Significance
Identifying the culprits of PM2.5 constituents that are most responsible for elevated risks of neurodegeneration is of paramount importance. We perform a US nationwide cohort study of the associations between PM2.5 constituents and dementia and AD. Long-term exposure to PM2.5 mass and major constituents, particularly from traffic and fossil fuel combustion sources, is significantly associated with elevated dementia or AD incidence. All constituents had largely linear concentration–response relationships at low concentrations for both end points, implying no safe level of air pollution for brain health. Using two independent exposure datasets allows us to examine the robustness of findings and thus strengthen the credibility of the evidence for the associations. Our results will facilitate targeted source-specific pollution control strategies.
Keywords: air pollution, PM2.5 constituents, dementia, Alzheimer’s disease, epidemiology
Abstract
Growing evidence suggests that fine particulate matter (PM2.5) likely increases the risks of dementia, yet little is known about the relative contributions of different constituents. Here, we conducted a nationwide population-based cohort study (2000 to 2017) by integrating the Medicare Chronic Conditions Warehouse database and two independently sourced datasets of high-resolution PM2.5 major chemical composition, including black carbon (BC), organic matter (OM), nitrate (NO3−), sulfate (SO42−), ammonium (NH4+), and soil dust (DUST). To investigate the impact of long-term exposure to PM2.5 constituents on incident all-cause dementia and Alzheimer’s disease (AD), hazard ratios for dementia and AD were estimated using Cox proportional hazards models, and penalized splines were used to evaluate potential nonlinear concentration–response (C-R) relationships. Results using two exposure datasets consistently indicated higher rates of incident dementia and AD for an increased exposure to PM2.5 and its major constituents. An interquartile range increase in PM2.5 mass was associated with a 6 to 7% increase in dementia incidence and a 9% increase in AD incidence. For different PM2.5 constituents, associations remained significant for BC, OM, SO42−, and NH4+ for both end points (even after adjustments of other constituents), among which BC and SO42− showed the strongest associations. All constituents had largely linear C-R relationships in the low exposure range, but most tailed off at higher exposure concentrations. Our findings suggest that long-term exposure to PM2.5 is significantly associated with higher rates of incident dementia and AD and that SO42−, BC, and OM related to traffic and fossil fuel combustion might drive the observed associations.
Globally, dementia is the seventh leading cause of death in the United States and a major cause of disability and dependency among older people, posing an urgent and significant public health challenge (1). More than 6 million Americans presently live with Alzheimer’s disease (AD) and dementia, leading to a massive economic burden; this number is projected to triple to approximately 14 million by 2060 (2). As medical treatments for the most common types of dementia remain challenging, identifying and mitigating modifiable risk factors are of paramount importance.
Growing evidence indicates that exposure to air pollution, specifically fine particulate matter (PM2.5), plays a crucial role in the pathogenesis of AD and AD-related dementias (ADRD). A recent critical review by Delgado-Saborit et al. (2021) (3) summarized the epidemiological evidence of the effects of air pollution on ADRD, and a positive association between long-term exposure to PM2.5 mass and increased ADRD risks was consistently reported across almost all studies. Relevant publications on air pollution and ADRD have doubled since this review, with the majority of studies finding positive associations between PM2.5 mass and either all-cause dementia or AD (4–6).
To facilitate the targeting of pollution control efforts, the National Academy of Sciences and the World Health Organization have placed a high priority on determining which constituents of the PM2.5 mass may be most hazardous (7, 8). Yang et al. (9) reviewed the literature regarding PM2.5 constituents and both short-term and long-term health effects, focusing on all-cause mortality and cardiorespiratory morbidity, and found consistent associations with both black carbon (BC) and organic carbon (OC). However, the relative contributions of individual PM2.5 constituents to ADRD risks remain largely unknown. Only one study to date has focused on PM2.5 constituents and ADRD, which looked at PM2.5 constituents and all-cause dementia in the northeastern United States (10).
Elucidating the potential relationship between PM2.5 constituents and dementia has been challenging because of the sparsity of available, speciated chemical composition measurements, and the chronic nature of neurodegeneration. To cope with these challenges, long-term, high-resolution spatiotemporal PM2.5 constituents’ estimates are needed, which require modeling with measurement constraints from ground observations. In addition, more complete health records, such as physician visits, inpatient visits, and outpatient visits, are needed to better capture disease incidence (5).
Here, we present a nationwide open cohort (i.e., dynamic cohort, meaning that members can leave or be added over time) study of the long-term PM2.5 constituents’ exposure with incident dementia and AD among US older adults during 2000 to 2017. We used two high-resolution, multiple-species air pollution datasets and all Medicare claims across the contiguous United States to estimate the effect of PM2.5 constituents on dementia risk.
Results
Study Population Characteristics.
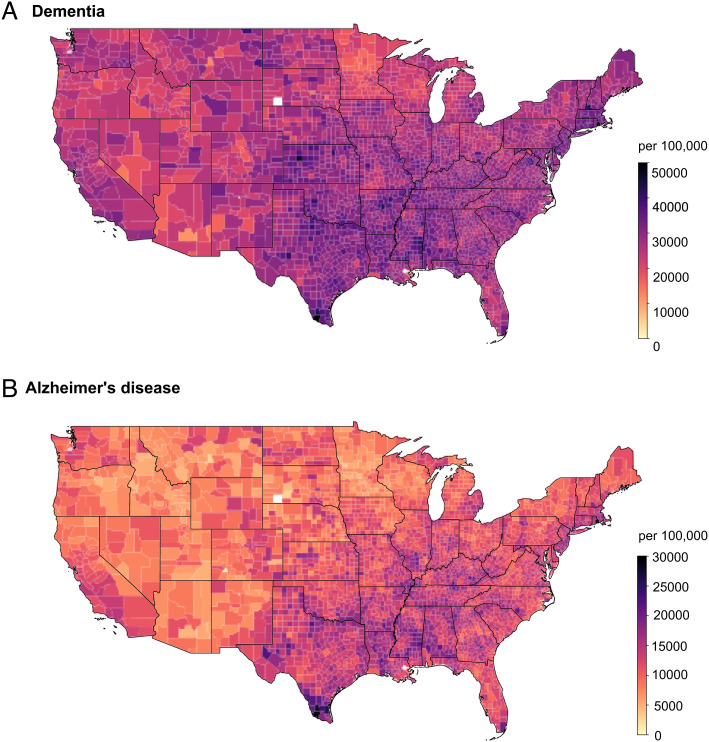
SI Appendix, Table S1 presents descriptive information on the dementia cohort and AD cohort between 2000 and 2017, with a 3-y clean period without events of interest. The dementia cohort included approximately 18.5 million individuals, and ~5.8 million individuals developed dementia. The AD cohort had approximately 19.2 million individuals, and ~2.8 million individuals developed AD. In both cohorts, most of the study population were White (~90%) and not eligible for Medicaid insurance (~89%), and about 60% were females. More detailed demographic characteristics on average and by PM2.5 mass quintiles are presented in SI Appendix, Tables S1 and S2. County-level occurrences of first dementia and AD events per 100,000 Medicare beneficiaries across the contiguous United States (2000 to 2017) are presented in Fig. 1.
