Significance
Combining rapid carbon dioxide fixation and growth, brown algae shape atmosphere chemistry that regulates climate. Brown algae exude dissolved organic molecules. Some of these molecules provide long-term carbon storage in the dissolved organic carbon pool, which holds substantial amounts of carbon. Accounting the carbon that comes from exudates requires analytic techniques to identify and quantify its molecules in the ocean. In this article, introducing quantitative tools for marinepolysaccharides, we show the extracellular matrix polysaccharide fucoidan to constitute up to half of brown algal exudates. This quantification of fucoidan can assess its contribution to carbon sequestration. Fucus vesiculosus contributes to carbon sequestration through the secretion of fucoidan without concomitant nutrient removal, expanding algal carbon dioxide removal to include secreted molecules.
Keywords: blue carbon, brown macroalgae, fucoidan, carbon sequestration, marine carbon cycle
Abstract
Brown algae annually convert gigatons of carbon dioxide into carbohydrates, including the complex extracellular matrix polysaccharide fucoidan. Due to its persistence in the environment, fucoidan is potentially a pathway for marine carbon sequestration. Rates of fucoidan secretion by brown algae remain unknown due to the challenge of identifying and quantifying complex polysaccharides in seawater. We adapted the techniques of anion exchange chromatography, enzyme-linked immunosorbent assay, and biocatalytic enzyme-based assay for detection and quantification of fucoidan. We found the brown alga Fucus vesiculosus at the Baltic Sea coast of south-west Finland to secrete 0.3% of their biomass as fucoidan per day. Dissolved fucoidan concentrations in seawater adjacent to algae reached up to 0.48 mg L−1. Fucoidan accumulated during incubations of F. vesiculosus, significantly more in light than in darkness. Maximum estimation by acid hydrolysis indicated fucoidan secretion at a rate of 28 to 40 mg C kg−1 h−1, accounting for 44 to 50% of all exuded dissolved organic carbon. Composed only of carbon, oxygen, hydrogen, and sulfur, fucoidan secretion does not consume nutrients enabling carbon sequestration independent of algal growth. Extrapolated over a year, the algae sequester more carbon into secreted fucoidan than their biomass. The global utility of fucoidan secretion is an alternative pathway for carbon dioxide removal by brown algae without the need to harvest or bury algal biomass.
Brown algae fix more carbon per area than terrestrial forests (1–4), and brown algal biomass is considered a potential carbon sink (5–9). However, algae release 14 to 35% of net primary production as dissolved molecules (10–15). Whether these exudates fuel microbial remineralization or contribute to carbon sequestration through recalcitrance or export to the deep sea remains under debate. Dissolved organic carbon concentrations decrease offshore with distance to kelp forests, but the high molecular weight polysaccharide fraction has shown considerable persistence (12, 16, 17). Remineralization rates of carbohydrates depend on the molecular structure (18). There is a need to constrain the molecular composition of brown algal exudates to understand their role in the ecosystem and in the global carbon cycle.
Primary producers, including algae, fix carbon into carbohydrates (19), which serve a multitude of functions. Brown algae mainly synthesize the polysaccharides laminarin, cellulose, alginate, and fucoidan: laminarin and cellulose from the immediate photosynthesis product glucose, alginate from uronic acids, and fucoidan from fucose. Fucoidan can be decorated with additional monosaccharides, as well as acetyl and sulfate groups giving fucoidan a net negative charge (20–22). While laminarin stores energy within cells (21), cellulose and alginate fulfill structural functions as cell wall constituents (20). Fucoidan builds the extracellular matrix or mucilage in brown algae (23, 24), increases in response to salinity (24, 25), and acts as an antimicrobial agent (26).
Fucoidan is delivered to the extracellular matrix by vesicle cells that secrete fucoidan into mucilage ducts (25, 27, 28). Thereby, brown algae maintain a mucilage barrier primarily consisting of fucoidan (20, 22, 27). Secretion of carbohydrates is a feature of epithelia and can mediate substantial carbon flux (29, 30). The high solubility of fucoidan and its location at the algae–seawater interface suggest that fucoidan has to be constantly resupplied.
Fucoidan shows considerable recalcitrance and has been found to persist for centuries in sediments. Dissolved molecules can sequester carbon if they are not degraded due to recalcitrance or if they are exported to below 1,000-m depth (31, 32), for example after aggregation (33–35). Dissolved energy storage polysaccharides like laminarin are preferentially targeted by bacteria of different classes (36–38) that need only three enzymes for complete degradation within hours (37, 39–41). In contrast, fucoidan can only be degraded by specialized bacteria that require dozens of different enzymes (35, 42). Fucoidan-targeting antibodies revealed fucose-containing sulfated polysaccharides from diatoms assemble and form particles (34, 35) that transport carbon to depth. Consistent antibody signals along sediment cores indicate that fucoidan persists during transport through the water column and in sediments for hundreds of years (43, 44). Thus, mucus secretion by brown algae could mediate carbon sequestration through the recalcitrance and assembly of fucoidan.
We hypothesized that brown algae secrete fucoidan into seawater based on reports of high carbohydrate content in dissolved organic carbon from brown algae (10, 12). Without methods for dissolved polysaccharide quantification, fucoidan concentrations in seawater remain unknown (35). We analyzed seawater from seagrass and brown algae incubations with approaches dedicated to specific and quantitative carbohydrate detection. Monosaccharide quantification, antibody binding, anion exchange chromatography, and enzymatic hydrolysis conclusively point toward the accumulation of dissolved fucoidan during algae incubations. Fucoidan carbon contributed substantially to the alga-released organic carbon. Our results suggest secreted fucoidan to sequester carbon at the same rate as carbon is stored in biomass.
Results
Accumulating Dissolved Organic Carbon from Brown Algae.
