentration was estimated by assigning them a nicotine concentration equivalent to the mode nicotine concentration value of other known liquids used in the same device type (eg. box mod liquids, pod mod liquids, and disposable liquids).
Analyses
Descriptive statistics were used to examine user and device characteristics including if participants reported knowing their device wattage and liquid nicotine concentration. Using device wattage and liquid nicotine concentration, nicotine flux (μg/s) was estimated using a previously established and validated formula,19 which was also used to estimate total nicotine yield in mg, and TPM emissions in mg assuming 10 “standard puffs” of 4 s per puff that are often used in clinical laboratory settings to examine ECIG use.21 The simplified steady-state model19 which can be applied to predict nicotine flux, nicotine yield, and TPM for any ECIG device type, requires wattage, nicotine concentration, and propylene glycol/vegetable glycerin ratio inputs. To calculate nicotine yield and TPM, nicotine flux and PM emitted per second were multiplied by 40 (ie, total puff duration from 10 4-second puffs). Descriptive statistics were examined for all primary devices and also were examined when ECIGs were grouped by device type. Device wattage, liquid nicotine concentration, nicotine flux, nicotine yield, and TPM emissions were compared between device types using independent samples analysis of variance with Scheffe post hoc t-tests using an alpha level of 0.05. One participant’s box mod device, liquid, nicotine flux, and nicotine and TPM yield data were excluded from analyses because of extreme values (ie, liquid nicotine concentration label was verified by research staff to be more than 8 times higher than other box mod device liquid nicotine concentrations and predicted nicotine flux 3.75 times higher than the next highest nicotine flux value).
Results
Participant Characteristics
Participants’ self-reported characteristics and primary device and liquid characteristics are displayed in Table 1. In summary, participants were mostly white (84.5%) non-Hispanic (94%) and approximately half were men (51.2%) and women (47.6%). Participants were on average 23.8 (SD = 9.6 range = 18–59) years old and 83.3% had completed high school and had attended some college. Most (77.4%) reported using ECIGs on a regular basis (every day or some days) for one year or more, and nearly three-quarters (70.2%) reported ECIG used “fairly frequently throughout the day” or “almost always throughout the day.” The most common primary ECIG device types as reported by participants were pod mods (40.5%), box mods/mechanical mods (40.5%; referred to as “box mods” hereinafter), and prefilled disposable/cig-alike (13.1%), however, some devices were recategorized by the research team. The most common discrepancy between categories assigned by the research team and participants’ self-reported categories was participants identifying prefilled/disposable devices as “pod mods” (likely because of the addition of the “disposable vape” category added after data collection had begun as these products became more popular over time). Mean E-Cigarette Dependence Scale26 score was 1.7 (SD = 0.8), slightly lower compared to E-Cigarette Dependence Scale scores reported elsewhere among daily ECIG users.27 Half of the participants (50.0%) reported smoking less than 100 cigarettes in their lifetime and less than a quarter (21.4%) reported currently smoking cigarettes every day or some days. Current (past 30-day) use of other tobacco products was low, with the most used other tobacco product being cigarillos or little cigars (19.0%).
Table 1.
Sample Demographics and ECIG/Tobacco Use Characteristics (N = 84)
| Characteristic | N | % |
|---|---|---|
| Age (M, SD) | 23.8 (9.6) | |
| Sex | ||
| Female | 41 | 47.6 |
| Male | 43 | 51.2 |
| Ethnicity | ||
