Abstract
Background
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) represents the most recent severe pandemic resulting in coronavirus disease 2019 (COVID-19). COVID-19 can damage the central nervous system, requiring admission to intensive care units (ICU) and aggressive treatments (long-term ventilatory assistance and sedation) to stabilize vitals. Most post-COVID-19 patients experience cognitive impairments and mood or stress disorders.
We aimed to study the frequency of cognitive deficits in COVID-19 survivors, the relationship between clinical factors in the acute phase and cognitive outcomes, affective states, and quality of life. We explored cognitive reserve (CR) role, as a post-COVID-19 resilience factor.
Methods
Twenty-nine COVID-19 inpatients were assessed using a neuropsychological battery, mood scales, quality of life, and social integration questionnaires. Twenty-five were retained through telephone follow-up to monitor cognitive sequelae, affective states, and reintegration levels roughly 8 months after hospital discharge. We administered the Cognitive Reserve Index questionnaire.
Results
We found most patients display no cognitive deficits. When they did, multi‐domain impairment occurred most frequently, especially involving executive functions. Results revealed a significant correlation between depression levels and the interval between ICU admission and tracheal tube removal. We found increased levels of depression and anxiety at follow-up, a significant relationship between resuming daily life activities, high CR, and executive functions.
Conclusions
These findings suggest the importance of psychological support in the long term and the modulating role of cognitive reserve in quality of life after infection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-023-06665-4.
Keywords: Severe COVID-19, Cognitive outcomes, Depression, Quality of life, Follow-up, Cognitive reserve
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that causes the coronavirus disease 2019 (COVID-19) represents the most recent severe pandemic worldwide, with high morbidity and mortality: over 614 million people infected since the beginning of the pandemic up to September 30, 2022, and 6,522,000 deaths [1].
Since the virus appeared, we observed several mutations in terms of transmissibility, lethality, and symptoms, resulting in extremely heterogeneous clinical pictures. This has made it harder to investigate the association between SARS-CoV-2 and neurological disorders. Thus far, sufficient findings are demonstrating the deleterious effects of SARS-CoV-2 on brain function [2], but the pathway is yet to be clarified. A considerable amount of evidence has been accumulated demonstrating that SARS-CoV-2 infection can damage the central nervous system (CNS) directly [3], via blood circulation and neuronal pathways, or indirectly [2], causing hypoxia. For example, viruses can invade the CNS through the olfactory membrane: they enter either the blood or lymph vessels and, consequently, can directly damage the brain by disrupting the blood–brain barrier [4]. Alternatively, viruses can migrate through the cribriform plate by infecting the trigeminal or vagal nerve [5]. Indirect neurologic complications should also be considered since SARS-CoV-2 often causes a severe respiratory viral illness that affects alveolar gas exchange, resulting in severe CNS damage [2]. These damages often require aggressive treatments, admission to intensive care units, long-term ventilatory assistance, and sedation to stabilize vitals. Moreover, most of these patients can experience dizziness, vertigo, anosmia, seizures, stroke, myopathies, encephalitis, Guillain–Barre syndrome, and delirium [6] during the process of weaning from mechanical ventilation or tracheal tube. Among the neurological alterations described, delirium is one of the main factors related to cognitive impairment [7], which is, in turn, linked to mood and post-traumatic stress disorders [8].
What about post-acute COVID-19 sequelae symptoms? To date, cognitive sequelae and psychological symptoms have been described in post-COVID‐19 patients, but only a few studies focused on the relationship between the severity of the acute-phase disease and post-acute COVID-19 sequelae symptoms [9–12]. To fulfill this gap, we assessed COVID-19 survivors admitted to the intensive care unit (ICU) during the initial stages of the pandemic. By using tests spanning across different cognitive domains, we aimed to (1) describe the frequency of deficits for specific cognitive domains, estimating the frequency of single- and multi-impairments, (2) investigate whether certain clinical factors related to the acute-phase disease severity were associated with cognitive deficits and the persistence of “brain fog” later on the acute event [13], (3) investigate the impact of the severity of the acute-phase disease on affective states and social life along with the (4) cognitive reserve (CR) role, as a plausible explanation of individual functional outcome and resilience to COVID-19 roughly 8 months after the discharge from the rehabilitation structure. CR refers to individual differences which allow people to better cope with brain pathology [14]. It has been demonstrated that CR has a protective role in several clinical and non-clinical populations [15]. To date and to the best of our knowledge, very few studies have already demonstrated the effect of CR in COVID-19 patients as a modulator on cognition and psychological symptoms [9, 16]. Here, we want to replicate these results in our sample.
