Abstract
Objective: While prolonged sedentary behaviors (SBs) increase cardiovascular disease (CVD) risk, interrupting prolonged sitting (PS) with frequent light exercise reduces arterial functional decline. Skeletal muscle electrical stimulation (EMS) enhances peripheral circulation through passive muscle contraction, suggesting that EMS reduces CVD risk by providing an alternative to active exercise for prolonged SBs. This study aimed to investigate the effects of EMS to skeletal muscles during PS on the endothelial function of the brachial artery (BA). Methods: Study participants included 12 healthy adult men who were subjected to 15 min of supine rest, followed by 1 h of PS only (control [CON] trial), or 20 min of EMS to the lower extremities at 50% of the maximum tolerance intensity during PS (EMS trial). Flow-mediated dilation (FMD) of the BA was measured before and 30 min after PS, and normalized FMD (nFMD) was calculated. Results: The nFMD of the CON trial significantly decreased 30 min after PS completion (6.21% ± 1.13%) compared with that before PS (7.26% ± 0.73%), and there was no significant change in the EMS trial before and after PS. The EMS trial showed a significant increase in the nFMD 30 min after PS completion (1.14 ± 0.77) compared with that before PS (0.84 ± 0.43). However, no significant difference was observed in the CON trials. Conclusion: Passive contraction of the lower extremity muscles by EMS increases BA nFMD, suggesting that prolonged sedentary lower extremity EMS use may reduce the risk of vascular endothelial dysfunction.
Keywords: Electrical muscle stimulation, Endothelial function, Flow-mediated vasodilation, Prolonged sitting
Most international physical activity guidelines recommend that adults engage in at least 150 minutes of moderate- intensity physical activity per week for health promotion and prevention of lifestyle-related diseases1). Those who do not adhere to this recommendation are considered physically inactive, accounting for 27.5% of the population in other countries2). Technological innovations and changes in the work environment, such as the mechanization of work, long hours of TV viewing or desk work, low-intensity physical activity, or sedentary behaviors (SBs), account for most of the waking hours in a day3).
The prevalence of such physical inactivity has been exacerbated by the recent increase in home-based work modules and restrictions on movement due to the COVID-19 pandemic4). Studies on SB have shown that they are directly related to hypertension, obesity5), decreased blood flow to the lower extremities, decreased shear stress (mechanical stress on the vascular wall that reflects vascular endothelial function) resulting in reduced flow-mediated dilation (FMD)6), and an increased risk of atherosclerosis and cardiovascular disease (CVD)7). Furthermore, since prolonged sitting (PS) increases the risk of total mortality, even when the recommended amount of physical activity is met8), it is urgent to promote the primary prevention of SB from the viewpoint of preventive and industrial rehabilitation.
FMD is an indicator of vascular endothelial function and an independent predictor of CVD9). It has been reported that vascular endothelial function is impaired by SB, hypertension, and obesity and can be improved by lifestyle modifications9). Endothelial dysfunction is one of the earliest pathological changes in the etiology of atherosclerosis and is associated with increased cardiovascular risk10,11), a valid and noninvasive physiological indicator widely used as a research tool to quantify endothelial function10). Increased blood flow velocity due to exercise induces mechanical and shear stress in vascular endothelial cells. These cells release endothelium-dependent vasodilators such as nitric oxide (NO), which relaxes vascular smooth muscle, causing vessels to dilate10). The effects of SB on vascular function have been reported previously.
Prolonged SB negatively affects vascular function and the development of CVD; however, interrupting SB with frequent light physical activity has been shown to reduce the risk of CVD. In a previous study, while the endothelial function of the superficial femoral artery decreased after 3 h of SB, this functional decline was suppressed by intervals of physical activity during SB12). Additionally, two physical activity sessions during breaks in prolonged SB significantly reduced FMD of the popliteal artery compared with the condition without physical activity13). However, the physical activities conducted to interrupt PS in these previous studies were intermittent aerobic exercises primarily designed for the lower extremities, involving treadmills, bicycle ergometers, and similar equipment. Additionally, it is difficult for workers who are forced to sit for long periods, such as those working at desks, to incorporate these activities into their daily lives because of the problems in securing the time, places, and equipment to perform the exercises. In contrast, the use of other dynamic exercises, such as electrical stimulation, as an alternative to active exercises may lead to a decrease in vascular endothelial function risk.
