Significance
The frequency discrimination important for animal communication and survival is achieved via a special functional arrangement of cells along the length of the cochlea. Hair cells at the base are tuned to high-frequency sounds, whereas those at the apex are tuned to lower frequencies. Gradual anatomical and physiological changes along the cochlea facilitate this frequency selectivity, but it remains unclear how this functional arrangement is established. Using mouse genetics, we found SHH specifies apical identity in the cochlear primordium. Then, SHH and FST together maintain cochlear cells with an apical fate. In the absence of FST or SHH, mice produce short cochleae lacking the apical domain, leading to low-frequency hearing loss.
Keywords: frequency discrimination, cochlea, tonotopy, follistatin
Abstract
The cochlea’s ability to discriminate sound frequencies is facilitated by a special topography along its longitudinal axis known as tonotopy. Auditory hair cells located at the base of the cochlea respond to high-frequency sounds, whereas hair cells at the apex respond to lower frequencies. Gradual changes in morphological and physiological features along the length of the cochlea determine each region’s frequency selectivity, but it remains unclear how tonotopy is established during cochlear development. Recently, sonic hedgehog (SHH) was proposed to initiate the establishment of tonotopy by conferring regional identity to the primordial cochlea. Here, using mouse genetics, we provide in vivo evidence that regional identity in the embryonic cochlea acts as a framework upon which tonotopy-specific properties essential for frequency selectivity in the mature cochlea develop. We found that follistatin (FST) is required for the maintenance of apical cochlear identity, but dispensable for its initial induction. In a fate-mapping analysis, we found that FST promotes expansion of apical cochlear cells, contributing to the formation of the apical cochlear domain. SHH, in contrast, is required both for the induction and maintenance of apical identity. In the absence of FST or SHH, mice produce a short cochlea lacking its apical domain. This results in the loss of apex-specific anatomical and molecular properties and low-frequency-specific hearing loss.
Complex environmental auditory stimuli comprise multiple sound frequencies, and precise frequency discrimination is critical for extracting information essential to animal communication and survival. The peripheral auditory organ, which is commonly referred to as the cochlea, achieves frequency discrimination via a specialized topography along its longitudinal axis known as tonotopy. Auditory hair cells in the basal (proximal) cochlea, closer to the vestibular organs, are tuned to high-frequency sounds, whereas hair cells in the apical (distal) cochlea are more sensitive to lower frequencies (1, 2).
Cochlear tonotopy arises from gradients in the mechanical properties of the basilar membrane along the length of the cochlea. Hair cells also exhibit graded changes in morphological and physiological characteristics, which facilitate the cochlea’s frequency selectivity (1, 2). Each hair cell has a bundle of stereocilia protruding from its surface that is responsible for detecting sound vibrations. These stereocilia are shorter in the hair cells at the cochlear base and longer toward the apex (3–5). In terms of number, hair cells at the base have more stereocilia per bundle than those at the apex (4, 6). In mammals, the angle of the V-shaped stereocilia on outer hair cells is wider at the base, growing gradually narrower toward the apex (7, 8). In addition, non-sensory cochlear structures including the spiral ligament, the tunnel of Corti, and the tectorial membrane display gradual changes along the tonotopic axis (9–11).
The expression of genes critical to auditory function also varies along the tonotopic axis. Kcnq4 and Kcna10, which encode voltage-gated potassium channels, are expressed in opposing gradients along the length of the cochlea (12, 13). Cacna1d, which encodes the calcium channel CaV1.3a1, and Calb2, which encodes the calcium-binding protein calbindin, are expressed at higher levels at the apex than at the base (14). Genes associated with non-syndromic hearing loss, such as Slc26a5, Tectb, and Otof, are also expressed at higher levels at the apex of the mouse cochlea, gradually tapering to lower levels at the base (13).
Although various morphological and molecular features associated with frequency discrimination have been identified, the underlying mechanisms by which tonotopy is established during development remain poorly understood. Recently, sonic hedgehog (SHH) signaling from the ventral midline (i.e., the notochord and floor plate) was proposed to initiate the establishment of tonotopy in both birds and mammals (4). An increasing gradient of SHH from base to apex confers regional identity to the primordial cochlea (4, 5). In chicken embryos, SHH establishes a gradient of bone morphogenetic protein 7 (BMP7) and subsequently of retinoic acid (RA) along the basilar papilla, which is analogous to the mammalian organ of Corti (4, 15, 16). Activation of SHH, BMP7, or RA in the embryonic basilar papilla promotes gene expression and hair cell morphology characteristic of the apex, whereas inhibition of BMP7 or RA induces basal phenotypes (4, 15, 16). Thus, BMP7 and RA appear to act downstream of SHH in tonotopic patterning in birds. It remains to be confirmed, though, whether tonotopic changes in the developing basilar papilla affect the ability of adults to respond to specific sound frequencies.
Unlike in chickens, Bmp7 expression in the developing mouse cochlea is neither activated by SHH nor expressed in a gradient (4). This suggests a divergence in the mechanisms that establish tonotopy between birds and mammals. It remains unclear, though, whether, and how regional identity specified by SHH in the developing mouse cochlea contributes to the tonotopic organization of the mature cochlea. Using a mouse genetic approach, we show in this study that once SHH specifies apical identity in the cochlear primordium, both SHH and follistatin (FST), an antagonist of TGFβ/BMP/activin signaling, play critical roles in the establishment of tonotopy by maintaining apical identity. Our observations of cochlear gene expression, hair bundle morphology, and hearing function indicate that the regional identity of the primordial cochlea provides a framework upon which the adult cochlea acquires the tonotopic properties essential for frequency selectivity.
Results
Apical Identity in the Developing Mouse Cochlea is Lost in the Absence of FST.
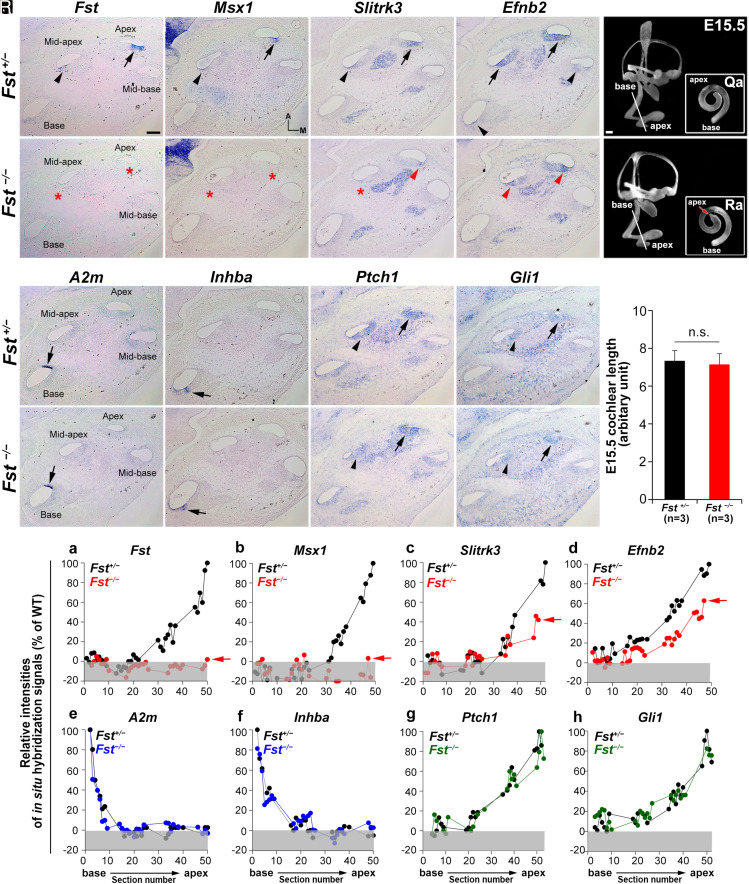
To determine whether FST plays a role in the tonotopic organization of the mammalian cochlea, we first asked whether the regional identity specified by SHH is compromised in the developing cochleae of Fst−/− embryos. We assessed regional identity via genes displaying an increasing or decreasing cochlear base-to-apex expression gradient throughout mouse embryogenesis (4, 5). In E15.5 cochleae, we found expression of Fst, Msx1, Slitrk3, and Efnb2 was strong at the apex and weaker toward the base, so we used these genes as apical cochlear markers (Fig. 1 A–D, arrows and arrowheads) (4). Then, because we found expression of A2m and Inhba was restricted to the base, we used these genes as basal cochlear markers (Fig. 1 I and J, arrows) (4). We visualized the graded expression patterns of these regional markers by performing in situ hybridizations on serial cochlear sections and plotting their signal intensities against the corresponding section numbers from base to apex (Fig. 1T and SI Appendix, Fig. S1) (5).
