Abstract
Objectives
Prior work demonstrates that co-cultured macrophages and fibroblasts from patients with SSc engage in reciprocal activation. However, the mechanism by which these cell types communicate and contribute to fibrosis and inflammation in SSc is unknown.
Methods
Fibroblasts were isolated from skin biopsies obtained from 7 SSc patients or 6 healthy age and gender-matched control subjects following written informed consent. Human donor-derived macrophages were cultured with exosomes isolated from control or SSc fibroblasts for an additional 48 h. Macrophages were immunophenotyped using flow cytometry, qRT-PCR and multiplex. For mutual activation studies, exosome-activated macrophages were co-cultured with SSc or healthy fibroblasts using Transwells.
Results
Macrophages activated with dermal fibroblast-derived exosomes from SSc patients upregulated surface expression of CD163, CD206, MHC Class II and CD16 and secreted increased levels of IL-6, IL-10, IL-12p40 and TNF compared with macrophages incubated with healthy control fibroblasts (n = 7, P < 0.05). Exosome-stimulated macrophages and SSc fibroblasts engaged in reciprocal activation, as production of collagen and fibronectin was significantly increased in SSc fibroblasts receiving signals from SSc exosome-stimulated macrophages (n = 7, P < 0.05).
Conclusion
In this work, we demonstrate for the first time that human SSc dermal fibroblasts mediate macrophage activation through exosomes. Our findings suggest that macrophages and fibroblasts engage in cross-talk in SSc skin, resulting in mutual activation, inflammation, and extracellular matrix (ECM) deposition. Collectively, these studies implicate macrophages and fibroblasts as cooperative mediators of fibrosis in SSc and suggest therapeutic targeting of both cell types may provide maximal benefit in ameliorating disease in SSc patients.
Keywords: SSc, exosomes, macrophages, fibrosis, inflammation, activation
Rheumatology key messages.
Exosomes secreted by SSc patients’ dermal fibroblasts are internalized by macrophages
SSc patient dermal fibroblast-secreted exosomes induce pro-fibrotic and inflammatory activation of macrophages
Fibroblasts and macrophages cooperatively mediate fibrosis and inflammation in SSc patients’ skin
Introduction
SSc is an autoimmune disease of unknown aetiology that affects multiple organ systems and is characterized by chronic inflammation, vasculopathy, and fibrosis. The standardized mortality ratio for SSc has not changed significantly in the past 40 years, and one in three patients dies within 10 years of disease diagnosis [1]. The development of novel treatment strategies has been hampered by an incomplete understanding of the causative molecular mechanism of pathogenesis.
Recent work suggests that aberrant fibroblast and immune activation mediated by macrophages results in fibrosis and inflammation that characterize SSc. In prior work, we demonstrated that macrophages from SSc patients produce elevated levels of pro-fibrotic cytokines compared with healthy age and gender-matched controls, and that co-cultured SSc-differentiated macrophages and SSc fibroblasts engage in reciprocal activation [2]. In support of a role for macrophages in SSc pathogenesis, global skin transcriptomic analysis of skin biopsies from patients with early diffuse cutaneous SSc (dcSSc) demonstrated upregulation of macrophage signatures that strongly correlated with higher modified Rodnan skin scores (mRSS) compared with healthy donors [3]. Increased expression of macrophage gene signatures is also observed in lung biopsies from SSc associated pulmonary arterial hypertension (SSc PAH) patients [4, 5]. However, little is known about the mechanisms that regulate macrophage and fibroblast interactions in SSc.
Intercellular communication mediated by extracellular vesicles has been implicated in the pathogenesis of many diseases, including rheumatological maladies and cancer [6]. Exosomes are secreted extracellular vesicles of endosomal origin that carry genetic material, proteins, lipids, and metabolites [7]. Notably, increased numbers of exosomes have been detected in SSc patient skin biopsies compared with healthy control skin [8, 9], and higher numbers of exosomes are secreted by monocytes, T cells, platelets, and endothelial cells in SSc patients [10].
We hypothesize that sustained paracrine activation between SSc macrophages and fibroblasts results in chronic inflammation and fibrosis. In our model, an inciting tissue injury induces monocyte mobilization and homing to skin, resulting in macrophage differentiation and activation. Activated macrophages then secrete pro-fibrotic mediators, resulting in the induction of fibroblast activation and the wound healing response. While macrophage and fibroblast activation subside as tissue injury is resolved in non-pathological conditions, we hypothesize that dermal macrophage activation in SSc is regulated and sustained by mediators released by aberrantly activated SSc fibroblasts. SSc fibroblast-activated macrophages then induce further activation of SSc fibroblasts, resulting in secretion of extracellular matrix (ECM) proteins and inflammatory mediators.
To address this hypothesis, we interrogated the ability of SSc patient-derived fibroblasts to induce human macrophage activation. We now show that stimulation of macrophages with SSc fibroblast-derived exosomes upregulated surface marker and secreted mediator expression consistent with the previously characterized SSc macrophage immunophenotype. Co-culture of SSc exosome-activated macrophages with SSc patient or healthy donor dermal fibroblasts increased expression of genes that mediate ECM formation in fibroblasts. Collectively, these results suggest macrophages and fibroblasts engage in mechanisms of mutual activation in SSc, resulting in inflammation and fibrosis.
