Abstract
Viruses impact host cells and have indirect effects on ecosystem processes. Plankton such as ciliates can reduce the abundance of virions in water, but whether virus consumption translates into demographic consequences for the grazers is unknown. Here, we show that small protists not only can consume viruses they also can grow and divide given only viruses to eat. Moreover, the ciliate Halteria sp. foraging on chloroviruses displays dynamics and interaction parameters that are similar to other microbial trophic interactions. These results suggest that the effect of viruses on ecosystems extends beyond (and in contrast to) the viral shunt by redirecting energy up food chains.
Keywords: virovory, viral shunt, microbial loop
Many known viruses cause diseases, and consequently, virology has long focused on viruses as pathogens. Viruses also affect ecosystem processes, however, by lysing microbes and causing the release of nutrients (i.e., the viral shunt) and through the indirect consequences of host mortality (1, 2). Both of these research domains place viruses as the top “predator” in their food chains, but like most predators, viruses also can serve as food.
Many foragers that swallow water, soil particles, or leaves routinely ingest virus particles. Given the small mass of virus particles relative to other foods, the consumption of viruses is thought to be calorically unimportant (3, 4) and not of sufficient magnitude to influence ecosystem processes. Nonetheless, viruses contain amino acids, nucleic acids, and lipids (5), and if consumed in sufficient quantities could influence the population dynamics of the species that consume them. Some ciliates and flagellates may ingest many viruses (3, 4, 6, 7), but the demographic impact of virus consumption (virovory) is unclear.
Here, we investigate the potential for virovory to fuel population growth and alter the pathways of energy flow in food webs. We measured the population growth of Halteria sp. and Paramecium bursaria in foraging trials with and without supplemental chloroviruses. We also tracked the reduction in chloroviruses and fit a classic trophic link model to the data to determine whether the Halteria–chlorovirus interaction can be viewed as a trophic interaction. Finally, we used fluorescent microscopy to confirm the ingestion of chloroviruses by ciliates.
Results
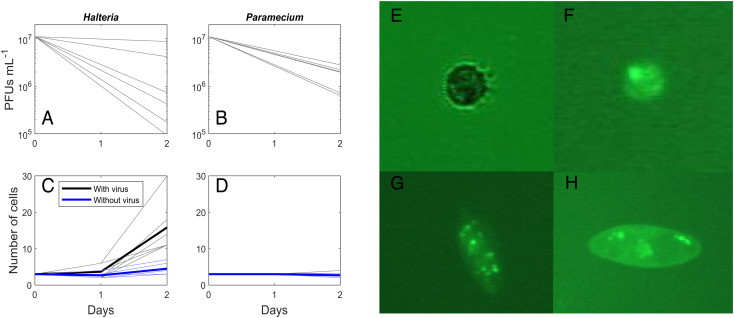
We found that both Halteria and Paramecium reduced chlorovirus plaque-forming units (PFUs) by up to two orders of magnitude in 2 d (Fig. 1 A and B). Fluorescent images showed chloroviruses in vacuoles (Fig. 1 E–H). The PFU density reduction and images together confirm a large flow of energy and matter from virus populations to a consumer. Moreover, our foraging trials demonstrated robust growth in the Halteria population with only chloroviruses as food (rint = 0.66 ± 0.26 [SD], black lines, Fig. 1A), with minimal to no growth in the controls (with chloroviruses filtered out; rint = 0.22 ± 0.12 [SD], blue lines, Fig. 1C). The abundance of the larger Paramecium did not increase in treatment or control trials (Fig. 1D), indicating that not all ciliates can grow on chloroviruses in these conditions, even when they consume them.
Fig. 1.
Halteria (A) and Paramecium bursaria (B) reduced plaque-forming unit density by twofold to two orders of magnitude in 2 d. Supplemental feeding of chloroviruses to the ciliate Halteria led to pronounced growth (C, black lines); in control dishes without virus, Halteria cell abundance was steady (C, blue lines). Paramecium (D) showed no cell growth in response to feeding with viruses. Light solid lines are individual replicates; thick lines are averages. Fluorescent micrographs of ciliates fed chloroviruses show Halteria with visible light (E) and with fluorescently labeled virus given as food (F). Two other ciliates, Euplotes sp. (G) and Paramecium caudatum(H), also show the indication of virus uptake. Aggregations of viruses are visible in the inside of vacuoles.
