Abstract
Objectives
Silver fibre gloves transport heat from the palm to the fingers, possibly reducing the burden of RP in SSc patients. We aim to evaluate the clinical efficiency of this intervention.
Methods
A multicentre, double-blind, randomized trial was performed, accounting for interindividual differences and external factors using a crossover design. Patients were randomized in two groups: group 1 wore 8% silver fibre gloves in period 1 and normal gloves in period 2 and group 2 vice versa. Each period lasted 6 weeks. The primary outcome was the Raynaud Condition Score (RCS) over time (minimal clinical important difference 1.4), assessed three times per week using an online questionnaire. Secondary outcomes included vascular complications and Scleroderma-Health Assessment Questionnaire (SHAQ). Outcomes were evaluated before unblinding using linear mixed models.
Results
A total of 85 SSc patients were included, with 76 completing the study. The mean RCS during 2 weeks before the study (i.e. without gloves) was 6.4 (s.d. 1.6). Both with silver fibre gloves and normal gloves the mean RCS decreased to 3.9 (s.d. 2.3) with a similar course over time. There was no difference in mean RCS over time between the type of gloves [β = 0.067 (95% CI −0.006, 0.19)]. Of secondary outcomes, total SHAQ [β = 0.036 (95% CI 0.026, 0.046)] was slightly higher with silver fibre gloves, which is clinically irrelevant. Three patients developed new digital ulcers with normal gloves vs one patient with silver fibre gloves [odds ratio 3.2 (95% CI 0.32, 31.1)].
Conclusions
Wearing gloves in SSc patients clearly decreases the RP burden. Our results do not support the hypothesis that increased heat transport of 8% silver fibre gloves is associated with less disease burden as measured in this study by the RCS compared with normal gloves.
Clinical trial registration number
Netherlands Trial register (https://www.trialregister.nl/) NL7904
Keywords: SSc, scleroderma, RP, silver fibre gloves, crossover trial
Rheumatology key messages
Using a crossover trial design accounts for interindividual differences and enables evaluating RP burden in SSc patients.
Wearing gloves decreases the burden caused by RP in SSc.
In SSc patients, 8% silver fibre gloves do not provide additional benefit to decrease RP burden compared with normal gloves.
Introduction
More than 90% of patients with SSc experience RP, usually as the first symptom of the disease [1–3]. RP often predates other signs and symptoms by several years [4]. RP is characterized by reversible and episodic attacks of vasospasm, mainly triggered by cold or emotional stress. Furthermore, RP secondary to SSc is linked with vascular alterations, resulting in narrowing of the blood vessels and blood flow impairment [5].
RP strongly influences the quality of life and reduction of RP symptoms results in better quality of life [6–8]. The management of RP requires a multifaceted approach, including vasoactive drug therapy, discontinuation of vasoconstrictors such as nicotine and lifestyle measures to avoid triggers [2]. Patients are advised to avoid exposure to cold by wearing warm clothes and gloves. In case of persistent complaints, electrically heated and silver fibre gloves are often tried [9, 10].
The use of silver for medical purposes has its origin in the antibacterial properties of silver. Wound care bandages with silver are known to decrease the risk of infections and stimulate the wound healing process [11]. In RP, silver fibres are thought to help by reflecting body warmth [12]. Only one study specifically evaluated the effect of silver fibre on microcirculation. This study concerned compression stockings in patients with chronic venous insufficiency and showed a slightly beneficial effect on skin perfusion with silver fibre stockings compared with normal stockings [13].
Clinical observations have suggested better efficacy in RP symptom reduction of silver fibre gloves compared with normal gloves [9, 14, 15]. SSc patients report that hand temperature increases and, as a consequence, disease burden related to RP decreases. Despite its generalized use among SSc patients in European countries, no objective evidence regarding its superiority for RP over normal gloves is available. Therefore we evaluated the added value of 8% silver fibre gloves compared with normal gloves in the treatment of patients with RP secondary to SSc.
Patients and methods
Study design and patients
This was a multicentre, double-blind, randomized, mirrored crossover trial that took place between December 2019 and June 2021 (Netherlands Trial register NL7904). Ethical approval was received from the independent ethics committees or institutional review boards of all participating centres (Leiden University Medical Center, Haga Hospital, Maasstad Hospital and Zuyderland Hospital) prior to study commencement (P18.240). The study was conducted in accordance with the Declaration of Helsinki [16].