| Hispanic/Latino(a) | 5 | 6.0 |
| Race | ||
| American Indian/Alaskan Native | 1 | 1.2 |
| Asian | 0 | 0.0 |
| Native Hawaiian/Pacific Islander | 1 | 1.2 |
| Black/African American | 5 | 6.0 |
| White/European American | 71 | 85.5 |
| More than one race | 5 | 6.0 |
| Education | ||
| Less than high school education | 5 | 6.0 |
| High school diploma or GED | 16 | 19.0 |
| Some college credit, but less than 1 year | 12 | 14.3 |
| 1 or more y of college, no degree | 37 | 44.1 |
| Associate’s degree | 7 | 8.3 |
| Bachelor’s degree | 7 | 8.3 |
| Regular ECIG use history1 | ||
| 0–3 months | 6 | 7.2 |
| 4–6 months | 7 | 8.4 |
| 7–12 months | 5 | 6.0 |
| Between 1–2 year | 20 | 24.1 |
| More than 2 years | 45 | 54.2 |
| ECIG frequency | ||
| At least once per day | 4 | 4.8 |
| Every once in a while throughout the day | 21 | 25.0 |
| Fairly frequently throughout the day | 35 | 41.7 |
| Almost always throughout most of the day | 24 | 28.6 |
| Regular ECIG device (self-report) | ||
| Prefilled disposable/Cig-alike | 11 | 13.1 |
| Vape pen/eGo style device | 2 | 2.4 |
| Box mod/mechanical mod | 34 | 40.5 |
| Pod mod such as JUUL | 34 | 40.5 |
| Other | 3 | 3.6 |
| Know ECIG liquid nicotine concentration (self-report) | ||
| Yes | 80 | 95.2 |
| Know ECIG device wattage (self-report) | ||
| Yes | 29 | 34.5 |
| ECIG liquid flavor preference (select all that apply) | ||
| Menthol or mint | 44 | 52.4 |
| Tobacco | 4 | 4.8 |
| Fruit | 62 | 73.8 |
| Desserts (such as ice cream, cake, and cookies) | 18 | 21.4 |
| Candy | 29 | 34.5 |
| Other (including clove, spice, nut, alcoholic drink, and coffee/tea) | 17 | 20.2 |
| I usually use multiple flavors | 6 | 7.1 |
| ECIG use after waking | ||
| After 60 min | 17 | 20.2 |
| 31–60 min | 16 | 19.1 |
| 6–30 min | 28 | 33.3 |
| Within 5 min | 23 | 27.4 |
| E-Cigarette Dependence Scale (M, SD) | 1.7 (0.8) | |
| Lifetime use of 100+ cigarettes | ||
| Yes | 41 | 49.4 |
| Current use of other tobacco products | ||
| Cigarettes | 20 | 21.4 |
| Cigar | 7 | 8.3 |
| Cigarillo or little cigar | 16 | 19.1 |
| Smokeless | 7 | 8.3 |
| Waterpipe | 9 | 10.7 |
Regular ECIG use was defined as using and ECIG “every day” or “some days.” E-Cigarette Dependence Scale scores were based on responses a 4-item scale.23
ECIG = electronic cigarette; GED = General Educational Development.
ECIG Device and Liquid Characteristics
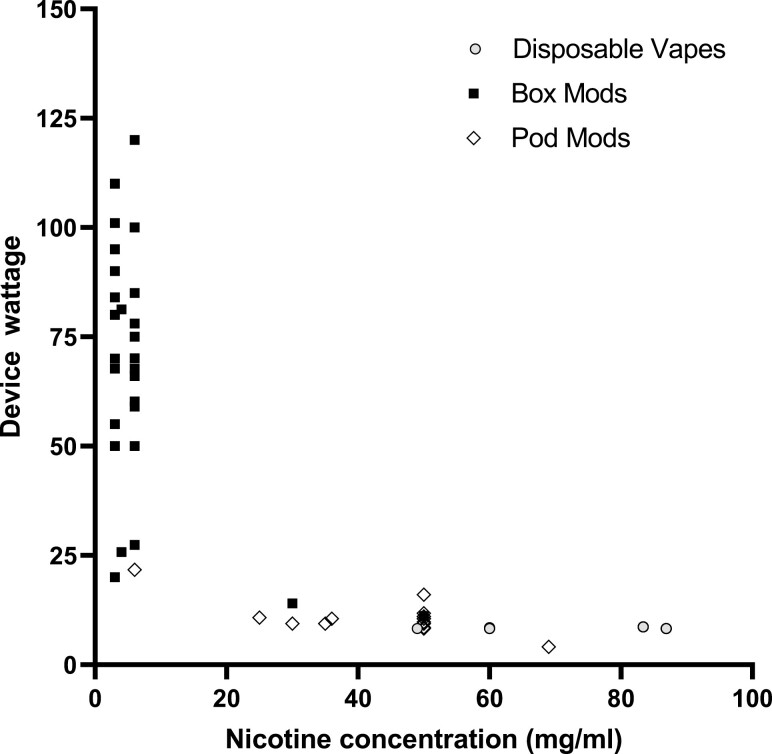
Participants’ primary devices fell into three device types (after the research team recategorized devices): Disposable vapes (20.2%), pod mods (36.9%), and box mods (42.9%). For disposable vapes, device wattage was based on multimeter measurement (n = 11), with the remaining devices (n = 6) being assigned the average multimeter value. For pod mods, wattage was determined by multimeter (n = 13), internet search (n = 2), and an average of multimeter and internet values (n = 16). For box mods, wattage was determined by multimeter (n = 2), display screen (n = 26), internet search (n = 1), and an average of multimeter, display screen, and internet values (n = 6). As displayed in Table 1, for devices where wattage could be calculated without using averages (n = 55), the mean was 39.9 W (SD = 36.1). When including estimated values based on devices in the same device type category, the mean device wattage for all devices (n = 83) was 33.7 W (SD = 33.6). The range was 4.1–120.0 W across all device types. However, disposable vapes had nearly identical wattages across all products (range = 8.3–8.7), box mod devices had the greatest range (11.1–120.0 W), and the range for pod mods was 4.1–21.7 W. There was a main effect of device type on wattage [F(2) = 118.4, p < .001]. Post hoc contrasts revealed that device power for disposable vapes (M = 8.4 W, SD = 0.2) and pod mods (M = 9.3 W, SD = 3.4) were significantly lower than mean device power for box mod devices (M = 67.5 W, SD = 26.1; p < .001).