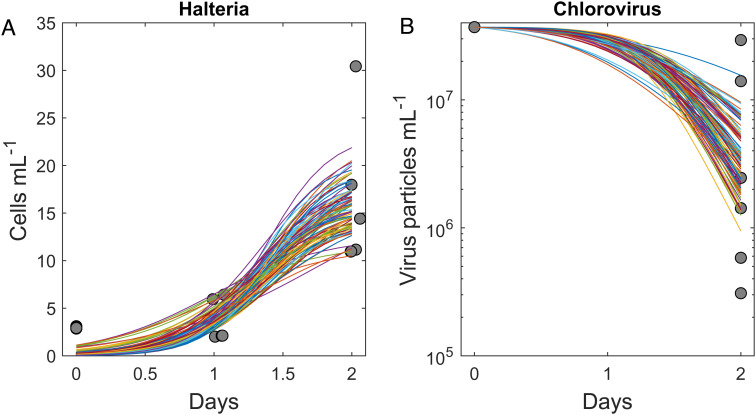
Dynamics of Halteria and chlorovirus abundances fit well to a trophic interaction model (Fig. 2 A and B) that also provides estimates of key interaction parameters (SI Appendix). For space clearance rate (a), the estimate was 0.20 mL per predator per day (CIs: 0.11 to 0.30), which is in the realm of expected values for other foraging protists (8). For conversion efficiency (e), the estimate was 4.6 × 10−7 (CIs: 3.1 × 10−7 to 6.3 × 10−7). After accounting for biovolume (SI Appendix), this translates to a gross growth efficiency of 17%, which compares well with estimates for other aquatic consumers (means are typically 10 to 30%) (9).
Fig. 2.
Predator–prey dynamics of Halteria foraging on chloroviruses. Halteria grew in abundance (A) as it decreased the abundance of chloroviruses (B). Individual colored lines are separate bootstrapped model fits.
Discussion
Our results show that some ciliates can consume enough virus particles to foster population growth at a level similar to protist growth generally (10). Small protists are themselves consumed by zooplankton, so this viral-derived energy and matter may move up through aquatic food webs, altering their structure and dynamics. Protists represent a large fraction of living biomass (11), and their grazing plays a major role in aquatic food webs (12), but current models of aquatic food webs and ecosystems do not include the trophic link between viruses and their consumers. Thus, current food web models are missing a critical interaction, echoing previous work demonstrating the importance of multicellular parasites in food webs more generally (13, 14).
Given the abundance of virus particles in water (1), the abundance of small aquatic protists, and the amount of water in the photic zone globally, the consumption of virus particles by protists could represent a significant and globally relevant trophic transfer. We estimate that each Halteria in our experiment ate ~104 to 106 viruses per day, suggesting that 1014 to 1016 virions could be consumed per day in a small pond (SI Appendix). The viral shunt is thought to limit the movement of energy up food webs by short-circuiting the grazer–microbe foraging interaction (1, 2). Our results indicate, however, that energy and nutrients from host microbes may pass through viruses into grazers and move up the food chain through virovory. This flow would depend on virion size and nutritional content, which varies among strains (15), but it is already clear that viruses of a wide range of sizes can be taken up by grazing protists (3, 4, 6, 7).
Our results suggest that virion persistence in the environment depends not only on environmental factors (16) but also on grazing by predators. It is therefore possible that grazers exert selective pressure and influence the evolution of viral phenotypes (17) in a way that interacts with pressure on viruses to effectively infect and replicate within hosts. At this point, however, the evolutionary effects of grazing on viruses are unknown.
Materials and Methods
We conducted foraging trials with Halteria and Paramecium as grazers. We created 0.4 mL foraging arenas on a 100-mm Petri dish lid and applied two treatments. In the virus treatment, we added 0.5 mL washed virus (2 × 107 PFUs per mL) to the drop with ciliates. In the control treatment, we added 0.5 mL of the virus concentrate cleared of virus with a 0.1-µm syringe filter. We conducted six replicate treatment and control trials at 22 °C. We counted ciliates after 1 and 2 d, and we then used the plaque assay to enumerate infectious chlorovirus particles for the initial virus preparation, the initial rinsed ciliate stocks, and the virus treatment drops at the end of the experiment.
We stained chloroviruses overnight at 6 °C with SYBR Green and washed the virus 10×. We used a Lumascope 400 (San Diego, CA) inverted microscope to image cells at 20×. We tested for uptake in Halteria and two other common ciliates, P. caudatum and Euplotes sp.
