Abstract
Recently, demand for fermented foods has increased due to their improved nutritional value, taste, and health-promoting properties. Worldwide consumption of these products is increasing. Fermented foods are generally safe for human consumption. However, some toxins, primarily biogenic amines (putrescine, phenylethylamine, histamine, tyramine, and cadaverine), mycotoxins (fumonisins, aflatoxins, ochratoxin A, zearalenone, and trichothecenes), and bacterial toxins (endotoxins, enterotoxins, and emetic toxins) can be produced as a result of using an inappropriate starter culture, processing conditions, and improper storage. These toxins can cause a multitude of foodborne illnesses and can lead to cardiovascular aberration and adverse gastrointestinal symptoms. Analytical techniques are in use for the detection of toxins in fermented foods for monitoring and control purposes. These include culture, chromatographic, immunoassays, and nano sensor-based techniques. These detection techniques can be used during the production process and along the food chain. On an industrial scale, HPLC is widely used for sensitive quantification of toxins in fermented foods. Recently, biosensor and nano sensor-based techniques have gained popularity due to accuracy, time efficiency, and simultaneous detection of multiple toxins. Other strategic methods being investigated for the removal of toxins from fermented foods include the use of specific starter cultures for bio-preservation, aflatoxin-binding, and biogenic amine-degradation agents that may help to appropriately manage the food safety concerns associated with fermented foods.
Keywords: Fermented food, HPLC, Nanosenors, PCR, Toxin
1. Introduction of fermented foods
Food fermentation is a food processing technology that uses the growth and metabolic activity of beneficial microorganisms naturally present or added to raw food materials for stabilization and transformation. Secondary metabolites formed as a result of fermentation contribute to the organoleptic properties of flavor and texture, functional properties, and the nutritive value of foods [1]. Based on the substrate used, the fermentation process is classified into the nine major groups of fermented cereals, fermented vegetables, fermented legumes, fermented roots/tubers, fermented milk products, fermented and preserved meat products, fermented, dried, and smoked fish products, miscellaneous fermented products, and alcoholic beverages [2]. Fermenting microorganisms can be incorporated into foods in two ways. First, wild or spontaneous fermentation is a natural phenomenon where naturally occurring microorganisms in food or present under processing conditions conduct the fermentation [3]. Examples include fermented soy foods, sauerkraut, and kimchi. A culture-dependent fermentation or “controlled fermentation” is a second process in which a starter culture is inoculated into a food product. Kefir and kombucha are examples of food products produced through starter culture inoculation [3].
Fermented foods provide beneficial effects to humans by two main mechanisms; modulation of gut microbiota, and/or formation of different bioactive compounds, such as exopolysaccharides, oligosaccharides, peptides, GABA-gamma aminobutyric acid, conjugated linoleic acids, and vitamins [3]. Fermentation of some foods also yields angiotensin-converting enzyme (ACE) inhibitory peptides which have an anti-hypertensive effect. Kefir, a yogurt-based drink, has been reported to improve protein digestibility [4]. Soy-based fermented foods including soy paste and soy sauce have shown anti-inflammatory effects both in vitro and in vivo [5].
The lactic acid bacteria (LAB) Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, and Weissella are the most common fermenting bacteria. Other bacterial consortia which can be isolated from fermented foods or are detected during fermentation include Bacillus in legume-based products, Bifidobacterium, Brachybacterium, Brevibacterium, and Propionibacterium in cheese, and Arthrobacter and Hafnia in meat products [1]. Several yeast taxa have also been isolated from fermented foods, including Candida, Debaryomyces, Geotrichum, Hansenula, Kluyveromyces, Pichia, Rhodotorula, Saccharomyces, Saccharomycopsis, Schizosaccharomyces, Torulopsis, Wickerhamomyces, and Zygosaccharomyces [1].
Owing to the benefits of fermented foods, there has been a remarkable increase in the worldwide consumption of fermented foods. A wide variety of indigenous fermented foods are consumed around the world. According to an estimate, there are approximately 5000 varieties of fermented foods and beverages globally [1]. The global market share of fermented foods was valued at approximately 149.5 billion US dollars in 2016 [6]. This value was based on industrial production only. Daily indigenous household preparation of fermented foods was excluded. Also, the availability of ready-to-eat fermented foods and beverages in the market enhances consumption due to accessibility for consumers. An increase in demand for fermented foods is expected at a compound annual growth rate of 4.3% during 2019–2024 and is expected to reach 205.5 billion US dollars by 2023 [6,7]. Of the different fermented foods available, fermented dairy products have the greatest demand worldwide. Among these, Kefir and drinking yogurts experienced significantly greater sales in recent years [7]. Out of the 172.2 million tons of milk produced by the European Union in 2018, 64.9 million tons (37.7%) were used for fermented cheese products, and 7.4 million tons (4.3%) were used for fermentation of other acidified milk products [3]. Although the dairy fermentation industry has had the greatest market value in the recent years, there are regional differences in the types of fermented foods consumed. Cereal-based fermented foods are widely consumed in the United States and Europe, while fermented dairy foods, fermented soybean, and fermented fish products are more commonly consumed in Asia [7,8].
Fermented foods consumption has recently increased due to their health benefits. However, fermented foods may pose a potential food safety risk due to various microbial toxins, including biogenic amines, mycotoxins, and bacterial toxins. These toxins may be produced during the fermentation process or may be added during the food processing stages.
2. Toxins in fermented foods-emerging challenges & public health concerns
Fermented foods are generally safe for human consumption but are not entirely devoid of potential health risks associated with toxins. Multiple factors account for the presence of toxins in fermented foods, including poor hygiene, an unfit starter culture, unsafe storage conditions, and the use of contaminated raw materials. Table 1 presents an overview of toxins that can be present in fermented foods. These toxins can be classified into three categories: biogenic amines, mycotoxins, and bacterial toxins [3,9–11].
Table 1.
Toxins in different fermented food products.
| Fermented food products | Toxins | Microbes | Harmful effects | Reference |
|---|---|---|---|---|
| Alcoholic beverages (South Africa-based) | Aflatoxinsa, Zearalenonea, Ochratoxina, Penicillic acida, Shiga toxinb | Aspergillus spp., Penicillium spp., Rhizopus spp., E-coli (STEC) | Neurotoxicity, nephrotoxicity, hemolytic uremic syndrome | [11,28] |
| Meju (fermented soybeans) | Aflatoxin B1a, Ochratoxin Aa | Aspergillus flavus, Aspergillus parasiticus | Cytotoxicity, carcinogenic, immunosuppression | [37] |
| Iru and ogiri (Nigerian fermented foods) | Aflatoxinsa, Endotoxinsb | Aspergillus spp., Penicillium spp., Rhizopus spp., Enterobacteriacae | Neurotoxicity, carcinogenic, immunosuppression, vomiting | [28] |
| Fish sausage (fermented) | Cadaverinec, putrescinec, histaminec, tyraminec | Enterobacteriacae | Acute and chronic toxicities, such as, vomiting | [72] |
| Sausages | Shiga toxinb | E. coli | Hemolytic uremic syndrome | [73] |
| Fermented sausages (dry) | Verotoxigenic (Shiga toxin)b | E. coli | Hemolytic uremic syndrome | [74] |
| Fermented milk products | Aflatoxins M1a | Aspergillus flavus, Aspergillus parasiticus | Neurotoxicity, nephrotoxicity, carcinogenic | [75] |
| Maize meal (fermented) | Fumonisin B1a, Aflatoxin B1a, Zearalenonea, Ochratoxin Aa | Fusarium graminearum | Neurotoxicity, carcinogenic, immunosuppression | [28] |
| Fermented pasta | Deoxynivalenola and T-2 toxina | Fusarium graminearum, F. asiaticum, F. meridionale, F. booth | Cytotoxicity, carcinogenic, immunosuppression, neurotoxicity, nephrotoxicity | [76] |
| Fermented soyabean (Doenjang) | Enterotoxinb | Bacillus cereus | Vomiting, diarrhea | [28] |
| Fermented soya sauce products | Deoxynivalenola, Enterotoxinb |
Fusarium spp. Bacillus cereus |
Immunosuppression, neurotoxicity, nephrotoxicity | [32] |
| Kimchi | Toxic trace elements (As, Cd, In, Pb, and Tl) | – | Neurotoxicity, neurodegenerative diseases | [77] |
| Pickled vegetables | Cadaverinec, tyraminec, Nitrosamines | Firmicutes, Leuconostoc spp. | Cardiac palpitations, headache, flushes, nausea | [78] |
| Milk tofu | Toxic elements (V, Ba, Sr, Pb, Cd), | – | Neurotoxicity, neurodegenerative diseases | [79] |
| Fumigated cheese | Citrinina, cyclopiazonic acida, roquefortine Ca | Aspergillus flavus, Penicillium roqueforti, Fusarium oxysporum | Neurotoxicity, nephrotoxicity, carcinogenic, immunosuppression, cytotoxicity | [80] |
| Ogi baba | Aflatoxins B1a | Aspergillus flavus | Carcinogenic, neurotoxicity | [28] |
| Ugba (fermented | Aflatoxins B1a, | Aspergillus spp., | Neurotoxicity, carcinogenic, | [28] |
| African oil bean seeds) | Fumonisin B1a, Sterigmatocystina | Fusarium spp. | immunosuppression, cytotoxicity | |
| Soumbala | Enterotoxinsb | Bacillus cereus | Vomiting, diarrhea | [28] |
| Bikalga | Enterotoxinsb | Bacillus cereus | Vomiting, diarrhea | [28] |
| Kenkey (fermented maize) | Aflatoxinsb | Aspergillus spp. | Neurotoxicity, carcinogenic | [81] |
| Yogurt | Aflatoxinsb | Aspergillus spp. | Neurotoxicity, carcinogenic | [75] |
Classification of mycotoxins.