Methods
Study design and participants
This is a prospective and observational cohort study performed in a tertiary hospital, Centro Cardinal Ferrari (CCF). We included 29 subjects admitted to our rehabilitative hospital, from April 5, 2020, to July 31, 2020, after SARS‐CoV‐2 infection. The inclusion criteria for this study were (a) having had COVID‐19 symptoms and confirmed positive for SARS‐CoV‐2 via polymerase chain reaction (PCR) and/or serology (anti‐SARS‐CoV2 IgM or IgG), (b) having been hospitalized in an Intensive Care Unit (ICU) with invasive mechanical ventilation (imv), and (c) being 18 + years old. The exclusion criterion was documented medical history of neurological or psychiatric conditions before the infection. Among the 29 initial patients, 25 were retained through follow-up. We performed a follow-up roughly 209 days after the discharge (209.44 ± 38.72; mode: 8 months) via phone interviews. Losses to follow-up included 4 individuals who declined to participate or were unresponsive to the invitation.
All patients gave informed consent for the study. This research has been approved by the Ethics Committee Area Vasta Emilia Nord (AVEN, protocol nr. 616/2020/OSS/AUSLPR, August 24, 2020).
Medical management and assessment
Medical treatments were performed at the admission to the CCF (Admission) until they were needed, as described below. Subsequently, cognitive and psychological evaluations were carried out (hospitalization). Psychological symptoms were also evaluated at discharge (discharge) and follow-up (follow-up). At follow-up, we also monitored the cognitive status and the ability to resume daily life activities. The timeline is depicted in Supplemental Information S1.
Medical treatments
All patients during the acute phase received medical care to both stabilize vital signs and led to COVID-symptoms resolution. To achieve the first goal, all the patients were admitted to ICU, and all of them needed tracheostomy, imv, and high-flow oxygen therapy. Subsequently, our well-integrated multi-professional team was able to wean patients from mechanical devices (imv, tracheostomy tube) and sedative drug therapies, namely, methadone, and benzodiazepines, by continuously monitoring SaO2 and daily arterial blood gas checks patients together to intensive respiratory therapy by assisted cough [17]. In the meantime, all patients underwent motor and functional rehabilitation. The exercises focused on muscle strengthening to gradually recover from generalized muscle weakness and increase fatigue tolerance, bed-to-chair mobility, wheelchair skills, pre-gait (sit-to-stand), and activities of daily living (ADL) training.
Neuropsychological assessment
Patients’ cognitive and psychological evaluations were performed after 3 criteria were met: (1) complete weaning from the sedative and antipsychotic drugs, (2) clinical stability defined by stabilized respiratory condition (PaO2/FiO2 > 300), and (3) score greater than 75 on the Galveston Orientation and Amnesia Test (GOAT). Tests were administered by expert neuropsychologists at CCF.
Cognitive aspects of mental function were evaluated by Addenbrooke’s Cognitive Examination Revised (ACE-R). A comprehensive neuropsychological assessment (NA) was available as well, and it included multiple tests for each of the following cognitive domains: attention, learning and short‐ and long‐term memory, and executive functions.
These tests were administered: Trail Making Test (TMT), Raven’s Progressive Matrices 1938 (PM38), Verbal Reasoning Test (VRT), Long-term Verbal Memory (Prose Memory), Rey auditory verbal learning test (15-Rey), Stroop test, Wisconsin Card Sorting Test (WCST), Block Tapping long-memory test (Corsi supraspan), Digit Span forward and backward, Block Tapping Short-Memory Test forward and backward (Corsi span), Rey-Osterrieth Complex Figure Test.
Neuropsychological scores were adjusted for age, sex, and education and were analyzed according to the method of equivalent scores (ESs), as described by Capitani and Laiacona [18]. The system of ESs provides, for each test, a result that ranges from 0 to 4: ESs = 0 accounts for the lower 5th percentile of the population; ESs = 1 indicates a score between the 5th and 20th percentiles; ESs = 2 and 3 indicates a score between the 20th and 50th percentiles; ESs = 4 corresponds to score above the 50th percentile. A clinical meaning for each point has also been proposed [19], and it allows us to classify the performance of the tests as “defective” (ESs = 0), “borderline” (ESs = 1), “low-end normal” (ESs = 2), and “normal” (ESs = 3 and 4). In addition to cognitive measures, patients were administered the Hospital Anxiety and Depression Scale (HADS) as a brief measure for anxiety and depression levels, beyond the World Health Organization Quality of Life (WHOQOL-100) and Impact of Event Scale (IES), evaluating the quality of life and social integration and post-traumatic stress levels, respectively. At discharge, on an average of 102.7 days since the ICU admission (102.69 ± 41.45 days; range: 54–234), we reassessed the affective state of patients by using the same scales (HADS, WHOQOL, and IES). A complete list with full test names and references is provided in Supplemental Information S2.
