Significance
ACT and CAR-T therapies have met significant obstacles in treating solid tumors. Here, we demonstrate a simple method to increase the efficacy of adoptively transferred T cells by conjugating low-dose antitumor cytokines onto cell surfaces. This method can be easily integrated into the current T cell manufacturing process without significant changes to the manufacturing process or time. This method allows local delivery of otherwise toxic cytokines, increases T cell infiltration into solid tumors, and induces antigen spreading by activating host immune responses. Overall, this method improves control over aggressive solid tumors and significantly reduces dosage of CAR-Ts needed for a curative response with minimal changes to the current processes for T cell manufacturing and adoptive T cell transfer therapies.
Keywords: nanoparticle, adoptive T cell transfer, CAR-T, metabolic labeling; anti-tumor cytokine
Abstract
Adoptive T cell transfer (ACT) therapies suffer from a number of limitations (e.g., poor control of solid tumors), and while combining ACT with cytokine therapy can enhance effectiveness, this also results in significant side effects. Here, we describe a nanotechnology approach to improve the efficacy of ACT therapies by metabolically labeling T cells with unnatural sugar nanoparticles, allowing direct conjugation of antitumor cytokines onto the T cell surface during the manufacturing process. This allows local, concentrated activity of otherwise toxic cytokines. This approach increases T cell infiltration into solid tumors, activates the host immune system toward a Type 1 response, encourages antigen spreading, and improves control of aggressive solid tumors and achieves complete blood cancer regression with otherwise noncurative doses of CAR-T cells. Overall, this method provides an effective and easily integrated approach to the current ACT manufacturing process to increase efficacy in various settings.
Adoptive cell transfer (ACT) of tumor-specific T cells has been shown to elicit tumor regression in patients in a variety of settings, but suffers from a number of limitations (1–4). In these therapies, a patient’s own T cells are typically isolated, activated, and expanded ex vivo and reinfused back into the patient. The therapeutic potential of ACT has been highlighted with the development of chimeric antigen receptor (CAR) T cell therapies, where T cells isolated from patients are genetically engineered to redirect their specificity against a tumor-specific cognate antigen (5, 6). However, while CAR-T therapies have been successful with certain hematological malignancies (7, 8), variable outcomes can result, and little success has been achieved to date with solid tumors due to poor T cell persistence and fitness, poor solid tumor penetration, and immunosuppression from the tumor microenvironment (5, 8–10).
A range of strategies have been explored to enhance adoptive T cell efficacy, including the design of CAR-T with a transgenic payload (T cells redirected for antigen-unrestricted cytokine-initiated killing, or TRUCKs), delivery vehicles for the ACT, and systemic delivery of antitumor cytokines and factors that modulate the immunosuppressive tumor microenvironment. Although TRUCK CAR-Ts were effective at inducing response at lower dosages, the expression of the transgenic payload was not limited to the tumor, resulting in substantial systemic toxicity observable in all major tissues (11–14). Various cytokines, including interleukin (IL)-2, IL-12, IL-21, and IL-15, have been systemically administered in animal models and clinically to improve the efficacy of ACT and CAR-T therapies (2, 3, 15–19). However, systemic delivery leads to toxicity and the rapid development of lethal inflammatory syndromes (15–17, 20). Additionally, the fast clearance rate of these agents and their untargeted distribution also resulted in minimal therapeutic activity in many clinical trials (21). Local delivery of these agents has been explored by backpacking cytokine onto T cells and other immune cells. However, these approaches required complicated processes to manufacture the backpacks and load the agents onto cells for delivery, resulting in prolonged manufacturing time in clinical use (22–24). Additionally, backpacking strategies may impair physiological functions of T cells due to long-term occupation of functional molecules on T cell surfaces (24).
Here, we demonstrate a simple and flexible approach to improve the efficacy of ACT therapies against solid tumors by conjugating antitumor cytokines directly onto T cells before adoptive transfers. T cells were metabolically labeled simply by adding nanoparticles comprised of unnatural azido sugars directly to the culture medium during cell expansion. This leads to labeling of the cellular glycocalyx with desired functional groups (25–27). Antitumor cytokines were then directly added to washed T cells for conjugation via click chemistry onto cell surfaces. We demonstrate that this simple and easily scalable method increases T cell effector function in vivo after adoptive transfer and can activate the endogenous immune system to encourage antigen spreading, allowing the recognition of additional tumor-specific antigens to enhance therapeutic efficacy.
Results
Nanoparticle-Mediated Metabolic Labeling Is Efficient and Durable and Doesn’t Affect T Cell Function or Phenotype.
