Abstract
The co-occurrence and accumulation of mycotoxin in food and feed constitutes a major issue to food safety, food security, and public health. Accurate and sensitive mycotoxins analysis can avoid toxin contamination as well as reduce food wastage caused by false positive results. This mini review focuses on the recent advance in detection methods for multiple mycotoxins, which mainly depends on immunoassay technologies. Advance immunoassay technologies integrated in mycotoxin analysis enable simultaneous detection of multiple mycotoxins and enhance the outcomes’ quality. It highlights toxicogenomic as novel approach for hazard assessment by utilizing computational methods to map molecular events and biological processes. Indeed, toxicogenomic is a powerful tool to understand health effects from mycotoxin exposure as it offers insight on the mechanisms by which mycotoxins exposures cause diseases.
Keywords: Analytical methods, Immunoassay, Mycotoxin detection, Multiple mycotoxins, Toxicogenomics
1. Introduction
Mycotoxins are naturally occurring toxins produced by certain fungi [1]. Pre-and post-harvest colonization of food commodities by fungi is largely influenced by various factors ranging from climatic and farm practice, handling and storage to processing and distribution of food [2]. Most mycotoxins are stable and survive food processing involves heat, physical, and chemical treatments [3]. Mycotoxin is one of the most dangerous contaminants of food and animal feed. Therefore, it is an important challenge and mission of many food safety specialists all over the world to protect humans or animals from dangerous contamination levels of various mycotoxins in the feeds or foods. Hazard analysis is a systematic approach to control food safety from biological, chemical, and physical hazards that can cause the end-product to be unsafe for consumption [4]. The analysis consists of identification, risk assessment, and the establishment of preventive measures toward the hazards and related causes [5]. Indeed, mycotoxin detection analysis plays an important role in reducing toxin exposure, wherein, improvement in technologies and techniques in the analytical process is crucial [6].
Mycotoxins exposure in humans may occur either directly by eating fungi-infected food or indirectly from animals that are fed with contaminated feed [7]. Of great significance, feed contamination poses an extra hazard for food safety due to the possible carry-over of mycotoxins to animal-derived products such as milk, meat, and egg, leading to mycotoxin intake by humans [8]. The negative effects on animal health and production have been documented in farmed animals such as poultry, swine, and cattle [9]. The phenomenon occurs due to the consumption of high levels of cereals and cereal by-products, that are prone to fungi infection in the daily diet of animals [10]. Animals showed varying susceptibilities to mycotoxins subjected to genetic, physiological, and environmental factors [11]. As animal species differ in terms of the absorption, metabolism, distribution, and excretion of mycotoxins, the sensitivity to the adverse effects of mycotoxins may vary [12]. Unfortunately, about 25% of the world’s harvested crops are contaminated by mycotoxins each year, leading to huge agricultural and industrial annual losses of around 1 billion metric tons of foods and food products [13].
The effects of some food-borne mycotoxins are acute with symptoms of severe illness appearing quickly after consumption of food products contaminated with mycotoxins [14]. Several mycotoxins are correlated with severe toxicological effects such as teratogenicity, hepatotoxicity, and carcinogenicity [15]. Other mycotoxins occurring in food have been linked to long-term effects on health, including immune deficiencies-related diseases [16]. Because of their severe effects on animal and human health, as well as their occurrences in food, a dozen have gained attention from the authorities. Hundreds of different mycotoxins have been identified, but the most observed mycotoxins that attract the concern to human health and livestock include aflatoxins, (AF), ochratoxin A (OTA), fumonisins (FUM), zearalenone (ZEN), and deoxynivalenol (DON) [17].
Mycotoxins have been detected in countless food commodities in every part of the world, thus poses critical challenge in regulation of mycotoxin contamination. Mycotoxins harm human and animal health, hamper economic development, as well as generate food wastage. Nonetheless, the co-occurrence of mycotoxins has further complicated the situation where the conventional mycotoxins analytical methods are no longer applicable. This review focuses on the recent advance in detection methods for multiple mycotoxins, which mainly depends on immunoassay technologies. Furthermore, it highlights toxicogenomic as a field which opens novel opportunities for hazard assessment by utilizing computational methods to map molecular events and biological processes.
2. Co-occurrence of mycotoxins
Risk assessments of mycotoxins in food done by the Joint FAO/WHO Expert Committee on Food Additives are used by governments and by the Codex Alimentarius Commission to establish maximum levels in food or provide other risk management advice to control or prevent contamination [18]. Codex standards are the international reference for national food supplies. For instance, total AFs in peanuts, almonds, hazelnuts, pistachios, Brazil nuts for further processing should not exceed 15 μg/kg. Meanwhile, OTA in raw wheat, barley, and rye should be lower than 5 μg/kg. The guidelines provided by the regulatory bodies only focus on the presence of single mycotoxin in food/feed [18].
However, the presence of multiple mycotoxins in raw ingredients and feeds is not a rare scenario. Such an incident is due to the simultaneous production of several types of mycotoxins by a single fungus. For instance, Fusarium spp. produces a range of trichothecenes (TCT; DON, nivalenol, T-2, and HT-2 toxins), ZEN, and FUMs [19]. In addition, contamination may be caused by different fungal species concurrently. The manufacturing of compound feed involved mixing and milling feed materials. Such a process increases the susceptibility of various mycotoxins present in the feed. According to Smith et al. [20], the mixtures of AFs + FUM, DON + ZEA, AFs + OTA, and FUM + ZEA were abundantly detected in cereal and derived cereal product samples world widely. Besides, AFs + OTA was found mainly in dried fruits, herbs, and spices [19]. Moreover, a search in the literature from 1987 to 2016 showed that binary mixtures are the most common, while AFs are primarily found together with OTA or fusariotoxins (mainly FUM and ZEA) [20] in all food/feed. Meanwhile, the regional distribution pattern of mycotoxins’ co-occurrence showed that AFs + OTA was the main mixture found in African samples. On the other hand, AFs + FUM mixture is the most prevalent in Africa, Asia, and South America [20]. AFs are a far greater problem in the tropics than in temperate zones of the world. However, because of the movement of agricultural commodities around the globe, no region of the world is AF-free. In more temperate and cold regions (Europe and North America), the mixture of TCTs or TCTs with ZEA are the most common, highlighting the importance of the climate conditions on fungal contamination, growth, metabolism, and thus mycotoxin production [21].
Such findings highlight the significance of mycotoxin co-occurrence-related study to provide the actual situations of health risks exposed to humans and animals. The combined toxicity effects are hardly predictable. Nevertheless, mycotoxin mixtures are found to have additive or synergistic effects, which are harmful to human and animal health. A previous study showed the mixture of DON and T-2 toxins significantly enhanced the mutagenic activity of AFB1 [22]. Although the screening of mycotoxins has been frequently performed, the legislation available over the world only considers homogenous mycotoxin exposure data and does not address the combined effects of mycotoxins.
3. Development of mycotoxin analytical methods
Mycotoxins can be detected via two main techniques: conventional quantification in the laboratory and rapid screening. Conventional methods include thin-layer chromatography (TLC) [23], gas chromatography- tandem mass spectrometry (GC–MS) [24], high-performance liquid chromatography (HPLC) [25], liquid chromatography-mass spectrometry (LC-MS) [26], liquid chromatography-tandem mass spectrometry (LC-MS/MS) [27], ultra-high liquid chromatography-tandem mass spectrometry (UHP-LC-MS/MS) [28]. Owing to their high accuracy, precision, and sensitivity, several conventional methods have been applied for the analysis of mycotoxins in many agricultural commodities and feedstuffs since the 1970s. LC and UHPLC were frequently used as these methods can identify regulated, unregulated, and emerging mycotoxins in one single run with small volume injection [29]. Yet, these conventional methods are deemed costly, tedious, complex, and impractical for fast screening in the field.
