Abstract
Objective:
Little is known about how changes in psychosocial factors impact changes in pain outcomes among patients with cancer and chronic pain. This longitudinal cohort study of cancer patients investigated the relationships between changes in psychosocial factors and changes in pain severity and interference over time.
Methods:
Data from patients with cancer and chronic pain (n = 316) treated at a tertiary pain clinic were prospectively collected. At their baseline visit (Time 1), patients provided demographic and clinical information, and completed validated psychosocial and pain assessments. Psychosocial and pain assessments were repeated at a follow-up visit (Time 2), on average 4.9 months later. Change scores (Time 2-Time 1) were computed for psychosocial and pain variables. Multivariable hierarchical linear regressions assessed the associations between changes in psychosocial factors with changes in pain outcomes over time.
Results:
Participants were an average age of 59 years, were 61% female, and 69% White. Overall, a decrease in pain severity (p ≤ 0.001), but not pain interference, was observed among the group over time. In multivariable analyses, increased pain catastrophizing was significantly associated with increased pain severity over time (β = 0.24, p ≤ 0.001). Similarly, increased pain catastrophizing (β = 0.21, p ≤ 0.001) and increased depression (β = 0.20, p ≤ 0.003) were significantly associated with increased pain interference over time. Demographic and clinical characteristics were not significantly related to changes in pain outcomes.
Conclusions:
Increased pain catastrophizing was uniquely associated with increased chronic pain severity and interference. Our findings indicate that cancer patients with chronic pain would likely benefit from the incorporation of nonpharmacological interventions, simultaneously addressing pain and psychological symptoms.
Keywords: cancer, cancer pain, Collaborative health outcomes information registry (CHOIR), depression, oncology, pain catastrophizing, psychosocial
1 |. INTRODUCTION
Pain persists as one of the most commonly reported symptoms among patients with cancer, with approximately 40%–90% reporting pain-related symptoms.1 Pain often results from tumor progression or from cancer treatment.1,2 Psychosocial factors (e.g., depression) predating one’s cancer experience, or symptoms following diagnosis and treatment, have also shown to contribute to cancer-related pain.3,4 If left undertreated, cancer-related pain can become chronic and has shown to negatively affect adherence to cancer treatments and quality of life, and even a desire for hastened death.5 Life expectancy is increasing along with the aging population, resulting in an increased prevalence of cancer.6 Concurrently, as cancer treatments improve, the number of cancer survivors has increased, along with the number of survivors living with chronic cancer-related pain.7,8 Understanding how patient-specific characteristics and changes in psychosocial factors across time contribute to worsened pain outcomes among patients with cancer is critical to identify those at highest risk of pain chronification so that targeted treatment efforts reach those with the greatest need.
While pharmacologic management of cancer pain is effective for the majority of patients, a subset of patients report continued pain due to inadequate pain management or they experience co-occurring chronic pain disorders, resulting in an increased risk for experiencing psychological distress.9,10 The biopsychosocial model of chronic pain11 offers a theoretical framework whereby differences in psychological and social factors, in addition to biological factors, contribute to the development and maintenance of pain. Indeed, psychological factors have shown to affect a patient’s reported pain, such that greater psychological distress and pain catastrophizing (negative, maladaptive pain-related cognitions including the magnification of, rumination about, and feeling helpless in the face of pain) are correlated with greater pain.10 Non-pharmacological interventions, such as behavioral and psychological treatments (e.g., cognitive behavioral therapy), have demonstrated to be important in conjunction with pharmacologic treatments, as well as stand-alone options, for reducing chronic malignant and non-malignant pain.3,10,12,13 Given the complexities of cancer pain, a multidimensional approach for proper pain assessment and management is necessary, with a particular focus on the role of psychosocial factors.9
Understanding how psychosocial factors change during cancer and affect chronic pain outcomes may also help to identify potential interventional targets. The collaborative health outcomes information registry (CHOIR) is a learning health care system that longitudinally collects multiple dimensions of physical, psychological, and social factors of patients who are receiving care at a large, tertiary pain management center. The CHOIR system uses validated questionnaires, including Patient-Reported Outcomes Measurement Information System (PROMIS) measures14 and legacy instruments. We previously conducted a cross-sectional analysis identifying several biopsychosocial factors that were associated with greater pain severity and interference among patients with chronic pain and cancer receiving pain care using the CHOIR system.15 This initial investigation highlighted how several modifiable psychological factors, including depression, sleep disturbance, and pain catastrophizing, were associated with worse pain, providing potential intervention targets to improve pain among patients with cancer. However, this analysis was conducted at one timepoint, and patients may experience fluctuating symptoms throughout cancer. Exploring how changes in psychosocial factors are associated with changes in chronic pain outcomes over time may reveal new opportunities to intervene. The goal of the present study was to expand upon our prior findings by evaluating how patient-specific characteristics and longitudinal changes in psychosocial factors contributed to changes in pain severity and pain interference in this sample of cancer patients with chronic pain.
