Abstract
An 8-month-old neutered female domestic mixed breed cat was presented to Dr. L. Kriaučeliūnas Small Animal Clinic due to coughing that persisted for 2 weeks. Lateral and dorsoventral chest radiographs revealed an unusual dome-shaped soft tissue opacity mass that had contact with the cranial part of the diaphragm. Together with heart and abdominal ultrasound findings, we decided that one of the differential diagnoses was a diaphragmatic hernia. During the diagnostic celiotomy, a vertical 4 cm in length diaphragmatic deficit was visualized. Left medial and lateral liver lobes were herniated, yet healthy-looking. Adhesions between the liver lobes and the pericardium sac were visualized and dissected. The pericardium was sutured with simple interrupted suture pattern. A herniorrhaphy was performed suturing the diaphragm with the continuous suture pattern. Successful surgical treatment resulted in fully resolved clinical symptoms.
Keywords: congenital hernia, diaphragmatic defect, caval foramen, heart ultrasound
Resumo
Um gato doméstico, fêmea, castrada de oito meses de idade, sem raça definida, foi apresentada à Clínica de Pequenos Animais do Dr. L. Kriaučeliūnas devido a uma tosse que persistiu por duas semanas. As radiografias lateral e dorsoventral do tórax revelaram uma massa incomum de opacidade de tecidos moles em forma de cúpula que tinha contato com a parte cranial do diafragma. Juntamente com os achados da ultrassonografia abdominal e cardíaca, decidimos que um dos diagnósticos diferenciais era uma hérnia diafragmática. Durante a celiotomia diagnóstica, foi visualizado um déficit diafragmático vertical de 4 cm de comprimento. Os lobos hepáticos medial e lateral esquerdos estavam herniados, mas com aparência saudável. As aderências entre os lobos hepáticos e o saco pericárdico foram visualizadas e dissecadas. O pericárdio foi suturado com padrão de sutura interrompida simples. Foi realizada herniorrafia suturando o diafragma com o padrão de sutura contínua. O tratamento cirúrgico bem-sucedido resultou em sintomas clínicos totalmente resolvidos.
Palavras-chave: hérnia congênita, defeito diafragmático, forame caval, ultrassom cardíaco
Introduction
The diaphragm is a thin, musculotendinous partition between abdominal and thoracic cavities. Its anatomy and embryology are quite complex. A central tendon is localized in a strong, tendinous center, where the caudal caval vein passes through the foramen. Costal, sternal, and lumbar muscles surround the tendinous center on all sides and attach to the inside part of the thoracic wall (Slatter, 2003).
There are numerous ways the diaphragm can herniate. All diaphragmatic hernias are divided into congenital and acquired. Acquired ones are usually traumatic, but medical studies conclude that the etiology of this pathology is yet poorly understood (Dalmer & Clugston, 2019). Research suggests that prevalence of congenital diaphragmatic hernias in human infants is only 1 in 3000 (Dalmer & Clugston, 2019). It is reported that such rare pathologies in both humans and animals are usually accompanied by confusion. Herniated tissues are mistaken for lung consolidations, abscesses, lung or mediastinal masses, cysts, and granulomas (Ng et al., 2006; Nonaka et al., 2008; Park et al., 2020; Voges et al., 1997). Therefore, the key to understanding this pathology and preventing further confusion is to study it and make the findings available to colleagues.
Case report
An 8-month-old neutered female domestic mixed breed cat weighing 3.8 kg presented with a 2-week history of coughing. The cough occurred when the animal stretched its neck and lowered its head. Coughing lasted for less than a minute, about 3 - 4 times a day. Also, the cat was lethargic and experienced episodes of mild asphyxia, wheezing, physical activity intolerance, and panting after it. The animal was dewormed and vaccinated a week prior to the first visit. The cat was spayed 5 weeks before in another clinic. According to the cat’s records from another clinic, clinical examination prior to sedation was within physiological limits. Laboratory tests or other diagnostic tools such as X-ray or ultrasound examination were not performed. No changes were detected during anesthesia. After the operation, the cat recovered perfectly within 8 days. The cat was fed with dry food, and occasionally given canned cat feed. Water was not limited. The cat was kept indoors and had no free exit to the outside. Owner denied any possibility of traumatic incidents at home. The only other animal it had contact with was another cat kept at home. This roommate cat sneezed and had a nasal discharge. An appointment with a pulmonologist and cardiologist was scheduled for cardiac and respiratory function testing.