As a result, technologies in mycotoxin analysis have been continuously revolutionized with new concepts and approaches. The rapid screening methods are reliable tests that offer more sensitive and specific evaluation at a cheaper cost. Such methods offer effective monitoring of various mycotoxin contamination in food/feed on the spot. Recently, an increasing trend has been observed for the development of simultaneous detection of mycotoxins arising from the rapid immunoassay concept [30]. Enzyme-linked immunosorbent assay (ELISA) is a classic immunoassay that has been widely used [31]. Sensitive microtiter plate ELISA-based immunoassays are readily available in the market to measure a range of mycotoxins. These kits mainly function based on a competitive, heterogeneous ELISA format. The binding between the labelled toxin and antibody can be affected by the presence of interference compound via attachment towards the labelled toxin/antibody, antibody denaturation, and enzyme inhibition [32]. ELISA may overestimate levels of mycotoxin due to cross-reactivities and matrix dependency, therefore it is only recommended for comprehensively researched samples and the presence of sufficient standards [33]. Besides, ELISA also requires costly detectors, tedious procedures, and skilled technicians. Hence, the development of rapid methods has been established at an accelerated pace owing to heavy demands. Advanced mycotoxin analyses developed in recent years are based on ELISA-inspired immunoassay theory.
4. Advance multi-mycotoxin immuno-based analysis
Emerging immunoassay platform technologies such as chemiluminescence (CL) immunoassay, fluorescence polarization (FP) immunoassay, lateral flow (LF) immunoassay, electrochemical (EC) immunosensor are useful for multiple mycotoxin detection [34]. The immunoassays used for multiplex mycotoxins analysis in recent years are summarized in Table 1. Immunoassay methods are developed based on antibody–antigen reactions. Mycotoxins are small non-immunogenic molecules [35]. The designing of specific antibodies against mycotoxins (analyte) and hapten is a crucial step for immunoassays. A hapten is a substance that can combine with a specific antibody but lacks antigenicity of its own [36]. An ideal hapten is closely similar to the target small molecule in terms of molecular structure and physicochemical properties with an attachment arm (4–6 carbon atoms) containing active group (–COOH and –NH2) to bind to the carrier protein [37].
Table 1.
List of the reviewed immunoassays for simultaneous detection of multiple mycotoxins in recent years.
| Matrix | Analyte | Sample Preparation | Format | Signal | LOD | Linear range | Analysis Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chemiluminescence Immunoassay | ||||||||
| Corn | AFB1, OTA, ZEA, FUMB1 | 75% acetonitrile:-water (84:16); 5 min | Competitive; Quantitative | strep-HRP with enhanced CL | AFB1 (0.21 ng/mL), OTA (0.19 ng/mL), ZEA (0.09 ng/mL), FUMB1 (0.24 ng/mL) | AFB1 (0.47–55.69 ng/mL), OTA (0.48–127.11 ng/mL), ZEA (0.22–31.36 ng/mL), and FUMB1 (0.56–92.57 ng/mL) | 60 | [44] |
| Red yeast rice | CIT, AFB1, OTA | 70% methanol solution (0.5 g sodium chloride); 30 min | Competitive; Quantitative | HRP with AuNP-IgG, tyramine, strep-HRP, CL substrate | CIT (0.00006 ng/mL), AFB1 (0.00008 ng/mL), OTA (0.00008 ng/mL) | 0.0001–1.0 ng/mL | 90 | [45] |
| Maize | AFB1, AFG1, OTA, ZEA, T-2 toxin, FUMB1, FUMB2, DON | 75% acetonitrile: methanol:-water (50:40:10); 10 min | Competitive; Quantitative | HRP with Investigador ™ EV 4065, Evidence Investigator Myco 7 | FB1+FB2 (250 μg/kg), AFB1 and AFG1 (1 μg/kg), OTA (1.5 μg/kg), ZEA (50 μg/kg), T-2 (25 μg/kg), DON (375 μg/kg) | FB1+FB2 (0–300 μg/kg), AFB1 (0–14 μg/kg), AFG1 (0–75 μg/kg), OTA (0–60 μg/kg), ZEA (0–150 μg/kg), T-2 (0–300 μg/kg), DON (0–7500 μg/kg) | 90 | [46] |
| Fluorescence Polarization Immunoassay | ||||||||
| Durum wheat | T-2 and HT-2 toxin | 90% methanol or water | Competitive; Quantitative | HT-2 tracers | T-2 and HT-2 toxin (10 μg/kg) | 50–200 μg/kg | 15 | [52] |
| Corn, soybean, sorghum, wheat, rice and oats | AFB1, ZEA, and OTA | 75% methanol:-water (70:30); 10 min | Competitive; Quantitative | chicken IgY with Europium NP | AFB1 (0.04 μg/kg), ZEN (0.20 μg/kg), and OTA (0.10 μg/kg) | AFB1 (0.00387–0.06924 mg/L), ZEN (0.01435–0.28789 mg/L) and OTA (0.0099–0.20423 mg/L) | 15 | [53] |
| Maize | AFB1, ZEA, DON, T-2, FUMB1 | 80% methanol in 0.02 M PB; 3 min | Competitive; Quantitative | AuNPs | Visible: AFB1 (10 μg/kg), ZEA (2.5 μg/kg), DON (1.0 μg/kg), T-2 (10 μg/kg), FUMB1 (0.5 μg/kg); Quantitative: AFB1 (0.59 μg/kg), ZEA (0.24 μg/kg), DON (0.32 μg/kg), T-2 (0.9 μg/kg), FUMB1 (0.27 μg/kg) | NA | 8 | [54] |
| Lateral Flow Immunoassay | ||||||||
| Wheat and wheat by-product | AFB1 and FUMB1 | 1% BSA and 2% PEG 10000 in PB; 2 min | Competitive; Qualitative | AuNPs | AFB1 (0.5 ng/mL) and FUMB1 (20 ng/mL) | NA | 10 | [60] |
| Buffer | AFB1, DON, FUMB1, T-2, and ZEA | NA | Competitive; Quantitative | Novel luminescent compound | AFB1 (1.3 ng/mL), DON (0.5 ng/mL), FUMB1 (0.4 ng/mL), T-2 (0.4 ng/mL), and ZEA (0.9 ng/mL) | AFB1 (5–40 ng/mL), DON (10–200 ng/mL), FUMB1 (0.5–10 ng/mL), T-2 (5–80 ng/mL), and ZEA (10–100 ng/mL) | NA | [61] |
| Maize | AFB1, DON, FUMB1, T-2, and ZEA | 70% methanol; 5 min | Competitive; Quantitative | 5,5-dithiobis-2-nitrobenzoic acid and 4-mercaptobenzoic acid | AFB1 (0.00096 ng/mL), DON (0.00011 ng/mL), FUMB1 (0.00026 ng/mL), T-2 (0.0086 ng/mL), and ZEA (0.0062 ng/mL) | AFB1 (0.0014–0.33 ng/mL), DON (0.14–33.3 ng/mL), FUMB1 (0.41–100 ng/mL), T-2 (0.014–33.3 ng/mL), and ZEA (0.015–3.7 ng/mL) | 20 | [62] |
| Electrochemical Immunosensor | ||||||||
| Corn | FUMB1 and DON | NA | Non-Competitive; Quantitative | AuNP and polypyrrole-electrochemically reduced graphene oxide | FUMB1 (4.2 ng/mL) and DON (8.8 ng/mL) | FUMB1 (200–4500 ng/mL) and DON (50–1000 ng/mL) | NA | [68] |
| Corn and wheat | AFB1, OTA, ZEN and DON | 20% methanol, 5 min | Non-Competitive; Quantitative | surface plasmon resonance (Hydrazone connection) | AFB1 (0.59 ng/mL), OTA (1.27 ng/mL), ZEA (7.07 ng/mL) and DON (3.26 ng/mL) | AFB1 (0.99–21.92 ng/mL), OTA (1.98–28.22 ng/mL), ZEA (10.37–103.31 ng/mL) and DON (5.31–99.37 ng/mL) | NA | [69] |
| Corn | FUMB1 and DON | 80% methanol, 15 min | Non-Competitive; Quantitative | Indium tin oxide and polydimethylsiloxane electrodes | FUMB1 (0.097 ng/mL) and DON (0.035 ng/mL) | FUMB1 (0.3–140 ng/mL; 0.2–60 ng/mL) | NA | [70] |