2 |. MATERIALS AND METHODS
2.1 |. Procedures
This was a retrospective, longitudinal study. The study sample included patients who completed a baseline survey (Time 1) prior to or at receipt of an initial medical evaluation at the Stanford Pain Management Center in Redwood City, California, as well as a follow-up visit (Time 2 survey). CHOIR is an open-source learning health care system platform (http://choir.stanford.edu) and integrates computerized adaptive testing, which allows for fewer items to be tested and yields greater precision in domain assessment. This system also affords the ability to comprehensively evaluate biopsychosocial patient-reported outcomes for pain disorders in real-world clinical samples.16,17 The CHOIR survey is completed at home via a secure email link up to 1 week before the initial visit or on a tablet during check-in at the initial visit. The self-reported questionnaires measure patients’ demographic and clinical characteristics (at baseline), medication use (at baseline), and psychosocial factors and pain symptoms (at both visits). Study procedures, which involved exclusively retrospective review of clinical data, were approved by the Institutional Review Board at the Stanford University School of Medicine under a protocol (IRB#28453).
We identified 841 patients with a cancer diagnosis identified through International Classification of Diseases, Ninth Revision (ICD-9) claim from the CHOIR dataset. Patients (n = 141) diagnosed with early stage nonmelanoma skin cancer, thyroid cancer, or carcinoma in situ were excluded because these conditions are uncommon causes of significant cancer-related pain. 700 patients completed the initial, baseline survey (T1). 316 patients completed the follow-up (T2) assessments and were used in the main analyses. Many patients travel from distant states/countries to attend Stanford’s pain clinic for an initial consultation to take recommendations back to their primary care team and therefore are not feasibly able to engage in longitudinal care.
2.2 |. Measures
Pain symptoms. Patients self-reported their average pain severity in the previous 7 days using a numeric rating scale (0 = no pain to 10 = worst pain imaginable) at both visits.18 The PROMIS Pain Interference scale14 was used to assess clinical pain interference in daily activities at both visits.
Psychosocial factors. The PROMIS14 measures were used to assess anxiety and depressive symptomology, sleep disturbance, and emotional support at both visits. The PROMIS measures have been used in prior samples of patients with cancer.15 Each assessment was standardized with a t-score of 50 to represent the population mean (SD = 10; range = 0–100). The 13-item Pain Catastrophizing Scale19 was used to assess magnification of, feeling helpless about, and rumination on pain at both visits, and has been validated in pain and control samples.20
Demographic and clinical characteristics. Self-reported demographic information included age, gender, race/ethnicity, and education at baseline. Using electronic medical records and the ICD-9 codes in medical claims (2016–2019), we extracted patients’ cancer types. Based on published categories for cancer diagnosis,21 we classified ICD-9 codes and characterized patients’ cancer as ‘poor prognosis’ if the code matched a cancer frequently diagnosed at an advanced stage and/or with a high mortality rate (e.g., pancreatic cancer, lung cancer, esophagogastric cancer, or acute myeloid leukemia) or if a non-lymphatic metastatic code was present (1960–1991; 20,970–20979).22–24 Patients self-reported opioid use before their initial appointment by answering “Are you currently taking any opioid medications? (Vicodin, Oxycontin, oxycodone, methadone, morphine, MS-Contin, codeine, Actiq, Duragesic, Dilaudid, Percocet, Opana, Nucynta, Stadol, Ultram, and Norco)”. Responses (0 = no, 1 = yes) were recorded for each medication separately. At the initial visit, patients self-reported previous or current pain interventions/treatments received, although whether interventions specifically targeted cancer pain is unknown (Supplemental Table S1).
2.3 |. Statistical analysis
Paired samples t-tests were used to examine changes in pain and psychosocial factors from Time 1 (baseline) to Time 2 (follow-up visit). Change scores for pain and psychosocial factors were calculated by subtracting scores at T1 from scores at T2, such that positive scores indicated an increase in that variable over time, and negative scores indicated a decrease. Associations between predictor variables (demographic and clinical characteristics, changes in psychosocial factors) and outcome variables (changes in pain severity and pain interference) were evaluated using univariable and multivariable linear regression models. We evaluated the associations between demographics, clinical characteristics, and changes in psychosocial factors with changes in pain severity and interference using simple linear regressions to individually assess the relationships of each predictor variable with the outcome variables (Supplemental Table S2). Hierarchical multivariable models were used to examine the variance in change in pain outcomes accounted for by our variables of interest, changes in psychosocial factors, after controlling for the impact of demographics, clinical characteristics, and the variability of time between assessments T1 and T2 (step 1: Demographics, clinical characteristics, and time between assessments, step 2: Changes in psychosocial factors). There were no violations of multicollinearity (VIFs ≤1.78, Tolerances ≥0.56).25,26 All statistical analyses were performed using SPSS v28.