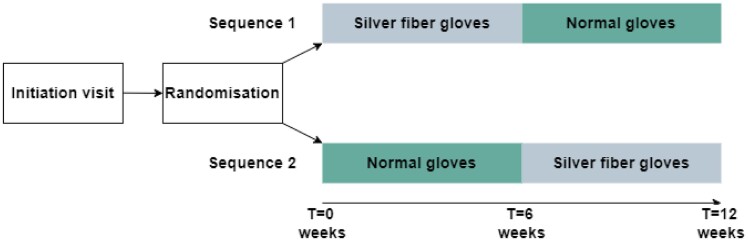
The study design is shown in Fig. 1. The study period consisted of two blocks of 6 weeks for each patient. Patients could be randomized to start with the silver fibre gloves in period 1, followed by the normal gloves in period 2 or (mirrored) first normal gloves in period 1 and then silver fibre gloves in period 2. A mirrored crossover design was chosen to account for interindividual differences and weather changes during the trial period. The study was conducted during winter and early spring (inclusion from October through February) to increase the likelihood of relevant RP burden.
Fig. 1.
Study design
In each glove period (6 weeks), patients received 18 questionnaires (three per week).
Eligible patients were ≥18 years old with a diagnosis of SSc according to the ACR/EULAR 2013 criteria [17]. Patients were required to have four or more RP attacks on three or more different days per week and a Raynaud Condition Score (RCS) at baseline ≥3.4 [18]. Moreover, vasoactive medication [calcium antagonists, angiotensin II receptor blockers, endothelin receptor antagonists and phosphodiesterase type 5 (PDE5) inhibitors] had to be stable for at least 2 weeks prior to start. We excluded patients with a past history of a sympathectomy, ongoing treatment with i.v. prostacyclin within 6 weeks of study start and/or a known allergy to silver and dyes used in textiles.
Blinding the gloves
Cotton gloves and silver fibre gloves were dyed to conceal the silver threads. Silver fibre gloves containing 8% silver were used in this study. Fifteen healthy controls were asked to assess which of the presented gloves contained silver threads, which were correctly identified in 50% of cases and misclassified in 50%, demonstrating the optic similarity of the dyed gloves.
Procedures
RP burden before the start of the trial was assessed based on self-report of the patients, asked by their treating physician at the outpatient clinic. If a patient fulfilled the inclusion criteria and was willing to participate, the study investigators called the patient with additional information. After 1 week, the patient was contacted again to confirm participation by signing informed consent. Included patients received instructions on how to complete the online questionnaires using Castor EDC (electronic data capture) software (Supplementary File, available at Rheumatology online) and how to wear the gloves, i.e. at least 10 h/day during the study period. After signing an informed consent, the first online questionnaire was sent. In this questionnaire patients had to complete general information, the RP burden of the past 2 weeks (prior RCS) and the Scleroderma Health Assessment Questionnaire (SHAQ). During the baseline visit, the number of digital ulcers and pitting scars were recorded and a nailfold capillaroscopy was performed.
During the study, data on RP attacks and the RCS were collected three times per week using automatically generated questionnaires that were sent by e-mail through Castor EDC. During the study, completeness of the online questionnaires was continuously monitored. If a patient missed more than one questionnaire in a row, he/she was called during that same week by the researcher.
At week 6, patients had to return the gloves of period 1 and were provided with the gloves of period 2. The gloves were wrapped in opaque foil, making it impossible to compare the gloves of both periods with each other. At weeks 6 and 12, the number of digital ulcers and pitting scars were recorded by physical inspection by the researcher, quality of life was measured using the SHAQ and a nailfold capillaroscopy was performed. At the end of the study, all participants were asked to state during which period they thought they had worn the silver fibre gloves.
Due to the coronavirus disease 2019 (COVID-19) pandemic, protocol alterations had to be made: a nailfold capillaroscopy before the study was not repeated if patients had a nailfold capillaroscopy <3 months prior to the start of the study. Moreover, if indicated by the pandemic regulations, the follow-up visits were performed remotely or by video call. As a consequence, gloves of period 1 were sent by post from the patients to the hospital and the gloves of period 2 were sent by post from the hospital to the patients. Follow-up assessments of nailfold capillaroscopy are lacking for the majority of patients. In addition, to check for development of digital ulcers, patients were requested to take photographs of both sides of both hands and send them digitally to the researchers for inspection for the presence of digital ulcers and/or pitting scars.