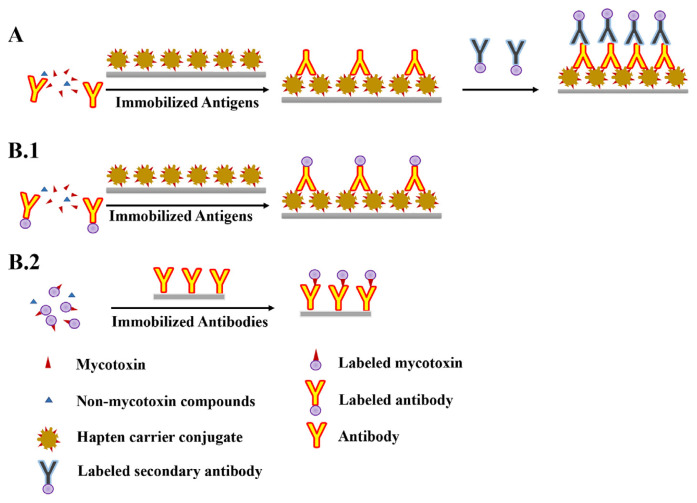
There are two categories of multiple mycotoxin analysis, competitive and non-competitive formats. The competitive format has been widely applied to develop rapid immuno-based methods due to the characteristic of mycotoxin (which contain merely one binding epitope) [38]. The main immunoassay formats for mycotoxins detection can be referred in Fig. 1. In the direct competitive mode, the mycotoxin from the sample competes for a limited number of antibody-binding sites with the toxin-enzyme conjugate. A lower signal indicates a higher concentration of mycotoxin in the sample, as the mycotoxin in the sample reduces the availability of the immobilized antibody to bind the labelled mycotoxin [39]. The detected signal is inversely associated with the concentration of mycotoxin. For indirect competitive format, mycotoxins in a test sample compete with immobilized labelled mycotoxins or their conjugates to bind with a limited amount of specific antibodies in the system [40].
Fig. 1.
Basic immunoassay concepts for mycotoxins detection: competitive indirect (A) and competitive direct (B.1 and B.2). Immunoassays are based on immunological principles that use antibodies to recognize and capture target antigens or haptens.
4.1. Chemiluminescence immunoassay
Mycotoxins detection using CL immunoassay employs simple optical equipment without an external light source [41]. Luminescence means that light is emitted by a substance when it returns from an excited state to a ground state [42]. A catalyst (enzyme, metal, and nanoparticles) is commonly used in a CL immunoassay to enhance the photon intensity. The catalyst added also acts as an enzyme protector and allows the reaction to maintain for a longer period [43]. Chemical reaction-induced CL intensity in the immunoassay is directly measured using a luminescent signal instrument. The luminous intensity is directly linked to the concentration of the measured substance.
Zhang and colleagues have successfully developed a simultaneous mycotoxin (AFB1, OTA, ZEN, and FUMB1) quantitative detection in corn samples [44]. The sensitivity of the CL immunoassay kit was enhanced by both the biotin-streptavidin system and enhanced CL. Results showed the detection limits for AFB1, OTA, ZEN, and FUMB1 were 0.21, 0.19, 0.09, and 0.24 ng/mL, with detection ranges of 0.47–55.69, 0.48–127.11, 0.22–31.36, and 0.56–92.57 ng/mL, respectively. The limit of detection (LOD) of this antibody microarray for AFB1, OTA, ZEN, and FUMB1 in corn was 5.25, 4.75, 2.25, and 6 μg/kg, respectively, with 79.2–113.4% recovery rates.
A novel CL immunoassay combined with a dual-signal amplification strategy was developed for rapid and ultrasensitive detection of multiple mycotoxins in herbal medicine [45]. The immunosensor array uses multi-HRP wrapped AuNPs as the primary signal tag to amplify the CL signals. The primary amplification is subjected to secondary amplification using the tyramine signal amplification (TSA) technique. Such an array tested on herbs containing citrinin, AFB1, and OTA provides excellent sensitivity (50–57-fold signal amplification and detection limits down to sub-pM level), small amounts of reagents (3.5 μL for each test), simple sample pretreatment [45].
In other study, Freitas et al. [46] have employed a biochip chemiluminescent immunoassay for multi-mycotoxins screening in maize. This immunoassay (Investigador™EV 4065, Evidence Investigator Myco 7) is based on the Evidence Investigator Biochip Array technology and uses a Randox Biochip containing immobilized antibodies specific to mycotoxins. Spiked samples were fortified at 250 μg/kg for FB1 + FB2, 1 μg/kg for AFB1 and AFG1, 1.5 μg/kg for OTA, 50 μg/kg for ZEA, 25 μg/kg for T2 and 375 μg/kg for DON. Besides, low false results rate (<5%) was achieved and the obtained precision data is in agreement with EU legislation performance criteria.
CL immunosensors have obtained extensive focus, as well as a large number of successful examples for the sensitive detection of mycotoxins. However, CL immunosensors are limited by high background signal and poor reagent stability [47].
4.2. Fluorescence polarization (FP) immunoassay
The FP immunoassay is based on the principle that when a fluorescent molecule in solution is exposed to polarized light at excitation wavelength the resulting emission is depolarized [48]. The orientation of the fluorescence emission (horizontal and vertical directions) determines the polarization [49]. Small-sized fluorescent molecules such as mycotoxins showed higher rotation and low polarization than larger molecules [50]. Meanwhile, the polarisation of mycotoxins can be increased via the interaction with antibodies. FP immunoassay involves the competition for binding sites on a mycotoxin-specific antibody between mycotoxin in the sample with a mycotoxin-fluorophore tracer [51]. The polarization value is inversely proportional to mycotoxin concentration. FP immunoassay is a non-enzymatic homologous immunoassay where the free and bound tracer needless to be separated. Removal of washing steps reduces assay time significantly.
For example, Lippolis and colleagues have developed a portable FP analyzer for the simultaneous determination of T-2 toxin, HT-2 toxin, and relevant glucosides in wheat [52]. Antibody used in the study showed high sensitivity (IC50 = 2.0 ng/mL) and cross-reactivity (100% for T-2 toxin and 80% for T-2 and HT-2 glucosides). The result revealed that the immunoassay developed displayed high sensitivity (LOD 10 μg/kg) with a recovery rate of 92–97%, which meet the criteria set by the European Union.
A multiplex quadruple-label time-resolved FP immunoassay has been established for simultaneous quantitative detection of AFB1, ZEN, and OTA in grains [53]. This assay was developed based on quadruple-label probes coupled with time-resolved fluorescent nanobeads. The probes were specific to chicken IgY-specific antibody (C line signal), AFB1, ZEN, and OTA monoclonal antibodies (T line signal). The ratios of T/C value determine the concentrations of mycotoxins. The LODs for AFB1, ZEN, and OTA were 0.04 μg/kg, 0.20 μg/kg, and 0.10 μg/kg in 6 grains (corn, soybean, sorghum, wheat, rice, and oats), respectively. The recovery rates ranged from 71.60-to 119.98%. The results obtained from this study were similar to that of LC-MS/MS [53].