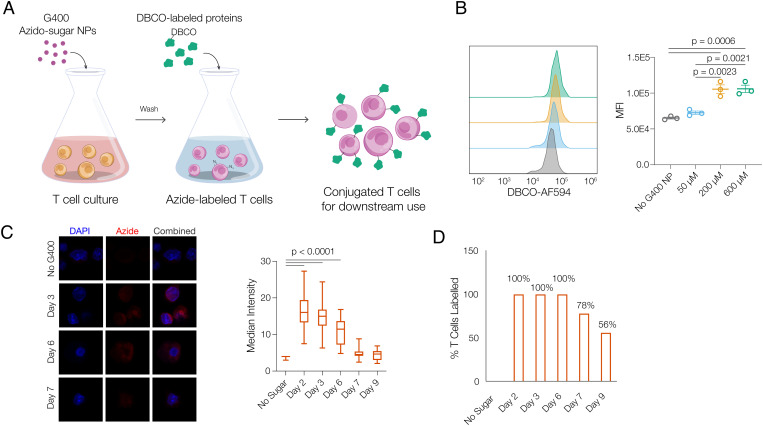
To conjugate cytokines onto T cells, T cells were first metabolically labeled with unnatural azido-sugar nanoparticles. G400 nanoparticles (G400 NP), a polymer of azido-sugar (n=400) derived from Ac4ManAz (28), were directly added to T cell cultures (Fig. 1A). After cellular uptake, G400 NP yields monomeric sugar-azide which is utilized in cellular metabolism and is integrated into membrane glycoproteins to provide azide groups on the T cell surface. Dibenzocyclooctyne (DBCO)-labeled cytokines were then added to washed T cells to conjugate cytokines onto T cell surfaces for downstream in vitro and in vivo use (Fig. 1A). The ability of unnatural azido-sugars to metabolically label the glycocalyx of T cells was first explored. 72 hours after G400 NPs were added to T cell cultures, T cells were found to be effectively metabolically labeled, as reflected by the increase in azide signaling. Furthermore, metabolic labeling was dose dependent, as reflected by cell-surface azide intensity quantification; saturation of the cell surface was reached at 200 µM of G400 NPs (Fig. 1B). Labeling was durable and efficient, as all T cells exhibited positive azide signal after 3 d, and more than 50% maintained a positive azide signal at least 9 d after initial labeling and G400 washout (Fig. 1 C and D). Importantly, exposure to high concentrations of G400 NP treatment did not significantly alter T cell proliferation, survival, or phenotype (SI Appendix, Fig. S1). 200 µM G400 NPs was used in all subsequent studies, as it provided maximum azido-labeling.
Fig. 1.
Azido-sugar nanoparticles metabolically label T cells with cell-surface azide groups. A, Schematic of metabolic labeling and cytokine conjugation of T cells with azido-sugar G400 NPs. Azido-sugar nanoparticles are directly added to T cell culture, enter T cells via endocytosis, and lead to presentation of azide group on T cell surfaces. After T cells are metabolically labeled, T cells are washed and DBCO-labeled proteins (e.g., cytokines) are directly added to produce conjugated T cells for downstream use. B, Median fluorescence intensity (MFI) of T cell surface azide signals after T cells are treated with G400 NPs at various concentrations for 3 d (n = 3, one-way ANOVA and Tukey’s test). C, Representative fluorescent imaging and quantification of maintenance of azide signal from T cells, and (D) percentage of T cells with positive azide signal over time. T cells were treated with 200 µM G400 NP for 3 d, after which G400 NP in the medium was removed (day 0) and T cells were subsequently cultured free of G400 NP (one-way ANOVA and Tukey’s test).
Cytokines Can Be Conjugated onto Azido-Labeled T Cells and Alter T Cell Function.
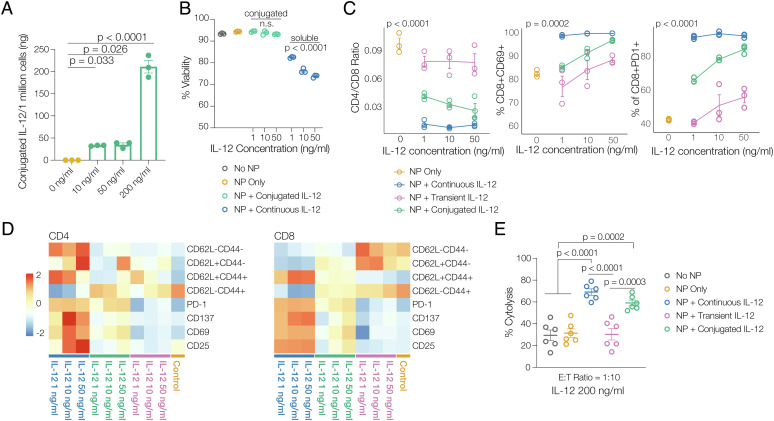
The ability of azido-labeling to mediate targeted conjugation of DBCO-modified cytokines to T cells and the impact of cytokine conjugation on the cells were next examined. A number of antitumor cytokines, including IL-12, IL-21, and tumor necrosis factor (TNF)-α, were first modified with dibenzocyclooctyne-sulfo-N-hydroxysuccinimidyl ester (DBCO-sulfo-NHS), and the modification was confirmed via matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) (SI Appendix, Fig. S2). DBCO-modified cytokines maintained the majority of their activity (SI Appendix, Fig. S3). Bioactivity was inversely correlated with the degree of modification, and 2-3 DBCO molecules per cytokine were used in all further studies to maintain activity while allowing for efficient conjugation to T cells. DBCO-modified cytokines were simply added to the medium of azido-labeled T cells to allow conjugation via click reaction (30 min at 4 °C), and T cells were subsequently washed to remove nonconjugated cytokine. As expected, cytokine conjugation via click chemistry was dose dependent, with higher concentrations of DBCO-cytokines resulting in higher percentage of cell-surface cytokine (SI Appendix, Fig. S4 A–C). The amount of DBCO-IL-12 loaded onto T cells was then quantified with enzyme-linked immunosorbent assay (ELISA) and was positively correlated with the concentration of cytokines added to the cells (Fig. 2A). With exposure to 200 ng/mL IL-12, ~210 ng IL-12 was conjugated per one million T cells (Fig. 2A).
Fig. 2.