Follow-up assessment
On an average of 8 months after the discharge, a telephone assessment of the cognitive status was performed by using the remote version of the Global Examination of Mental State (tele-GEMS). To monitor the affective state and the individual’s ability to resume daily life activities within the community, we administered HADS and the Reintegration to Normal Living Index (RNLI), respectively. We also made a brief qualitative assessment to evaluate subjective cognitive failure and/or psychological symptoms. Finally, we administered the Cognitive Reserve Index questionnaire (CRIq).
Statistical analysis
Descriptive statistics were reported for socio-demographic and clinical variables for the whole sample. Absolute numbers and percentages were used for categorical variables, while mean and standard deviation were used for continuous ones.
The HADS, WHOQOL-100, IES, RNLI, and CRIq (total and sub-scores) have been considered as dependent variables of interest separately.
First, we analyzed the frequency of the presence or absence of cognitive deficits based on neuropsychological test scores as a function of three categories: pathological (ESs = 0), limit (ESs = 1), and normal (ESs > / = 2). The distribution of test scores was analyzed by grouping them into cognitive domains. To define cognitive domains, we ran a principal component analysis (PCA) that included all the test scores from NA [20]. To run the PCA, we transformed individual raw scores from each test into percentiles.
Subsequently, we analyzed the frequency of cognitive deficits in patients by classifying neuropsychological tests according to the domains defined by the PCA. A cognitive domain was classified as affected if it had at least one score with ESs = 0. This analysis aimed to see whether single- or multi-domain impairment occurs after SARS‐CoV‐2 infection. Additionally, we aimed to explore the most frequent associations of cognitive deficits. Finally, we performed analyses to explore the impact on cognition of the acute-phase disease severity, evaluated through the variable hospitalization and tracheal tube removal, along with the affective scores.
All statistical analyses were performed using MATLAB. The significance level was set to p < 0.05 for all analyses.
Results
Patient sample characteristics
Our sample (Supplemental Information S3) was composed of 19 males (65%) and 10 females (35%), with a mean age of 59.6 years (59.62 ± 8.4; range: 43–75) and mean level of education of 11 years (11.03 ± 3.81; range: 5–18). All patients were hospitalized for an average of 55 days (55.00 ± 30.28 days; range: 28–137) before being admitted to CCF. All entered the Intensive Care Unit (ICU) (51.31 ± 27.86 days; range: 23–128) and were intubated. The tracheal tube was removed roughly 61 days after the ICU admission (61.41 ± 30.51 days; range: 16–157). We performed the neuropsychological assessment on an average of 78.24 days (SD = 28.17; range: 30–137) since the admission to ICU.
In the following sections, the results of the neuropsychological assessment and psychological functioning, together with their associations with clinical factors and the cognitive reserve, will be described.
Cognitive status and test scores distribution
We found normal overall cognitive functioning (Supplemental Information S4). General cognitive decline was observed exclusively in one patient (3.4%) having a pathological ACE-R score. An item-by-item analysis showed that this patient scored low in these domains: attention/orientation, memory, and lexical fluency.
Regarding NA, since we used several tests with many scores, we grouped them into cognitive domains to better describe our results in terms of cognitive processes that are affected or spared in our sample. To identify the most involved cognitive domains, we ran a PCA including the percentiles from all tests used in NA, excluding the ACE-R since it spans multiple cognitive domains as a screening tool. The result of the PCA showed that three main factors (Figure S5) explained most of the variance (79.7%): Learning and Long‐Term Memory (L & LTM), including all scores on Corsi Supraspan, Prose Memory, 15-Rey, and recall Rey–Osterrieth complex figure; Attention (A), including scores on TMT and Stroop test; Executive Functioning (EF), including scores on VRT, PM38, WCST, Verbal Fluency Test, Rey–Osterrieth complex figure (copy).
The scores from the digit and Corsi spans (forward and backward) showed low loadings and were not associated with any of the three main factors, so we grouped them under the category “Short‐Term and Working Memory” (ST&WM). Since one score on the VRT remained outside the main factor EF but was from the same test, it was included in the same factor for the analyses.
The number of test scores suggestive of cognitive deficits (ESs = 0) varied across tests and domains (Table 1 and Supplemental Information S6). Specifically, the percentage of abnormal test scores ranged from 10 to 31% depending on the test in the L<M factor, from 7 to 17% for ST&WM, from 7 to 21% for A, and from 3 to 28% for EF. If we include borderline scores (ESs = 1), an increase of cognitive domains affected can be observed, namely L<M (20–38%), ST&WM (14 to 38%), A (21–38%), and EF (6–35%). Overall, 14% of the patients obtained a pathological score while 74% of them obtained a normal score. This percentage can be increased up to 86% if borderline scores were added.