Materials and methods
Isolation and culture of human fibroblasts
Human dermal control and SSc patient fibroblasts were isolated from clinically affected skin of 7 SSc patients (3 SSc patients with early, active disease (<3 years since diagnosis) and 4 SSc patients with late-stage disease (>3 years since diagnosis)) or 6 healthy age and gender-matched control subjects following written informed consent. Three patients had GI involvement and two were diagnosed with lung disease; organ involvement information was not available for the remaining two patients. Ethics approval was provided by the Tufts Medical Center Institutional Review Board (IRB), protocol # 12773 and by the Northwestern University Institutional Review Board (IRB), protocol # STU00080328. Patient and healthy control characteristics and patient clinical data are described in Table 1. SSc and healthy control dermal fibroblasts (SScDFs and NDFs) were isolated from skin biopsies as described [11], and used at passages 4–9. Fibroblasts were cultured with ATCC Fibroblast Basal Medium, 10 U/ml penicillin, and 10 μg/ml streptomycin supplemented with Fibroblast Growth Kit and 2% FBS (HyClone).
Table 1.
Demographic and clinical characteristics of human primary cells and plasma used in this study
| Cell Line | Age | Gender | Race | Clinical Subtype |
|---|---|---|---|---|
| Norm02 | – | – | White/non-Hispanic | Healthy |
| Norm15-4 | 23 | F | White/non-Hispanic | Healthy |
| CF7.2 | – | – | Black/African American | Healthy |
| CF8.2 | 68 | F | Asian | Healthy |
| CF11.2 | 72 | F | Black/African American | Healthy |
| CF13.2 | 62 | F | White/non-Hispanic | Healthy Healthy |
| Hama03 | 45 | F | White/non-Hispanic | Limited |
| Hama04 | 62 | F | White/non-Hispanic | Diffuse |
| SF1.2 | 51 | F | White/non-Hispanic | Diffuse |
| SF3.2 | 72 | F | White/non-hispanic | Diffuse |
| SF5.2 | 65 | F | – | Diffuse |
| SF15.2 | 54 | F | White/non-Hispanic | Diffuse |
| SF16.2 | 77 | F | White/non-Hispanic | Diffuse |
Isolation and culture of human peripheral blood monocytes
Healthy donor peripheral blood mononuclear cells (PBMCs) were obtained from whole blood following written informed consent. Ethics approval was provided by the Geisel School of Medicine Committee for the Protection of Human Subjects (protocol number 17011). Mononuclear cells were separated on Ficoll-Paque Premium (density 1.077; GE Healthcare) and enriched for CD14+ monocytes by magnetic bead selection (Miltenyi Biotec). Monocyte purity was assessed at ≥95% using cytospin, Wright-Giemsa staining, and flow analysis of CD14 expression. To generate healthy macrophages, CD14+ monocytes were cultured in complete HEPES-buffered RPMI 1640, 1% penicillin/streptomycin supplemented with 10% FBS, 0.025M HEPES, and 20 ng/ml macrophage colony-stimulating factor (M-CSF; Peprotech) for 4 days prior to activation with exosomes.
Exosome isolation and characterization
SSc patient or healthy donor control fibroblasts were cultured until cells gained ∼25% confluency. Media was then aspirated and cells were washed with serum-free media. Cultures were incubated in Fibroblast Basal Medium with Fibroblast Growth Kit (ATCC) and 2% exosome-depleted FBS (ThermoFisher). After 72 h, exosomes were purified from supernatants of confluent fibroblast cultures using the ExoQuick-TC EV isolation kit (System Biosciences). Fibroblast were activated with 5 ng/ml TGF-β for 24 h prior to exosome isolation. Suspended cells and debris were removed from supernatants by centrifugation at 3000 x g and passed through 0.22 µm filter units (Millipore Sigma). Pre-cleared supernatants (25 ml) were concentrated to 600 µl with 30K Amicon Ultra Centrifugal Filter Units (Millipore, Billerica, MA), mixed with 120 µl of ExoQuick-TC polymer, and incubated overnight at 4°C. Exosomes were precipitated by centrifugation at 1500 x g for 30 min, resuspended in 100 µl PBS, and quantified using nanoparticle tracking analysis (NTA, NanoSight NS300, Malvern Panalytical Ltd, Malvern, UK). Particle size and concentration were calculated using NanoSight NTA 3.4. software. Purity of isolated exosomes by immunoblot analysis of CD63 (System Biosciences), HSP70 (System Biosciences), CD81 (System Biosciences), and CD9 (System Biosciences). Images were acquired using Bio-Rad ChemiDoc XRS and processed using Bio-Rad Image Lab Version 6.0.1.
Exosome labelling and internalization
To monitor exosome trafficking, purified exosomes were labelled using PKH26 (Sigma-Aldrich). Briefly, exosomes were mixed with diluent C and PKH26 dye with gentle agitation for 30 s, rested at room temperature for 5 min, quenched with 10% BSA, and transferred to a polycarbonate tube. Serum-free media was added, followed by addition of 0.971M sucrose to the bottom of the tube, and centrifugation for 2 h at 190 000 × g at 4°C in a Sorvall WX80+ ultracentrifuge. Supernatants were aspirated and labelled pellets were rinsed with PBS and resuspended in RPMI 1640 media containing exosome-depleted FBS. Resuspended pellets were transferred into Amicon 10 kDa MWCO filter units and centrifuged at 3000 x g for 50 min at 4°C. Labelled exosomes were resuspended in PBS, quantified using nanoparticle tracking analysis, and stored at -20°C.
To observe exosome uptake by macrophages, CD14+ monocytes were differentiated in complete media for 4 days and seeded on coverslips coated with poly-d-lysine (ThermoFisher). Macrophages were adhered to coverslips for 24 h, and then washed and cultured in complete media containing exosome-depleted FBS and 1 × 109 PKH26- labelled exosomes for 16 h. Macrophages were washed, fixed for 15 min with 2% formaldehyde, and blocked with 2% BSA for 1 h. Cells were treated with TrueVIEW Autofluorescence Quenching Kit (Vector Laboratories) and ProLong Gold antifade Mountant with DAPI (ThermoFisher) was added before placing coverslips on slides. Images were taken using Zeiss Axio Observer Z1 and Zeiss ZEN 2012 software. Fluorescence intensity of dyes was quantified using ImageJ.