Fig. 1.
Specification of apical cochlear regional identity is compromised in Fst−/− cochleae. (A–P) In situ hybridization analysis with apical (Fst, Msx1, Slitrk3, and Efnb2) and basal (A2m and Inhba) regional markers and readouts of SHH signaling (Ptch1 and Gli1) in Fst+/− and Fst−/− embryos at E15.5. Arrows and arrowheads indicate relatively strong and weak expression, respectively. Red asterisks and red arrowheads indicate absence or down-regulation of expression, respectively. (Q–S) Paint-fill analysis of the Fst−/−inner ear (Q, R) and cochlear lengths of control and Fst−/−at E15.5(S). (T) Relative in situ hybridization signal intensities along the cochlear duct in Fst+/− and Fst−/− embryos. Red arrows indicate the apical end of the cochleae of Fst−/− embryos. Gray boxes below 0% in each graph indicate background signal. Representative graphs are presented from one Fst+/− and one Fst−/− cochlea for each gene. Additional samples are in SI Appendix, Fig. S1. The scale bar in A, 100 μm, also applies to B–P. The scale bar in Q, 100 μm, also applies to R.
When we examined the cochleae of E15.5 Fst−/− embryos, we found the overall inner ear structure and cochlear length were similar to those of controls (Fst+/−), except for a subtle malformation of the apical end (Fig. 1 Q and R). Fst−/−cochleae had undetectable Msx1 expression at the apex and mid-apex (Fig. 1 F and T, b and SI Appendix, Fig. S1). Mutant cochleae also showed greatly reduced Slitrk3 and Efnb2 expression compared to controls (Fig. 1 G, H, and T, c and d and SI Appendix, Fig. S1), although their expression gradients were maintained. In contrast, Fst−/− cochleae showed normal basal expression of A2m and Inhba (Fig. 1 M, N, and T, e and f and SI Appendix, Fig. S1). These results indicate a selective down-regulation of apical but not basal markers in the developing cochleae of Fst−/−mutants, suggesting a loss of apical identity. This was particularly true in the most apical region.
Since apical cochlear identity is specified by strong SHH activity (4, 5, 17), we asked whether the loss of apical identity in Fst−/− cochleae was due to abnormal SHH signaling. We found expression of Ptch1 and Gli1, indicators of SHH activity, was comparable between Fst+/− and Fst−/− cochleae (Fig. 1 K, L, O, P, and T, g and h and SI Appendix, Fig. S1). This suggests the loss of apical identity in Fst−/− cochleae was not due to defective SHH signaling. Together, these results indicate FST is critical for apical cochlear specification.
FST is Insufficient to Induce Apical Identity or Inhibit Basal Identity.
Because Fst expression is regulated by SHH signaling (4, 18), and because both SHH and FST are required for apical cochlear specification (Fig. 1) (4), we hypothesized that FST functions downstream of SHH. To test this idea, we asked whether ectopic FST expression promotes apical identity at the expense of basal identity, like ectopic SHH signaling (4). To this end, we used transgenic mice (R26-FST) that express the human FST transgene under the control of the ubiquitous R26 promoter in the presence of doxycycline (SI Appendix, Fig. S2A) (19). We administered doxycycline to timed pregnant female mice starting at E10.5 (SI Appendix, Fig. S2B) and confirmed FST overexpression in the entire cochlea at E15.5 (SI Appendix, Fig. S2H, red arrows). We noted that the expression gradients of the apical markers Msx1, Slitrk3, and Efnb2 were maintained (SI Appendix, Fig. S2 D–F and I–K), as was normal expression of the basal marker Inhba (SI Appendix, Fig. S2 G–L). These results indicate ectopic FST expression is insufficient to induce apical identity or inhibit basal identity. Thus, although SHH and FST are both required for apical cochlear specification, only SHH is sufficient to induce apical identity.
FST overexpression in R26-FST embryos inhibits activin signaling in the developing cochlea, leading to delayed hair cell differentiation (19). Since FST overexpression in R26-FST embryos does not disrupt regional identity, it is possible that activin A signaling is not required for regional specification in the cochlea. We tested this idea by examining the expression of regional markers in Inhba−/− mutants, which lack activin signaling. We found Inhba−/− cochleae at E18.5 exhibited delayed hair cell differentiation and ectopic inner hair cell production (SI Appendix, Fig. S3 A–H), as previously reported (19). We did not see any change, however, in the expression of the apical markers Fst and Msx1 or the basal marker A2m (SI Appendix, Fig. S3 I–P). These results suggest activin A signaling is not required for the specification of regional cochlear identity.
FST is Essential for Maintaining Apical Cochlear Identity.
Although our loss- and gain-of-function experiments indicated a requirement for FST in apical cochlear specification, FST, unlike SHH, proved insufficient to promote apical identity or inhibit basal identity (Fig. 1 and SI Appendix, Figs. S1 and S2). To better understand FST’s role in apical specification, we examined the cochleae of Fst−/− mutants at earlier embryonic stages, starting from E11.75, when expression of the apical marker Msx1 is first detected at the tip of the cochlear primordium (4, 17). In control (Fst+/−) embryos at E11.75, we found strong expression of the apical markers Fst and Msx1 and the SHH signaling indicator Ptch1 at the apex but not the base of the cochlear primordium (SI Appendix, Fig. S4 A, a–c and i–k). In the cochlear primordia of E11.75 Fst−/− mutants, although Fst expression was absent (SI Appendix, Fig. S4 A, m), the apical expression of Msx1 (SI Appendix, Fig. S4 A, n) and Ptch1 was unaffected (SI Appendix, Fig. S4 A, o). These results indicate FST is not required for the initial specification of apical identity. Msx1 expression in Fst−/− cochleae began to drop at E12.5 (SI Appendix, Fig. S4 B, f ) and was almost completely absent by E13.5 (SI Appendix, Fig. S4 C, f ). We also found that the Ptch1 expression gradient—an indicator of the SHH signaling gradient—was similar between Fst+/− and Fst−/− cochleae during these stages (SI Appendix, Fig. S4 B, c and g and C, c and g). We did not observe any terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells in the apical region where Msx1 was down-regulated during these stages (SI Appendix, Fig. S4 A, p; B, h; and C, h). This suggests the Msx1 down-regulation we observed was not secondary to a loss of Msx1-expressing cells via cell death. These results indicate that although FST is dispensable for the initial specification of apical cochlear identity, it is required for its maintenance (Fig. 1 and SI Appendix, Fig. S4).