3 |. RESULTS
3.1 |. Cohort characteristics
Patients were on average 59.1 years (SD = 15.1, range = 19–92), 61% female (n = 192), and 69% White (n = 214) (Table 1). On average, 4.9 months elapsed between the baseline and follow-up visits (SD = 7.1 months, median = 2 months, range = 0–42 months), although time varied across patients. In total, 53% (n = 166) had a poor prognosis, 14% (n = 44) had hematologic malignancies, and 60% (n = 188) reported using prescription opioids at baseline.
TABLE 1.
Demographic and clinical characteristics
| N (%) | |
|---|---|
| Age, mean (SD), years | 59.1 (15.1) |
| Sex | |
| Male | 124 (39%) |
| Female | 192 (61%) |
| Education | |
| ≤High school | 38 (12%) |
| Some college | 98 (31%) |
| Bachelor’s degree | 77 (24%) |
| Master’s degree | 57 (18%) |
| Doctorate | 41 (13%) |
| Race/ethnicity | |
| White | 214 (69%) |
| African American | 7 (2%) |
| American Indian/Alaska Native | 4 (1%) |
| Asian | 37 (12%) |
| Another race | 14 (5%) |
| Hispanic | 33 (11%) |
| Prognosis | |
| Poor prognosis | 166 (53%) |
| Hematologic malignancies | 44 (14%) |
| Opioid use | 188 (60%) |
| Time between surveys, mean (SD), months | 4.9 (7.1) |
3.2 |. Changes in psychosocial factors and pain symptoms
At baseline (T1), patients reported a mean pain severity score of 5.58 (SD = 2.19, range = 0–10) and a mean pain interference t-score score of 63.53 (SD = 6.11, range = 38–80). At follow-up (T2), patients reported a mean pain severity score of 5.02 (SD = 2.35, range = 0–10)and a mean pain interference t-score of 63.37 (SD = 7.63, range = 38–83). Over time, there was a significant decrease in pain severity (t (315) = 4.90, p ≤ 0.001), but no significant change in pain interference over time was observed (t (315) = 0.40, p = 0.69) (Table 2, Figure 1).
TABLE 2.
Changes in pain and psychosocial factors over time
| Time 1 | Time 2 | t-test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M(SD) | Median | Range | M(SD) | Median | Range | P | t | Effect size [95% CI] | |
| Changes in pain | |||||||||
| Pain severity | 5.58 (2.19) | 6.00 | 0–10 | 5.02 (2.35) | 5.00 | 0–10 | <0.001 | 4.90 | 0.28 [0.16, 0.39] |
| Pain interference | 63.53 (6.11) | 64.00 | 38–80 | 63.37 (7.63) | 64.00 | 38–83 | 0.69 | 0.40 | 0.02 [−0.09, 0.13] |
| Changes in psychosocial factors | |||||||||
| Depression | 52.81 (9.69) | 53.00 | 34–84 | 54.50 (9.79) | 55.00 | 34–84 | <0.001 | −3.61 | −0.20 [−0.31, −0.09] |
| Anxiety | 54.20 (9.23) | 55.00 | 32–84 | 55.87 (9.67) | 57.00 | 32–84 | <0.001 | −3.84 | −0.22 [−0.33, −0.10] |
| Sleep disturbance | 55.99 (9.01) | 56.00 | 26–83 | 55.24 (9.62) | 55.00 | 26–83 | 0.10 | 1.65 | 0.09 [−0.02, 0.20] |
| Pain catastrophizing | 21.03 (12.20) | 20.00 | 0–52 | 18.11 (12.88) | 16.00 | 0–52 | <0.001 | 5.13 | 0.29 [0.18, 0.40] |
| Emotional support | 50.25 (9.57) | 50.00 | 25–66 | 50.56 (9.89) | 48.00 | 20–66 | 0.50 | −0.67 | −0.04 [−0.15, 0.07] |
Note: PROMIS = pain interference(range: 0–100), depression(range: 0–100), anxiety(range: 0–100), sleep disturbance(range: 0–100), and emotional support(range: 0–100); Pain Catastrophizing Scale = pain catastrophizing(range: 0–52).
FIGURE 1.