Classification of bacterial toxins.
classification of endotoxins.
2.1. Biogenic amines
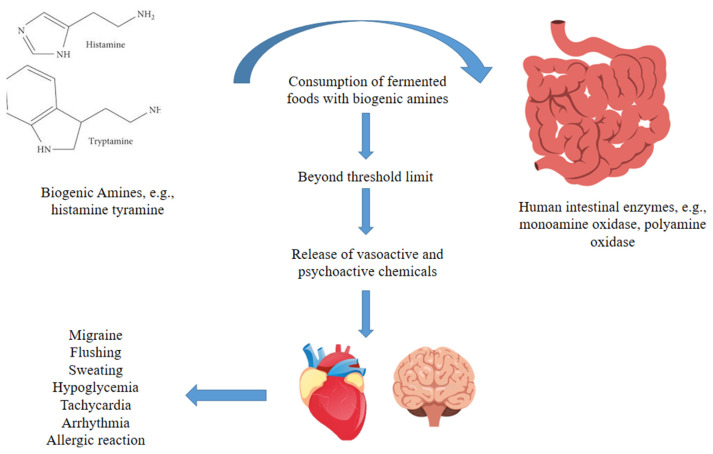
In fermented foods, LAB used in the fermentation process are the main reasons for the production of biogenic amines. LAB produce amino-acid decarboxylase enzymes which convert amino acids to biogenic amines. The presence of biogenic amines in foods can cause intoxication symptoms such as heart palpitations, redness, headaches, vertigo, and altered blood pressure and can be fatal at high concentrations [12] (Fig. 1).
Fig. 1.
The harmful health effects of biogenic amines from fermented foods and the detoxification process by the intestinal enzymes in the body.
Biogenic amines are most commonly reported in wine, beer, fermented soybeans, meat, dairy, and vegetable products. Soy-based foods, such as soy sauce, miso, natto, stinky tofu, and tempeh, have been reported to cause biogenic amine toxicity (Table 1). The dietary groups with the maximum average values for the total concentration of biogenic amines include fish sauce with an average total biogenic amine concentration of 582–588 mg/kg followed by fermented vegetables (375–390 mg/kg), cheese (177–334 mg/kg), and fermented sausages (281–283 mg/kg) [13]. The primary biogenic amines produced during the fermentation of these products are putrescine, phenylethylamine, histamine, tyramine, and cadaverine. Histamine and tyramine are the most common and important concerning food safety [11]. Biogenic amines are thermostable, non-volatile compounds with a low molecular weight that resist heat treatments applied during food processing.
Histamine, produced from histidine, is a notorious causative agent for food poisoning outbreaks. Cheese (mainly gouda, Swiss, cheddar, gruyere, and Cheshire) and wine are among the reported outbreak sources of histamine poisoning. In these outbreaks, the histamine content of cheeses ranged between 850 and 1870 mg/kg [14]. In wine, a histamine concentration of 2 mg/l was the permissible limit for import and export purposes [15]. In Taiwan, histamine content was reported to be greater than 50 mg/kg in kimchi [16]. The Commission Regulation (EC) 2073/2005 has established the permissible limits for histamine only for fish and fish products in terms of food safety. These permissible limits range between 100 mg/kg and 200 mg/kg. For fish products that are subjected to enzymatic treatment in brine solution have a bit higher permissible histamine between 200 mg/kg and 400 mg/kg [13].
Histamine poisoning can have adverse health effects [17]. Symptoms of histamine poisoning include abdominal pains, arrhythmia, extrasystoles, urticaria, redness, headache, nasal discharge, respiratory failure, asthma, hypotension, hemoconcentration and eyelid swelling [14]. Microbial strains responsible for histamine production in fermented foods are Oenococcus oeni, Pediococcus parvulus, Pediococcus pentosaceous, Tetragenococcus species, Leuconostoc species, Ligilactobacillus saerimneri 30a, Lentilactobacillus hilgardii, Lentilactobacillus buchneri and Lactobacillus curvatus [18].
Streptococcus thermophilus is an important thermophilic LAB used as a starter culture in cheese making. In order to make cheese from pasteurized milk, it is combined with thermophilic Lactobacilli. It is also commonly used in many natural whey cultures for making classic raw milk cheeses. Research has shown that S. thermophilus contains histidine decarboxylase (hdcA) gene, which has novel properties and may considerably increase the histamine accumulation in cheese [19].
Tyramine is another potential biogenic amine that can be found in cheese, fermented vegetables, alcoholic beverages, and fermented sausages at toxicologically relevant levels. Tyramine indirectly increases the blood noradrenaline concentration and acts as a vasoconstrictor leading to hypertension, migraine, brain hemorrhage, and heart failure [17,20]. Concentrations higher than 100 mg/kg of food weight are considered harmful to humans [21].
A study was conducted for the quantitative determination of various biogenic amines in twenty-two different food products from the Belgian market. The most significant biogenic amines present in blue cheeses were tyramine and cadaverine, with tyramine contents up to 1306 mg/kg [22].
In another study conducted in Switzerland, tyramine was detected in 46 fermented sausage samples out of 62 samples with a maximum concentration of 785.2 mg/kg [14]. Similarly, tyramine accumulation has been reported in the Thai fermented shrimp Kung-som because fermented shrimp is rich in tyrosine (10.78 mg/g dry mass), which is a precursor for tyramine formation [23]. Korean fermented foods, such as doenjang (fermented soybean paste), have also been reported for tyramine toxicity with concentrations up to 1430.7 mg/kg [24]. The Gram-positive bacteria Enterococcus (E. faecalis and E. faecium) and Lactobacillus species (Latilactobacillus curvatus and Levilactobacillus brevis) are the major groups responsible for tyramine formation and accumulation in fermented foods [12]. Carnobacterium, Leuconostoc, Staphylococcus, and Lactococcus can also contribute to tyramine toxicity in fermented foods. L. brevis, L. hilgardii, L. plantarum, and Leuconostoc species can cause tyramine production in fermented beverages [25].
2.2. Mycotoxins
Mycotoxins are also a potential food safety concern in fermented foods [26]. Mycotoxins are secondary toxic metabolites produced by fungi that primarily contaminate agricultural products, such as cereals, grains, wheat, maize, rye, oats, and legumes including peanuts, beans, peas, lentils, and soybean. Therefore, cereal-based fermented foods are vulnerable to mycotoxin contamination [9]. Mycotoxins are thermostable molecules, which increase the difficulty of elimination once produced in or after entry into any food.
Beer is vulnerable to contamination by mycotoxins during all stages of brewing [27]. Aflatoxins, fusarium toxins, and zearalenone may enter the malt from cereal grains due to thermostability, or directly contaminate produced beer due to water solubility [27]. A study conducted in Nigeria showed that 82% of fermented food samples (maize gruel Ogi, sorghum gruel Ogi-baba, melon seed Ogiri, locust bean Iru) contained mycotoxins, mainly aflatoxin B1, fumonisin B1, and sterigmatocystin [28]. Aspergillus, Fusarium, Penicillium alternaria, and Cladosporium were prominent contaminant microorganisms. These fungal taxa produce several toxins, including fumonisins, aflatoxins, ochratoxin A, zearalenone, and trichothecenes, which can be nephrotoxic, carcinogenic, and immunotoxic [28]. The blue mold cheese fermenting fungi Penicillium roqueforti produces mycotoxins, cyclopiazonic acid, and rugulovasine A and B [29]. Aspergillus flavus and species of Fusarium are responsible for aflatoxin B1 and trichothecene T-2 toxins in the fish sauce during fermentation [30]. Deoxynivalenol, 3-acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol are harmful for both human and animals and present in grains and wheat products. Based on research on animals and clinical findings involving humans, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) decided that the provisional highest tolerable intake of 1 g/kg body weight/day for the total amount of deoxynivalenol and its acetyl derivatives [31]. However, China has set a limit of 1000 μg/kg for deoxynivalenol in cereal-based products, both 3- acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol legal upper limits have not yet been established [32].