Both SHH and FST Are Required for the Maintenance of Apical Cochlear Identity.
Genetic ablation of SHH signaling after specification of apical identity leads to low-frequency-specific hearing loss (20). This suggests SHH may also have a maintenance function in the apical cochlea in addition to its role in initial specification. Thus, we analyzed gene expression in Emx2Cre/+; Smolox/lox mutants, in which the SHH transducer Smo is deleted starting at E11.5 after the specification of regional identity (20). In Emx2Cre/+; Smolox/lox cochleae, we found Ptch1 expression was reduced and almost completely abolished by E16.5, indicating a progressive loss of SHH activity (SI Appendix, Fig. S5 A, f; B, f; C, f; F, and G). At E12.5, Fst and Msx1 were specifically expressed at the apex but not at the base in both control (Smolox/lox) and Emx2Cre/+; Smolox/lox mutant mice (SI Appendix, Fig. S5A), indicating normal specification of apical identity. Msx1 expression, however, was greatly reduced at E15.5 (SI Appendix, Fig. S5 B, e and D) and completely lost by E16.5 (SI Appendix, Fig. S5 C, e and E). Interestingly, Fst expression was not reduced in Emx2Cre/+; Smolox/lox mutant cochleae at these stages (SI Appendix, Fig. S5 A, d; B, d; and C, d), indicating that apical identity was progressively lost despite normal FST expression. These results, along with the fact that Fst−/− mutants showed progressive loss of apical identity with intact SHH signaling (Fig. 1 and SI Appendix, Fig. S4), indicate that both FST and SHH are required to maintain apical cochlear identity, with neither alone being sufficient.
SHH’s Capacity to Induce Ectopic Apical Identity Requires FST.
Since both FST and SHH are essential for the maintenance of apical identity, we next asked whether FST participates in SHH’s ability to ectopically induce apical identity at the base of the cochlea. To this end, we examined regional markers in the cochleae of Pax2-Cre; SmoM2 mutant mice expressing the constitutively active SHH transducer R26-SmoM2 in the presence or the absence of FST (Fst+/+or Fstlox/lox) (4). As previously demonstrated (4), Pax2-Cre; SmoM2 cochleae exhibited up-regulation of the SHH target genes Ptch1 and Gli1 (SI Appendix, Fig. S6 C and D, red arrows), down-regulation of the basal markers A2m and Inhba (SI Appendix, Fig. S6 I and J), and up-regulation of the apical markers Fst, Msx1, Slitrk3, and Efnb2 across the entire cochlea (SI Appendix, Fig. S6 Q–T).
We next asked whether SHH’s ability to induce apical identity depends on FST. In Pax2-Cre; SmoM2; Fstlox/lox mutant cochleae, strong SHH activity is maintained along the entire cochlea even in the absence of Fst expression (SI Appendix, Fig. S6 E and F–U). The basal markers A2m and Inhba remained completely down-regulated, indicating that strong SHH activity inhibits basal identity independent of FST (SI Appendix, Fig. S6 K and L). Surprisingly, however, in the absence of FST, the ectopic expression of the apical markers Msx1 and Slitk3 at the cochlear base was restored to the wild-type gradient increasing from base to apex (SI Appendix, Fig. S6 V and W). This indicates that SHH’s ability to induce ectopic apical identity at the base depends on FST. Interestingly, Pax2-Cre; SmoM2; Fstlox/lox cochleae continued to exhibit ectopic up-regulation of the apical marker Efnb2 (SI Appendix, Fig. S6X, red arrows), suggesting that, unlike Msx1 and Slitrk3, Efnb2 expression depends solely on SHH.
FST Supports the Expansion of an Apical Cochlear Cell Population.
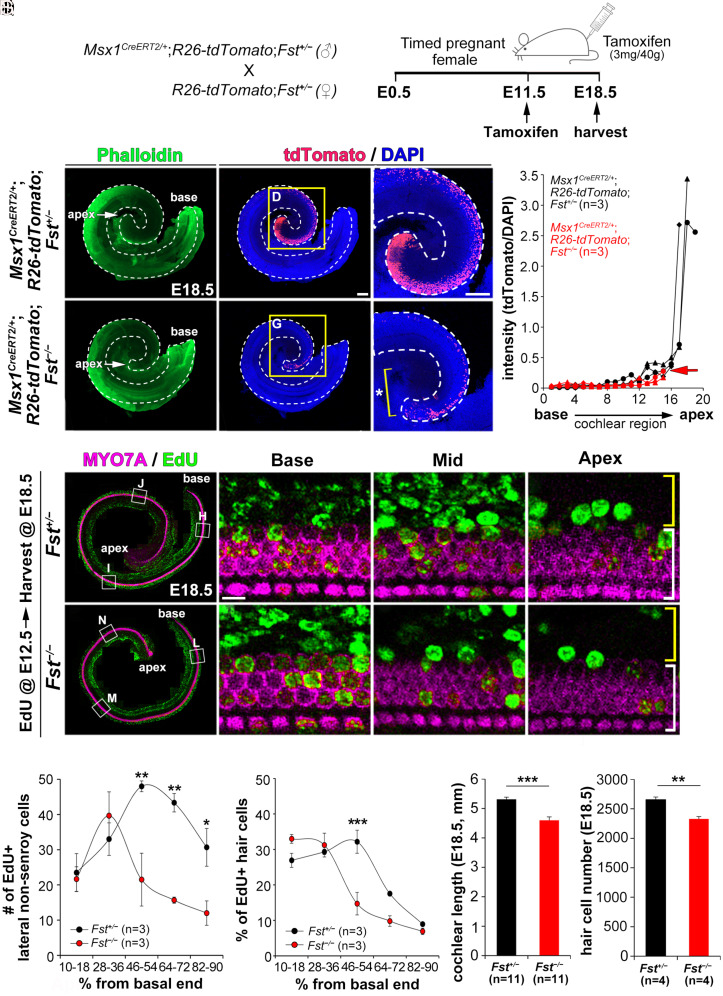
Our results indicate that FST plays an essential role in maintaining apical cochlear identity (Fig. 1 and SI Appendix, Fig. S4). To clarify the consequences of the loss of apical identity, we fate-mapped the lineage of the apical cell population (Msx1-positive) by crossing Msx1CreERT2/+ mice with R26-tdTomato reporter mice in the presence or the absence of Fst (Fst+/− or Fst−/−) (21, 22). After injecting tamoxifen into pregnant female mice at E11.5, when Msx1 expression is comparable between Fst+/− and Fst−/−embryos (SI Appendix, Fig. S4), we examined Msx1-lineage cell fate at E18.5 (Fig. 2A). In control Msx1CreERT2/+; R26-tdTomato; Fst+/− embryos, we found a dense population of Msx1-lineage cells in the lateral compartment of the apical cochlea, with fewer cells spread along the cochlear duct toward the base (Fig. 2 C and D). The loss of FST (in Msx1CreERT2/+; R26-tdTomato; Fst−/− embryos), however, greatly reduced the Msx1-lineage cell population (Fig. 2 F and G) and shortened the cochlear duct because of the loss of the apical domain in which the dense Msx1-lineage population would have otherwise resided (Fig. 2G, asterisk). When we plotted tdTomato-fluorescence intensity along the cochlear duct for control mice, we found the strongest signal clustered in the apical cochlear regions, with the remaining regions exhibiting only weaker signals (Fig. 2H, black lines). Msx1CreERT2/+; R26-tdTomato; Fst−/−mutant mice did not exhibit any strong fluorescence at the apex, showing only weak signal along the cochlear duct (Fig. 2H, red lines and red arrow). These results suggest that FST is crucial for the formation of the apical cochlear domain because of its role in expanding the apical (Msx1-positive) cell population.