Outcome measurements
The primary efficacy endpoint of this study was the burden of RP, which was measured using the RCS, a scale from 1 (no symptoms) to 10 (extreme symptoms) [19]. The minimal clinically important difference (MCID) of the RCS is 1.4 [18]. Secondary efficacy endpoints included the frequency and duration of RP attacks, impact of RP attacks on daily functioning [numeric rating scale (NRS) 1–10] and a numeric rating scale (1–10) score for warmth of the hands. An RP attack was defined as an episode of at least a two-phase colour change in the fingers in response to cold exposure or emotion, consisting of pallor and/or cyanosis and reactive hyperaemia associated with local discomfort.
Early termination took place if patients developed vascular complications or experienced an increase in RP burden indicating the need for a change in vasoactive medication as evaluated by the treating physician or patients developed an allergic reaction to the gloves.
Statistical analysis
Power and sample size were determined using the MCID of the RCS of 14 (s.d. 26) for change from baseline, based on the study of Khanna et al. [18]. With an α level of 0.05, we required 72 participants in total to attain a power of 90%. Accounting for an expected 10% loss to follow-up, we originally sought to include 80 participants. During the study, loss to follow-up was higher than the expected 10%, partly due to the COVID-19 pandemic. Therefore an amendment to the study protocol was designed and inclusion was increased to 85 patients.
Analyses were performed on data coded for the sequences of the gloves, with unblinding postponed until after interpreting the results. Amendments were made to the original study protocol, as linear mixed models are superior to area under the curve calculation in case of missing data [19] (see Supplementary File, available at Rheumatology online). The primary analysis was therefore a linear mixed model with RCS as the dependent variable and type of gloves as the independent variable, with a random intercept and slope to cluster on patient identity on the intention-to-treat set, adjusted for prior RCS. In addition, a completers analysis was performed by calculating the area under the curve for the RCS and a dependent t-test was used to compare the gloves. Moreover, to evaluate if RCS increased (more symptoms) after switching from silver to normal and subsequently decreased (fewer symptoms) after switching from normal to silver, we compared the RCS of the last three questionnaires of period 1 and the first three questionnaires of period 2.
Secondary endpoints were analysed with similar linear mixed models. To assess whether vascular complications occurred more often in the normal gloves than in the silver fibre gloves, we performed a generalized linear mixed model with the vascular complications as the dependent variable and the type of gloves as the independent variable, adjusted for the number of study visits.
Subsequently we performed exploratory analyses to test whether effect modification occurred with the following variables: presence of digital ulcers or pitting scars (yes/no), frequency of RP attacks, smoking status (current/no), use of vasoactive medication (yes/no), late SSc pattern on nailfold capillaroscopy (yes/no), median prior RCS ≥7.0 (yes/no), wearing the gloves for ≥10 h (yes/no) and correct identification of the silver fibre gloves (after completing the study period). Effect modification by these variables was evaluated for the primary and secondary outcome measures.
Finally, we compared the baseline characteristics of patients who responded to the intervention (RCS change >MCID of RCS) and patients who did not (RCS change <MCID of RCS).
Analyses were performed in Stata SE 16 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
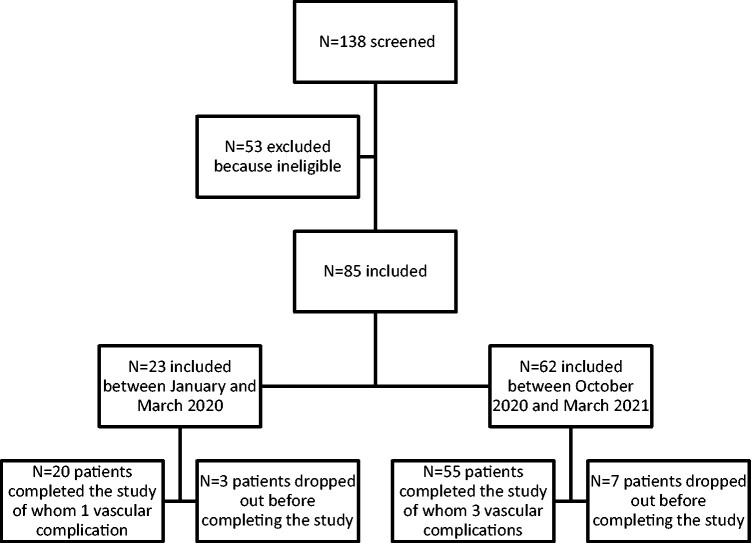
In total, 85 patients were included, of which 75 (88%) completed the study (Fig. 2). Ten patients prematurely ended the study for the following reasons: too heavy burden of participation (n = 3), withdrawal of consent (n = 2), allergic reaction to the gloves (n = 2), unclear reasons (n = 2) and indication to start treatment with cyclophosphamide because of SSc disease activity (n = 1).