Azido labeling of T cells allows conjugation of DBCO cytokines to generate potent cytokine-dependent inflammatory phenotypes. A, ELISA quantification of the amount of DBCO-IL-12 conjugated onto one million T cells at various DBCO-IL-12 concentrations (n = 3, one-way ANOVA and Tukey’s test). B, Viability data for T cells receiving no IL-12, conjugated with DBCO IL-12 and presented with soluble IL-12 (no DBCO label) in media in in vitro culture for 7 d (n = 3). C, Representative phenotyping data and (D) heatmap data showing memory phenotype markers and activation and exhaustion markers, for T cells conjugated with DBCO-IL-12 (“NP + conjugated IL-12;” green), treated with continuously soluble IL-12 (“NP + continuous IL-12;” blue), or temporarily exposed to IL-12 for duration of reaction time (“NP + transient IL-12;” purple) at different cytokine concentrations cultured in vitro for 7 d (n = 3, two-way ANOVA). E, Anti-B16-F10 tumor cell cytolytic activities of Pmel-1 T cells conjugated with DBCO-IL-12 (“NP + conjugated IL-12;” green), treated with soluble IL-12 (“NP + continuous IL-12;” blue), or temporarily exposed to IL-12 (“NP + transient IL-12;” purple) (n = 6, one-way ANOVA and Tukey’s test).
The impact of conjugated cytokines was next assessed. While continuous exposure to antitumor cytokines typically leads to significant cellular toxicity at high doses, DBCO cytokines conjugated onto T cell exhibited minimal cytotoxicity (Fig. 2B and SI Appendix, Fig. S5A). As a control, T cells were exposed to unmodified cytokines for the same amount of time used for the cytokine coupling reaction and were then washed away (NP + transient IL-12); this transient cytokine exposure had modest impact on long-term T cell phenotype. In contrast, conjugated cytokines (NP + conjugated IL-12) directed T cell differentiation in a dose-dependent manner even at 7 d after conjugation, similarly but to a lesser extent than continuous exposure to unmodified cytokine at the same concentration as used in cytokine conjugation (NP + continuous IL-12) (Fig. 2 C and D and SI Appendix, Fig. S5 B and C). The change in T cell phenotype with cytokine conjugation translated to functional changes, as reflected by increased cytolysis ability of Pmel-1 T cells against B16-F10 melanoma tumor cells (Fig. 2E and SI Appendix, Fig. S3D). IL-21 and TNF-α were also successfully conjugated to T cells with the same approach (SI Appendix, Fig. S5). Considering the biofunction of DBCO-modified cytokines, the conjugation efficiency, and the ability for conjugated cytokines to direct T cell differentiation and cytolytic activities, DBCO-IL-12 was used in subsequent studies. Overall, these findings demonstrate that cytokine conjugation leads to long-term impact on T cell phenotype, without the toxicity associated with continuous exposure to high concentrations of soluble cytokine.
Cytokine Conjugation Promotes T Cell Persistence and Effector Differentiation In Vivo.
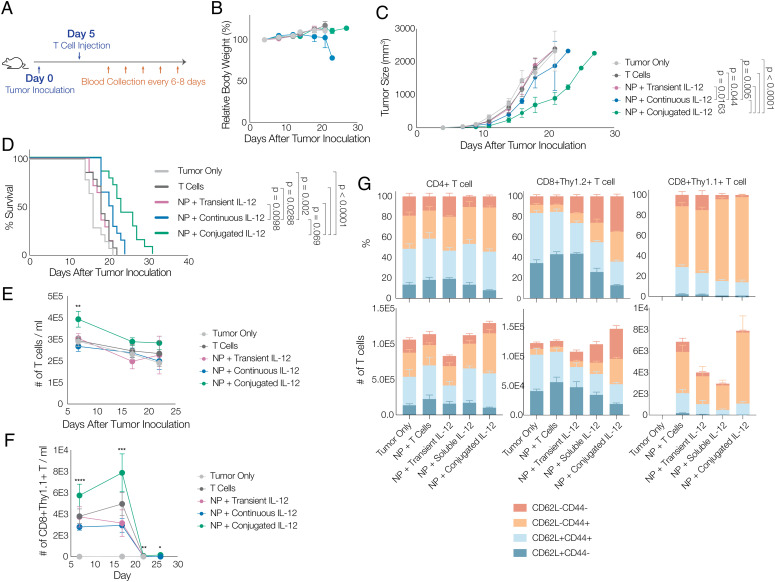
Next, the effects of antitumor cytokine conjugation on T cells were examined in vivo, via the adoptive transfer of a subcurative dose of Thy1.1+ Pmel-1 T cell receptor (TCR)-transgenic gp100-specific T cells in a B16-F10 melanoma model (Fig. 3A). Adoptively transferred T cell conditions included: unmodified T cells (T cells), T cells treated with G400 NPs and exposed to unmodified cytokine for the reaction time (NP + transient IL-12), T cells treated with G400 NPs and conjugated with DBCO-IL-12 (NP + conjugated IL-12), or T cells treated with G400 NPs and injected together with the same amount of control, non-DBCO IL-12 (1.47 µg) that was conjugated to T cells in the NP + conjugated IL-12 condition (NP + soluble IL-12). We did not observe weight loss over the course of treatment except in mice receiving unconjugated, systemic IL-12 (NP + soluble IL-12), indicating that the IL-12-conjugated T cells were well tolerated by mice (Fig. 3B). The unmodified T cells alone, or T cells with soluble IL-12, offered minimal benefit in controlling tumor growth and prolonging mouse survival. However, IL-12 conjugation onto T cells significantly delayed tumor growth and prolonged life span by ~50% (Fig. 3 C and D); it also significantly increased the circulating total number of T cells and number of adoptively transferred tumor-specific T cells at peak response (Fig. 3 E and F). These effects were significantly greater than found in animals that received soluble IL-12 with ACT. Additionally, mice receiving T cells with IL-12 conjugation had a higher population of effector-like and effector-memory-like T cells, not only among the adoptively transferred T cells (Thy1.1+), but also among endogenous T cells (Thy1.2+, Fig. 3G).