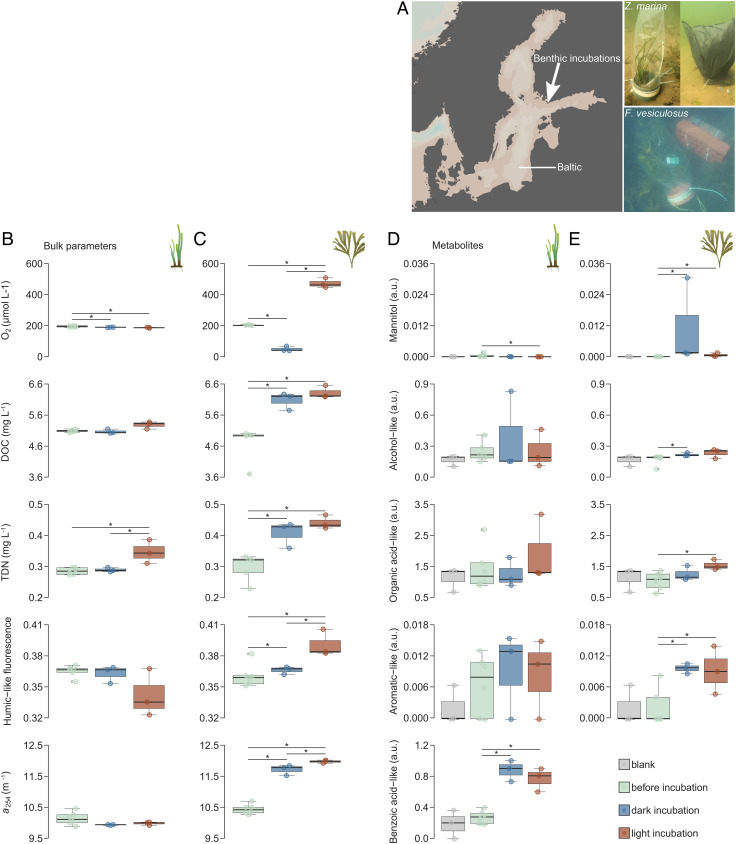
We incubated subtidal bladderwrack, Fucus vesiculosus, and co-occurring eelgrass, Zostera marina, during August 2020 in the coastal Baltic Sea over 5 h in transparent or dark bags (Fig. 1A) and analyzed seawater from incubations. Z. marina served as a control species that secretes organic carbon, but not fucoidan. We found differing impacts on seawater chemistry (Fig. 1 B and C and SI Appendix, Tables S1 and S2). In response to light and dark treatments (SI Appendix, Fig. S1), oxygen concentrations in algae incubations surpassed solubility in light and decreased to ~50 µmol L−1 in dark (Fig. 1C, first panel). Dissolved organic carbon (mean ± SE) increased from 4.71 ± 0.23 mg C L−1 to 6.32 ± 0.12 mg C L−1 in light incubations of algae and to 6.08 ± 0.16 mg C L−1 in the dark (Fig. 1C, second panel). Concurrently, total dissolved nitrogen concentrations, humic-like fluorescence (FDOM), and absorption at 254 nm (CDOM) dissolved compounds increased (Fig. 1C, third to fifth panel). Dissolved oxygen, FDOM, and CDOM in light incubations exceeded dark incubations. The statistical results are summarized in SI Appendix, Table S1. In contrast to F. vesiculosus, dissolved oxygen concentrations decreased in both Z. marina incubations. Dissolved organic carbon concentrations increased slightly in light, while dissolved nitrogen increased significantly in the light incubations compared with dark incubations (SI Appendix, Table S2). Overall, the incubations of F. vesiculosus reproduced previously observed effects of brown algae on bulk seawater organic chemistry (10–12, 15, 45, 46)
Fig. 1.
Dissolved organic carbon accumulated during brown algae incubations in the Baltic Sea in south-west Finland (A, white arrow in Left panel). Eelgrass, Zostera marina (A, Top Right panel), and bladderwrack, Fucus vesiculosus (A, Bottom Right panel), were incubated for 5 h in transparent bags with and without shading. Before and after incubations, filtered seawater oxygen (O2), dissolved organic carbon (DOC), and total dissolved nitrogen (TDN) concentrations along with humic-like fluorescence (peak c) and chromophoric dissolved organic matter (CDOM) absorption coefficient a(254) were determined for Z. marina (B) and F. vesiculosus (C). Accumulation of mannitol and four unidentified metabolites (in arbitrary units, a.u.) in incubations could be observed using SeaMet, a derivatization and gas chromatography–mass spectrometry approach for marine samples (D and E). Asterisks indicate statistical significance (Benjamini–Hochberg adjusted P < 0.05) according to Conover–Iman test (B and C) and Wilcoxon signed rank test (D and E).
Metabolomics on seawater samples from incubations showed that small molecules increased in concentration but did not drive the increase in dissolved organic carbon observed in algae incubations (Fig. 1E and SI Appendix, Tables S3 and S4). Signals of derivatized metabolites on a gas chromatography-coupled mass spectrometer were minimal compared with signals from a standard chemical mixture at 0.4 mM (SI Appendix, Table S5). Mannitol was the only metabolite out of four significantly accumulating compounds that could be identified by matching to the standard chemical mixture (Fig. 1E, first panel). It showed the highest accumulation in algal incubations, with average signal increases of ~1190-fold and ~70-fold after the dark and light incubations, respectively. Previous studies have described mannitol accumulation up to 400 mg L−1 during brown algae fragmentation (17) and mannitol release in response to osmotic shock of up to 6 g kg−1 dry weight within 2 min (47). Mannitol from brown algae is completely consumed in seawater within days (17). Metabolomics also detected significant signal increases of an alcohol-like, an organic acid-like, and an aromatic-like metabolite from ~1.5-fold to ~10-fold after incubation of algae (Fig. 1E, second to fifth panel). In seagrass incubations, a benzoic acid-like metabolite accumulated that could not be found in algae incubations (Fig. 1D, fifth panel). In terms of carbon mass, mannitol and the three unidentified metabolites contributed minor quantities to the increase in dissolved organic carbon during algae incubations.
Monosaccharide Composition, Antibody Binding, and Enzymatic Digestion Identify Dissolved Fucoidan.
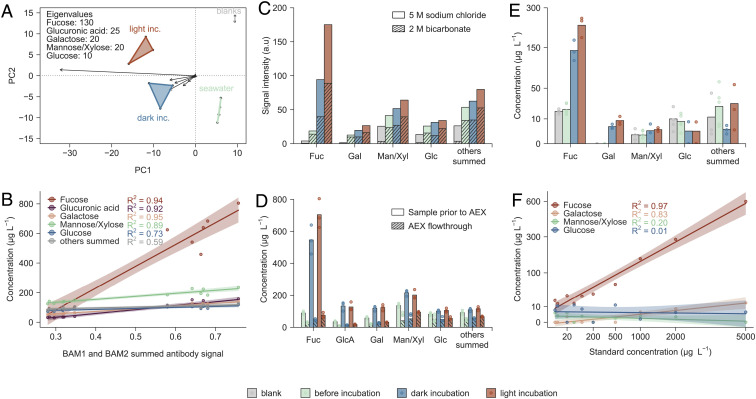
We identified dissolved fucoidan in ambient and incubation seawater via two complementary approaches, monosaccharide composition and antibody-based techniques. After acid hydrolysis, the monosaccharides fucose, glucuronic acid, mannose and xylose, and galactose separated sample groups of blank samples, ambient seawater, and light and dark incubations of F. vesiculosus in a principal component analysis (Fig. 2A). These monosaccharides compose F. vesiculosus fucoidan (42). Additionally, the concentrations of fucose, glucuronic acid, mannose and xylose, and galactose correlated with the summed signal intensity of fucoidan-specific antibodies BAM1 and BAM2 (24)(analyzed by ELISA) with adjusted R2 ~ 0.9 or higher (Fig. 2B). Mannuronic acid in the range of 5 to 20 µg L−1 indicated the presence of dissolved alginate, however ~30-fold lower concentrated than fucoidan (SI Appendix, Fig. S2). Monosaccharide composition and antibody binding supported a major contribution of fucoidan to carbohydrates in seawater from algae incubations.