Also displayed in Table 1, the nicotine concentration was determined for 78 liquids (M = 36.5 mg/ml, SD = 29.8). For disposable vapes, values were based on chemical analysis (n = 11) and internet search (n = 6). For pod mods, nicotine concentration values were based on chemical analysis (n = 5), product labels (n = 16), internet search (n = 7), and three were assigned mode values (50 mg/ml). For box mods, most nicotine concentrations were based on product labels (n = 33) and two were assigned mode values (6 mg/ml). For all devices, including those for which data were estimated from other liquids in the same device category, the mean liquid nicotine concentration was 36.0 mg/ml (SD = 29.3). There was a main effect of device type on liquid nicotine concentration [F(2) = 203.8, p < .001]. From highest to lowest liquid nicotine concentration, disposable vapes had a mean nicotine concentration of 73.4 mg/ml (SD = 14.5), followed by pod mods (M = 48.6 mg/ml, SD = 13.6) and box mods (M = 6.7 mg/ml, SD = 8.8). Post hoc contrasts indicated mean liquid nicotine concentrations used in each device type were significantly different (ps < .001).
Around one-third (34.5%) of all participants reported knowing their device wattage. However, this knowledge was associated with the device type. Specifically, 75% of box mod users reported knowing their device wattage, whereas only 4.2% of participants who did not use a box mod reported knowing it (χ2(1) = 45.7, p < .001). This observation did not hold for liquid nicotine concentration: Nearly all participants (95.2%) reported knowing their liquid nicotine concentration, and there was no association based on device type, likely because most ECIG products display nicotine concentrations on product packaging. Figure 1 displays distributions of device wattage and liquid nicotine concentration by device type.
Figure 1.
Distribution of ECIG device wattage and nicotine concentration by device type. The cluster of points at points at 9.38 W and 50 mg/ml nicotine concentration are seven pod mod devices that wattage was unknown and thus were assigned the pod mod mean wattage.
Predicted Nicotine Flux, Total Nicotine Emissions, and TPM Emissions
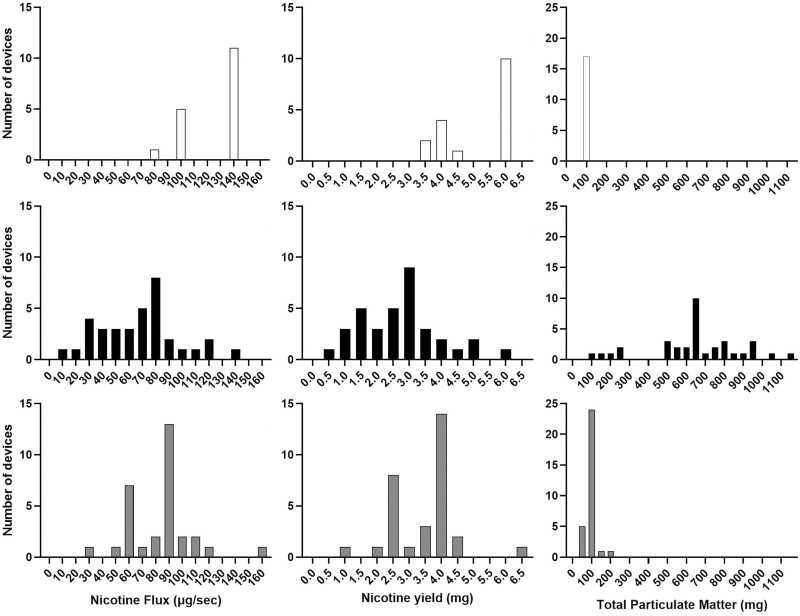
There were 51 participants whose device wattage and liquid nicotine concentration were able to be determined using device displays or product labeling, direct measurement, or internet searches. For these devices and liquids, mean predicted nicotine flux ranged from 12.0 to 160.1 μg/s (M = 83.5 μg/s, SD = 41.3). As displayed in Table 2, when including all devices and liquids with estimated wattage and nicotine concentration values, the mean predicted nicotine flux was 85.6 μg/s (SD = 34.3), similar to the nicotine flux of combustible cigarettes calculated from previous studies (87.1 μg/s28–35). The distribution of nicotine flux by device type is displayed in Figure 2. Disposable vapes had the highest mean nicotine flux (M = 124.1 μg/s, SD = 25.9), followed by pod mods (M = 84.0 μg/s, SD = 25.3), and lastly box mods (M = 68.5 μg/s, SD = 30.1). There was a main effect of device type on predicted nicotine flux [F(2) = 23.5, p < .0] with post hoc Scheffé contrasts indicating disposable vapes had significantly greater predicted nicotine flux relative to pod mods and box mods (ps < .001).