Fig. 1.
Nationwide occurrences of first dementia events (A) and first Alzheimer’s disease events (B) per 100,000 Medicare beneficiaries across the contiguous United States (2000 to 2017), with a 3-y clean period considered.
Air Pollution Levels.
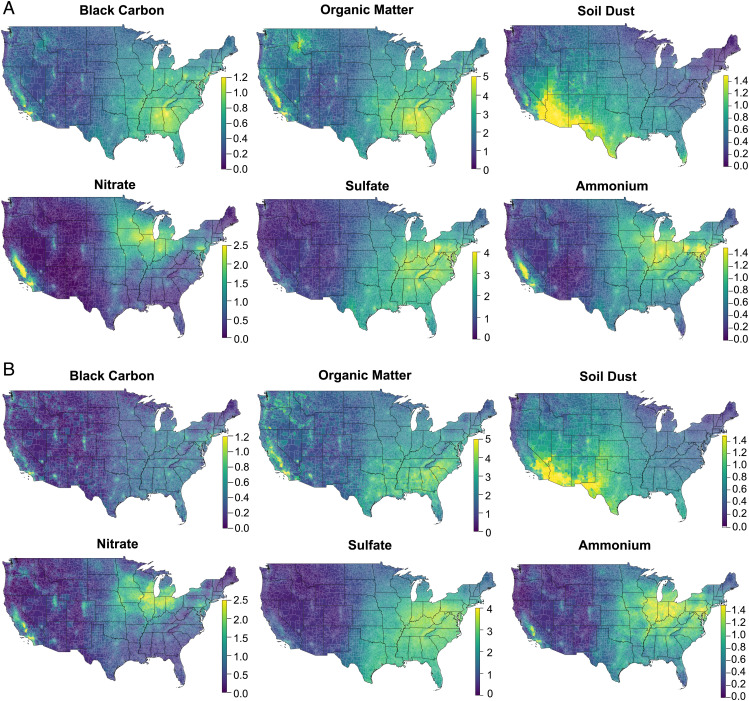
We accessed two high-resolution, speciated air pollution datasets of the contiguous United States from 2000 to 2017 from two independent sources, including BC, organic matter (OM), nitrate (NO3−), sulfate (SO42−), ammonium (NH4+), and soil dust (DUST) (see Methods). Using exposure I, the dementia cohort had an average PM2.5 mass concentration of 9.58 µg/m3, with an interquartile range (IQR) of 3.68 µg/m3 (11). The average concentrations of PM2.5 major constituents and the corresponding IQRs are listed in SI Appendix, Table S1. The exposure II data showed similar means and IQRs for all PM2.5 constituents of interest except for BC, which showed lower levels than exposure I, albeit with overlapping distributions (12). The AD cohort shared a similar exposure with the dementia cohort (SI Appendix, Table S1). Two speciated air pollution datasets tend to have similar spatial distributions of major PM2.5 constituents across the United States (Fig. 2). SI Appendix, Fig. S1 lists the correlation matrix among PM2.5 mass and its six major constituents. PM2.5 mass was highly correlated with NH4+, SO42−, BC, and OM in both exposure sets (r values range from 0.60 to 0.83). BC and OM (r = 0.74 and 0.78 for exposures I and II, respectively) as well as SO42− and NH4+ (r = 0.84 and 0.86) also showed strong correlations in both exposure sets. The temporal trends of the annual population-weighted mean concentration of six PM2.5 constituents are presented in SI Appendix, Fig. S2 of supplementary materials. Overall, from 2000 to 2017, national annual average concentrations showed a drastic downward trend for SO42−, NH4+, and NO3− in both exposure datasets, with more variability observed in SO42− concentrations from year to year, while other constituents showed a slighter temporal variation in different exposure datasets.
Fig. 2.
Average concentrations of PM2.5 major constituents (µg/m3), respectively derived from exposure I [van Donkelaar et al. (11)] (A) and exposure II [Amini et al. (12)] (B) across the contiguous United States from 2000 to 2017.
Health Effect Estimates.