Fig. 2.
Polysaccharide-targeting techniques enabled specific quantification of dissolved fucoidan. (A) Acid hydrolyzed samples from algae incubations are plotted in a principal component analysis of monosaccharide concentrations with eigenvalue lengths of dominant monosaccharides. (B) Monosaccharide concentrations after acid hydrolysis are plotted against the summed signal absorbance at 450 nm, measured by ELISA, of two fucoidan-specific antibodies. (C) Washing with 2 M bicarbonate buffer removed considerable amounts of monosaccharides (striped bars) prior to elution with 5 M NaCl (solid bars). (D) Monosaccharide concentrations in acid hydrolyzed samples (solid bars) exceeded monosaccharides in the flow-through of AEX (striped) several folds. (E) Monosaccharides in samples, released using enzymes in the soluble cell fraction of fucoidan-metabolizing Lentimonas sp. (F) Calibration curves for monosaccharides from enzymatic digestion of fucoidan from F. vesiculosus. Abbreviations: incubation (inc.), arbitrary units (a.u.), anion exchange chromatography (AEX), fucose (Fuc), galactose (Gal), mannose/xylose (Man/Xyl), glucose (Glc), and glucuronic acid (GlcA).
A fucose-dominated fraction from filtered seawater eluted after application of 5 M NaCl to anion exchange chromatography (AEX) columns (Fig. 2C). Prior washing with 2 M bicarbonate eluted a fraction with a lower relative abundance of fucose, and monosaccharides that are not present in F. vesiculosus fucoidan. (Fig. 2C, striped bars). Binding to the AEX column showed that 70 to 80% of fucose, galactose, and glucuronic acid were contained in anionic polymers (Fig. 2D). As in seawater samples, the signal from fucoidan-specific antibodies BAM1 and BAM2 (analyzed by carbohydrate microarrays) correlated with fucose concentration in AEX elutions from brown algae incubations and ambient seawater (SI Appendix, Fig. S3). For comparison, samples from Z. marina incubations showed no binding of BAM1 and BAM2 fucoidan-specific antibodies, but in some replicates binding of BAM7 antibody that recognizes alginate, and has reported cross-reactivity with pectin and to a lesser extent with fucoidan (48) (SI Appendix, Fig. S3). BAM7 antibody signal on Z. marina correlated linearly with galactose (P < 0.001, R2 = 0.98), fucose (P < 0.001, R2 = 0.91), and xylose (P < 0.001, R2 = 0.66). This correlation may indicate the presence of anionic polysaccharides of seagrass origin, including sulfated galactans, which also contain xylose (49), or arabinogalactan-proteins (50). AEX elutions obtained with 5 M NaCl contained strongly anionic fucoidan, while the washing step eluted some fucose-containing and other anionic carbohydrates.
Detection of fucoidan monosaccharides as products of enzymatic activities confirmed the presence of F. vesiculosus fucoidan in seawater. Digests were performed with enzymes extracted from the fucoidan-metabolizing Lentimonas sp. CC4 grown on F. vesiculosus fucoidan. Enzymatic digestion of F. vesiculosus fucoidan from standards and environmental samples yielded quantitative amounts of fucose and galactose (Fig. 2 E and F). In a Bray–Curtis dissimilarity analysis, enzymatic hydrolysis of filtered seawater and acid hydrolysis of AEX elutions showed higher specificity than acid hydrolysis of filtered seawater (SI Appendix, Fig. S4). We used this enzymatic approach for fucoidan-specific hydrolysis to produce the most specific quantitative assessment of fucoidan. Enzymatic hydrolysis of filtered and dialyzed seawater confirmed the accumulation of F. vesiculosus fucoidan during incubations.
Specific Quantification Reveals the Accumulation of Dissolved Fucoidan in Brown Algae Incubations.
The seawater collected from the F. vesiculosus incubations after 5 h was enriched in dissolved fucoidan (Table 1 and SI Appendix, Table S6). Fucan-specific monoclonal antibody binding qualitatively verified the presence of fucoidan in all seawater samples, and showed accumulation during algae incubations compared with control samples. Quantitative estimates of fucoidan in seawater from algae incubations range between 1,333 and 3,755 µg L−1 in light and between 614 and 2,913 µg L−1 in dark. These concentrations exceed fucoidan levels prior to incubations (62 to 476 µg L−1) in ambient seawater. Fucoidan accumulated more under light conditions compared with dark conditions, a trend that was consistent across methods.
Table 1.
Combined polysaccharide-specific quantification confirms fucoidan accumulation during brown algae incubations
| Method | Blank | Initial | Dark inc. | Light inc. | Specific | Quantitative |
|---|---|---|---|---|---|---|
| Antibody BAM1 | 0.07 ± 0.00a | 0.23 ± 0.01b | 0.38 ± 0.01c | 0.41 ± 0.03c | ++ | o |
| Antibody BAM2 | 0.07 ± 0.00a | 0.08 ± 0.00a | 0.28 ± 0.01b | 0.27 ± 0.01b | ++ | o |
| Acid hydrolysis | 0.5 ± 0.3a | 476 ± 15.5b | 2913 ± 279c | 3755 ± 282c | o | ++ |
|
Acid hydrolysis of 5 M NaCl elution |
42.6 ± 8.0a | 61.9 ± 9.35a | 614 ± 170b | 1333 ± 94c | + | + |
| Enzymatic hydrolysis | 136 ± 8.9a | 154 ± 19.5a | 1135 ± 145b | 1832 ± 182b | ++ | + |
Relative signal intensities (mean ± SE) of two fucoidan-binding monoclonal antibodies (BAM1 and BAM2) from ELISA on filtered seawater samples on algae incubations. Calculated concentrations (µg L−1mean ± SE) of dissolved fucoidan in algae incubation samples based on acid hydrolysis, AEX followed by acid hydrolysis and enzymatic hydrolysis. Fucose from hydrolyzed fucoidan standard was the best quantitative predictor (SI Appendix, Fig. S6), and used to calculate sample fucoidan concentrations. Superscripted letters indicate significant differences between groups (blank, initial, dark inc., and light inc.) within each method (SI Appendix, Tables S6 and S7). How specific and quantitative approaches are is indicated from low (o) to high (++) by symbols. Abbreviations: incubation (inc.), anion exchange chromatography (AEX).