Table 1.
Distribution of test scores according to the three main categories (pathological, ESs = 0; limit, ESs = 1; and normal ES > / = 2) and of mean percentage of patients who scored ESs = 0, 1, or 2–4 across all tests
| Distribution of test scores (n, %) | |||
|---|---|---|---|
| Pathological | Limit | Normal | |
| Attention (A) | |||
| Stroop — time | 6 (20.69%) | 1 (3.45%) | 22 (75.86%) |
| Stroop — error | 6 (20.69%) | 0 | 23 (79.31%) |
| TMT — A | 2 (6.9%) | 4 (13.79%) | 23 (79.31%) |
| TMT — B | 5 (17.24%) | 6 (20.69%) | 18 (62.07%) |
| Executive functions (FE) | |||
| Phonemic fluency | 1 (3.45%) | 1 (3.45%) | 27 (93.10%) |
| Semantic fluency | 1 (3.45%) | 3 (10.34%) | 25 (86.21%) |
| P/S alternate fluency | 2 (6.9%) | 4 (13.79%) | 23 (79.31%) |
| VRT | 6 (20.69%) | 2 (6.9%) | 21 (72.41%) |
| PM38 | 1 (3.45%) | 7 (24.14%) | 21 (72.41%) |
| WCST | 7 (24.14%) | 3 (10.34%) | 19 (65.52%) |
| Rey-Osterrieth Complex Figure (copy) | 8 (27.58%) | 2 (6.9%) | 19 (65.52%) |
| Learning and long-term memory (L<M) | |||
| Prose Memory — hierarchic score | 4 (13.79%) | 7 (24.14%) | 18 (62.07%) |
| Prose Memory — non-hierarchic score | 3 (10.34%) | 4 (13.79%) | 22 (75.86%) |
| 15-Rey (immediate) | 3 (10.34%) | 3 (10.34%) | 23 (79.32%) |
| 15-Rey (delayed) | 5 (17.2%) | 2 (6.9%) | 22 (75.9%) |
| Corsi supraspan | 5 (17.2%) | 2 (6.9%) | 22 (75.9%) |
| Rey-Osterrieth Complex Figure (recall) | 9 (31.1%) | 1 (3.4%) | 19 (65.5%) |
| Short-term and working memory (ST&WM) | |||
| Digit span — forward | 3 (10.3%) | 1 (3.4%) | 25 (86.3%) |
| Digit span — backward | 2 (6.9%) | 8 (27.6%) | 19 (65.5%) |
| Corsi span — forward | 0 | 11 (37.9%) | 18 (62.1%) |
| Corsi span — backward | 5 (17.2%) | 2 (6.9%) | 22 (75.9%) |
| Distribution of mean percentage (%) | 13.8 | 12.2 | 74.0 |
Patients with cognitive deficits by domains
Our results show that almost half of the tested patients (12/29) did not pathologically score (ESs = 0) in any of the NA tests. Most of the remaining patients (n = 17) did not show signs of cognitive deficits either. Specifically, an average of 22% of subjects scored ESs = 0 across tests, 14% scored ESs = 1, and 64% scored ESs > 1. When impairment occurred, multiple‐domain than single-domain impairment was more frequent (76.5% vs 23.5%) (χ2(1) = 9.53, p = 0.002). Attention deficits were the most frequent types of deficits in patients with single‐domain impairment (18.0%), significantly exceeding deficits in EF (p < 0.001) and L<M (p < 0.001). Executive function was the cognitive domain most frequently impaired in conjunction with other domains in patients with multiple‐domain impairment, especially with L<M (Supplemental Information S5).
Clinical variables and cognitive reserve
We assessed the affective states of inpatients (depression, anxiety, quality of life, and post-traumatic stress), but rarely we found pathological results. Subsequently, we performed further analyses to explore the effect of ICU hospitalization on cognition and affective states. First, correlations between individual durations of hospitalization in the ICU and the composite scores for each cognitive domain were not significant (A: r = 0.30, p = 0.15; L<M: r = 0.1, p = 0.52; EF: r = 0.1, p = 0.51), suggesting that there is no effect of this variable on the magnitude of cognitive impairment. Second, we correlated the composite scores of the cognitive domains and the number of days before the tracheal tube removal. The results did not show any significant relationship between this association. On the contrary, significant correlations were found between the sub-score “depression” of the HADS scale and the number of elapsed days since the ICU admission to the tracheal tube removal (Fig. 1, r = 0.69, p = 0.002).