Fig. 3.
Conjugating IL-12 on T cell surfaces increases the efficacy of subsequent ACT therapies. A, Schematic of animal study timeline; B16-F10 tumors were inoculated on day 0, followed by tail vein injection of T cells on day 5; blood was collected every 6 to 8 d for flow cytometry analysis after T cell injection. Animals were untreated (“tumor only;” light gray), treated with T cells without labeling and without IL-12 conjugation (“T cells;” dark gray), treated with T cells metabolically labeled and transiently exposed to non-DBCO-conjugated IL-12 prior to transfer (“NP + transient IL-12;” purple), treated with T cells that were labeled and transferred with the same quantity of soluble IL-12 (not DBCO conjugated) as was conjugated to T cells (“NP + continuous IL-12;” blue), or treated with T cells that were metabolically labeled and then conjugated with IL-12 prior to transfer (“NP + conjugated IL-12;” green). B, Body weight of different treatment groups over time, normalized to that of 4 d after tumor inoculation. C, Average tumor volume, and (D) mouse survival data over therapeutic study (n = 7, Mantel–Cox test). E, Total number of T cells per 1 mL of blood and (F) number of CD8+Thy1.1+ Pmel-1 T cells per 1 mL of blood over therapeutic study (n = 7, one-way ANOVA and Tukey’s test, *P < 0.05, **P < 0.01, ***P < 0.001). G, percentage and count of T cells expressing markers indicative of different memory populations in blood on day 17 (n = 7). Total CD4+ T cells, host CD8+ T cells (Thy1.2+), and adoptively transferred CD8+ T cells (Thy 1.1+) were analyzed.
IL-12 Conjugation Enhances T Cell Solid Tumor Penetration and Endogenous Th1 Response.
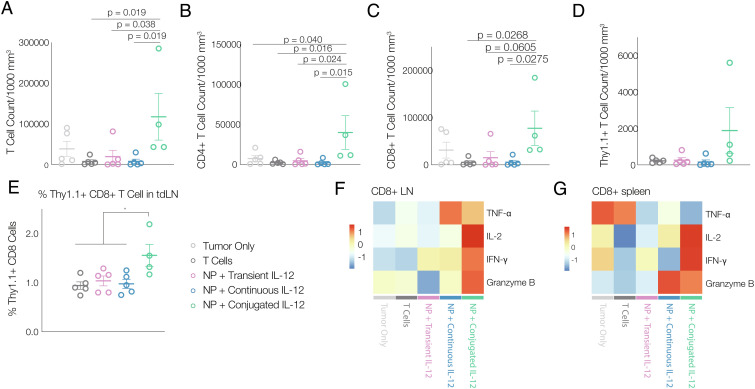
As the increase in total T cell number in mice receiving NP+ conjugation was higher than the number of transferred Pmel-1 T cells, we next explored whether the adoptively transferred T cells activated the endogenous immune system. Nine days after adoptive transfer of T cells, dendritic cells (DCs) were increased in tumors in all conditions (SI Appendix, Fig. S6A). However, IL-12 conjugation significantly increased both the absolute count of T cell-infiltrating tumors, and the average T cell count when normalized to tumor volume, as compared to the other conditions (Fig. 4A and SI Appendix, Fig. S6B). This increase was observed in both CD4+ T helper cells and CD8+ cytotoxic T cells (Fig. 4 B and C). IL-12 conjugation also significantly increased the number of antigen-specific Pmel-1 T cells in the tumor and tdLNs (Fig. 4 D and E). It is worth noting that the increase in the total influx of T cells into the tumor (Fig. 4A) is far greater than the increase in the number of transferred Pmel-1 T cells (Fig. 4D). Additionally, IL-12 conjugation also led to larger spleens (SI Appendix, Fig. S6C) as well as an increased percentage of Thy1.1+ Pmel-1 T cells in tdLNs and spleens (Fig. 4E and SI Appendix, Fig. S6 D and E).
Fig. 4.
Conjugating IL-12 on T cell surfaces increases T cell infiltration in solid tumors and effector function. A, Total number of T cells per 1,000 mm3 tumor (n = 5). B, Total number of CD4+ and (C) CD8+ T cells per 1,000 mm3 tumor (n = 5). D, Number of transferred CD8+Thy1.1+ T cells per 1,000 mm3 tumor (n = 5). E, Percentage of transferred Thy1.1+ CD8+ T cells among all T cells in lymph nodes (n = 5). F, heatmap of average expression level of Th1 cytokines in CD8+ T cells isolated from lymph nodes and (G) from spleen following ex vivo antigen restimulation and FAC analysis (n = 5). Animals were untreated (“tumor only;” light gray), treated with T cells without labeling and without IL-12 conjugation (“T cells;” dark gray), treated with T cells metabolically labeled and transiently exposed to non-DBCO-conjugated IL-12 prior to transfer (“NP + transient IL-12;” purple), treated with T cells that were labeled and transferred with the same quantity of soluble IL-12 (not DBCO conjugated) as was conjugated to T cells (“NP + continuous IL-12;” blue), or treated with T cells that were metabolically labeled and then conjugated with IL-12 prior to transfer (“NP + conjugated IL-12;” green); all data was collected 9 d after ACT. All statistics were calculated with one-way ANOVA and Tukey’s test, *P < 0.05.