Electrical muscle stimulation (EMS) of skeletal muscles has been reported to glucose metabolism and energy consumption14,15) in transient and interventional studies. Similarly, EMS has been shown to activate endothelial nitric oxide synthase (eNOS), a vasodilator; induce endothelium-derived vasodilation; and increase blood flow16). Therefore, it is suggested that EMS during prolonged sedentary activities, as an alternative to active exercises, such as bicycle pedaling and jogging, can reduce the risk of decline in vascular endothelial function.
Therefore, this study aimed to investigate the assumption that EMS of the lower extremity can reduce the endothelial function of the brachial artery (BA) during PS.
Methods
Participants
The study participants were 12 healthy adult men (age: 21.6 ± 1.8 years, height: 173.7 ± 6.6 cm, weight: 67.4 ± 6.2 kg, body mass index: 22.3 ± 1.6 kg/m2) who had never smoked or taken any regular medications. This study was approved by the Research Ethics Committee of the Department of Physical Therapy, Faculty of Health Science, Osaka Yukioka College of Health Science (#33-0005), Japan. Additionally, the participants were provided with an oral explanation of the content and purpose of the study, refusal, withdrawal, and interruption of participation, and the study was initiated with their consent.
Protocol
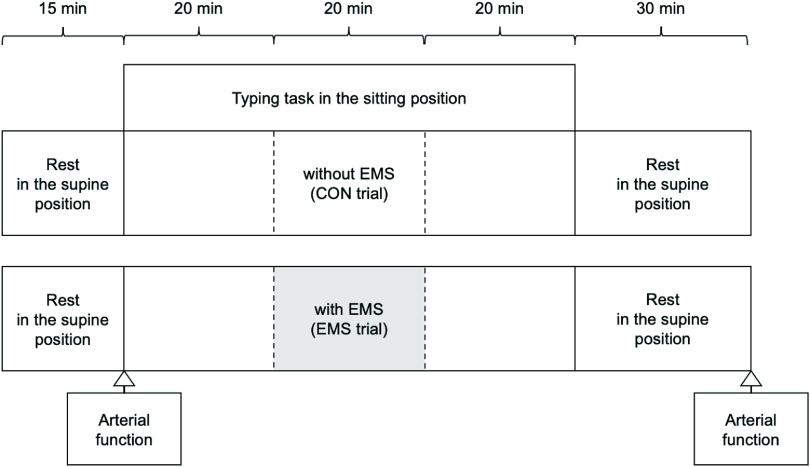
The protocol for each trial is shown in Figure 1. All participants were randomly assigned to either EMS of the skeletal muscle or the control (CON) trial, with a minimum interval of one week between the two trials. In the EMS trial, EMS was administered to the lower extremities in the seated position from 20 to 40 min after the start of the seated behavior, whereas in the CON trial, the seated behavior was continued without EMS. The electrode belts were placed at five locations: waist, bilateral thighs, and bilateral ankle joints. The maximum tolerance of all participants was measured before the stimulation intensity was set.
Fig. 1.
Experimental protocol of test sessions
All subjects performed each test in random order.
Arterial function measurements: SBP, DBP, HR, Di, FV, and FMD
EMS, electrical muscle stimulation; CON, control; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; Di, vessel diameter; FV, blood flow velocity; FMD, flow-mediated dilation
EMS
In the EMS trial, belt electrode-skeletal muscle electrical stimulation (G-TES; Homer Ion, Tokyo, Japan) was performed at a frequency of 4 Hz, pulse width of 250 µs17), and exponentially increasing waves with a stimulation intensity of 50% of the maximum tolerance. EMS was applied to the calf and thigh muscles, including the quadriceps femoris, hamstrings, gastrocnemius, and hip adductor muscles using a stimulator. A frequency of 4 Hz was adopted because high-frequency EMS induces tonic contraction of skeletal muscles and induces muscle fatigue more easily than low-frequency EMS18). The value of 4 Hz was selected because this study aimed to promote peripheral circulation through aerobic exercises19). One silicon-rubber electrode band (5.3 × 93.3 cm) was wrapped around the lumbar region, two bands (5.3 × 69.6 cm) were wrapped around both distal parts of the thighs, and two bands (5.3 × 54.6 cm) were applied to both ankles (Fig. 2A and 2B). As the stimulation cycles of the bilateral thighs and lower legs were synchronized, the bilateral lower-extremity muscle groups were stimulated simultaneously. The stimulation was set to 50% of the maximum tolerance without causing discomfort (39–82 mA on the thighs, below 30 mA on the ankles).
Fig. 2.