Fig. 2.
Failure of apical cochlear expansion caused by reduced cell proliferation in Fst−/− cochleae. (A–H) Fate-mapping of Msx1-lineage cells using Msx1CreERT2/+; R26-tdtomato mice in the presence (Fst+/−) or absence (Fst−/−) of FST. After tamoxifen was injected into pregnant female mice at E11.5, the embryos were harvested at E18.5 (A). Quantification of tdTomato-fluorescence intensity along the cochlear duct (H). (I–R) Comparing EdU-labeled cells in Fst+/− and Fst−/− embryos. After EdU was injected into pregnant female mice at E12.5, EdU-labeled cells (green) were counted among the lateral non-sensory cells (yellow brackets) and MYO7A-positive hair cells (white brackets) (I–P). The number of EdU-positive cells in the lateral compartment (Q) and the percentage of EdU-positive hair cells (R) were plotted against the distance along the cochlear duct divided into five regions from base to apex. (S–T) Comparisons of cochlear length and hair cell number between E18.5 Fst+/− and Fst−/− embryos. Data in Q–T are presented as means ± SE. Statistical comparisons were determined via two-way ANOVA for the EdU analysis and unpaired t tests with Bonferroni corrections for the cochlear length measurements (*P < 0.05, **P < 0.01, and ***P < 0.001). The scale bar in C, 200 μm, also applies to B–C, E–F, and I–M. The scale bar in D, 200 μm, also applies to G. The scale bar in J, 10 μm, also applies to K–L and N–P.
FST Promotes Cell Proliferation in both Sensory and Non-Sensory Cochlear Cells.
MSX1 controls cell lineage development by promoting cell proliferation in the dental and limb mesenchyme (23, 24). We therefore asked whether loss of Msx1 in Fst−/− cochleae affects cell proliferation. After injecting timed pregnant female mice with 5-ethynyl-2′-deoxyuridine (EdU) at E12.5, which is when Msx1 expression begins to fall in Fst−/−embryos (SI Appendix, Fig. S4), we harvested the resulting embryos at E18.5. Fst−/− mutants showed far fewer EdU-positive cells in the sensory and lateral non-sensory compartments of the apical and middle cochlea than Fst+/− controls (Fig. 2 I–P). We quantified EdU-positive cells by dividing the length of the cochlea into five regions from the basal end (0%) to the apical end (100%) in both the lateral non-sensory cell region and in the hair cell region. Fewer EdU-positive cells appeared in the lateral compartment of the middle-to-apical cochlear regions than in controls (Fig. 2Q). EdU-positive hair cells were also significantly reduced in the middle cochlear region (Fig. 2R). Less than 10% of the hair cells in the apex of both genotypes were EdU-positive, probably because the cells at the apex had already exited the cell cycle before EdU injection (25). This reduced cell proliferation resulted in a significant reduction of cochlear length and total hair cell number in Fst−/− embryos (Fig. 2 S and T). Together, these results indicate FST plays a crucial role in the expansion of the apical cochlear domain by promoting cell proliferation.
We next asked whether the reduced cell proliferation of Fst−/− mutants also affects hair cell differentiation (SI Appendix, Fig. S7). In E15.5 controls (Fst+/+), we confirmed the base-to-apex progression of hair cell differentiation by noting the presence of cortical condensation in inner and outer hair cells at the base (SI Appendix, Fig. S7B, brackets) but not the apex (SI Appendix, Fig. S7D, asterisks). In Fst−/− mutants, cortical condensation was evident across the entire cochlea, including the apex (SI Appendix, Fig. S7 E–H, brackets). At E18.5, Fst−/− mutants exhibited more organized cortical condensation in the apex than controls (SI Appendix, Fig. S7 L–P). Fst−/− cochleae also frequently possessed an extra row of outer hair cells at the apex (SI Appendix, Fig. S7P, arrowhead). This has been observed before in mutant cochleae exhibiting premature cell cycle exit (5, 26). These results are consistent with a previous report that FST maintains pro-sensory cells in a proliferative and undifferentiated state (19). Our results also indicate that FST’s regulation of cell proliferation extends to non-sensory cells in the lateral cochlear compartment (Fig. 2 I–R). Together, these results confirm FST contributes to apical cochlear expansion by regulating cell proliferation.
Loss of Apical Identity Results in Loss of Apical Hair Bundle Morphology.
Our developmental gene expression and fate-mapping analyses suggested that FST plays a role in maintaining and expanding apical cell populations (Figs. 1–2 and SI Appendix, Fig. S4). We next asked how this early role of FST in the embryonic cochlea contributes to the tonotopic organization of the mature cochlea. Because the Fst−/− mutation is neonatal lethal in mice, we generated an inner ear-specific Fst conditional knockout (Fst cKO) by crossing Pax2-Cre mice with Fstlox/lox mice (27, 28). We confirmed that Fst cKO (Pax2-Cre; Fstlox/lox) cochleae exhibit the same changes as Fst−/− cochleae in the expression of apical (Fst, Msx1, Slitrk3, and Efnb2) and basal markers (A2m and Inhba) (SI Appendix, Fig. S8). Fst cKO mice were viable, fertile, and survived to at least 3 mo of age. In 4-wk-old Fst cKO mice, although we did not observe any apparent hair cell disorganization or degeneration, we frequently observed an extra row of apical outer hair cells (Fig. 3 A–F ) like those we observed in embryonic cochleae (SI Appendix, Fig. S7).
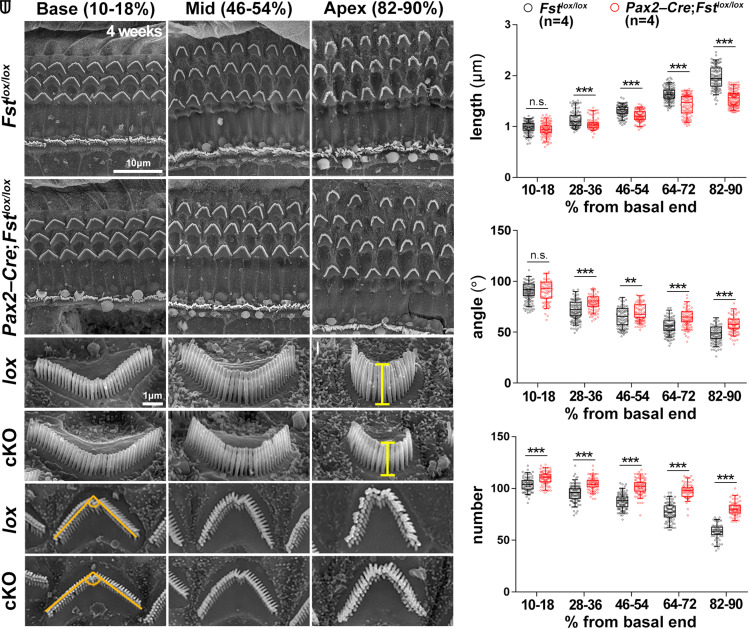
Fig. 3.