Fig. 2.
Inclusion process
The included patients had a mean age of 60 years (s.d. 12), 80% were female and 60% had lcSSc. The median RP duration was 132 months [interquartile range (IQR) 60–240] and 67% used (stable dose) vasoactive medication during the study (Table 1). Ninety percent of all questionnaires were completed by the included patients. Forty-one percent of the included patients wore the gloves for at least 10 h/day.
Table 1.
Baseline characteristics of included SSc patients (N = 85)
| Characteristics | Values |
|---|---|
| Age, years, mean (s.d.) | 60 (12) |
| Female, n (%) | 68 (80) |
| Currently smoking, n (%) | 10 (12) |
| Disease duration, months, median (IQR) | 72 (33, 132) |
| RP duration, months, median (IQR) | 132 (60, 240) |
| lcSSc, n (%) | 51 (60) |
| Digital ulcers (current), n (%) | 6 (7) |
| Pitting scars (current), n (%) | 36 (42) |
| SSc pattern on NC, n (%) | 66 (78) |
| Early SSc pattern, n | 10 |
| Active SSc pattern, n | 27 |
| Late SSc pattern, n | 29 |
| Vasoactive medication, n (%) | 57 (67) |
| Prior RCS scorea (1–10), mean (s.d.) | 6.4 (1.6) |
Vasoactive medication included the use of calcium channel blockers, endotheline-1 antagonists and PDE5 inhibitors.
Mean RCS during the 2 weeks before the start of the trial (without wearing gloves).
NC: nailfold capillaroscopy.
Blinding of the gloves
Forty-seven percent of patients correctly identified the period of silver fibre gloves and 53% did not, demonstrating that blinding of the gloves was successful.
Primary efficacy endpoint
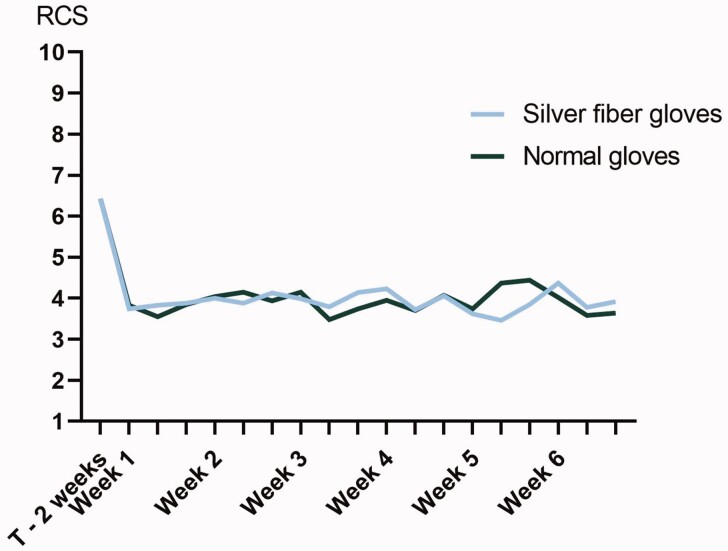
The mean RCS during the 2 weeks before the study (i.e. without gloves) was 6.4 (s.d. 1.6). Over time, with the silver fibre gloves, the mean RCS decreased to 3.9 (s.d. 2.3) and with the normal gloves it decreased to 3.9 (s.d. 2.3; Fig. 3). Using linear mixed model analysis, there was no statistically significant difference in the RCS during the study period between the normal and silver fibre gloves [β = -0.067 (95% CI −0.19, 0.059); Table 2].