A smartphone-based quantitative dual detection mode device has been developed by Liu et al. [54]. The device is integrated with gold nanoparticles and time-resolved fluorescence microspheres. The signals were detected using visible light and fluorescence, then presented in the smartphone’s application. The device can be used to detect AFB1, ZEN, DON, T-2, and FB1. The visible LODs were 10/ 2.5/1.0/10/0.5 and 2.5/0.5/0.5/2.5/0.5 μg/kg for the two methods, respectively. The quantitative limits of detection (qLODs) were 0.59/0.24/0.32/0.9/0.27, 0.42/ 0.10/0.05/0.75/0.04 μg/kg, respectively. The recoveries of both immunoassays (visible light and fluorescence) ranged from 84.0% to 110.0%. It is worth noting that validation of FP immunoassay is required for every food commodity as the polarisation of tracers can be affected by the matrix.
FL offers great advantages and broad application. Nevertheless, some challenges or limitations are yet to be solved. The background fluorescence and complex matrix components of the tested samples may interfere the fluorescence signals [55]. Moreover, the corresponding antibody of multiple mycotoxins need to be prepared for simultaneous detection. Hence, the development of novel fluorescent reporters will be the focused in future FL immunoassay.
4.3. Lateral flow immunoassay
LF immunoassay is a simple one-step immunochromatographic paper assay that does not require complex instruments [56]. Its outstanding advantages of convenience and rapidity are especially suitable for on-site monitoring of mycotoxin contamination in food. These test strips are mainly labelled with gold or enzyme [57]. The basic LF immunoassay equipment consists of sample coating pads, conjugate-release pads, absorbent pads, and nitrocellulose membranes [58]. Typically, LF immunoassay is a competitive-based mycotoxin detection assay where mycotoxin in the sample and the mycotoxin-conjugate immobilized on the test line compete for the specific antibody. The absence of colour on the test line indicates the presence of an analyte, while the colour developed on the test line and the control line indicates a negative result [59]. Some existing LF immunoassays have been considerably improved by combining different kinds of nanosensors or strategies for increasing sensitivity or efficiency.
LFIA-based multiplex detection of AFB1 and FUM in a single test line with multi-coloured gold nanoparticles signals has been reported [60]. The nanoparticles (red and blue) were attached to antibodies against the analytes. The detection of AFB1 and FUM contamination in raw and processed food was achieved with visual cut-off levels at 1 ng/mL and 50 ng/mL, respectively. Another study has combined microarray with LF immunoassay for simultaneous mycotoxins detection (AFB1, DON, FUMB1, T-2, and ZEA) [61]. A strong fluorescence organic compound that can be read under UV light was utilized. The LOD of AFB1, DON, FUMB1, T-2, and ZEA were 1.3, 0.5, 0.4, 0.4, and 0.9 ppb, respectively. The recoveries of these five mycotoxins were 70.7–119.5% and 80.4–124.8% for intra-assay and inter-assay, respectively. The device offers high specificity and high sensitivity.
A multiplex LFIA-based on surface-enhanced Raman scattering was established to detect six main fungal toxins in maize [62]. The Au@Ag nuclear shell nanoparticles used in the LFIA are composed of two types of Raman reporter molecules, 5,5-dithiobis-2-nitrobenzoic acid and 4-mercaptobenzoic acid, which distributed on three test lines of nitrocellulose membrane. Upon optimisation, the limits of detection were as low as 0.96 pg/mL for AFB1, 6.2 pg/mL for ZEN, 0.26 ng/mL for FUMB1, 0.11 ng/mL for DON, 15.7 pg/mL for OTA, and 8.6 pg/mL for T-2 toxin, respectively. The spiking experiment showed high accuracy with recovery of 78.9–106.2% and satisfactory assay precision with the coefficient of variations below 16%.
A recent review has summarized the recent development of LFIA for both single and mutiplex mycotoxin analysis [63]. The development of LFIA provides a promising technique for multiplex, highly sensitive, and on-site detection of mycotoxins.
4.4. Electrochemical immunosensor
The EC immunosensor, a type of biosensor, employs the antibody as a capture agent and quantitatively measures the electrical signal resulting from the binding event between the antibody and analyte [64]. EC sensors are characterized by simple operation, outstanding sensitivity, low cost, and facile miniaturization and have become a promising strategy for addressing specificity and sensitivity in detection. EC sensors are solid-state devices containing specific recognition elements, signal transducer, and electrochemical display [65]. The EC compounds are produced upon interactions between mycotoxin and antibody/aptamer on the transducer surface [66]. Aptamers are short, single-stranded DNA or RNA (ssDNA or ssRNA) molecules that can selectively bind to the analyte [67]. Electrochemical signal transducers, on the other hand, are normally made of Au, indium tin oxide, and carbon.
The first mycotoxin multiplex EC immunosensor was reported for the detection of FUMB1 and DON [68]. A disposable screen-printed carbon electrode modified by nanoparticles (Au and polypyrrole-electrochemically reduced graphene oxide) was used as sensing platform. Such modification enhanced anti-toxin antibody immobilization, electrical conductivity, and biocompatibility. The LODs were 4.2 ppb for FUMB1 and 8.6 ppb for DON.
In other study, Wei et al. [69] established a simultaneous detection method of multi-residue using surface plasmon resonance technique to measure AFB1, OTA, ZEN and DON in corn and wheat with high sensitivity, accuracy and specificity. Surface plasmon resonance sensor chip was fabricated based on self-assembled monolayer. The LODs for AFB1 (0.59 ng/mL), OTA (1.27 ng/mL), ZEA (7.07 ng/mL) and DON (3.26 ng/mL) were identified. In addition, low cross-reactivity for all four mycotoxins were demonstrated in the study.
A dual-channel three-electrode electrochemical sensor pattern was etched on a transparent indium tin oxide-coated glass via photolithography and was integrated with capillary-driven polydimethylsiloxane microfluidic channel [70]. The two working electrodes were functionalized with gold nanoparticles and anti-FB1 and anti-DON antibodies. Tests were performed by incubating the working electrodes in a sample solution introduced in the PDMS channel. The formation of toxin-antibody immunocomplexes on the working electrode surface produced electrochemical signal responses, which were recorded and compared with the control signal to quantify individual mycotoxin concentrations. Such dual-channel ITO-microfluidic electrochemical immunosensor able to achieve LODs of 97 pg/mL and 35 pg/mL, respectively for FB1 and DON, and their corresponding linear ranges of detection were 0.3–140 ppb and 0.2–60 ppb.
EC immunosensor provides reliable quantitative results at low cost of assay and equipment. However, EC immunosensor is sensitive towards pH, ions and temperature of the samples [71]. Therefore, such factors should be taken into account during mycotoxin quantification.
5. Pitfalls of mycotoxin analysis
Various problems have been found in the analytical chemistry of mycotoxin. The accuracy of the mycotoxin analysis is greatly dependent on the accuracy of sampling. The distribution of analytes in the food/feed commodities is highly heterogeneous [72]. A previous study showed that the coefficient of variation in the determination of AF concentration in peanuts is between 60% and 120% for sampling, dropped to 20% for subsampling, and remained 10% for the analysis [73]. Inappropriate sampling techniques may lead to inaccurate determination of mycotoxin contents in the food/feed. Such items available in the market pose a huge health risk to consumers. The common issues in sample preparation include inadequate purification, presence of artifacts, loss of analyte, and false recovery calculation [74]. The introduction of internal standards in the sample before the extraction and clean-up may solve part of the problem.