Fig. 1.
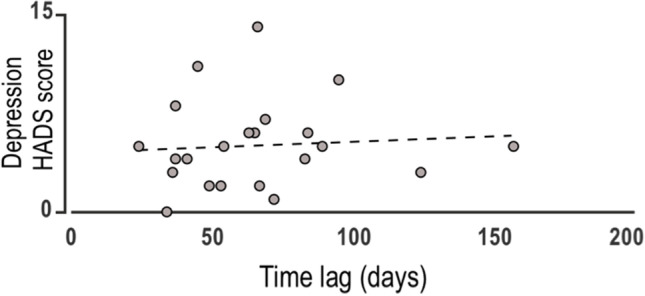
Significant correlations between the score at the Depression subscale of HADS and the number of elapsed days when the tracheal tube was removed starting from the admission to the ICU; r = 0.69, p < 0.05
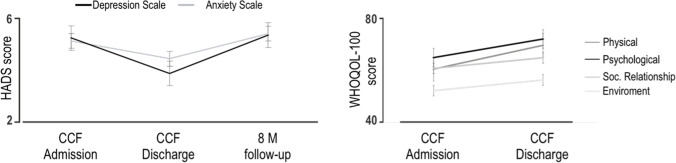
This finding suggests a positive relationship between depression levels and a variable, such as tracheal tube removal, which could be considered an illness indicator. Third, correlations between anxiety and depression sub-scores from the HADS and all domain factors were performed. There were no significant correlations between anxiety sub-scores and cognitive domains. We only observed that the whole sample showed a lower depression sub-score at the hospital discharge than the one displayed at the admission (Fig. 2, left, p = 0.036).
Fig. 2.
Scores at scales assessing the affective states. Left, HADS scores at the admission to our unit (CCF Admission), at the hospital discharge (CCF Discharge), and during the follow-up (8 M follow-up). Right, the WHOQOL-100 scale was administered at admission and discharge. Bar and line graphs show mean ± s.e.m.; p < 0.05
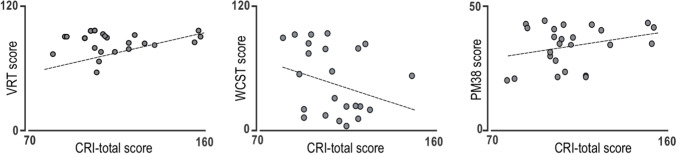
Similarly, lower scores were displayed on physical area (p = 0.01) and psychological area (p = 0.009) of the WHOQOL-100 scale at the hospital discharge compared with the one at the admission (Fig. 2, right). Finally, we further investigated the relationship between the cognitive reserve and the performance of patients on NA tests. Remarkably, we found a positive correlation between the CRIq score and EF domain (VRT, WCST, and PM38, Fig. 3).
Fig. 3.
Correlation between logical-verbal reasoning (VRT), cognitive flexibility (WCST), logical-operative reasoning (PM38), and the total score at CRIq; p < 0.05
Cognitive and affective states in the follow‐up of patients with COVID‐19
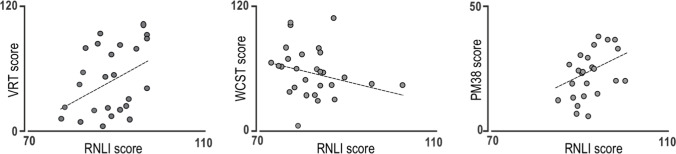
At follow-up, we assessed the global cognitive status of patients by tele-GEMS, as it can be administered by phone. We found that 3 out of 25 recruited patients scored below the cut-off. However, the relatively small sample of the present follow-up does not permit the statistical testing of the significance of this cognitive decay. Regarding the HADS scale, we found that the score increased up to the initial level for both the Anxiety and Depression scales (Fig. 2, left), even if this increase is not statistically significant. Finally, we found a significant relationship between the RNLI scores and the ones on VRT, WCST, and PM38 tests (Fig. 4).
Fig. 4.
Correlation between logical-verbal reasoning (VRT), cognitive flexibility (WCST), logical-operative reasoning (PM38), and the RNLI score; p < 0.05
CRIq and RNLI scores are reported in Supplemental Information S4.