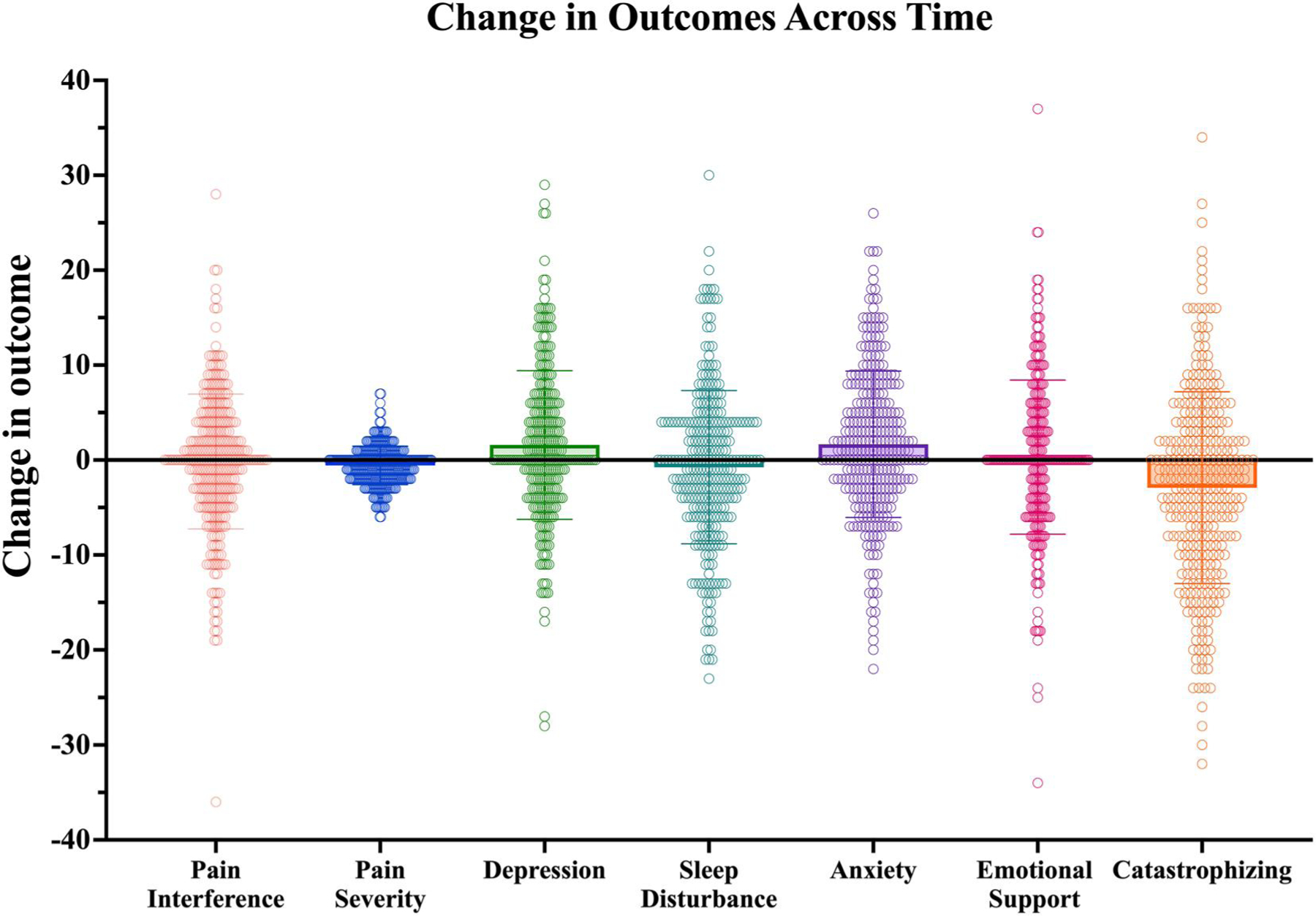
Change scores for pain outcomes and psychosocial predictors. Possible ranges for each variable: pain interference (range: 0–100); pain severity (range: 0–10); depression (range: 0–100); sleep disturbance (range: 0–100); anxiety (range: 0–100); emotional support (range: 0–100); catastrophizing (range: 0–52)
Interestingly, pain catastrophizing significantly decreased over time (p ≤ 0.001), whereas symptoms of depression and anxiety significantly (p ≤ 0.001) increased over time (Table 2, Figure 1). No significant change in sleep disturbance or emotional support was observed over time (p ≥ 0.05).
3.3 |. Predicting change in pain severity
Change scores for pain outcomes and psychosocial factors, which were calculated by subtracting scores at T1 from scores at T2 (positive scores indicate an increase in that variable over time, negative scores indicate a decrease), were used to explore univariable associations among psychosocial factors and pain. Univariable analysis indicated that demographic and clinical characteristics were not significantly associated with the directionality or extent of change in pain severity between visits (p ≥ 0.05) (Supplemental Table S2). Changes in psychological factors including increased depression, anxiety, sleep disturbance, and pain catastrophizing were significantly associated with increased pain severity (βs = 0.14–0.29, p ≤ 0.001). Change in emotional support was not significantly related to change in pain severity (p ≥ 0.05).
