Significance
Many insects have evolved a developmental arrest called diapause to overcome unfavorable seasonal changes. In pupal diapause species, the fat body is structurally intact in diapause pupae for months, whereas it undergoes cell dissociation after pupation in nondiapause pupae. In the cotton bollworm, cathepsin L activates matrix metalloproteinases that degrade extracellular matrix proteins, resulting in fat body cell dissociation and lipid metabolism. The physiological process is indispensable for pupal development, and its stimulation might moderately avert pupal diapause. This study reveals a crucial role played by the fat body in regulating insect development and diapause.
Keywords: Pupal development, pupal diapause, fat body cell dissociation, cathepsin L, matrix metalloproteinase
Abstract
In Lepidoptera and Diptera, the fat body dissociates into single cells in nondiapause pupae, but it does not dissociate in diapause pupae until diapause termination. Using the cotton bollworm, Helicoverpa armigera, as a model of pupal diapause insects, we illustrated the catalytic mechanism and physiological importance of fat body cell dissociation in regulating pupal development and diapause. In nondiapause pupae, cathepsin L (CatL) activates matrix metalloproteinases (Mmps) that degrade extracellular matrix proteins and cause fat body cell dissociation. Mmp-induced fat body cell dissociation activates lipid metabolism through transcriptional regulation, and the resulting energetic supplies increase brain metabolic activity (i.e., mitochondria respiration and insulin signaling) and thus promote pupal development. In diapause pupae, low activities of CatL and Mmps prevent fat body cell dissociation and lipid metabolism from occurring, maintaining pupal diapause. Importantly, as demonstrated by chemical inhibitor treatments and CRISPR-mediated gene knockouts, Mmp inhibition delayed pupal development and moderately increased the incidence of pupal diapause, while Mmp stimulation promoted pupal development and moderately averted pupal diapause. This study advances our recent understanding of fat body biology and insect diapause regulation.
Diapause is an actively induced developmental arrest that has evolved in a large number of insect species. This physiological and behavioral adaptation facilitates insect survival under unfavorable environmental conditions, such as adverse weather changes (1). The two most remarkable characteristics of insect diapause are developmental arrest and profound metabolic suppression, which can decrease by as much as 90% (2). One reason for this metabolic suppression could be the breakdown of mitochondria via mitophagy (3). The cotton bollworm, Helicoverpa armigera (Lepidoptera), is one of the most devastating pests worldwide (4–6). Its larvae respond to short day length and low temperature by entering a pupal period lasting up to 3 mo (diapause). Diapause pupae exhibit extremely prolonged lifespans compared to their nondiapause pupal counterparts, which develop into adults within approximately 3 wk (7, 8). The pupal diapause state in H. armigera is accompanied by a major shutdown in metabolic activity, which is especially obvious in the brain and fat body, and this shutdown is directly linked to low ecdysone levels in the hemolymph (7). It is well established that high ecdysone levels promote pupal development and low ecdysone levels result in pupal diapause (1, 9). In most, if not all insects that undergo pupal diapause, the inhibition in mitochondrial respiration or impaired insulin signaling are two important characteristics of diapause (10, 11), and moreover, decreasing mitochondrial respiration or insulin signaling can extend the lifespan via an increase in reactive oxygen species (ROS) (8, 12). It seems that ROS elicits distinct responses at different developmental stages: promoting aging in older individuals while extending life span in younger individuals (8). Most insects do not feed during pupal diapause; they slowly use nutrient reserves accumulated prior to diapause as fuel during the long diapause period (12). Previous studies have shown that intermediates of the tricarboxylic acid (TCA) cycle released from the fat body act in the brain, causing ecdysone biosynthesis in the prothoracic glands and terminating pupal diapause (7). Lipids deposited in the fat body during larval feeding are the primary energy sources for the diapause pupae (13–16). Nevertheless, the “cause–effect” relationships between lipid metabolism in the fat body and pupal diapause remain unclear.
The fat body is a multifunctional tissue and constitutes a considerable percentage of the fresh weight in an insect (more than 50% in some cases). The fat body performs roles similar to two major tissues involved in lipid metabolism, e.g., the liver and fat tissues in vertebrates. Nutrients are stored in fat body cells during the feeding larval stage, then released into the hemolymph and ultimately absorbed from there by imaginal organs during the nonfeeding pupal phase. Moreover, by producing humoral factors, the fat body coordinates the growth of multiple organs with the energy demands of the organism (17–19). In the early stages of the metamorphosis of holometabolous insects, the fat body transforms from an organized thin-layer tissue to individual fat body cells, known as fat body cell dissociation. Thus far, the catalytic mechanism of the process is contentious and its physiological importance remains hypothetical (19). In H. armigera, the fat body dissociates in nondiapause pupae but remains structurally intact in diapause pupae, and starts to dissociate when the diapause is terminated, implying that fat body cell dissociation is closely related to pupal development and diapause (20). In the tobacco hornworm, Manduca sexta (Lepidoptera), fat body releases an unknown stimulatory factor(s) promoting the prothoracic gland to produce ecdysone (21). In the corn earworm, Heliothis zea (Lepidoptera), implantation of fat body from 24-h-old nondiapause pupae stimulates diapause termination in diapause pupae, indicating a diapause terminating factor(s) is released from the fat body into the hemolymph. This factor does not exert a direct effect on prothoracic gland but is necessary for neuropeptide-stimulated ecdysone production (22). These unknown factors may be metabolites released from dissociated fat body cells, and therefore, it is crucial to determine how fat body cell dissociation is involved in pupal development and diapause.
As demonstrated in the fruit fly, Drosophila melanogaster (Diptera), the enzymes responsible for extracellular matrix (ECM) breakdown are matrix metalloproteinases (Mmps) (23, 24). Mmps show the ability to degrade all major protein components in the ECM and basement membranes under physiological conditions (25). In addition to other researchers (26), we have identified that Mmps induce fat body cell dissociation in D. melanogaster (27, 28). We have also found that Mmps promotes fat body cell dissociation in the domestic silkmoth, Bombyx mori (Lepidoptera) (29). Moreover, the actions of Mmps are suppressed in vivo by a family of proteinase inhibitors called tissue inhibitors of metalloproteinases (Timps) (30). The overexpression of Timp blocks fat body cell dissociation in both D. melanogaster and B. mori (26–29). By contrast, precocious fat body cell dissociation is observed in the Timp-mutant D. melanogaster (28). Furthermore, in three lepidopterans, H. armigera, B. mori, and the Chinese silk moth, Antheraea pernyi, the lysosomal cysteine proteinase Cathepsin L (CatL) are involved in fat body cell dissociation (20, 31, 32). Although CatL might be released into the ECM and thus degrade certain ECM components (33), Mmps are considered the main proteinases that degrade most ECM components in mammals (23, 24, 30). It is well known that mammalian cathepsins could directly activate MMPs through proteinase cleavage and could indirectly activate MMPs by cleaving TIMPs and thus relieving the TIMPs’ inhibitory effect on MMPs (34, 35). However, nothing is known about the genetic and biochemical interactions between CatL and Mmps in insects.
To understand the molecular mechanism and cause–effect relationship of fat body cell dissociation and pupal diapause, using H. armigera as a model of pupal diapause insects, we show that CatL acts upstream of Mmps and that Mmps degrade ECM proteins and cause fat body cell dissociation. More importantly, although it has long been assumed that fat body cell dissociation is a downstream part of pupal development or diapause, we reveal that Mmp-induced fat body cell dissociation promotes pupal development and might moderately avert pupal diapause by activating lipid metabolism. This study significantly advances our understanding of fat body cell dissociation, especially its regulatory roles in two different developmental trajectories: pupal development or pupal diapause.
Results
A Comparison of Fat Body Cell Dissociation between Nondiapause and Diapause Pupae.