To better understand the function of T cells in the presence of tumor antigens, isolated T cells from spleen and tdLNs were cocultured with B16-F10 melanoma cells for 4 h and were stained for intracellular cytokines. In mice receiving Pmel-1 T cells with IL-12 conjugation, both CD4+ and CD8+ T cells isolated from tdLNs and spleens demonstrated higher expression levels of cytotoxic granzyme B and Th1 cytokines than T cells in the other experimental conditions (IL-2, interferon (IFN)-γ, etc.) (Fig. 4 F and G and SI Appendix, Fig. S6F). Additionally, the increased production of IFN-γ could also induce increased expression of C-X-C motif chemokine ligand (CXCL) 9 and CXCL10 in surrounding monocytes and improve T cell recruitment into the tumor (29), which may explain the significant increase in total T cell infiltration in solid tumors (Fig. 4 A and D).
IL-12 Conjugation to T Cells Activated Endogenous DCs and Led to Antigen Spread.
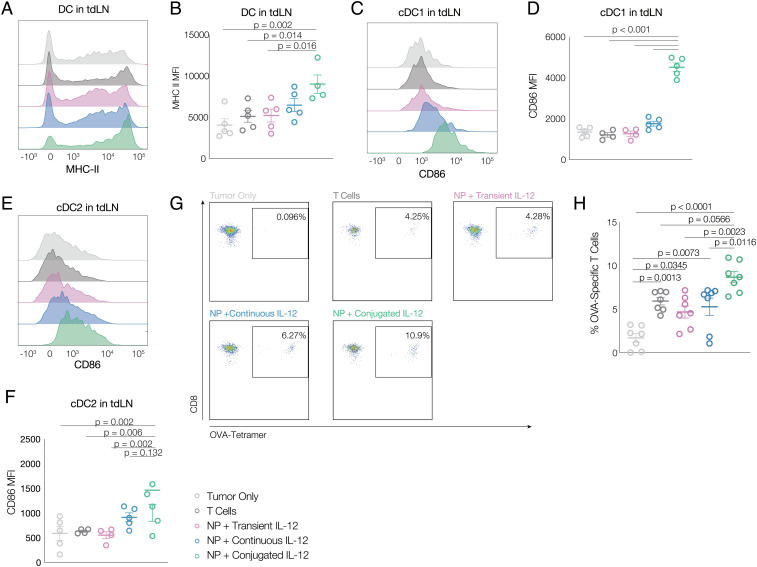
To further understand the impact of IL-12-conjugated Pmel-1 T cells, the phenotype of tdLN DCs and antigen spread were analyzed. Mice receiving IL-12-conjugated T cells had significantly higher MHC-II expression on DCs in tdLNs than found in other conditions (Fig. 5 A and B). Additionally, DCs in spleen also demonstrated higher levels of the costimulatory molecule CD86 (Fig. 5 C–F) in both cDC1 and cDC2, suggesting higher levels of DC activation with IL-12 conjugation, better crosspresentation to CD8+ cytotoxic T cells, and better activation of CD4+ T cell responses. B16 melanoma tumors with ovalbumin over-expression (B16-OVA) were next inoculated in mice (Thy1.2+) for 5 d before Thy1.1+ Pmel-1 T cells were adoptively transferred intravenously. Tumor growth and survival curve were similar to those previously observed in B16-F10 models, where IL-12-conjugated Pmel-1 T cells significantly controlled tumor growth and extended life span (SI Appendix, Fig. S7 A and B). While there were initially few OVA-specific circulating T cells in any condition, a significant increase was found at day 7 in mice receiving IL-12-conjugated T cells, compared to the other conditions (Fig. 5 G and H and SI Appendix, Fig. S7 C and D).
Fig. 5.
Conjugating IL-12 on T cell surfaces activates host DCs, increases antigen presentation, and promotes antigen spreading. A, Representative flow cytometry histograms, and (B) summary MFI data for MHC-II expression in DCs in tumor draining lymph nodes (n = 4 to 5). Representative flow cytometry histograms, and summary MFI data for CD86 expression in cDC1 (C and D) and cDC2 (E and F) in tumor draining lymph nodes (n = 4 to 5) 9 d after T cell adoptive transfer. G, Representative flow cytometry plots and (H) summary flow cytometry data for OVA-specific CD8+ T cells on day 10 after T cell adoptive transfer (n = 7). Animals were untreated (“tumor only;” light gray), treated with T cells without labeling and without IL-12 conjugation (“T cells;” dark gray), treated with T cells metabolically labeled and transiently exposed to non-DBCO-conjugated IL-12 prior to transfer (“NP + transient IL-12;” purple), treated with T cells that were labeled and transferred with the same quantity of soluble IL-12 (not DBCO conjugated) as was conjugated to T cells (“NP + continuous IL-12;” blue), or treated with T cells that were metabolically labeled and then conjugated with IL-12 prior to transfer (“NP + conjugated IL-12;” green). All statistics were calculated with one-way ANOVA and Tukey’s test.
Coconjugation of Cytokines Enhances ACT, and IL-12 Conjugation Improves Subcurative CAR-T Therapy.
Finally, the versatility of this approach to combine cytokines and enhance CAR-T therapy was explored. IL-15/IL-15Rα and IL-12 were together conjugated onto T cells, to simultaneously support T cell proliferation and T cell differentiation toward a Th1 response, respectively (30). T cells were able to proliferate in vitro in the absence of IL-2 only with IL-15/IL-15Rα conjugation (SI Appendix, Fig. S8 A and B). Additionally, T cells conjugated with both cytokines expressed a Th1 phenotype similar to T cells treated with soluble IL-12, indicating no detrimental impact of IL-15/IL-15Rα coconjugation (SI Appendix, Fig. S8C). In the B16-F10 melanoma model, transfer of T cells conjugated with both IL-15/IL-15Rα and IL-12 led to better control over tumor growth and prolonged mouse survival, as compared to conjugation of either single cytokine, suggesting a synergistic effect between the proliferative ability of IL-15/IL-15Rα and the Th1-directing ability of IL-12 (SI Appendix, Fig. S9 A and B). Conjugation of both cytokines also significantly increased circulating T cell numbers (SI Appendix, Fig. S9C).