The employed approaches differed in how specific and how quantitative polysaccharide detection is achieved (Table 1). Acid hydrolysis reported the highest values, followed by enzymatic hydrolysis and finally acid hydrolysis of 5 M NaCl elutions. Using acid hydrolysis of filtered seawater to estimate dissolved fucoidan yielded the lowest levels of contamination and fucoidan concentrations in ambient seawater distinct from blanks. Acid hydrolysis of 5 M NaCl elutions produced fucoidan estimates lower than estimates from direct acid hydrolysis of filtered, dialyzed seawater due to losses during AEX. Yet, selectivity and the concentration factor associated with this method revealed a significant influence of treatment (SI Appendix, Table S7; light vs. dark, P = 0.035) on fucoidan estimates. Enzymatic hydrolysis of filtered, dialyzed seawater produced estimates in-between acid hydrolysis and AEX values. Specificity and quantitative signal of enzymatic hydrolysis suggest this to be a potential stand-alone method for fucoidan quantification. The three quantitative approaches converge on a clear pattern of fucoidan content in samples, with comprehendible differences in absolute values.
Fucoidan Secretion Drives Dissolved Carbon Flux.
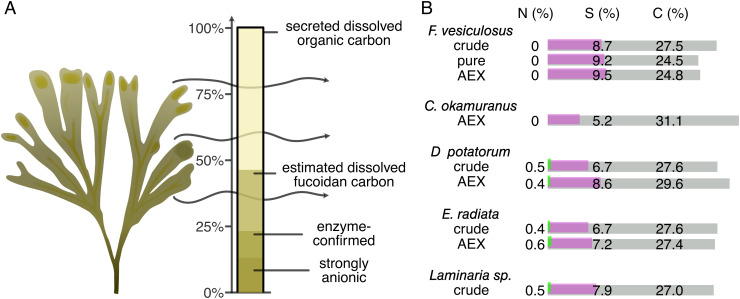
Fucoidan contributed substantially to the accumulation of dissolved organic carbon, but not to the accumulation of dissolved nitrogen (Fig. 3A). Enzyme-confirmed fucoidan quantification accounted for 25.4 ± 3.2% of organic carbon exudation in light incubations and 18.0 ± 4.5% in dark incubations. Based on acid hydrolysis, fucoidan secretion accounted for 49.5 ± 1.8% of the organic carbon exudation in light, and 43.8 ± 4.4% in dark. Carbon content of fucoidan was based on elemental analysis of pure F. vesiculosus fucoidan. Brown algal fucoidans consisted of 25 to 30% carbon, 5 to 10% sulfur, and 0 to 0.6% nitrogen (Fig. 3B). Absorbance of fucoidans showed a distinct difference between crude and pure or AEX-purified standards, with crude standards showing a higher absorbance of light between 250 and 450 nm in all cases (SI Appendix, Fig. S5). Molecules associated with fucoidan such as phlorotannins likely contributed to the increased CDOM and FDOM signals but may fall short of explaining nitrogen accumulation (15). Fucoidan explained 18 to 25% or 44 to 50% of the dissolved organic carbon that accumulated during algae incubations, based on enzymatic hydrolysis and acid hydrolysis, respectively.
Fig. 3.
Up to 50% of F. vesiculosus-secreted dissolved organic carbon was fucoidan. (A) Fucoidan obtained via AEX accounted for 13%, enzyme-confirmed fucoidan for 22% and fucoidan calculated from acid hydrolysis for 47% of secreted DOC. (B) Elemental composition of fucoidans from various brown algae shows carbon and sulfur content along with near absence of nitrogen. Abbreviations: anion exchange chromatography (AEX), Fucus (F.), Cladosiphon (C.), Durvillea (D.), Ecklonia (E.).
Secretion rates could be calculated by direct assessment of dissolved fucoidan. Secretion rates of fucoidan carbon per kg dry biomass under light conditions amounted to 19.9 ± 5.08 mg C kg−1 h−1 based on enzyme hydrolysis and 39.2 ± 10.37 mg C kg−1 h−1 based on acid hydrolysis. Under dark conditions, algae secreted 11.5 ± 3.46 and 28.3 ± 7.10 mg C kg−1 h−1 based on enzyme and acid hydrolysis, respectively. Dry algal thalli biomass, excluding epibiota, varied between 16.5 and 29.7 g, while bag volumes ranged between 4.8 and 6.8 L. Rates of organic carbon exudation of 45.6 to 106.8 mg C kg−1 h−1 as determined here are lower than previously reported rates for F. vesiculosus of 127.2 and 298.8 mg C kg−1 h−1, but within the range of values reported for brown algae (summarized in ref. 51). There was no significant correlation between fucoidan accumulation and algal biomass or algal surface area. For a day with 15-h daylight, we estimated a rate of fucoidan secreted per dry biomass at 360 mg C kg−1 d−1 based on enzymatic hydrolysis and at 789 mg C kg−1 d−1 based on acid hydrolysis. This compares with a mean biomass accumulation of 240 mg C kg−1 d−1 for a 4-y-old algal stock (52, 53). Carbon removal by fucoidan secretion is on the same order of magnitude as carbon accumulation in biomass.
Discussion
Brown algae secrete a substantial fraction of fixed carbon as organic material, of which we found up to 50% to be in the form of fucoidan. Algae incubations led to an accumulation of dissolved organic carbon from 4.7 mg L−1 to 6.2 mg L−1. We performed acid hydrolysis, anion exchange chromatography, enzyme-linked immunosorbent assay, and a biocatalytic enzyme-based assay on seawater samples. The combination of methods enabled identification and quantification of complex fucoidan, accounting for up to half of the accumulated organic matter. Despite different analytical windows, the techniques converged on a consistent pattern comparing treatments, with higher accumulation in light (Table 1). The findings reported relieve the major analytical constraint that has until now prohibited quantification of rates of fucoidan release by brown algae. The substantial secretion rates suggest that fucoidan production and release contribute to carbon sequestration by brown algae.
