Significance
Following bloodstream infection, Staphylococcus aureus can invade internal tissues where it forms and persists in abscesses. Using a mouse model for chronic staphylococcal infection of the kidney, we demonstrate that IL-17A-producing γδ T cells accumulate in infected kidneys and acquire a kidney-resident phenotype. Depletion of γδ T cells results in increased renal bacterial numbers in chronic infection and following reinfection. Thus, in the mouse, kidney-resident γδ T cells are nonredundant in limiting local S. aureus growth during chronic infection and provide acquired protection against reinfection.
Keywords: γδ T cells, Staphylococcus aureus, IL-17, tissue residency, T cell memory
Abstract
γδ T cells are involved in the control of Staphylococcus aureus infection, but their importance in protection compared to other T cells is unclear. We used a mouse model of systemic S. aureus infection associated with high bacterial load and persistence in the kidney. Infection caused fulminant accumulation of γδ T cells in the kidney. Renal γδ T cells acquired tissue residency and were maintained in high numbers during chronic infection. At day 7, up to 50% of renal γδ T cells produced IL-17A in situ and a large fraction of renal γδ T cells remained IL-17A+ during chronic infection. Controlled depletion revealed that γδ T cells restricted renal S. aureus replication in the acute infection and provided protection during chronic renal infection and upon reinfection. Our results demonstrate that kidney-resident γδ T cells are nonredundant in limiting local S. aureus growth during chronic infection and provide enhanced protection against reinfection.
The gram-positive pathogen Staphylococcus aureus represents a prime example for a pathobiont, being a frequent commensal of healthy human skin and nasal mucosa but also being able to cause severe invasive disease, e.g., skin and soft tissue infections, pneumonia, endocarditis, or osteomyelitis. S. aureus is also a frequent cause of bacterial sepsis associated with high mortality. Infections are increasingly caused by antibiotic-resistant strains such as methicillin-resistant S. aureus strains that impede effective antibacterial treatment (1).
The innate immune response against S. aureus is characterized by the recruitment of neutrophils and formation of abscesses in infected tissues. Neutropenic patients or patients with defective neutrophil function are particularly susceptible to S. aureus infections (2, 3). Depletion of neutrophils in mice confirms the protective role of neutrophils in S. aureus infection (4, 5). Adaptive immunity to S. aureus is thought to rely on CD4+ T cells. S. aureus-specific CD4+ T cells, particularly Th17 cells, are frequently found in healthy humans (6–8). Hyper-IgE syndrome patients with impaired Th17-cell responses due to STAT3 mutation suffer from S. aureus infections indicating a main function of these cells in protection (9). In line with their role in promoting neutrophil responses, type-3 cytokines produced by Th17 cells are crucial for the control of S. aureus. Patients with defects in IL-17A, IL-17F, or in IL-17R signaling are more susceptible to S. aureus infections (10), and mice with deficiencies in IL-17A, IL-17F, or IL-17R show impaired control of the pathogen (11–16). The role of Th1 cells in S. aureus infection is less clear (12, 17, 18); however, in mice vaccine-induced Th1 cells can provide some protection against subsequent S. aureus infection (8, 19, 20).
Several mouse studies could demonstrate a role of γδ T cells in the control of S. aureus (12, 14, 16, 21–25). In contrast to conventional αβ T cells, γδ T cells have a limited T cell receptor (TCR) repertoire and are not restricted by major histocompatibility complex class I or class II proteins. In mice, γδ T cells with specific Vγ chains differ in phenotype, cytokine profile, and tissue location. While Vγ5+ and Vγ7+ T cells are exclusively found in the epidermis and the intestinal epithelium, respectively, Vγ1+, Vγ4+, and Vγ6+ T cells are widely distributed in lymphoid and nonlymphoid tissues and retain some migratory capacity. [Of note, the Vγ nomenclature of Heilig and Tonegawa is used in this study (26)]. Upon activation, Vγ1+ and Vγ4+ T cells mainly produce IFN-γ, but Vγ4+ T cells are also able to secrete IL-17A. In contrast, the cytokine response of Vγ6+ T cells is uniformly dominated by type-3 cytokines such as IL-17A, IL-17F, and IL-22. γδ T cells can be activated via their TCR, although antigen recognition by the γδ TCR is still largely enigmatic and only a limited number of antigens have been identified in mice and humans (27, 28). However, γδ T cells can effectively respond to signals from a stressed or inflamed environment. γδ T cells express toll-like receptors (TLR) and receptors for stressed cells and respond to inflammatory cytokines such as IL-1β, IL-18, IL-6, IL-12, and IL-23 (28–30). Therefore, γδ T cells are also regarded as innate T cells.
γδ T cell responses to microbial pathogens have been described in rodents and humans, and γδ T cells protect against a variety of pathogens in mouse infection models (27, 28). In these models, γδ T cells mostly show rapid production of type-1 and type-3 cytokines and provide protection mainly in the early phase of acute infection, consistent with a rapid and less restricted response of innate T cells. The role of γδ T cells in chronic and secondary infection is less clear. Following oral Listeria monocytogenes infection, Vγ6+ T cells accumulate in the intestinal mucosa. Upon oral challenge with L. monocytogenes, but not with Salmonella typhimurium, these γδ T cells rapidly respond with extensive proliferation and IL-17A and IFN-γ production and thereby support the protection provided by conventional memory αβ T cells (31). Thus, γδ T cells can display key features of adaptive responses, namely the formation of pathogen-specific memory and the rapid response to secondary infection.
For S. aureus infection, a protective role of γδ T cells could be demonstrated in murine models for skin and wound infection but also in bacterial pneumonia and peritonitis models (12, 14, 16, 21–25). In these models, γδ T cells provide protection in the very early phase of infection, underlining the role of innate effector T cells for immediate response after infection. Protection is accomplished by rapid production of type-3 cytokines and accelerated recruitment of neutrophils (12, 21–23). In lung and peritoneal infection, early responses are accompanied by local accumulation of Vγ1+ and Vγ4+ T cells (22, 23). In the skin and peritoneum, responses at later time points are dominated by Vγ6+ T cells, which then are main producers of type-3 cytokines (16, 23). There is also an indication for a role of γδ T cells in acquired protection against S. aureus. In IL-1β-deficient mice, γδ T cells are mainly responsible for protection against skin reinfection (24), and repeated peritoneal infection causes local expansion of Vγ6+ T cells, which protect against reinfection and can transfer protection to naive recipient mice (23), raising the question whether there is a general systemic or tissue-specific adaptation of γδ T cells to S. aureus and whether such γδ T cell responses could confer innate-like protective memory.
Here, we used a mouse model of systemic S. aureus infection to analyze the function of γδ T cells in the acute and chronic phases of the disease. After intravenous (i.v.) application, S. aureus grew to particularly high numbers in the kidney and persisted in this organ for more than 2 mo. Infection resulted in a 100-fold expansion of the renal γδ T cell population, which remained enlarged during chronic infection. After initial proliferation, renal γδ T cells acquired a CD69+ tissue-resident phenotype and continuously produced IL-17A. Depletion experiments revealed that γδ T cells restricted renal S. aureus proliferation in the acute stage of infection but also provided protection during chronic infection and upon reinfection. In conclusion, our results demonstrate that in systemic infection, γδ T cells accumulate to high numbers in infected tissues and continuously restrict local S. aureus growth in the acute and chronic stages of infection.
Results
Systemic S. aureus Infection in Mice Leads to a Massive Accumulation of γδ T Cells in the Kidney.