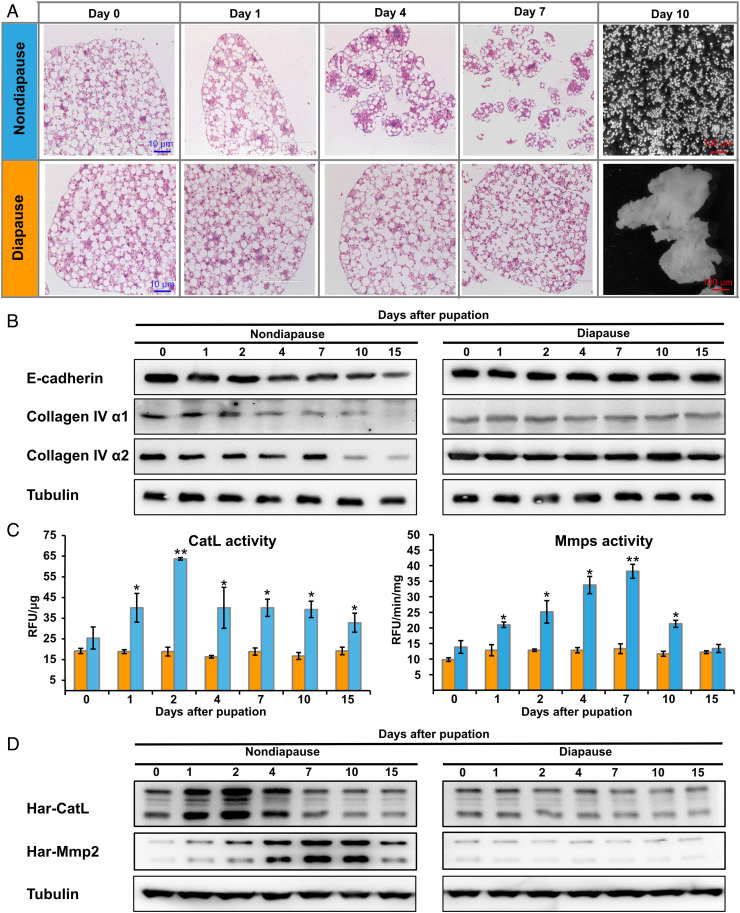
It has been reported that the fat body dissociates during pupal development in H. armigera, but it does not dissociate in diapause pupae until diapause termination (20). Here we used histological sectioning and H&E staining to compare H. armigera fat body cell dissociation between nondiapause and diapause pupae. Consistent with the previous report (20), the nondiapause pupal fat body showed progressive cell dissociation, commencing 1 to 2 d after pupation and completed 10 d after pupation, at which time, individual fat body cells were distributed throughout the pupal body cavity. In contrast, diapause pupal fat body cells maintained a tight arrangement and did not dissociate, even 10 d after pupation (Fig. 1A).
Fig. 1.
A comparison of fat body cell dissociation between nondiapause and diapause pupae from 0 to 15 d after pupation. (A) Histological sections and H&E staining analysis of morphological changes. (B) Western blot showing patterns of degradation for three ECM protein components (E-cadherin, Collagen IV α1, and Collagen IV α2). (C) Developmental changes in the enzymatic activity of two ECM proteinases (CatL and Mmps). Bars represent the mean ± SEM. Significant differences were calculated using Student’s t test (*P < 0.05; **P < 0.01) according to three biological replicates. (D) Western blot showing changes in Har-CatL and Har-Mmp2 protein levels.
Each insect fat body tissue sheet is encased in a thin basement membrane (19). Collagen IV comprises two α1 chains and one α2 chain. In addition to being the most abundant constituent of the basement membrane, collagen IV mediates cell–cell adhesion via its intercellular concentrations (36, 37). E-cadherin is the main component of adherens junctions and mediates cell–cell adhesion in the fat body (27). Western blot analyses demonstrated that the three aforementioned ECM protein components were gradually degraded as fat body cell dissociation progressed in the nondiapause pupae, whereas these components remained intact in the diapause pupal fat body (Fig. 1B and SI Appendix, Fig. S9A), confirming that the fat body lost structural integrity during the dissociation process. Thus, the degradation degree of these ECM protein components reflects the degree of fat body cell dissociation.
As introduced above, CatL and Mmps are involved in fat body cell dissociation in a variety of insect species (20, 26–29, 31, 32). One CatL gene (named Har-CatL), three Mmp genes (named Har-Mmp1, Har-Mmp2, and Har-Mmp3) and one Timp gene (named Har-Timp) were identified in the H. armigera genome (SI Appendix, Fig. S1A). We first compared the developmental patterns of enzymatic activity in the fat body of nondiapause and diapause pupae. In nondiapause pupal fat body, the CatL activity increased from 0 d after pupation and peaked 2 d after pupation (prior to discernible changes in fat body cell–cell adhesion), and then decreased slowly. Mmp activity gradually increased from 0 d after pupation, reaching the maximum level 7 d after pupation, and then decreased steadily. In general, the CatL activity peak is ahead of the Mmp activity peak. In diapause pupal fat body, the activities of both CatL and Mmps remained unchanged with significantly low levels (*P < 0.05; **P < 0.01) (Fig. 1C). Further quantitative real-time PCR and Western blot analyses of temporal expression patterns of Har-CatL and Har-Mmps showed that their mRNA and protein levels exhibited trends similar to their enzymatic activities (Fig. 1D and SI Appendix, Figs. S1B and S9A). These developmental changes suggest that CatL and/or Mmps degrade ECM components and cause fat body cell dissociation.
CatL Activates Mmps and Mmps Dissociate Fat Body Tissues.
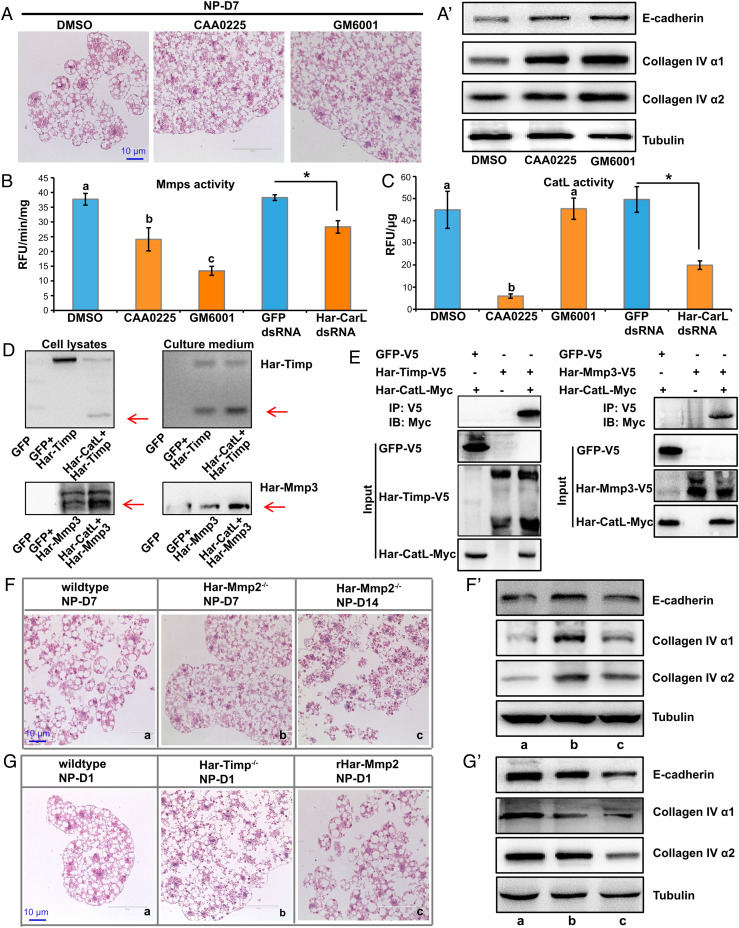
To clarify the catalytic mechanisms of CatL and Mmps in fat body cell dissociation in H. armigera, we assessed the degree of fat body cell dissociation after injecting nondiapause pupae with the CatL inhibitor CAA0225 or the Mmps inhibitor GM6001 0 d after pupation. As detected 7 d after pupation, both inhibitors blocked fat body cell dissociation and prevented the degradation of ECM protein components (Fig. 2 A and A’ and SI Appendix, Fig. S9C).