Fig. 3.
RCS during the study period. T–2 weeks: mean RCS during the 2 weeks before start of the trial (without wearing gloves)
Table 2.
Primary and secondary efficacy outcomes
| Outcomes | β | 95% CI |
|---|---|---|
| Primary | ||
| RCS | −0.067 | −0.19, 0.059 |
| Secondary | ||
| Raynaud attacks frequency | 0.48 | −0.25, 1.21 |
| Raynaud attacks duration | −39.80 | −115.65, 36.05 |
| NRS hand warmth | 0.086 | −0.041, 0.21 |
| Impact Raynaud | −0.088 | −0.21, 0.035 |
| HAQ_DI | −0.036 | −0.046, −0.026 |
NRS: numeric rating scale.
The reference category was normal gloves.
Linear mixed models were performed with the primary and secondary outcomes as dependent variables, the type of gloves as the independent variable, adjusted for prior RCS.
A β of −0.067 means that wearing 8% silver fibre gloves gave a 0.067 lower RCS compared with normal gloves, which was a nonsignificant difference.
In the completer analysis of patients who completed 100% of the questionnaires (n = 20), there was no statistically significant difference in the area under the curve of RCS over time between the silver fibre gloves and normal gloves (78.3 vs 76.6, respectively; P = 0.726). The clinical characteristics of these completers (n = 20) did not differ from those of the other patients (n = 65; Supplementary Table S1, available at Rheumatology online).
Moreover, after switching from silver fibre to normal gloves, seven patients reported an increase in the RCS (more symptoms) and two patients a decrease (fewer symptoms). After switching from normal to silver fibre gloves, seven patients reported an increase in the RCS and three patients a decrease. Fifty-four patients had a stable RCS after switching from one type of glove to the other.
Secondary efficacy endpoints
For frequency of RP attacks, duration of RP attacks, NRS of warmth of the hands and NRS impact of RP, no statistically significant differences were found between the silver fibre and normal gloves (Table 2). Only for the HAQ was a statistically significant difference in RCS between silver fibre and normal gloves found [β = −0.036 (95% CI −0.046, −0.026); Table 2], which was not clinically meaningful [20].
Adverse events
Two cutaneous allergic reactions (silver fibre gloves, n = 1; normal gloves, n = 1) were reported, both requiring withdrawal from the trial. Vascular complications necessitating a change of medication occurred in four patients: three while wearing normal gloves and one while wearing silver fibre gloves [OR 0.31 (95% CI 0.31, 3.1)].
Effect modification
Subgroup analyses for the presence of digital ulcers or pitting scars, frequency of RP attacks, smoking status, the use of vasoactive medication, late SSc pattern on nailfold capillaroscopy, median prior RCS ≥7.0, wearing the gloves for ≥10 h and correctly identifying the period of silver fibre gloves did not show statistically significant differences in the primary (Table 3) or secondary outcomes (Supplementary Table S2–S6, available at Rheumatology online).
Table 3.
Subgroup analyses for RCS
| Characteristics | Number of SSc patients | β | 95% CI |
|---|---|---|---|
| Vasoactive medication | 57 | −0.072 | −0.23, 0.090 |
| No vasoactive medication | 28 | −0.064 | −0.27, 0.14 |
| DU and PS on baseline | 37 | −0.039 | −0.42, 0.34 |
| No DU and PS on baseline | 48 | −0.073 | −0.21, 0.061 |
| Smoker | 10 | 0.160 | −0.20, 0.52 |
| Non-smoker | 73 | −0.12 | −0.25, 0.020 |
| Late SSc pattern on baseline | 29 | −0.061 | −0.29, 0.17 |
| Other pattern on baseline | 55 | −0.072 | −0.22, 0.079 |
| Prior RCSa ≥7 | 47 | −0.15 | −0.31, 0.024 |
| Prior RCSa <7 | 38 | 0.040 | −0.15, 0.23 |
| Wearing the gloves ≥10 h | 35 | −0.18 | −0.37, 0.011 |
| Wearing the gloves <10 h | 34 | −0.040 | −0.23, 0.15 |
| Correctly identifying the period of silver fibre gloves | 45 | −0.16 | −0.34, 0.014 |
| Not correctly identifying the period of silver fibre gloves | 40 | 0.040 | −0.14, 0.22 |
For each subgroup, shown per row, a linear mixed model was performed with the RCS as the dependent variable, the type of gloves as the independent variable, adjusted for prior RCS, and a random slope was added. The reference category was normal gloves. For example, in the SSc patients with vasoactive medication, a β of −0.072 means that wearing 8% silver fibre gloves gave a 0.072 lower RCS compared with normal gloves in SSc patients with vasoactive medication, which was a non-significant difference.