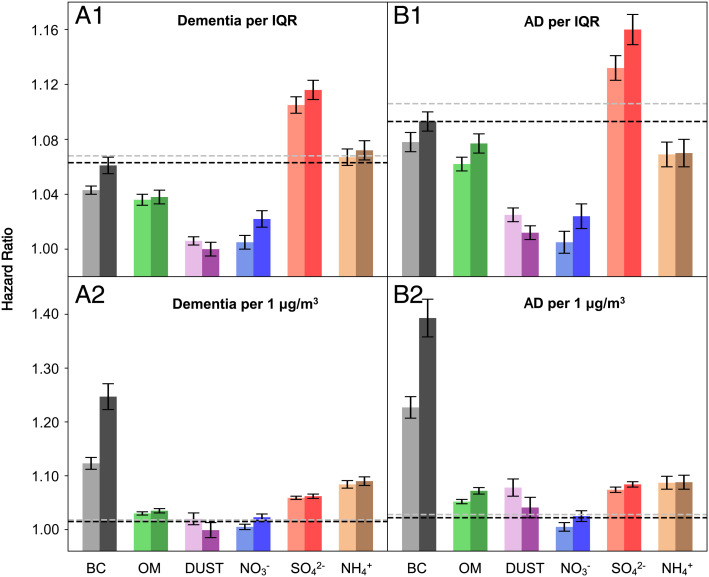
Fig. 3 provides the main results from the single-constituent Cox proportional hazards models stratified by individual characteristics, adjusting for neighborhood-level socioeconomic status (SES), behavioral risk factors, health-care capacity variables, and residual temporal and spatial trends (see Methods). Higher PM2.5 mass and major constituents of interest were all consistently observed to be associated with increased dementia and AD incidence using exposure I. A null association was observed between DUST and dementia using exposure II. Per IQR increase in each pollutant in exposure I, hazard ratios (HRs) of dementia were 1.068 (95% CI: 1.063, 1.072) for PM2.5 mass, 1.043 (1.040, 1.047) for BC, 1.036 (1.032, 1.039) for OM, 1.006 (1.003, 1.010) for DUST, 1.105 (1.099, 1.011) for SO42−, 1.005 (1.000, 1.010) for NO3−, and 1.067 (1.061, 1.073) for NH4+ (SI Appendix, Table S4). Corresponding HRs of AD were 1.106 (1.099, 1.114) for PM2.5 mass, 1.078 (1.071, 1.084) for BC, 1.062 (1.057, 1.068) for OM, 1.025 (1.020, 1.030) for DUST, 1.132 (1.123, 1.141) for SO42−, 1.005 (0.997, 1.103) for NO3−, and 1.069 (1.060, 1.078) for NH4+ (SI Appendix, Table S5), respectively. Exposure II in general yielded slightly larger effect estimates than exposure I for both end points, and the association between NO3− and AD became significant using exposure II. On a per 1 µg/m3 basis, BC had the highest associations with both end points across exposure datasets, followed by NH4+ and SO42−. Per 1 µg/m3 increase in BC, HRs of dementia were 1.123 (1.112, 1.135) using exposure I and 1.247 (1.223, 1.272) using exposure II (SI Appendix, Table S4), and HRs of AD were 1.227 (1.207, 1.247) using exposure I and 1.393 (1.358, 1.429) using exposure II (SI Appendix, Table S5).
Fig. 3.
Hazard ratios of (A) dementia or (B) Alzheimer’s disease (AD) associated with per IQR or per 1 µg/m3 increase in annual mean concentration of each PM2.5 major constituent, respectively, including BC, OM, soil dust (DUST), NO3−, SO42−, and NH4+. The dotted lines stand for the corresponding results for PM2.5 mass. The estimated hazard ratios were obtained from single-constituent models, and error bars stand for the 95% CIs. The light and dark colors are used to distinguish air pollutants derived from two exposure models, with the light one indicating exposure I data (11) and the dark one indicating exposure II data (12). The corresponding hazard ratio values could be found in SI Appendix, Tables S3 and S4 (Model 1).
Concentration–Response (C-R) Relationships.
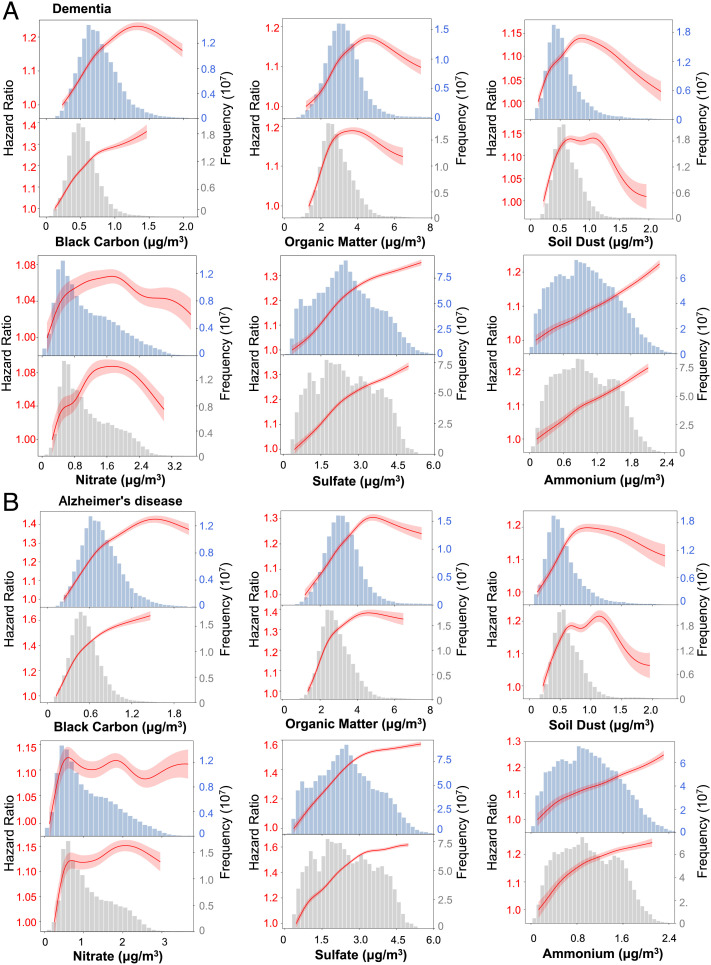
Fig. 4 presents the C-R relationships between each PM2.5 constituent of interest and outcomes of incident dementia and AD from single-constituent models. Linear relationships were observed with BC (exposure II), SO42−, and NH4+, with no sign of threshold for both outcomes. The curves for BC with exposure I are essentially near linear for both end points until high and rarely occurring concentrations. Near-linear relationships were observed with OM (<5 µg/m3) and DUST (<1 µg/m3) in middle concentrations and then leveled out for higher concentrations for both end points. The C-R curves showed positive linear associations between both dementia and AD outcomes and NO3− at low concentrations; however, the relationships became unstable above about 1 µg/m3.
Fig. 4.
The concentration–response curves for each PM2.5 constituent and dementia (A) and Alzheimer’s disease (B). The concentration–response curves, derived from the single-constituent models, are shown for the concentration ranges between 0.5th and 99.5th percentiles of the pollutants, i.e., with 1% poorly constrained extreme values excluded. For each constituent, the top panel used exposure I data (11) and the bottom panel used exposure II data (12).
Effect Modifications.