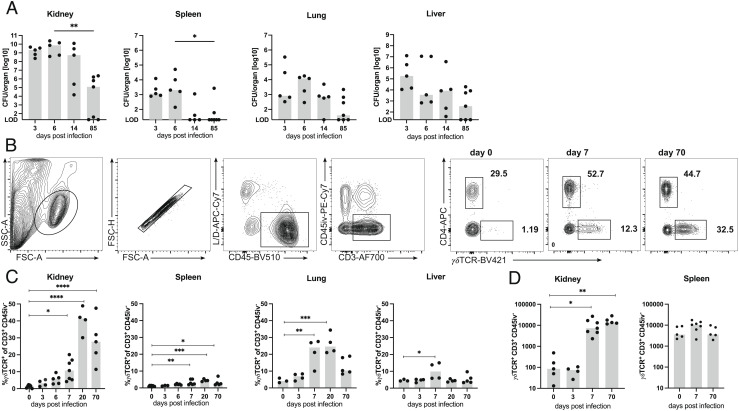
To characterize the γδ T cell response against systemic infection, mice were i.v. infected with 107 colony-forming units (CFU) of the S. aureus strain SH1000, and numbers of viable bacteria in kidneys, lung, liver, and spleen were determined at different time points postinfection (p.i.). At days 3 and 6 p.i., we detected particularly high bacterial numbers of up to 1010 CFU in the kidneys. In the spleen, lung, and liver, the numbers of bacteria were substantially lower (Fig. 1A). After 2 wk, the numbers slowly declined. Although mice could restrict the S. aureus infection, viable bacteria were still detected in various tissues of about half of the animals on day 85 p.i. Due to the particularly high bacterial load in the kidneys, we focused our analysis on this organ. Renal sections of infected mice were stained with anti-CD44 monoclonal antibodies (mAb) to determine the extent of the inflammatory reaction (SI Appendix, Fig. S1). In the kidneys of naive mice, only scarce staining for CD44 was detected, which is not visible in the overviews. Following infection, CD44-positive areas were initially found in the medulla and subsequently spread into the cortex. By day 14, large areas of the kidneys were CD44 positive. On day 85, kidneys were mostly CD44-negative with sporadic CD44-positive spots.
Fig. 1.
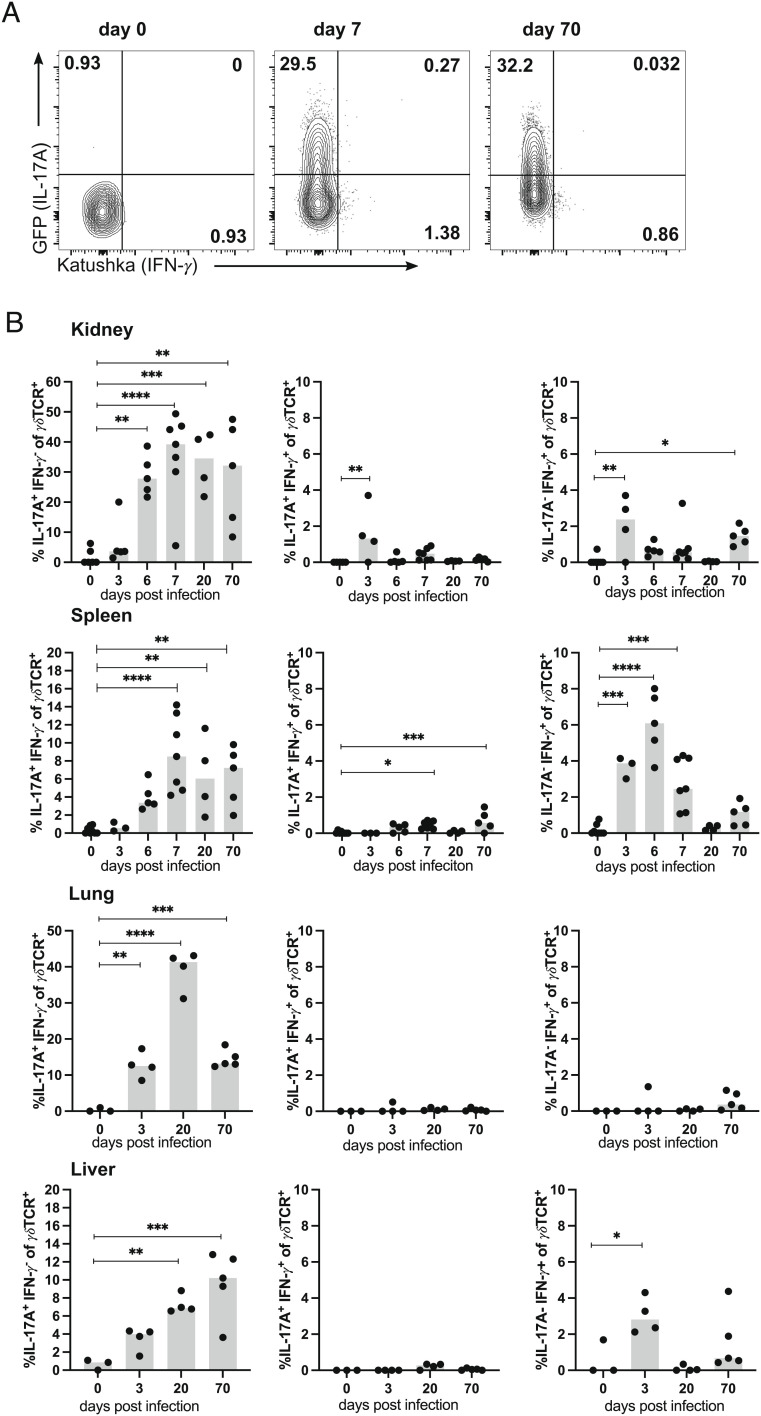
S. aureus causes chronic infection and induces a strong γδ T cell response. (A) TCRdH2BeGFP mice were infected i.v. with 107 CFU of S. aureus. On indicated days p.i., bacteria numbers in the kidney, spleen, lung, and liver were determined. Data are from one of two independent experiments (n = 5 to 7 mice per time point), symbols represent numbers from individual mice, and bars show the median. (LOD, limit of detection). (B–D) Foxp3RFP×Il17aeGFP×IfngKat mice were infected i.v. with 107 CFU of S. aureus or remained without infection. On indicated days p.i., leukocytes from kidneys, spleen, lungs, and liver were isolated and directly analyzed by flow cytometry. Three minutes prior to collecting organs, mice received i.v. fluorochrome-conjugated anti-CD45 mAb to label vascular cells. (B) Gating strategy shown for renal leukocytes and representative dot plots of renal γδ T cells and CD4+ T cells on days 0, 7, and 70 p.i. (C) Percentages of γδ T cells in the kidney, spleen, lung, and liver. (D) γδ T cell counts in the kidney and spleen. (C, D) Results from one of two independent experiments with three to seven animals per time point. Symbols represent individual mice, and bars show median values. Statistical analyses were performed by Kruskal–Wallis test and Dunn’s multiple comparisons posttest (A) or by one-way ANOVA test and Dunnett’s multiple comparisons posttest (C, D).
Next, γδ T cells in the spleen, kidneys, liver, and lung were determined at different time points p.i. To exclude intravascular cells from analysis, we intravenously injected anti-CD45 mAb 3 min before tissue extraction. Nonvascular cells were then identified by the absence of CD45 i.v. staining. Peripheral organs from healthy mice contained only low percentages of γδ T cells (Fig. 1 B and C). Following S. aureus infection, the proportion of γδ T cells dramatically increased in the kidney with a maximum on day 20 p.i. γδ T cells also strongly accumulated in the lung, while only a small increase was observed in the liver and spleen. Interestingly, the percentages of γδ T cells in the kidney were still elevated on day 70 p.i. Accumulation of γδ T cells in the spleen and kidney was also evident when total numbers were calculated (Fig. 1D). In the kidney, we observed a 100-fold expansion of the γδ T cell population at day 7 p.i., which remained substantially enlarged at day 70 p.i. In parallel to γδ T cells, accumulation of CD4+ T cells was determined in the spleens and kidneys of infected mice. As we described before (32), the percentages and numbers of CD4+ T cells in the kidneys of infected mice also remained elevated at day 70 p.i. (SI Appendix, Fig. S2).
TCRdH2BeGFP mice coexpressing a histone–enhanced green fluorescent protein (eGFP) fusion protein from the Tcrd gene locus (33) were used to localize γδ T cells in the kidney (SI Appendix, Fig. S3). In naive mice, we could not detect GFP+ cells in kidney sections. At day 3, γδ T cells were sporadically found but mostly localized intravascularly. The numbers of renal γδ T cells increased with few cells on day 6 and very frequent cells on day 14 mainly in the tubulointerstitial space. At this time point, γδ T cells were concentrated in CD44-positive inflamed areas. Overall, the histological results correlated well with results derived from flow cytometric analysis and indicated that γδ T cells accumulate at the sites of renal S. aureus infection.