RNA extraction, cDNA synthesis, and qRT-PCR
Total RNA was obtained using the Quick RNA microprep kit (Zymo Research). Complementary DNA (cDNA) was synthesized from 60 ng total RNA and random hexamers using qScript XLT cDNA SuperMix (QuantaBio). Quantitative real-time PCR (qRT-PCR) was performed using TaqMan Probe single tube assays (ThermoFisher). The StepOnePlus Real-Time PCR System (Applied Biosystems) was used for amplification and detection. Threshold cycle number was determined using Opticon software. Thermal cycling conditions and mRNA level normalization to β-actin were performed as described [2]. Product accumulation was measured during the extension phase and samples were run in duplicate.
Multi-plex cytokine assay and ELISA
Monocytes were plated at 5 × 105 cells/well in 12-well tissue culture dishes and differentiated into macrophages in complete RPMI media with 20 ng/ml M-CSF for 4 days. Macrophages were washed with fresh serum-free media and treated with 2 × 109 healthy donor or SSc patient fibroblast-derived exosomes for an additional 48 h. Cell-free supernatants were then collected, aliquoted and stored at −80°C. Secreted protein production was assessed using a Milli-Plex suspension array system using fluorescent-dyed Luminex microspheres (beads) (EMD Millipore) or ELISA (Thermofisher) for CCL2 quantification. The fluorescence intensity for each bead was measured using a Bio-Plex array reader. Bio-Plex manager software with 5-parameter curve fitting was used for data analysis. The level of detection of each analyte was 7.8 pg/ml.
Flow cytometry
All fluorophore-conjugated antibodies were obtained from BioLegend. Cell staining was performed for 30 min at 4°C, with 2 mg/ml Globulins Cohn fraction II, III (Sigma) to reduce non-specific binding. Adherent cells were stained and doublets excluded by forward scatter A (FSC-A) vs FSC-H gating. Cells were analysed using an 8-colour MACSQuant 10 (Miltenyi Biotec) with 3 laser sources (405 nm, 488 nm, 635 nm) and FlowJo 10.7.1 software.
Co-culture experiments
Healthy monocytes (6 × 105 cells/well) were seeded alone in 12-well plates for 4 days. On day 5, monocyte-differentiated macrophages were washed and treated with 2 × 109 of either SSc patient or healthy donor control fibroblast-derived exosomes for 48 h in complete RPMI media supplemented with exosome-depleted FBS. On day 6, macrophage/fibroblast co-cultures were established by placing polyethylene terephthalate treated-Transwell inserts (Falcon) seeded with (6 × 103 cells/well) SSc patient or healthy donor control fibroblasts cultured with complete media in exosome-depleted FBS on top of macrophages. For mono-cultures, SSc or healthy dermal fibroblasts (6 × 103 cells/well) were treated with either SSc or healthy control fibroblast-derived exosomes. Fibroblasts were confluent at conclusion of the experiment on day 8. RNA was isolated from fibroblasts and macrophages for analysis of gene expression.
Statistical analysis
Figures are representative of at least three independent experiments. All experiments were repeated at least three times, unless otherwise noted, and at least 3 technical replicates of each analyte were included in each assay. Results are described as mean (s.d.) and were analysed by unpaired student’s t test or one-way ANOVA for multiple comparison (as indicated in Figure Legends) using GraphPad Prism 8. Significance was achieved at P < 0.05.
Results
Healthy donor and SSc fibroblast-derived exosomes are internalized by macrophages
Recent studies demonstrate that cargo transported by exosomes induces macrophage activation, highlighting their role as paracrine intracellular signalling mediators. Because our prior work suggested that macrophages and SSc fibroblasts engage in reciprocal activation [2], we hypothesized that exosomes secreted by dermal fibroblasts confer local activation of macrophages in SSc patient skin.
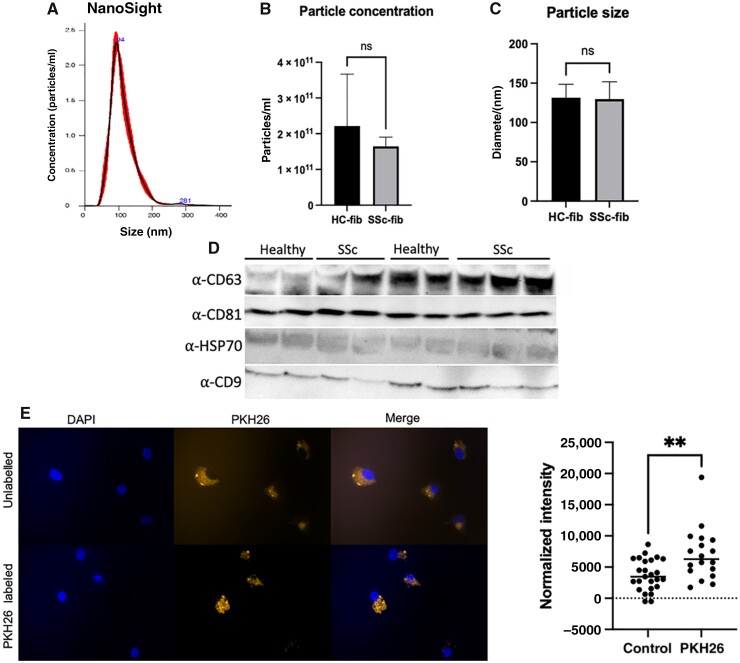
To test this, exosomes were isolated from conditioned culture media of SSc patient and healthy donor dermal fibroblasts activated with 5 ng/ml TGF-β for 24 h. Nanoparticle tracking analysis was used to quantify purified exosomes (Fig. 1A), and showed an average exosome size of 130 nm (range 112-160nm) [12]. As observed in Fig. 1B and C, there were no significant differences in either the size or concentration of exosomes secreted by SSc patient fibroblasts compared with healthy donor fibroblasts (Fig 1B and C). Immunoblot analysis demonstrated significant enrichment of canonical exosome markers, including CD9, CD63. CD81 and HSP70, confirming exosome isolation (Fig. 1D).