The subgroup-specific HRs in single-constituent models are presented in SI Appendix, Fig. S3. We found that BC and SO42− were always positively associated with both dementia and AD across effect modifiers. The results obtained using estimates from exposure I and exposure II mostly showed similar patterns across the effect modifiers. For dementia, effect estimates for NO3−, SO42−, and NH4+ were higher among relatively older populations and males. Black individuals had a higher risk of dementia associated with NO3− and NH4+, while races other than Blacks and Whites had a stronger association of dementia with DUST and SO42−. Additionally, for DUST, SO42−, and NH4+, people eligible for Medicaid were at a significantly greater risk of dementia than those not eligible for Medicaid. Similar patterns were also observed in the association between AD and these constituents.
Sensitivity Analysis.
Our results were robust in a series of sensitivity analyses. First, for both outcomes, multiconstituent models yielded similar results for most PM2.5 constituents, except that NO3− was observed to have a negative impact on dementia or AD after adjusting for other PM2.5 constituents using both exposure datasets (SI Appendix, Tables S4 and S5). The adjustment for the residual PM2.5 mass (i.e., subtracting the constituent of interest from total PM2.5 mass) yielded similar results, compared to the multiconstituent models (SI Appendix, Tables S4 and S5). Second, we considered the possible effect of outcome misclassification by 1) fitting a linear regression model based on incidence rates and 2) estimating the true number of cases within strata based on prior estimates of Medicare sensitivity and specificity, and the results of both methods remained largely stable or slightly larger compared to the main analysis, indicating that potential outcome misclassification may have biased the results toward the null (SI Appendix, Tables S6 and S7). Third, a stricter “clean” period of 5 y yielded roughly consistent results with the main analyses (SI Appendix, Table S8). In addition, the results of the nonmover cohort suggest little bias from residential mobility (SI Appendix, Table S9). Furthermore, the effect estimates remained unchanged regardless of the form of adjustment for “year” in the main models (SI Appendix, Table S10). Last, after accounting for differing exposure measurement error, we found that the relative effect estimates across the major PM2.5 constituents were consistent with our main analyses, and the conclusions were not affected, although the magnitudes were attenuated (SI Appendix, Table S11).
Discussion
Using two sets of high-resolution air pollution datasets, we consistently observed that long-term exposure to PM2.5 mass and its major constituents were associated with higher risks of dementia and AD among older adults in a large US cohort. More specifically, higher exposure to four (i.e., SO42−, NH4+, BC, and OM) of the six constituents explored were consistently associated with higher dementia and AD risks. SO42− and BC were associated with the highest dementia or AD risks, while NO3− and DUST had relatively lower impacts. Overall, we observed stronger associations for AD than dementia. The relatively greater influence of particulate matter on AD may be because dementia encompasses a wide variety of disorders with different etiologies, some of which may be unrelated to fine particle pollution. The results from the multiconstituent models were only modestly changed from those in the single constituent models except for NO3− suggesting that these associations were not confounded by other constituents.
The effect estimates per IQR increase on dementia and AD are the largest for SO42− and lowest for NO3−. Our single-constituent and multiconstituent models showed inconsistent results for NO3−, and we interpret this as evidence that there is no sufficient statistical evidence for the harmful effect of NO3−, and the protective effect seen in the multiconstituent models may be due to collinearity. Another plausible mechanism to explain the protective effect of NO3− is that the presence of NO3− is associated with lower aerosol acidity, which may reduce the solubility and bioavailability of transition metals (13). The per IQR effect estimates for NH4+ are in between, as expected, as NH4+ is chemically associated with SO42− and NO3−. The principal sources of SO2 (i.e., SO42− precursor) are fossil fuel combustion (14). SO42− in the form of (NH4)2SO4 or NH4HSO4 does not have strong acute neurotoxicity based on toxicological assessments (15). However, SO42− may serve as a proxy for other combustion-emitted pollutants subsequently processed in the atmosphere, such as oxygenated organic species, which are known to cause oxidative stress (16). Excessive oxidative stress has been linked with the production of α-synuclein, amyloid-β, and hyperphosphorylated tau protein and may play a role in ADRD etiology (17).
Another plausible explanation is that the high aerosol acidity associated with SO42− may enhance the solubility and bioavailability of trace metals (18) and further generate reactive oxygen species in vivo by redox cycling and lead to oxidative stress (19). Studies on AD pathology found that the olfactory bulbs participate in the neuroinflammatory process related to exposure to metals in fine particles (20). The role of the olfactory nerve as a direct pathway of exposure to PM2.5 is supported by rodent studies that have demonstrated exacerbation of AD-related pathology following exposure to well-characterized ambient air pollution (21). As rodents are obligate nose breathers, this exposure scenario isolates the olfactory system as a direct route through which PM2.5 and its constituents could access the brain and contribute to AD pathology. In studies using the wild type and rats that express human AD risk genes, exposure to traffic-related air pollution resulted in an increase in PM2.5 particles in the hippocampus of wild-type and transgenic rats, with a concomitant increase in AD-related pathology in both genotypes (22). Although focused on PM2.5, these findings provide clear evidence for the olfactory nerve to serve as a conduit for PM2.5 and its associated components to contribute to AD pathogenesis. Moreover, the synergistic effect of (NH4)2SO4 and the presence of ultrafine particles may accelerate the aggregation of peptides that impact progression of neurodegenerative diseases (23). Collectively, these studies provide support for our findings about the relatively higher magnitude of SO42− compared to NO3−.