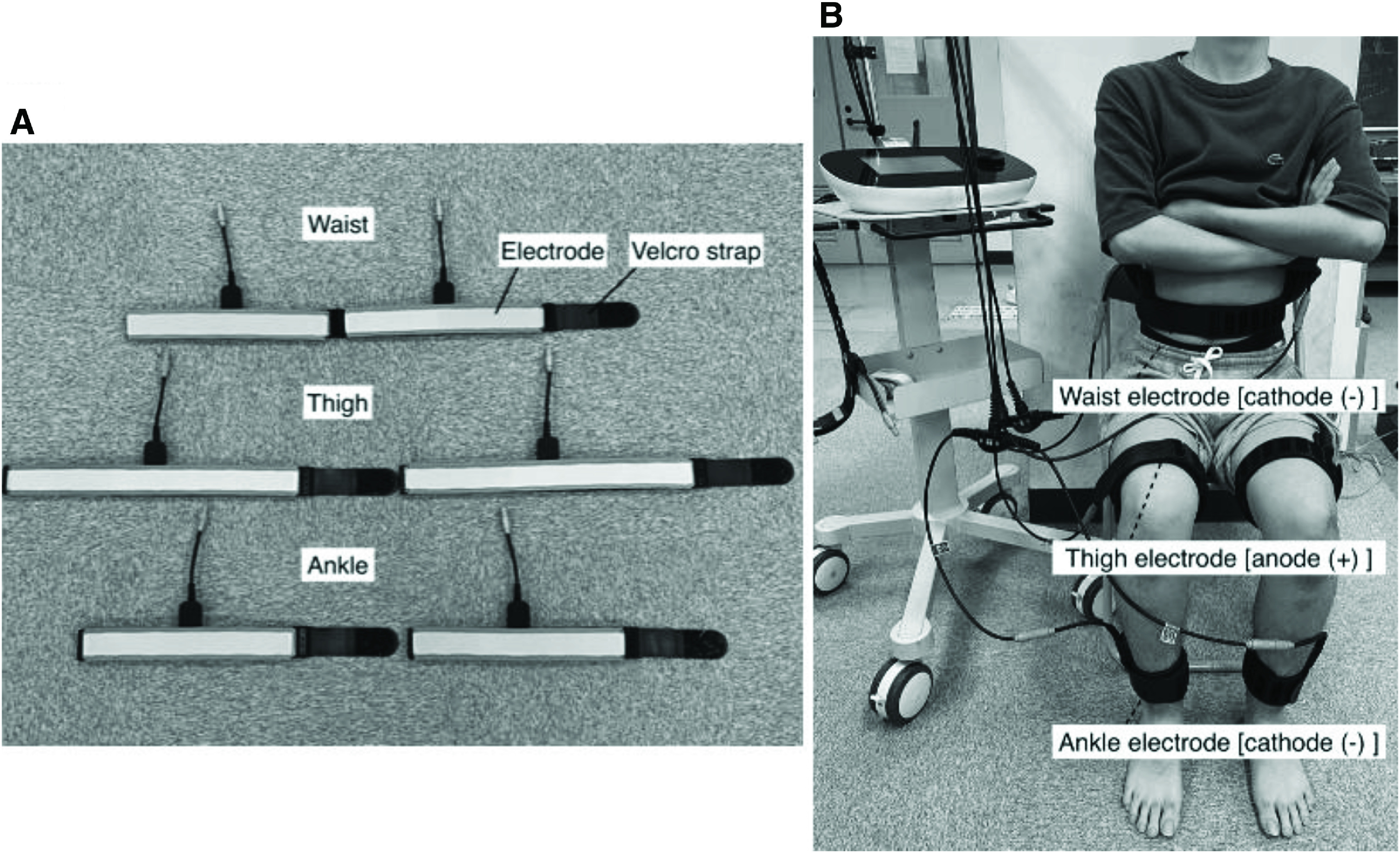
(A) Belt and stimulation electrodes, and (B) belt electrodes (anode and cathode) were attached to the waist, bilateral distal parts of the thigh, and ankle with a strap.
Measurements
BA endothelial function
In this study, the endothelial function of the BA was assessed. The endothelial function of the BA is an indicator of systemic endothelial function11), and FMD of the BA is highly significant because it is a predictor of CVD20).