Diagnostics
During heart auscultation no murmurs were detected, the cat had a regular, well heard sinus rhythm, with a heart rate of 176 x/min. No rhythm abnormalities were detected, the cat had normal levocardia. Breathing was gentle, and the cat did not have any inspiration or expiration difficulties. The cat had no nasal discharge. Clinical examination was insignificant. The cat had its front right leg and abdominal area shaved. Blood samples were taken from v. cephalica using a vacuum blood collection system. A urine sample was taken by cystocentesis with ultrasound control. Complete blood count (IDEXX ProCyte Dx; Laboratories Inc, Hoofddorpe, Netherlands), serum biochemistry (IDEXX Catalyst One; IDEXX Laboratories Inc., Hoofddorpe, Netherlands), urinalysis (Thinka RT ‒ 4010; Arkray; Amstelveen, Netherlands), and feline leukemia virus together with feline immunodeficiency virus serology (Rapid Test Kit; J&G Biotech LTD, London, United Kingdom) were performed. Leukocytes 19.08 x10˄g/L (reference interval [RI] 2.87 - 17.02) and lymphocytes 8.99 x10˄g/L (reference interval [RI] 0.92 - 6.88) were mildly increased. Aspartate aminotransferase (71IU/L; reference interval [RI] 2 - 62 IU/L), alanine aminotransferase (192IU/l; [RI] 19-100IU/L) were also mildly increased. Urinalysis showed proteinuria, which was clinically insignificant. All other laboratory testing results were insignificant.
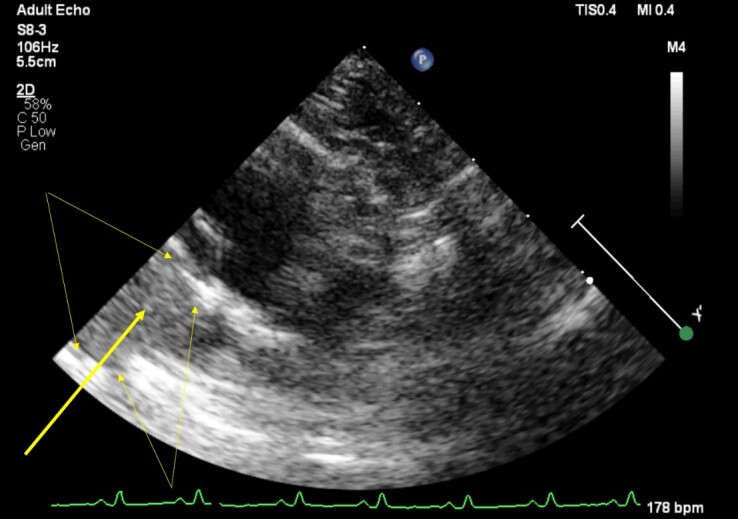
A heart ultrasound (Philips EPIQ CVx; WA, USA; S8-3 3-8 MHz Sector Array transducer) examination was performed for a non-sedated animal. Before examination the hair from the chest area were clipped and shaved. No cardiac pathologies were detected. When evaluating the base of the heart in the short axis (right parasternal position), the right side of the heart looked unusually enlarged. Otherwise, the echocardiogram was normal. No dilatation of the cardiac chambers was observed, both mitral and pulmonary artery valves were able to close tightly. The ultrasound pictures made it look like a hepatic lobe pressed the right atrium and CCV from the outside, however, no changes in blood flow were observed. This liver lobe size was 2.8 cm in the echograms. The pericardial sac had adhesion to the liver lobe, however no effusion was observed (Figure 1).
Figure 1. Heart ultrasound image of the thoracoabdominal region of an 8-month-old neutered female domestic mixed breed cat with persistent coughing, mild asphyxia, nasal discharge, and physical activity intolerance via a subcostal window. Yellow arrow indicates hepatic parenchyma tissue and two-way yellow arrows indicate its edges.
Thoracic x-rays and abdominal ultrasound were requested for further investigation due to - non-specific clinical and heart ultrasound examination findings.