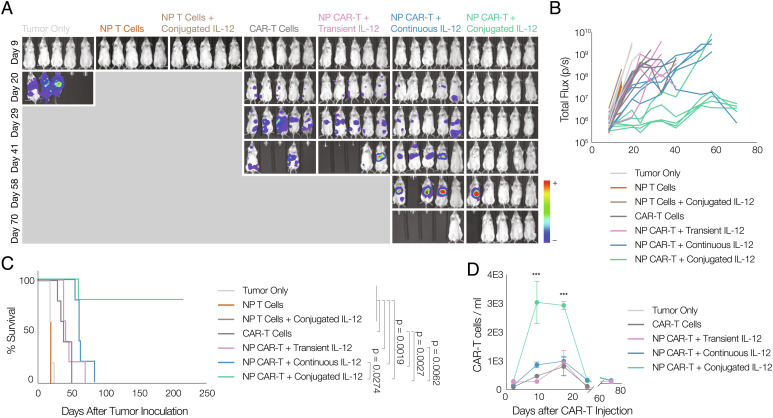
The application of cytokine conjugation was next explored in the context of CAR-T therapy. NSG mice were inoculated with Raji-luc lymphoma before CD19-specific CAR-T cells were injected systemically at half of the curative dose. T cells that have no CAR vector expression showed no effect in controlling tumor growth. In comparison, metabolically labeled CAR-T cells conjugated with IL-12 significantly controlled tumor development and prolonged mice survival (Fig. 6 A–C). CAR-T cells with IL-12 conjugation also showed better proliferation in mice compared with mice receiving untreated T cells or T cells with systemic cytokine treatment (Fig. 6D).
Fig. 6.
IL-12 conjugation improves the efficacy of CAR-T therapy. A, Bioluminescence image showing tumor burden in Raji xenograft model (n=5). B, Quantification of bioluminescent signal, and (C) mouse survival data for Raji xenograft model (n = 5, Mantel–Cox test). D, Number of anti-CD19 CAR-T cells per 1 mL of blood over time (n = 5). Animals were untreated (“tumor only;” light gray), treated with metabolically labeled T cells without CAR vector expression (“NP T cells;” dark orange), treated with metabolically labeled and IL-12-conjugated T cells without CAR vector expression (“NP T cells + conjugated IL-12;” brown), treated with CAR-T cells without labeling but without IL-12 conjugation (“CAR-T;” dark gray), treated with CAR-T cells metabolically labeled and transiently exposed to non-DBCO-conjugated IL-12 prior to transfer (“NP + transient IL-12;” purple), treated with CAR-T cells that were labeled and transferred with the same quantity of soluble IL-12 (not DBCO conjugated) as was conjugated to CAR-T cells (“NP + continuous IL-12;” blue), or treated with CAR-T cells that were metabolically labeled and then conjugated with IL-12 prior to transfer (“NP + conjugated IL-12;” green).
Discussion
Here, we demonstrate a simple and scalable nanotechnology strategy to increase the efficacy of adoptive T cell transfer (ACT) therapies. Nanoparticles fabricated from a polymer of synthetic sugar provided a potent approach to efficiently label T cells for cytokine conjugation and direct T cell differentiation and function. T cells can be easily metabolically labeled and conjugated with antitumor cytokines, or other agents, by simply adding nanoparticles and cytokines sequentially into normal T cell culture, without disrupting the conventional manufacturing processes for ACT and CAR-T therapies. T cells conjugated with the antitumor cytokine IL-12 demonstrated significantly better control over solid tumor growth, likely by increasing the number of adoptively transferred T cells both in circulation and penetrating the tumors. Additionally, IL-12 conjugation led to activation of the endogenous immune system toward a Th1 type response, enabling the recognition of tumor antigens beyond those targeted by the ACT. Finally, this approach was shown to be useful in CAR-T and human tumor models, setting the stage for future clinical use and also serving as a versatile platform for the conjugation of multiple cytokines.
The artificial sugar nanoparticles utilize the T cell metabolic pathways to efficiently label cell surface glycoproteins with azide groups, without otherwise significantly altering the T cells, providing a chemical handle for the conjugation of various agents onto T cell surfaces. T cells were azide labeled at ~100% efficiency, with the level of azide incorporation proportional to the sugar dose added to the culture medium. Various cytokines could, also in a dose-dependent manner, be conjugated to the T cell surface, and maintain their bioactive functions, as demonstrated by an analysis of a number of phenotypic and functional features. These findings support the broad utility of metabolic labeling to enable modification of T cells.