Fig. 1.
Exosome isolation, characterization, and internalization
(A) Nanoparticle tracking analysis (NanoSight) of particle size distribution and concentration in exosomes purified from conditioned media (CM) of human healthy control (HC-fib) and SSc patient (SSc-fib) fibroblasts. (B and C) Comparison of concentration and diameter of exosomes derived from CM of healthy control and SSc patient fibroblasts as analysed by NanoSight. (D) Representative image of immunoblot of canonical exosome proteins (CD63, CD81, HSP70 and CD9) in healthy control and SSc patient fibroblast-derived exosome lysates. Healthy human macrophages were cultured with unlabeled or PKH26-labelled exosomes isolated from fibroblast CM of healthy control (HC) or SSc patients (SSc) for 16 h. (E) Representative image depicting internalization. Imaging was performed using Zeiss Axio Observer Z1 and Zeiss ZEN 2012 (blue edition) software (left), and quantification of fluorescence intensity was measured using ImageJ (right), **P < 0.01.
To study exosome uptake, we labelled secreted fibroblast-derived exosomes with a red fluorescent cell linker, PKH26, that labels lipophilic membranes, and incubated macrophages with the labelled exosomes. Cell nuclei were stained with DAPI. As demonstrated in Fig. 1E, human macrophages increase uptake of labelled vs unlabelled exosomes over time, confirming exosome internalization.
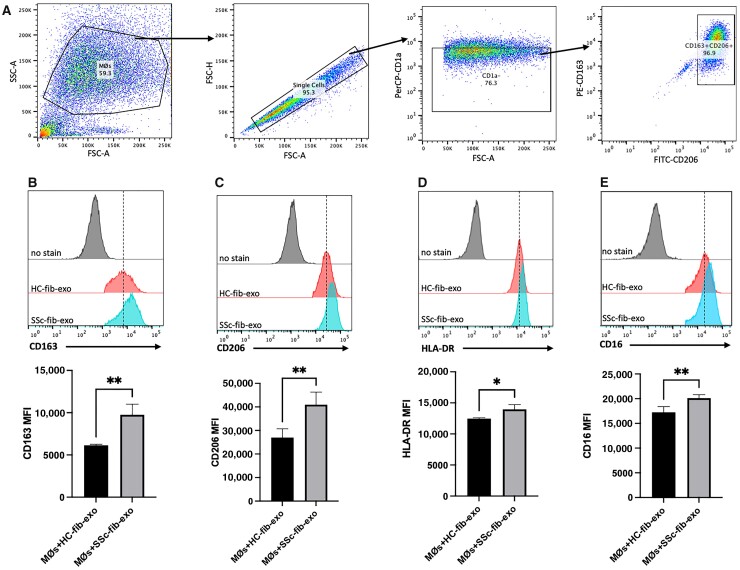
SSc fibroblast-derived exosomes induce CD163, CD206, and MHC class II surface expression on healthy macrophages
After validating macrophage uptake of fibroblast-derived exosomes, we next interrogated the effect of SSc fibroblast-derived exosomes on macrophage activation. The gating strategy is shown in Fig. 2A. Surface levels of CD163, CD206, and HLA-DR were significantly increased on macrophages activated with exosomes derived from SSc fibroblasts (Fig. 2B–D) compared with macrophages treated with healthy donor-derived fibroblast exosomes. This pattern of expression is consistent with the previously characterized human SSc macrophage surface marker profile [2]. In addition to CD163, CD206, and MHC Class II, CD16 levels were also elevated on macrophages stimulated with SSc exosomes compared with healthy control exosomes (Fig. 2E). Furthermore, surface levels of CD80 and CD64 did not vary dependent on fibroblast exosome origin (Supplementary Fig. S1, available at Rheumatology online), in accordance with our prior report [2]. These results suggest that SSc fibroblast-derived exosomes contribute to the SSc macrophage immunophenotype.
Fig. 2.
SSc fibroblast-derived exosomes upregulate surface marker expression characteristic of the SSc macrophage immunophenotype
Monocyte-derived human macrophages were stimulated for 48 h with 2 x 109 exosomes purified from CM of healthy control or SSc patient fibroblasts that had been activated with TGF-beta for 24 h prior to harvest. Following incubation, macrophages were washed, and surface marker expression was analysed using flow cytometry. (A) Flow cytometry gating strategy of CD1a-CD163+CD206+ macrophage selection. (B–E) Analysis of CD163, CD206, HLA-DR and CD16 macrophage surface marker expression. Surface levels were quantified using multi-colour flow cytometry and are presented in mean fluorescence intensity (MFI) units. *P < 0.05, **P < 0.01, macrophages treated with SSc patient fibroblast-derived exosomes vs macrophages treated with healthy donor fibroblast-derived exosomes. Data are representative of results obtained from analysis of 4 healthy donors and 5 SSc patients. Three separate experiments were performed for comparison. Error bars represent s.d.