Table 2.
ECIG Device and Liquid Characteristics and Predicted Nicotine Flux, Nicotine Yield, and TPM Yield
| Devices and liquids with complete dataa | Devices and liquids with estimates includedb | |||||
|---|---|---|---|---|---|---|
| Range | Mean (SD) | Median | Range | Mean (SD) | Median | |
| All devices (n = 83) | ||||||
| Device wattage | 4.1–120.0 | 39.9 (36.1) | 20.0 | 4.1–120.0 | 33.7 (33.6) | 10.5 |
| Liquid nicotine concentration (mg/ml) | 3.0–86.9 | 36.5 (29.8) | 49.5 | 3.0–86.9 | 36.0 (29.3) | 50.0 |
| Nicotine flux (μg/sec) | 12.0–160.1 | 83.5 (41.3) | 75.6 | 12.0–160.1 | 85.6 (34.3) | 84.2 |
| Nicotine yield (mg) | 0.48–6.40 | 3.34 (1.65) | 3.03 | 0.48–6.40 | 3.42 (1.37) | 3.37 |
| TPM yield (mg) | 39.3–1154.7 | 368.3 (343.8) | 154.0 | 39.3–1154.7 | 324.1 (323.5) | 101.0 |
| Disposable vapes (n = 17) | ||||||
| Device wattage | 8.3–8.7 | 8.5 (0.2) | 8.7 | 8.3–8.7 | 8.4 (0.2) | 8.3 |
| Liquid nicotine concentration (mg/ml) | 49.0–86.9 | 73.4 (14.5) | 83.4 | 49.0–86.9 | 73.4 (14.5) | 83.4 |
| Nicotine flux (μg/sec) | 82.8–144.7 | 138.8 (18.6) | 144.7 | 81.2–144.7 | 124.1 (25.6) | 144.0 |
| Nicotine yield (mg) | 3.31–5.79 | 5.55 (0.74) | 5.79 | 3.25–5.79 | 4.98 (1.01) | 5.76 |
| TPM yield (mg) | 79.7–83.4 | 81.7 (2.0) | 83.4 | 79.7–83.4 | 81.2 (1.8) | 79.7 |
| Rebuildable/box mod devices (n = 35) | ||||||
| Device wattage | 11.1–120.0 | 67.5 (28.7) | 70.0 | 11.1–120.0 | 67.5 (26.1) | 67.7 |
| Liquid nicotine concentration (mg/ml) | 3.0–50.0 | 6.8 (9.2) | 6.0 | 3.0–50.0 | 6.7 (8.8) | 6.0 |
| Nicotine flux (μg/sec) | 12.0–144.1 | 65.0 (31.8) | 60.6 | 12.0–144.1 | 68.4 (30.1) | 70.8 |
| Nicotine yield (mg) | 0.48–5.76 | 2.60 (1.27) | 2.42 | 0.48–5.76 | 2.73 (1.21) | 2.83 |
| TPM yield (mg) | 107.2–1154.7 | 640.7 (279.5) | 673.6 | 107.2–1154.7 | 649.6 (250.7) | 649.5 |
| Pod mod devices (n = 31) | ||||||
| Device wattage | 4.1–21.7 | 9.4 (4.1) | 9.8 | 4.1–21.7 | 9.3 (3.4) | 9.4 |
| Liquid nicotine concentration (mg/ml) | 6.0–69.00 | 48.4 (14.6) | 50.0 | 6.0–69.0 | 48.6 (13.6) | 50.0 |
| Nicotine flux (μg/sec) | 26.1–160.1 | 76.2 (33.2) | 56.3 | 26.1–160.1 | 84.0 (25.3) | 93.8 |
| Nicotine yield (mg) | 1.04–6.40 | 3.04 (1.33) | 2.25 | 1.04–6.40 | 3.36 (1.01) | 3.75 |
| TPM yield (mg) | 39.3–209.1 | 88.2 (47.9) | 87.9 | 39.3–209.1 | 89.8 (33.0) | 90.3 |
aIncludes participants’ data for participants’ ECIG devices (n = 51) that wattage and nicotine concentration was able to be determined as described in the methods. Both device wattage and liquid nicotine concentration was able to be determined for 11 disposable devices, 27 rebuildable/box mod devices, and 13 pod mod devices.
bIncludes participants’ data for all participants (n = 83) including those participants whose device wattage determined based on average wattage of other devices in the same device type or mode nicotine concentrations of liquids from the same device type (n = 32).