Model 1 of the hierarchical linear regression (Table 3), including only demographics, clinical characteristics, and time between assessments, did not significantly explain change in pain severity (F (8, 302) = 1.12, R2 = 0.029, p = 0.348). Model 2, which added changes in psychosocial factors, significantly predicted change in pain severity (F (13, 297) = 5.62, R2 = 0.113, p < 0.001). Adding changes in psychosocial factors to the model accounted for an additional 8.4% of the unique variance in change in pain severity (p < 0.001), relative to only taking demographics, clinical characteristics, and time between assessments into account. In the multivariable analysis, increased pain catastrophizing over time was the sole unique, significant predictor of increased pain severity (β = 0.24, p ≤ 0.001). Demographic and clinical characteristics were not significantly associated with change in pain severity, nor were changes in depression, anxiety, sleep disturbance, or emotional support (p ≥ 0.05).
TABLE 3.
Multivariable linear regressions of changes in pain severity and pain interference
| Change in pain severity | Change in pain interference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Unstandardized coefficient B (95% Cl) | Standardized β | R 2 | F-change | P | Unstandardized coefficient B (95% Cl) | Standardized β | R 2 | F-change | P |
| Model 1 | 0.029 | 1.12 | 0.348 | 0.039 | 1.53 | 0.156 | ||||
| Age | −0.01 (−0.02, 0.01) | −0.06 | 0.305 | 0.04 (−0.01, 0.10) | 0.09 | 0.137 | ||||
| Female gender | −0.44 (−0.92, 0.03) | −0.11 | 0.068 | 0.60 (−1.05, 2.25) | 0.04 | 0.474 | ||||
| White race | −0.17 (−0.69, 0.35) | −0.04 | 0.523 | −1.75 (−3.54, 0.05) | −0.11 | 0.056 | ||||
| College degree | −0.18 (−0.65, 0.29) | −0.04 | 0.446 | −1.38 (−2.99, 0.23) | −0.10 | 0.092 | ||||
| Prognosis hematologic | −0.28 (−1.02, 0.45) | −0.05 | 0.447 | −1.47 (−4.02, 1.07) | −0.07 | 0.256 | ||||
| Poor prognosis | −0.24 (−0.75, 0.28) | −0.06 | 0.372 | −0.60 (−2.38, 1.19) | −0.04 | 0.513 | ||||
| Opioid use | 0.39 (−0.09, 0.87) | 0.10 | 0.109 | 0.90 (−0.76, 2.57) | 0.06 | 0.286 | ||||
| Time | 0.01 (−0.02, 0.05) | 0.04 | 0.461 | 0.07 (−0.05, 0.18) | 0.07 | 0.241 | ||||
| Model 2 | 0.113 | 5.62 | <0.001 | 0.226 | 14.40 | <0.001 | ||||
| Demographic and Clinical Characteristics | ||||||||||
| Age | −0.01 (−0.02, 0.01) | −0.05 | 0.360 | 0.04 (−0.02, 0.08) | 0.07 | 0.175 | ||||
| Female gender | −0.33 (−0.79, 0.14) | −0.08 | 0.169 | 1.05 (−0.47, 2.56) | 0.07 | 0.174 | ||||
| White race | −0.06 (−0.57, 0.44) | −0.02 | 0.801 | −1.50 (−3.13, 0.13) | −0.10 | 0.070 | ||||
| College degree | −0.16 (−0.62, 0.29) | −0.04 | 0.474 | −1.01 (−2.48, 0.46) | −0.07 | 0.175 | ||||
| Prognosis hematologic | −0.09 (−0.81, 0.62) | −0.02 | 0.798 | −0.45 (−2.77, 1.88) | −0.02 | 0.706 | ||||
| Poor prognosis | −0.16 (−0.66, 0.34) | −0.04 | 0.534 | −0.50 (−2.14, 1.14) | −0.04 | 0.547 | ||||
| Opioid use | 0.35 (−0.13, 0.82) | 0.08 | 0.151 | 0.99 (−0.54, 2.52) | 0.07 | 0.203 | ||||
| Time | 0.01 (−0.02, 0.04) | 0.04 | 0.528 | 0.02 (−0.08, 0.13) | 0.02 | 0.660 | ||||
| Changes in Psychosocial actors | ||||||||||
| Depression | 0.00 (−0.04, 0.04) | 0.01 | 0.913 | 0.18 (0.06, 0.31) | 0.20 | 0.003 | ||||
| Anxiety | 0.00 (−0.03, 0.04) | 0.02 | 0.824 | 0.09 (−0.04, 0.21) | 0.09 | 0.163 | ||||
| Sleep disturbance | 0.02 (−0.01, 0.05) | 0.06 | 0.287 | 0.09 (−0.01, 0.18) | 0.10 | 0.071 | ||||
| Pain catastrophizing | 0.05 (0.02, 0.07) | 0.24 | <0.001 | 0.15 (0.06, 0.23) | 0.21 | <0.001 | ||||
| Emotional support | −0.02 (−0.05, 0.01) | −0.08 | 0.195 | 0.09 (−0.00, 0.18) | 0.10 | 0.056 | ||||
Note: PROMIS = pain interference, depression, anxiety, sleep disturbance, and emotional support; Pain Catastrophizing Scale = pain catastrophizing; Time = time between the initial and follow-up visits.