Different mycotoxins have specific detrimental effects on the health of an individual. The major mycotoxins include aflatoxins, ochratoxin A, patulin and zearalenone. Even small amounts of mycotoxins in foods can pose a considerable health risk. Aflatoxins have carcinogenic potentials and mainly affect the liver, causing hepatotoxicity. Ochratoxin A is nephrotoxic and accelerates the formation of free radicals, which are harmful to the human body. Patulin toxicity can lead to adverse gastrointestinal conditions and neurotoxicity. Zearalenone has carcinogenic and teratogenic potentials [33]. Regulatory authorities in many countries have defined the permissible limits of mycotoxins in different foodstuffs. For example, the Commission of the European Communities (EC regulation No. 1881/2006) has set an upper limit for mycotoxins in some fermented foods at 50 μg/kg of patulin in apple cider and other apple-based fermented drinks, 0.050 μg/kg of aflatoxin M1 in raw milk and milk-based products, and 2.0 μg/kg of ochratoxin A in wine [27]. The Codex Committee on Food Additives and Contaminants has specified 0.5 mg/kg for aflatoxin M1 in milk, whereas the US Food and Drug Administration (FDA) has defined 20 ppb as the permissible limit for aflatoxin M1 in milk [34].
2.3. Bacterial toxins
Another potential food safety risk in fermented foods is the presence of bacterial toxins. Some bacteria and their toxins become incorporated into fermented products via substrate contamination. Studies have specifically highlighted the presence of bacterial toxins in both meat and vegetable-based fermented foods [28]. A review of studies showed that several vegetable-based fermented foods, such as cucumbers, mustard greens, young melons, cabbage, Chinese cabbage, papayas, and bamboo shoots are at high risk of microbial contamination due to indirect contamination via a raw substrate [9]. Nem Chua (fermented pork sausage) consumed in Vietnam has a high risk of causing food intoxication due to processing without exposure to heat or any cooking. Thus, Nem Chua is vulnerable to contamination and growth of toxin-producing bacteria, such as Staphylococcus aureus [9]. New Zealand mussel (Perna canaliculus) is a traditional fermented food in New Zealand that is commonly contaminated with Clostridium botulinum. Clostridium spores survive during fermentation due to tolerance of anaerobic conditions and germination and growth of vegetative cells, leading to the production of heat-resistant toxins that cause food intoxication. Traditionally consumed fermented foods in the Northeast regions of India have also been investigated for potential foodborne toxin contamination. A significant microbial load of the toxin-producing bacteria Bacillus cereus, P. mirabilis, and C. botulinum was found in soybean, fish, and pork-based fermented foods [35].
Cell membranes of Gram-negative bacteria contain lipopolysaccharide complexes, known as endotoxins that are heat stable and, thus, can survive processing conditions and pose a significant risk of food-borne illnesses. The presence of bacterial endotoxins has been reported in fermented ogiri, ugba, iru, ogi, and ogi baba in Africa [28]. Among the fermented foods tested, iru, a condiment derived from alkaline fermentation of locust beans, had the highest endotoxin concentration at 5.5 × 104 endotoxin units/g. Sphingomonas, paucimobilis, and Escherichia coli were the dominant Gram-negative taxa responsible for endotoxin production. A significant correlation between microbial load and toxin concentration was also revealed [28].
Sorghum is an important staple crop in Africa made edible through fermentation. Several studies have reported the occurrence of opportunistic bacteria and their toxins in sorghum-based fermented foods [26]. B. cereus, Clostridium perfringens, E. coli, Listeria monocytogenes, and Enterococci contaminate fermented weaning cereals. Gowé, a traditional cereal-based fermented beverage in Benin, has food safety concerns due to contamination with E. coli and Enterobacteriaceae [10].
Bacillus species are predominant in oil-bean seeds. These taxa produce less worrisome, heat-labile enterotoxins in ugba and more hazardous, emetic, heat-stable toxins in baobab seeds [36]. B. cereus sensu lato is a foodborne pathogen in some Korean fermented soybean products, such as doenjang, gochujang, ssamjang, and chokochujang [37]. Shiga toxin-producing E. coli (STEC) is another causative agent of foodborne illnesses worldwide. In a study conducted in Nigeria, STEC was detected in 2% of raw milk samples, 6% of fresh local cheese, and 9.6% of fresh local cheese. All these bacterial toxins can lead to food poisoning, have deleterious effects on health, and cause adverse gastrointestinal symptoms [38].
3. Analytical techniques
3.1. Techniques for biogenic amines detection
Detection of biogenic amines in fermented foods is crucial. Early detection of biogenic amines in foods allows timely preventive measures to be implemented for avoiding food intoxication. In addition to toxin detection, it is imperative to detect bacteria containing decarboxylase enzymes to facilitate a preliminary risk estimation of biogenic amine formation in fermented foods and to take measures to counter accumulation.
Traditionally, culture-dependent techniques have been used to detect the presence of biogenic amineforming bacteria in fermented foods (Table 2). These techniques are based on pH changes in differential growth media. Another culture-based method relies on detecting carbon dioxide produced by the enzymatic action of bacterial decarboxylases [17]. Other culture-based techniques involve growing LAB on a culture media followed by DNA extraction and sequencing to evaluate the genetic potential of these bacteria for the production of biogenic amines [39]. Culture-based processes are labor-intensive, time-consuming, and sometimes yield false-positive due to the development of alkali-based compounds or false-negative results due to the acid produced during fermentation along with biogenic amines, causing interference with the accuracy of toxin detection [40].
Table 2.
Analytical techniques with merits and demerits used for detection in fermented foods.
| Analytical Technique | Fermented Food | Toxin/Bacteria Detected | Merits | Demerits | Reference |
|---|---|---|---|---|---|
| Culture-dependent method | Stinky Tofu, Chinese traditional fermented food (grasshopper sub shrimp paste), Japanese sake, Chinese Luzhou-flavor liquor, Grape wine, Vietnamese alcoholic beverage | Biogenic amines | Investigation of microbial diversity at the species level | Laborious, Time-consuming, Culture media may not be suitable for every bacterium, False-positive results | [17,40] |
| HPLC | Cheese, Wine, Cider, Fermented fish sauce, Thai fermented pork | Biogenic amines | Reliable, sensitive quantification of toxins, commercially available | Tedious, Time-consuming | [25,48] |
| Red, rose, and white wine, Beer, Cheese, Red yeast rice, Cereal-based ferments, Maize gruel (ogi), Sorghum gruel (ogi-baba), Melon seed (ogiri), Locust bean (iru), African oil bean seed (ugba) | Mycotoxins | [33,34,53,54,60] | |||
| Fermented seed condiments | Bacillus sp. | [82] | |||
| TLC | Wine, Cider | Biogenic amines | Effective separation of toxins | Time-consuming | [25,83,84] |
| Mycotoxins | [53] | ||||
| PCR | Wine, Chinese traditional fermented food grasshopper sub shrimp paste | Biogenic amine-producing bacteria | Convenient Sensitive High differentiation |
Less sensitive compared to biosensors False positive results |
[40,85] |
| Fermented locust bean, Fermented melon | Mycotoxins | [41,60] | |||
| Alkaline fermented seed condiments (tayohounta, soumbala, Ogiri, Ugba), Doenjang, a Korean fermented soybean paste, Fermented fish (Pla Som and Pla Ra), Indian fermented pork and fish, Iru, ogi and ogi baba | Bacillus sp., Enterobacter sp., Endotoxins | [28,82] | |||
| Fermented beverages (mahewu and umqombothi) | |||||
| Biosensors/Nano sensors | Wine, Yogurt, Cheese, Roquefort, Monascus fermented food, soybean | Biogenic amines, Mycotoxins, Enterotoxins, Diarrheal toxins (Nhe and Hb1), P. mirabilis, B. cereus | Rapid, Latest technique, Highly sensitive and allows simultaneous detection of toxins | Not common at industrial scale | [33,35,36,46,53,60,64] |
Compared to conventional culture-dependent methods, modern molecular methods (Table 2) for detecting bacteria containing decarboxylases are more rapid and reliable [40]. These methods enhance the early detection of biogenic amine-producing bacteria. Molecular techniques include DNA hybridization and polymerase chain reaction (PCR) [41]. DNA hybridization involves aligning nucleotide sequences to identify similar lines. Primers are then designed using these similar sequences to amplify a DNA fragment that is then used as a probe in a whole-cell dot-blot hybridization assay. However, this method also yields false results [12].