Discussion
This study has been conceived to characterize the extent of cognitive impairment in post‐COVID‐19 severely affected patients. Cognitive impairments have been observed in hospitalized and non‐hospitalized subjects [21], mild cases [22], and asymptomatic patients [23]. To our knowledge, little evidence supports the idea that cognitive impairment is most pronounced in people severely affected, such as people who were admitted to the Intensive Care Unit (ICU) [9–11]. On the contrary, Alemanno et al. [12] demonstrated that patients who benefited from the most invasive respiratory assistance were the ones who showed the most conserved cognitive functions, even if they were also the youngest. Here, we aimed to investigate how far‐reaching the cognitive sequelae of COVID‐19 were in our sample of patients. All of them had been ICU-hospitalized and had needed a tracheostomy. To this end, we assessed their overall cognitive status, the specific cognitive functions, the affective states, and the role of the cognitive reserve (CR) in protecting them from COVID-19 effects.
We found that virtually all the patients (96%) showed normal cognitive functioning at first-level assessment. Regarding the second-level assessment, our results demonstrate that most of the patients (86%) did not show signs of cognitive deficits. This is in line with recent studies [12, 20] and against many pieces of evidence accumulated thus far. A possible explanation for the discrepancy between our results and the existing literature is that we recruited only severely affected patients, as suggested by Priftis et al. [20]. Since patients were ICU hospitalized, they most likely received prompt and adequate treatments. This has prevented them from having an increased risk of neurological and cognitive deficits. Another plausible explanation is that we did not include patients with neurological issues provoked by SARS-CoV-2. Finally, because all patients were referred for evaluation, regardless of whether they had objective cognitive necessity or subjective cognitive complaints, this overall result is not surprising; however, it does help to clarify why there are controversial conclusions about the presence of post-acute COVID-19 sequelae symptoms.
Another finding highlighted by the study is that, among the subjects reporting cognitive deficits, a larger proportion of multi‐domain (82.6%) rather than single‐domain (17.4%) impairment occurs, confirming García‐Sánchez and coworkers’ results [24]. In this respect, we observed that attentional deficits were the most frequent in patients with single‐domain impairment (75%), coherently with the literature [25, 26]. We also found that the most common domain affected overall is executive functions (EF, 70.6%), even if no one demonstrated single‐domain impairment in EF. According to the literature [24, 27], EF was always altered in combination with other cognitive domains, most likely because of its role in controlling and regulating the proper functioning of other cognitive domains and behaviors [28]. The second most affected domain overall was learning and long-term memory (64.7%) which was also the most common deficit in conjunction with either attention or EF. This is reasonable, considering that it is known that both attention and executive functions can affect the amnestic process.
In addition to the associations between cognitive domains, we examined whether other clinical factors (ICU hospitalization, tracheal tube removal) might be linked to cognitive performance and affective states. Intriguingly, we found that neither hospitalization nor tracheal tube removal significantly affected test performance. Given that much of the current literature has focused specifically on COVID patients that were hospitalized [29], our study contributes to emerging evidence [23] that ICU hospitalized patients may not suffer from post‐COVID cognitive sequelae and may have better performance compared to ones of not-hospitalized patients or mild cases [24]. Depression and anxiety were not linked to cognitive performance, as well. This discrepancy with the literature disappears if we consider the qualitative assessment: few of our patients (3/5), who complained of psychological symptoms, also reported cognitive deficits, coherently with the literature [16, 30, 31]. Surprisingly, we observed a significant correlation between the depression score (HADS scale) and the number of elapsed days from ICU admission to tracheal tube removal, meaning those who scored higher on the depression subscale at admission, their tracheal tube was removed later. Most likely, this could be explained since having a tube through the own throat may be hard to tolerate, also because of its symbolic representation of illness. However, the scores on the HADS scale were rarely pathological in our sample. As we might expect, hospitalization represented a protective factor from developing psychological sequelae after COVID-19 [16, 32]. This could also explain why the level of depression is further reduced at the discharge, and why the scores at both physical and psychological areas of the WHOQOL scale are lower, when we compare them with the scores obtained at the admission. We hypothesized that being hospitalized may have induced patients to feel safer. Conversely, being at home could have made individuals feel more vulnerable. These feelings may have been overemphasized by restraining measures, which were still on, according to Stanton et al. [33]. Finally, this could also explain why we found increased levels of depression and anxiety, although not statistically significant, at the 8-month follow-up, coherently with the literature [34].
The second set of results obtained from the follow-up revealed that having been affected by COVID-19 does not affect the overall cognitive status (88% of patients scored at or above the cut-off for tele-GEMS) and the capability to resume daily life activities. It might be argued that we tested the subjects several months later COVID-19 onset. However, it is not the case since we assessed the cognitive status at the admission obtaining similar results. Furthermore, García-Sánchez et al. [24] tested patients roughly 8 months later COVID-19 onset, and they still observed several neuropsychological disorders. Regarding abilities to resume daily life activities, we found a significant relationship between those abilities and some executive functions (logical-operative/logical-verbal reasoning, cognitive flexibility). Interestingly, those executive functions also play a role in the activation of alternative strategies and problem-solving. Hence, it is reasonable to believe that individuals with these skills are most likely to replace outdoor activities with indoor activities (home workouts, cooking, gardening, reading).