3.4 |. Predicting change in pain interference
Univariable analysis indicated that demographic and clinical characteristics were not significantly associated with change in pain interference (p ≥ 0.05). Changes in psychological factors including increased depression, anxiety, sleep disturbance, and pain catastrophizing were significantly associated with increased pain interference (βs = 0.22–0.36, p ≤ 0.001). Change in emotional support was not significantly related to change in pain interference (p ≥ 0.05).
Model 1 of the hierarchical linear regression (Table 3), including only demographics, clinical characteristics, and time between assessments, did not significantly predict change in pain interference (F (8, 302) = 1.53, R2 = 0.39, p = 0.146). Model 2, which added changes in psychosocial factors, significantly predicted change in pain interference (F (13, 297) = 14.40, R2 = 0.226, p < 0.001). Adding changes in psychosocial factors to the model accounted for an additional 18.7% of the unique variance in pain interference (p < 0.001). Increased pain catastrophizing (β = 0.21, p ≤ 0.001) and increased depression (β = 0.20, p ≤ 0.003) were the only two significant predictors of increased pain interference. Demographic and clinical characteristics were not significantly associated with change in pain interference, nor were changes in anxiety, sleep disturbance, or emotional support (p ≥ 0.05).
4 |. DISCUSSION
In this retrospective, longitudinal study of cancer patients with chronic pain, we sought to identify how changes in biopsychosocial factors were associated with changes in pain severity and pain interference over time. Changes in several psychological factors were significantly associated with worsened pain outcomes over time, including increased pain catastrophizing, depression, anxiety, and sleep disturbance. Demographic and clinical characteristics were unrelated to changes in pain severity and interference. Notably, when changes in all biopsychosocial variables were simultaneously included in the models, increased pain catastrophizing was the sole psychosocial variable consistently associated with increased pain severity and interference over time. These findings highlight the importance of understanding and considering changes in pain-specific psychological processing of pain. Specifically, increases in catastrophic thinking about pain was associated with both worse reported severity and interference of that pain. This association suggests that symptoms of pain catastrophizing may be particularly important targets for pain treatment among patients with cancer.
Patients’ pain severity significantly decreased from T1 to T2, but interestingly there was not a significant change in pain interference over time. Although pain catastrophizing significantly decreased over time, and depression and anxiety symptoms increased, these psychological symptoms were still greatly predictive of worsened pain outcomes between visits. This also suggests that while patients may have experienced less severe levels of pain over time, possibly due to multidimensional pain treatment received during visits, this decrease in pain severity did not positively impact their ability to engage in meaningful daily activities, possibly explained by elevated depressive and anxiety symptoms. Alternatively, there may not have been enough time between the two survey assessments for a true treatment effect to occur for pain interference.
Across both pain outcomes, change in pain catastrophizing emerged as the only significant, independent psychosocial predictor in multivariable models. While psychological factors have shown to relate to greater pain in prior work including our own,15,27–29 our findings highlight the unique role pain catastrophizing may have in explaining pain changes over time for patients with chronic pain and cancer. Pain catastrophizing is recognized as a maladaptive cognitive-emotional process in response to anticipated or actual pain and is commonly identified as related to three cognitive subtypes including rumination, magnification, and helplessness surrounding the pain experience. While an increase in these negative pain-related cognitions was related to worsened pain outcomes, we are unable to infer the directional relationship of these associations. For example, increases in catastrophizing may have led to increased pain severity and interference, or vice vera.
4.1 |. Clinical implications
To our knowledge, this is one of the first studies to evaluate the relative contributions of changes in psychosocial factors on pain outcomes over time, while accounting for demographic and clinical characteristics, in a large, diverse sample of patients with cancer and chronic pain. Our findings suggest that changes in psychological factors, particularly pain catastrophizing, are important to identify when treating chronic pain in cancer patients. Pain symptoms in the context of cancer can be particularly alarming as they may signify and are often perceived as indicating worsening disease, disease recurrence, or the presence other underlying etiology.30,31 Pharmacologic treatment of cancer pain with opioids remains one of the most common management strategies,9 although some suggest that the long-term effectiveness of opioids is limited.12,32,33 Concurrently, several barriers to adequate cancer pain management persist, including patients intentionally avoiding opioids because of the stigma around them and difficult side effects, or barriers due to physician reluctance.9 Anxiety and depression increased over time in this sample, and although these symptoms did not seem to influence pain intensity or interference over time, this may suggest that patients with cancer have other ongoing, unmet psychological symptoms that could benefit from intervention. Our findings provide evidence for the need for behavioral interventions or other nonpharmacological treatments that target pain catastrophizing and other psychological pain-related symptoms – symptoms opioids cannot and are not meant to treat, to improve pain for those with cancer and chronic pain.34–36
Cancer patients rarely have access to behavioral pain treatment, particularly those that specifically target catastrophizing during and following cancer treatment.3,10 Future research should focus on developing and evaluating brief, accessible psychological pain interventions that target pain catastrophizing in cancer patients, as has been done with non-cancer chronic pain samples.34–36 Tailored interventions that are integrated with patients’ cancer pain care are needed to meet the specific needs of patients with cancer-related pain, in addition to identifying which patients may benefit most from such interventions, and when exactly during one’s cancer trajectory behavioral pain treatment might be most effective.