The participants were asked to rest in the supine position for at least 15 min to obtain resting arm systolic blood pressure (SBP) and diastolic blood pressure (DBP) using a standard sphygmomanometer on their left arm. An occlusion cuff was placed around the right forearm while two ECG leads were attached to the wrists to measure heart rate (HR). FMD was quantified by high-resolution ultrasonography (UNEXEF 38G; UNEX, Nagoya, Japan) to measure endothelial function. The BA was scanned longitudinally 5–10 cm proximal to the elbow joint. To occlude blood flow, the cuff was inflated 50 mmHg above the SBP for 5 min. Upon cuff deflation, the blood flow velocity and artery diameter were measured for an additional 3 min, and the change in BA diameter was immediately expressed as the percentage change relative to the vessel diameter before cuff inflation. FMD was calculated as the baseline value (Dibase) before the cuff was released to the peak value after cuff release (Dipeak). FMD was calculated using the following equation: FMD (%) = {(Dipeak - Dibase)/Dibase} × 100. A detailed description of the measurements is provided in a previous study21).
All participants were instructed to limit alcohol consumption, caffeine intake, and strenuous exercise from the day before to the end of the experiment.
In this study, to compare FMD under different trials, we calculated the peak shear rate (PSR) from the vessel diameter and blood flow rate. The blood flow velocity was calculated from the color Doppler data and displayed as a waveform in real time. PSR was calculated as the difference in flow velocity between the hyperemic response (peak after cuff deflation: FVpeak) and baseline (FVbase) divided by the baseline BA diameter. Subsequently, normalized FMD (nFMD) was calculated using the following equation22):
All measurements were performed after 15 min of supine rest and 30 min after PS. The HR during PS was measured every 10 min using thoracic bipolar induction (POLAR H10; Polar, Tokyo, Japan).
Work efficiency
To examine the effect of EMS on work efficiency, the participants typed a specified novel on a personal computer (PC) for 60 min. The same novel was used for both trials, and different chapters were typed for each trial. The text and typing paper were set up as a 400-word manuscript, and the percentage of correct responses was calculated from the number of words typed and the number of words missed during the 60-min task.
Statistical analysis
The results of this study were analyzed for normality using the Shapiro–Wilk test to confirm the normal distribution of data. The measurements for each trial were compared using two-way ANOVA with repeated measures to test for the presence or absence of an interaction, and the Bonferroni test was performed for posterior analysis. All measurements are expressed as mean and standard deviation and were considered statistically significant at a significance level of <5%.
Results
HR during each trial
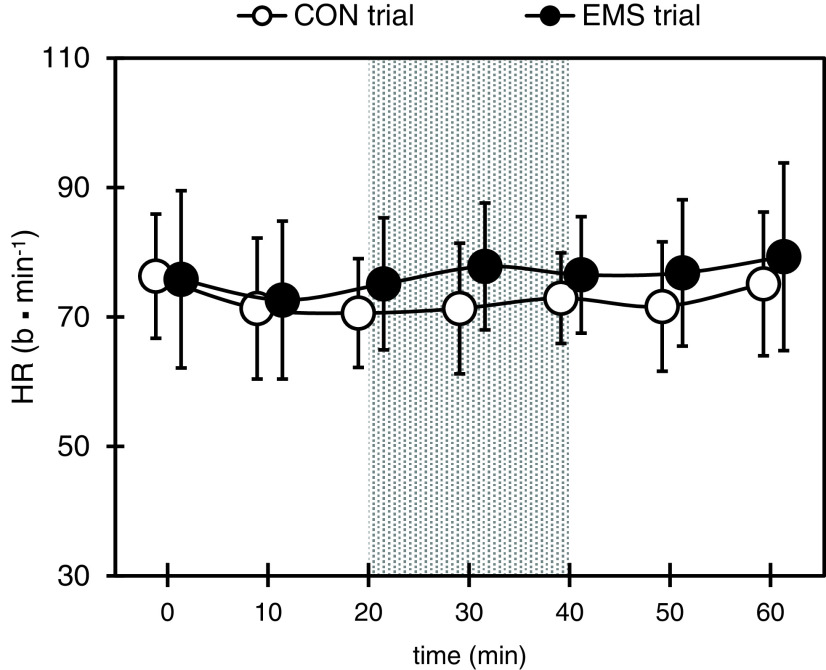
The changes in HR during the two trials are shown in Figure 3. No significant differences were observed between the two trials in all tests.
Fig. 3.
Changes in HR during each trial
Gray areas represent EMS trials during electrical stimulation.
Values are presented as mean ± SD.
CON, control; EMS, electrical muscle stimulation; HR, heart rate; SD, standard deviation
Work efficiency after each trial
The number of characters that could be typed in the novels in the EMS and CON trials was 1944.6 ± 467.1 in the EMS trial and 1962.8 ± 364.5 in the CON trial, with no significant differences between the trials. The percentage of correct responses was 96.4% in the EMS trial and 98.2% in the CON trial, with no significant differences between the trials.