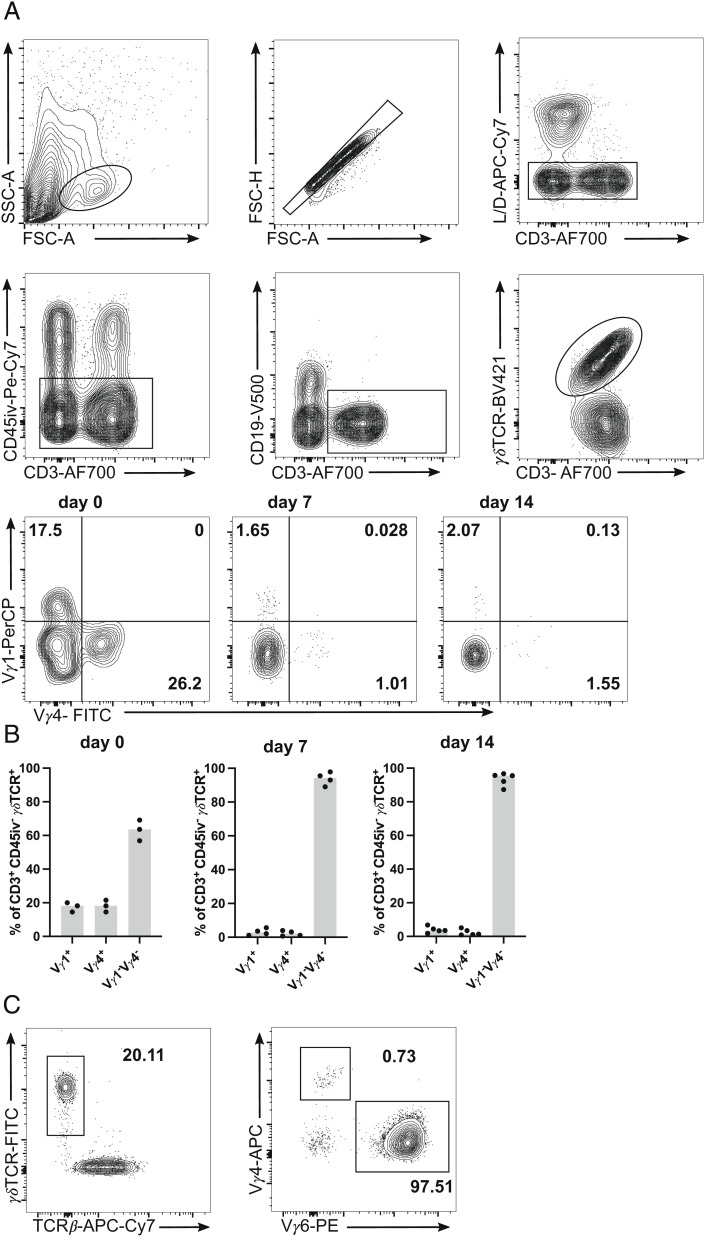
In the kidneys of naive mice, each 15 to 20% of γδ T cells were Vγ1+ or Vγ4+ (Fig. 2 A and B). Upon infection, the percentages of both populations declined and the response was dominated by >90% of Vγ1−Vγ4− γδ T cells. Based on previous studies (34, 35), we suspected that Vγ1−Vγ4− γδ T cells were Vγ6+ γδ T cells. Staining for Vγ6+ in selected samples proved that Vγ1−Vγ4− γδ T cells were indeed mainly Vγ6+ (Fig. 2C). Thus, Vγ6+ cells were the most abundant renal γδ T cell subset during infection with S. aureus.
Fig. 2.
Vγ repertoire of renal γδ T cells. C57BL/6 mice were infected i.v. with 107 CFU of S. aureus or remained without infection. At the indicated time points, leukocytes were isolated from the kidney, and directly analyzed by flow cytometry. Three minutes prior to collecting organs, mice received i.v. fluorochrome-conjugated anti-CD45 mAb to label vascular cells. (A) Renal γδ T cells were identified as CD45iv− CD3+ γδTCR+ cells and analyzed Vγ1 and Vγ4 expression. Gating strategy for renal γδ T cells and representative dot plots for Vγ1 and Vγ4 staining of γδ T cells. (B) Percentages of renal Vγ1+, Vγ4+, and Vγ1−Vγ4− γδ T cells. (C) Representative dot plots for Vγ4 and Vγ6 staining of renal γδ T cells. Results in (B) are representative of one of two independent experiments with three to five animals per time point. Symbols represent individual mice and bars show median values.
Renal γδ T Cells Proliferate and Acquire a Tissue-Resident Phenotype after S. aureus Infection.
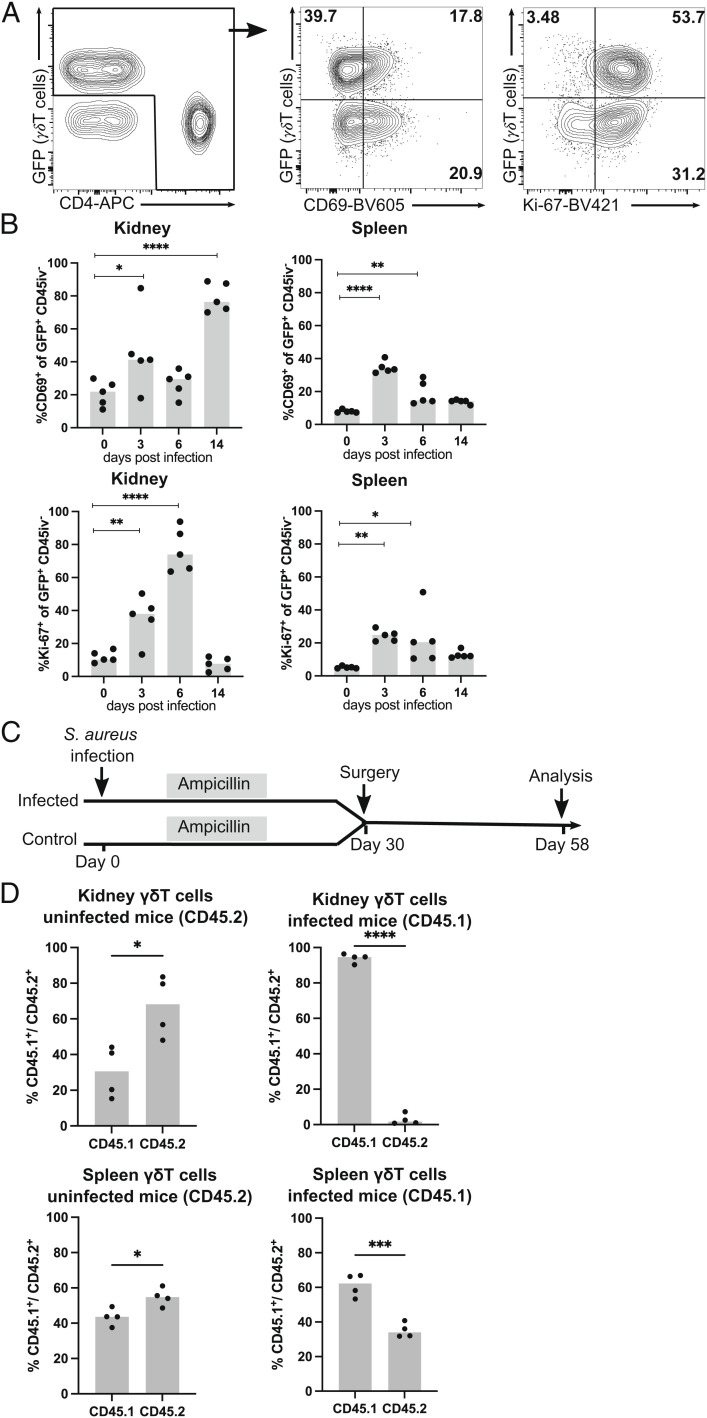
Next, we phenotypically and functionally characterized γδ T cells in the spleen and kidneys following systemic S. aureus infection. TCRdH2BeGFP mice were infected and the percentage of CD69+ γδ T cells was determined. CD69+ is transiently expressed following T cell activation. In addition, tissue-resident memory T cells (Trm cells) constitutively express CD69 (36). In naive mice, approx. 10% and 20% of γδ T cells and 20% and 30% of CD4+ T cells in spleen and kidney, respectively, expressed CD69 and most likely represented tissue-resident T cell populations (Fig. 3 A and B and SI Appendix, Fig. S4A). Three days p.i., we observed an increase in the percentage of CD69+ γδ T cells and CD69+ CD4+ T cells in both tissues. In kidneys but not in spleen, percentages of CD69+ γδ T cells and CD69+ CD4+ T cells massively increased at day 14 p.i., and the vast majority of both cell populations were CD69+, which was likely due to the acquisition of a Trm-cell phenotype (32).