Fig. 2.
Functional analysis of CatL and Mmps in fat body cell dissociation. (A and A’) Fat body cell dissociation (A) and degradation of three ECM protein components (A’) were inhibited by injection of the CatL inhibitor CAA0225 and the Mmp inhibitor GM6001 in nondiapause pupae. (B and C) Mmps activity was downregulated by GM6001, CAA0225, or Har-CatL double-stranded RNA (dsRNA) (B); CatL activity was downregulated by CAA0225 and Har-CatL dsRNA, but not affected by GM6001 (C). Bars represent the mean ± SEM. Significant differences were calculated using ANOVA (bars labeled with different lowercase letters are P < 0.05) or Student’s t test (*P < 0.05) according to three biological replicates. (D) Co-overexpression of Har-CatL and Har-Timp or Har-Mmp3 showed cleavage of Har-Timp or Har-Mmp3 by Har-CatL. Har-Timp and Har-Mmp3 were detected by using V5 epitope tag antibody. (E) Co-immunoprecipitation of Har-CatL and Har-Timp or Har-Mmp3 showed direct binding of Har-Timp or Har-Mmp3 by Har-CatL. Har-CatL was detected by using Myc epitope tag antibody. (F and F’) Fat body cell dissociation (F) and degradation of three ECM protein components (F’) were inhibited in Har-Mmp2−/− mutants. (G and G’) Fat body cell dissociation (G) and degradation of three ECM protein components (G’) were enhanced in Har-Timp−/− mutants or pupae injected with recombinant Har-Mmp2.
To understand the detailed molecular mechanism how CatL and Mmps interact in the induction of fat body cell dissociation, we performed pharmaceutical, biochemical, and genetic experiments. As expected, CAA0225 inhibited CatL activity, and GM6001 inhibited Mmps activity. Importantly, Mmp activity in the fat body was significantly inhibited upon CatL inhibition, whereas CatL activity in the fat body was unaffected upon Mmp inhibition (Fig. 2 B and C), suggesting that CatL acts upstream of Mmps during fat body cell dissociation in H. armigera. The same results were obtained in two other lepidopterans, B. mori and the fall armyworm, Spodoptera frugiperda (SI Appendix, Fig. S2 A–F), indicating that a similar catalytic mechanism of fat body cell dissociation occurs during pupal development in Lepidoptera.
By co-overexpressing Har-CatL and Har-Timp in HaEpi cells derived from H. armigera, we found that Har-CatL cleaved Har-Timp into two fragments (Fig. 2D and SI Appendix, Fig. S9B). Each of the three Har-Mmps and Har-CatL was co-overexpressed in HaEpi cells. Har-Mmp1 and Har-Mmp2 showed no obvious changes after overexpression alone or after overexpression with Har-CatL (SI Appendix, Fig. S3A). However, a greater proportion of Har-Mmp3 precursor was cleaved into a mature peptide (Fig. 2D and SI Appendix, Fig. S9B), implying that Har-CatL triggered the activation of the latent Har-Mmp3 precursor. By contrast, Har-Mmps did not cleave or affect the activity of Har-CatL (SI Appendix, Fig. S3B). The physical interaction between Har-CatL and Har-Timp or Har-Mmp3 was further confirmed by co-immunoprecipitation within HaEpi cells, and the results showed that Har-CatL-Myc bound to Har-Timp-V5 and Har-Mmp3-V5, while Har-CatL did not interact with GFP-V5 alone (Fig. 2E).
We then overexpressed Har-CatL and each Har-Mmp tissue specifically in the D. melanogaster fat body using the GAL4/UAS system. The overexpression of Har-CatL exerted no apparent effect on fat body cell dissociation, but overexpression of Har-Mmp2 induced dramatic precocious fat body cell dissociation. In addition, overexpression of Har-Mmp1 or Har-Mmp3 also induced precocious fat body cell dissociation with a less efficiency than Har-Mmp2 (SI Appendix, Fig. S4). These results reveal significant differences in the catalytic mechanisms of fat body cell dissociation between Lepidoptera and D. melanogaster regarding to the role of CatL. In D. melanogaster, neither loss-of-function nor gain-of-function of Dm-CatL exerted effect on fat body cell dissociation (SI Appendix, Fig. S4C). These results also strongly support the idea that Har-Mmp2 is the major Mmp gene responsible for fat body cell dissociation in H. armigera.
Next, we constructed Har-Mmp2 and Har-Timp mutants by employing a dual sgRNA-directed CRISPR-Cas9 system. A Har-Mmp2−/− mutant line with a 426-bp sequence deleted and a Har-Timp−/− mutant line with a 340-bp sequence deleted were obtained. The genomic PCR detection and Western blot analyses confirmed the deletion of aforementioned sequences in Har-Mmp2 and Har-Timp (SI Appendix, Fig. S5 A–B’’). The Har-Mmp2−/− mutants routinely survived to the pharate adult stage but failed to emerge as adults. Har-Timp−/− mutants emerged with twisted wings and died within 3 d; moreover, the surviving adults were unable to mate and reproduce (SI Appendix, Fig. S5 C–C’’). Under nondiapause conditions, in comparison with wild-type pupae, the Har-Mmp2−/− pupae showed significantly delayed fat body cell dissociation and ECM component degradation (Fig. 2 F and F’). Although fat body cell dissociation was only slightly accelerated in the Har-Timp−/− pupae, a high level of ECM component degradation compared to that in the wild-type animals was observed. Moreover, the injection of recombinant Har-Mmp2 promoted fat body cell dissociation and ECM component degradation, confirming the predominant role played by Har-Mmp2 in these processes (Fig. 2 G and G’ and SI Appendix, Fig. S9C). Taken together, the data show that during fat body cell dissociation in H. armigera, CatL directly activates Mmp3, CatL also releases the Timp-induced inhibition on Mmps, and then, Mmps induce fat body cell dissociation by degrading ECM components.
Mmp-Induced Fat Body Cell Dissociation Regulates Pupal Development and Diapause.
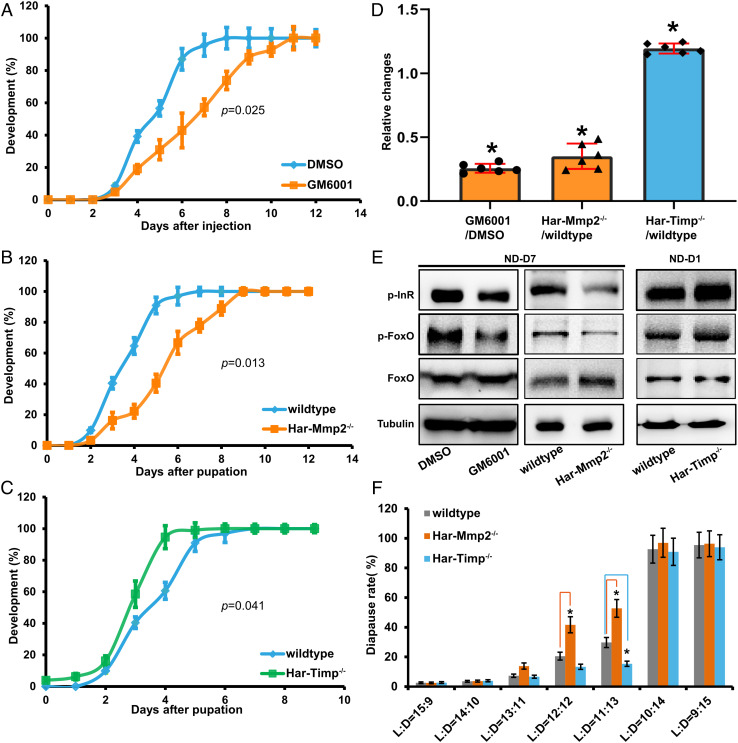
We then investigated whether and how Mmp-induced fat body cell dissociation regulates pupal development and diapause in H. armigera. Stemmata migration is a well-established morphological marker used for monitoring the development rate in H. armigera pupae (38, 39). Importantly, pupal stemmata migration was delayed approximately 2 d after the injection of the Mmp inhibitor GM6001 (P = 0.025, Kolmogorov–Smirnov test), and it was similarly delayed in the Har-Mmp2−/− mutant (P = 0.013), but accelerated approximately 1 d earlier in the Har-Timp−/− mutant (P = 0.041) (Fig. 3 A–C). These results demonstrate that fat body cell dissociation promotes pupal development.