Among all examined components of PM2.5, BC has the largest effect estimates on dementia and AD per 1 µg/m3 increase. We note that exposure II has higher effect estimates per 1 µg/m3 than exposure I, mainly because of the discrepancy in estimated BC concentrations between two datasets (SI Appendix, Table S1). This discrepancy in BC estimates can be explained by the fact that these two exposure sets relied on monitor data based on different techniques (i.e., thermal method vs. optical methods). (24, 25) BC are combustion-related particles, mainly derived from tailpipe traffic emissions and biomass burning (26). Our results are consistent with the literature that suggests an association between traffic-related air pollution (measured as BC levels) and poor cognition levels in older men (27). Possible explanations of the neurotoxicity of BC include increased levels of inflammatory mediators, markers of oxidative damage to DNA, and β-amyloid deposition as well as evidence of blood–brain barrier disruption (28). BC particles and other ultrafine particles can also be small enough to pass through the olfactory nerve, bypass the blood–brain barrier, and translocate to the brain (29), leading to oxidative stress and neuroinflammation (30). A recent study also highlights the translocation of fine BC particles from the lung to the brain through the inhalation/circulation/brain route (31). In addition, BC could act as a carrier for highly toxic OM species such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), (32) and BC can be coemitted with nontailpipe traffic-related pollutants, such as road dust and metals and organics from brake and tire abrasion (33). These species can have adverse neurotoxic effects (34). On a per 1-µg/m3 basis, NH4+ and SO42− also had high effect estimates on dementia and AD. However, as SO42− is usually highly correlated with NH4+, the HR per 1 µg/m3 of (NH4)2SO4 would be lower than the values reported for each individual constituent.
OM is another important PM2.5 constituent that increases the risks of dementia and AD in our study. OM makes up a substantial fraction of PM2.5 mass, consisting of primary OM directly emitted from combustion emissions and other sources and secondary OM formed by atmospheric oxidation of gas-phase species (35). Due to the complexity of OM in terms of sources and constitutions, the toxicity of some OM compounds has motivated greater scrutiny than other PM2.5 compounds in health studies (36). OM includes highly toxic species such as PAHs (including products of atmospheric processing, such as oxy- and nitro-PAHs) and PCBs and contributes a large fraction of aerosol oxidative potential (37, 38). Ultrafine particles from traffic sources also largely consist of carbonaceous species, including OM and BC (39). However, research on the neurological effects of atmospheric OM pollutants is scarce, and more studies are warranted.
The linear relationships of BC (exposure II), SO42−, and NH4+ with both outcomes were observed with no sign of threshold, and these results were consistent with the linear C-R relationships in the previously published “Northeastern” study (40). Interestingly, “bell-shaped” C-R curves were observed for the relationship between OM, DUST, and NO3− exposure and dementia/AD, suggesting that the C-R curves are relatively steep at very low to moderate levels of exposure and leveled down at high levels of exposure. Numerous hypotheses have sought to explain the mechanisms leading to a nonlinear C-R relationship; these include errors in calculating exposure levels of pollution at higher concentrations, the existence of competing risks, and preferential avoidance based on symptoms (41). Moreover, the difference in population distributions across different constituents may also lead to different C-R curves.
Previous epidemiology studies have also demonstrated the associations between short-term and long-term exposure to PM2.5 constituents for different morbidity and mortality. One recently published literature review has systematically reviewed 35 epidemiological studies (25 time series and 10 cohorts), and the authors observed the most robust and consistent associations between both BC and OC for all-cause mortality and cardiovascular mortality and morbidity. They also reported that NO3− and SO42− were relevant for adverse cardiovascular and respiratory health outcomes. Since that review, another large city-level daily time series study of all-cause mortality has recently been published (42), covering 210 cities in 16 countries. They found that NH4+ had the largest effect estimates, while SO42− also increased daily mortality, but less markedly.
Strengths and Limitations.
Our study has several strengths. This is a nationwide, population-based, open cohort study characterizing the health effects of ambient PM2.5 constituent exposure on dementia and AD incidence. The large sample size gives us ample statistical power to identify the effects of long-term PM2.5 constituent exposure on neurodegeneration. This study provides insights into composition-specific health effects of PM2.5, while most studies focus on total PM2.5 mass. Second, two independently sourced state-of-the-art exposure datasets allow us to examine the robustness of results against measurement error, thus strengthening the credibility of the findings. Third, using comprehensive Medicare claims (including physician visits) can better reflect incidence, as they can capture more cases, particularly earlier diagnosed cases that are often missed in hospitalization records.
Despite these strengths, we acknowledge that our study has several limitations. First, exposure measurement error has been inevitably introduced when using predicted ambient concentrations, albeit two datasets showed consistent effect estimates. Additionally, coarse exposure concentrations at the ZIP code level may not allow some constituents with high spatial variability to be well characterized. However, due to the limitations in Medicare data resolution, health outcome information at the ZIP code level is the most granular information that can be obtained. Thus, analysis using higher resolution health information matched with fine PM2.5 constituents’ exposure estimates is needed in future studies. Second, outcome misclassification is likely to occur when relying on administrative records. AD cases account for 48% of dementia diagnoses in our database, suggesting the undiagnosed nature of AD using administrative records (43). Moreover, dementia and AD have a long and insidious onset, and the exact timing of the disease onset is unknown. Another limitation is that we are able to adjust for only potential confounders that can be estimated based on neighborhood-level characteristics, and future work should incorporate individual-level characteristics associated with ADRD (44, 45). In addition, it is unclear whether the high effects of BC and SO42− are due to their intrinsic neurotoxicity or other culprit pollutants that are coemitted and correlated. Last, although PM2.5 constituents provide useful information about sources, it is important to assess more source-specific effects of PM2.5, as they can be readily translatable into effective abatement strategies.
In conclusion, long-term exposure to fine particle pollution is associated with higher risks of dementia and AD, and individual PM2.5 constituents are associated with differences in risk. BC and SO42− have the strongest associations. Our findings imply that policies that target the reduction in ambient PM2.5 concentrations, particularly primary and secondary particulate pollutants from sources such as traffic and sulfur-containing fossil fuel combustion, have a significant public health impact.
Materials and Methods
Study Population.
We analyzed two nationwide, privacy-protected and publicly available databases from the Centers for Medicare and Medicaid Services (CMS), including the Medicare denominator file and the Medicare Chronic Conditions Warehouse (CCW), based on which we constructed separate cohorts for all-cause dementia and AD subtype in 2000 to 2017. The denominator file contains enrollment records for each Medicare beneficiary, including demographics, Medicaid insurance status (a proxy for SES), the date of death (if any), and ZIP code of residence, which were updated annually. The CCW claims data include predefined indicators for chronic conditions among the fee-for-service (FFS) Medicare beneficiaries and provides the date of the first occurrence with a diagnosis code for a specific condition.