Fig. 3.
After S. aureus infection, renal γδ T cells proliferate and acquire a tissue-resident phenotype. (A, B) TCRdH2BeGFP mice were infected with 107 CFU of S. aureus or remained without infection. γδ T cells from the spleen and kidney were isolated and analyzed directly by flow cytometry. Three minutes prior to collecting organs, mice received i.v. fluorochrome-conjugated anti-CD45 mAb to label vascular cells. (A) Representative anti-CD4 and GFP staining of renal CD45iv− CD3+ T cells (Left) at day 6 p.i. and of CD69 (Middle) and Ki-67 (Right) expression of gated CD4+ (Lower Quadrants) and γδ T cells (Upper Quadrants). (B) Percentages of CD69+ and of Ki-67+ GFP+ γδ T cells in the spleen and kidney analyzed at the indicated days p.i. Results are from one of two independent experiments with four to five animals per time point. (C, D) CD45.1 mice were infected with S. aureus. After 2 wk, infected and CD45.2 control mice were treated with ampicillin. On day 30 p. i., infected and control mice were surgically joined, and after further 28 d, mice were killed and cells in the spleen and kidney were analyzed. (C) Experimental scheme. (D) Percentages of CD45.1+ and CD45.2+ γδ T cells in the spleen and kidney of mice with and without prior S. aureus infection. Symbols represent individual mice and bars show the median. Statistical analysis was performed with one-way ANOVA test and Dunnett’s multiple comparisons posttest (B) or with Student t test (D).
In order to directly test the tissue residency of γδ T cells, we performed parabiosis experiments. CD45.1 congenic C57BL/6 mice were infected with S. aureus. To prevent the spread of infection between mice, parabiosis partners were pretreated with ampicillin resulting in the eradication of the infection (32). After 4 wk, circulations of previously infected mice were connected to those of naive CD45.2 mice. After 4 wk of parabiosis, we observed almost equal distribution of CD45.1+ and CD45.2+ γδ T cells in spleens of naive CD45.2 mice but a 2:1 excess of CD45.1+ γδ T cells in spleens of previously infected CD45.1 mice (Fig. 3 C and D). In the kidneys of naive mice, we detected some accumulation of γδ T cells from their infected partners. In contrast, the kidneys of infected CD45.1 mice contained almost exclusively CD45.1+ γδ T cells, indicating that γδ T cells that had accumulated in the kidney during S. aureus infection were largely sessile and not replaced by circulating cells.
T cells from the spleen and kidney were also stained for the proliferation marker Ki-67 (Fig. 3 A and B). We observed a significant increase in the percentages of Ki-67+ γδ T cells, which at day 6 p.i. reached up to 70% of renal γδ T cells and was consistent with the massive expansion of the γδ T cell populations at this time point. At day 14, the percentages of Ki-67+ γδ T cells had declined to the level observed prior to infection. Renal CD4+ T cells also showed strong Ki-67 upregulation at day 6 p.i. (SI Appendix, Fig. S4B); however, with 40% Ki-67+ cells, proliferation was less pronounced than that observed in renal γδ T cells.
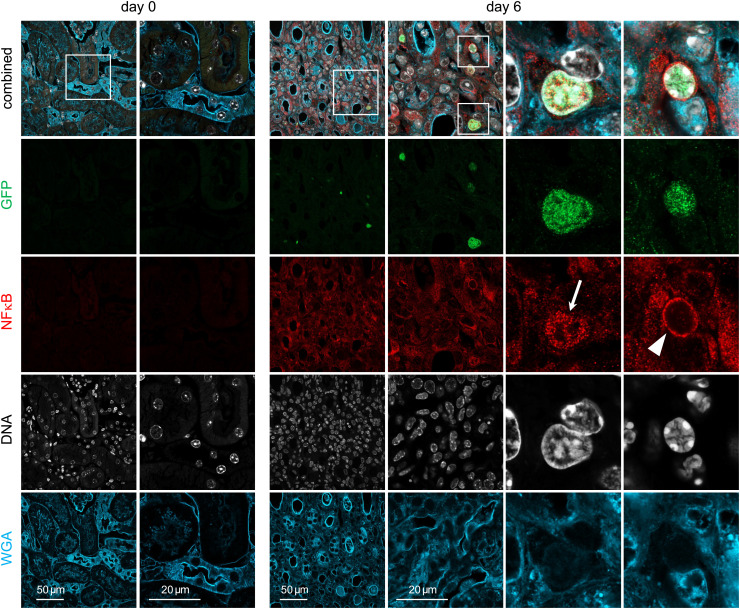
To identify signals that cause local activation of γδ T cells, we determined the NFκB expression and localization in these cells. NFκB is translocated to the nucleus in response to TCR activation but also to TLR activation or to stimulation with cytokines including TNF-α and members of the IL-1 cytokine family (37). Renal sections of TCRdH2BeGFP mice with nuclear GFP expression in γδ T cells were stained for NFκB at different time points post S. aureus infection (Fig. 4 and SI Appendix, Fig. S5). In sections of naive mice, neither γδ T cells (GFP+) nor NFκB staining was detected. There was a low level of ubiquitous NFκB expression on day 3 p.i., and the few renal γδ T cells showed a perinuclear NFκB staining pattern. NFκB staining had significantly increased on day 6. Staining in γδ T cells was mainly perinuclear, however, we also sporadically observed γδ T cells with a nuclear NFκB staining pattern. On day 14, there was still strong ubiquitous NFκB staining but γδ T cells presented almost exclusively with perinuclear NFκB pattern. In conclusion, these results show upregulation and transient nuclear localization of NFκB in γδ T cells at day 6 p.i., which correlates with the strong proliferation of cells at this time point.
Fig. 4.
NFκB response in renal γδ T cells following S. aureus infection. TCRdH2BeGFP mice were infected with 107 CFU of S. aureus or remained without infection. Renal sections from mice at days 0 and 6 p.i. were stained with anti-GFP Ab to identify GFP+ γδ T cells (green; due to the histone 2B eGFP fusion protein, the staining is localized in the nucleus), anti-NFκB Ab (red), DNA (Hoechst, white), and wheat germ agglutinin (WGA, blue). Representative staining for sections from days 0 and 6 are shown (original magnification ×600). Large magnifications on the right show nuclear GFP+ γδ T cells with nuclear (arrow) and perinuclear (arrow head) NFκB staining. Additional sections for days 3 and 14 are presented in SI Appendix, Fig. S5. Sections are representative for five to seven mice per time point.
Renal γδ T Cells Produce IL-17A during Acute and Chronic S. aureus Infection.
γδ T cells are a potent source of IL-17A during bacterial infections (38–40). To investigate IL-17A and IFN-γ production by γδ T cells in response to systemic S. aureus infection, we used Foxp3RFP×Il17aeGFP×IfngKat mice that express the fluorescent proteins eGFP, RFP, and Katushka under the control of the Il17a, the Foxp3 and the Ifng promoters, respectively. Mice were infected, and γδ T cells were isolated from the spleen, kidney, lung, and liver and analyzed for the expression of eGFP and Katushka without further in vitro stimulation. Thus, the expression of the fluorescent protein presented the in vivo cytokine production of cells at the time point of analysis. In naive mice, we detected only marginal percentages of γδ T cells producing either IL-17A or IFN-γ (Fig. 5 A and B). Infection caused a massive increase of IL-17A+ γδ T cells in all tissues, with up to 40% of IL-17A+ γδ T cells in the kidney and lung. Interestingly, a large fraction of γδ T cells remained IL-17A+ in the chronic phase of infection at day 70. We also observed an increase in percentages of IFN-γ+ γδ T cells, particularly in the spleen. However, IFN-γ production was only transient and percentages were substantially lower than those of IL-17A+ γδ T cells. In the kidney and spleen, IL-17A+IFN-γ+ γδ T cells were also detected but remained at low percentages at all analyzed time points. IL-17A production at late time points of infection was dependent on the chronic presence of S. aureus since we observed lower percentages of IL-17A+ γδ T cells and lower expression levels of the IL-17A reporter eGFP in IL-17A+ cells in mice treated with ampicillin (SI Appendix, Fig. S6 A–C).