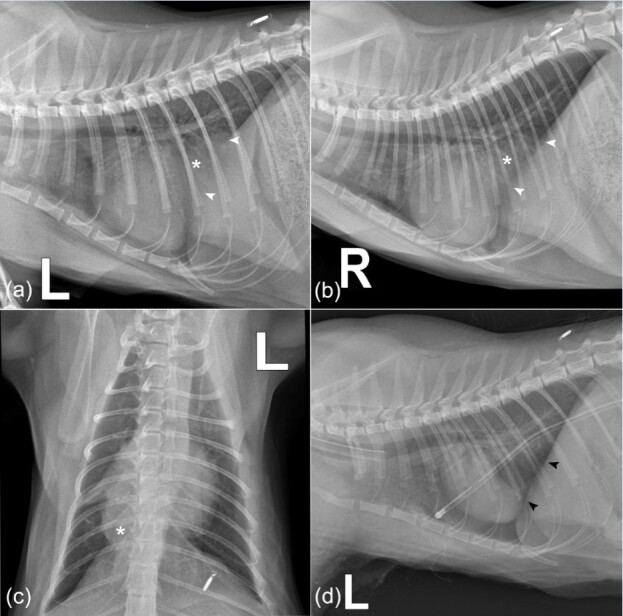
Thoracic x-rays (Optica 20; Varex, The Netherlands) of left, right lateral (LL) and dorsoventral (DV) projections were performed in lateral and sternal recumbencies. Examination was also performed for a non-sedated animal. Plain thoracic radiography revealed a broad-based, round dome shaped, shadow in the chest cavity (Figure 2). The shadow partially covered the heart, forming a mass effect. Ventrally to location of caudal vena cava, x-ray images did not show a characteristic lung tissue pattern and vena cava was not visible. In the location of vena cava, lung tissue was more contrast enhanced. This contrast-enhanced tissue was broad-based and was in contact with the adjacent diaphragm (Figure 2).
Figure 2. Thoracic radiographs of an 8-month-old neutered female domestic mixed breed cat with persistent coughing, mild asphyxia, nasal discharge, and physical activity intolerance. (a) Left lateral, (b) right lateral recumbency and (c) dorsoventral projections. Panel a-c shows an enlarged cardiac silhouette with cardiomegaly before surgical intervention. The cardiac silhouette merges with the diaphragmatic outline and loss of diaphragmatic border. There is a lobulated soft tissue opacity in the caudal thorax just right of the midline at mid-height, with a broad base to the diaphragm (asterisk). White arrowheads indicate the loss of diaphragmatic margins. The caudal vena cava is not visible. Left lateral (d) recumbency thoracic radiograph after herniorrhaphy and thoracic tube placement. Black arrowheads indicate the integrity of the diaphragm after the surgical procedure.
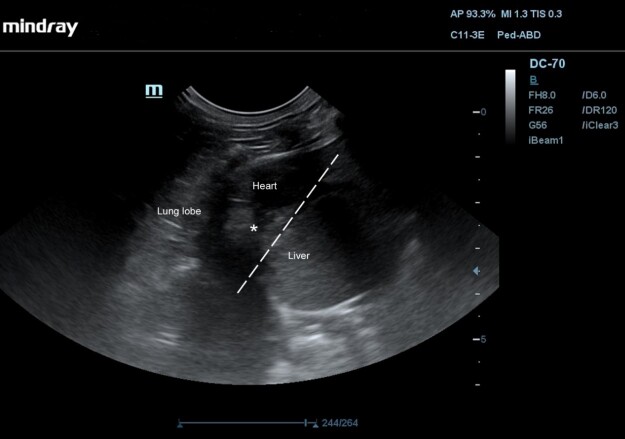
Abdominal ultrasound (Mindray DC-70; Hamburg, Germany; C11-3e 2.6-12.8 MHz Micro-Convex Array transducer) via subcostal (transabdominal) approach through the liver was performed. Observing the diaphragm, it was visible absence of the echogenic diaphragmatic landmark centrally and identified cranial displacement of a portion of the liver into the caudal thorax, continuation of the liver parenchyma attached to the pericardial sac (Figure 3).
Figure 3. Ultrasound image of the thoracoabdominal region of an 8-month-old neutered female domestic mixed breed cat with persistent coughing, mild asphyxia, nasal discharge, and physical activity intolerance via a subcostal window. There is an interruption in the diaphragmatic interface (dotted line), with herniation of hepatic parenchyma (asterisk).