PCR is another molecular method that is faster and more sensitive than other methods. PCR facilitates specific detection of amino acid decarboxylase genes, which are amplified from biogenic amine-producing bacterial strains. Thus, PCR enables prior risk assessment of biogenic amine formation by designing oligonucleotide primers comparable to the nucleotide sequences in decarboxylase genes [12]. This method has been used to detect histidine decarboxylases (pyruvic-dependent and pyridoxal phosphate-dependent) and tyrosine decarboxylases. These molecular techniques have been modified to fit conditions for fermented food products, including cheese and sausages [41].
Recent advancements have enabled simultaneous detection of histamine, tyramine, and putrescine-producing LAB using multiplex PCR (mPCR) [17]. Successful implementation of real-time PCR (q-PCR) for quantitative estimation of histamine-producing LAB [42] and tyramine-producing strains [43] in cheese has also been documented. Putrescine is another common biogenic amine found in dairy products. It is synthesized when LAB deaminates agmatine, which is made from arginine in milk. A culture-independent multiplex qPCR technique based on the specific amplification of the particular domain of the agmatine deaminase gene cluster (AGDIc) has been developed for the detection, quantification, and identification of LAB capable of producing putrescine from agmatine [44].
The cider samples were examined using qPCR methods designed for the quantification of biogenic amine producers from cheese and wine. A strong correlation was found between the amount of biogenic amine and the presence of microorganisms that produce biogenic amine [45]. However, PCR may also yield false-positive results if the selected genes have been taken from the database in which the function is assigned by homology but not by function. False-positive results may also occur if any biogenic amine-producing microorganism has not yet identified and described. Other detection methods include enzymatic assays that are exclusively histamine-specific. The most commonly used assay uses the formation of hydrogen peroxide in response to the enzymatic activity of oxidase on histamine. However, this method also yields false positives because other cultures also produce hydrogen peroxide exclusive to histamine. Another form of the enzymatic assay using chromogen is leucocrystal violet. This method effectively screens out histamine-producing bacterial strains but does not provide the concentration of histamine produced because of culture media interference. This drawback has been rectified by filtering the culture medium and replacing leucocrystal violet with chromogen 3,3-Diaminobenzidine, which paved the way for quantifying histamine produced by bacterial strains [17].
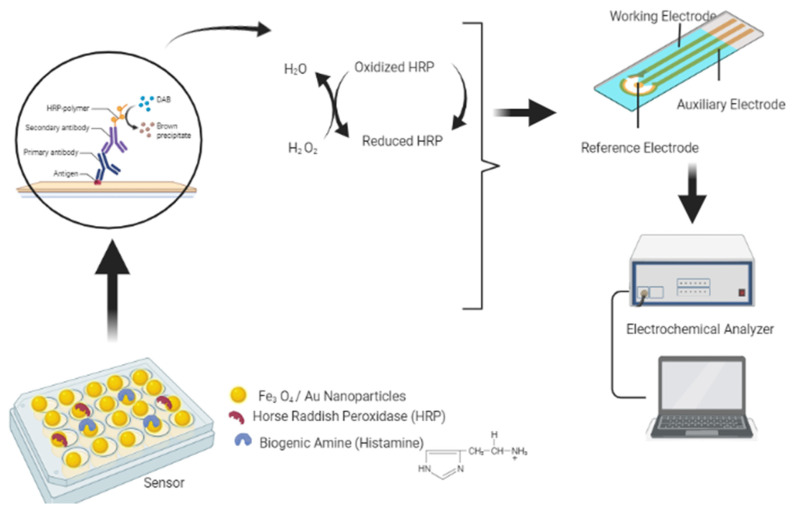
Novel methods of detecting biogenic amines in fermented foods use biosensors and nano-sensors [46] (Fig. 2). Enzyme sensors are also used to detect biogenic amines in fermented foods. Sensors have been used to detect the biogenic amines histamine, tyramine, and putrescine in food products [46]. Recently, enzyme-based sensors have been investigated for sensitivity to the detection of biogenic amines in foods. These enzyme-based biosensors work by detecting signals generated due to changes in physicochemical properties after the interaction of specific amine oxidases with biogenic amines. Changes in pH, heat generation, and gas production are detected by the biosensors. Another type of biosensor is based on the detection of light emitted due to interaction between the enzyme and an analyte. The major biosensors for biogenic amine detection are enzyme-based electrochemical biosensors. These enzyme sensors act swiftly and can detect biogenic amines in food within 20 min [46]. A novel fluorescence sensing method for tyramine detection has also been developed that integrates molecular imprinting and the Quantum Dot-Graphene technique for tyramine detection. This method has been used for cost-effective and rapid tyramine detection in rice wine with a high sensitivity for tyramine [47].
Fig. 2.
Mechanism of electrochemical detection of biogenic enzymes using nanosensors: the shift in the redox potential of the HRP enzyme after reaction with biogenic amines is detected by an electrode attached to the electrochemical analyzer, which displays the electrical signals indicating the presence of biogenic amines in fermented foods.
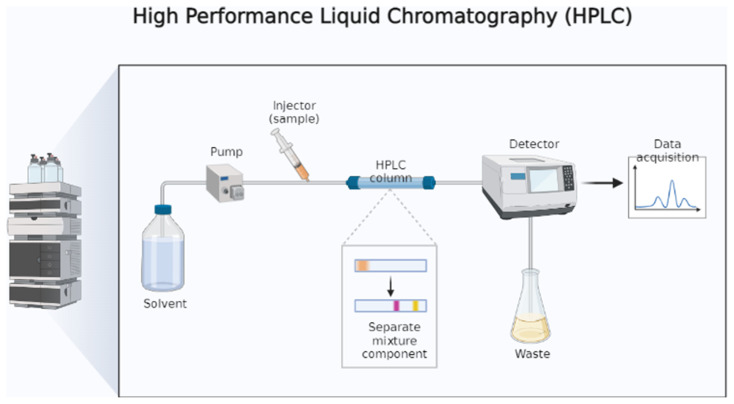
Chromatographic techniques are also used to detect biogenic amines. Thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) are the most commonly used [17]. TLC works by separating biogenic amines from samples in the form of spots on layers of silica gel/cellulose attached to glass plates or aluminum sheets. The highly polar nature of amines can alter the chromatographic resolution by forming streaks. The amines are, therefore, first converted into derivatives using dansyl chloride. The relatively stable sulphonamide derivates improve fluorescence and provide satisfactory chromatographic results [17]. The biogenic contents of the Japanese soy-based fermented food natto and the Italian blue cheese gorgonzola were successfully detected using HPLC with fluorescence detection [48].
The most popular chromatographic technique for detecting biogenic amines in food is HPLC (Fig. 3). HPLC was used to measure the variety and amount of biogenic amines in fermented milk from cows and goats [49]. The ultra-high performance liquid chromatography (UHPLC) technique had also been used to determine biogenic amines in a variety of cheese samples. Particularly, high-resolution mass spectrometry and UHPLC using columns packed with sub-2 m tiny particles have provided significant opportunities to improve accuracy, sensitivity, and quickness of biogenic amine measurement in food samples [50]. This method uses pre- and post-column derivatization and then uses fluorescence or UV (ultraviolet) radiation for detection. Different derivatization reagents are used for the pre and post-columns, including dansyl chloride, benzoyl chloride, fluoresceine, 9- fluorenylmethyl chloroformate, ophthaladehyde, naphthalene-2,3-carboxaldehyde, n-acetylcysteine, and 2- mercaptoethanol [17]. Reversed-phase-HPLC, multichannel UV detection, post-column derivatization, and fluorescence detection have been used to identify biogenic amines in fermented sausages and meat [17]. Douchi, sufu, fermented sausage, yulu, and shrimp paste are few examples of traditional Chinese fermented dishes. The biogenic amine content of these food had also been determined using reversed-phase HPLC-DAD using an Inertsil ODS-SP column after pre-column derivatization with dansyl chloride [51]. HPLC is the official reference method for the detection of histamine (Regulation EC No 2073/2005, microbiological criteria for foodstuffs) [17]. There is potential with available analytical methods for biogenic amine detection in fermented foods. Each method has merits and demerits. However, the latest molecular techniques using nanosensors have shown better reliability and less time [46].
Fig. 3.
Mechanism of high-performance liquid chromatography (HPLC).