Our study population comprised all Medicare beneficiaries enrolled at age 65 y or above living in the contiguous United States from 2000 to 2017, with continuous enrollment 1) in the Medicare FFS program and 2) in both Medicare Part A (hospital insurance) and Part B (medical insurance) over the follow-up period. These inclusion criteria were used because the CCW relies on FFS, Part A, and Part B to identify cases. In addition, we further required a clean period of 3 y of enrollment without dementia or AD to better approximate “incidence,” i.e., removing potentially prevalent cases in their first 3 y of follow-up. Hence, study subjects entered the cohort on January 1st of the year following the clean period and were followed until the first diagnosis of dementia or AD across all available Medicare claims, death, end of enrollment in either of the mentioned Medicare programs, or end of follow-up. Our research was approved by Emory’s IRB and the CMS under the data use agreement.
Outcome Classification.
All-cause dementia and AD subtype were the two primary outcomes of this study. AD is a specific single disease that falls under the dementia umbrella, and AD is the major type of dementia that represents ~60 to 80% of dementia cases. The diagnoses of dementia and AD were identified and recorded as two distinct indicators using an algorithm created by CCW that incorporated information across the available Medicare claims, including inpatient and outpatient claims, Carrier files (primarily doctor visits), skilled nursing facility, and home health-care claims. This algorithm has been shown to be reasonably accurate in classifying diseases based on validation studies such as those by Taylor et al. (46, 47) who found high sensitivity for dementia (0.85) but lower for AD (0.65). CCW provides the date of the first occurrence with a dementia or AD diagnosis code. In the dementia cohort, the outcome was defined as the first occurrence of a dementia diagnosis code. In the AD cohort, the outcome was defined as either (1) the first occurrence of an AD diagnosis with no prior dementia diagnosis or (2) the first occurrence of a dementia diagnosis when there was a subsequent AD diagnosis (given that the original dementia diagnosis was probably AD). Given that previous studies (5) have found a greater effect of long-term PM2.5 exposure on AD compared to dementia, it is likely that some causes of dementia may be less associated with air pollution, while AD with distinct disease assessment had a stronger association. The specific effects of different PM2.5 constituents on dementia and AD were evaluated separately in this study to further assess whether AD progression is more related to PM2.5 pollution compared to all-cause dementia.
Exposure Assessment.
We accessed two high-resolution, speciated air pollution datasets of the contiguous United States from 2000 to 2017 from two independent sources. The first set of annual mean concentrations including total PM2.5 mass and its six major constituents (exposure I) was estimated at 1-km resolution using a previously validated PM2.5 composition prediction model (11). Briefly, mean satellite-derived PM2.5 total mass concentrations were first estimated at 1-km resolution by combining satellite retrievals of aerosol optical depth, chemical transport modeling (CTM), and ground-based observations. The six major constituents of PM2.5 were calculated by decomposing the PM2.5 total mass into individual chemical constituents using CTM output and further calibrated using ground-based observations. Our annual predictions of each constituent achieved good long-term spatial agreements compared with ground measurements, with cross-validated R2 of 0.59 for BC, 0.86 for NO3−, 0.96 for SO42−, 0.90 for NH4+, 0.57 for OM, and 0.60 for DUST.
The second set of annual mean predictions for PM2.5 constituents (exposure II) was estimated using superlearning and ensemble weighted averaging models, with a 50-m spatial resolution in urban areas and a 1-km resolution in nonurban areas. Details about exposure II dataset can be found elsewhere (12). Briefly, PM2.5 constituent training data were collected from 987 monitoring sites, and hundreds of predictors were used for six superlearning models and an ensemble weighted-averaging model. This approach achieved excellent model performance, with cross-validated R2 for individual components used in this study ranging from 0.856 (OM) to 0.957 (SO42−). In addition to PM2.5 constituents, we have previously used a similar ensemble model that integrated multiple machine learners and hundreds of predictors to estimate PM2.5 mass concentrations across the contiguous United States, with cross-validated R2 of 0.89 for annual predictions (48). This PM2.5 mass dataset has been widely used in previous epidemiological studies (5, 49, 50).
We finally averaged these gridded predictions for each constituent at the ZIP code level (i.e., the finest spatial resolution of Medicare data) for each year. Two sets of ZIP code-level annual means were assigned to each Medicare beneficiary, and any residential mobility changes by ZIP code were considered annually.
Covariates.
Individual-level demographics (age, sex, and race) and Medicaid insurance status were obtained from the Medicare denominator file. We also included in the model neighborhood-level covariates, including ZIP code-level SES variables (population density, median household income, % Black population, % population living in rental house or apartment, % population aged 65 or above living below the poverty line, and % population with less than a high school education), county-level behavioral risk factors (smoking prevalence and mean body mass index), county-level health care capacity variables (number of hospitals and active medical doctors per 1,000 people), and a geographical region indicator. All covariates were included as linear terms in the models unless otherwise noted. Details and data sources of covariates are described in Shi et al. (2021) (49).
Statistical Analysis.
We fit stratified Cox proportional hazards models with a generalized estimating equation (GEE) to estimate the associations between time-varying annual mean concentrations of PM2.5 constituents on dementia or AD among older adults, with years of follow-up as the time scale. Given the potential multicollinearity among PM2.5 constituents, we fit single-constituent models in the main analyses and estimated HRs per IQR increase and per 1 µg/m3 increase in the annual mean concentrations of each PM2.5 constituent. GEE allowed us to adjust for residual autocorrelation within the ZIP code and thus obtain more statistically robust CIs for the effect estimates. All models were stratified by age at entry (1-y age categories), race (White, Black, and other), sex, and Medicaid insurance status and adjusted for the neighborhood-level covariates (see Covariates). To adjust for potential residual temporal and spatial trends, a linear term for calendar years and an indicator for geographical regions were included.
To account for possible nonlinearity in the C-R relationships between each PM2.5 constituent and dementia or AD, we fit penalized splines for each constituent in single-constituent models. The models adjusted for the same covariates as our main model. We further assessed potential effect modification by introducing an interaction term between the constituent and a modifier of individual-level characteristics, including age group (≤75 y vs. >75 y), race, sex, and Medicaid eligibility.