Differential diagnoses included pulmonary mass, caudal mediastinal mass (including neoplasia or granuloma), lung lobe torsion and diaphragmatic hernia (caval foramen hernia, pleuroperitoneal hernia).
Treatment and management
Midline exploratory celiotomy was scheduled. A 22-gauge venous catheter was inserted into the patient's v. cephalica. The cat began receiving 12.5 mg amoxicillin - clavulanate (Synulox, Zoetis, Belgium) SC q24h and Ringer lactate IV fluid therapy at a 5 mL/kg/h maintenance rate. Since the cat had episodes of asphyxia, coughing and wheezing, it was classified as a Class 4 patient in Physical Status Classification, proposed by the American Society of Anesthesiologists.
Preoperative clinical examination was performed: mucous membranes were pink, CRT - 1 s, femoral pulse was strong, rhythmic, heart rate was 144 x/min, breathing rate- 40 x/min, and rectal temperature was 38.9C. Premedication was performed with 0.04 mg/kg atropine sulfate 1 mg/kg (PharmaSwiss, Czech Republic) and maropitant (Cerenia; Pfizer, United Kingdom) IV. Sedation was performed with 0.4 mg/kg methadone hydrochloride (Richter Pharma, Austria), 0.4 mg/kg midazolam (B. Braun, Germany) IV. Induction was performed with 4 mg/kg propofol (Fresenius Kabi, Germany) IV, 2 mcg/kg fentanyl (Polfa, Poland) IV. The animal was intubated with a cuffed 3.5 mm size endotracheal tube. The surgical area was prepared according to aseptic and antiseptic requirements.
Anesthesia was performed with sevoflurane 2% minimum alveolar concentration (MAC) anesthetic gasses and 15 mL/kg/100% O2/min was maintained. Fentanyl was administered by constant rate infusion (CRI). During the operation, into 250 mL NaCl 0.9 percent solution bottle, 40 percent 30.0 mL of glucose solution and 6.0 mL of fentanyl (0.3 mg/kg) were injected. The solution was infused at 3.0 mL/kg/h dose during the operation. The cat was mechanically ventilated during the procedure: applicable PCV: Pinsp. 10 cm H2O, RR - 8 rpm, I:E 1:2, T-0.5 s.
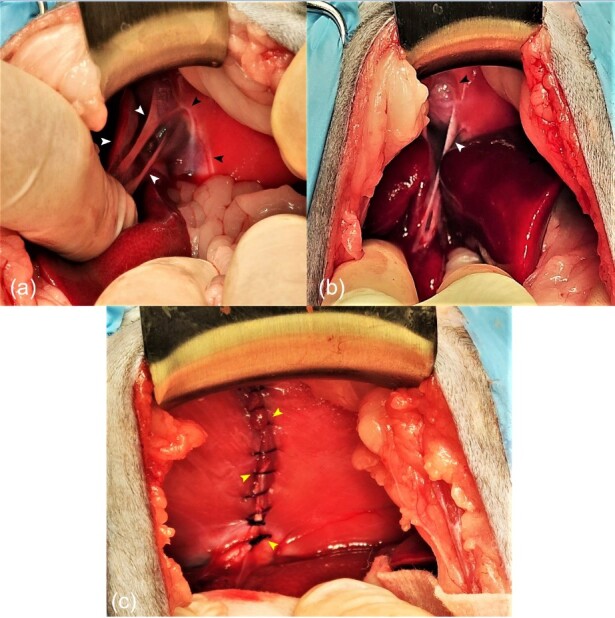
A midline celiotomy was performed. The diaphragm was visualized. A vertical 4 cm defect was identified cranially to the bundles of right diaphragmic crus in the central tendon area, at the level caval foramen. The left medial and lateral liver lobes were herniated. Budd-Chiari-like syndrome has not been identified. There was also no compression to the CCV. The lobes had adhesions to the pericardium, yet it was intact. Liver adhesions from the pericardium were bluntly dissected to enable their repositioning back into the peritoneal cavity. After the dissection the pericardium had a 1 cm defect, so it was sutured using simple interrupted suture pattern with 4/0 polydioxanone (PDX; Atramat, Mexico) suture material. Edges of the diaphragm were scarified with the scalpel on both sides. The caval foramen defect was opposed with simple continuous suture pattern using 3/0 polydioxanone (PDX; Atramat, Mexico) suture material. The abdominal cavity was flushed with sterile NaCl 0.9% solution at the dose of 50 mL/kg. After the closure of the abdominal wall, a thoracostomy was performed. A size 10 chest drain (Kruuse; Havretoften, Denmark) was inserted between 7th and 8th intercostal spaces. Negative chest cavity pressure was restored and maintained (Figure 4).