Fig. 3.
Mmp-induced fat body cell dissociation promotes pupal development and might moderately avert pupal diapause. (A–C) Changes of pupal development under manipulation of fat body cell dissociation in nondiapause pupae. Delayed pupal development was caused by injection of GM6001 (A) or by Har-Mmp2−/− mutation (B). Precocious pupal development was caused by Har-Timp−/− mutation (C). Bars represent the mean ± SEM. Significant differences were determined by using Kolmogorov–Smirnov test according to three biological replicates. (D) Relative changes in brain COX activity were decreased in GM6001- injected pupae (ND-D7) and Har-Mmp2−/− mutant pupae (ND-D7), but increased in Har-Timp−/− mutant pupae (ND-D1). Bars represent the mean ± SEM. Significant differences were calculated using Student’s t test (*P < 0.05) according to three biological replicates. (E) Levels of phosphorylated InR and FoxO in the pupal brain were decreased in GM6001-injected pupae (ND-D7) and Har-Mmp2−/− mutant pupae (ND-D7), but increased in Har-Timp−/− mutant pupae (ND-D1). (F) The incidence of diapause was increased in Har-Mmp2−/− mutant pupae (L: D = 12: 12 or 11: 13) and decreased in Har-Timp−/− mutant pupae (L: D = 11: 13).
Previous studies have showed that low brain mitochondrial activity (Cytochrome oxidase/COX activity) and low insulin signaling are associated with H. armigera pupal diapause (7, 8, 10, 38). Therefore, we detected the brain COX activity and insulin signaling after injecting the inhibitor GM6001 or DMSO, and compared their levels among wildtype, Har-Mmp2−/−, and Har-Timp−/− pupae. Brain COX activity was downregulated upon the inhibition of fat body cell dissociation but upregulated upon its promotion (Fig. 3D). The phosphorylation levels of InR (Y1220/Y1221) and FoxO (S191) reflect the activity of insulin signaling (8, 40). Western blot analyses revealed that insulin signaling changed in a manner similar to the change in brain COX activity in the aforementioned animals (Fig. 3E and SI Appendix, Fig. S9D). These experimental data demonstrate that fat body cell dissociation activates brain mitochondrial activity and insulin signaling during pupal development, implying a possible role in regulating pupal diapause.
To this end, the effect of fat body cell dissociation on the incidence of pupal diapause was evaluated by rearing newly hatched larvae in different photoperiods at 20 °C until diapause was identified. It is necessary to note that the incidence of pupal diapause in both the Har-Mmp2−/− and Har-Timp−/− mutants was not affected upon the strictly short day length during the light: dark (L: D) photoperiods of 15: 9 or 14: 10 and the strictly long day length during the L: D=10: 14 or 9: 15 photoperiod. Nevertheless, the incidence of pupal diapause was increased in the Har-Mmp2−/− mutants during the L: D=12: 12 or 11: 13 photoperiod, while it was reduced in the Har-Timp−/−mutant during the L: D=11: 13 photoperiod (Fig. 3F). Thus, in certain (near-critical or threshold) photoperiod conditions, the inhibition of Mmp-induced fat body cell dissociation might moderately increase the incidence of pupal diapause, while its stimulation might moderately avert pupal diapause.
Mmp-Induced Fat Body Cell Dissociation Promotes Lipid Mobilization.
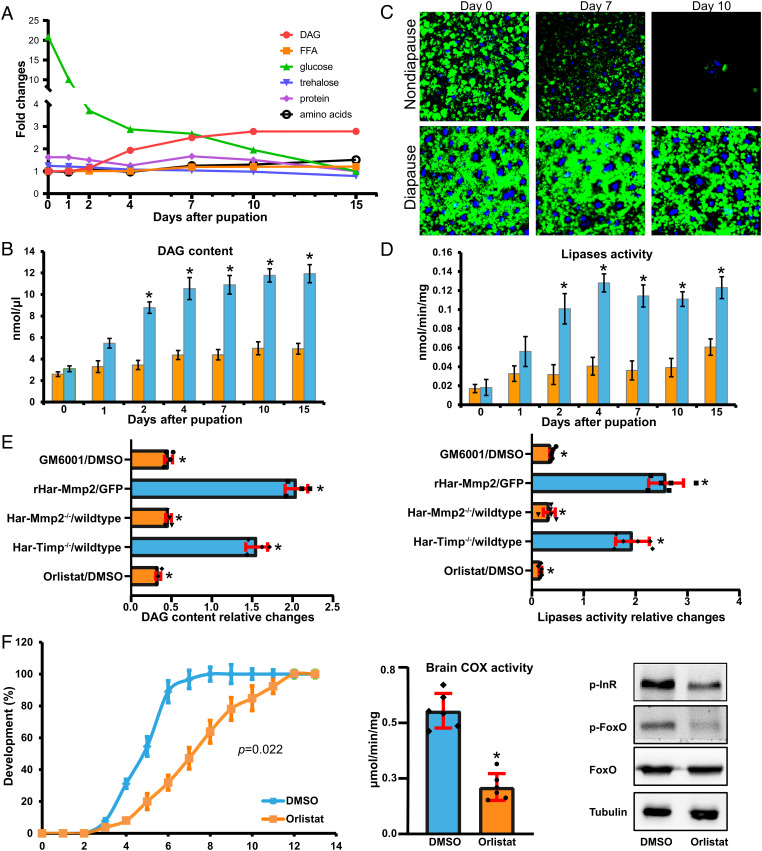
As the fat body is a major energy reservoir for promoting pupal development (19) and terminating pupal diapause (13–16), we have hypothesized that fat body cell dissociation regulates pupal development or diapause by releasing nutrients stored during the feeding larval stages. We compared changes in the main nutrients (diacylglycerol [DAG], free fatty acid [FFA], glucose, trehalose, protein, and amino acids) in the hemolymph as well as lipids and glycogen in the fat body between nondiapause pupae and diapause pupae. In nondiapause pupae, the glucose content decreased gradually from 0 to 15 d after pupation. The DAG content remained unchanged during the first 2 d after pupation, increased from 2 to 10 d after pupation, and then remained at a high level from 10 to 15 d after pupation, suggesting that fat body lipid mobilization accompanies fat body cell dissociation during pupal development. In addition, other nutrients in the hemolymph were relatively stable during pupal development. Importantly, in diapause pupae, the DAG content remained constant or slowly increased, trehalose levels were higher than nondiapause pupae beginning from 7 d after pupation, while the other nutrients were not significantly different from nondiapause pupae (Fig. 4 A and B and SI Appendix, Fig. S6). Lipid droplets in fat body cells are involved in energy production and signaling (41). Intracellular lipolysis can be indicated by a decrease in lipid droplet size (42). Staining by BODIPY, a fluorescence dye indicator of lipid droplets, revealed that lipid droplets in fat body cells gradually decreased in size during pupal development, but remained unchanged or slightly decreased in diapause pupae (Fig. 4C). Moreover, lipases activity in the fat body progressively increased in nondiapause pupae but was constantly low in diapause pupae with a slight increasing trend (Fig. 4D). Thus, the changes of lipid droplets and lipase activity in the fat body result in the significant differences of DAG content in the hemolymph between nondiapause and diapause pupae.
Fig. 4.