All analytical methods of mycotoxins should be fully validated by establishing compliance to various criteria: selectivity, linearity, the limit of detection, the limit of quantification, decision limit, detection capability, intra-/interday precision, recovery, accuracy, and robustness [75]. It is worth noting that the golden standard of validations and certified reference materials are necessary for the process of method validation. Besides, interlaboratory studies and comparisons could assist in identifying possible analytical issues with mycotoxin analysis [76].
Noteworthy, mycotoxins may be converted into modified forms by plant detoxification systems [77]. Such modifications lead to modified chromatographic profiles, epitope conformation, or polarity, these mycotoxin derivatives usually escape conventional analytical methods and are not regulated by legislation and thus are called “masked” mycotoxins.
6. Toxicogenomics
Toxicogenomics is a new paradigm of toxicology inspired by the rapid development of microarray technologies in toxicology study. Today the field has expanded to include proteomics, genomics, transcriptomics, metabolomics, as well as epigenomics [78]. It is a sub-discipline of toxicology that deals with hazard identification, mechanistic toxicology, and risk assessment. Various toxicogenomics technologies measure hundreds to thousands of molecules (eg, DNA, RNA, proteins, lipids, and metabolites) from non-clinical and environmental toxicity studies. Since its introduction, toxicogenomics is frequently applied as a biomarker discovery tool that diagnoses or predicts disease. Furthermore, it has been extremely useful to shed light on the cellular and molecular mechanisms that are responsible for the toxicity [79]. With the presence of advanced developments in bioinformatics and analytical technologies, omics studies analyses can now be employed to understand the roles of biomolecules. Besides, the technologies help to recognize the relationships, systemic effects, and mechanisms of actions of compounds/agents [80].
These molecular techniques can be categorized into targeted and non-targeted [81]. Untargeted experiments intend to quantify the broadest range of changes in proteome, genome, transcriptome, or metabolome found in an extracted sample without prior knowledge of the possible outcomes. Undeniably, the results can be affected by the methods of extraction and analysis. The complex data gained from the results need computational software and knowledge to recognize and link the biomolecules between samples. At the same time, their interconnectivity in pathways related to the phenotype or aberrant process can be examined [82]. Meanwhile, targeted omics experiments provide higher selectivity and sensitivity compared to untargeted experiments [83]. The biomolecules analyzed are based on information gathered from the literature review. Besides, optimal methods are established for the analysis of targeted biomolecules and pathways. This review focus on untargeted omic toxicogenomic studies performed in recent years.
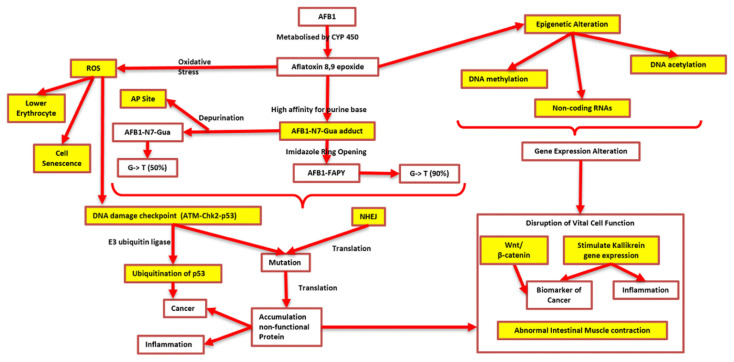
A recent study revealed that ZEA treatment for up to 40 μg/mL resulted in significant increase in generation time and decrease in egg production of Caenorhabditis elegans [84]. There were 3149 gene expressions altered by ZEA. The most affected genes were involved in development and reproduction. The genes responsible for collagen synthetic pathway was 20 folds lower compared to untreated C. elegans. The results demonstrate that disruption of the collagen biosynthetic pathway might be a key mechanism in ZEA-induced reproductive and developmental toxic effects in C. elegans. Another study by Liew et al. [85] also revealed that AFB1 altered gut proteomes of the rats via several pathways related to the occurrence of inflammation, cancer, and ROS generation as described in Fig. 2. Proteomic profiling in the study was performed using LC/MS/MS. Results showed that AFB1 downregulated five pathways and upregulated 19 pathways found in Reactome database.
Fig. 2.
Reactome pathways related to AFB1-induced toxicity (highlighted in yellow). Adopted from Liew et al. [85] https://doi.org/10.1016/J.FCT.2022.112808, with permission from Elsevier.
The effects of multiple mycotoxins on human and animals have attracted the attention of researchers. A study through integrated metabolic pathways has suggested that compared with individual mycotoxins, the combination of DON and ZEN group weakens the toxic effect in mouse liver, indicating the antagonistic effect of DON+ZEN treatment [86]. The mice were subjected to combined 2 mg/kg DON and 20 mg/kg ZEN for 21 days. The metabolic pathway analysis was performed with MetaMapp and drawn by CytoScape. The metabolic pathway analysis demonstrated that the combined DON and ZEN treatment could down-regulate the valine, leucine and isoleucine biosynthesis, glycine, serine and threonine metabolism, and O-glycosyl compounds related glucose metabolism in liver tissue. The metabolic profiling in serum confirmed the finding that the combined DON and ZEN treatment has an “antagonistic effect” on liver metabolism of mice. On the other hand, Gao et al. performed transcriptomic (RNA) and proteomic profiling (iTRAQ) on intestinal epithelial cells (Caco-2 cells), stimulated with aflatoxin M1 (AFM1) (4 μg/mL) and OTA (4 μg/mL) [87]. Such cross-omics analysis identified several mechanisms induced by AFM1+OTA. Up to 10,906 genes were altered by AFM1+OTA, where 10,004 genes were downregulated. The highest number of genes were related to inflammation. Meanwhile, transcriptomic analysis demonstrated that AFM1 and OTA primarily activated the signaling pathways involved in immunity/inflammation, including complement and coagulation cascades and the TNF, transforming growth factor (TGF)-β, T cell receptor, B cell receptor, and Notch signaling pathways. The transcriptomic and proteomic profiling results in this study also showed that the genes and proteins altered by single mycotoxins is different from combination of mycotoxins [87]. This highlights the need for toxicogenomic applications in co-occurrence of mycotoxins.
Toxicogenomic studies provide a new aspect in the assessment of environmental exposure. These studies offer valuable information at molecular level which can be used to identify the mode of toxicity mechanism of mycotoxin. Due of their sensitivity, toxicogenomic technologies are expected to reveal more than has been possible to date about the potential effects of exposure to toxic substances even in the early stage. Such data also improves the understanding of the variability in reactions of humans and animals towards mycotoxins.
7. Conclusions and perspectives
Mycotoxins in food currently present a global threat and require sensitive and selective detection. Importantly, the isolation and extraction method of mycotoxin should be optimized and reproducible for further detection. The market today for mycotoxin test kits is already a competitive one with multiple companies selling variations of similar products. Conventional mycotoxin detection methods used in regulatory agencies has been deemed unsuitable for the current situation where multiple mycotoxins are present in the food/feed. Immunoassay techniques able to improve the situation by providing higher sensitivity, precision, and simplicity as well as cost-effective. Growing progress in immunoassay technologies is keeping its pace over the past 5 years for reliable mycotoxin detection. Although pioneering studies have recently been performed, immunoassays with specificity and high throughput for multiple mycotoxin detection need to be further investigated.
Based on the literature, on-site monitoring of multiple mycotoxins using hand-held digital biosensors is the key development to control risk assessment. Nonetheless, immunoassays are still the current detection method of choice. Besides, toxicogenomic is likely to play a role in mycotoxin regulation and litigation. In fact, the development of tools and approaches for elucidating the mechanisms involved in toxic responses helps to enhance risk assessment by regulatory authority.
Supplementary Information
Acknowledgments
Both authors have made substantial contributions to the framework, preparation and writing of manuscript.