Loss of apical region stereocilia morphology in 4-wk-old Fst cKO mutants. (A–R) Scanning electron micrographs of the organ of Corti from control (lox) (Fstlox/lox) and inner ear-specific Fst cKO (Pax2-Cre; Fstlox/lox) mice. The lengths of the outer hair cell stereocilia were measured at the vertex of the V-shaped hair bundles from the lateral side (G–L). The angle of the V-shaped stereocilia and the number of stereocilia per outer hair cell were measured using a top-down view (M–R). The scale bar in A, 10 μm, also applies to B–F. The scale bar in G, 1 μm, also applies to H–R. (S–U) Quantification of the length, angle, and number of outer hair cell stereocilia along the tonotopic axis in each of the five cochlear regions beginning with the basal end. These represent the base (10 to 18%), mid-base (28 to 36%), mid (46 to 54%), mid-apex (64 to 72%), and apex (82 to 90%) regions. Statistical comparisons were determined via two-way ANOVA with Bonferroni corrections for multiple comparisons (n.s., non-significant, **P < 0.01, and ***P < 0.001).
Next, we evaluated tonotopic changes in outer hair cell morphology, noting stereocilia length, angle of the stereocilia V-shaped arrangement, and number of stereocilia per hair cell. Each of these characteristics gradually changes along the tonotopic axis (3, 5, 7, 8). First, we divided the cochlear duct into five tonotopic regions—base, mid-base, mid, mid-apex, and apex—with each defined as a percentage of the overall length. In 4-wk-old control mice (Fstlox/lox), stereocilia length increased gradually from base to apex (Fig. 3 G–I and S). Although Fst cKO cochleae maintained this gradient, stereocilia length was significantly reduced along the length of each cochlea, except at the base (Fig. 3 J–L and S). In control mice, the angle of the stereocilia V-shaped arrangement is widest at the base, gradually narrowing toward the apex (Fig. 3 M–O and T). In Fst cKO cochleae, this angle was significantly wider along the length of the cochlea, except at the base (Fig. 3 P–R and T). The base of control cochleae has more stereocilia per hair cell than those at the apex, and Fst cKO cochleae show significantly higher numbers than controls across their entire length (Fig. 3U). These results suggest the tonotopic organization of cochlear stereocilia morphology in Fst cKO mice is shifted basally.
Because Fst−/− mutants have shorter cochleae due to apical truncation (Fig. 2), we compared stereocilia morphology at equivalent distances from the base in control and Fst cKO mice (SI Appendix, Fig. S9A). Interestingly, we only found a significant difference in stereocilia length and angle at the apex of the cochlear duct (SI Appendix, Fig. S9 B and C). Control and Fst cKO outer hair cells also had similar numbers of stereocilia along the cochlear duct, although there were some regions with differences (SI Appendix, Fig. S9D). These results suggest the failure to maintain apical identity in embryonic Fst cKO mutant cochleae results in a loss of the morphological properties characteristic of apical stereocilia.
We next asked whether the loss of apical identity secondary to abnormal SHH signaling in Emx2Cre/+; Smolox/lox mutants (SI Appendix, Fig. S5) was also responsible for the similar phenotypes we observed in Fst cKO mutants. When we compared stereocilia length, angle, and number along the tonotopic axis of control (Smolox/lox) and mutant (Emx2Cre/+; Smolox/lox) cochleae at P15, we observed a similar tonotopic shift toward more basal properties like we saw in Fst cKO mutant cochleae (SI Appendix, Fig. S10). Similar to what we observed in Fst cKO mice, control and Smo cKO mice showed similar hair bundle morphology at equivalent distances from the base. In Smo cKO mice, however, the apical end was absent (SI Appendix, Fig. S11). Together, these results indicate that loss of apical identity in the embryonic cochlea due either to loss of FST or SHH leads to a loss of apical tonotopic characteristics in the mature cochlea.
Loss of the Apical Cochlea is Accompanied by Changes in Gene Expression.
We next used RNA sequencing to investigate changes in gene expression in Fst cKO mutants associated with the loss of the apical cochlear domain. After isolating basal, middle, and apical regions from the cochleae of 4-wk-old control and Fst cKO mice (SI Appendix, Fig. S12A), we examined their global gene expression profiles and compared them via a principal components analysis. In control, cochleae, basal, middle, and apical gene expression profiles were distinctly separate along the PC1 axis, which accounted for 55.18% of the overall variability (SI Appendix, Fig. S12B, black circles). In Fst cKO cochleae, however, while the basal and middle gene expression profiles were clearly separate, the apical and middle profiles clustered close to one another (SI Appendix, Fig. S12B, red circles). This suggests that in Fst cKO mutants, the gene expression profile of the apical cochlea has shifted toward that of the middle cochlea. This most likely reflects the fact that the portion of the cochlea collected as the “apex” contains more of the middle cochlear region since Fst cKO mutants lack a true apical end.
Next, we looked for genes and pathways affected by apical loss in Fst cKO mutants. To focus on genes differentially expressed along the tonotopic axis, we selected genes that increased or decreased by at least 1.5-fold from the base to the apex in control cochleae. We classified these genes as the UP (612 genes) or DOWN (730 genes) groups, respectively (SI Appendix, Fig. S12 C and D, and Datasets S1-S2). We found Fst was included in the UP group, indicating that it was expressed in a graded pattern from E10.5 to 4 wk of age (Dataset S1) (8). Most of the gene ontology (GO) terms enriched in the UP group were related to the inner ear and hearing. These terms included sensory perception of sound, inner ear morphogenesis, stereocilium, and potassium ion transport (SI Appendix, Fig. S13 and Dataset S3). In contrast, the GO terms enriched in the DOWN group were associated with broad developmental and biological processes, including extracellular matrix organization, cell adhesion, and multicellular organism development (SI Appendix, Fig. S13 and Dataset S3). Interestingly, the UP and DOWN groups from experiments with chicken basilar papillae showed enrichment of similar GO terms (29). These results suggest genes whose expression increases from the base to the apex of the cochlea are important for the functional aspects of tonotopy, such as sound perception, whereas genes whose expression decreases toward the apex are associated more with the structural properties of tonotopy, such as the extracellular matrix in the basilar membrane (29).
Next, we further classified genes in the UP group that were significantly down-regulated at the apex or had a less steep gradient of expression in Fst cKO mice than controls, referring to them as UP-1 genes (SI Appendix, Fig. S12C and Datasets S1 and S2). We reasoned that UP-1 genes would be the most affected by the loss of the apical cochlea in Fst cKO mutants. Similarly, we further classified genes in the DOWN group that were significantly up-regulated at the apex or that had a less steep gradient of expression in Fst cKO mice than controls, referring to them as DOWN-1 genes (SI Appendix, Fig. S12D and Dataset S2). Plotting heatmaps of the expression of 128 UP-1 genes and 269 DOWN-1 genes in Fst cKO cochleae revealed changes in gene expression levels at the apex that were still associated with an expression gradient (SI Appendix, Fig. S12 E and F and Datasets S1 and S2). As expected, we found a significant reduction in Fst expression in Fst cKO cochleae (SI Appendix, Fig. S12 E, a and Dataset S1). The UP-1 group included genes like Tectb, Otof, and Otogl that are associated with hearing loss, the potassium ion channel-related genes Kcnip4 and Kcna10 and the calcium-binding protein gene Calb2. The DOWN-1 group also included genes related to hearing loss (e.g., Slc4a10 and Gjb6), as well as genes like Car2, Adra2a, Smpd3, Tnmd, and Dcn, which are known to be expressed in a gradient (SI Appendix, Fig. S12 F, a and Dataset S2). To identify the cascade of upstream regulators that may cause the gene expression changes we observed in the UP-1 and DOWN-1 groups, we performed an upstream regulator analysis using Ingenuity Pathway Analysis tools (SI Appendix, Fig. S12G and Dataset S4). For the DOWN-1 group, we noted TGFβ signaling components such as TGFB1, SMAD3, and INHA among the identified upstream regulators (SI Appendix, Fig. S12G and Dataset S4). Together, these results suggest the global gene expression profile of the apex of Fst cKO cochleae shifts toward that of the middle cochlea via a mechanism that may be downstream of aberrant TGFβ/BMP signaling.