3.2. Techniques for mycotoxins detection
Different methods of mycotoxin detection have been investigated for efficacy and accuracy (Table 2). These include the chromatographic techniques of gas chromatography (GC), HPLC, TLC, and immunological assays coupled with immunosensors [52].
One method for mycotoxin detection in fermented foods is HPLC. It is often the standard method for mycotoxin detection at the industrial level, and numerous protocols are in place for the detection of mycotoxins, particularly for the estimation of ochratoxin A amounts [53]. HPLC is often coupled with UV and fluorescence detectors (FD) for the detection of mycotoxins, particularly aflatoxins. Also, the use of 2.6 μm core–shell particles in the chromatography column has improved the analytical performance of HPLC. The use of HPLC coupled with tandem mass spectrometry (LC-MS/MS) has been used for a more sensitive and specific screening of mycotoxins in food samples [54]. Utilizing liquid chromatography-tandem mass spectrometry, twenty-three mycotoxins, including aflatoxin B1 (AFB1), fumonisin B1 (FB1), and sterigmatocystin (STE) have been estimated in fermented food samples from Southwest Nigeria, including maize gruel (ogi), sorghum gruel (ogibaba), melon seed (ogiri), locust bean (iru) [55].
UHPLC (ultrahigh-performance liquid chromatography) is another technique for mycotoxin detection. It is a solid-phase extraction procedure used for the separation of particles. Mycotoxins are then detected using multiple reaction modeling (MRM) in positive electrospray ionization mode. Multiple mycotoxins can be detected using this method, including aflatoxins B1, B2, G1, G2, ochratoxin A, fumonisins B1, and B2, zearalenone, deoxynivalenol, T-2 toxin, and HT-2 toxin. This method has been used in the brewing industry for mycotoxin analysis of wine and beer [54]. Fusarium mycotoxins in beers and spices have also been quantified using this technique in Nigeria [56]. TLC is also a common technique for detecting mycotoxins, especially aflatoxins. Modifications in
TLC have improved its efficacy, compared to HPLC with 5000 theoretical plates for a 5 cm migration in high-performance TLC (HPTLC) compared with 5000 for a 20 cm long HPLC column. The method uses formation of plates with an adsorption material, such as silica gel, followed by a volatile solvent for spotting purposes. Mycotoxins are then quantified using fluorescence, color, or UV adsorption. The TLC method can be efficiently used for screening purposes by ruling out negative samples. Multi-toxin detection is also possible by applying extracts to different TLC plates and grouping the mycotoxins according to chromatographic properties. This method can be applied in the detection of aflatoxins, ochratoxin A, citrinin, sterigmatocystin, zearalenone, trichothecenes, patulin, and penicillic acid [52]. Mycotoxigenicity of Spanish fermented meat sausage has been investigated by TLC [57].
GC can also be used for mycotoxin detection in food products when the mycotoxins are converted to volatile compounds. The GC system is linked to an MS (mass spectrophotometry) flame ionization detector (FID) or Fourier transform infrared spectroscopy (FTIR) to detect volatile mycotoxins [53]. GC–MS is a suitable method for detection of mycotoxins in aromatic fermented foods, such as Chinese horse bean chilli paste [58].
An enzyme-linked immunosorbent assay (ELISA) is another method for mycotoxin detection. The citrinin level in monascus (red yeast rice) has been detected using ELISA [59]. In contrast to conventional ELISA, the latest immunoassays use biosensors or immunosensors. These are broadly classified into labeled and label-free. Labeled immune sensors working on the principle of competitive immunoassay involve a sample analyte competing with a conjugated or labeled analyte for attachment to antibodies. On the contrary, in labeled non-competitive (sandwich) immunoassay, secondary antibodies generate a signal when an antigen is captured. The sandwich-type labeled assay is commonly used for aflatoxin B1 estimation. Alternatively, in the label-free immunosensor, noncompetitive method, the transducer reads the interaction between the analyte and immobilized antibodies [60]. However, this method gives false-positive results due to nonspecific binding. Instead of immune sensors, mycotoxins can also be detected using aptasensors that involve the use of aptamers, which are synthetic oligonucleotide ligands (either single-stranded DNA or RNA) containing 10–50 variable bases. These aptamers have high specificity, stability, and cost-effectiveness and can, thus, be valuable replacements for antibodies [60]. Ochratoxin A was detected in wine using enzyme-linked aptamer assays (ELAAS) based on a competition format [61]. All of these methods can be used for mycotoxin detection in fermented foods. However, the TLC, HPLC, and immunosensor methods offer significant reliability for industrial applications.
3.3. Techniques for bacterial toxins detection
Considering the risk of foodborne illnesses associated with bacterial contamination, use of highly sensitive analytical techniques for detection is mandatory. Culture-based techniques facilitate detection of bacterial strains, while some methods are explicitly focused on detection of bacterial toxins. Detection of endotoxins of Gram-negative bacteria by chromogenic limulus amoebocyte lysate (LAL) assay has been performed [28]. This technique uses the LAL test kit that contains a standard endotoxin, the chromogenic substrate LAL, and endotoxin-free water used as a blank. A microplate reader (wavelength set at 405 nm) is used to measure the absorbance of the sample mixture. The endotoxin concentration is determined using a standard curve for a known concentration of endotoxin standards in endotoxin-free water [28]. Microbiological quality of fermented foods (ogiri, ugba, iru, ogi and ogi baba) and beverages (mahewu and umqombothi) from selected Nigerian and South African markets has been evaluated using LAL kits [62].
For phenotypic detection of toxic shock syndrome toxin (TSST-1) produced by coagulase-negative Staphylococci, a reversed passive latex agglutination (RPLA) test kit has been used [63]. This technique has also been used to detect hemolysin BL (Hbl ) enterotoxin produced by B. cereus [36]. Using this method, a positive result is indicated by the formation of a lattice structure (agglutination) after adding a test sample to the latex suspension of each super-antigenic toxin control [63]. A Bacillus diarrheal enterotoxin visual immunoassay (BDEVIA) toxin detection kit has also been used specifically for the detection of the non-hemolytic (Nhe) enterotoxin complex [36]. B. cereus enterotoxins from doenjang, a Korean fermented soybean paste was analyzed using BDEVIA and RPLA kits [64].
Novel approaches for bacterial toxin detection also aim at the identification of genes responsible for toxin production. Such techniques help in the preliminary detection of the toxigenic potential of bacterial strains. Toxin-producing genes are identified using mPCR [65]. This method has been successfully used for the detection of the four toxin-producing genes hbl, nhe, cytK2, and cesB in B. cereus [66]. The genes responsible for TSST-1 (sea, seb, and sec) production have also been detected using this method [63]. The mPCR technique requires DNA extraction, followed by a comparison with specific primers for gene detection. Also, modification of mPCR has allowed simultaneous amplification of all the toxin genes. The nucleotide sequences are then compared with the GenBank database to identify the specific toxin genes [63].
The PCR technique has been used to detect bacterial strains in fermented foods. B. cereus, P. mirabilis, and C. botulinum were isolated from indigenous fermented soybean, fish, and pork foods in India using qPCR (quantitative PCR) (Table 2). The diarrheal toxins (Nhe and Hbl ) and enteric toxins (cereulide) were also detected in soybean samples through qPCR and immunoassay [35].
In contrast to conventional labor-intensive culture-based methods, modern molecular methods using PCR offer rapid, convenient, and early estimation of the food safety risks associated with foodborne pathogenic bacteria. Also, PCR has higher sensitivity and specificity, thus, producing reliable and accurate results [63].
4. Recent strategies for minimizing toxins in fermented foods
Apart from early detection of toxins during the production stage and application of good manufacturing practices (GMPs), other methods have been implemented to reduce amount of toxins in fermented foods. One recent approach is biopreservation, which includes the use of an effective starter culture to improve microbial control of toxins in fermented foods [9]. For instance, heat-treated Bacillus subtilis (a GRAS bacterium), isolated from kimchi has been investigated as a biocontrol agent to reduce ochratoxin A levels in fermented foods [67]. This bacterium disrupted the hyphae of the toxin-producing species A. ochraceus and A. carbonarius. B. subtilis also produced lipopeptides (surfactins, iturins, and fengycins) that causes the degradation of ochratoxin A [67]. Miso prepared using L. plantarum as a starter culture had a reduced biogenic amine content and produced KL-1, a potent bacteriocin that inhibits the growth of the amine-producing microorganisms Latilactobacillus sakei, Leuconostoc mesenteroides, and E. faecalis [68].