Abbreviations
- AFB1
aflatoxin B1
- OTA
ochratoxin A
- FUM
fumonisins
- ZEN
zearalenone
- DON
deoxynivalenol
- TCT
trichothecenes
Funding Statement
The authors would like to thank Ministry of Higher Education, Malaysia for the financial support under Fundamental Research Grant Scheme (FRGS/1/2018/SKK06/UPM/02/02).
Footnotes
Financial support
The authors would like to thank Ministry of Higher Education, Malaysia for the financial support under Fundamental Research Grant Scheme (FRGS/1/2018/SKK06/UPM/02/02).
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Disclaimer
This manuscript is a part of PhD thesis by Winnie-Pui-Pui Liew submitted to Universiti Putra Malaysia.
Conflict of interest
All authors do not have conflict of interest.
References
- 1. Anfossi L, Giovannoli C, Baggiani C. Mycotoxin detection. Curr Opin Biotechnol. 2016 doi: 10.1016/j.copbio.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 2. Moretti A, Pascale M, Logrieco AF. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci Technol. 2019 doi: 10.1016/j.tifs.2018.03.008. [DOI] [Google Scholar]
- 3. Liu L, Xie M, Wei D, Liu L, Xie M, Wei D. Biological detoxification of mycotoxins: current status and future advances. Int J Mol Sci. 2022;23:1064. doi: 10.3390/IJMS23031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panghal A, Chhikara N, Sindhu N, Jaglan S. Role of food safety management systems in safe food production: a review. J Food Saf. 2018;38:e12464. doi: 10.1111/JFS.12464. [DOI] [Google Scholar]
- 5. Art P. Handbook of food science and technology 3. Handb Food Sci Technol. 2016;3 doi: 10.1002/9781119296225. [DOI] [Google Scholar]
- 6. Singh J, Mehta A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: a review. Food Sci Nutr. 2020;8:2183–204. doi: 10.1002/FSN3.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ladeira C, Frazzoli C, Orisakwe OE. Engaging one health for non-communicable diseases in Africa: perspective for mycotoxins. Front Public Health. 2017 doi: 10.3389/fpubh.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alshannaq A, Yu JH. Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Publ Health. 2017 doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haque MA, Wang Y, Shen Z, Li X, Saleemi MK, He C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: a review. Microb Pathog. 2020;142:104095. doi: 10.1016/J.MICPATH.2020.104095. [DOI] [PubMed] [Google Scholar]
- 10. Pinotti L, Ottoboni M, Giromini C, Dell’Orto V, Cheli F. Mycotoxin contamination in the EU feed supply chain: a focus on Cereal Byproducts. Toxins. 2016 doi: 10.3390/TOXINS8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iheshiulor OOM, Esonu BO, Chuwuka OK, Omede AA, Okoli IC, Ogbuewu IP. Effects of mycotoxins in animal nutrition: a review. Asian J Anim Sci. 2011 doi: 10.3923/ajas.2011.19.33. [DOI] [Google Scholar]
- 12. Tkaczyk A, Jedziniak P. Mycotoxin biomarkers in pigs—current state of knowledge and analytics. Toxins. 2021;13:586. doi: 10.3390/TOXINS13080586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matny N. Fusarium head blight and crown rot on wheat & barley: losses and health risks. Adv Plants Agric Res. 2015 doi: 10.15406/apar.2015.02.00039. [DOI] [Google Scholar]
- 14. Frazzoli C, Gherardi P, Saxena N, Belluzzi G, Mantovani A. The hotspot for (global) one health in primary food production: aflatoxin M1 in dairy products. Front Public Health. 2017 doi: 10.3389/fpubh.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar D, Barad S, Sionov E, Keller NP, Prusky DB. Does the host contribute to modulation of mycotoxin production by fruit pathogens? Toxins. 2017 doi: 10.3390/toxins9090280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furian AF, Fighera MR, Royes LFF, Oliveira MS. Recent advances in assessing the effects of mycotoxins using animal models. Curr Opin Food Sci. 2022;47:100874. doi: 10.1016/J.COFS.2022.100874. [DOI] [Google Scholar]
- 17. Mavrommatis A, Giamouri E, Tavrizelou S, Zacharioudaki M, Danezis G, Simitzis PE, et al. Impact of mycotoxins on animals’ oxidative status. Antioxidants. 2021;10:214. doi: 10.3390/ANTIOX10020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taniwaki MH, Pitt JI, Copetti Mv, Teixeira AA, Iamanaka BT. Understanding mycotoxin contamination across the food chain in Brazil: challenges and opportunities. Toxins. 2019;11 doi: 10.3390/TOXINS11070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolawole O, de Ruyck K, Greer B, Meneely J, Doohan F, Danaher M, et al. Agronomic factors influencing the scale of Fusarium mycotoxin contamination of oats. J Fungi. 2021;7:965. doi: 10.3390/JOF7110965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith MC, Madec S, Coton E, Hymery N. Natural Co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins. 2016;8:94. doi: 10.3390/TOXINS8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benkerroum N. Aflatoxins: producing-molds, structure, health issues and incidence in southeast Asian and sub-saharan African countries. Int J Environ Res Publ Health. 2020;17:1215. doi: 10.3390/IJERPH17041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alshannaq A, Yu JH. Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Publ Health. 2017;14 doi: 10.3390/IJERPH14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott PM, Lawrence JW, van Walbeek W. Detection of mycotoxins by thin-layer chromatography: application to screening of fungal extracts. Appl Microbiol. 1970;20:839–42. doi: 10.1128/AM.20.5.839-842.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berthiller F, Dall’Asta C, Schuhmacher R, Lemmens M, Adam G, Krska AR. Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid Chromatography–Tandem mass spectrometry. J Agric Food Chem. 2005;53:3421–5. doi: 10.1021/JF047798G. [DOI] [PubMed] [Google Scholar]
- 25. Rajakylá E, Laasasenaho K, Sakkers PJD. Determination of mycotoxins in grain by high-performance liquid chromatography and thermospray liquid chromatography—mass spectometry. J Chromatogr A. 1987;384:391–402. doi: 10.1016/S0021-9673(01)94686-2. [DOI] [PubMed] [Google Scholar]
- 26. Rundberget T, Wilkins AL. Determination of Penicillium mycotoxins in foods and feeds using liquid chromatography–mass spectrometry. J Chromatogr A. 2002;964:189–97. doi: 10.1016/S0021-9673(02)00698-2. [DOI] [PubMed] [Google Scholar]
- 27. Sulyok M, Krska R, Schuhmacher R. A liquid chromatography/ tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal Bioanal Chem. 2007;389:1505–23. doi: 10.1007/S00216-007-1542-2/FIGURES/4. [DOI] [PubMed] [Google Scholar]
- 28. Romero-González R, Garrido Frenich A, Martínez Vidal JL, Prestes OD, Grio SL. Simultaneous determination of pesticides, biopesticides and mycotoxins in organic products applying a quick, easy, cheap, effective, rugged and safe extraction procedure and ultra-high performance liquid chromatography– tandem mass spectrometry. J Chromatogr A. 2011;1218:1477–85. doi: 10.1016/J.CHROMA.-2011.01.034. [DOI] [PubMed] [Google Scholar]