Finally, our last aim was to shed light on the protective role of CR. It has been well-established that CR is involved in moderating clinical outcomes regardless of pathology [15], demonstrating that individuals with high CR cope with brain damage better than individuals with low CR [14]. Here, we hypothesized CR could play a protective effect against cognitive and psychological deficits in post-COVID-19 survivors. Noteworthy, we found a significant association between high CR and executive function domain. Those subjects with better executive functions, namely, logical-operative/logical-verbal reasoning, and cognitive flexibility, also displayed high CR, suggesting that CR, even if indirectly, might positively affect the ability to resume normal social activities. Altogether, these results support our initial hypothesis and confirm existing data in the literature [9, 16].
We acknowledge that our study has some limitations. First, the small number of patients, but we chose the opportunity to study a uniform sample over the chance to recruit a larger number of patients, including mild cases or patients hospitalized later. Second, our study lacks a control group, such as ICU patients with a different diagnosis or less severe COVID-19 infection. Therefore, we cannot conclude whether our results can be explained by the severity of COVID-19 infection per se or by several factors associated with ICU admission. Third, we cannot monitor patients’ neuropsychological profiles over time since we used two different screening tools to assess the overall cognitive status at admission and follow-up. Thus, further investigations are needed to address these issues and long-term follow-up or multiple follow-ups to establish if these patients could have an increased risk for neurodegenerative diseases as pointed out by some researchers [10, 35].
Conclusion
We believe the present study’s findings add new knowledge to the growing research field investigating sequelae post-COVID-19. Not always severely affected COVID-19 patients report cognitive deficits or psychological symptoms, even if subjective complaints can be reported several months later the onset of the infection. For this reason, psychological support should be guaranteed to these patients when discharged, as they received it during the hospitalization.
Supplementary Information
Below is the link to the electronic supplementary material.
Data Availability
The data set generated and analyzed in the current study are available from the corrisponding author on a reasonable request.
Declarations
Ethics approval and consent to participate
All the participants gave informed consent for the study. This research has been approved by the Ethics Committee Area Vasta Emilia Nord (AVEN, protocol nr. 616/2020/OSS/AUSLPR, August 24, 2020).
Conflict of interest
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: The initial online version needs corrections. The sentence [removed for blind review] should be replaced with Centro Cardinal Ferrari (CCF) when it appears in the section Study design and participants. In the remaining cases, it should be replaced with CCF.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/16/2023
A Correction to this paper has been published: 10.1007/s10072-023-06747-3
References
- 1.World Health Organization (2022) WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai I, Manchanda R, Kumar N, Tiwari A, Kumar M. Neurological manifestations of coronavirus disease 2019: exploring past to understand present. Neurol Sci. 2021;42:773–785. doi: 10.1007/s10072-020-04964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilvers MA, McKean M, Rutman A, et al. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 2001;18:965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 5.Bhola S, Trisal J, Thakur V, Kaur P, Kulshrestha S, Bhatia SK, Kumar P. Neurological toll of COVID-19. Neurol Sci. 2022;43(4):2171–2186. doi: 10.1007/s10072-022-05875-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatoon F, Prasad K, Kumar V. Neurological manifestations of COVID-19: available evidences and a new paradigm. J Neurovirol. 2020;26(5):619–630. doi: 10.1007/s13365-020-00895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotfs K, Roberson SW, Wilson JE, et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24:1–9. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi J, Gao Y, Zhao L, et al. Prevalence of delirium, depression, anxiety, and post-traumatic stress disorder among COVID-19 patients: protocol for a living systematic review. Syst Rev. 2020;9:258. doi: 10.1186/s13643-020-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costas-Carrera A, Sánchez-Rodríguez MM, Cañizares S, Ojeda A, Martín-Villalba I, Primé-Tous M, et al. Neuropsychological functioning in post-ICU patients after severe COVID-19 infection: the role of cognitive reserve. Brain Behav Immun Health. 