Amine-negative Pediococcus pentosaceus strains isolated from cheonggukjang (Korean soybean fermented food) and fermented fish sausage can be used as a starter culture because these have been effective in reducing histamine and tyramine contents in soy-based ferments by 14.7–23.7% and 15.7–25.9%, respectively. These strains prevent the development of biogenic amine-producing bacteria and, thus, inhibit formation of biogenic amines in fermented sausages and soybean [68]. B. subtilis and Bacillus amyloliquefaciens isolated from the Korean soybean fermented foods doenjang, cheonggukjang, and gochujang also showed biogenic amine degradation potentials as these taxa contain the enzyme amino-oxidase that catalyzes deamination biogenic amines [15]. Nine amine oxidases have been identified in L. plantarum, CAU 3823 strain, which can degrade 40% of the biogenic amines in Chinese rice wine at the end of fermentation process [69]. Some specific L. paracasei strains can also lower the levels of histamine and tyramine in cheese [70].
Saccharomyces cerevisiae was reported to be an effective aflatoxin binder in dairy-based fermented products, such as yogurt. The aflatoxin adheres to the mannan bacterial cell wall component, which is a bioactive glycoprotein that binds aflatoxin [71].
Specific starter cultures are preferred for indigenous preparation of fermented foods that are at high risk for toxin contamination. These starter cultures exhibit a broad range of function as bio-preservatives, aflatoxin-binders, and degraders of biogenic amines. Further research is required regarding the choice of starter cultures to eliminate the risk of toxin formation in fermented foods. Also, the latest technologies are now targeting preparation of genetically designed starter cultures [68].
5. Conclusion and future perspectives
Biogenic amines, mycotoxins, and bacterial toxins are a significant hazard in fermented foods. Foodborne illnesses due to toxins have been documented in fermented foods and beverages, including biogenic amines (particularly, histamine and tyramine) in cheeses, soy foods, and wine, mycotoxins in cereal-based fermented foods and alcoholic beverages, and bacterial toxins in sausages and sorghum- based fermented foods. To combat the risk of toxins associated with fermented foods, it is important to apply the latest detection techniques at the industrial level. The conventional, labor-intensive, and time-consuming culture-based techniques that have been used for the estimation of toxins in fermented foods sometimes yield false-positive results. Modern analytical techniques have been investigated for sensitivity and specificity in detection of toxins in fermented foods, including chromatography, PCR, and immunoassays linked with biosensors and nano-sensors. The chromatographic techniques of HPLC and TLC are mostly used at industrial scales. A safer and more desirable fermentation technique uses specially designed starter cultures at an industrial scale to produce amine degrading enzymes, aflatoxin binding proteins, and bacteriocins for bio-preservation. Owing to the growing demand for fermented foods, it is imperative that the latest techniques for toxin detection are industrialized, GMPs are implemented, proper storage conditions are maintained, and the choice of a starter culture is made wisely.
Supplementary Information
Acknowledgements
The authors would like to thank the Department of Food Science and Human Nutrition, University of Veterinary and Animal Sciences, Lahore for their support.
Funding Statement
The authors would like to thank the Department of Food Science and Human Nutrition, University of Veterinary and Animal Sciences, Lahore for their support.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1. Tamang JP, Watanabe K, Holzapfel WH. Review: diversity of microorganisms in global fermented foods and beverages. Front Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mota de Carvalho N, Costa EM, Silva S, Pimentel L, Fernandes TH, Estevez Pintado M. Fermented foods and beverages in human diet and their influence on gut microbiota and health. Fermentation. 2018;4 doi: 10.3390/fermentation4040090. [DOI] [Google Scholar]
- 3. Voidarou C, Antoniadou M, Rozos G, Tzora A, Skoufos I, Varzakas T, et al. Fermentative foods: microbiology, biochemistry, potential human health benefits and public health issues. Foods. 2021;10(1):69. doi: 10.3390/foods10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosa DD, Dias MMS, Grześkowiak ŁM, Reis SA, Conceição LL, Maria do Carmo GP. Milk kefir: nutritional, microbiological and health benefits. Nutr Res Rev. 2017;30:82–96. doi: 10.1017/S0954422416000275. [DOI] [PubMed] [Google Scholar]
- 5. Das D, Sarkar S, Borsingh Wann S, Kalita J, Manna P. Current perspectives on the anti-inflammatory potential of fermented soy foods. Food Res Int. 2022;152:110922. doi: 10.1016/j.foodres.2021.110922. [DOI] [PubMed] [Google Scholar]
- 6. Kårlund A, Gómez-Gallego C, Korhonen J, Palo-Oja OM, El-Nezami H, Kolehmainen M. Harnessing microbes for sustainable development: food fermentation as a tool for improving the nutritional quality of alternative protein sources. Nutrients. 2020:12. doi: 10.3390/NU12041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ilango S, Antony U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci Technol. 2021:118. doi: 10.1016/j.tifs.2021.05.034. [DOI] [Google Scholar]
- 8. Kumari R, Sanjukta S, Sahoo D, Rai AK. Functional peptides in Asian protein rich fermented foods: production and health benefits. Syst Microbiol Biomanufact. 2022;2:1–13. doi: 10.1007/s43393-021-00040-0. [DOI] [Google Scholar]
- 9. Anal AK, Perpetuini G, Petchkongkaew A, Tan R, Avallone S, Tofalo R, et al. Food safety risks in traditional fermented food from South- East Asia Food Control. 2020:109. doi: 10.1016/j.foodcont.2019.106922. [DOI] [Google Scholar]
- 10. Adinsi L, Mestres C, Akissoé N, Vieira-Dalodé G, Anihouvi V, Durand N, et al. Comprehensive quality and potential hazards of gowe, a malted and fermented cereal beverage from West Africa. A diagnostic for a future re-engineering. Food Control. 2017;82:18–25. doi: 10.1016/j.foodcont.2017.06.019. [DOI] [Google Scholar]
- 11. Sivamaruthi BS, Kesika P, Chaiyasut C. Toxins in fermented foods: prevalence and preventions—a mini review. Toxins. 2019;11 doi: 10.3390/toxins11010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visciano P, Schirone M.Update on biogenic amines in fermented and non-fermented beverages. 2022. [DOI] [PMC free article] [PubMed]
- 13. (BIOHAZ) EP on BH. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:2393. [Google Scholar]
- 14.Schirone M, Visciano P, Tofalo R, Suzzi G. Histamine food poisoning. In: Hattori Y, Seifert R, editors. Histamine histamine recept Heal Dis. Cham: Springer International Publishing; 2017. pp. 217–35. [DOI] [Google Scholar]
- 15. Alvarez MA, Moreno-arribas MV. The problem of biogenic amines in fermented foods and the use of potential biogenic microorganisms as a solution. Trends Food Sci Technol. 2014;39:146–55. doi: 10.1016/j.tifs.2014.07.007. [DOI] [Google Scholar]
- 16. Tsai Y-H, Kung H-F, Lin Q-L, Hwang J-H, Cheng S-H, Wei C-I, et al. Occurrence of histamine and histamine-forming bacteria in kimchi products in Taiwan. Food Chem. 2005;90:635–41. doi: 10.1016/j.foodchem.2004.04.024. [DOI] [Google Scholar]
- 17. Marcobal A, De Las Rivas B, Muñoz R. Methods for the detection of bacteria producing biogenic amines on foods: a survey. J Fur Verbrauchersc Und Leb. 2006;1:187–96. doi: 10.1007/s00003-006-0035-0. [DOI] [Google Scholar]
- 18. Lucas PM, Wolken WAM, Claisse O, Lolkema JS, Lonvaud-Funel A. Histamine-producing pathway encoded on an unstable plasmid in Lactobacillus hilgardii 0006. Appl Environ Microbiol. 2005;71:1417–24. doi: 10.1128/AEM.71.3.1417-1424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gardini F, Rossi F, Rizzotti L, Torriani S, Grazia L, Chiavari C, et al. Role of Streptococcus thermophilus PRI60 in histamine accumulation in cheese. Int Dairy J. 2012;27:71–6. doi: 10.1016/j.idairyj.2012.07.005. [DOI] [Google Scholar]