ECIG device and liquid characteristics include measured values and values that are estimated based on mean values from devices and liquids with known characteristics. Nicotine flux, nicotine yield, and TPM yield are predicted for 10 4-second puffs using an established formula.17 One participant’s ECIG device and liquid is not included due to extreme values.
ECIG = electronic cigarette; TPM = total particulate matter.
Figure 2.
Predicted nicotine flux as well as nicotine yield and total particulate matter (TPM) associated with 10 4-second puffs, from participants’ electronic cigarette devices and liquids. Panels display number of devices that had predicted values separately for each of the device types that were used by participants (disposable vapes, box mods, and pod mods).
The predicted nicotine yield associated with 10 4-second puffs from the 51 participants for whom device wattage and liquid nicotine concentration could be determined was 3.34 mg (SD = 1.65) and ranged from 0.48 to 6.40 mg. This range included values below and above the nicotine yield associated with a single cigarette of 1.6–1.9 mg reported elsewhere.36 Including all devices and liquids with estimated wattage and nicotine concentration values, the mean predicted nicotine yield from all devices was 3.42 mg (SD = 1.37; see Table 2). Because nicotine yield is directly correlated with nicotine flux when puffing behaviors are held constant, predicted nicotine yield associated with 10 4-second puffs followed the same pattern observed for flux. Specifically, there was a main effect of device type on predicted nicotine yield [F(2) = 24.1, p < .001]. Disposable vapes had the highest predicted nicotine yield (M = 4.98 mg, SD = 1.01), followed by pod mods (M = 3.36 mg, SD = 1.01), and lastly box mods (M = 2.73 mg, SD = 1.21) and post hoc contrasts indicated disposable vapes had significantly higher predicted nicotine yields relative to pod mods and box mods (p < .001). All disposable ECIG devices had predicted nicotine yields from 10 4-second puffs that exceeded the yield of a single cigarette.36
For the devices that both wattage and liquid nicotine concentration were determined, predicted TPM yield ranged from 39.3 to 1154.7 mg (M = 368.3 mg, SD = 343.8). Including all devices with determined and estimated wattage and nicotine concentration data, the mean predicted TPM yield was 324.1 mg (SD = 322.5; see Figure 2). TPM yield ranges were smallest for disposable vapes (79.7–83.4 mg), followed by pod mods (39.3–209.1 mg), and the greatest for box mods (107.2–1154.7 mg). There was a main effect of device type on predicted TPM yield [F(2) = 118.3, p < .001]. Post hoc contrasts revealed that predicted TPM yields from disposable vapes (M = 81.2 mg, SD = 1.8) and pod mods (M = 89.8 mg, SD = 33.0) did not differ significantly, and that both were significantly less than predicted TPM yields from box mods (M = 649.6 mg, SD = 250.7; ps < .001).
Discussion
This study found that ECIG device wattage and liquid nicotine concentration from devices and liquids used by ECIG users in “real-world” settings varied considerably and were associated with ECIG device type. Patterns emerged across different device types: as displayed in Figure 1, box mod users had great variability in their device wattages, but limited variability in ECIG liquid nicotine concentration. Conversely, pod mod and disposable vape users had less variability in device wattage, but much greater variability in ECIG liquid nicotine concentration. Predicted nicotine flux and total nicotine yield using a mathematical model37 also varied greatly, with some products having predicted nicotine fluxes much less than that of a combustible cigarette, which ranges from 51.4 to 131.7 μg/s μg/s,28–35 while others had predicted nicotine fluxes much greater than that of a combustible cigarette. Higher model-calculated TPM was associated with higher wattage, however, TPM was not associated with a specific nicotine flux. Rather, high nicotine flux and TPM emissions were predicted from high wattage box mod devices, while disposable vapes also had high model-predicted nicotine fluxes and total nicotine emissions, but also the lowest TPM emissions.
Because nicotine flux can be compared across products, understanding the nicotine flux of ECIG devices may aid researchers and policymakers in understanding how ECIG use is associated with key public health outcomes. Specifically, because nicotine flux can be compared across any product that yields nicotine (eg, ECIGs, cigarettes, smokeless tobacco, nicotine patch, etc.), regulators can focus on a performance standard. That is, regulators can identify ECIG products that have the potential for the greatest public health benefit, such as emitting enough nicotine to suppress withdrawal symptoms in cigarette smokers, and not delivering nicotine in amounts that make them more addictive than cigarettes, all while not being appealing to youth and nontobacco users.