Loss of the Apical Cochlea Leads to Low-Frequency-Specific Hearing Loss.
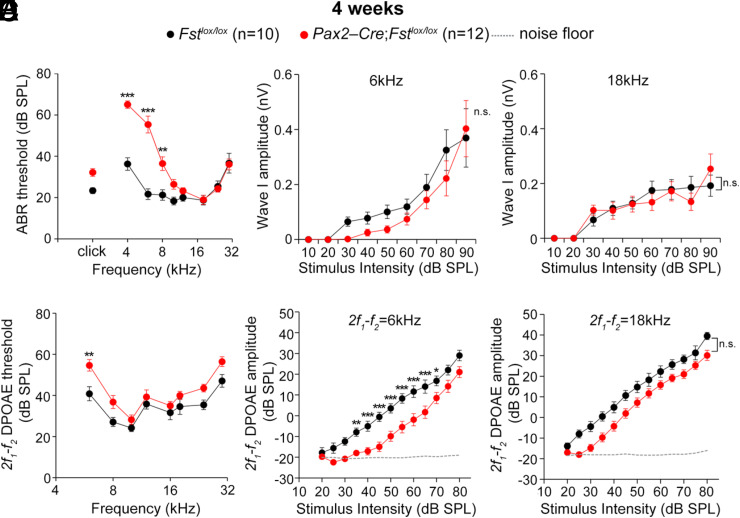
The loss of apical identity in embryonic cochleae we observed leads to tonotopic changes in stereocilia morphology and gene expression in mature cochleae. We next asked whether these structural and molecular changes in tonotopy affect the cochlea’s ability to respond to specific sound frequencies. Because Fst cKO mutants lack the cochlear apex, we expected a deficiency in low-frequency hearing. We therefore assessed hearing sensitivity by measuring auditory brainstem response (ABR) thresholds in 4-wk-old control and Fst cKO mice in response to broadband click stimuli or pure tones. Although we did not observe significant changes in the ABR thresholds to broadband click stimuli (Fig. 4A), we found that the ABR thresholds to pure tone stimuli were significantly higher at low frequencies (4 to 8 kHz) but not high frequencies (Fig. 4A). These results demonstrate that Fst cKO mutant mice are less sensitive to low-frequency sounds than high-frequency sounds.
Fig. 4.
Low-frequency-specific hearing loss in 4-wk-old Fst cKO mutants. (A–C) ABR analyses of 4-wk-old Fstlox/lox and Pax2-Cre; Fstlox/lox mice. ABR thresholds of Fstlox/lox and Pax2-Cre; Fstlox/lox mice to click stimuli and individual frequencies (A). I/O function analyses of wave I amplitudes from the ABRs to low (6 kHz) and high (18 kHz) frequencies (B, C). (D–F ) DPOAE analyses of 4-wk-old Fstlox/lox and Pax2-Cre; Fstlox/lox mice. 2f1-f2 DPOAE thresholds of Fstlox/lox and Pax2-Cre; Fstlox/lox mice (D). DPOAE I/O function analyses at 6 kHz and 18 kHz (E, F ). Data are presented as means ± SE. Statistical comparisons were determined via two-way ANOVA with Bonferroni corrections for multiple comparisons (n.s., non-significant, *P < 0.05, **P < 0.01, and ***P < 0.001).
Last, we asked whether these elevated ABR thresholds were caused by a defect in the inner hair cells activating spiral ganglion neurons or in the outer hair cells acting as the cochlear amplifier. The ABR wave I amplitudes, which represent the summed auditory nerve fiber response, were similar in control and Fst cKO mice to low (6 kHz) and high (18 kHz) frequency sounds at 4 wk (Fig. 4 B and C). In contrast, distortion-product otoacoustic emission (DPOAE) thresholds, which reflect the cochlear amplifier function of the outer hair cells, were significantly higher at 6 kHz than 18 kHz (Fig. 4D). We confirmed this reduction in Fst cKO mice at low frequencies via an input/output (I/O) function analysis of the DPOAE amplitudes (Fig. 4 E and F ). These results suggest the low-frequency-specific hearing loss we observed in Fst cKO mice is likely caused by defective outer hair cell function disrupting the cochlear amplifier for low-frequency sounds. When we tracked this low-frequency hearing loss of Fst cKO mice at later time points, we found that it continued out as far as 12 wk of age (SI Appendix, Fig. S14). Together, these results suggest FST is crucial for the establishment of tonotopy via its role in establishing and patterning the features necessary for low-frequency hearing in the cochlear apex.
Discussion
Sequential Progression of the Establishment of Tonotopic Organization in the Mammalian Cochlea.
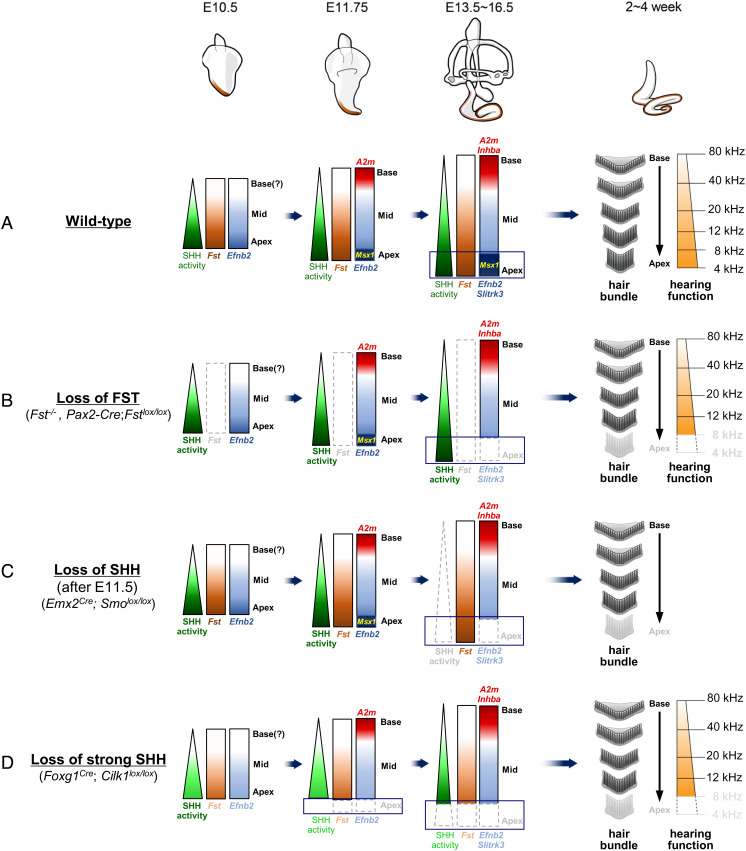
Observations from our current study and from previous studies indicate that the establishment of tonotopic organization proceeds sequentially during cochlear development (Fig. 5A). An extrinsic SHH signaling gradient confers regional identity to the developing cochlea by promoting apical identity and suppressing basal identity (4, 5, 17). This regional identity is evident in increasing base-to-apex expression gradients of the apical markers Fst and Efnb2 as soon as an out-pocketing of the cochlear primordium becomes visible at the ventral otocyst in E10.5 mice (Fig. 5A). By E11.75, the slightly elongated cochlear primordium expresses Msx1 and A2m in the apical and basal-most regions, respectively (Fig. 5A). Then, two more expression gradients emerge: an increasing base-to-apex gradient of Slitrk3 and an increasing apex-to-base gradient of Inhba (Fig. 5A). These differential gradients are maintained during cochlear elongation throughout embryogenesis independent of the apex-to-base wave of cell cycle exit and the base-to-apex wave of hair cell differentiation (4, 5). This regional identity of the cochlear primordium provides a framework for the tonotopic organization of the mature cochlea (Fig. 5A).