Figure 4. Hernial appearance during exploratory celiotomy of an 8-month-old neutered female domestic mixed breed cat with persistent coughing, mild asphyxia, nasal discharge, and physical activity intolerance. (a) and (b) Prior to hernia reduction: there is a defect in the central tendon of the diaphragm with reduced liver mass in the abdominal cavity. White arrowheads indicate adhesions. Black arrowheads show the edges of the hernia. Following herniorrhaphy (c): liver lobes have been reduced into the abdominal cavity. Simple continues suture pattern oppose the edges of the hernial ring defect (yellow arrowheads).
Outcome
After the surgery, the cat recovered perfectly. The cat has been placed in the ICU unit for 5 days. Postoperatively, fentanyl constant rate infusion was stopped and replaced with 0.9% NaCl saline solution at the dose of 5 mL/kg/h intravenously for 2 days. Postoperative analgesia was achieved with buprenorphine 0.03 mg/kg (Richter Pharma AG, Austria) IV q12h and robenacoxib (Elanco GmbH, Germany) was induced at 2 mg/kg SC q24h for 4 days. Cat also received 12.5 mg amoxicillin-clavulanate (Synulox, Zoetis, Belgium) q24h SC for 4 days. The thoracostomy tube was removed after 48 h postoperatively. After the operation, the animal had a great appetite. Cough episodes and respiratory disorders did not recur. Two weeks postoperatively, the animal tolerated physical activity well, had no panting after it and had no episodes of asphyxia. After 3 months recheck, the parameters of the animal clinical examination were excellent and within the normal value range with no respiratory disorders.
Discussion
All diaphragmatic hernias are divided into congenital and acquired. There are reported pathways that contribute toward the development of the hernias. In human medicine, these factors are fetal male gender, maternal age above 40 years, vitamin deficiencies, smoking, consumption of alcohol and chemicals during the pregnancy (Haroun, 2018). Studies in veterinary medicine have concluded that retinoids have an impact on the development of congenital diaphragmatic hernias. Pathology was present in 25 - 70% of the offspring that were fed with Vitamin A deficient feeds. The rate of herniation decreased when Vitamin A was introduced into the diet and was totally prevented when retinoids were given on the 10th and 11th days of gestation (Montedonico et al., 2008).
Speaking of anatomical areas of diaphragmatic hernias, they can be hiatal, pleuroperitoneal, sternocostal, central, caval, or caused by the eventration. When the congenital diaphragmatic defects are unusual, the exact type of hernia can rarely be determined. Based on our knowledge, this cat most probably had a congenital diaphragmatic defect. The reason for this opinion is that the cat had no history of trauma, lived strictly indoors, was still young, and during the celiotomy, we were not able to find any traumatic changes whatsoever. Based on existing scientific literature, similar congenital hernias were diagnosed accordingly (Ng et al., 2006; Voges et al., 1997).
Diaphragmatic hernias might result in compromised lung volume, lung hypoplasia, pulmonary hypertension, respiratory distress, liver incarceration, hepatic hypoxia, and Budd-Chiari-like syndrome (Evans & Biery, 1980; Losty, 2014; Siow et al., 2020). Although such severe complications are rarely reported. In most cases, hernias are found accidentally or during routine screenings (Kim et al., 2016). Quite mild symptoms are seen in dogs and cats. Patients are ill with acute cough, reverse sneezing, and chronic cough for 5 weeks (Park et al., 2020; White et al., 2003). Our cat had a cough too, that resolved soon after the surgical treatment.
Some studies state that chest X-rays are highly valuable in the diagnosis of diaphragmatic hernias. Kim et al, 2016 reported that right lateral radiographies revealed caval foramen hernias in 6 dogs out of 7. Nevertheless, more specific diagnostic images such as a CT scan are highly recommended for a better understanding of the pathology and better surgical treatment options (Kim et al., 2016). Performing procedures such as thoracoscopic diaphragmatic defect repairs would be a challenge without the high-resolution computed tomography images (Park et al., 2020).