A combination of techniques is required to quantify complex polysaccharides such as fucoidan. We examined three approaches for fucoidan quantification. 1) Acid hydrolysis combined with antibody identification showed fucoidan release by F. vesiculosus. Acid indiscriminately hydrolyzed glycosidic and ester bonds, yielding ~90% of quantifiable monosaccharide constituents (37). Monoclonal antibodies provided specificity with a semi-quantitative assessment (24, 35, 43). Backed by identification on a structural level by antibodies, acid hydrolysis provides an upper boundary of fucoidan concentrations. 2) AEX narrowed the analytical window by selectively extracting anionic polysaccharides from seawater. As binding strength increases with fucoidan length, fucoidan molecules of very high molecular weight may bind irreversibly to the AEX resin. AEX underestimated fucoidan as low molecular weight compounds were washed away (Fig. 2C) and elution was potentially incomplete. 3) Enzymatic hydrolysis specifically yielded monosaccharides from F. vesiculosus fucoidan, providing confidence in the molecular identity. The soluble cell fraction of Lentimonas sp. CC4 grown on F. vesiculosus fucoidan showed activity on cellulose, alginate, and F. vesiculosus fucoidan, and lower to no activity on galactomannan, pectin, and Laminaria hyperborea fucoidanin line with previous results (42). Species-specific fucoidan quantification is thus theoretically possible with enzymatic tools. Incomplete hydrolysis of fucoidan and possible conversion of fucose by downstream enzymes likely decrease the yield of this method. Lower molecular weight of the fucoidan standard compared with samples may have led to an additional underestimation of fucoidan concentrations. Technical noise likely caused by fucoidan substrate remaining in the soluble cell fraction impaired quantification below 0.1 mg L−1 fucoidan. It is possible to concentrate fucoidan from seawater by AEX and detect fucoidan in extracts by enzymatic hydrolysis (SI Appendix, Fig. S6). The combination of carbohydrate-targeting techniques affirmed the identification and quantification of dissolved F. vesiculosus fucoidan, paving the way for fucoidan tracing in marine environments.
The molecular composition of brown algae exudates reported elsewhere suggests fucoidan secretion to be a common feature. Brown algae release fucoidan into mucilage ducts, which deliver the polysaccharide to the thallus surface (23, 24, 28) from where it dissolves (24, 35, 54). In comparison with alginate, fucoidan undergoes less bridging with divalent cations and exhibits lower viscosity (20, 22, 54). Dedicated vesicle cells for fucoidan production and secretion support the notion of fucoidan loss to the surrounding seawater (23, 27, 28). Green and red algae lack this dedicated machinery of fucoidan production (22). In direct comparison, brown, green, and red algae show similar exudation rates, but widely different molecular compositions (55). Compared with green and red algae, brown alga-exuded organics contain four times as much carbohydrate carbon, of which 40% is in fucose (55). With vesicle cells and mucilage constituting a widespread feature of brown algae and reports of carbohydrate-rich, fucose-dominated exudates, fucoidan secretion appears to occur across this cosmopolitan taxon.
Fucoidan secretion in combination with constrained microbial degradation indicates sequestration of brown algal exudates in the ocean. In the laboratory with replete nutrients and no competition or pathogens, adapted bacteria degrade about 40% of fucoidan within days (56). In nature under suboptimal conditions, fucoidan degradation takes more time (57). About 10 to 30% of organic matter moved and stored in the ocean is glycan and within this glycan mixture there is about 10% fucose, the major building block of fucoidan (57). Fucose-containing sulfated polysaccharides are dissolved, present in particles (34, 58), and exported showing it is stable enough to reach the deep sea and underlying sediment (43, 44). How much carbon fucoidan removes becomes measurable only with these or other techniques that provide sufficient molecular resolution. Several studies established resistance of carbohydrate-rich brown algal exudates to microbial degradation, either due to intrinsic recalcitrance or inhibition of microbes. Bacteria grown on exudates of Turbinaria ornata exhibit lower growth and growth efficiency than bacteria growing on exudates of green or red algae from the same habitat (55). In degradation studies, between 30 and 85% of organic carbon exuded by Ecklonia cava remained after 30 d (16), while almost 40% of Saccharina japonica exudates and 78% of Sargassum horneri exudates persisted for 150 d (3, 45).
This study focused on fucoidan secretion of F. vesiculosus, and we acknowledge the limited experimental setting and single sampling location. The dissolved organic carbon exudation rates and abundant carbohydrates reported here, particularly fucose, are consistent with previous studies (10, 12, 55). This study only measured fucoidan secretion for a short interval during one day in August when exudation rates are lower compared with other months (10, 12). The incubated algae potentially experienced stress due to experimental manipulation, but stress also occurs in nature and neither stress molecules nor indicators of decay were detected. Our sampling location in the Baltic Sea differs from most marine environments with a lower salinity of approximately 6 psu, which may have affected the degree of fucoidan sulfation (22) and secretion rates (14). Fucoidan secretion as a protection may change with tidal changes, desiccation, and other stressors. The examined techniques allow quantification of fucoidan secretion rates across the cosmopolitan taxon of brown algae under different environmental conditions.
Fucoidan secretion by F. vesiculosus expands conventional carbon dioxide removal valuation, which considers algal biomass carbon, to include secreted molecules. While organic carbon secretion by brown algae has been known for decades (17, 28, 59), the lack of analytics for marine samples has constrained molecular quantification (35, 37, 39, 60). Natural release of dissolved fucoidan by brown algae eases the necessity to dump biomass in current approaches. Farmed algae biomass could supply applications in agriculture, pharmaceutics, and materials. Harvesting of farmed algal biomass benefits eutrophied systems but would reduce primary productivity in oligotrophic waters including most of the open ocean due to nutrient depletion (61). In turn, brown algae likely continue to secrete fucoidan despite low nutrient concentrations as long as light and carbon dioxide are available. Secreted fucoidan contributes to carbon sequestration by brown algae and does not induce significant nutrient removal (Fig. 3B) (52).
Conclusion
We adapted and compared techniques to quantify dissolved fucoidan, a complex polysaccharide that is difficult to degrade for microbes and accumulates in the ocean. Fucoidan quantification was enabled by combining acid hydrolysis, anion exchange chromatography, and enzymatic digestion, and its presence was confirmed by monoclonal antibody binding. Fucoidan comprised 18 to 50% of dissolved organic carbon exuded by the brown algae Fucus vesiculosus. Dissolved fucoidan likely stems from dissipation of mucilage. Fucoidan does not contain relevant amounts of nitrogen nor other growth-limiting elements, enabling nutrient-independent release of fucoidan carbon. As brown algae secrete 14 to 35% of their net primary production, they inject substantial amounts of fucoidan carbon into the 660 Gt dissolved organic carbon pool in the ocean. Fucoidan can accumulate in particles and could, through the biological carbon pump, reach the deep ocean where carbon is sequestered. While acknowledging the limited scale of our experiment, we conclude that dissolved fucoidan secretion is likely an overlooked contribution of brown algae to carbon dioxide removal.