- 29. Vargas Medina DA, Bassolli Borsatto JV, Maciel EVS, Lanças FM. Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. TrAC, Trends Anal Chem. 2021;135:116156. doi: 10.1016/J.TRAC.2020.116156. [DOI] [Google Scholar]
- 30. Adunphatcharaphon S, Elliott CT, Sooksimuang T, Charlermroj R, Petchkongkaew A, Karoonuthaisiri N. The evolution of multiplex detection of mycotoxins using immunoassay platform technologies. J Hazard Mater. 2022;432:128706. doi: 10.1016/J.JHAZMAT.2022.128706. [DOI] [PubMed] [Google Scholar]
- 31. Zhu F, Zhang B, Zhu L. An up-converting phosphor technology-based lateral flow assay for rapid detection of major mycotoxins in feed: comparison with enzyme-linked immunosorbent assay and high-performance liquid chromatography-tandem mass spectrometry. PLoS One. 2021;16:e0250250. doi: 10.1371/JOURNAL.PONE.0250250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Su Z, Li T, Wu D, Wu Y, Li G. Recent progress on single-molecule detection technologies for food safety. J Agric Food Chem. 2022;70:458–69. doi: 10.1021/ACS.JAFC.1C06808/ASSET/IMAGES/MEDIUM/JF1C06808_0006.GIF. [DOI] [PubMed] [Google Scholar]
- 33. Zhu L, Li S, Sun L, Zhao J, Huang J, Jiang Y, et al. Development and validation of a specific sandwich ELISA for determination of soybean allergens and its application in processed foods. Process Biochem. 2022;117:134–41. doi: 10.1016/J.PROCBIO.2022.03.022. [DOI] [Google Scholar]
- 34. Jia M, Liao X, Fang L, Jia B, Liu M, Li D, et al. Recent advances on immunosensors for mycotoxins in foods and other commodities. TrAC, Trends Anal Chem. 2021;136:116193. doi: 10.1016/J.TRAC.2021.116193. [DOI] [Google Scholar]
- 35.Harshavardhan S, Rajadas SE, Vijayakumar KK, Durai WA, Ramu A, Mariappan R.Electrochemical immunosensorsvols. Bioelectrochemical Interface Engineering. 2019. pp. 343–69. [DOI]
- 36.Kelly HG, Kent SJ, Wheatley AK.Immunological basis for enhanced immunity of nanoparticle vaccines. 2019. [DOI] [PubMed]
- 37. Fernández M, Orozco J. Advances in functionalized photosensitive polymeric nanocarriers. Polymers. 2021;13:2464. doi: 10.3390/POLYM13152464. . 2021;13:2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rovina K, Shaeera SN, Vonnie JM, Yi SX. Recent biosensors technologies for detection of mycotoxin in food products. Mycotox Food Saf. 2019 doi: 10.5772/INTECHOPEN.89022. [DOI] [Google Scholar]
- 39. Rahman HU, Yue X, Yu Q, Xie H, Zhang W, Zhang Q, et al. Specific antigen-based and emerging detection technologies of mycotoxins. J Sci Food Agric. 2019;99:4869–77. doi: 10.1002/JSFA.9686. [DOI] [PubMed] [Google Scholar]
- 40. Nolan P, Auer S, Spehar A, Elliott CT, Campbell K. Current trends in rapid tests for mycotoxins. 2019;36:800–14. doi: 10.1080/19440049.2019.1595171. [DOI] [PubMed] [Google Scholar]
- 41. Zong C, Jiang F, Wang X, Li P, Xu L, Yang H. Imaging sensor array coupled with dual-signal amplification strategy for ultrasensitive chemiluminescence immunoassay of multiple mycotoxins. Biosens Bioelectron. 2021;177:112998. doi: 10.1016/J.BIOS.2021.112998. [DOI] [PubMed] [Google Scholar]
- 42. Fu Z, Wang K, Zou B. Recent advances in organic pressure-responsive luminescent materials. Chin Chem Lett. 2019;30:1883–94. doi: 10.1016/J.CCLET.2019.08.041. [DOI] [Google Scholar]
- 43. Calabretta MM, Zangheri M, Calabria D, Lopreside A, Montali L, Marchegiani E, et al. Paper-based immunosensors with bio-chemiluminescence detection. Sensors. 2021;21:4309. doi: 10.3390/S21134309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang X, Wang Z, Fang Y, Sun R, Cao T, Paudyal N, et al. Antibody microarray immunoassay for simultaneous quantification of multiple mycotoxins in corn samples. Toxins. 2018;10:415. doi: 10.3390/TOXINS10100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zong C, Jiang F, Wang X, Li P, Xu L, Yang H. Imaging sensor array coupled with dual-signal amplification strategy for ultrasensitive chemiluminescence immunoassay of multiple mycotoxins. Biosens Bioelectron. 2021;177:112998. doi: 10.1016/J.BIOS.2021.112998. [DOI] [PubMed] [Google Scholar]
- 46. Freitas A, Barros S, Brites C, Barbosa J, Silva AS. Validation of a biochip chemiluminescent immunoassay for multi-mycotoxins screening in maize (Zea mays L.) Food Anal Methods. 2019;12:2675–84. doi: 10.1007/S12161-019-01625-1. [DOI] [Google Scholar]
- 47. Calabretta MM, Zangheri M, Calabria D, Lopreside A, Montali L, Marchegiani E, et al. Paper-based immunosensors with bio-chemiluminescence detection. Sensors. 2021;21:4309. doi: 10.3390/S21134309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hendrickson OD, Taranova NA, Zherdev Av, Dzantiev BB, Eremin SA. Fluorescence polarization-based bioassays: new horizons. Sensors. 2020;20:7132. doi: 10.3390/S20247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hofmann A, Schmid M, Brütting W. The many facets of molecular orientation in organic optoelectronics. Adv Opt Mater. 2021;9:2101004. doi: 10.1002/ADOM.202101004. [DOI] [Google Scholar]
- 50. Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in analysis and detection of major mycotoxins in foods. Foods. 2020;9:518. doi: 10.3390/FOODS9040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rahman HU, Yue X, Yu Q, Xie H, Zhang W, Zhang Q, et al. Specific antigen-based and emerging detection technologies of mycotoxins. J Sci Food Agric. 2019;99:4869–77. doi: 10.1002/JSFA.9686. [DOI] [PubMed] [Google Scholar]
- 52. Lippolis V, Porricelli ACR, Mancini E, Ciasca B, Lattanzio VMT, de Girolamo A, et al. Fluorescence polarization immunoassay for the determination of T-2 and HT-2 toxins and their glucosides in wheat. Toxins. 2019;11:380. doi: 10.3390/TOXINS11070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chang X, Zhang Y, Liu H, Tao X. A quadruple-label time-resolved fluorescence immunochromatographic assay for simultaneous quantitative determination of three mycotoxins in grains. Anal Methods. 2020;12:247–54. doi: 10.1039/C9AY02309G. [DOI] [Google Scholar]
- 54. Liu Z, Hua Q, Wang J, Liang Z, Li J, Wu J, et al. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens Bioelectron. 2020:158. doi: 10.1016/J.BIOS.2020.112178. [DOI] [PubMed] [Google Scholar]
- 55.Lin X, Yu W, Tong X, Li C, Duan N, Wang Z, et al. Application of nanomaterials for coping with mycotoxin contamination in food safety: from detection to control. 2022. [DOI] [PubMed]
- 56. Qu H, Qu B, Cheng J, Zhang Y, Zeng W, Wang Q, et al. Development of a one-step lateral flow immunoassay for rapid detection of icariin. Curr Pharmaceut Anal. 2016;14 doi: 10.2174/1573412913666161214125948. [DOI] [Google Scholar]
- 57. Zhang J, Yu Q, Qiu W, Li K, Qian L, Zhang X, et al. Gold-platinum nanoflowers as a label and as an enzyme mimic for use in highly sensitive lateral flow immunoassays: application to detection of rabbit IgG. Microchim Acta. 2019;186:6. doi: 10.1007/S00604-019-3464-Z. . 2019;186:1-9. [DOI] [PubMed] [Google Scholar]