Fig. 5.
Renal γδ T cells retain IL-17A production during chronic S. aureus infection. Foxp3RFP×Il17aeGFP×IfngKat mice were infected with 107 CFU of S. aureus or remained without infection. At the indicated days p.i., cells were isolated from the spleen, kidney, lung, and liver, and CD45iv− γδTCR+ CD3+ cells were directly analyzed for cytokine reporter proteins. Three minutes prior to collecting of organs, mice received i.v. fluorochrome-conjugated anti-CD45 mAb to label vascular cells. (A) Representative dot plots for Katushka (IFN-γ) and GFP (IL-17A) expression in renal γδ T cells. (B) Percentages of IL-17A+IFN-γ−, IL-17A+IFN-γ+, and IL-17A−IFN-γ+ γδ T cells in organs. Representative result of two independent experiments with four to seven mice per group and time point. Symbols represent individual mice, and bars show median values. Statistical analysis was performed by one-way ANOVA test and Dunnett’s multiple comparisons posttest.
To identify the subtype of IL-17A-producing γδ T cells, renal T cells from S. aureus-infected mice were polyclonally stimulated and cytokine expression in Vγ1+, Vγ4+, and Vγ1−Vγ4− γδ T cells was determined (SI Appendix, Fig. S6D). Vγ1+ and Vγ4+ showed strong production of IFN-γ but only approx. 5% of cells were IL-17A+. In contrast, the vast majority of Vγ1−Vγ4− γδ T cells was IL-17A+, and a small subset of these cells was IFN-γ+IL-17A+ indicating that Vγ1−Vγ4− γδ T cells and thus most likely Vγ6+ cells were the main IL-17A-producing γδ T cells in infected kidneys. In contrast, renal Vγ1+ and Vγ4+ γδ T cells were more capable of producing IFN-γ. In conclusion, renal Vγ1−Vγ4− (Vγ6+) γδ T cells become rapidly activated, proliferate, and secrete IL-17A after systemic S. aureus infection. During the chronic phase, renal γδ T cells acquire a CD69+ Trm phenotype and continuously produce IL-17A.
Cytokine production by CD4+ T cells was analyzed in parallel (SI Appendix, Fig. S6 E–G). In the spleen and kidney, we observed a peak of IL-17A+ CD4+ T cells at day 7 p.i. Thereafter, the percentages of IL-17A+ CD4+ T cells declined. However, in the chronic phase at day 70 p.i., some mice showed high percentages of IL-17A+ CD4+ T cells in the kidney. IL-17A production by renal CD4+ T cells at late time points of infection was dependent on the presence of S. aureus (SI Appendix, Fig. S6 A–C). We also detected IFN-γ+ CD4+ T cells at days 3 to 7 p.i., and IL-17A+IFN-γ+ CD4+ T cells remained at marginal levels at all time points of analysis.
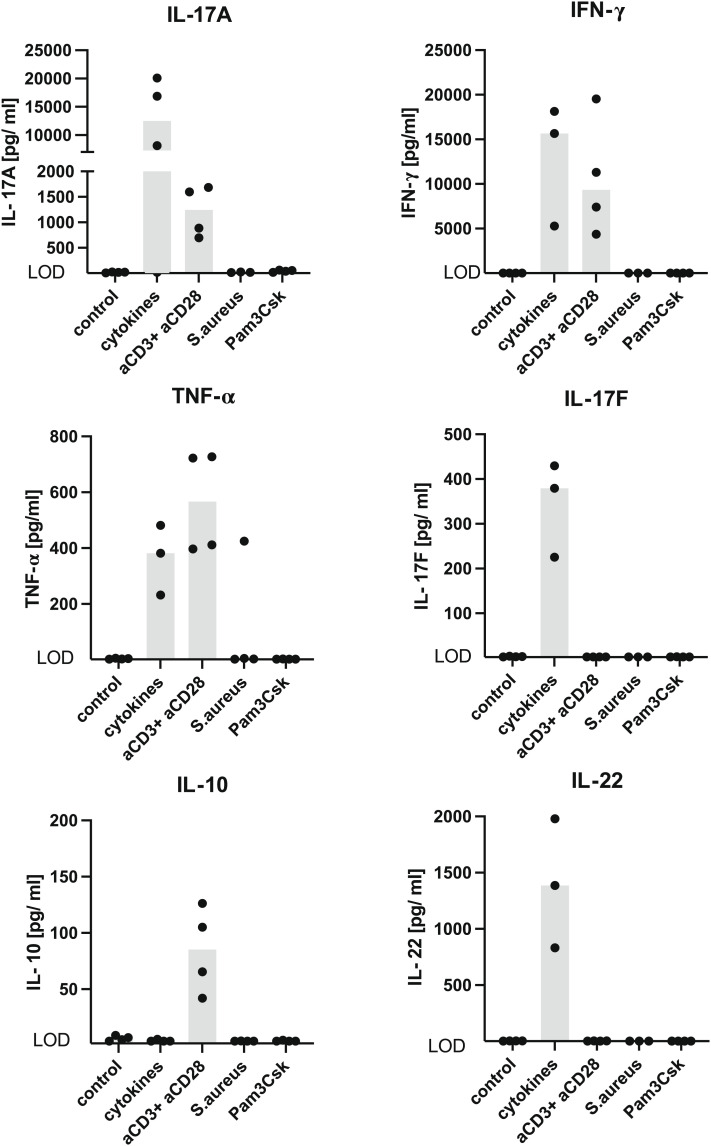
γδ T cells can respond to inflammatory signals from the environment with cytokine production (29, 30, 41). In order to test this capacity in S. aureus-induced renal γδ T cells, TCRdH2BeGFP mice were infected with S. aureus and after 4 wk treated with ampicillin to clear the infection. After further 6 wk, GFP expression was used to sort γδ T cells from kidneys. Cells were stimulated with either anti-CD3 and anti-CD28 mAb, a combination of inflammatory cytokines (IL-1β, IL-6, and IL-23), heat-killed S. aureus, or the TLR2 agonist Pam3Cys-Ser-(Lys)4 (32). After 3 d, cytokines in the supernatant were determined (Fig. 6). Stimulation with anti-CD3 and anti-CD28 mAbs caused the production of IL-17A, IFN-γ, and TNF-α. Incubation with cytokines induced similar levels of IFN-γ and TNF-α, but in contrast to TCR stimulation, we found 10-fold higher concentrations of IL-17A. In addition, IL-17F and IL-22 were only detected after cytokine stimulation. No cytokine production was observed following stimulation with heat-killed S. aureus or Pam3Cys-Ser-(Lys)4. Thus, renal resident γδ T cells were able to produce large amounts of type-3 cytokines in response to inflammatory cytokines.
Fig. 6.
Renal γδ T cells produce type-3 cytokines in response to inflammatory cytokines. TCRdH2BeGF mice were infected with 107 CFU of S. aureus. After 4 wk, mice were treated with ampicillin for 2 wk. Leucocytes were isolated from the kidney and after T cell enrichment by magnetic negative selection, γδ T cells were isolated based on their GFP expression by flow cytometry. γδ T cells were stimulated either with cytokines (IL-1β, IL-6, and IL-23), anti-CD3, and anti-CD28 mAb, heat-killed S. aureus, or Pam3Cys-Ser-(Lys)4. After 72 h, cytokines were determined in the supernatant. Cells were stimulated and analyzed in triplicates or quadruplicates. One representative experiment out of two is shown. LOD: IL-17A 2.0 pg/ml, IFN-γ 1.7 pg/ml, TNF-α 1.5 pg/ml, IL-22 1.8 pg/ml, IL-17F 1.9 pg/ml, IL-10 1.8 pg/ml.