Materials and Methods
Samples were collected in August 2020 at the coastal Ångbåtsbryggan monitoring site (link, (62–64)) in the Baltic Sea (59° 50′ 28.284′′ N, 23° 14′ 58.308′′ E). 800 mL ambient seawater from 1- and 3-m depth and from 5-h incubations of subtidally growing Zostera marina and Fucus vesiculosus was filtered at 0.7 µm. Dissolved organic carbon, total dissolved nitrogen, and CDOM/FDOM were measured at the Tvärminne Zoological Station, Hanko, Finland. The experimental setup had a strong effect on oxygen concentrations in the incubations, beyond accurate measurability. Therefore, we do not report primary productivity rates. For the identification of small molecules, water samples were derivatized and analyzed by gas chromatography–mass spectrometry (GC–MS) following a protocol for seawater samples (60). Anionic polysaccharides were extracted and purified from 400 mL of the filtered seawater (ambient and incubations) using anion exchange chromatography (AEX) and eluted with 5 M NaCl. 5 M NaCl elutions and remaining filtered seawater were transported frozen to the Max Planck Institute for Marine Microbiology in Bremen, Germany, for further purifications, monosaccharide quantification, GC–MS, antibody binding, and enzymatic digestion.
Filtered seawater and 5 M NaCl elutions were dialyzed through 1-kDa membranes against MilliQ-water, dried, and resuspended. Monosaccharides were quantified after acid hydrolysis (1 M HCl, 100 °C, 24 h) using anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). Filtered seawater was analyzed by ELISA adding aliquots into polymer-binding 96-well plates and measuring antibody binding via spectrophotometric signal, while the 5 M NaCl elutions were analyzed with carbohydrate microarrays by printing aliquots onto nitrocellulose membrane and measuring antibody binding via colorimetric signal (35). Crude and purified fucoidan from seven brown algae species were subjected to elemental analysis for carbon, nitrogen, and sulfur content. To calculate daily fucoidan exudation rates, five of the daylight hours were considered at 100% light levels (exudation rates from light incubations), nine were considered to be in the dark (exudation rates from dark incubations), while we estimated 10 h operating at 50% (averaged exudation rates from light and dark incubations) based on the average relative solar altitude.
Polysaccharide Extraction.
Anionic polysaccharides were extracted from seawater using anion exchange columns. To this end, 100 mL filtered seawater samples passed through 5 mL HiTrap ANX (high sub) FF cartridges (Cytiva), which had been conditioned with 40 mL MilliQ-water, at 4 mL min−1 flow rate. For washing, 20 mL MilliQ-water was applied after the sample and combined with the sample flow through. To elute weakly bound anionic molecules, 12 mL of a 2:1 mixture of 2 M ammonium bicarbonate and 2 M ammonium carbonate was passed over the cartridges at 2 mL min−1 and collected in a 15-mL tube. To elute strongly anionic molecules, 30 mL 5 M NaCl was applied and collected in a 50-mL tube. The 15-mL and 50-mL tubes were frozen at −20 °C until further processing.
Enzymatic Digestion.
Enzymes were obtained from a fucoidan-degrading bacterial culture. A Lentimonas sp. CC4 culture (42) was grown on 0.2% fucoidan from Fucus vesiculosus (Merck/SigmaAldrich, Germany) as the only carbon source in 200 mL MOPS-buffered minimal medium (42). Fourteen days after inoculation, the culture had reached an optical density of 0.714 at 600-nm wavelength. Cells were pelleted by centrifugation at 30,000 × g for 20 min at 4 °C. After centrifugation, the supernatant was decanted and the cell pellet stored at −20 °C. The cell pellet was resuspended in lysis buffer composed of a 5-mL Bug Buster protein extraction kit (Merck/Sigma-Aldrich), 0.5 mg mL−1 lysozyme from chicken egg white (Merck/SigmaAldrich), 0.1 mg mL−1 DNAse (Merck/SigmaAldrich), and 1 × complete protease inhibitors (Merck/SigmaAldrich). The cell suspension was incubated for 15 min on ice with pipetting up and down and centrifuged at 24,000 × g for 30 min at 4 °C to remove cell debris. The supernatant containing the soluble cytosolic proteins was transferred into clean tubes and kept on ice until digestion assays. The total protein concentration was measured using a bicinchoninic acid protein assay kit (Merck/SigmaAldrich) following the manufacturer’s guidelines.
Dialyzed samples were combined with cell lysate containing catabolic enzymes specific to sulfated fucan to break down the polysaccharide into quantifiable monosaccharides. A 100 µL reaction mixture included 80 µL dialyzed sample, 0.7% sea salt, 10 mM CaCl2, and 10 µg of total proteins. A negative control contained heat-inactivated cell lysate to account for residual monosaccharides endogenous to bacterial cells. Samples were incubated for 3 h at 37 °C. The reaction was stopped by heating the samples to 99 °C for 15 min. Digests were then spun down to remove precipitates. Supernatant containing digestion products was collected, frozen, and dried under vacuum. For monosaccharide quantification, dried samples were dissolved in 80 µL MilliQ-water, vortexed, and centrifuged at 21 000 × g for 3 min to remove the insoluble fraction. For HPAEC-PAD analysis, samples were further diluted 10 times with MilliQ-water.
Fucose was the monosaccharide product of which concentration was significantly correlated with fucoidan concentration of the input (Fig. 2F and SI Appendix, Fig. S7). Thus, fucose concentration was used to estimate fucoidan concentrations in samples. Even though galactose and glucuronic acid have strong correlation, their constitution was low. Thus, galactose and glucuronic acid are less sensitive estimators for fucoidan especially in environmental samples and in enzymatic digestion assays. Therefore, fucoidan concentration is calculated based on fucose content only.
Further details can be found in the SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Joanna Norkko, Laura Kauppi, Sara Karvo, Jaana Koistinen, and Kia Rautava from the Tvärminne Zoological Station for their assistance in project preparation, field work and lab work, technicians at the Max Planck Institute for Marine Microbiology, Bremen, namely Marvin Weinhold for metabolomic analyses, Alek Bolte for HPAEC-PAD measurements and Tina Trautmann for Lentimonas cultivation, and Kai Bischoff and Inga Hellige for comments on the manuscript. We thank the scientist reviewers who proposed to discuss fucoidan secretion as a common process in brown algae and to include evidence for the stability of fucoidan in the environment. H.B.-W. and M.A.A. acknowledge support from the European Union’s Horizon 2020 research and innovation program under grant agreement No 730984, ASSEMBLE Plus project. H.B.-W., N.P.N., M.B., S.V.-M. and J.-H.H. thank the Max Planck Society. J.-H.H. received funding from the DFG (HE 7217/1-1) and the Cluster of Excellence initiative (EXC-2077–390741603). H.B.-W., M.L. and J.-H.H. also thank the sea4society, CDRmare campaign in the German Marine Research Alliance.