A first step in this process is viewing ECIGs as a diverse class of products made up of many different products. Some “closed system” ECIGs that users cannot modify easily are more uniform in terms of nicotine flux, such as disposable vapes. However, other ECIGs allow users to modify virtually all devices and liquid characteristics, such as box mods. In addition to the challenges presented by device and liquid heterogeneity within ECIG-type subgroups, some ECIG users may not classify their devices into “correct” categories. For example, four participants in the current study presented with a single type of device (Trinity Alpha: a pod mod device), but participants self-reported that this device was a prefilled disposable, pod mod, and a box mod device. Because the device type category does not provide details on an ECIG user’s device wattage and nicotine concentration, the device type category is not often useful for predicting nicotine flux or other product emissions.
Even knowing an ECIG user’s exact ECIG device and liquid is sometimes insufficient for being able to predict the nicotine flux of this device. Indeed, five different participants presented with the exact same ECIG device (VooPoo Drag). However, this one ECIG product had four unique predicted nicotine fluxes because the participants used different combinations of device wattage and liquid nicotine concentrations. The nicotine fluxes of these device and liquid combinations included values below, similar to, and above the nicotine flux of a combustible cigarette because of participants modifying their ECIG device or liquid characteristics. Thus, the heterogeneity of ECIG devices and the almost infinite combinations of ECIG devices and liquids available present considerable challenges for regulators whose goal is to develop regulatory policies to promote public health.
Methods are needed to determine the nicotine flux of ECIG users’ devices despite the identified challenges. For example, cigarette smokers looking to switch completely to ECIGs may be unlikely to transition to a device that emits nicotine at a much lower rate than that of a combustible cigarette. Similarly, ECIGs that emit nicotine at a very low rate may serve as “starter products” for youth because they are less aversive. A concerning finding of the current study was that many of the disposable vape products that are popular among youth and young adults, such as Puff Bars,8 had some of the highest nicotine flux values observed, which may increase the risk of dependence. This possibility represents a major concern given the emerging evidence of potential negative health effects associated with ECIG use,38–45 and the increased risk of progression to cigarette use among youth who use ECIGs.46–48
This study had several limitations. Because a convenience sample was used and researchers attempted to limit repeated ECIG devices, the sample likely included a greater prevalence of box mod users than other device types. As a result, participant characteristics, including that 95.2% of participants reported knowing their liquid nicotine concentration or that approximately half of the participants reported smoking less than 100-lifetime cigarettes in 2019–2020, may not be generalizable to all ECIG users. Not all ECIGs were able to be measured using the multimeter and thus researchers had to rely on device displays or information from manufacturer/retailer websites. ECIG liquid nicotine concentration was determined based on product labeling for some products and a small number of liquids were estimated using mode values of other liquids from other ECIGs of the same device type. Additionally, nicotine concentration on product labels may be inaccurate.49 This may have resulted in differences in recorded and actual device wattage and liquid nicotine concentration for some products. Nicotine flux, nicotine yield, and TPM emissions were predicted based on 10 4-second puffs21,50 using a mathematical model that has been shown to predict values that are correlated highly (r = 0.84) with empirical data.19 The model was validated using different device types that utilized tanks and cartridges, liquids with a range of nicotine concentrations, and a range of device wattages.19 However, emissions predicted in the current study were not validated with measured emissions and thus may not reflect actual exposures among ECIG users due to variability in puffing behavior across individuals and nuances in ECIG devices that may increase or decrease emissions. Future research on ECIG nicotine and other toxicant emissions that considers unique ECIG user puffing behaviors, device characteristics (such as temperature controls, coil variability), and liquid characteristics likely will improve on the external validity of the predicted values reported here.
Conclusions
Variation in nicotine, PM, and other toxicants emitted from ECIGs likely contribute to differences in abuse potential, dependence, health risks, and other health outcomes. This study demonstrates the need for measurement approaches that can determine the nicotine flux with greater accuracy than current methods, especially self-reports based on device type. Once ECIG emissions can be determined for ECIG products more effectively, there will be greater opportunity for empirically informed, health-promoting regulatory policies.
Supplementary Material
Contributor Information
Eric K Soule, Department of Health Education and Promotion, East Carolina University, Greenville, NC, USA; Center for the Study of Tobacco Products, Virginia Commonwealth University, Richmond, VA, USA.
Shannon Mayne, Department of Health Education and Promotion, East Carolina University, Greenville, NC, USA.
William Snipes, Department of Health Education and Promotion, East Carolina University, Greenville, NC, USA.
Elizabeth K Do, Health Behavior and Policy, Virginia Commonwealth University, Richmond, VA, USA; Schroeder Institute, Truth Initiative, Washington, DC, USA.
Travis Theall, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA, USA.
Christoph Höchsmann, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA, USA.
Soha Talih, Center for the Study of Tobacco Products, Virginia Commonwealth University, Richmond, VA, USA; Mechanical Engineering Department, Maroun Semaan Faculty of Engineering and Architecture, American University of Beirut, Beirut, Lebanon.
Corby K Martin, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA, USA.