Cytokine conjugation onto T cells via G400 NP significantly boosted the efficacy of both ACTs against solid tumors and human CAR-T cells to hematologic cancer, even at significantly lower doses than previously used. The ability of IL-12 conjugation to significantly delay the progression of aggressive melanoma was likely related to the increased number of adoptively transferred T cells in circulation at peak response, their direction toward a more effector-memory like phenotype, and significantly increased infiltration of the solid tumor. In the CAR-T context, IL-12 conjugation allowed tumor control with a subcurative dose and similarly enhanced the number of circulating CAR-T cells postinfusion. The effects in both models were found with the conjugation of only 1.47 µg IL-12 and a single T cell injection. This is a significantly lower dose as compared with previous animal studies (~3 to 10 µg over 10 injections) and clinical trials where subjects generally receive multiple cytokine injections each week or even daily throughout the treatment (15, 16, 24, 31, 32). Compared with previous strategies, our method also provided better tumor targeting and tumor infiltration of cytokine-conjugated T cells without inducing noticeable systemic toxicity. Previous strategies increased cytokine tumor-targeting ability mostly by fusing cytokines to tumor-targeting modalities such as antibodies. However, those agents often still induced systemic toxicity given that many tumor antigens are also expressed in nontumorous cells (33–35). The findings here suggest clinical relevance, in potentially improving the treatment of solid tumors, allowing treatment for patients with less available T cells, or reducing the time needed for CAR-T proliferation ex vivo.
Strikingly, IL-12 conjugation had a significant impact on the endogenous immune system and orchestrated a type 1 immune response and antigen spread. This is reflected in elevated levels of Th1 cytokines in T cells isolated from spleens and tdLNs, increased activation and antigen presentation activities of DCs, and enhanced numbers of host T cells specific to antigens not targeted by the ACT. The antigen spread may result from the adoptively transferred T cells being more effective at killing cancer cells, resulting in the release of more cancer-associated antigen, altered cytokine signaling by the IL-12-conjugated T cells, or by direct engagement of host immune cells with the IL-12 presented by the transferred T cells from their surface. In any case, this finding suggests that the immediate debulking of tumors resulting from ACT can be enhanced by long-term immunosurveillance via mobilization of host anticancer immune responses resulting from the cytokine conjugation to the adoptively transferred T cells. Mobilization of host immune response and antigen spreading has been previously identified as a major contributor to ACT and CAR-T efficacy and was observed both in patients bearing metastatic lesions and in preclinical animal models. However, activation of the host immune system and antigen spreading is generally achieved with the addition of another therapy. For example, chemotherapy and radiation in conjunction with CAR-T therapy helped recruit T cells to solid tumors although this could induce strong side effects (36, 37). CAR-T cells have also been combined with bacterial infections or checkpoint blockade, or genetically engineered to express ligands for activating cell-surface molecules (38–40). In contrast to these other approaches, cytokine conjugation via metabolic labeling can mobilize a host immune response with little modification to current T cell-manufacturing procedures, and without the need to orchestrate multiple therapeutic modalities.
Altogether, our findings demonstrate a simple approach to conjugate antitumor cytokines onto T cells and the resulting, dramatic boost this provides to the efficacy of ACT therapies at low cytokine doses. This method could be readily integrated into the current ACT- and CAR-T-manufacturing processes by simply adding the sugar nanoparticles to the cell culture medium during ex vivo T cell expansion and including a short cytokine conjugation and wash step before transfer. The ability to conjugate multiple cytokines provides the possibility to exploit various combinations of cytokines for maximum efficacy in the future. The easy integration and versatility of this platform could transform current ACT and CAR-T therapies.
Materials and Methods
Detailed descriptions of the experimental procedures are provided in SI Appendix, Materials and Methods.
Monomers of G400 NPs were synthesized as previously described. G400 NPs were then polymerized with azobisisobutyronitrile (AIBN) and poly(ethylene glycol) methyl ether 2-(dodecylthiocarbonothioylthio)-2-methylpropionate (PEG DDMAT), nanoprecipitated, and stored for future use.
Mouse T cells and human T cells were isolated from fresh mouse spleen and human peripheral blood mononuclear cells (PBMCs), respectively, with magnetic-based cell sorting. T cells were then activated with anti-CD3/CD28 Dynabead T cell activator and cultured for downstream use. T cells were azido labeled by adding G400 NP solution directly to T cell cultures at various concentrations for 72 h after activation, and azide signaling was detected with flow cytometry and fluorescent microscopy.
DBCO cytokines were modified with DBCO-sulfo-NHS and verified with MALDI-TOF. DBCO-modified cytokines were conjugated onto T cells by reacting at 4 °C for 30 min. Phenotype changes of T cells were examined with flow cytometry, and functional changes were analyzed with cytolysis assays in vitro.
Mouse B16-F10 melanoma models were established by injecting 100k B16-F10 tumor cells subcutaneously. Tumor growth was monitored over time. T cells were adoptively transferred via tail vein injection on day 5. Xenograft lymphoma models were established by injecting 500k luciferized Raji cells intravenously. CAR-T cells were manufactured by transducing T cells with CAR-vector-containing lentivirus and were adoptively transferred intravenously on day 4. Adoptively transferred T cells were tracked by collecting blood from mouse tail vein and phenotyped with flow cytometry.
T cells were isolated from tumor and tumor-draining lymph nodes by breaking the tissue down, digesting with 200 IU collagenase type I, passing through a filter, and centrifuging with gradiented Percoll. For intracellular cytokine staining, isolated T cells from tumor, spleen, and lymph nodes were cocultured with B16-F10 melanoma T cells. After 1 h, GolgiPlug was added according to manufacturer’s protocol. After 3 h, T cells were processed for flow cytometry.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We are thankful to Dr. A. Najibi, Dr. M. Dellacherie, and Dr. S. Badrinath for sharing their knowledge on critical pieces of experiments. We thank M. Perez, T. Ferrante, and E. Zigon for technical support and discussions. We acknowledge funding from the NIH (R01 CA238039), the Food and Drug Administration (R01 FD006589), and the Wyss Institute.