- 58.Asghari S, Ekrami E, Barati F, Avatefi M, Mahmoudifard M.The role of the nanofibers in lateral flow assays enhancement: a critical review. 2022. [DOI]
- 59. Gandhi K, Sharma N, Gautam PB, Sharma R, Mann B, Pandey V. Lateral Flow Assay. 2022:249–70. doi: 10.1007/978-1-0716-1940-7_12. [DOI] [Google Scholar]
- 60. di Nardo F, Alladio E, Baggiani C, Cavalera S, Giovannoli C, Spano G, et al. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta. 2019;192:288–94. doi: 10.1016/J.TALANTA.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 61. Charlermroj R, Phuengwas S, Makornwattana M, Sooksimuang T, Sahasithiwat S, Panchan W, et al. Development of a microarray lateral flow strip test using a luminescent organic compound for multiplex detection of five mycotoxins. Talanta. 2021;233:122540. doi: 10.1016/J.TALANTA.2021.122540. [DOI] [PubMed] [Google Scholar]
- 62. Zhang W, Tang S, Jin Y, Yang C, He L, Wang J, et al. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J Hazard Mater. 2020:393. doi: 10.1016/J.JHAZMAT.2020.122348. [DOI] [PubMed] [Google Scholar]
- 63. Zhou S, Xu L, Kuang H, Xiao J, Xu C. Immunoassays for rapid mycotoxin detection: state of the art. Analyst. 2020;145:7088–102. doi: 10.1039/D0AN01408G. [DOI] [PubMed] [Google Scholar]
- 64. Chen X, Wu H, Tang X, Zhang Z, Li P. Recent advances in electrochemical sensors for mycotoxin detection in food. Electroanalysis. 2021 doi: 10.1002/ELAN.202100223. [DOI] [Google Scholar]
- 65. Erkmen C, Unal DN, Kurbanoglu S, Uslu B. Basics of electrochemical sensors. Eng Mater. 2022:81–99. doi: 10.1007/978-3-030-98021-4_5/COVER. [DOI] [Google Scholar]
- 66. Chen X, Wu H, Tang X, Zhang Z, Li P. Recent advances in electrochemical sensors for mycotoxin detection in food. Electroanalysis. 2021 doi: 10.1002/ELAN.202100223. [DOI] [Google Scholar]
- 67. Kim DM, Go MJ, Lee J, Na D, Yoo SM. Recent advances in micro/nanomaterial-based aptamer selection strategies. Molecules. 2021;26:5187. doi: 10.3390/MOLECULES26175187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu L, Seenivasan R, Wang YC, Yu JH, Gunasekaran S. An electrochemical immunosensor for rapid and sensitive detection of mycotoxins fumonisin B1 and deoxynivalenol. Electrochim Acta. 2016;213:89–97. doi: 10.1016/J.ELECTACTA.2016.07.096. [DOI] [Google Scholar]
- 69. Wei T, Ren P, Huang L, Ouyang Z, Wang Z, Kong X, et al. Simultaneous detection of aflatoxin B1, ochratoxin A, zear-alenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019;300:125176. doi: 10.1016/J.FOODCHEM.2019.125176. [DOI] [PubMed] [Google Scholar]
- 70. Lu L, Gunasekaran S. Dual-channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta. 2019;194:709–16. doi: 10.1016/J.TALANTA.2018.10.091. [DOI] [PubMed] [Google Scholar]
- 71. Evtugyn G, Porfireva A, Kulikova T, Hianik T. Recent achievements in electrochemical and surface plasmon resonance aptasensors for mycotoxins detection. Chemosensors. 2021;9:180. doi: 10.3390/CHEMOSENSORS9070180. [DOI] [Google Scholar]
- 72. Zhao X, Wise MA, Waldhoff ST, Kyle GP, Huster JE, Ramig CW, et al. The impact of agricultural trade approaches on global economic modeling. Global Environ Change. 2022;73:102413. doi: 10.1016/J.GLOENVCHA.2021.102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Perrone G, Ferrara M, Medina A, Pascale M, Magan N. Toxigenic fungi and mycotoxins in a climate change scenario: ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms. 2020;8:1496. doi: 10.3390/MICROORGANISMS8101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. ting Yan X, Zhang Y, Zhou Y, hui Li G, song Feng X. Technical overview of orbitrap high resolution mass spectrometry and its application to the detection of small molecules in food (update since 2012) 2020;52:593–626. doi: 10.1080/10408347.2020.1815168. [DOI] [PubMed] [Google Scholar]
- 75. Kenngott KGJ, Albert J, Meyer-Wolfarth F, Schaumann GE, Muñoz K. Fusarium mycotoxins in maize field soils: method validation and implications for sampling strategy. Toxins. 2022;14:130. doi: 10.3390/TOXINS14020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Steiner D, Humpel A, Stamminger E, Schoeberl A, Pachschwoell G, Sloboda A, et al. An interlaboratory comparison study of regulated and emerging mycotoxins using liquid chromatography mass spectrometry: challenges and future directions of routine multi-mycotoxin analysis including emerging mycotoxins. Toxins. 2022;14:405. doi: 10.3390/TOXINS14060405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kizis D, Vichou AE, Natskoulis PI. Recent advances in mycotoxin analysis and detection of mycotoxigenic fungi in grapes and derived products. Sustainability. 2021;13:2537. doi: 10.3390/SU13052537. [DOI] [Google Scholar]
- 78.Burczynski ME.An introduction to toxicogenomics. 2003. [DOI]
- 79.Koedrith P, Kim HL, Weon J, Il, Seo YR.Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int J Hyg Environ Health. 2013. [DOI] [PubMed]
- 80. Guillemin N, Horvatić A, Kuleš J, Galan A, Mrljak V, Bhide M. Omics approaches to probe markers of disease resistance in animal sciences. Mol Biosyst. 2016 doi: 10.1039/c6mb00220j. [DOI] [PubMed] [Google Scholar]
- 81.Horgan RP, Kenny LC, Mrcpi M, Phd M.SAC review “omic” technologies: genomics, transcriptomics, proteomics and metabolomics clinical Research fellow and specialist registrar in obstetrics and gynaecology. Obstetrician & Gynaecologist. 2011. [DOI]
- 82. Misra BB, Langefeld C, Olivier M, Cox LA. Integrated omics: tools, advances and future approaches. J Mol Endocrinol. 2018 doi: 10.1530/jme-18-0055. [DOI] [PubMed] [Google Scholar]
- 83.Bell M, Blais JM.Omics” workflow for paleolimnological and geological archives: a review. Science of the Total Environment. 2019. [DOI] [PubMed]
- 84. Yang Z, Xue KS, Sun X, Williams PL, Wang JS, Tang L. Toxicogenomic responses to zearalenone in Caenorhabditis elegans reveal possible molecular mechanisms of reproductive toxicity. Food Chem Toxicol. 2018;122:49–58. doi: 10.1016/J.FCT.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 85. Liew WPP, Sabran MR, Than LTL, Abd-Ghani F. Metagenomic and proteomic approaches in elucidating aflatoxin B1 detoxification mechanisms of probiotic Lactobacillus casei Shirota towards intestine. Food Chem Toxicol. 2022;160:112808. doi: 10.1016/J.FCT.2022.112808. [DOI] [PubMed] [Google Scholar]
- 86. Ji J, Zhu P, Cui F, Pi F, Zhang Y, Li Y, et al. The antagonistic effect of mycotoxins deoxynivalenol and zearalenone on metabolic profiling in serum and liver of mice. Toxins. 2017;9:28. doi: 10.3390/TOXINS9010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gao Y, Ye Q, Bao X, Huang X, Wang J, Zheng N. Transcriptomic and proteomic profiling reveals the intestinal immunotoxicity induced by aflatoxin M1 and ochratoxin A. Toxicon. 2020;180:49–61. doi: 10.1016/J.TOXICON.2020.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.