BA function before and after each trial
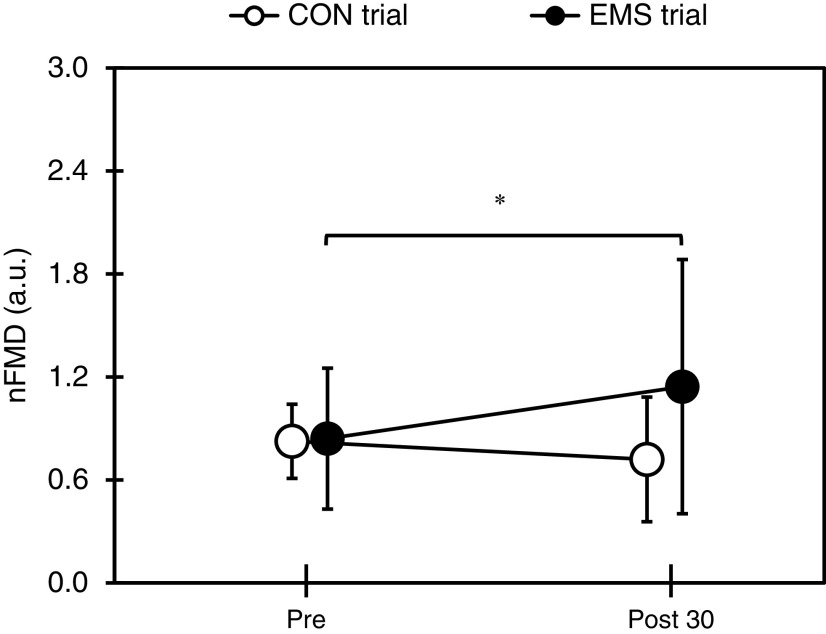
The changes in SBP, DBP, HR, Dibase, Dipeak, FVbase, FVpeak, PSR, and FMD before and after each trial are shown in Table 1. In both trials, DBP showed a significant increase 30 min after PS compared to before PS (p <0.05), and HR decreased significantly after each trial (p <0.01). There was no significant main effect or interaction between SBP, Dibase, Dipeak, FVbase, FVpeak, and PSR before PS and 30 min after PS completion in both trials. There was a significant interaction for FMD (F(1,11) = 5.258, p <0.05), and in the CON trial, there was a significant decrease 30 min after PS completion compared with that before PS (p <0.05). The changes in nFMD before PS and 30 min after PS completion are shown in Figure 4 (0.84 ± 0.43 and 1.14 ± 0.77 in the EMS trial and 0.84 ± 0.43 and 0.74 ± 0.55 in the CON trial, respectively; F(1,11) = 6.884, p <0.05). In the EMS trial, a significant increase was observed 30 min after PS completion compared with that before PS (p <0.05).
Table 1.
Characteristics of SBP, DBP, HR, Di, FV, PSR, and FMD before and after each trial
| EMS trial | CON trial | ||||||
|---|---|---|---|---|---|---|---|
| Pre | Post 30 | Time effect within group p-value |
Pre | Post 30 | Time effect within-group p-value |
Group × time interaction p-value |
|
| *p <0.05, **p <0.01 vs. Pre | |||||||
| SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; Dibase, diameter baseline; Dipeak, diameter peak line; FVbase, flow volume base; FVpeak, flow-volume peak; PSR, peak shear rate; FMD, flow-mediated dilation; EMS, electrical muscle stimulation; CON, control | |||||||
| SBP (mmHg) | 119.7 ± 6.5 | 117.2 ± 6.5 | 0.10 | 116.5 ± 6.8 | 115.3 ± 8.6 | 0.50 | 0.23 |
| DBP (mmHg) | 64.7 ± 3.4 | 67.6 ± 4.7 | 0.01** | 65.7 ± 7.7 | 70.0 ± 7.7 | 0.01** | 0.60 |
| HR (b⋅min–1) | 64.9 ± 8.8 | 59.5 ± 7.0 | 0.01** | 64.7 ± 8.5 | 61.1 ± 7.5 | 0.01** | 0.04* |
| Dibase (mm) | 3.8 ± 0.3 | 3.8 ± 0.2 | 0.28 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.85 | 0.48 |
| Dipeak (mm) | 4.1 ± 0.3 | 4.1 ± 0.3 | 0.29 | 4.2 ± 0.3 | 4.1 ± 0.3 | 0.57 | 0.97 |
| FVbase (cm⋅sec–1) | 13.2 ± 5.8 | 11.8 ± 5.0 | 0.20 | 14.0 ± 8.5 | 9.9 ± 4.3 | 0.06 | 0.21 |
| FVpeak (cm⋅sec–1) | 52.4 ± 18.7 | 46.4 ± 20.7 | 0.08 | 50.3 ± 16.2 | 47.8 ± 14.8 | 0.61 | 0.43 |
| PSR (s–1) | 10.4 ± 4.4 | 9.3 ± 5.6 | 0.80 | 9.4 ± 2.8 | 9.8 ± 3.3 | 0.77 | 0.26 |
| FMD (%) | 7.2 ± 0.9 | 7.3 ± 1.1 | 0.17 | 7.3 ± 0.7 | 6.2 ± 1.1 | 0.04* | 0.04* |
Fig. 4.