4.2 |. Study limitations
Several limitations are important to consider. Although increased catastrophizing was associated with greater increases in pain outcomes, we cannot infer causality between the reported associations. We were also unable to distinctly qualify the underlying etiology of patients’ pain in this sample that could be due to either cancer or another co-occurring pain disorder; yet our findings help to illuminate “real world” complications of managing cancer pain – as many patients experience co-occurring chronic pain disorders unrelated to their cancer that further complicate cancer pain treatment.37 In supplemental Table S1, we report on the relatively low treatment rates that patients self-reported receiving prior to completing the baseline survey. Treatments that patients may have received between the baseline and follow-up visits as part of their multidisciplinary pain care is unknown. While pain treatments (e.g., non-opioid pain medications, physical therapy, and psychological evaluations) were available to all patients within this care model, patients often do not receive such treatments between their first few visits as treatment is focused on completing a thorough pain evaluation before providing recommendations. Future studies should aim to distinctly capture treatments for both pain and psychological needs. Additionally, medical reconciliation, or maintaining an accurate medication list for patients, is an ongoing challenge.38 Although we accounted for use of opioids at baseline, we do not have data on the dosage or how usage may have changed over time.
5 |. CONCLUSIONS
The present study highlights the importance of identifying and treating patients’ psychological symptoms, especially pain catastrophizing, when providing chronic pain interventions in the context of cancer. Our findings indicate that cancer patients with chronic pain would likely benefit from the incorporation of nonpharmacological interventions that can simultaneously address pain and psychological symptoms.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by Redlich Pain Endowment (SM) and the National Institutes of Health [R35GM128691 (KLS), K24NS126781 (SM), K24DA053564 (BD) and National Palliative Care Research Center (DRA)]. Study procedures, which involved exclusively retrospective review of clinical data, were approved by the Institutional Review Board at the Stanford University School of Medicine under a protocol (IRB#28453).
Funding information
National Palliative Care Research Center; National Institutes of Health; Redlich Pain Endowment
Footnotes
CONFLICTS OF INTEREST
The authors declare none.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.van den Beuken-van Everdingen M Chronic pain in cancer survivors: a growing issue. J Pain Palliat Care Pharmacother. 2012;26(4):385–387. 10.3109/15360288.2012.734908 [DOI] [PubMed] [Google Scholar]
- 2.Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer. 2011;117(9):1994–2003. 10.1002/cncr.25761 [DOI] [PubMed] [Google Scholar]
- 3.Gorin SS, Krebs P, Badr H, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30(5):539–547. 10.1200/jco.2011.37.0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Psychological distress of patients with advanced cancer: influence and contribution of pain severity and pain interference. Cancer Nurs. 2006;29(5):400–405. 10.1097/00002820-200609000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Paice JA. Cancer pain management: strategies for safe and effective opioid prescribing. J Natl Compr Cancer Netw. 2016;14(5S):695–697. 10.6004/jnccn.2016.0195 [DOI] [PubMed] [Google Scholar]
- 6.den Beuken-van Everdingen V, van Kuijk S, Janssen D, Joosten E. Treatment of pain in cancer: towards personalised medicine. Cancers. 2018;10(12):502. 10.3390/cancers10120502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. WHO Report on Cancer: setting Priorities, Investing Wisely and Providing Care for All; 2020.