To determine whether the virus and Halteria dynamics were consistent with a trophic interaction, we fit a trophic link model to the data (SI Appendix). We used the PottersWheel Toolbox in MATLAB 2021a to fit the model to 100 bootstrapped datasets and then used the median of the fitted parameters as an estimate of the system-level parameters.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank K. Lyons and two anonymous reviewers for helpful suggestions on this draft and C. Akwani and J. Miller for laboratory assistance.
Author contributions
J.P.D. and D.D.D. designed research; J.P.D. and D.D.D. performed research; J.P.D., J.L.V.E., Z.A.-A., and I.V.A. contributed new reagents/analytic tools; J.P.D. analyzed data; and J.P.D., J.L.V.E., I.V.A., and D.D.D. wrote the paper.
Competing interest
The authors declare no competing interest.
Data, Materials, and Software Availability
Experimental data for ciliate growth and virus consumption, images, and analysis code are hosted on the Zenodo public repository at https://doi.org/10.5281/zenodo.7410482 (18).
Supporting Information
References
- 1.Wilhelm S. W., Suttle C. A., Viruses and nutrient cycles in the sea. BioScience 49, 781–788 (1999). [Google Scholar]
- 2.Weitz J. S., Wilhelm S. W., Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol. Rep. 4, 17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hennemuth W., et al. , Ingestion and inactivation of bacteriophages by Tetrahymena. J Eukaryot Microbiol. 55, 44–50 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Deng L., et al. , Grazing of heterotrophic flagellates on viruses is driven by feeding behaviour. Env. Microbiol. Rep. 6, 325–330 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Jover L. F., Effler T. C., Buchan A., Wilhelm S. W., Weitz J. S., The elemental composition of virus particles: implications for marine biogeochemical cycles. Nat. Rev. Microbiol. 12, 519–528 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Olive M., Moerman F., Fernandez-Cassi X., Altermatt F., Kohn T., Removal of waterborne viruses by Tetrahymena pyriformis is virus-specific and coincides with changes in protist swimming speed. Environ. Sci. Technol. 56, 4062–4070 (2022), 10.1021/acs.est.1c05518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González J., Suttle C., Grazing by marine nanoflagellates on viruses and virus-sized particles: Ingestion and digestion. Mar. Ecol. Prog. Ser. 94, 1–10 (1993). [Google Scholar]
- 8.Uiterwaal S. F., Lagerstrom I. T., Lyon S. R., DeLong J. P., FoRAGE database: A compilation of functional responses for consumers and parasitoids. Ecology 103, e3706 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Straile D., Gross growth efficiencies of protozoan and metazoan zooplankton and their dependence on food concentration, predator-prey weight ratio, and taxonomic group. Limnol. Ocean 42, 1375–1385 (1997). [Google Scholar]
- 10.Rose J. M., Caron D. A., Does low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnol. Oceanog 52, 886–895 (2007). [Google Scholar]
- 11.Bar-On Y. M., Phillips R., Milo R., The biomass distribution on Earth. Proc. Natl. Acad. Sci. U.S.A. 115, 6506–6511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherr E., Sherr B., Significance of predation by protists in aquatic microbial food webs. Antonie van Leeuwenhoek 81, 293–308 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Hudson P. J., Dobson A. P., Lafferty K. D., Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 21, 381–385 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Lafferty K. D., Dobson A. P., Kuris A. M., Parasites dominate food web links. Proc. Natl. Acad. Sci. U.S.A. 103, 11211–11216 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jover L. F., Effler T. C., Buchan A., Wilhelm S. W., Weitz J. S., The elemental composition of virus particles: Implications for marine biogeochemical cycles. Nat. Rev. Microbiol. 12, 519–528 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Long A. M., Short S. M., Seasonal determinations of algal virus decay rates reveal overwintering in a temperate freshwater pond. ISME J. 10, 1602–1612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLong J. P., et al. , Towards an integrative view of virus phenotypes. Nat. Rev. Microbiol. 20, 83–94 (2021). [DOI] [PubMed] [Google Scholar]
- 18.DeLong J. P., Van Etten J. L., Al-Ameeli Z., Agarkova I. V., and Dunigan D. D., jpdelong/Virovory: Data and code for DeLong et al PNAS. Zenodo. 10.5281/zenodo.7410482. Deposited 08 December 2022. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Experimental data for ciliate growth and virus consumption, images, and analysis code are hosted on the Zenodo public repository at https://doi.org/10.5281/zenodo.7410482 (18).