Herniorrhaphy is a recommended surgical management option. We performed the procedure by closing the diaphragmatic defect with single interrupted sutures and placing a thoracic drain. Our successful outcome is compatible with other studies that reported resolution of respiratory symptoms as well (White et al., 2003).
Conclusions
This report presents a case of diagnosis and surgical treatment of congenital diaphragmatic hernia in a cat. After performing extensive diagnostics, the hernia was recognized as caval foramen type. The case of this cat is followed by a successful surgical treatment that led to resolved respiratory system symptoms.
Footnotes
How to cite: Kvitka, D., Juodžentė, D., Rudenkovaitė, G., & Burbaitė, E. (2023). Successful early diagnosis and surgical treatment of congenital caval foramen hernia in an 8-month-old mixed breed cat. Brazilian Journal of Veterinary Medicine, 45, e005622. https://doi.org/10.29374/2527-2179.bjvm005622
Ethics statement: All procediments were consented by the animal owner.
Financial support: None.
Availability of complementary results: On readers’ request we can share the details of the case by mail.
The study was carried out at Dr. L. Kriaučeliūnas small animal clinic, Veterinary Faculty, Lithuanian University of Health Sciences, Kaunas, Lithuania.
References
- Dalmer T. R., Clugston R. D. Gene ontology enrichment analysis of congenital diaphragmatic hernia-associated genes. Pediatric Research. 2019;85(1):13–19. doi: 10.1038/s41390-018-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. M., Biery D. N. Congenital peritoneopericardial diaphragmatic hernia in the dog and cat: A literature review and 17 additional case histories. Veterinary Radiology. 1980;21(3):108–116. doi: 10.1111/j.1740-8261.1980.tb00589.x. [DOI] [Google Scholar]
- Haroun H. S. Congenital diaphragmatic hernias: A review article. Anatomy Physiol Biochem Int J. 2018;4(2):555635 [Google Scholar]
- Kim J., Kim S., Jo J., Lee S., Eom K. Radiographic and computed tomographic features of caval foramen hernias of the liver in 7 dogs: Mimicking lung nodules. The Journal of Veterinary Medical Science. 2016;78(11):1693. doi: 10.1292/jvms.16-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losty P. D. Congenital diaphragmatic hernia: Where and what is the evidence? Seminars in Pediatric Surgery. 2014;23(5):278–282. doi: 10.1053/j.sempedsurg.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Montedonico S., Nakazawa N., Puri P. Congenital diaphragmatic hernia and retinoids: Searching for an etiology. Pediatric Surgery International. 2008;24(7):755–761. doi: 10.1007/s00383-008-2140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. S., Lee T. W., Wan S., Yim A. P. Caval foramen hernia masquerading as a thoracic mass. Canadian Journal of Surgery. 2006;49(1):64–65. [PMC free article] [PubMed] [Google Scholar]
- Nonaka Y., Takashima K., Yamane T., Yamane Y. Caval foramen hernia in a dog. Dobutsu Rinsho Igaku. 2008;17:81–85. [Google Scholar]
- Park J., Lee H. B., Jeong S. M. Caval foramen hernia in a dog: Preoperative diagnosis and surgical treatment. The Journal of Veterinary Medical Science. 2020;82(11):1602. doi: 10.1292/jvms.19-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow J. W., Hoon Q. J., Jenkins E., Heblinski N., Makara M. Caval foramen hernia in a cat. Journal of Feline Medicine and Surgery Open Reports. 2020;6(2):2055116920964021. doi: 10.1177/2055116920964021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatter D. H. Textbook of small animal surgery. Saunders; 2003. pp. 471–487. [Google Scholar]
- Voges A. K., Bertrand S., Hill R. C., Neuwirth L., Schaer M. True diaphragmatic hernia in a cat. Veterinary Radiology & Ultrasound. 1997;38(2):116–119. doi: 10.1111/j.1740-8261.1997.tb00825.x. [DOI] [PubMed] [Google Scholar]
- White J. D., Tisdall P. L. C., Norris J. M., Malik R. Diaphragmatic hernia in a cat mimicking a pulmonary mass. Journal of Feline Medicine and Surgery. 2003;5(3):197–201. doi: 10.1016/S1098-612X(02)00069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]