Changes in nFMD before and after each trial
*Significantly different from Pre (p <0.05)
CON, control; EMS, electrical muscle stimulation; nFMD, normalized flow-mediated dilation
Discussion
This study investigated the effects of EMS during PS on vascular endothelial function in healthy adult men. The results showed that the EMS trial with EMS to the lower extremities increased the nFMD of BA 30 minutes after the end of the trial compared to before PS. It was found that giving EMS during PS had a beneficial effect on nFMD.
Vranish et al.23) reported that blood flow and shear stress in the lower extremities decreased within 10 min of the start of sitting, indicating that these are factors that induce a decrease in FMD in the lower extremities due to PS. In contrast, walking for 5 min during 3 h of SB suppressed the decline in FMD, indicating that aerobic exercise during SB suppresses the decline in vascular endothelial function12). Overall, these results indicate that physical activity during prolonged SB suppresses vascular endothelial dysfunction.
Most importantly, this study indicates that nFMD increased significantly in the EMS trial 30 min after PS completion compared to that before PS. This may be due to the increase in blood flow caused by the dynamic skeletal muscle contraction in response to EMS; vasodilation caused by the enhanced production of NO, lactate, and H+; metabolic products of muscle contraction; and the inhibition of muscle sympathetic nerve activity (MSNA). The rhythmic rise and fall of intramuscular pressure due to skeletal muscle contraction increases blood flow24); increases shear stress, which is a mechanical stress on vascular endothelial cells; and promotes the production of NO, resulting in a vasodilator response. Previous studies have shown that eNOS is activated during EMS and exercise16). In addition, previous studies have shown that endothelium-derived vasodilatation occurs in patients with heart failure, causing an increase in the blood flow. Janssen et al.25) reported that EMS to the lower extremity muscles at a frequency of 3 Hz and maximum tolerance intensity increased blood flow in the common femoral artery. In the present study, we used a low frequency of 4 Hz, which may have increased the blood flow in the lower extremities, as in a previous study. Furthermore, compared with voluntary exercise, EMS provides electrical stimulation to the entire lower extremity and produces muscle contraction; thus, blood flow can be expected to increase. EMS stimulation also releases substance P and calcitonin gene-related peptide from nociceptive C fibers; these substances have vasodilating effects and act on skin vasodilation26). Furthermore, a comparison of changes in skin blood flow under high-frequency stimulation (110 Hz) and low- frequency stimulation (4 Hz) revealed that low-frequency stimulation increases skin blood flow at stimulation sites27). Since these increases in cutaneous blood flow increase blood flow in conduit arteries, increased cutaneous blood flow is one possible mechanism for increasing FMD in conduit arteries. It is, therefore, a factor in the increased blood flow in the conduit arteries of the lower extremities, the site of EMS stimulation. In voluntary exercise, type I fibers are sequentially mobilized, whereas, in EMS, mobilization begins with type II fibers, which have a lower threshold, and the mode of mobilization is different28). Because of the different energy metabolism characteristics, metabolic vasodilators that inhibit sympathetic nerve activity such as lactate, H+, and acidosis are easily produced in the lower limbs, which are active muscles. Kimura et al. have shown that EMS at an intensity that is not uncomfortable increases blood lactate concentration29). In the present study, the same effect may have occurred because of MSNA inhibition. Venous blood stasis induced by sitting posture causes an increase in MSNA30), which dominates the vascular smooth muscle; an increase in blood pressure31); and a decrease in FMD32). However, in large muscles, such as the lower- extremity muscles, MSNA innervating the vascular smooth muscle is suppressed by a low-intensity bicycle pedaling exercise of approximately 50 W33). In addition, it has been shown that light-intensity activity and walking also suppress MSNA by inducing skeletal muscle contraction and promoting venous return34). These results suggest that the EMS trial in this study promoted venous return, suppressed blood flow congestion, and inhibited MSNA-induced vasoconstriction, whereas vasodilation was not inhibited.