Mean RCS during the 2 weeks before the start of the trial (without wearing gloves).
DU: digital ulcers; PS: pitting scars.
Responder analysis
In 56 SSc patients, wearing gloves resulted in a relevant improvement of RCS (>MCID). Of these patients, 49 showed relevant improvement with both types of gloves, 3 showed relevant improvement with the normal gloves only and 4 showed relevant improvement with the silver fibre gloves only. Patients reaching improvement >MCID of the RCS with both types of gloves had a statistically significant higher prior RCS compared with patients who did not reach the MCID of the RCS (7.0 vs 4.7; P < 0.001; Supplementary Table S7, available at Rheumatology online). All other clinical variables were comparable between groups (Supplementary Table S7, available at Rheumatology online).
Discussion
This is the first multicentre, double-blind, randomized crossover trial to evaluate the efficacy of silver fibre gloves on reducing the RP burden in SSc patients. The results of this study show that wearing gloves decreases RP burden in SSc patients. No additional clinical benefit of wearing silver fibre gloves compared with normal gloves was found regarding the outcome measures we used.
Hypothetically, silver fibre gloves might show benefit by improved containment of bodily warmth [12]. Despite a lack of previous studies on the application of silver fibre gloves in RP, these gloves are recommended in clinical practice [9, 14, 15]. Clinical experience indicates that some patients who previously had insufficient benefit from normal gloves experience improvement of RP when wearing silver fibre gloves. However, none of our results provide an objective basis for recommendation of silver fibre gloves over the use of normal gloves in improving the burden of RP in SSc.
One could hypothesize that uncontrollable factors such as the weather could have influenced our findings. However, to reduce bias by interindividual differences and external factors like weather we chose to perform a crossover study. By limiting the study period to the winter months with mean temperatures <15°C (Supplementary Fig. S1, available at Rheumatology online) and patients being exposed to both types of gloves during the complete study period, we have accounted for external factors. Moreover, patient-related factors, such as the duration of wearing the gloves, were taken into account by blinding the intervention. We chose to evaluate the effect of each type of gloves for 6 weeks. As the RCS improves quickly with gloves and is relatively stable over time, future studies evaluating gloves and with RCS as the primary outcome could consider a shorter study duration (Fig. 3).
An additional point to consider is the outcome measures used. Our primary outcome was a patient-reported outcome on the burden of RP, which might not be sufficiently sensitive to identify changes in microcirculation or bodily warmth. However, the RCS is a widely used measurement in RP trials. Additionally, all evaluated secondary outcomes also did not show any effect. Finally, from a patient care perspective, it can be questioned whether subtle changes in bodily warmth that are not experienced by the patient are of clinical importance. Therefore we conclude that there is no additional benefit of 8% silver fibre gloves regarding the outcome measures we used.
Previous reports suggest an antimicrobial effect of silver coatings [11, 21], which could prevent infections in patients with vascular complications. In our study, a small difference in the frequency of vascular complications was noted: three with normal gloves and one with silver fibre gloves. In our study, we did not look at infections and this study is underpowered to determine whether there is a causal relation between vascular complications and silver fibre gloves.
In this study, 8% silver fibre gloves were used, but currently 12% silver fibre gloves are also available. We cannot rule out that we would have detected a difference between normal and 12% silver fibre gloves. Furthermore, in daily practice patients who reported additional benefit from silver gloves also often use silver fibre T-shirts and/or socks that may account for overall clinical improvement. We can only speculate that a comparison between normal warm body garments and silver fibre garments may show similar results as those now found for gloves.