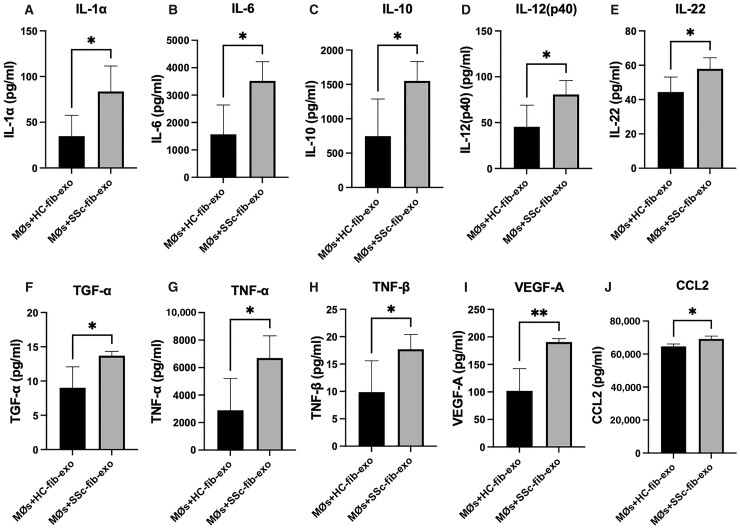
Macrophages cultured with SSc patient fibroblast-derived exosomes upregulate production of pro-inflammatory and pro-fibrotic cytokines
To assess the influence of SSc fibroblast-derived exosomes on the macrophage secretome, cell supernatants were collected from macrophages stimulated with either healthy donor or SSc patient fibroblast-derived exosomes and analysed using Luminex. As demonstrated in Fig. 3, macrophages treated with SSc-fibroblast derived exosomes increased expression of both pro- and anti-inflammatory cytokines relative to macrophages treated with exosomes from healthy fibroblasts.
Fig. 3.
Macrophages cultured with SSc patient fibroblast-derived exosomes upregulate pro-inflammatory and pro-fibrotic cytokines
Human monocytes were cultured in complete media with 20 ng/ml M-CSF for 5 days. On the 5th day, cells were washed and complete media with exosome-depleted FBS were added to macrophages prior to treatment with healthy or SSc-fibroblast-derived exosomes for 48 h. At the conclusion of activation, macrophage supernatants were collected and analysed by multiplex for secreted protein production. Release of IL-1α (A), IL-6 (B), IL-10 (C), IL-12 (p40) (D), IL-22 (E), TGF-α (F), TNF (G), TNF-β (lymphotoxin) (H), VEGF-A (I) and CCL2 (J) were increased by macrophages stimulated with SSc patient fibroblast-derived exosomes compared with healthy control fibroblast-derived exosomes. *P < 0.05, **P < 0.01; data shown are representative of results obtained from analysis of supernatant collected from macrophages stimulated with fibroblast-derived exosomes from 4 healthy donors or 5 SSc patients. Error bars represent s.d.
Known regulators of fibrosis, including IL-6, IL-10, IL-12p40, IL-22, VEGF, and CCL2 (Fig. 3B–E, I and J), were released at significantly higher levels when macrophages were stimulated with SSc patient fibroblast exosomes compared with healthy control exosomes. Similarly, inflammatory mediators including IL-1α, TNF and lymphotoxin (TNF-β) (Fig. 3A, G and H), were also upregulated, consistent with inflammation as an inducer of fibrosis.
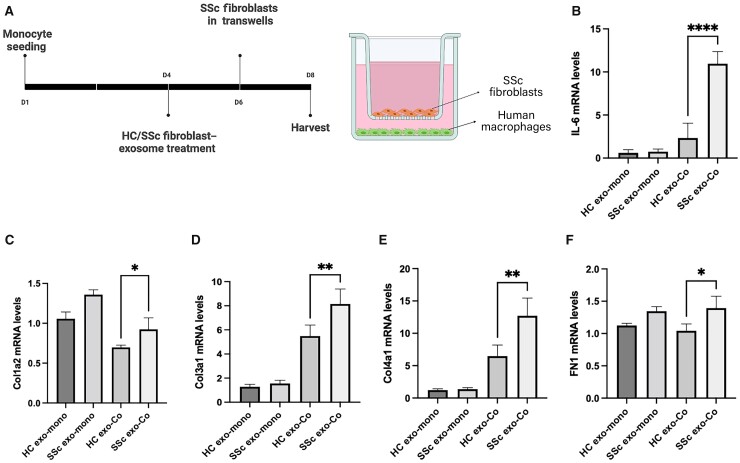
Macrophages stimulated with SSc fibroblast-derived exosomes induce SSc fibroblast activation
Because prior studies have shown that mediators released by activated macrophages drive fibrosis [13–16], we next interrogated the ability of exosome-activated macrophages to induce SSc fibroblast activation. Human monocytes were differentiated with M-CSF for 4 days, followed by activation with either healthy or SSc fibroblast-derived exosomes for an additional 2 days. Exosome-stimulated macrophages were then co-cultured with SSc fibroblasts using Transwell chambers for 2 additional days (Fig. 4A). As demonstrated in Fig. 4B–E, SSc fibroblasts co-cultured with SSc exosome-activated macrophages produced significantly increased mRNA levels of IL-6, Collagen Type I, Collagen Type III, Collagen Type IV and fibronectin compared with SSc fibroblasts cultured with macrophages activated with healthy control exosomes. Monocultured SSc fibroblasts treated with SSc fibroblast-derived exosomes in the absence of macrophages showed no significant changes in pro-fibrotic mediator expression (Fig. 4B, D–F). Furthermore, co-cultured macrophages retained the surface marker expression profile established with SSc fibroblast-derived exosome activation (compare Fig. 2vsSupplementary Fig. S2, available at Rheumatology online). These data implicate exosome-activated macrophages in SSc fibroblast activation.
Fig. 4.