γδ T Cells Provide Protection against Systemic S. aureus Infection.
Next, we asked, whether γδ T cells contribute to S. aureus control during systemic infection. Tcrd−/− mice deficient in γδ T cells and Tcrd+/− control mice were infected with S. aureus and the bacterial load in the spleen and kidneys was determined on day 7 p.i. (SI Appendix, Fig. S7 A and B). Tcrd−/− mice had significantly higher bacterial numbers in the kidneys. Thus, renal γδ T cells participated in protection against S. aureus. This result is consistent with earlier studies in which Tcrd−/− mice showed reduced protection against pulmonary and dermal S. aureus infections (21, 22).
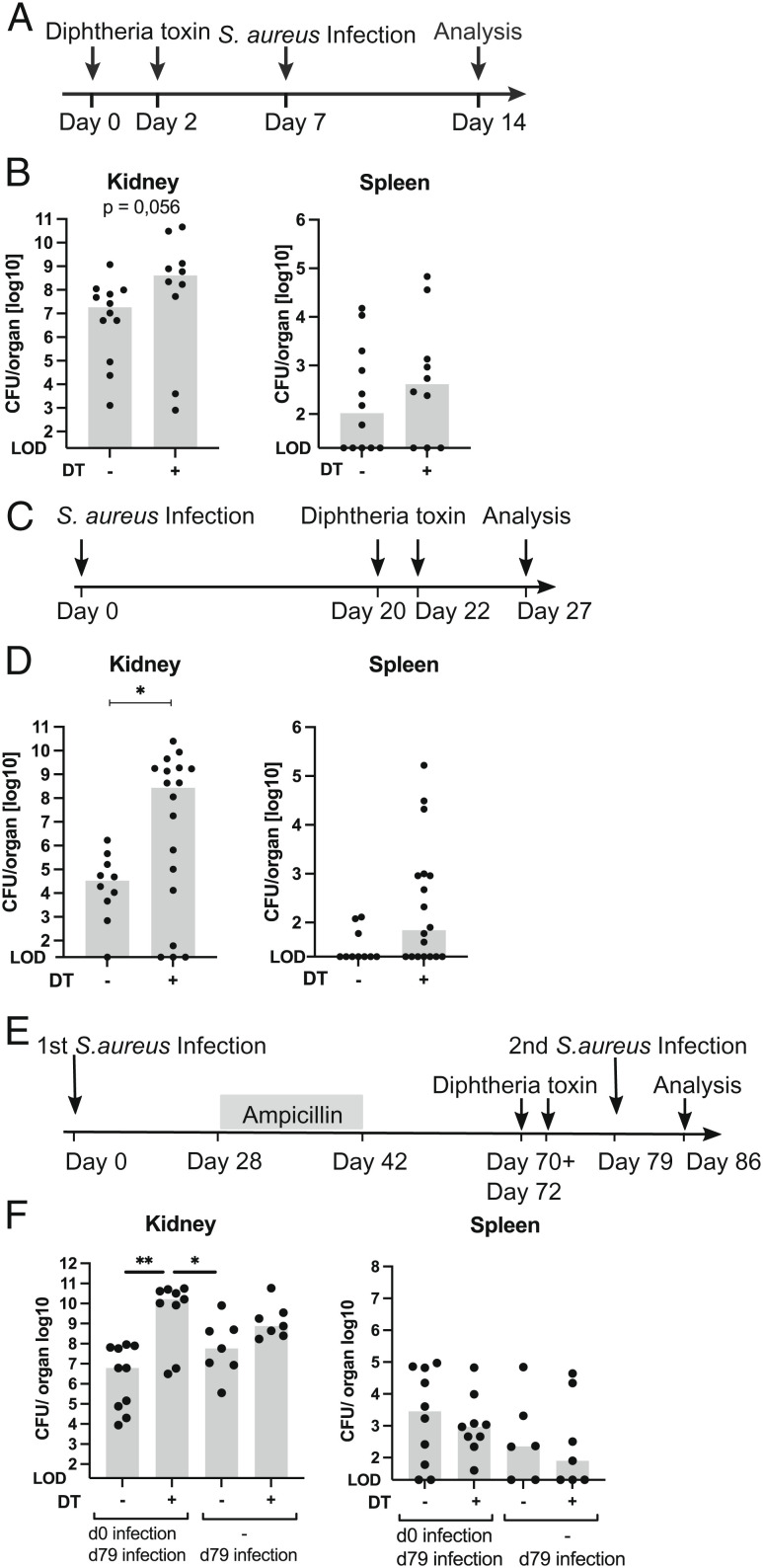
To exclude compensatory mechanisms by other cells taking over functions of γδ T cells in mice born without these cells, we used TcrdGLD mice that express the diphtheria toxin (DT) receptor on γδ T cells and allow depletion of γδ T cells with DT (42). TcrdGLD mice received two doses of 1 µg DT intraperitonealy (i.p.) within a 48-h interval. Seven days later, mice were infected with S. aureus, and after further 7 d, bacterial load in different organs was determined (Fig. 7 A and B and SI Appendix, Fig. S7C). Compared to TcrdGLD mice pretreated with phosphate buffered saline (PBS), mice treated with DT had significantly higher bacterial numbers in the lung and liver and close to significantly higher numbers (P = 0.056) in the kidneys. At this time point, DT-treated mice had still only marginal percentages of renal GFP+ T cells (SI Appendix, Fig. S7 D and E). This result was in line with our findings using Tcrd−/− mice and confirmed the nonredundant protective role of γδ T cells in early systemic S. aureus infection.
Fig. 7.
Depletion of γδ T cells during chronic S. aureus infection and prior to reinfection results in loss of bacterial control. (A, B) On days 0 and 2, TcrdGLD mice received 1 µg DT in PBS i.p. Control animals received PBS only. On day 7, mice were infected i.v. with 107 CFU of S. aureus. Seven days later, bacterial numbers in the kidney, lung, spleen, and liver as well as percentages of renal γδ T cells were determined. (A) Experimental scheme. (B) Bacterial counts in the kidney and spleen. (C, D) TcrdGLD mice were infected i.v. with 107 CFU of S. aureus. On days 20 and 22, mice were treated i.p. with 1 µg DT in PBS or with PBS only. On day 27, bacterial numbers in the kidney, lung, spleen, and liver were determined. (C) Experimental scheme. (D) Bacterial counts in kidney and spleen. (E, F) TcrdGLD mice were infected i.v. with 107 CFU of S. aureus (d0) or remained without infection (−). After 4 wk, all mice were treated with ampicillin in drinking water for 2 wk. On days 70 and 72, mice were treated i.p. with 0.5 µg DT in PBS or with PBS only. On day 79, mice of all groups were infected i.v. with 107 CFU of S. aureus. Seven days later bacterial numbers in the kidney, spleen, liver, and lung were determined. (E) Experimental scheme. (F) Bacterial counts in the kidney and spleen. (B, D, F) Each dot represents one mouse. For each setting, data are pooled from two independent experiments with n = 4 to 10 per group and experiment. Bars show median values. LOD = 20 CFU. Statistics were performed with the Mann–Whitney U test (B, D) or Kruskal–Wallis test and Dunn’s multiple comparisons posttest (F).
γδ T cells produce IL-17A during chronic S. aureus infection indicating a continuous response of these cells. To investigate whether γδ T cells are required to restrict S. aureus replication in the chronic stage of infection, TcrdGLD mice were infected with S. aureus and treated with DT or PBS on days 20 and 22 p.i. (Fig. 7 C and D and SI Appendix, Fig. S7F). When the bacterial load was determined on day 27 p.i., we observed a significant increase in bacterial numbers in the kidney from DT-treated mice. Analysis of renal T cells confirmed the depletion of γδ T cells regarding both GFP+ and γδ TCR+ cells (SI Appendix, Fig. S7 G and H). Thus, renal γδ T cells contribute to the local bacterial control during chronic S. aureus infection.