Fig. 5.
Summary diagram illustrating tonotopic organization in the mammalian cochlea. (A) The tonotopic organization of the cochlea is established sequentially. First, apical-to-basal regional identity is established. This then provides a framework upon which the basal-to-apical tonotopic characteristics tuned to specific frequencies appear along the mature cochlea. (B–D) Loss or gain of FST or SHH function perturbs this framework in the developing cochlea, resulting in changes in the tonotopic characteristics of the mature cochlea. See the Discussion section for details on each genotype.
Once the tonotopic framework is established in the cochlear primordium, both FST and SHH work to maintain apical identity as indicated by Msx1 expression. This apical identity is progressively lost in the absence of either FST or SHH during cochlear development (Fig. 5 B and C). FST also plays a role in the expansion of the apical cell population by promoting cell proliferation in both the sensory and non-sensory compartments (Fig. 2) (19). This increase in apical cells contributes to the extension of the apical cochlear domain (Fig. 2). In this extended apical cochlea, hair cells differentiate to adopt apical tonotopic characteristics optimized for responses to low-frequency sounds (Fig. 5A). In the absence of FST, the cochlear duct is shortened because it lacks the apical domain. This leads to the loss of the apical hair bundles and apical gene expression that supports low-frequency hearing (Fig. 5B).
It should be noted that failure to maintain apical identity in the absence of FST results in tonotopic changes nearly identical to those observed in the ciliary mutant Foxg1Cre; Cilk1lox/lox (Fig. 5D), which result from a failure to induce apical identity secondary to abnormal SHH signaling (5). The phenotypes these mutants share include loss of the apical cochlear region, apical hair bundle characteristics, and low-frequency-specific hearing (Fig. 5 B–D) (5). Using a mouse genetic approach, we have clearly demonstrated that a change in regional identity in the embryonic cochlea influences tonotopy in the corresponding region of the adult cochlea, leading to a frequency-specific hearing defect.
The Functional Relationship between SHH and FST in the Development of Tonotopy.
Although Fst expression is activated by SHH signaling (SI Appendix, Fig. S6) (4), our analyses of the cochleae of mice with single or compound mutations of SHH and FST suggest a complex functional relationship rather than a simple signaling cascade (Fig. 5 and SI Appendix, Fig. S15). Unlike SHH, FST is neither sufficient (as tested in R26-FST mice) nor necessary (as tested in Fst−/−mice) to induce apical identity (SI Appendix, Figs. S2 and S4). Nor is the activin A signaling antagonized by FST required for regional cochlear patterning (as tested in Inhba−/− mice) (SI Appendix, Fig. S3). Thus, it is unlikely that SHH specifies regional identity in the mouse cochlea by regulating TGFβ/BMP signaling. The situation is somewhat different, though, in the chicken basilar papilla. There, SHH regulates regional patterning by establishing an expression gradient of BMP7 (4, 15). While BMP7 promotes apical identity, BMP inhibition via CHRDL1 (chordin-like 1) promotes basal identity (15). Bmp7 is not, however, activated by SHH signaling, nor are Bmp7 and Chrdl1 expressed in gradients in the mouse cochlea (4, 8). If SHH function is mediated by other TGFβ/BMP ligands or other signaling pathways, it remains unclear. We hope in the future to explore the effects on the mouse cochleae of the conditional loss of common mediators of TGFβ/BMP signaling like SMAD4. We also expect that transcriptomic analyses along the tonotopic axis during cochlear development will provide further insight into this phenomenon.
Instead of acting in a signaling cascade, both SHH and FST are required for apical cochlear patterning. Loss of FST (Pax2-Cre; Fstlox/lox) or SHH (Emx2Cre; Smolox/lox) causes similar tonotopic changes, namely apical cochlear truncation and loss of apical tonotopic characteristics (Fig. 5 B and C). It remains unclear whether SHH and FST pattern the apical cochlea cooperatively or independently. The expression of Msx1—a marker of apical identity—is abolished by E13.5 in Fst−/− mutants (SI Appendix, Fig. S4) but remains until E16.5 in the absence of SHH function (Foxg1Cre; Shhlox/− and Emx2Cre; Smolox/lox) (SI Appendix, Fig. S5) (4). Thus, SHH and FST’s maintenance of apical identity may have different temporal requirements. Furthermore, although both FST and SHH regulate cell proliferation and contribute to apical cochlear extension (Fig. 2) (5, 19, 26), SHH activity is stronger in the medial cochlear compartment and FST expression is stronger in the lateral compartment (Fig. 1 and SI Appendix, Fig. S4). Thus, SHH and FST do seem to contribute to cochlear cell proliferation via distinct roles in the medial and lateral compartments, respectively.
Signals Promoting Basal Cochlear Identity.
In contrast to our progress with apical tonotopic patterning, we do not yet understand how the basal cochlea is specified and patterned. Basal cochlear identity is effectively suppressed by strong SHH activity, so it is specified in the region where SHH activity is low (SI Appendix, Fig. S6) (4). It is possible that low SHH activity is sufficient to induce basal identity, but it is also possible that another signaling pathway either actively promotes basal identity, inhibits apical identity, or both via a gradient opposing that of SHH. Indeed, the presence of such a signal is implied from the phenotypes of cochlea that combined gain of SHH function and loss of FST function (SI Appendix, Figs. S6 and S15). We found a restoration of increasing base-to-apex expression gradients of apical markers such as Msx1 and Slitrk3 under constitutive SHH activation (Pax2-Cre; SmoM2) in the absence of FST (Pax2-Cre; SmoM2; Fstlox/lox) (SI Appendix, Figs. S6 and S15). This was somewhat surprising, because Pax2-Cre; SmoM2; Fstlox/lox cochleae activate SHH ubiquitously and lack FST, meaning both the SHH and FST gradients that are so important for establishing regional identity are essentially abolished. Yet, regional patterning of the cochlea appears to occur in these mutant mice, indicating that signals other than SHH and FST are involved in regional patterning. As this unknown signal inhibits SHH-induced gene expression at the base but not the apex, it is likely expressed in a gradient that decreases from base to apex (SI Appendix, Fig. S15C). In addition, since its inhibitory action on SHH-induced gene expression is relieved by FST, this signal is likely related to TGFβ/BMP/activin signaling. Although activin A signaling falls into this category (19), activin A cannot be the unknown signal because we were able to confirm normal regional specification in the absence of activin A signaling (Inhba−/− mice) (SI Appendix, Fig. S3). We hope soon to identify this ligand expressed in a decreasing base-to-apex gradient that inhibits apical gene expression and is antagonized by FST. This and any other signals that induce basal identity and suppress apical identity independent of SHH or FST will give insight into basal identity specification.