- 20. Komprda T, Burdychová R, Dohnal V, Cwiková O, Sládková P, Dvořáčková H. Tyramine production in Dutchtype semi-hard cheese from two different producers. Food Microbiol. 2008;25:219–27. doi: 10.1016/j.fm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 21. Özogul Y, Özogul F. Biogenic amines formation, toxicity, regulations in food 2019. [Google Scholar]
- 22. Dabadé DS, Jacxsens L, Miclotte L, Abatih E, Devlieghere F, De Meulenaer B. Survey of multiple biogenic amines and correlation to microbiological quality and free amino acids in foods. Food Control. 2021;120:107497. [Google Scholar]
- 23. Saelao S, Maneerat S, Thongruck K, Watthanasakphuban N, Wiriyagulopas S, Chobert JM, et al. Reduction of tyramine accumulation in Thai fermented shrimp (kung-som) by nisin Z-producing Lactococcus lactis KTH0-1S as starter culture. Food Control. 2018;90:249–58. doi: 10.1016/j.food-cont.2018.03.003. [DOI] [Google Scholar]
- 24. Cho T-Y, Han G-H, Bahn K-N, Son Y-W, Jang M-R, Lee C-H, et al. Evaluation of biogenic amines in Korean commercial fermented foods 2006. 2006:730–7. [Google Scholar]
- 25. Coton M, Romano A, Spano G, Ziegler K, Vetrana C, Desmarais C, et al. Occurrence of biogenic amine-forming lactic acid bacteria in wine and cider. Food Microbiol. 2010;27:1078–85. doi: 10.1016/J.FM.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 26. Adebo OA. African sorghum-based fermented foods: past, current and future prospects. Nutrients. 2020:12. doi: 10.3390/nu12041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pascari X, Ramos AJ, Marín S, Sanchís V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res Int. 2018;103:121–9. doi: 10.1016/j.foodres.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 28. Adekoya I, Obadina A, Olorunfemi M, Akande O, Landschoot S, De Saeger S, et al. Occurrence of bacteria and endotoxins in fermented foods and beverages from Nigeria and South Africa. Int J Food Microbiol. 2019;305 doi: 10.1016/j.ijfoodmicro.2019.108251. [DOI] [PubMed] [Google Scholar]
- 29. Adekoya I, Obadina A, Phoku J, De Boevre M, De Saeger S, Njobeh P. Fungal and mycotoxin contamination of fermented foods from selected South African markets. Food Control. 2018;90:295–303. [Google Scholar]
- 30. Lin X, Tang Y, Hu Y, Lu Y, Sun Q, Lv Y, et al. Sodium reduction in traditional fermented foods: challenges, strategies, and perspectives. J Agric Food Chem. 2021:69. doi: 10.1021/acs.jafc.1c01687. [DOI] [PubMed] [Google Scholar]
- 31. FOOD ROFVDIN. Joint FAO/WHO Expert committee on food Additives (JECFA) 1987 [Google Scholar]
- 32. Yan P, Liu Z, Liu S, Yao L, Liu Y, Wu Y, et al. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins. 2020;12:200. doi: 10.3390/toxins12030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oancea S, Stoia M. Mycotoxins : a review of toxicology, analytical methods and health risks MYCOTOXINS : a review of toxicology. 2015 [Google Scholar]
- 34. Anukul N, Vangnai K, Mahakarnchandkul W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J Food Drug Anal. 2013;21:227–41. doi: 10.1016/j.jfda.2013.07.009. [DOI] [Google Scholar]
- 35. Keisam S, Tuikhar N, Ahmed G, Jeyaram K. Toxigenic and pathogenic potential of enteric bacterial pathogens prevalent in the traditional fermented foods marketed in the Northeast region of India. Int J Food Microbiol. 2019;296:21–30. doi: 10.1016/j.ijfoodmicro.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 36. Ouoba LII, Thorsen L, Varnam AH. Enterotoxins and emetic toxins production by Bacillus cereus and other species of Bacillus isolated from Soumbala and Bikalga, African alkaline fermented food condiments. Int J Food Microbiol. 2008;124:224–30. doi: 10.1016/j.ijfoodmicro.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 37. Yim JH, Kim KY, Chon JW, Kim DH, Kim HS, Choi DS, et al. Incidence, antibiotic susceptibility, and toxin profiles of Bacillus cereus sensu lato isolated from Korean fermented soybean products. J Food Sci. 2015;80:M1266–70. doi: 10.1111/1750-3841.12872. [DOI] [PubMed] [Google Scholar]
- 38. Thorsen L, Abdelgadir WS, Rønsbo MH, Abban S, Hamad SH, Nielsen DS, et al. Identification and safety evaluation of Bacillus species occurring in high numbers during spontaneous fermentations to produce Gergoush, a traditional Sudanese bread snack. Int J Food Microbiol. 2011;146:244–52. doi: 10.1016/j.ijfoodmicro.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 39. Gu J, Liu T, Hou J, Pan L, Sadiq FA, Yuan L, et al. Analysis of bacterial diversity and biogenic amines content during the fermentation processing of stinky tofu. Food Res Int. 2018;111:689–98. doi: 10.1016/j.foodres.2018.05.065. [DOI] [PubMed] [Google Scholar]
- 40. Sang X, Ma X, Hao H, Bi J, Zhang G, Hou H. Evaluation of biogenic amines and microbial composition in the Chinese traditional fermented food grasshopper sub shrimp paste. LWT (Lebensm-Wiss & Technol) 2020;134:109979. doi: 10.1016/j.lwt.2020.109979. [DOI] [Google Scholar]
- 41. El Sheikha AF, Hu DM. Molecular techniques reveal more secrets of fermented foods. Crit Rev Food Sci Nutr. 2020:60. doi: 10.1080/10408398.2018.1506906. [DOI] [PubMed] [Google Scholar]
- 42. Ladero V, Linares DM, Fernández M, Alvarez MA. Real time quantitative PCR detection of histamine-producing lactic acid bacteria in cheese: relation with histamine content. Food Res Int. 2008;41:1015–9. [Google Scholar]
- 43. Torriani S, Gatto V, Sembeni S, Tofalo R, Suzzi G, Belletti N, et al. Rapid detection and quantification of tyrosine decarboxylase gene (tdc) and its expression in Gram-positive bacteria associated with fermented foods using PCR-based methods. J Food Protect. 2008;71:93–101. doi: 10.4315/0362-028X-71.1.93. [DOI] [PubMed] [Google Scholar]
- 44. Ladero V, Cañedo E, Pérez M, Martín MC, Fernández M, Alvarez MA. Multiplex qPCR for the detection and quantification of putrescine-producing lactic acid bacteria in dairy products. Food Control. 2012;27:307–13. [Google Scholar]
- 45. Ladero V, Coton M, Fernández M, Buron N, Martín MC, Guichard H, et al. Biogenic amines content in Spanish and French natural ciders: application of qPCR for quantitative detection of biogenic amine-producers. Food Microbiol. 2011;28:554–61. doi: 10.1016/j.fm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 46. Ahangari H, Kurbanoglu S, Ehsani A, Uslu B. Latest trends for biogenic amines detection in foods: enzymatic biosensors and nanozymes applications. Trends Food Sci Technol. 2021;112:75–87. doi: 10.1016/j.tifs.2021.03.037. [DOI] [Google Scholar]
- 47. Wang Q, Zhang D. A novel fluorescence sensing method based on quantum dot-graphene and a molecular imprinting technique for the detection of tyramine in rice wine. Anal Methods. 2018;10:3884–9. doi: 10.1039/c8ay01117f. [DOI] [Google Scholar]
- 48. Ishimaru M, Muto Y, Nakayama A, Hatate H, Tanaka R. Determination of biogenic amines in fish meat and fermented foods using column-switching high-performance liquid chromatography with fluorescence detection. Food Anal Methods. 2019;12:166–75. doi: 10.1007/s12161-018-1349-0. [DOI] [Google Scholar]
- 49. Costa MP, Balthazar CF, Rodrigues BL, Lazaro CA, Silva ACO, Cruz AG, et al. Determination of biogenic amines by high-performance liquid chromatography (HPLC-DAD) in probiotic cow’s and goat’s fermented milks and acceptance. Food Sci Nutr. 2015;3:172–8. doi: 10.1002/fsn3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mayer HK, Fiechter G. UHPLC analysis of biogenic amines in different cheese varieties. Food Control. 2018;93:9–16. doi: 10.1016/j.foodcont.2018.05.040. [DOI] [Google Scholar]
- 51. Gong X, Wang X, Qi N, Li J, Lin L, Han Z. Determination of biogenic amines in traditional Chinese fermented foods by reversed-phase high-performance liquid chromatography (RP-HPLC) Food Addit Contam. 2014;31:1431–7. doi: 10.1080/19440049.2014.926402. [DOI] [PubMed] [Google Scholar]