Mmp-induced fat body cell dissociation induces lipid mobilization. (A) Relative changes in diglyceride, free fatty acids, glucose, trehalose, total protein, and total amino acids in hemolymph from day 0 to 15 d after pupation in nondiapause pupae. (B) Developmental pattern of hemolymph diglyceride content in nondiapause and diapause pupae. (C) BODIPY staining of lipid droplets (green) in the fat body of nondiapause pupae. The nuclei were stained with DAPI (blue). (D) Developmental patterns of fat body lipases activity in nondiapause and diapause pupae. (E) Relative changes in hemolymph diglyceride content and fat body lipases activity in GM6001-injected pupae (ND-D7), pupae injected with recombinant Har-Mmp2 (NP-D1), Har-Mmp2−/− mutant pupae (ND-D7), Har-Timp−/− mutant pupae (ND-D1), and the lipases inhibitor Orlistat-injected pupae (ND-D7). (F) Delayed pupal development, decreased brain COX activity, and decreased levels of phosphorylated InR and FoxO in nondiapause pupae caused by Orlistat injection. Bars represent the mean ± SEM. Significant differences were calculated using Student’s t test (*P < 0.05) or Kolmogorov–Smirnov test according to three biological replicates.
Moreover, fat body lipases activity and hemolymph DAG content were decreased upon the inhibition of fat body cell dissociation by injection of GM6001 or mutation of Har-Mmp2, whereas they were increased upon promotion by injection of recombinant Har-Mmp2 (rHar-Mmp2) and mutation of Har-Timp (Fig. 4E). The results show that fat body cell dissociation increases fat body lipases activity and hemolymph DAG content. Importantly, the injection of Orlistat, a general lipase inhibitor, decreased fat body lipases activity and hemolymph DAG content (Fig. 4E). Likewise, the injection of Orlistat led to a delay of pupal development (P = 0.022, Kolmogorov–Smirnov test) and decrease levels in brain COX activity and insulin signaling (Fig. 4F). In conclusion, Mmp-induced fat body cell dissociation induces lipid mobilization to enter pupal development, whereas lipid mobilization is attenuated in the structurally intact fat body to maintain pupal diapause.
Fat Body Cell Dissociation Activates Lipid Metabolism through Transcriptional Regulation.
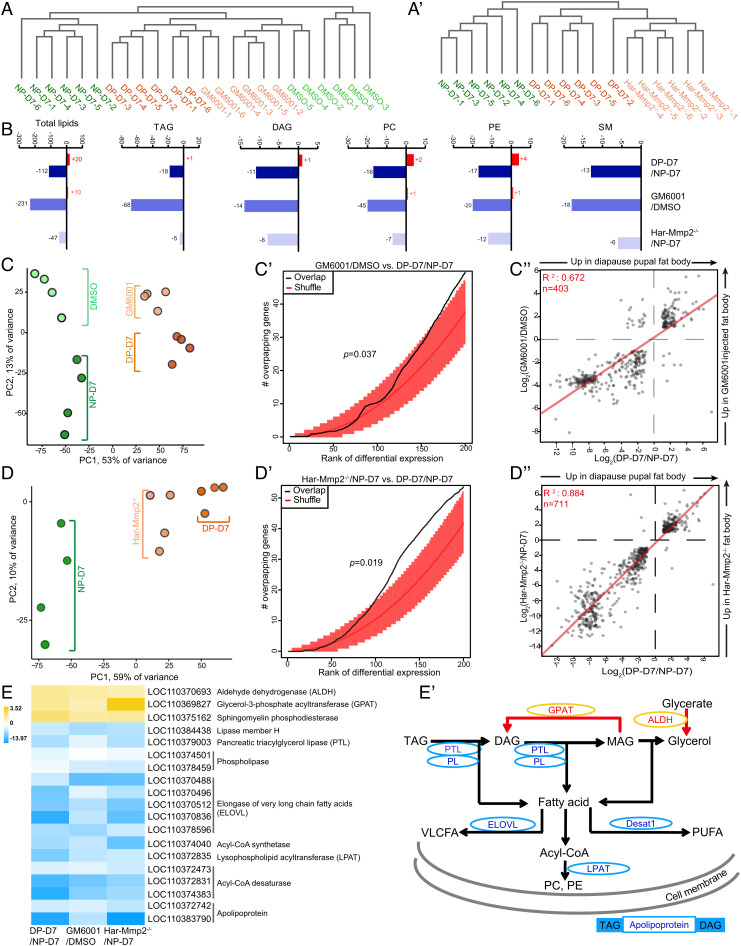
To understand the details how fat body cell dissociation promotes lipid mobilization, we performed a lipidomic analysis with hemolymph samples collected from nondiapause pupae (on days 1 and 7), diapause pupae (on day 7), DMSO- or GM6001-injected pupae (on day 7), Har-Mmp2−/− pupae (on day 7), and Har-Timp−/− pupae (on day 1). A total of 621 independent lipid species were identified in the pupal hemolymph samples by lipidomics, and the majority of these lipid species were triacylglycerol (TAG), DAG, phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin (SM) (Dataset S1). The lipid profiles revealed extensive changes as demonstrated by a partial least squares discriminant analysis (SI Appendix, Fig. S7 A–A’’) and unsupervised hierarchical clustering (Fig. 5 A and A’ and SI Appendix, Fig. S7B). The samples were separated according to the degree of fat body cell dissociation, and moreover, the inhibition of fat body cell dissociation-converted hemolymph lipid profile tended to be diapause-like, whereas the promotion of fat body cell dissociation on day 1 converted hemolymph lipid profile tended to be day 7 nondiapause like. Furthermore, levels of the total lipid and each lipid species decreased dramatically in the hemolymph after the inhibition of fat body cell dissociation (Fig. 5B and SI Appendix, Fig. S7 C and C’), while the TAG and DAG levels were increased in the Har-Timp−/− pupal hemolymph (SI Appendix, Fig. S7D).
Fig. 5.
Combined analyses of the lipidome and transcriptome showing that Mmp-induced fat body cell dissociation activates lipid metabolism through transcriptional regulation. (A and A’) Unsupervised cluster analysis of samples based on the abundance of 621 lipid species profiled in hemolymph isolated from nondiapause and diapause pupae as well as pupae upon the inhibition of fat body cell dissociation. (B) Changes in total lipid species and various lipid groups with differential abundance following the inhibition of fat body cell dissociation. Red bars represent the number of lipids at increased levels, while blue bars represent the number of lipids at decreased levels. (C–C’’) Injection of GM6001 caused diapause-like gene expression in fat body. PCA based on the 500 genes with the most variable expression showing nondiapause pupae and diapause pupae (full circles) and nondiapause pupae after DMSO or GM6001 injections (semitransparent circles) (C). Overlapping of genes with expression affected by GM6001 injections among genes differentially expressed in diapause and nondiapause pupae (black line). The P value was obtained by comparison of 1,000 random permutations of the gene lists (“shuffle,” orange line) (C’). Log2-fold-changes in gene expression that responded to GM6001 injections over DMSO (y-axis) and diapause versus nondiapause pupae (x-axis) (C”). (D–D’’) Mutation of Har-Mmp2 caused diapause like gene expression in fat body. PCA based on the 500 most variable genes showing nondiapause pupae and diapause pupae (full circles) and Har-Mmp2−/− pupae under nondiapause conditions (semitransparent circles) (D). Overlapping of genes with expression affected by Har-Mmp2 mutation with those differentially expressed in diapause and nondiapause pupae (black line) (D’). Comparison of log2-fold-change for DEGs present in both the diapause pupae versus nondiapause pupae comparison (x-axis) and the Har-Mmp2−/− pupae versus nondiapause pupae (y-axis) (D”). (E and E’) Changes of lipogenesis and lipolysis pathways upon manipulation of fat body cell dissociation. Heatmap of log2-fold-change for DEGs in the lipogenesis and lipolysis genes (E). Schematic representation of glycerolipid metabolism affected by fat body cell dissociation (E’). PC, phosphatidylcholine; PE, phosphatidylethanolamine.