We conducted several sensitivity analyses to assess the robustness of our main results. First, we fit multiconstituent models by including multiple PM2.5 constituents in one model simultaneously. Considering the high correlations between BC and OM as well as between SO42− and NH4+ (SI Appendix, Fig. S1), we fit two separate multiconstituent models, 1) by including BC, DUST, SO42−, and NO3− simultaneously and 2) by including OM, DUST, SO42−, and NO3− simultaneously. Second, in single-constituent models, we additionally adjusted for the residual PM2.5 mass (i.e., subtracting the constituent of interest from total PM2.5 mass) other than the constituent of interest. Third, we assessed the impact of possible outcome misclassification in two ways: 1) fitting linear regression models for incidence rates of dementia or AD with GEE, which yielded additive effect estimates that were less sensitive to bias, because random misclassification of the outcome would be absorbed into the residual errors of the linear model for the true rates of events (51) and 2) considering the possible effect of outcome misclassification following methods similar to those described by Fox et al. (52) and adjusting the observed outcomes for each stratum based on the estimates of Medicare sensitivity and specificity from Taylor et al. (47) to estimate the expected true number of cases. In addition, we applied a clean period of 5 y by excluding anyone diagnosed with dementia or AD in their first 5 y of follow-up. Compared with the main analysis using a 3-y clean period, this would increase the possibility to capture the first diagnosis of dementia or AD, at a cost-reducing sample size. Moreover, we conducted a nonmover cohort analysis for subjects who did not move during the follow-up period to account for potential measurement error related to a change in the residential address. To further explore the impact of the temporal variation of exposure, we tested two other sets of adjustments for time trend, including 1) adding year as a spline term in the model with 4 degrees of freedom and 2) adding year as a categorical variable in the model. Finally, to investigate the impact of differing exposure measurement error across constituents on dementia and AD, we first extracted the regression mean square error for each constituent from the two exposure assessment models and then added an error term (following a log-normal distribution) to the original data and generated 20 perturbed datasets. Then, each dataset was used to fit the single-constituent models, and the results were summarized (53). To improve the computation efficiency, we bootstrapped 100 times for each perturbed dataset.
R software, version 4.0.2, was used for all analyses. Statistical significance was determined by two-sided P < 0.05.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We want to specially thank the Centers for Medicare & Medicaid Services for giving us access to the Medicare claims to conduct this study. We also gratefully acknowledge Caroline Owens for editorial support. This study was supported by NIH (R01 AG074357, R21 ES032606), the HERCULES Center (P30 ES019776), and the Emory Goizueta Alzheimer’s Disease Research Center (P50 AG025688). J.S. and H.A. were supported by U.S. EPA (RD-83587201) and NIH (P30 ES000002, R01 ES032418-01). H.A. was supported by Novo Nordisk Foundation (NNF17OC0027812). R.V.M. was supported by NASA HAQAST (Grant 80NSSC21K0508).
Author contributions
L.S. and P.L. designed research; L.S., Y.W., H.Z., and P.L. performed research; L.S., Q.Z., Y.W., H.H., H.Z., J.S., T.M., H.L., H.H.C., J.Z.L., and P.L. analyzed data; and L.S., Q.Z., Y.W., H.H., H.Z., J.S., H.A., A.v.D., R.V.M., K.S., J.A.S., W.M.C., T.M., H.H.C., J.Z.L., T.W., X.M., A.G.R., R.J.W., and P.L. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. G.T. is a guest editor invited by the Editorial Board.
Contributor Information
Liuhua Shi, Email: liuhua.shi@emory.edu.
Pengfei Liu, Email: pengfei.liu@eas.gatech.edu.
Data, Materials, and Software Availability
The Medicare dataset was stored and analyzed in the Emory Rollins School secure cluster environment, with Health Insurance Portability and Accountability Act compliance. The rules governing the Medicare dataset prohibit any sharing of the health datasets being used for our epidemiologic research. Restricted by our Data Use Agreement with the US Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available from us. Academic and nonprofit researchers who are interested in using Medicare data should contact the US Centers for Medicare & Medicaid Services directly to obtain their own datasets upon completion of a Data Use Agreement.
Supporting Information
References
- 1.World Health Organization, “Global status report on the public health response to dementia” (World Health Organization, Geneva, 2021). [Google Scholar]
- 2.A. Association, 2022 Alzheimer ‘s disease facts and figures. Alzheimer’s Dement. 15, 321–387 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Delgado-Saborit J. M., et al. , A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci. Total Environ. 757, 143734 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Sakhvidi M. J. Z., et al. , Outdoor air pollution exposure and cognitive performance: Findings from the enrolment phase of the constances cohort. Lancet Planet. Health 6, e219–e229 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Shi L., et al. , A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat. Commun. 12, 1–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., et al. , Association of improved air quality with lower dementia risk in older women. Proc. Natl. Acad. Sci. U.S.A. 119, e2107833119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Research Council, Research Priorities for Airborne Particulate Matter: IV. Continuing Research Progress (The National Academies Press, Washington, DC, 2004). [Google Scholar]
- 8.World Health Organization, “Health relevance of particulate matter from various sources: Report on a WHO workshop, Bonn, Germany 26–27 March 2007” (WHO Regional Office for Europe, Copenhagen, 2007). [Google Scholar]
- 9.Yang Y., et al. , Short-term and long-term exposures to fine particulate matter constituents and health: A systematic review and meta-analysis. Environ. Pollut. 247, 874–882 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Li J., et al. , Long-term effects of PM2. 5 components on blood pressure and hypertension in Chinese children and adolescents. Environ. Int. 161, 107134 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Van Donkelaar A., Martin R. V., Li C., Burnett R. T., Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ. Sci. Technol. 53, 2595–2611 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Amini H., et al. , Hyperlocal super-learned PM2. 5 components across the contiguous US. [Preprint] (2022). 10.21203/rs.3.rs-1745433/v1. accessed 16 January 2022 [DOI]
- 13.Fang T., et al. , Highly acidic ambient particles, soluble metals, and oxidative potential: A link between sulfate and aerosol toxicity. Environ. Sci. Technol. 51, 2611–2620 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Mazzei F., et al. , Characterization of particulate matter sources in an urban environment. Sci. Total Environ. 401, 81–89 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi J., et al. , The effect of ammonium sulfate injection on peripheral nerve. J. Reconstr. Microsurg. 13, 389–396 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Bates J. T., et al. , Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 53, 4003–4019 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Tönnies E., Trushina E., Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 57, 1105–1121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber R. J., Guo H., Russell A. G., Nenes A., High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nat. Geosci. 9, 282–285 (2016). [Google Scholar]