We did see an improvement in the RCS just by wearing gloves. This provides evidence for previous expert opinion statements indicating gloves should be used as part of conservative measures to improve RP burden in SSc [9, 14, 15]. As our study was not designed to detect a difference between wearing gloves and not wearing gloves, we cannot claim to have scientifically proven the benefit of wearing any type of gloves. Regression to the mean has to be considered. It is noteworthy, however, that a constant decrease of the RCS was observed over all 18 different questionnaires while patients wore gloves.
Given the number of outcomes tested, the possibility of statistical significance by chance in one of the tests has to be considered. Of the five secondary endpoints tested, the HAQ showed a significant difference between the silver fibre and normal gloves. The effect size was very small: the HAQ decreased by 0.036 while wearing silver fibre gloves. This difference is not clinically relevant, as it is far smaller than the estimated MCID of the HAQ in other diseases, such as 0.22 in RA [20].
This study has some limitations. RP burden before the start of the trial was assessed based on self-reporting of the patients asked by their treating physician at the outpatient clinic, thus recall bias could have occurred. However, based on the randomized crossover design, we expect that potential recall bias affected the results with both normal and silver gloves equally. Before the start of the study, patients were asked to wear the gloves at least 10 h/day during the study period, which only 35 patients fulfilled. However, the subgroup analysis stratified for the duration of wearing the gloves did not show different results. In total, only 20 patients completed all 36 questionnaires. Still, our overall response rates were high with a median response of 94% (IQR 83–100). This is a remarkable result considering the fact that patients were asked to complete online questionnaires thrice weekly and also during the, for some patients, stressful COVID-19 pandemic. By using linear mixed models we were able to include all completed questionnaires for each patient. Finally, we did not observe a difference in the clinical characteristics of patients completing all questionnaires and patients who did not.
In conclusion, although the results of this study show that wearing gloves reduced the burden of RP secondary to SSc, no additional benefit from 8% silver fibre gloves compared with normal gloves could be demonstrated. Based on this study, there is no basis for rheumatologists to specifically recommend 8% silver fibre gloves over normal gloves to decrease the burden of RP, as measured in this study by the RCS, in SSc patients.
Supplementary Material
Acknowledgements
The authors would like to thank the included patients for participating in this study, Skafit for providing the gloves, Femke Drost-Hoekstra and Belinda Jilderda for helping with the study visits, Joze Krol-van Berkel for designing the Castor database and Rory Monahan for diligently proofreading the manuscript. The HANDS ON study was approved by the medical ethics committee of each participating centre and all patients signed informed consent. E.M.H., J.V.B., C.F.A. and T.W.J.H. were responsible for the study concept and design. E.M.H., S.I.E.L., J.V.B., F.B.M., C.M.C. and A.S. were responsible for the acquisition of data. S.I.E.L., S.A.B., J.V.B. and C.A.L. were responsible for the analysis and interpretation of data and drafting the manuscript. All authors were responsible for critically revising the manuscript and read and approved the final manuscript.
Funding: The 8% silver fibre gloves used in this study were sponsored by Skafit. The authors, not the sponsors, were responsible for the study design; the collection, analyses and interpretation of all data; writing of the article and the decision to publish.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Sophie I E Liem, Department of Rheumatology, Leiden University Medical Center, Leiden.
Eva M Hoekstra, Department of Rheumatology, Leiden University Medical Center, Leiden.
Femke Bonte-Mineur, Department of Rheumatology and Clinical Immunology, Maasstad Ziekenhuis, Rotterdam.
César Magro Checa, Department of Rheumatology, Zuyderland Medical Center, Heerlen.
Anne Schouffoer, Department of Rheumatology, Haga Ziekenhuis, The Hague, The Netherlands.
Cornelia F Allaart, Department of Rheumatology, Leiden University Medical Center, Leiden.
Tom W J Huizinga, Department of Rheumatology, Leiden University Medical Center, Leiden.
Sytske Anne Bergstra, Department of Rheumatology, Leiden University Medical Center, Leiden.
Jeska K de Vries-Bouwstra, Department of Rheumatology, Leiden University Medical Center, Leiden.