- 52.Cole RJ. Modern methods in the analysis and structural elucidation of mycotoxins. Elsevier Science; 2012. [Google Scholar]
- 53. Turner NW, Subrahmanyam S, Piletsky SA. Analytical methods for determination of mycotoxins: a review. Anal Chim Acta. 2009;632:168–80. doi: 10.1016/j.aca.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 54. Al-Taher F, Banaszewski K, Jackson L, Zweigenbaum J, Ryu D, Cappozzo J. Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J Agric Food Chem. 2013;61:2378–84. doi: 10.1021/jf304729f. [DOI] [PubMed] [Google Scholar]
- 55. Adekoya I, Njobeh P, Obadina A, Chilaka C, Okoth S, De Boevre M, et al. Awareness and prevalence of mycotoxin contamination in selected Nigerian fermented foods. Toxins. 2017;9:363. doi: 10.3390/toxins9110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chilaka CA, De Boevre M, Atanda OO, De Saeger S. Quantification of Fusarium mycotoxins in Nigerian traditional beers and spices using a multi-mycotoxin LC-MS/MS method. Food Control. 2018;87:203–10. doi: 10.1016/j.foodcont.2017.12.028. [DOI] [Google Scholar]
- 57. Lopez-Diaz T-M, Santos J-A, Garcia-Lopez M-L, Otero A. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium isolates. Int J Food Microbiol. 2001;68:69–74. doi: 10.1016/s0168-1605(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 58. Lu Y, Yang L, Yang G, Chi Y, Sun Q, He Q. Insight into the fermentation of Chinese horse bean-chili-paste. Food Rev Int. 2021;37:683–705. [Google Scholar]
- 59.S-W Wu, Y-A Yu, Liu B-H, Yu F-Y.toxins Article. 2018. [DOI]
- 60.Chauhan R, Singh J, Sachdev T, Basu T, Malhotra BD.Recent advances in mycotoxins detection. 2016. [DOI] [PubMed]
- 61. Barthelmebs L, Jonca J, Hayat A, Prieto-Simon B, Marty JL. Enzyme-Linked Aptamer Assays (ELAAs), based on a competition format for a rapid and sensitive detection of Ochratoxin A in wine. Food Control. 2011;22:737–43. doi: 10.1016/J.FOODCONT.2010.11.005. [DOI] [Google Scholar]
- 62. Adekoya I, Obadina A, Olorunfemi M, Akande O, Landschoot S, De Saeger S, et al. Occurrence of bacteria and endotoxins in fermented foods and beverages from Nigeria and South Africa. Int J Food Microbiol. 2019;305:108251. doi: 10.1016/j.ijfoodmicro.2019.108251. [DOI] [PubMed] [Google Scholar]
- 63. Brien KO. Page 1 of 24. Soc Change. 2007:1–24. [Google Scholar]
- 64. Lee N, Kim MD, Chang HJ, Choi SW, Chun HS. Genetic diversity, antimicrobial resistance, toxin gene profiles, and toxin production ability of Bacillus cereus isolates from doenjang, a Korean fermented soybean paste. J Food Saf. 2017;37:1–7. doi: 10.1111/jfs.12363. [DOI] [Google Scholar]
- 65. Ranjbar R, Safarpoor Dehkordi F, Sakhaei Shahreza MH, Rahimi E. Prevalence, identification of virulence factors, O-serogroups and antibiotic resistance properties of Shigatoxin producing Escherichia coli strains isolated from raw milk and traditional dairy products. Antimicrob Resist Infect Control. 2018;7:1–11. doi: 10.1186/s13756-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sánchez-Chica J, Correa MM, Aceves-Diez AE, Castañeda-Sandoval LM. A novel method for direct detection of Bacillus cereus toxin genes in powdered dairy products. Int Dairy J. 2020:103. doi: 10.1016/j.idairyj.2019.104625. [DOI] [Google Scholar]
- 67. Shukla S, Park JH, Kim M. Efficient, safe, renewable, and industrially feasible strategy employing Bacillus subtilis with alginate bead composite for the reduction of ochratoxin A from wine. J Clean Prod. 2020;242:118344. doi: 10.1016/j.jclepro.2019.118344. [DOI] [Google Scholar]
- 68. Park YK, Lee JH, Mah JH. Occurrence and reduction of biogenic amines in traditional Asian fermented soybean foods: a review. Food Chem. 2019;278:1–9. doi: 10.1016/j.foodchem.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 69. Niu T, Li X, Guo Y, Ma Y. Identification of a lactic acid bacteria to degrade biogenic amines in Chinese rice wine and its enzymatic mechanism. Foods. 2019;8 doi: 10.3390/foods8080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guarcello R, de Angelis M, Settanni L, Formiglio S, Gaglio R, Minervini F, et al. Selection of amine-oxidizing dairy lactic acid bacteria and identification of the enzyme and gene involved in the decrease of biogenic amines. Appl Environ Microbiol. 2016;82:6870–80. doi: 10.1128/AEM.01051-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Corassin CH, Bovo F, Rosim RE, Oliveira CAF. Efficiency of Saccharomyces cerevisiae and lactic acid bacteria strains to bind aflatoxin M1 in UHT skim milk. Food Control. 2013;31:80–3. doi: 10.1016/j.foodcont.2012.09.033. [DOI] [Google Scholar]
- 72. Kongkiattikajorn J. Potential of starter culture to reduce biogenic amines accumulation in som-fug, a Thai traditional fermented fish sausage. J Ethn Foods. 2015;2:186–94. [Google Scholar]
- 73. Holck A, Axelsson L, McLeod A, Rode TM, Heir E. Health and safety considerations of fermented sausages. J Food Qual. 20172017 [Google Scholar]
- 74. Balamurugan S, Ahmed R, Gao A, Strange P. Comparison of the fate of the top six non-O157 shiga-toxin producing Escherichia coli (STEC) and E. coli O157:H7 during the manufacture of dry fermented sausages. Int J Food Microbiol. 2017;259:14–21. doi: 10.1016/j.ijfoodmicro.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 75. Nadira A, Rosita J, Norhaizan ME, Redzwan SM. Screening of aflatoxin M1 occurrence in selected milk and dairy products in Terengganu, Malaysia. Food Control. 2017;73:209–14. [Google Scholar]
- 76. González-Osnaya L, Cortés C, Soriano JM, Moltó J, Mañes J. Occurrence of Deoxynivalenol and T-2 toxin in bread and pasta commercialised in Spain. Food Chem. 2011;124:156–61. doi: 10.1016/j.foodchem.2010.06.002. [DOI] [Google Scholar]
- 77. Hwang IM, Yang J-S, Jung J-H, Lee H-W, Lee HM, Seo H-Y, et al. Dietary intake assessment of macro, trace, and toxic elements via consumption of kimchi in South Korea. J Sci Food Agric. 2019;99:6474–81. doi: 10.1002/jsfa.9926. [DOI] [PubMed] [Google Scholar]
- 78. Yu Y, Li L, Xu Y, An K, Shi Q, Yu Y, et al. Evaluation of the relationship among biogenic amines, nitrite and microbial diversity in fermented mustard. Molecules. 2021;26:6173. doi: 10.3390/molecules26206173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Paz S, Rubio C, Gutiérrez ÁJ, González-Weller D, Hardisson A. Human exposure assessment to potentially toxic elements (PTEs) from tofu consumption. Environ Sci Pollut Res. 2021;28:33522–30. doi: 10.1007/s11356-021-13076-5. [DOI] [PubMed] [Google Scholar]
- 80. Taniwaki MH, Hocking AD, Pitt JI, Fleet GH. Growth of fungi and mycotoxin production on cheese under modified atmospheres. Int J Food Microbiol. 2001;68:125–33. doi: 10.1016/S0168-1605(01)00487-1. [DOI] [PubMed] [Google Scholar]
- 81. Atter A, Ofori H, Anyebuno G, Amoo-Gyasi M, kofi Amoa-Awua W. Safety of a street vended traditional maize beverage, ice-kenkey, in Ghana. Food Control. 2015;55:200–5. [Google Scholar]
- 82.Owusu-Kwarteng J, Parkouda C, Adedeji Adewumi G, Iréne Ivette Ouoba L, Jespersen L.Technologically relevant Bacillus species and microbial safety of West African traditional alkaline fermented seed condiments. 2020. [DOI] [PubMed]
- 83. Landete JM, de las Rivas B, Marcobal A, Mũoz R. Molecular methods for the detection of biogenic amine-producing bacteria on foods. Int J Food Microbiol. 2007;117:258–69. doi: 10.1016/j.ijfoodmicro.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 84. Costantini A, Cersosimo M, Del Prete V, Garcia-Moruno E. Production of biogenic amines by lactic acid bacteria: screening by PCR, thin-layer chromatography, and high-performance liquid chromatography of strains isolated from wine and must. J Food Protect. 2006;69:391–6. doi: 10.4315/0362-028X-69.2.391. [DOI] [PubMed] [Google Scholar]
- 85. Ladero V, Calles-Enriquez M, Fernandez M, Alvarez MA. Toxicological effects of dietary biogenic amines. Curr Nutr Food Sci. 2010;6:145–56. doi: 10.2174/157340110791233256. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.