2022;21:100425. doi: 10.1016/j.bbih.2022.100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negrini F, Ferrario I, Mazziotti D, Berchicci M, Bonazzi M, de Sire A, et al. Neuropsychological Features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for postacute rehabilitation. Arch Phys Med Rehabil. 2021;102(1):155–158. doi: 10.1016/j.apmr.2020.09.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One. 2021;16(2):e0246590. doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristillo V, Pilotto A, Piccinelli SC, Gipponi S, Leonardi M, Bezzi M, Padovani A. Predictors of “brain fog” 1 year after COVID-19 disease. Neurol Sci. 2022;43(10):5795–5797. doi: 10.1007/s10072-022-06285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menardi A, Bertagnoni G, Sartori G, Pastore M, Mondini S. Past life experiences and neurological recovery: the role of cognitive reserve in the rehabilitation of severe post-anoxic encephalopathy and traumatic brain injury. J Int Neuropsychol Soc. 2020;26(4):394–406. doi: 10.1017/S1355617719001231. [DOI] [PubMed] [Google Scholar]
- 16.Devita M, Di Rosa E, Iannizzi P, Bianconi S, Contin SA, Tiriolo S, et al. Risk and protective factors of psychological distress in patients who recovered from COVID-19: the role of cognitive reserve. Front Psychol. 2022;13:852218. doi: 10.3389/fpsyg.2022.852218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen TM, Hov B, Halvorsen T, Røksund OD, Vollsæter M. Upper airway assessment and responses during mechanically assisted cough. Respir Care. 2021;66(7):1196–1213. doi: 10.4187/respcare.08960. [DOI] [PubMed] [Google Scholar]
- 18.Capitani E, Laiacona M. Outer and inner tolerance limits: their usefulness for the construction of norms and the standardization of neuropsychological tests. Clin Neuropsychol. 2017 doi: 10.1080/13854046.2017.1334830. [DOI] [PubMed] [Google Scholar]
- 19.Aiello EN, Depaoli EG (2022) Norms and standardizations in neuropsychology via equivalent scores: software solutions and practical guides. Neurol Sci 43(2):961–966. 10.1007/s10072-021-05374-0. [DOI] [PMC free article] [PubMed]
- 20.Priftis K, Velardo V, Vascello M, Villella S, Galeri S, Spada MS et al (2022) Limited evidence for neuropsychological dysfunction in patients initially affected by severe COVID-19. Neurol Sci 43(12):1–3. 10.1007/s10072-022-06373-5 [DOI] [PMC free article] [PubMed]
- 21.Hampshire A, Trender W, Chamberlain SR, Jolly AE, Grant JE, Patrick F, et al. Cognitive deficits in people who have recovered from COVID-19 relative to controls: an N = 84285 online study. MedRxiv. 2021 doi: 10.1101/2020.10.20.20215863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amalakanti S, Arepalli KVR, Jillella JP. Cognitive assessment in asymptomatic COVID-19 subjects. Virusdisease. 2021;32:146–149. doi: 10.1007/s13337-021-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Sánchez C, Calabria M, Grunden N, Pons C, Arroyo JA, Gómez-Anson B, et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 2022;12(3):e2508. doi: 10.1002/brb3.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeria M, Cejudo JC, Sotoca J, Deus J, Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miskowiak K, Johnsen S, Sattler S, Nielsen S, Kunalan K, Rungby J, et al. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuster JM. Executive frontal functions Exp brain res. 2000;133(1):66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- 29.Alnefeesi Y, Siegel A, Lui LMW, Teopiz KM, Ho RCM, Lee Y, et al. Impact of SARS-CoV-2 infection on cognitive function: a systematic review. Front Psychiatry. 2021;11:621773. doi: 10.3389/fpsyt.2020.621773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santangelo G, Baldassarre I, Barbaro A, Cavallo ND, Cropano M, Maggi G, et al. Subjective cognitive failures and their psychological correlates in a large Italian sample during quarantine/self-isolation for COVID-19. Neurol Sci. 2021;42:2625–2635. doi: 10.1007/s10072-021-05268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill NL, Mogle J, Wion R, Munoz E, DePasquale N, Yevchak AM, et al. Subjective cognitive impairment and affective symptoms: a systematic review. Gerontologist. 2016;56:e109–e127. doi: 10.1093/geront/gnw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton R, To QG, Khalesi S, Williams SL, Alley SJ, Thwaite TL, et al. Depression, anxiety and stress during COVID-19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environ Res Public Health. 2020;17(11):4065. doi: 10.3390/ijerph17114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanderlind WM, Rabinovitz BB, Miao IY, Oberlin LE, Bueno-Castellano C, Fridman, , et al. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr Opin Psychiatry. 2021;34(4):420–433. doi: 10.1097/YCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frontera JA, Boutajangout A, Masurkar AV, Betensky RA, Ge Y, Vedvyas A, et al. Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or Alzheimer’s dementia. Alzheimer’s Dementia: J Alzheimer's Assoc. 2022;18:899–910. doi: 10.1002/alz.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set generated and analyzed in the current study are available from the corrisponding author on a reasonable request.