Author contributions
H.B.-W., M.A.A., and J.-H.H. designed research; H.B.-W., M.A.A., N.P.N., M.B., E.A., S.V.-M., and C.G. performed research; M.L. contributed new reagents/analytic tools; H.B.-W., M.A.A., M.B., E.A., S.V.-M., M.L., C.G., and J.-H.H. analyzed data; and H.B.-W., M.A.A., and J.-H.H. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
The data that support the findings of this study have been deposited to PANGAEA-Data Publisher for Earth & Environmental Science (https://www.pangaea.de) (65) and mass spectrometry data deposited to MetaboLights as PDI-32766.
Supporting Information
References
- 1.Gao K., McKinley K. R., Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 6, 45–60 (1994). [Google Scholar]
- 2.Keenan T. F., Williams C. A., The terrestrial carbon sink. Annu. Rev. Environ. Resour. 43, 219–243 (2018). [Google Scholar]
- 3.Watanabe K., et al. , Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosciences 17, 2425–2440 (2020). [Google Scholar]
- 4.Tsai D.D.-W., Chen P. H., Ramaraj R., The potential of carbon dioxide capture and sequestration with algae. Ecol. Eng. 98, 17–23 (2017). [Google Scholar]
- 5.Krumhansl K. A., Scheibling R. E., Production and fate of kelp detritus. Mar. Ecol. Prog. Ser. 467, 281–302 (2012). [Google Scholar]
- 6.Braeckman U., et al. , Degradation of macroalgal detritus in shallow coastal Antarctic sediments. Limnol. Oceanogr. 64, 1423–1441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill R., et al. , Can macroalgae contribute to blue carbon? An Australian perspective. Limnol. Oceanogr. 60, 1689–1706 (2015). [Google Scholar]
- 8.Krause-Jensen D., Duarte C. M., Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 9, 737–742 (2016). [Google Scholar]
- 9.Trevathan-Tackett S. M., et al. , Comparison of marine macrophytes for their contributions to blue carbon sequestration. J. Ecology 96, 3043–3057 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Wada S., et al. , Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula. Japan. J. Exp. Mar. Biol. Ecol. 349, 344–358 (2007). [Google Scholar]
- 11.Wada S., Hama T., The contribution of macroalgae to the coastal dissolved organic matter pool. Estuar. Coast. Shelf Sci. 129, 77–85 (2013). [Google Scholar]
- 12.Abdullah M. I., Fredriksen S., Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway. J. Mar. Biol. Assoc. U. K. 84, 887–894 (2004). [Google Scholar]
- 13.Khailov K. M., Burlakova Z. P., Release of dissolved organic matter by marine seaweeds and distribution of their total organic production to inshore communities. Limnol. Oceanogr. 14, 521–527 (1969). [Google Scholar]
- 14.Sieburth J., Studies of algal substances in the sea III. The production of extracellular organic matter by littoral marine algae. J. Exp. Mar. Biol. Ecol. 3, 290–309 (1969). [Google Scholar]
- 15.Powers L. C., et al. , Sargassum sp. act as a large regional source of marine dissolved organic carbon and polyphenols. Global Biogeochem. Cycles 33, 1423–1439 (2019). [Google Scholar]
- 16.Wada S., et al. , Bioavailability of macroalgal dissolved organic matter in seawater. Mar. Ecol. Prog. Ser. 370, 33–44 (2008). [Google Scholar]
- 17.Lucas M. I., Newell R. C., Velimirov B., Heterotrophic utilisation of mucilage released during fragmentation of kelp (Ecklonia maxima and Laminaria pallida). II. Differential utilisation of dissolved organic components from kelp mucilage. Mar. Ecol. Prog. Ser. 4, 43–55 (1981). [Google Scholar]
- 18.Arnosti C., et al. , The biogeochemistry of marine polysaccharides: Sources, inventories, and bacterial drivers of the carbohydrate cycle. Annu. Rev. Mar. Sci. 13, 81–108 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Nevins D. J., Sugars: Their origin in photosynthesis and subsequent biological interconversions. Am. J. Clin. Nutr. 61, 915S–921S (1995). [DOI] [PubMed] [Google Scholar]
- 20.Deniaud-Bouët E., et al. , Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 114, 1203–1216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadam S. U., Tiwari B. K., O’Donnell C. P., Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. 50, 24–31 (2015). [Google Scholar]
- 22.Domozych D., "Algal cell walls" in eLS (JohnWiley & Sons, Ltd: Chichester, 2019), 10.1002/9780470015902.a0000315.pub4, pp. 1–11. [DOI] [Google Scholar]
- 23.Callow M. E., Evans L. V., Localization of sulphated polysaccharides by X-ray microanalysis in Laminaria saccharina. Planta 131, 155–157 (1976). [DOI] [PubMed] [Google Scholar]
- 24.Torode T. A., et al. , Monoclonal antibodies directed to fucoidan preparations from brown algae. PLoS One 10, e0118366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skriptsova A. V., Fucoidans of brown algae: Biosynthesis, localization, and physiological role in thallus. Russ. J. Mar. Biol. 41, 145–156 (2015). [Google Scholar]
- 26.Ayrapetyan O. N., et al. , Antibacterial properties of fucoidans from the brown algae Fucus vesiculosus L. of the barents sea. Biology 10, 67 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans L. V., Callow M. E., Polysaccharide sulphation in Laminaria. Planta 117, 93–95 (1974). [DOI] [PubMed] [Google Scholar]
- 28.Evans L. V., Simpson M., Callow M. E., Sulphated polysaccharide synthesis in brown algae. Planta 110, 237–252 (1973). [DOI] [PubMed] [Google Scholar]
- 29.Wild C., et al. , Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428, 66–70 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Schroeder B. O., Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep. (Oxf) 7, 3–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampitt R. S., et al. , Ocean fertilization: A potential means of geoengineering? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 366, 3919–3945 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Passow U., Carlson C. A., The biological pump in a high CO2 world. Mar. Ecol. Prog. Ser. 470, 249–271 (2012). [Google Scholar]
- 33.Engel A., Thoms S., Riebesell U., Rochelle-Newall E., Zondervan I., Polysaccharide aggregation as a potential sink of marine dissolved organic carbon. Nature 428, 929–932 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Huang G., et al. , Secretion of sulfated fucans by diatoms may contribute to marine aggregate formation. Limnol. Oceanogr. 66, 3768–3782 (2021). [Google Scholar]