- 19.Huang L., Liu Y., Jin W., Ji X., Dong Z., Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKCγ–ERK signaling pathway in the developing brain. Brain Res. 1476, 164–171 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Calderón-Garcidueñas L., et al. , Alzheimer’s disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults≤ 40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ. Res. 166, 348–362 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Bein K. J., Wallis C. D., Silverman J. L., Lein P. J., Wexler A. S., Emulating near-roadway exposure to traffic-related air pollution via real-time emissions from a major freeway tunnel system. Environ. Sci. Technol., 10.1021/acs.est.1c07047 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patten K. T., et al. , The effects of chronic exposure to ambient traffic-related air pollution on Alzheimer’s disease phenotypes in wildtype and genetically predisposed male and female rats. Environ. Health Perspect. 129, 057005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaumbekova S., Torkmahalleh M. A., Shah D., Impact of ultrafine particles and secondary inorganic ions on early onset and progression of amyloid aggregation: Insights from molecular simulations. Environ. Pollut. 284, 117147 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Bauer J. J., Yu X.-Y., Cary R., Laulainen N., Berkowitz C., Characterization of the sunset semi-continuous carbon aerosol analyzer. J. Air Waste Manag. Assoc. 59, 826–833 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Hansen A., Rosen H., Novakov T., The aethalometer—an instrument for the real-time measurement of optical absorption by aerosol particles. Sci. Total Environ. 36, 191–196 (1984). [Google Scholar]
- 26.Penner J. E., Hegg D., Leaitch R., Unravelling the role of aerosols in climate change. Environ. Sci. Technol. 35, 332A–340A (2001). [DOI] [PubMed] [Google Scholar]
- 27.Power M. C., et al. , Traffic-related air pollution and cognitive function in a cohort of older men. Environ. Health Perspect. 119, 682–687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderón-Garcidueñas L., et al. , Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol. Pathol. 32, 650–658 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Hopkins L. E., et al. , Repeated iron–soot exposure and nose-to-brain transport of inhaled ultrafine particles. Toxicol. Pathol. 46, 75–84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elder A., et al. , Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 114, 1172–1178 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi Y., et al. , Passage of exogeneous fine particles from the lung into the brain in humans and animals. Proc. Natl. Acad. Sci. U.S.A. 119, e2117083119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelissen G., et al. , Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 39, 6881–6895 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Fussell J. C., et al. , A review of road traffic-derived non-exhaust particles: Emissions, physicochemical characteristics, health risks, and mitigation measures. Environ. Sci. Technol. 56, 6813–6835 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferri C. P., et al. , Global prevalence of dementia: A delphi consensus study. Lancet 366, 2112–2117 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q., et al. , Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 34, L13801 (2007). [Google Scholar]
- 36.Mauderly J. L., Chow J. C., Health effects of organic aerosols. Inhalation Toxicol. 20, 257–288 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Samara C., On the redox activity of urban aerosol particles: Implications for size distribution and relationships with organic aerosol components. Atmosphere 8, 205 (2017). [Google Scholar]
- 38.Santiago-De La Rosa N., et al. , Emission factors of polycyclic aromatic hydrocarbons and oxidative potential of fine particles emitted from crop residues burning. Polycyclic Aromat Compd. 42, 5123–5142 (2022). [Google Scholar]
- 39.Sandradewi J., et al. , Using aerosol light absorption measurements for the quantitative determination of wood burning and traffic emission contributions to particulate matter. Environ. Sci. Technol. 42, 3316–3323 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Li J., et al. , Long-term effects of PM2. 5 components on incident dementia in the Northeastern United States. Innovation 3, 100208 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pope C. A. III, et al. , Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: Shape of the exposure-response relationship. Circulation 120, 941–948 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Masselot P., et al. , Differential mortality risks associated with PM2. 5 components: A multi-country, multi-city study. Epidemiology 33, 167–175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alzheimer’s Association, 2008 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 4, 110–133 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Moore A. A., Whiteman E. J., Ward K. T., Risks of combined alcohol/medication use in older adults. Am. J. Geriatr. Pharmacother. 5, 64–74 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reid M. C., Boutros N. N., O’Connor P. G., Cadariu A., Concato J., The health-related effects of alcohol use in older persons: A systematic review. Subst. Abus. 23, 149–164 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Taylor D. H. Jr., Fillenbaum G. G., Ezell M. E., The accuracy of medicare claims data in identifying Alzheimer’s disease. J. Clin. Epidemiol. 55, 929–937 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Taylor D. H. Jr., Østbye T., Langa K. M., Weir D., Plassman B. L., The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. J. Alzheimer’s Dis. 17, 807–815 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Q., et al. , An ensemble-based model of PM2. 5 concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 130, 104909 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi L., et al. , Low-concentration air pollution and mortality in American older adults: A National cohort analysis (2001–2017). Environ. Sci. Technol. 56, 7194–7202 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi L., et al. , Long-term effects of PM2· 5 on neurological disorders in the American Medicare population: A Longitudinal cohort study. Lancet Planet. Health 4, e557–e565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutcheon J. A., Chiolero A., Hanley J. A., Random measurement error and regression dilution bias. BMJ 340, c2289 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Fox M. P., Lash T. L., Greenland S., A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int. J. Epidemiol. 34, 1370–1376 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Goldman G. T., et al. , Impact of exposure measurement error in air pollution epidemiology: Effect of error type in time-series studies. Environ. Health 10, 1–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The Medicare dataset was stored and analyzed in the Emory Rollins School secure cluster environment, with Health Insurance Portability and Accountability Act compliance. The rules governing the Medicare dataset prohibit any sharing of the health datasets being used for our epidemiologic research. Restricted by our Data Use Agreement with the US Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available from us. Academic and nonprofit researchers who are interested in using Medicare data should contact the US Centers for Medicare & Medicaid Services directly to obtain their own datasets upon completion of a Data Use Agreement.