Following the lipidomic analysis, we performed a comprehensive transcriptomic analysis of fat body tissues isolated from the aforementioned seven groups of pupae (Dataset S2). Considering the possibility that the inhibition of fat body cell dissociation might promote diapause-biased gene expression in the fat body, we compared the fat body transcriptomes from nondiapause and diapause pupae with those obtained from pupae treated with DMSO or GM6001. A principal component analysis (PCA) revealed that the greatest variance separated the nondiapause pupal fat body from the diapause pupal fat body (Fig. 5C, x-axis). Strikingly, the second PCA component captured the similarities between diapause and GM6001-induced states, with a clear correspondence of nondiapause pupal fat body and DMSO on the one hand, and diapause pupal fat body and GM6001 on the other hand (Fig. 5C, y-axis). Consistent with this observation, the expression of diapause-biased genes overlapped extensively with genes responsive to GM6001 (P = 0.037) (Fig. 5C’). More specifically, genes with expression downregulated in diapause pupal fat body also exhibited downregulated expression in response to GM6001 (Fig. 5C’’). Similarly, the Har-Mmp2−/− pupal fat body exhibited a diapause like transcriptional state (P = 0.019) (Fig. 5 D–D’’), whereas the Har-Timp−/− pupal fat body transcriptome on day 1 was shifted toward a day 7 nondiapause-like state (SI Appendix, Fig. S7 E–E’’).
A Venn diagram revealed that 266 genes showed common downregulated expression and 73 genes showed common upregulated expression after fat body cell dissociation inhibition (SI Appendix, Fig. S8 A and B). KEGG pathway analysis of common differential expressed genes (DEGs) showed that the pathways of “glycerolipid metabolism,” “fatty acid metabolism,” and “sphingolipid metabolism” were among the 20 most-enriched KEGG pathways (SI Appendix, Fig. S8 A’ and B’). Then we assessed the expression of DEGs with respect to lipid metabolism. Upon the inhibition of fat body cell dissociation, the gene expression of two key enzymes involved in TAG synthesis (ALDH and GPAT) was upregulated; notably, the gene encoding SMPD, which converts sphingomyelin into ceramide, was also upregulated. Moreover, the gene expression of several enzymes involved in lipolysis, such as PTL, LipH, and phospholipases, was downregulated. Furthermore, we observed that many genes involved in the biosynthesis of very long chain fatty acids (VLCFAs), unsaturated fatty acids (UFAs), PC, and PE were downregulated (Fig. 5 E and E’). A Venn diagram and KEGG pathway analyses performed in the Har-Timp−/− versus NP-D1 group confirmed that “glycerolipid metabolism” and “fatty acid metabolism” were affected by the degree of fat body cell dissociation (SI Appendix, Fig. S8 C–D’). In brief, fat body cell dissociation activates lipid metabolism through transcriptional regulation and thus promotes pupal developmental and might moderately avert pupal diapause.
Discussion
A Comparison of Catalytic Mechanisms of Fat Body Cell Dissociation between Lepidoptera and D. melanogaster.
It is well known that Mmps induce fat body cell dissociation in D. melanogaster and B. mori, and the membrane-bound Mmp2 plays major roles (26–29). It has been reported that CatL participates in fat body cell dissociation in three lepidopterans, H. armigera, B. mori, and A. pernyi (20, 31, 32). The present study confirmed that CatL functioned in fat body cell dissociation in three lepidopterans: H. armigera, B. mori, and S. frugiperda (Fig. 2 and SI Appendix, Fig. S2). To gain further insights into this fundamental phenomenon in insect development, we established transgenic fly lines and overexpressed Har-CatL and three Har-Mmp genes specifically in the D. melanogaster fat body. The results showed that overexpression of the Har-Mmps, especially Har-Mmp2, but not Har-CatL, induced notable increase in fat body cell dissociation in D. melanogaster (SI Appendix, Fig. S4), which was consistent with previously reported results (26, 27). Although we cannot rule out the possibility that CatL directly degrade certain ECM components, we infer that insect Mmps are proteinases with direct functions in fat body cell dissociation by degrading ECM components. In H. armigera and likely other lepidopterans, CatL participates in fat body cell dissociation in two ways: by binding, degrading, and inactivating Har-Timp, and by binding, cleaving, and activating Har-Mmp3 (Fig. 6). In general, this outcome in H. armigera fat body cell dissociation is consistent with the reports in mammals’ ECM remodeling (25, 30). Nevertheless, these two degradation pathways are not found in D. melanogaster, in which neither loss-of-function nor gain-of-function of Dm-CatL exerted effect on fat body cell dissociation. Collectively, CatL plays important roles in fat body cell dissociation in Lepidoptera but not in D. melanogaster. Thus, the catalytic mechanism of fat body cell dissociation in Lepidoptera is significantly different from and more complex than that in D. melanogaster.
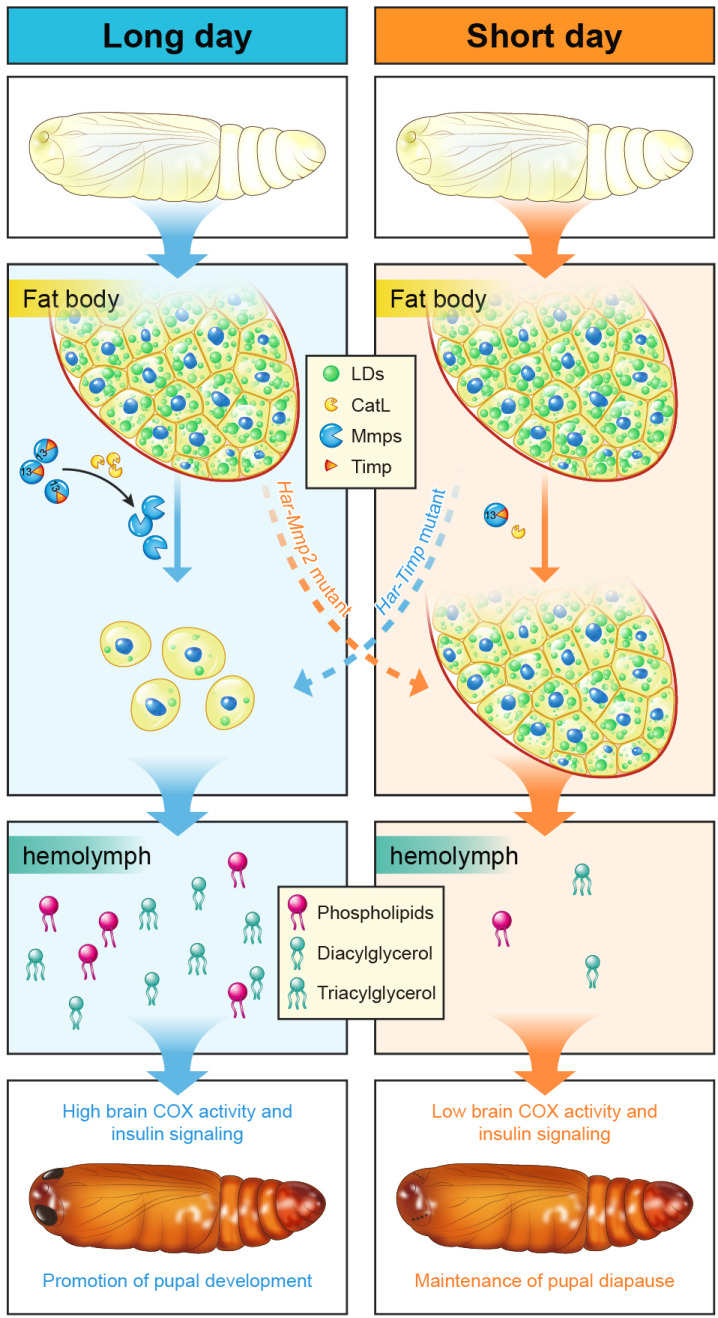
Fig. 6.
A model shows the roles played by fat body cell dissociation in pupal development and diapause. In nondiapause individuals, high CatL and Mmps levels cause fat body cell dissociation and lipid metabolism, promoting pupal development during metamorphosis. In contrast, low CatL and Mmps levels in the diapause pupae result in that fat body cell dissociation and lipid metabolism do not occur, maintaining pupal diapause.