- 35.Vidal-Melgosa S., et al. , Diatom fucan polysaccharide precipitates carbon during algal blooms. Nat. Commun. 12, 1150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappelmann L., et al. , Polysaccharide utilization loci of North Sea Flavobacteriia as basis for using SusC/D-protein expression for predicting major phytoplankton glycans. ISME J. 13, 76–91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker S., et al. , Laminarin is a major molecule in the marine carbon cycle. Proc. Natl. Acad. Sci. U.S.A. 117, 6599–6607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruger K., et al. , In marine Bacteroidetes the bulk of glycan degradation during algae blooms is mediated by few clades using a restricted set of genes. ISME J. 13, 2800–2816 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker S., Scheffel A., Polz M. F., Hehemann J.-H., Accurate quantification of laminarin in marine organic matter with enzymes from marine microbes. Appl. Environ. Microbiol. 83, e03389–e03316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnosti C., Microbial extracellular enzymes and the marine carbon cycle. Annu. Rev. Mar. Sci. 3, 401–425 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Alderkamp A.-C., Van Rijssel M., Bolhuis H., Characterization of marine bacteria and the activity of their enzyme systems involved in degradation of the algal storage glucan laminarin. FEMS Microbiol. Ecol. 59, 108–117 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Sichert A., et al. , Verrucomicrobia use hundreds of enzymes to digest the algal polysaccharide fucoidan. Nat. Microbiol. 5, 1026–1039 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Salmeán A. A., Willats W. G. T., Ribeiro S., Andersen T. J., Ellegaard M., Over 100-year preservation and temporal fluctuations of cell wall polysaccharides in marine sediments. Front. Plant Sci. 13, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidal-Melgosa S., et al. , Not digested: Algal glycans move carbon dioxide into the deep-sea. bioRxiv [Preprint] (2022). 10.1101/2022.03.04.483023, 2022.2003.2004.483023. Accessed 4 March 2022. [DOI] [Google Scholar]
- 45.Gao Y., et al. , Dissolved organic carbon from cultured kelp Saccharina japonica: Production, bioavailability, and bacterial degradation rates. Aquac. Environ. Interact. 13, 101–110 (2021). [Google Scholar]
- 46.Reed D. C., et al. , Patterns and controls of reef-scale production of dissolved organic carbon by giant kelp Macrocystis pyrifera. Limnol. Oceanogr. 60, 1996–2008 (2015). [Google Scholar]
- 47.Reed R., Wright P. J., Release of mannitol from Pilayella littoralis (Phaeophyta. Ectocarpales) in response to hypoosmotic stress. Mar. Ecol. Prog. Ser. 29, 205–208 (1986). [Google Scholar]
- 48.Torode T. A., et al. , Dynamics of cell wall assembly during early embryogenesis in the brown alga Fucus. J. Exp. Botany 67, 6089–6100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva J. M. C., et al. , Biological activities of the sulfated polysaccharide from the vascular plant Halodule wrightii. Revista Brasileira de Farmacognosia 22, 94–101 (2012). [Google Scholar]
- 50.Pfeifer L., Shafee T., Johnson K. L., Bacic A., Classen B., Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Sci. Rep. 10, 8232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paine E. R., Schmid M., Boyd P. W., Diaz-Pulido G., Hurd C. L., Rate and fate of dissolved organic carbon release by seaweeds: A missing link in the coastal ocean carbon cycle. J. Phycol. 57, 1375–1391 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Balina K., Romagnoli F., Blumberga D., Chemical composition and potential use of fucus vesiculosus from gulf of riga. Energy Procedia 95, 43–49 (2016). [Google Scholar]
- 53.Kautsky L., Qvarfordt S., Schagerstrom E., Fucus vesiculosus adapted to a life in the Baltic sea: Impacts on recruitment, growth, re-establishment and restoration. Bot. Mar. 62, 17–30 (2019). [Google Scholar]
- 54.Mariani P., Tolomio C., Braghetta P., An ultrastructural approach to the adaptive role of the cell wall in the intertidal alga Fucus virsoides. Protoplasma 128, 208–217 (1985). [Google Scholar]
- 55.Nelson C. E., et al. , Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. ISME J. 7, 962–979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sichert A., et al. , Verrucomicrobia use hundreds of enzymes to digest the algal polysaccharide fucoidan. Nat. Microbiol. 5, 1026–1039 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Bligh M., Nguyen N., Buck-Wiese H., Vidal-Melgosa S., Hehemann J. H., Structures and functions of algal glycans shape their capacity to sequester carbon in the ocean. Curr. Opin. Chem. Biol. 71, 102204 (2022). [DOI] [PubMed] [Google Scholar]
- 58.Vidal-Melgosa S., et al. , Diatom fucan polysaccharide precipitates carbon during algal blooms. Nat. Commun. 12, 1150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newell R. C., Lucas M. I., Velimirov B., Seiderer L. J., Quantitative significance of dissolved organic losses following fragmentation of kelp (Ecklonia maxima and Laminaria pallida). Mar. Ecol. Prog. Ser. 2, 45–59 (1980). [Google Scholar]
- 60.Sogin E. M., Puskás E., Dubilier N., Liebeke M., Marine metabolomics: A method for nontargeted measurement of metabolites in seawater by gas chromatography-mass spectrometry. mSystems 4, e00638–00619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bach L. T., et al. , Testing the climate intervention potential of ocean afforestation using the Great Atlantic Sargassum Belt. Nat. Commun. 12, 2556 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gustafsson C., Norkko A., Quantifying the importance of functional traits for primary production in aquatic plant communities. J. Ecol. 107, 154–166 (2019). [Google Scholar]
- 63.Asmala E., et al. , Role of eelgrass in the coastal filter of contrasting Baltic Sea environments. Estuaries Coasts 42, 1882–1895 (2019). [Google Scholar]
- 64.Gammal J., Järnström M., Bernard G., Norkko J., Norkko A., Environmental context mediates biodiversity-ecosystem functioning relationships in coastal soft-sediment habitats. Ecosystems 22, 137–151 (2018). [Google Scholar]
- 65.Buck-Wiese H., et al. , Incubation experiment of Fucus vesiculosus and Zostera marina specimen sampled from Tvärminne Zoological Station, Hanko Finland. PANGAEA. https://doi.pangaea.de/10.1594/PANGAEA.950878. Deposited 18 August 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The data that support the findings of this study have been deposited to PANGAEA-Data Publisher for Earth & Environmental Science (https://www.pangaea.de) (65) and mass spectrometry data deposited to MetaboLights as PDI-32766.