On the other hand, FMD in the CON trial in the present study significantly decreased 30 min after PS completion compared with that before PS, and no significant change was observed in nFMD. In a previous study, a significant decrease in FMD was observed in the superficial femoral artery 1 h after PS initiation, whereas no significant change in FMD was observed in the BA35). In Thosar et al. study, upper- extremity activity during PS was not quantified because of the high degree of freedom of the upper extremities during PS, such as psychologically stress-free reading and PC use35). However, since the present study assumed desk work for workers, typing work using a PC was conducted during PS, suggesting that vasoconstriction was affected by prolonged mental tension and sympathetic hyperactivity. In the CON trial of the present study, the FVbase showed a decreasing trend 30 min after the end of sedentary activity compared with that before sedentary activity, suggesting that FMD decreased in the CON trial, whereas nFMD, which takes into account the blood flow velocity, did not show a significant change. In this study, all participants performed typing tasks using a PC with PS to assess work efficiency, but there were no significant differences between the two tests. Thus, the results suggest that the decrease in vascular function is suppressed without a decrease in work efficiency.
In summary, our results suggest that EMS to the lower extremities during sedentary work improves vascular endothelial function. Compared with other countries, workers in Japan, especially office workers, mainly work at desks and spend a lot of time in the sitting position36). In addition, the results of this study may provide basic data for comparing electrical stimulation tools that can be implemented during sedentary activities such as desk work.
This study has some limitations. Due to the transient nature of this study, the long-term impact of the intervention remains to be investigated. In the future, the effects of different intensities of EMS and the production of vasoactive substances, such as NO, could be examined, but this is unknown because biochemical tests have not been performed, nor have the effects of PS and EMS on the sympathetic nervous system. In addition, because the intensity of EMS stimulation is determined subjectively by the individual, there is a possibility that individual differences may occur in the amount of muscle contraction. Furthermore, factors such as exercise habits and age were predicted to cause differences in the vascular responses to the protocol used in this study. Future studies should consider differences in EMS intensity, duration, frequency, and amount of muscle contraction considering these factors.
Conclusion
These results suggest that the use of EMS as an alternative to aerobic exercises may reduce the risk of endothelial dysfunction when it is difficult to interrupt PS and exercises.
Acknowledgments
We acknowledge all participants and staff members who participated in this study.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1). World Health Organization [Internet] . WHO guidelines on physical activity and sedentary behaviour [cited 2022 Feb 18]. Available from: https://www.who.int/publications/i/item/9789240015128 [PubMed]
- 2). Guthold R, Stevens GA, et al. : Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018; 6: e1077–e1086. [DOI] [PubMed] [Google Scholar]
- 3). Dunstan DW, Kingwell BA, et al. : Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012; 35: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Fukushima N, Machida M, et al. : Associations of working from home with occupational physical activity and sedentary behavior under the COVID-19 pandemic. J Occup Health. 2021; 63: e12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Hu FB, Li TY, et al. : Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003; 289: 1785–1791. [DOI] [PubMed] [Google Scholar]
- 6). Padilla J, Sheldon RD, et al. : Impact of acute exposure to increased hydrostatic pressure and reduced share rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol. 2009; 297: H1103–H1108. [DOI] [PubMed] [Google Scholar]
- 7). Malek AM, Alper SL, et al. : Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999; 282: 2035–2042. [DOI] [PubMed] [Google Scholar]
- 8). van der Ploeg HP, Chey T, et al. : Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012; 172: 494–500. [DOI] [PubMed] [Google Scholar]
- 9). Matsuzawa Y, Sugiyama S, et al. : Successful diet and exercise therapy as evaluated on self-assessment score significantly improves endothelial function in metabolic syndrome patients. Circ J. 2013; 77: 2807–2815. [DOI] [PubMed] [Google Scholar]