Finally, to test whether a local increase of renal γδ T cells would provide protection against reinfection, TcrdGLD mice were infected with S. aureus and after 4 wk, mice received ampicillin in the drinking water. On days 70 and 72, mice were treated with DT or PBS, and on day 79, mice were again infected with S. aureus. Analysis of bacterial load revealed similar numbers in the spleen, lung, and liver of mice with and without DT treatment (Fig. 7 E and F and SI Appendix, Fig. S7 I–K). In mice without prior infection, γδ T cell depletion resulted in higher bacterial load in the kidney which, however, did not reach a significant level in this experiment. γδ T cell depletion prior to secondary S. aureus infection caused a significantly higher renal bacterial load, indicating that local protection provided by infection-induced tissue-resident renal γδ T cells could not be fully compensated by other acquired mechanisms in the kidney. In summary, results from γδ T cell depletion experiments demonstrate that γδ T cells provide protection during the initial and chronic stages of kidney infection as well as against secondary infection.
Discussion
This study revealed an enormous expansion and long-lasting accumulation of IL-17-producing γδ T cells in the kidneys after systemic infection with S. aureus, thereby posing the question why this phenomenon was kidney-specific and far less pronounced in other tissue such as the spleen, liver, or lung. It could be a consequence of lower initial and chronic S. aureus load in these tissues since S. aureus grew to particularly high numbers and persisted as a bacterial reservoir in the kidney for the entire observation period of our experiments. Persistence of high numbers of γδ T cells could also be a result of an “empty niche” for innate T cells in naive kidneys. Mice kept under clean condition have generally low numbers of resident immune cells in nonlymphoid tissues, particularly in nonbarrier sites such as the kidney (43). Thus, T cells recruited to the inflamed kidney during S. aureus infection would find abundant space to fill and to consequently remain as Trm cells in large numbers even after eradication of the pathogen.
The dynamics of renal γδ T cell expansion after S. aureus infection supported the hypothesis that acute infection induced a strong initial burst of γδ T cell proliferation followed by long-term persistence. Currently, it is unclear to which extent the response originates from γδ T cells present in the kidney prior to infection or from γδ T cells recruited early in infection. We observed up to 100-fold expansion of the renal γδ T cell population, which was mainly constituted by invariant Vγ6+ cells similar to skin and peritoneal infection models (16, 23). Over time, the majority of renal γδ T cells acquired a CD45iv−CD69+ tissue-resident phenotype, and renal γδ T cells proved to be sessile in parabiosis. At day 6 p.i., a large fraction of γδ T cells also became GFP+, indicating IL-17A production in situ. In contrast to ceasing cell proliferation, γδ T cells continued to produce IL-17A in the chronic phase of infection. We conclude that IL-17A production in the chronic phase was somehow but not exclusively caused by a persisting reservoir of S. aureus, since ampicillin treatment reduced GFP+ expression in renal γδ T cells. Still, γδ T cells retained a low level of IL-17A expression after clearance of the pathogen. The cause of this IL-17A production is currently unclear, but it might be involved in renal tissue regeneration. Overall, the γδ T cell response can be separated into two phases. In the acute response, γδ T cells show fulminant proliferation and cytokine production most likely due to the widespread renal distribution of S. aureus and extensive local inflammation. In the subsequent chronic response, γδ T cells continue to produce IL-17A and probably other cytokines but have ceased to proliferate. The switch from acute to chronic response could be due to regulatory mechanisms aimed at preventing immunopathology but also to local confinement of S. aureus and reduced inflammatory signals sufficient for induction of IL-17A but insufficient for proliferation of γδ T cells.
As antigens recognized by mouse γδ TCR are largely unknown, it remains unclear what activates the γδ T cells during S. aureus infection. In the first days of renal infection, we observed upregulation of NFκB expression, and at day 6, but not at day 14, γδ T cells with nuclear NFκB were detected. Thus, nuclear localization correlated with proliferation but not with cytokine production of γδ T cells. TCR stimulation causes activation of NFκB (37); therefore, nuclear localization could indicate antigen recognition by γδ TCR at this time point. On the other hand, γδ T cells from infected kidneys produced large amounts of cytokines in response to inflammatory cytokines, and for type-3 cytokines IL-17A, IL-17F, and IL-22, levels induced by cytokines were even higher than those after TCR stimulation. Thus, IL-17A secretion in the kidney could well be a consequence of local inflammation. However, NFκB should also become activated after stimulation of receptors for TNF-α or IL-1 family cytokines (37). Currently, we have no explanation for the only transient NFκB activation in the acute infection. Inflammatory signals in chronic infection could trigger NFκB activation below the detection level of our assay, or NFκB translocation could be actively restricted to prevent immunopathology.
Acute depletion of γδ T cells in TcrdGLD mice before S. aureus infection confirmed previous results from Tcrd−/− mice and indicated that other cells cannot fully compensate for the absence of γδ T cells. This suggests that γδ T cells are crucial for controlling the early phases of S. aureus infection. Likewise, depletion of γδ T cells during chronic infection caused a massive increase in renal staphylococci numbers. Thus, γδ T cells were essential for continuously restricting the growth of S. aureus, and CD4+ Th17 cells which also accumulated to high numbers in the infected kidney or other cells induced by the S. aureus infection could not replace γδ T cells in this function. Five days after DT treatment, we still observed almost complete absence of γδ T cells in the kidney, despite substantially increased bacterial numbers. Thus, the thymic output of γδ T cells was not sufficient for rapid replacement of renal γδ T cells, and this insufficiency might even be worsened because Vγ6+ T cells, which largely built the response, are exclusively generated in late embryonic development (42).
Finally, we addressed whether increased numbers of Vγ6+ T cells could provide protection against reinfection. DT-mediated removal of γδ T cells prior to reinfection caused a strong increase in renal S. aureus numbers, and this increase was more pronounced than that after depletion of cells before primary infection. Overall, these results suggest that γδ T cells can provide acquired or adaptive protection. Acquired protection by γδ T cells has been studied in several bacterial infection models. Following lung infection with Bordetella pertussis, Vγ4+ T cells accumulate in the lung and upon reinfection respond with proliferation and rapid IL-17A production (44). In vitro, these Vγ4+ T cells respond to killed B. pertussis but not to other bacteria, suggesting a specific recognition of B. pertussis. Oral L. monocytogenes infection causes the accumulation of IL-17A-secreting Vγ6+ T cells in the mesenteric lymph nodes that protect against oral but not systemic listeria reinfection or oral infection with salmonella, indicating the formation of a local acting listeria-specific memory Vγ6+ T cell population (31, 45). Acquired protection by Vγ6+ T cells was also suggested for skin and peritoneal infection models with S. aureus (23, 24). In these and in our S. aureus infection model, it is not clear whether the protection provided by γδ T cells is specific for S. aureus. In our study, S. aureus infection causes the accumulation and persistence of large numbers of renal γδ T cells which show low basal IL-17A production under homeostatic conditions and strong production of type-3 cytokines after stimulation with inflammatory cytokines. Thus, the enlarged population of renal γδ T cells could also restrict the replication of S. aureus and other invading microbes by an innate type of response to local inflammation.
γδ T cells from mice differ substantially from those of humans. In response to bacterial infections, rapid and sometimes profound expansion of human γδ T cells, particularly of Vγ9Vδ2+ T cells, is observed (46). Vγ9Vδ2+ T cells comprise the majority of γδ T cells in human peripheral blood. They have a semiinvariant TCR repertoire and respond to microbial phosphoantigens in a butyrophilin (BTN)-3A1- and BTN-2A1-dependent manner (24, 47, 48). In healthy individuals, Vγ9Vδ2+ T cells can display a type-3 profile with the expression of RORγt, IL-23R, and CCR6 but rarely produce IL-17A. However, inflammatory cytokines can induce differentiation of Vγ9Vδ2+ T cells to IL-17A+ cells in vitro and increased percentages of IL-17A+ Vγ9Vδ2+ T cells are found in the blood of children with bacterial meningitis (24, 48–51). In a humanized mouse model, Vγ9Vδ2+ T cells provide rapid protection against S. aureus infection (46) and Vγ9Vδ2+ T cells cocultured with S. aureus-infected cells become activated and secrete IFN-γ but little IL-17A (52). Overall, results so far suggest that human γδ T cells contribute to protection against S. aureus; however, the protective mechanism and particularly the role of IL-17A produced by γδ T cells require further investigation.