SSc exosome-stimulated macrophages activate SSc fibroblasts
Monocyte-derived human macrophages were plated in the bottom chamber of Transwell plates, and were differentiated and activated with 2 × 109 healthy (HC exo) or SSc patient fibroblast-derived exosomes (SSc exo) for 48 h. At conclusion of activation, Transwells containing SSc patient fibroblasts were inserted into wells. (A) Schematic diagram of culture conditions. (B–F) Total RNA was extracted from mono- (HC/SSc exo-mono) or co-cultured (HC/SSc exo-Co) SSc-fibroblasts. Relative mRNA levels were measured using Taqman real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.0005, ****P < 0.0001; data shown are representative of results obtained from analysis of 4 healthy donors, 4 SSc patients. Two technical replicates were analysed in three separate experiments. Error bars represent s.d.
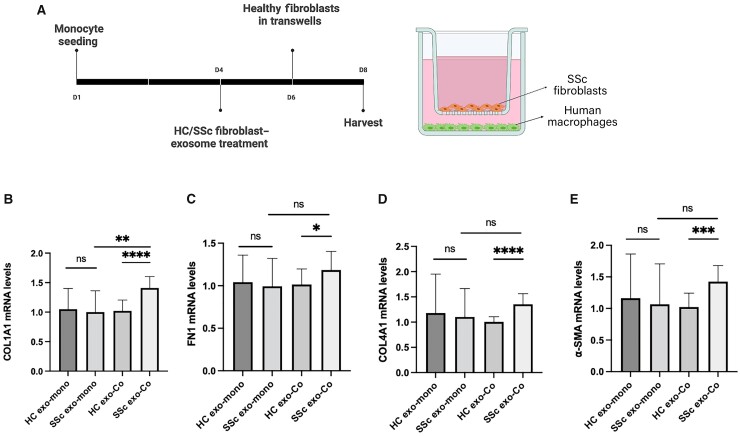
SSc phenotype is not induced in healthy fibroblasts by co-culture with SSc exosome-activated macrophages
Stimulation with antibodies, growth factors, and cytokines can contribute to activation of healthy fibroblasts [17–19]. To assess the potential contribution of SSc exosome-activated macrophages to healthy fibroblast activation, monocytes were differentiated and activated with healthy or SSc patient exosomes as in Fig. 4 and co-cultured with dermal fibroblasts from healthy donors (Fig. 5A). In contrast to observations with SSc patient fibroblasts, ECM protein expression was not induced by SSc exosomes compared with healthy exosomes, regardless of culture condition (Fig. 5B), with the notable exception of COL1A1. While COL1A4, fibronectin, and α-SMA mRNA levels were elevated in healthy fibroblasts co-cultured with macrophages activated with SSc exosomes compared with heathy exosomes, expression levels in either co-culture condition were not significantly different from monocultures of healthy fibroblasts stimulated with SSc or healthy exosomes (Fig. 5B). These results are consistent with prior observations [2] and suggest disease-specific differences in fibroblast subset representation may underlie response to macrophage activation in SSc.
Fig. 5.
SSc phenotype is not conferred to healthy fibroblasts by co-culture with SSc exosome-activated macrophages
Monocyte-derived human macrophages were plated in the bottom chamber of Transwell plates and were differentiated for 4 days in the completed media followed by activation with 2 × 109 healthy (HC exo) or SSc patient fibroblast-derived exosomes (SSc exo) for 48 h. At conclusion of activation, Transwells containing healthy fibroblasts were inserted into wells. (A) Schematic diagram of culture conditions. (B–E) Total RNA was extracted from mono- (HC/SSc exo-mono) or co-cultured (HC/SSc exo-Co) healthy fibroblasts. Relative mRNA levels were measured using TaqMan real-time PCR. *P < 0.05, ***P < 0.0005, ****P < 0.0001; data shown are representative of results obtained from analysis of 4 healthy donors, 4 SSc patients. Two technical replicates were analysed in three separate experiments. Error bars represent s.d..
Discussion
While activated macrophages play a critical role in wound healing through interaction with fibroblasts, sustained macrophage activation can result in inflammation and fibrosis [20]. Significantly, macrophages and fibroblasts are co-localized in the skin of SSc patients [21, 22]. Because macrophages derive activation from local micro-environmental cues and given the proximity of macrophages and fibroblasts in skin, we hypothesized that SSc fibroblast-derived mediators contribute to sustained pro-fibrotic activation of dermal SSc macrophages.
We previously identified a pro-fibrotic immunophenotype of human SSc macrophages consisting of elevated surface expression of CD163, CD206, HLA-DR and increased production of inflammatory mediators, including CCL2 and IL-6. We now demonstrate that exosomes derived from SSc dermal fibroblasts elicit a similar activation profile from macrophages, and show that exosomes also induce surface expression of CD16, consistent with prior reports in SSc patient monocytes [23, 24]. Notably, CD16+ myeloid cells are implicated in dermal and liver fibrosis and Crohn’s disease [24–26]. CD16, which is expressed on non-classical monocytes, is induced by IL-10 [27], which is upregulated in SSc [28]. Binding of immunoglobulin G (IgG) to CD16 induces survival signals to macrophages, leading to accumulation and differentiation of macrophages in inflamed tissue. Because IgG auto-antibodies are present in SSc patients [29, 30], increased IgG-bound CD16 may enhance retention and survival of pathological macrophages in SSc tissues.
Exosome-activated macrophages produced increased TNF, which is elevated in SSc patients and is correlated with pulmonary fibrosis and decreased vital capacity [31]. Similarly, IL-12p40 and IL-22 were also increased by SSc exosome-mediated activation. Serum IL-12p40 is positively correlated with TNF in SSc patients [32], and IL-22 enhances the pro-inflammatory effect of TNF in SSc dermal fibroblasts [33]. Coordinate regulation of these cytokines may synergistically induce inflammation and fibrotic activation. While IL-1α is upregulated in SSc patient skin-derived fibroblasts, IL-1α inhibition decreases IL-6 and collagen synthesis in SSc fibroblasts, suggesting IL-1α plays a role in the development of the SSc fibroblast phenotype [34]. Thus, macrophage-derived IL-1α may mediate SSc fibroblast activation.