- 10). Celermajer DS, Sorensen KE, et al. : Noninvasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992; 340: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 11). Widlansky ME, Gokce N, et al. : The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003; 42: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 12). Thosar SS, Bielko SL, et al. : Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc. 2015; 47: 843–849. [DOI] [PubMed] [Google Scholar]
- 13). Ishikawa M, Miura H, et al. : Effect of difference in exercise frequency during breaks during prolonged sitting behavior on vascular endothelial function. Health Care. 2019; 61: 281–285. (in Japanese) [Google Scholar]
- 14). Hamada T, Hayashi T, et al. : Electrical stimulation of human lower extremities enhances energy consumption, carbohydrate oxidation, and whole body glucose uptake. J Appl Physiol. 2004; 96: 911–916. [DOI] [PubMed] [Google Scholar]
- 15). Hamada T, Sasaki H, et al. : Enhancement of whole body glucose uptake during and after human skeletal muscle low-frequency electrical stimulation. J Appl Physiol. 2003; 94: 2107–2112. [DOI] [PubMed] [Google Scholar]
- 16). Hambrecht R, Fiehn E, et al. : Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998; 98: 2709–2715. [DOI] [PubMed] [Google Scholar]
- 17). Watanabe K, Taniguchi T, et al. : Metabolic and cardiovascular responses during voluntary pedaling exercise with electrical muscle stimulation. Eur J Appl Physiol. 2014; 114: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 18). Muro M, Nagata A, et al. : Observation of high and low frequency muscle fatigue by means of 31P nuclear magnetic resonance. Ann Physiol Anthropol. 1986; 5: 89–96. [DOI] [PubMed] [Google Scholar]
- 19). Miyamoto T, Kamada H, et al. : Low-Intensity electrical muscle stimulation induces significant increases in muscle strength and cardiorespiratory fitness. Eur J Sport Sci. 2016; 16: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 20). Yeboah J, Crouse JR, et al. : Brachial flow-mediated dilation predicts incident cardiovascular events in older adults. Circulation. 2007; 115: 2390–2397. [DOI] [PubMed] [Google Scholar]
- 21). Corretti MC, Anderson TJ, et al. : Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol. 2002; 39: 257–265. [DOI] [PubMed] [Google Scholar]
- 22). Tarro Genta F, Eleuteri E, et al. : Flow-mediated dilation normalization predicts outcome in chronic heart failure patients. J Card Fail. 2013; 19: 260–267. [DOI] [PubMed] [Google Scholar]
- 23). Vranish JR, Young BE, et al. : Brief periods of inactivity reduce leg microvascular, but not macrovascular, function in healthy young men. Exp Physiol. 2018; 103: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 24). Kagaya A: Muscle blood flow during exercise in man. Jpn J Phys Educ Health Sport Sci. 2001; 46: 429–442. (in Japanese) [Google Scholar]
- 25). Janssen TW, Hopman MT: Blood flow response to electrically induced twitch and tetanic lower-limb muscle contractions. Arch Phys Med Rehabil. 2003; 84: 982–987. [DOI] [PubMed] [Google Scholar]
- 26). Petrofsky JS, Al-Malty AM, et al. : Relationship between multiple stimuli and skin blood flow. Med Sci Monit. 2008; 14: CR399–405. [PubMed] [Google Scholar]
- 27). Cramp AF, Gilsenan C, et al. : The effect of high- and low-frequency transcutaneous electrical nerve stimulation upon cutaneous blood flow and skin temperature in healthy subjects. Clin Physiol. 2000; 20: 150–157. [DOI] [PubMed] [Google Scholar]
- 28). Hamada T, Kimura T, et al. : Selective fatigue of fast motor units after electrically elicited muscle contractions. J Electromyogr Kinesiol. 2004; 14: 531–538. [DOI] [PubMed] [Google Scholar]
- 29). Kimura T, Matsumoto K, et al. : Percutaneous electrical muscle stimulation attenuates postprandial hyperglycemia in obese and pre-obese Japanese men. Int J Sport Health Sci. 2010; 8: 1–6. [Google Scholar]
- 30). Cui J, McQuillan PM, et al. : Limb venous distension evokes sympathetic activation via stimulation of the limb afferents in humans. Am J Physiol Heart Circ Physiol. 2012; 303: H457–H463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Shvartz E, Gaume J, et al. : Hemodynamic responses during prolonged sitting. J Appl Physiol. 1983; 54: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 32). Hijmering ML, Stroes ES, et al. : Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002; 39: 683–688. [DOI] [PubMed] [Google Scholar]
- 33). Saito M, Mano T: Exercise mode affects muscle sympathetic nerve responsiveness. Jpn J Physiol. 1991; 41: 143–151. [DOI] [PubMed] [Google Scholar]
- 34). Saito M, Tsukanaka A, et al. : Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993; 75: 663–667. [DOI] [PubMed] [Google Scholar]
- 35). Thosar SS, Bielko SL, et al. : Differences in brachial and femoral artery responses to prolonged sitting. Cardiovasc Ultrasound. 2014; 12: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Bauman A, Ainsworth BE, et al. : IPS Group: The descriptive epidemiology of sitting: a 20-country comparison using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med. 2011; 41: 228–235. [DOI] [PubMed] [Google Scholar]