- 8.Wild CP, Stewart BW, Wild C. World Cancer Report 2014. World Health Organization; 2014. [Google Scholar]
- 9.Kwon JH. Overcoming barriers in cancer pain management. J Clin Oncol. 2014;32(16):1727–1733. 10.1200/jco.2013.52.4827 [DOI] [PubMed] [Google Scholar]
- 10.Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32(16):1703–1711. 10.1200/jco.2013.54.4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The bio-psychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- 12.Eaton LH, Brant J, McLeod K, Hsing Yeh C. Nonpharmacologic Pain Interventions: a review of evidence-based practices for reducing chronic cancer pain. Clin J Oncol Nurs. 2017;21(3):54–79. 10.1188/17.cjon.s3.54-70 [DOI] [PubMed] [Google Scholar]
- 13.Thomas EM, Weiss SM. Nonpharmacological interventions with chronic cancer pain in adults. Cancer Control. 2000;7(2):157–164. 10.1177/107327480000700206 [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 (Suppl 1)):S3–S11. 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azizoddin DR, Schreiber K, Beck MR, et al. Chronic pain severity, impact, and opioid use among patients with cancer: an analysis of bio-psychosocial factors using the CHOIR learning health care system. Cancer. 2021;127(17):3254–3263. 10.1002/cncr.33645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturgeon JA, Darnall B, Kao M, Mackey S. Physical and psychological correlates of fatigue and physical function: a Stanford-NIH open source pain registry study. J Pain Official J Am Pain Soc. 2015;16(3):291. 10.1016/j.jpain.2015.01.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan JS, Hah JM, Mackey SC. Effects of smoking on patients with chronic pain: a propensity-weighted analysis on the Collaborative Health Outcomes Information Registry. Pain. 2019;160(10): 2374–2379. 10.1097/j.pain.0000000000001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. 10.1016/s0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 19.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 20.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96(3):319–324. 10.1016/s0304-3959(01)00463-8 [DOI] [PubMed] [Google Scholar]
- 21.Allsop MJ, Ziegler LE, Mulvey MR, Russell S, Taylor R, Bennett MI. Duration and determinants of hospice-based specialist palliative care: a national retrospective cohort study. Palliat Med. 2018; 32(8):1322–1333. 10.1177/0269216318781417 [DOI] [PubMed] [Google Scholar]
- 22.Enzinger AC, Ghosh K, Keating NL, Cutler DM, Landrum MB, Wright AA. US Trends and Racial/ethnic Disparities in Opioid Access Among Patients with Poor Prognosis Cancer at the End of Life (EOL). American Society of Clinical Oncology; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA A Cancer J Clin. 2011;61(4):212–236. 10.3322/caac.20121 [DOI] [PubMed] [Google Scholar]
- 24.Obermeyer Z, Powers BW, Makar M, Keating NL, Cutler DM. Physician characteristics strongly predict patient enrollment in hospice. Health Aff. 2015;34(6):993–1000. 10.1377/hlthaff.2014.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayers R Classical and Modern Regression with Applications. PWSKENT Publ. Co.; 1990. [Google Scholar]
- 26.Menard S Applied Logistic Regression Analysis. 106. Sage; 2002. [Google Scholar]
- 27.Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J pain. 2013;14(10):1185–1195. 10.1016/j.jpain.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 28.Schreiber KL, Martel MO, Shnol H, et al. Persistent pain in post-mastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. PAIN®. 2013;154(5):660–668. 10.1016/j.pain.2012.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. J Pain Symptom Manag. 2002;24(5):526–542. 10.1016/s0885-3924(02)00497-9 [DOI] [PubMed] [Google Scholar]
- 30.Hackett J, Godfrey M, Bennett MI. Patient and caregiver perspectives on managing pain in advanced cancer: a qualitative longitudinal study. Palliat Med. 2016;30(8):711–719. 10.1177/0269216316628407 [DOI] [PubMed] [Google Scholar]
- 31.Luigjes-Huizer YL, Tauber NM, Humphris G, et al. What is the prevalence of fear of cancer recurrence in cancer survivors and patients? A systematic review and individual participant data meta-analysis. Psycho Oncol. 2022;31(6):879–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vitzthum LK, Riviere P, Sheridan P, et al. Predicting persistent opioid use, abuse, and toxicity among cancer survivors. JNCI J Natl Cancer Inst. 2020;112(7):720–727. 10.1093/jnci/djz200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah R, Chou L, Kuo Y, Raji MA. Long-term opioid therapy in older cancer survivors: a retrospective cohort study. J Am Geriatr Soc. 2019;67(5):945–952. 10.1111/jgs.15945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darnall BD, Sturgeon J, Kao MC, Hah J, Mackey S. From catastrophizing to recovery: a pilot study of a single-session treatment for pain catastrophizing. J Pain Res. 2014;7:219. 10.2147/jpr.s62329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darnall BD, Roy A, Chen AL, et al. Comparison of a single-session pain management skills intervention with a single-session health education intervention and 8 sessions of cognitive behavioral therapy in adults with chronic low back pain: a randomized clinical trial. JAMA Netw Open. 2021;4(8):e2113401. 10.1001/jamanetworkopen.2021.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziadni MS, Gonzalez-Castro L, Anderson S, Krishnamurthy P, Darnall BD. Efficacy of a single-session “Empowered relief” zoom-delivered group intervention for chronic pain: randomized controlled trial conducted during the COVID-19 pandemic. J Med Internet Res. 2021;23(9):e29672. 10.2196/29672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruppert LM, Cohn ED, Keegan NM, et al. Spine pain and metastatic prostate cancer: defining the contribution of nonmalignant etiologies. JCO Oncol Pract. 2022;18(6):e938–e947. 10.1200/op.21.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose AJ, Fischer SH, Paasche-Orlow MK. Beyond medication reconciliation: the correct medication list. JAMA. 2017;317(20):2057–2058. 10.1001/jama.2017.4628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