Notably, SSc fibroblast-derived exosomes induced specific but not global macrophage activation. Expression of IL-1β, IL-17, IL-4, and IFN-γ [35–38], which have been implicated in the pathogenesis of SSc, was not significantly different in macrophages treated with healthy or SSc fibroblast-derived exosomes (Supplementary Fig. S3, available at Rheumatology online). This result suggests that either other cellular populations are responsible for production of these cytokines in SSc and/or that macrophages require stimulation with other factors besides/in addition to exosomes to elicit production of these mediators.
Our prior work implicated SSc patient plasma in the induction of macrophage activation, and we now demonstrate a role for dermal SSc patient fibroblast-derived exosomes. While SSc plasma and exosome-activated macrophages share many phenotypic characteristics, the activation profiles generated by these distinct mediators are not identical. One potential explanation for differences in these immunophenotypes is peripheral vs tissue activation. In our model, an inciting tissue injury induces monocyte mobilization, activation, and homing to skin, resulting in macrophage differentiation. Activated macrophages then secrete pro-fibrotic mediators, resulting in fibroblast activation and the wound healing response. Fibroblast secretion of exosomes sustains macrophage activation, and prolonged engagement of upregulated CD16, possibly by autoantibodies, results in increased persistence of macrophages in SSc patient skin. In this model, sustained paracrine activation between SSc macrophages and fibroblasts results in chronic inflammation and fibrosis.
Supporting this, we now show that SSc exosome-activated macrophages induce fibroblast activation. SSc fibroblasts co-cultured with SSc fibroblast exosome-activated macrophages produced higher levels of Type I, Type III and Type IV collagens compared with healthy controls. Collagen type 1 α2 is overexpressed in SSc lung fibroblasts; turnover of Type III collagen is associated with fibrosis in SSc and is correlated with mRSS; and turnover of Type IV collagen is associated with SSc vasculopathy [39]. We also demonstrated that fibronectin, which is overexpressed in SSc fibroblasts and mediates myofibroblast differentiation [40], is upregulated in co-cultured fibroblasts. Moreover, co-culture with exosome-activated macrophages resulted in increased IL-6 levels in SSc fibroblasts. IL-6 overexpression induces excessive collagen production by SSc fibroblasts [41], which can be attenuated by anti-IL-6 blocking antibodies [42].
Recent scRNA-seq studies of SSc patients’ skin demonstrate the presence of multiple dermal fibroblast subsets [43, 44] that may play distinct roles in SSc pathogenesis. SSc exosome-activated macrophages elicited production of many but not all ECM mediators implicated in SSc pathogenesis (Supplementary Fig. S4, available at Rheumatology online), suggesting fibroblast heterogeneity. We now show that SSc exosome-stimulated macrophages can induce activation of SSc patient but not healthy donor fibroblasts (Fig. 5). Our results are consistent with a model of SSc pathogenesis in which recruited monocytes infiltrate skin, differentiate and become activated by exosomes secreted by discrete SSc-associated fibroblast subsets, resulting in further expansion and sustained pathological fibroblast activation. While exosome-activated macrophages are not sufficient to confer an SSc phenotype to healthy fibroblasts, they do provide disease-specific activation to distinct SSc fibroblast subsets.
This study has several limitations. Patients with lcSSc and dcSSc were grouped together in our analyses (Table 1), and we were also unable to study a completely treatment-naïve population. Furthermore, this study was underpowered to further subdivide the analyses by disease duration or treatment status.
Nevertheless, these findings are the first to implicate exosomes as mediators of cross-talk between macrophages and fibroblasts in SSc, and suggest these cells engage in mechanisms of cooperative activation to induce inflammation and fibrosis. Identification of exosome cargo responsible for pro-fibrotic macrophage and fibroblast activation may provide therapeutic targets for SSc patients.
Supplementary Material
Acknowledgements
We would like to acknowledge assistance from our colleagues in the Department of Microbiology and Immunology at the Geisel School of Medicine for their invaluable advice with exosome isolation, and training with and access to the NanoSight NS300 (Dr Bruce Stanton) and for assistance with imaging and use of the Zeiss Axio Observer Z1 (Dr David Leib). Flow cytometry was carried out in DartLab, the Immune Monitoring and Flow Cytometry Resource at the Norris Cotton Cancer Center at Dartmouth with NCI Cancer Center Support Grant 5P30CA023108-37.
Funding: This work was supported by the National Institutes of Health National Institute of Allergy and Infectious Disease (1R21AI169420-01 to PAP and MLW. PAP received support from the Geisel School of Medicine at Dartmouth’s Center for Quantitative Biology from National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM130454 (MLW). The CQB Data Analytics Core performed analyses for the project under award P20GM130454. These studies also received funding from National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases Small Business Innovative Research Grants (1R43AR072170-01 and 2R44AR072170-02 to PAP, JAG, and MLW); by Established Investigator Research Grants from the Scleroderma Foundation (PAP, JAG, and MLW); and by funding from the Scleroderma Research Foundation (MLW). RB received support from the John Osborn Polak Endowment.
Disclosure statement: Patricia Pioli, Jonathan Garlick, and Michael Whitfield have received National Institutes of Health Small Business Innovative Research Grant Awards. Michael Whitfield has received consulting funding from Bristol-Myers Squibb, Boehringer Ingelheim, Corbus Pharmaceuticals, Third Rock Ventures, Celdara Medical, LLC, and UCB Biopharma in the area of SSc research. All other authors declare no competing interests.
Contributor Information
Rajan Bhandari, Department of Microbiology and Immunology.
Heetaek Yang, Department of Microbiology and Immunology; Department of Biomedical Data Science, Geisel School of Medicine at Dartmouth, Lebanon, NH.