Thomas Eissenberg, Center for the Study of Tobacco Products, Virginia Commonwealth University, Richmond, VA, USA.
Bernard F Fuemmeler, Health Behavior and Policy, Virginia Commonwealth University, Richmond, VA, USA; Massey Cancer Center, Virginia Commonwealth University, Richmond, VA, USA.
Funding
This research was supported by grant number R21CA239188 from the National Cancer Institute of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration, and partial support was provided by NIH grants U54 GM104940 from the National Institute of General Medical Sciences, which funds the Louisiana Clinical and Translational Science Center, and NORC Center Grant P30 DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by NIDDK. Thomas Eissenberg, Eric Soule, and Soha Talih’s research also is supported by grant number U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration. Eric Soule’s effort also is supported by grant number R15ES032138 from the National Institute of Environment Health Sciences of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or the FDA.
Declaration of Interest
EKS, CKM, TE, and BFF are named on a patent application for a smartphone app that determines electronic cigarette devices and liquid characteristics. Thomas Eissenberg is a paid consultant in litigation against the tobacco and electronic cigarette industry and is named on one patent for a device that measures the puffing behavior of electronic cigarette users and another patent application for a smoking cessation intervention. All others have no conflicts to report.
Authors’ Contributions
EKS contributed to the conceptualization of the study, data collection, and analysis, and led the writing of the manuscript. SM and WS contributed to data collection and analysis and provided a critical review of the manuscript. ST assisted with mathematical model calculations and interpretations. EKD, TT, CH, CKM, TE, and BFF contributed to the conceptualization of the study and provided a critical review of the manuscript. All authors approved the final version of the manuscript.
Data Availability
De-identified data are available at reasonable request.
References
- 1. Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci. 2017;1394(1):5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anic GM, Sawdey MD, Jamal A, Trivers KF.. Frequency of use among middle and high school student tobacco product users—United States, 2015–2017. MMWR Morb Mortal Wkly Rep. 2018;67(49):1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students--United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361–367. [DOI] [PubMed] [Google Scholar]
- 5. Park-Lee E, Ren C, Sawdey MD, et al. Notes from the field: e-cigarette use among middle and high school students - National Youth Tobacco Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1387–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students - National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(5):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. King BA, Gammon DG, Marynak KL, Rogers T.. Electronic cigarette sales in the United States, 2013–2017. JAMA. 2018;320(13):1379–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delnevo C, Giovenco DP, Hrywna M.. Rapid proliferation of illegal pod-mod disposable e-cigarettes. Tob Control. 2020;29(e1):e150–e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartmann-Boyce J, McRobbie H, Lindson N, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2020;10(10):CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibrahim S, Habiballah M, Sayed IE.. Efficacy of electronic cigarettes for smoking cessation: a systematic review and meta-analysis. Am J Health Promot. 2021;35(3):442–455. [DOI] [PubMed] [Google Scholar]
- 11. Kalkhoran S, Glantz SA.. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P.. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. [DOI] [PubMed] [Google Scholar]
- 13. The National Academies of Sciences Engineering Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 14. Wang RJ, Bhadriraju S, Glantz SA.. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111(2):230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cobb CO, Lopez AA, Soule EK, et al. Influence of electronic cigarette liquid flavors and nicotine concentration on subjective measures of abuse liability in young adult cigarette smokers. Drug Alcohol Depend. 2019;203:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Hellani A, Salman R, El-Hage R, et al. Nicotine and Carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics Nicotine Tob Res. 2018;20(2):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shihadeh A, Eissenberg T.. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob Res. 2015;17(2):158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eissenberg T, Shihadeh A.. Nicotine flux: a potentially important tool for regulating electronic cigarettes. Nicotine Tob Res. 2015;17(2):165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talih S, Balhas Z, Salman R, et al. Transport phenomena governing nicotine emissions from electronic cigarettes: model formulation and experimental investigation. Aerosol Sci Technol. 2017;51(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shihadeh A, Eissenberg T.. Nicotine flux, a potentially powerful tool for electronic cigarette regulation. Paper presented at: Annual Meeting of the Society for Research on Nicotine and Tobacco; March 2020; New Orleans, LA.