Mmp-Induced Fat Body Cell Dissociation Promotes Pupal Development by Activating Lipid Metabolism.
It has been long assumed that the larval fat body stores lipids to support pupal development during insect metamorphosis. As shown in B. mori, lipid droplets are accumulated in the larval fat body and utilized after pupation (43). Here, our experimental results further demonstrate that blockage of lipid mobilization in the fat body delays pupal development. The underlying mechanisms controlling lipid mobilization from the pupal fat body are not well studied previously, although it has been suggested that autophagy plays a fundamental role in this process (19). We suppose that both fat body cell dissociation and autophagy are involved. In this study, we discovered that the Har-Mmp2−/− and Har-Timp−/− mutants showed delayed and precocious fat body cell dissociation, respectively; and moreover, the Har-Mmp2−/− exhibited phenotypic defects similar to chemical inhibition on CatL and Mmps activities. Further research demonstrates that Mmp-induced fat body cell dissociation promotes pupal development by activating lipid metabolism through transcriptional regulation (Fig. 6). However, the transcriptionally regulatory mechanism, including transcription factors and their activation, requires future investigation.
It is usually considered that fat body TAGs are secreted into the hemolymph as DAGs (44). We found that besides DAGs, many TAG and PL species were also released into the hemolymph by fat body cell dissociation (Fig. 5B). TAGs are the main caloric reserve; PLs are membrane component; acting as signal molecules, DAGs might also activate PKCs and PKDs that play vital roles in regulation of glucose and lipid homeostasis (45). In D. melanogaster, lipoprotein particles (apoliprotein plus lipids) are able to cross the blood–brain barrier to promote neuroblast proliferation and increase systemic insulin signaling (46, 47). Our results collectively show that fat body cell dissociation increases brain metabolic activity (i.e., mitochondria respiration and insulin signaling) and thus promote pupal development. Whether lipoprotein particles directly act in the brain is worthy of future studies. Although we cannot rule out the possibility that CatL and Mmps also directly regulate brain activity, it is very likely that activated by fat body cell dissociation, lipid metabolism promotes pupal development through providing energy resources and acting as signal molecules.
Fat Body Cell Dissociation Plays a Moderate Role in Regulating Pupal Diapause.
As a peripheral organ, it is well known that low levels of ecdysone in diapause pupae prevents fat body cell dissociation from occurring, the structurally intact fat body is the consequence of diapause. The main reason should be that the low 20E signaling is not able to induce high expression levels of CatL and Mmps that cause fat body cell dissociation (Fig. 6) (20, 28). In fact, a previous study has implied that the brain, prothoracic gland, and fat body form a regulatory loop in the regulation of pupal diapause (7). Our study suggests that fat body cell dissociation is a crucial fat body component in the regulatory loop. The molecular mechanism as demonstrated in promoting pupal development above should be conserved in averting pupal diapause. The lipid species released through fat body cell dissociation might provide energy resources and act as signal molecules to increase brain metabolic activity, which causes ecdysone production in the prothoracic glands and eventually averts pupal diapause (Fig. 6). Recently, abundant studies in D. melanogaster have demonstrated that the larval fat body plays a central role in the integration of hormonal and nutritional signals to regulate growth and development by releasing fat body signals to remotely control the brain (19, 48, 49). Our study reveals a similar role played by the pupal fat body, but fat body cell dissociation just plays a moderate role in regulating pupal diapause only during the near-critical photoperiod (L: D = 12: 12 or 11: 13). Interestingly, a recent study showed that mitochondrial degradation and biogenesis in another peripheral organ-flight muscle played vital roles in adult diapause regulation (3).
As demonstrated in this study, under long or short photoperiod, the incidence of diapause was unaffected by fat body cell dissociation. However, under intermediate (near-critical) photoperiod, the incidence of diapause is increased upon the inhibition of fat body cell dissociation via mutation of Har-Mmp2 and is reduced upon the promotion of fat body cell dissociation via mutation of Har-Timp, suggesting that fat body cell dissociation moderately averts pupal diapause (Fig. 3F). This assertion was also supported by a recently study about 20E triggered pupal diapause termination in A. pernyi. They showed fat body cell dissociation preceded eye pigmentation, an indicator for the beginning of pupal–adult transition. They also found that Mmps and CatL were rapidly upregulated upon 20E injection, and metabolism-related genes displayed a slower response (50). Our subsequent lipidomic and transcriptomic analyses revealed that hemolymph lipids and fat body gene expression in diapause pupae shared great similarity with nondiapause pupae upon the inhibition of fat body cell dissociation (Fig. 5). Because the activation of fat body cell dissociation and lipid metabolism might moderately avert pupal diapause, keeping fat body intact is absolutely necessary for maintaining pupal diapause. It is of note that the hemolymph contents of DAG, trehalose, and amino acids slowly and gradually increase in the diapause pupae (Fig. 4B and SI Appendix, Fig. S6). We assume that fat body coordinately releases DAG, trehalose, and amino acids, and possibly intermediates of the TCA cycle for terminating pupal diapause, and fat body cell dissociation is one important process of the fat body in regulating pupal diapause.
Materials and Methods
A detailed description of the materials and methods used in this study is provided in SI Appendix, SI Materials and Methods. H. armigera, B. mori, S. frugiperda, and several strains of transgenic D. melanogaster were used. Western blot analysis, dsRNA injection, multiple staining methods, enzymatic activity and metabolite contents assay, and lipidomic and transcriptomic analyses were performed. The pupal developmental status was determined by observing disappearance of the pupal stemmata. Chemicals, cell culture, and qPCR were described. See SI Appendix, Table S1 for a list of all primers used.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Acknowledgments
We are grateful for Prof. Yi-Dong Wu and Wei-Hua Xu providing insects and for Prof. Xiao-Fan Zhao providing HaEpi cells. This study was supported by the Laboratory of Lingnan Modern Agriculture Project (NT2021003 to S.L.), the National Natural Science Foundation of China (31930014 to S.L., 32200398 to Q.J.), the Department of Science and Technology in Guangdong Province (2019B090905003 and 2019A0102006 to S.L.), the Natural Science Foundation in Guangdong Province (Grant No. 2022A1515010938 to Q.J.), and the Basic and Applied Basic Research of Guangzhou City (Grant No. 202102020722 to Q.J.).
Author contributions
Q.J. and S.L. designed research; Q.J. performed research; Q.J. analyzed data; and Q.J. and S.L. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix. The raw lipidomics data have been deposited to the Metabolights database (https://www.ebi.ac.uk/metabolights/MTBLS5830) (51). The RNA-seq raw data have been deposited to National Center for Biotechnology Information’s Sequence Read Archive database under accession numbers PRJNA876068 (52).