In conclusion, we showed that γδ T cells strongly accumulate in S. aureus-infected kidneys and are required to restrict bacterial growth in the acute but also in the chronic phase of infection. Furthermore, long-term persistence of invariant IL-17A-producing γδ T cells after bacterial clearance conferred an acquired protection against reinfection, thus establishing the concept of kidney-resident innate-like memory γδ T cells.
Materials and Methods
Wild-type and transgenic mice were infected with 1 to 5 × 107 CFU of S. aureus in 100 µl sterile PBS via the tail vein. Bacterial clearance was achieved by adding ampicillin (1g/l) to the drinking water for 2 wk. Bacterial numbers in the kidney, lung, liver, and spleen were quantified by plating serial dilutions of homogenized organs on lysogeny broth agar. Details on depletion of γδ T cells, parabiosis, and analysis of T cell responses of mice are given in SI Appendix, Material and Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Dr. Friedrich Koch-Nolte for providing anti-Art2b nanobodies. This study was supported by grants from Deutsche Forschungsgemeinschaft (CRC 1192 to N.G., U.P., C.F.K., C.M.-S, and H.-W.M. and PR727/11-2 and PR727/13-1 to I.P.), ANID/BASAL/FB210008 (to M.R.B), and Conicyt/FONDEQUIP/EQM140016 (to M.R.B).
Author contributions
T.B., D.R., N.G., U.P., C.F.K., I.P., and H.-W.M. designed research; T.B., D.R., N.C.L., C.S., J.S., L.C.H., P.B., G.R., S.Z., M.H., P.B., S.N., M.V.R., M.R.B., and C.M.-S. performed research; H.R., N.G., I.S., and I.P. contributed new reagents/analytic tools; T.B., D.R., C.M.-S., and H.-W.M. analyzed data; and T.B. and H.-W.M. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Tong S. Y., Davis J. S., Eichenberger E., Holland T. L., Fowler V. G. Jr., Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang C. I., et al. , Bloodstream infections in adult patients with cancer: Clinical features and pathogenic significance of Staphylococcus aureus bacteremia. Support Care Cancer. 20, 2371–2378 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Buvelot H., Posfay-Barbe K. M., Linder P., Schrenzel J., Krause K. H., Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. F.E.M.S. Microbiol. Rev. 41, 139–157 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Mölne L., Verdrengh M., Tarkowski A., Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 68, 6162–6167 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson C. M., et al. , Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J. Surg. Res. 150, 278–285 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielinski C. E., et al. , Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Bacher P., et al. , Regulatory T cell specificity directs tolerance versus allergy against aeroantigens in humans. Cell 167, 1067–1078 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Bröker B. M., Mrochen D., Péton V., The T cell response to Staphylococcus aureus. Pathogens 5, 31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong P. F., et al. , An update on the hyper-IgE syndromes. Arthritis. Res. Ther. 14, 228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Casanova J. L., Puel A., Mucocutaneous IL-17 immunity in mice and humans: Host defense vs. excessive inflammation. Mucosal Immunol. 11, 581–589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishigame H., et al. , Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Cho J. S., et al. , IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudva A., et al. , Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J. Immunol. 186, 1666–1674 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher B. M., et al. , Nlrp-3-driven interleukin 17 production by γδ T cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect. Immun. 81, 4478–4489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan L. C., et al. , Nonredundant roles of interleukin-17A (IL-17A) and IL-22 in murine host defense against cutaneous and hematogenous infection due to methicillin-resistant Staphylococcus aureus. Infect. Immun. 83, 4427–4437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchitto M. C., et al. , Clonal Vγ6+Vδ4+ T cells promote IL-17-mediated immunity against Staphylococcus aureus skin infection. Proc. Natl. Acad. Sci. U.S.A. 116, 10917–10926 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y. X., Tarkowski A., Impact of interferon-gamma receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. J. Immunol. 155, 5736–5742 (1995). [PubMed] [Google Scholar]
- 18.McLoughlin R. M., Lee J. C., Kasper D. L., Tzianabos A. O., IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 181, 1323–1332 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Gaudreau M. C., Lacasse P., Talbot B. G., Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine. 25, 814–824 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Zhang F., et al. , Protection against Staphylococcus aureus colonization and infection by B- and T-cell-mediated mechanisms. mBio. 9, e01949–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mölne L., Corthay A., Holmdahl R., Tarkowski A., Role of gamma/delta T cell receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus. Clin. Exp. Immunol. 132, 209–215 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P., et al. , Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 13, 38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy A. G., et al. , Staphylococcus aureus infection of mice expands a population of memory γδ T cells that are protective against subsequent infection. J. Immunol. 192, 3697–3708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillen C. A., et al. , Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J. Clin. Invest. 128, 1026–1042 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baral P., et al. , Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 24, 417–426 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas W., Pereira P., Tonegawa S., Gamma/delta cells. Annu. Rev. Immunol. 11, 637–685 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Ribot J. C., Lopes N., Silva-Santos B., gammadelta T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 21, 221–232 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Hayday A. C., γδ T cell update: Adaptate orchestrators of immune surveillance. J. Immunol. 203, 311–320 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Martin B., Hirota K., Cua D. J., Stockinger B., Veldhoen M., Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Sutton C. E., et al. , Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Sheridan B. S., et al. , γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 39, 184–195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krebs C. F., et al. , Pathogen-induced tissue-resident memory TH17 (TRM17) cells amplify autoimmune kidney disease. Sci. Immunol. 5, eaba4163 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Prinz I., et al. , Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat. Immunol. 7, 995–1003 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Reinhardt A., et al. , Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in Mice. Arthritis Rheumatol. 68, 2476–2486 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Wilharm A., et al. , Microbiota-dependent expansion of testicular IL-17-producing Vγ6+ γδ T cells upon puberty promotes local tissue immune surveillance. Mucosal Immunol. 14, 242–252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner J.-E., Becker M., Mittrücker H.-W., Panzer U., Tissue-resident lymphocytes in the kidney. J. Am. Soc. Nephrol. 29, 389–399 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruland J., Mak T. W., Transducing signals from antigen receptors to nuclear factor kappaB. Immunol. Rev. 193, 93–100 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Lockhart E., Green A. M., Flynn J. L., IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177, 4469–4662 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Shibata K., Yamada H., Hara H., Kishihara K., Yoshikai Y., Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178, 4466–4472 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Wilharm A., et al. , Mutual interplay between IL-17-producing γδ T cells and microbiota orchestrates oral mucosal homeostasis. Proc. Natl. Acad. Sci. U.S.A. 116, 2652–2661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas J. D., et al. , CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur. J. Immunol. 39, 3488–3497 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Sandrock I., et al. , Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing γδ T cells. J. Exp. Med. 215, 3006–3018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beura L. K., et al. , Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misiak A., Wilk M. M., Raverdeau M., Mills K. H., IL-17-producing innate and pathogen-specific tissue resident memory γδ T cells expand in the lungs of Bordetella pertussis-infected mice. J. Immunol. 198, 363–374 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Romagnoli P. A., Sheridan B. S., Pham Q. M., Lefrançois L., Khanna K. M., IL-17A-producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc. Natl. Acad. Sci. U.S.A. 113, 8502–8507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Kamath A., Das H., Li L., Bukowski J. F., Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J. Clin. Invest. 108, 1349–1357 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gay L., et al. , Role of Vγ9vδ2 T lymphocytes in infectious diseases. Front. Immunol. 13, 928441 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ness-Schwickerath K. J., Jin C., Morita C. T., Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J. Immunol. 184, 7268–7280 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan L., et al. , A fetal wave of human type 3 effector γδ cells with restricted TCR diversity persists into adulthood. Sci. Immunol. 6, eabf0125 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Caccamo N., et al. , Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood 118, 129–138 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Moens E., et al. , IL-23R and TCR signaling drives the generation of neonatal Vgamma9Vdelta2 T cells expressing high levels of cytotoxic mediators and producing IFN-gamma and IL-17. J. Leukoc. Biol. 89, 743–752 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Cooper A. J. R., Lalor S. J., McLoughlin R. M., Activation of human Vδ2+ γδ T cells by Staphylococcus aureus promotes enhanced anti-Staphylococcal adaptive immunity. J. Immunol. 205, 1039–1049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.