In summary, our present study provides in vivo evidence that tonotopic organization in the mammalian cochlea is established sequentially after the initial formation of a framework in the cochlear primordium. This framework provides positional information for the development of the molecular, structural, and functional characteristics that underlie tonotopy in the mature cochlea. Perturbation of regional identity in the embryonic cochlea affects the ability of the mature cochlea to discriminate the corresponding sound frequencies. It remains unclear, though, how cochlear cells and tissues with specific regional identities acquire their respective tonotopic properties in concert with complex and seemingly unrelated developmental processes, such as continuous cochlear extension and opposing waves of cell cycle exit and hair cell differentiation.
Materials and Methods
The mice used in this study were generated as previously described (4, 19, 20, 27, 28, 30, 31). Immunofluorescence staining, scanning electron microscopy, in situ hybridization, paint-fill injections, and EdU and TUNEL staining were performed as previously described (5, 26, 32–34). ABRs and DPOAEs were measured in a sound-proof chamber using Tucker-Davis Technologies (TDT) RZ6 digital signal processing hardware and the BioSigRZ software package as previously described (33). A more detailed account of the materials and methods used in this study appears in the SI Appendix, Materials and Methods section.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Acknowledgments
We thank Dr. Doris Wu for her critical reading of the manuscript, Drs. Jeong-Oh Shin, Ji-Hyun Ma, and Harinarayana Ankamreddy for their help beginning this study, and Mr. Dong-Su Jang (Medical Illustration & Design), part of the Medical Research Support Services group of Yonsei University College of Medicine, for artistic support. This work was supported by the National Research Foundation of Korea (NRF-2014M3A9D5A01073865, NRF-2016R1A5A2008630, and NRF-2022R1A2C3007281 to J.B.; NRF-2022M3E5F2017487 to U.-K.K.), Samsung Science and Technology Foundation (SSTF-BA2101-11 to J.B.), Team Science Award of Yonsei University College of Medicine (6–2021–0004 to J.B.), and by the National Institutes of Health (DC016538 to M.P-D.; DC011571 to A.D.; P20GM103436 and P30ES030283 to J.W.P; HD032067 to M.M.M.).
Author contributions
H.Y.K., J.W.P., A.D., U.-K.K., and J.B. designed research; H.Y.K., M.-A.K., M.P.-D., and K.S.K. performed research; H.Y.K., M.-A.K., H.M., J.Y.H., and K.S.K. analyzed data; and H.Y.K., M.M.M., A.D., and J.B. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix. All the raw data of our RNA-seq analyses have been deposited in the Gene Expression Omnibus data repository under accession code: GSE203228.
Supporting Information
References
- 1.Mann Z. F., Kelley M. W., Development of tonotopy in the auditory periphery. Hear. Res. 276, 2–15 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Davis R. L., Gradients of neurotrophins, ion channels, and tuning in the cochlea. Neuroscientist 9, 311–316 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Wright A., Dimensions of the cochlear stereocilia in man and the guinea pig. Hear. Res. 13, 89–98 (1984). [DOI] [PubMed] [Google Scholar]
- 4.Son E. J., et al. , Conserved role of Sonic Hedgehog in tonotopic organization of the avian basilar papilla and mammalian cochlea. Proc. Natl. Acad. Sci. U.S.A. 112, 3746–3751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon K. H., et al. , Dysregulation of sonic hedgehog signaling causes hearing loss in ciliopathy mouse models. Elife 9, e56551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilney L. G., Saunders J. C., Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J. Cell Biol. 96, 807–821 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim D. J., Functional structure of the organ of Corti: A review. Hear. Res. 22, 117–146 (1986). [DOI] [PubMed] [Google Scholar]
- 8.Son E. J., et al. , Developmental gene expression profiling along the tonotopic axis of the mouse cochlea. PLoS One 7, e40735 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehret G., Frankenreiter M., Quantitative analysis of cochlear structures in the house mouse in relation to mechanisms of acoustical information processing. J. Comp. Physiol. 122, 65–85 (1977). [Google Scholar]
- 10.Keiler S., Richter C. P., Cochlear dimensions obtained in hemicochleae of four different strains of mice: CBA/CaJ, 129/CD1, 129/SvEv and C57BL/6J. Hear. Res. 162, 91–104 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Morell M., et al. , Ultrastructure of the Odontocete organ of Corti: Scanning and transmission electron microscopy. J. Comp. Neurol. 523, 431–448 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Beisel K. W., et al. , Differential expression of KCNQ4 in inner hair cells and sensory neurons is the basis of progressive high-frequency hearing loss. J. Neurosci. 25, 9285–9293 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura H., et al. , Deafness gene expression patterns in the mouse cochlea found by microarray analysis. PLoS One 9, e92547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamura S., Adams J. C., Immunolocalization of peptide 19 and other calcium-binding proteins in the guinea pig cochlea. Anat. Embryol. (Berl) 194, 407–418 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Mann Z. F., et al. , A gradient of Bmp7 specifies the tonotopic axis in the developing inner ear. Nat. Commun. 5, 3839 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiede B. R., et al. , Retinoic acid signalling regulates the development of tonotopically patterned hair cells in the chicken cochlea. Nat. Commun. 5, 3840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bok J., et al. , Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 134, 1713–1722 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Muthu V., et al. , Genomic architecture of Shh-dependent cochlear morphogenesis. Development. 146, dev181339 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prajapati-DiNubila M., Benito-Gonzalez A., Golden E. J., Zhang S., Doetzlhofer A., A counter gradient of Activin A and follistatin instructs the timing of hair cell differentiation in the murine cochlea. Elife 8, e47613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tateya T., et al. , Hedgehog signaling regulates prosensory cell properties during the basal-to-apical wave of hair cell differentiation in the mammalian cochlea. Development 140, 3848–3857 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Madisen L., et al. , A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lallemand Y., Moreau J., Cloment C. S., Vives F. L., Robert B., Generation and characterization of a tamoxifen inducible Msx1(CreERT2) knock-in allele. Genesis 51, 110–119 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Feng X. Y., Zhao Y. M., Wang W. J., Ge L. H., Msx1 regulates proliferation and differentiation of mouse dental mesenchymal cells in culture. Eur. J. Oral. Sci. 121, 412–420 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., et al. , Phosphorylation of Msx1 promotes cell proliferation through the Fgf9/18-MAPK signaling pathway during embryonic limb development. Nucleic Acids Res. 48, 11452–11467 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y. S., Liu F., Segil N., A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 133, 2817–2826 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Bok J., Zenczak C., Hwang C. H., Wu D. K., Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc. Natl. Acad. Sci. U.S.A. 110, 13869–13874 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohyama T., Groves A. K., Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 38, 195–199 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Jorgez C. J., Klysik M., Jamin S. P., Behringer R. R., Matzuk M. M., Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol. Endocrinol. 18, 953–967 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Koo H., et al. , Position specific alternative splicing and gene expression profiles along the tonotopic axis of chick cochlea. Front. Mol. Biosci. 8, 726976 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matzuk M. M., et al. , Multiple defects and perinatal death in mice deficient in follistatin. Nature 374, 360–363 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Matzuk M. M., et al. , Functional analysis of activins during mammalian development. Nature 374, 354–356 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Ankamreddy H., et al. , Region-specific endodermal signals direct neural crest cells to form the three middle ear ossicles. Development 146, dev167965 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Han W., et al. , Distinct roles of stereociliary links in the nonlinear sound processing and noise resistance of cochlear outer hair cells. Proc. Natl. Acad. Sci. U.S.A. 117, 11109–11117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morsli H., Choo D., Ryan A., Johnson R., Wu D. K., Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 18, 3327–3335 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Data Availability Statement
All study data are included in the article and/or SI Appendix. All the raw data of our RNA-seq analyses have been deposited in the Gene Expression Omnibus data repository under accession code: GSE203228.