Supporting Information
References
- 1.Denlinger D. L., Regulation of diapause. Annu. Rev. Entomol. 47, 93–122 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Denlinger D. L., Wilis J. H., Fraenkel G., Rates and cycles of oxygen consumption during pupal diapause in Sarcophaga flesh flies. J. Insect Physiol. 18, 871–882 (1972). [DOI] [PubMed] [Google Scholar]
- 3.Lebenzon J. E., et al. , Reversible mitophagy drives metabolic suppression in diapausing beetles. Proc. Natl. Acad. Sci. U.S.A. 119, e2201089119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y. X., Xu W. H., Proteomic and phosphoproteomic analysis at diapause initiation in the cotton bollworm, Helicoverpa armigera. J. Proteome Res. 9, 5053–5064 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Lu Y. X., Xu W. H., Integrated proteomic and metabolomic analysis of larval brain associated with diapause induction and preparation in the cotton bollworm, Helicoverpa armigera. J. Proteome Res. 11, 1042–1053 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q., Lu Y. X., Xu W. H., Proteomic and metabolomic profiles of larval hemolymph associated with diapause in the cotton bollworm, Helicoverpa armigera. BMC Genomics 14, 751 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W. H., Lu Y. X., Denlinger D. L., Cross-talk between the fat body and brain regulates insect developmental arrest. Proc. Natl. Acad. Sci. U.S.A. 109, 14687–14692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X. S., Wang T., Lin X. W., Denlinger D. L., Xu W. H., Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 114, E7832–E7840 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y. X., Zhang Q., Xu W. H., Global metabolomic analyses of the hemolymph and brain during the initiation, maintenance, and termination of pupal diapause in the cotton bollworm, Helicoverpa armigera. PLoS One 9, e99948 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin X. W., Tang L., Yang J. H., Xu W. H., HIF-1 regulates insect lifespan extension by inhibiting c-Myc-TFAM signaling and mitochondrial biogenesis. Biochim. Biophys. Acta 1863, 2594–2603 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Ragland G. J., Denlinger D. L., Hahn D. A., Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. U.S.A. 107, 14909–14914 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C., Mahar R., Merritt M. E., Denlinger D. L., Hahn D. A., ROS and hypoxia signaling regulate periodic metabolic arousal during insect dormancy to coordinate glucose, amino acid, and lipid metabolism. Proc. Natl. Acad. Sci. U.S.A. 118, e2017603118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn D. A., Denlinger D. L., Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 53, 760–773 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Hahn D. A., Denlinger D. L., Energetics of insect diapause. Annu. Rev. Entomol. 56, 103–121 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Lehmann P., et al. , Energy and lipid metabolism during direct and diapause development in a pierid butterfly. J. Exp. Biol. 219, 3049–3060 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Güney G., et al. , A look into Colorado potato beetle lipid metabolism through the lens of lipid storage droplet proteins. Insect. Biochem. Mol. Biol. 133, 103473 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Arquier N., Léopold P., Fly foie gras: Modeling fatty liver in Drosophila. Cell Metab. 5, 83–85 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Hoshizaki D. K., “Fat body” in The Insects: Structure and Function, Simpson S. J., Douglas A. E., Eds. (Cambridge University Press, ed. 5, 2015), pp. 132–145. [Google Scholar]
- 19.Li S., Yu X., Feng Q., Fat body biology in the last decade. Annu. Rev. Entomol. 64, 315–333 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., et al. , A regulatory pathway, ecdysone-transcription factor relish-cathepsin L, is involved in insect fat body dissociation. PLoS Genet. 9, e1003273 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruetzmacher M. C., Gilbert L. I., Bollenbacher W. E., Indirect stimulation of the prothoracic gland of Manduca sexta by juvenile hormone: Evidence for a fat body stimulatory factor. J. Insect Physiol. 30, 771–778 (1984). [Google Scholar]
- 22.Gray R., Meola R., Holman G. M., Fat body stimulation of ecdysone synthesis in Heliothis zea. J. Insect Physiol. 33, 325–331 (1987). [Google Scholar]
- 23.Page-McCaw A., Ewald A. J., Werb Z., Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page-McCaw A., Remodeling the model organism: Matrix metalloproteinase functions in invertebrates. Semin. Cell Dev. Biol. 19, 14–23 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabral-Pacheco G. A., et al. , The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21, 9739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bond N. D., et al. , βFTZ-F1 and matrix metalloproteinase 2 are required for fat-body remodeling in Drosophila. Dev. Biol. 360, 286–296 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Jia Q., Liu Y., Liu H., Li S., Mmp1 and Mmp2 cooperatively induce Drosophila fat body cell dissociation with distinct roles. Sci. Rep. 4, 7535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia Q., et al. , Juvenile hormone and 20-hydroxyecdysone coordinately control the developmental timing of matrix metalloproteinase-induced fat body cell dissociation. J. Biol. Chem. 292, 21504–21516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia Q., et al. , Matrix metalloproteinases promote fat body cell dissociation and ovary development in Bombyx mori. J. Insect Physiol. 111, 8–15 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Nagase H., Visse R., Murphy G., Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Yang H., et al. , Cathepsin-L is involved in degradation of fat body and programmed cell death in Bombyx mori. Gene. 760, 144998 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Sun Y. X., et al. , Cathepsin L-like protease can regulate the process of metamorphosis and fat body dissociation in Antheraea pernyi. Dev. Comp. Immunol. 78, 114–123 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Sudhan D. R., Siemann D. W., Cathepsin L targeting in cancer treatment. Pharmacol. Ther. 155, 105–116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy N., Lynch M. A., Activation of the P2X7 receptor induces migration of glial cells by inducing cathepsin B degradation of tissue inhibitor of metalloproteinase 1. J. Neurochem. 123, 761–770 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Padamsey Z., et al. , Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron 93, 132–146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai J., Ma M., Feng Z., Pastor-Pareja J. C., Inter-adipocyte adhesion and signaling by collagen IV intercellular concentrations in Drosophila. Curr. Biol. 27, 2729–2740 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Luo W., et al. , Juvenile hormone signaling promotes ovulation and maintains egg shape by inducing expression of extracellular matrix genes. Proc. Natl. Acad. Sci. U.S.A. 118, e2104461118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin X. W., Xu W. H., Hexokinase is a key regulator of energy metabolism and ROS activity in insect lifespan extension. Aging (Albany NY) 8, 245–259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng S. L., Zhang X. S., Xu W. H., COXIV and SIRT2-mediated G6PD deacetylation modulate ROS homeostasis to extend pupal lifespan. FEBS J. 288, 2436–2453 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Yuan D., et al. , The AMPK-PP2A axis in insect fat body is activated by 20-hydroxyecdysone to antagonize insulin/IGF signaling and restrict growth rate. Proc. Natl. Acad. Sci. U.S.A. 117, 9292–9301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arena R., et al. , Lipid droplets in mammalian eggs are utilized during embryonic diapause. Proc. Natl. Acad. Sci. U.S.A. 118, e2018362118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng H., et al. , Premature remodeling of fat body and fat mobilization triggered by platelet-derived growth factor/VEGF receptor in Drosophila. FASEB J. 31, 1964–1975 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Peng J., et al. , Comparative transcriptome analysis provides novel insight into morphologic and metabolic changes in the fat body during silkworm metamorphosis. Int. J. Mol. Sci. 19, 3525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinclair B. J., Marshall K. E., The many roles of fats in overwintering insects. J. Exp. Biol. 221, jeb161836 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Kolczynska K., Loza-Valdes A., Hawro I., Sumara G., Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: A review. Lipids Health Dis. 19, 113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brankatschk M., Eaton S., Lipoprotein particles cross the blood-brain barrier in Drosophila. J. Neurosci. 30, 10441–10447 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brankatschk M., Dunst S., Nemetschke L., Eaton S., Delivery of circulating lipoproteins to specific neurons in the Drosophila brain regulates systemic insulin signaling. Elife 3, e02862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parra-Peralbo E., Talamillo A., Barrio R., Origin and development of the adipose tissue, a key organ in physiology and disease. Front. Cell Dev. Biol. 9, 786129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Texada M. J., et al. , A fat-tissue sensor couples growth to oxygen availability by remotely controlling insulin secretion. Nat. Commun. 10, 1955 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du J., et al. , Pupal diapause termination and transcriptional response of Antheraea pernyi (Lepidoptera: Saturniidae) triggered by 20-hydroxyecdysone. Front. Physiol. 13, 888643 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia Q., Li S., Helicoverpa armigera pupal hemolymph lipidomics raw data. MetaboLights. https://www.ebi.ac.uk/metabolights/MTBLS5830. Deposited 2 September 2022.
- 52.Jia Q., Li S., Helicoverpa armigera pupal fat body RNA-seq raw data. NCBI. https://www.ncbi.nlm.nih.gov/sra/PRJNA876068. Deposited 18 September 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Data Availability Statement
All study data are included in the article and/or SI Appendix. The raw lipidomics data have been deposited to the Metabolights database (https://www.ebi.ac.uk/metabolights/MTBLS5830) (51). The RNA-seq raw data have been deposited to National Center for Biotechnology Information’s Sequence Read Archive database under accession numbers PRJNA876068 (52).