Author contributions
Y.L., H.W., K.W.W., and D.J.M. designed research; Y.L., K.A.-B., J.M.B., M.P., and D.K.Y.Z. performed research; Y.L., J.Z., J.W.P., H.W., and K.W.W. contributed new reagents/analytic tools; Y.L. analyzed data; D.J.M. supervison, funding acquisition; and Y.L. and D.J.M. wrote the paper.
Competing interest
The authors declare competing interest. The authors have organizational affiliations to disclose, K.W.W. serves on the scientific advisory board of SQZ Biotech, Nextechinvest, Bisou Bioscience Company, and T-Scan Therapeutics, the authors have stock ownership to disclose, K.W.W. is a scientific co-founder of Immunitas Therapeutics, the authors have patent filings to disclose, Y.L., H.W., and D.J.M. have applied through Harvard University for patents on this technology, the authors have research support to disclose, K.W.W. receives sponsored research funding from Novartis.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Kochenderfer J. N., et al. , Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg S. A., et al. , Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl. J. M ed. 319, 1676–1680 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S. A., et al. , Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl. J. Med. 313, 1485–1492 (1985). [DOI] [PubMed] [Google Scholar]
- 4.Donohue J. H., et al. , The systemic administration of purified interleukin 2 enhances the ability of sensitized murine lymphocytes to cure a disseminated syngeneic lymphoma. J. Immunol. 132, 2123–2128 (1984). [PubMed] [Google Scholar]
- 5.Jackson H. J., Rafiq S., Brentjens R. J., Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June C. H., O’Connor R. S., Kawalekar O. U., Ghassemi S., Milone M. C., CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Atrash S., Bano K., Harrison B., Abdallah A. O., CAR-T treatment for hematological malignancies. J. Investig. Med. 68, 956–964 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Gill S., Maus M. V., Porter D. L., Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev. 30, 157–167 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Valkenburg K. C., de Groot A. E., Pienta K. J., Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 15, 366–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanitis E., Irving M., Coukos G., Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 33, 55–63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., et al. , Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 21, 2278–2288 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmielewski M., Abken H., TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 15, 1145–1154 (2015), https://doi-org.ezp-prod1.hul.harvard.edu/10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 13.Chmielewski M., Kopecky C., Hombach A. A., Abken H., IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 71, 5697–5706 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Fu R., et al. , Delivery techniques for enhancing CAR T cell therapy against solid tumors. Adv. Funct. Mater. 31, 2009489 (2021). [Google Scholar]
- 15.Motzer R. J., et al. , Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin. Cancer Res. 4, 1183–1191 (1998). [PubMed] [Google Scholar]
- 16.Sangro B., et al. , Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J. Clin. Oncol. 22, 1389–1397 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Bortolanza S., et al. , Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol. Ther 17, 614–622 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo N., et al. , Study of recombinant human interleukin-12 for treatment of complications after radiotherapy for tumor patients. World J. Clin. Oncol. 8, 158–167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia S., et al. , Recombinant interleukin-21 plus sorafenib for metastatic renal cell carcinoma: A phase 1/2 study. J. ImmunoTher. Cancer 2, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlon K. C., et al. , Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 33, 74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floros T., Tarhini A. A., Anticancer cytokines: Biology and clinical effects of interferon-α2, interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin. Oncol. 42, 539–548 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotz C., et al. , Local delivery of mRNA-encoding cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 13, 7804 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Wyatt Shields C., et al. , Cellular backpacks for macrophage immunotherapy. Sci. Adv. 6, 6579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang L., et al. , Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxon E., Bertozzi C. R., Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Prescher J. A., Dube D. H., Bertozzi C. R., Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Du J., et al. , Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 19, 1382–1401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H., et al. , Metabolic labeling and targeted modulation of dendritic cells. Nat. Mater. 19, 1244–1252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokunaga R., et al. , CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy. Cancer Treat Rev. 63, 40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato N., Patel H. J., Waldmann T. A., Tagaya Y., The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc. Natl. Acad. Sci. U.S.A. 104, 588–593 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth M. J., Taniguchi M., Street S. E. A., The anti-tumor activity of IL-12: Mechanisms of innate immunity that are model and dose dependent. J. Immunol. 165, 2665–2670 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Chiodoni C., et al. , Different requirements for α-galactosylceramide and recombinant IL-12 antitumor activity in the treatment of C-26 colon carcinoma hepatic metastases. Eur. J. Immunol. 31, 3101–3110 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Fallon J. K., Vandeveer A. J., Schlom J., Greiner J. W., Enhanced antitumor effects by combining an IL-12/anti-DNA fusion protein with avelumab, an anti-PD-L1 antibody. Oncotarget 8, 20558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue D., Hsu E., Fu Y. X., Peng H., Next-generation cytokines for cancer immunotherapy. Antib. Ther. 4, 123–133 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pogue S. L., et al. , Targeting attenuated interferon-α to myeloma cells with a CD38 antibody induces potent tumor regression with reduced off-target activity. PLoS One 11, e0162472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava S., et al. , Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell 39, 193–208.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geyer M. B., et al. , Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight 4, e122627 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapuis A. G., et al. , T-cell therapy using interleukin-21–primed cytotoxic T-cell lymphocytes combined with cytotoxic T-cell lymphocyte antigen-4 blockade results in long-term cell persistence and durable tumor regression. J. Clin. Oncol. 34, 3787 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin G., et al. , Pathogen-boosted adoptive cell transfer therapy induces endogenous antitumor immunity through antigen spreading. Cancer Immunol. Res. 8, 7–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etxeberria I., et al. , Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8+ T cells. Cancer Cell 36, 613–629.e7 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.