- 21. Hiler MM, Breland AB, Spindle TR, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol. 2017;25(5):380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rudy A, Leventhal AM, Goldenson NI, Eissenberg T.. Assessing electronic cigarette effects and regulatory impact: challenges with user self-reported device power. Drug Alcohol Depend. 2017;179:337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morean ME, Krishnan-Sarin S, Sussman S, et al. Psychometric evaluation of the Patient-Reported Outcomes Measurement Information System (PROMIS)Nicotine Dependence Item Bank for use with electronic cigarettes. Nicotine Tob Res. 2020;22(11):2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talih S, Salman R, El-Hage R, et al. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob Control. 2019;28(6):678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Talih S, Salman R, Soule E, et al. Electrical features, liquid composition and toxicant emissions from ‘pod-mod’-like disposable electronic cigarettes [published online ahead of print May 12, 2021]. Tob Control. 2021. doi: 10.1136/tobaccocontrol-2020-056362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morean ME, Krishnan-Sarin S, Sussman S, et al. Psychometric evaluation of the E-Cigarette Dependence Scale. Nicotine Tob Res. 2019;21(11):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morean ME, Krishnan-Sarin S, O’Malley S.. Assessing nicotine dependence in adolescent E-cigarette users: the 4-item Patient-Reported Outcomes Measurement Information System (PROMIS) Nicotine Dependence Item Bank for electronic cigarettes. Drug Alcohol Depend. 2018;188:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armitage AK, Alexander J, Hopkins R, Ward C.. Evaluation of a low to middle tar/medium nicotine cigarette designed to maintain nicotine delivery to the smoker. Psychopharmacology (Berl). 1988;96(4):447–453. [DOI] [PubMed] [Google Scholar]
- 29. Hammond D, Fong GT, Cummings KM, et al. Cigarette yields and human exposure: a comparison of alternative testing regimens. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1495–1501. [DOI] [PubMed] [Google Scholar]
- 30. Melikian AA, Djordjevic MV, Hosey J, et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res. 2007;9(3):377–387. [DOI] [PubMed] [Google Scholar]
- 31. Watson CV, Richter P, de Castro BR, et al. Smoking behavior and exposure: results of a menthol cigarette cross-over study. Am J Health Behav. 2017;41(3):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pauwels CGGM, Boots AW, Visser WF, et al. Characteristic human individual puffing profiles can generate more TNCO than ISO and Health Canada regimes on smoking machine when the same brand is smoked. Int J Environ Res Public Health. 2020;17(9):3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gee J, Prasad K, Slayford S, et al. Assessment of tobacco heating product THP1.0. Part 8: Study to determine puffing topography, mouth level exposure and consumption among Japanese users. Regul Toxicol Pharmacol. 2018;93:84–91. [DOI] [PubMed] [Google Scholar]
- 34. Jones J, Slayford S, Gray A, et al. A cross-category puffing topography, mouth level exposure and consumption study among Italian users of tobacco and nicotine products. Sci Rep. 2020;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Djordjevic MV, Stellman SD, Zang E.. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92(2):106–111. [DOI] [PubMed] [Google Scholar]
- 36. Connolly GN, Alpert HR, Wayne GF, Koh H.. Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005. Tob Control. 2007;16(5):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Talih S, Balhas Z, Salman R, et al. A mathematical model to predict nicotine emissions from electronic cigarettes: model formulation and a multi-factor experimental investigation. Aerosol Sci Tech.. 2016;51(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Antoniewicz L, Brynedal A, Hedman L, Lundbäck M, Bosson JA.. Acute effects of electronic cigarette inhalation on the vasculature and the conducting airways. Cardiovasc Toxicol. 2019;19(5):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carnevale R, Sciarretta S, Violi F, et al. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150(3):606–612. [DOI] [PubMed] [Google Scholar]
- 40. Ghosh A, Coakley RC, Mascenik T, et al. Chronic E-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med. 2018;198(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. King JL, Reboussin BA, Wiseman KD, et al. Adverse symptoms users attribute to e-cigarettes: results from a national survey of US adults. Drug Alcohol Depend. 2019;196:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li D, Sundar IK, McIntosh S, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob Control. 2020;29(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moheimani RS, Bhetraratana M, Peters KM, et al. Sympathomimetic effects of acute E-cigarette use: role of nicotine and non-nicotine constituents. J Am Heart Assoc. 2017;6(9):e006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reidel B, Radicioni G, Clapp PW, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197(4):492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soule EK, Nasim A, Rosas S.. Adverse effects of electronic cigarette use: a concept mapping approach. Nicotine Tob Res. 2016;18(5):678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pierce JP, Chen R, Leas EC, et al. Use of E-cigarettes and other tobacco products and progression to daily cigarette smoking. Pediatrics. 2021;147(2):e2020025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berry KM, Fetterman JL, Benjamin EJ, et al. Association of electronic cigarette use with subsequent initiation of tobacco cigarettes in US youths. JAMA Netw Open. 2019;2(2):e187794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buettner-Schmidt K, Miller DR, Orr M, et al. Electronic cigarette refill liquids: nicotine content, presence of child-resistant packaging, and in-shop compounding. J Pediatr Nurs. 2021;59:45–54. [DOI] [PubMed] [Google Scholar]
- 50. Spindle TR, Talih S, Hiler MM, et al. Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug Alcohol Depend. 2018;188:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data are available at reasonable request.