Data availability statement
Data are available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Meier FM, Frommer KW, Dinser R. et al. Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2012;71:1355–60. [DOI] [PubMed] [Google Scholar]
- 2. Hughes M, Ong VH, Anderson ME. et al. Consensus best practice pathway of the UK Scleroderma Study Group: digital vasculopathy in systemic sclerosis. Rheumatology (Oxford) 2015;54:2015–24. [DOI] [PubMed] [Google Scholar]
- 3. Cappelli L, Wigley FM.. Management of Raynaud phenomenon and digital ulcers in scleroderma. Rheum Dis Clin North Am 2015;41:419–38. [DOI] [PubMed] [Google Scholar]
- 4. Matucci-Cerinic M, Kahaleh B, Wigley FM.. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum 2013;65:1953–62. [DOI] [PubMed] [Google Scholar]
- 5. Sunderkötter C, Riemekasten G.. Pathophysiology and clinical consequences of Raynaud’s phenomenon related to systemic sclerosis. Rheumatology (Oxford) 2006;45(Suppl 3):iii33–5. [DOI] [PubMed] [Google Scholar]
- 6. Milio G, Corrado E, Genova C. et al. Iloprost treatment in patients with Raynaud’s phenomenon secondary to systemic sclerosis and the quality of life: a new therapeutic protocol. Rheumatology (Oxford) 2006;45:999–1004. [DOI] [PubMed] [Google Scholar]
- 7. Wigley FM, Flavahan NA.. Raynaud’s phenomenon. N Engl J Med 2016;375:556–65. [DOI] [PubMed] [Google Scholar]
- 8. van Leeuwen NM, Ciaffi J, Liem SIE, Huizinga TWJ, de Vries-Bouwstra JK.. Health-related quality of life in patients with systemic sclerosis: evolution over time and main determinants. Rheumatology (Oxford) 2021;60:3646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Roon AM, Abdulle AE, Zhang D, Stel AJ, Kuijpers M, (Udo) Mulder DJ.. Sympathicotomie bij medicatieresistent fenomeen van Raynaud. Ned Tijdschr Geneeskd 2021;165:D5630. [PubMed] [Google Scholar]
- 10.Z. Damen NHG-Behandelrichtlijn Fenomeen van Raynaud 2019. https://richtlijnen.nhg.org/behandelrichtlijnen/fenomeen-van-raynaud#volledige-tekst.
- 11. MacKeen PC, Person S, Warner SC, Snipes W, Stevens SE Jr.. Silver-coated nylon fiber as an antibacterial agent. Antimicrob Agents Chemother 1987;31:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu P-C, Liu X, Liu C. et al. Personal thermal management by metallic nanowire-coated textile. Nano Lett 2015;15:365–71. [DOI] [PubMed] [Google Scholar]
- 13. Jaccard Y, Singer E, Degischer S. et al. Effect of silver-threads-containing compression stockings on the cutaneous microcirculation: a double-blind, randomized cross-over study. Clin Hemorheol Microcirc 2007;36:65–73. [PubMed] [Google Scholar]
- 14. Herrick AL. Evidence-based management of Raynaud’s phenomenon. Ther Adv Musculoskelet Dis 2017;9:317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stöcker JK, Schouffoer AA, Spierings J. et al. Evidence and consensus-based recommendations for non-pharmacological treatment of fatigue, hand function loss, Raynaud’s phenomenon, and digital ulcers in patients with systemic sclerosis. Rheumatology (Oxford) 2022;61:1476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 17. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 18. Khanna PP, Maranian P, Gregory J, Khanna D.. The minimally important difference and patient acceptable symptom state for the Raynaud’s condition score in patients with Raynaud’s phenomenon in a large randomised controlled clinical trial. Ann Rheum Dis 2010;69:588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merkel PA, Herlyn K, Martin RW. et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum 2002;46:2410–20. [DOI] [PubMed] [Google Scholar]
- 20. Pope JE, Khanna D, Norrie D, Ouimet JM.. The minimally important difference for the health assessment questionnaire in rheumatoid arthritis clinical practice is smaller than in randomized controlled trials. J Rheumatol 2009;36:254–9. [DOI] [PubMed] [Google Scholar]
- 21. Bonilla-Gameros L, Chevallier P, Sarkissian A, Mantovani D.. Silver-based antibacterial strategies for healthcare-associated infections: processes, challenges, and regulations. An integrated review. Nanomedicine 2020;24:102142. [DOI] [PubMed] [Google Scholar]
- 22. Twisk JW, Rijnhart JJ, Hoekstra T. et al. Intention-to-treat analysis when only a baseline value is available. Contemp Clin Trials Commun 2020;20:100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.