Noelle N Kosarek, Department of Microbiology and Immunology; Department of Biomedical Data Science, Geisel School of Medicine at Dartmouth, Lebanon, NH.
Avi E Smith, Department of Diagnostic Science, Tufts University School of Dental Medicine, Boston, MA.
Jonathan A Garlick, Department of Diagnostic Science, Tufts University School of Dental Medicine, Boston, MA.
Monique Hinchcliff, Division of Rheumatology, Allergy, and Immunology, Department of Internal Medicine, Yale School of Medicine, New Haven, CT, USA.
Michael L Whitfield, Department of Biomedical Data Science, Geisel School of Medicine at Dartmouth, Lebanon, NH.
Patricia A Pioli, Department of Microbiology and Immunology.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V.. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:208–19. [DOI] [PubMed] [Google Scholar]
- 2. Bhandari R, Ball MS, Martyanov V. et al. Profibrotic activation of human macrophages in systemic sclerosis. Arthritis Rheumatol 2020;72:1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skaug B, Khanna D, Swindell WR. et al. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann Rheum Dis 2020;79:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang T, Huang C, Luo H. et al. Identification of key genes and immune profile in limited cutaneous systemic sclerosis-associated pulmonary arterial hypertension by bioinformatics analysis. Life Sci 2021;271:119151. [DOI] [PubMed] [Google Scholar]
- 5. Christmann RB, Hayes E, Pendergrass S. et al. Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum 2011;63:1718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colletti M, Galardi A, De SM. et al. Exosomes in systemic sclerosis: messengers between immune, vascular and fibrotic components? Int J Mol Sci 2019;20:4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalluri R, LeBleu VS.. The biology, function, and biomedical applications of exosomes. Science 2020;367:eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura K, Jinnin M, Fukushima S, Ihn H.. Exosome expression in the skin and sera of systemic sclerosis patients, and its possible therapeutic application against skin ulcer. J Dermatol Sci 2016;84:e97–8. [Google Scholar]
- 9. Nakamura K, Jinnin M, Harada M. et al. Altered expression of CD63 and exosomes in scleroderma dermal fibroblasts. J Dermatol Sci 2016;84:30–9. [DOI] [PubMed] [Google Scholar]
- 10. Guiducci S, Distler JHW, Jüngel A. et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum 2008;58:2845–53. [DOI] [PubMed] [Google Scholar]
- 11. Huang M, Cai G, Baugh LM. et al. Systemic sclerosis dermal fibroblasts induce cutaneous fibrosis through Lysyl oxidase-like 4: new evidence from three-dimensional skin-like tissues. Arthritis Rheumatol 2020;72:791–801. [DOI] [PubMed] [Google Scholar]
- 12. Doyle LM, Wang MZ.. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019;8:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration (Oxf) 2017;4:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song E, Ouyang N, Hörbelt M. et al. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol 2000;204:19–28. [DOI] [PubMed] [Google Scholar]
- 15. Meznarich J, Malchodi L, Helterline D. et al. Urokinase plasminogen activator induces pro-fibrotic/m2 phenotype in murine cardiac macrophages. PLoS One 2013;8:e57837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang I-H, Rose GE, Ezra DG, Bailly M.. Macrophages promote a profibrotic phenotype in orbital fibroblasts through increased hyaluronic acid production and cell contractility. Sci Rep 2019;9:9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aden N, Nuttall A, Shiwen X. et al. Epithelial cells promote fibroblast activation via IL-1alpha in systemic sclerosis. J Invest Dermatol 2010;130: 2191–200. [DOI] [PubMed] [Google Scholar]
- 18. Zhou X, Tan FK, Milewicz DM. et al. Autoantibodies to fibrillin-1 activate normal human fibroblasts in culture through the TGF-beta pathway to recapitulate the “scleroderma phenotype”. J Immunol 2005;175: 4555–60. [DOI] [PubMed] [Google Scholar]
- 19. Takamura N et al. PDGF promotes dermal fibroblast activation via a novel mechanism mediated by signaling through MCHR1. Front Immunol 2021;12:745308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carulli MT, Ong VH, Ponticos M. et al. Chemokine receptor CCR2 expression by systemic sclerosis fibroblasts: evidence for autocrine regulation of myofibroblast differentiation. Arthritis Rheum 2005;52:3772–82. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Z, Wu Y, Wu B. et al. DZ2002 ameliorates fibrosis, inflammation, and vasculopathy in experimental systemic sclerosis models. Arthritis Res Ther 2019;21:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bielecki M, Kowal K, Lapinska A. et al. Peripheral blood mononuclear cells from patients with systemic sclerosis spontaneously secrete increased amounts of vascular endothelial growth factor (VEGF) already in the early stage of the disease. Adv Med Sci 2011;56:255–63. [DOI] [PubMed] [Google Scholar]
- 23. Schneider L, Marcondes NA, Hax V. et al. Flow cytometry evaluation of CD14/CD16 monocyte subpopulations in systemic sclerosis patients: a cross sectional controlled study. Adv Rheumatol 2021;61:27. [DOI] [PubMed] [Google Scholar]
- 24. Lescoat A, Lecureur V, Roussel M. et al. CD16-positive circulating monocytes and fibrotic manifestations of systemic sclerosis. Clin Rheumatol 2017;36:1649–54. [DOI] [PubMed] [Google Scholar]
- 25. Zimmermann HW, Seidler S, Nattermann J. et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One 2010;5:e11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koch S, Kucharzik T, Heidemann J, Nusrat A, Luegering A.. Investigating the role of proinflammatory CD16+ monocytes in the pathogenesis of inflammatory bowel disease. Clin Exp Immunol 2010;161:332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang ZQ, Bapat AS, Rayanade RJ, Dagtas AS, Hoffmann MK.. Interleukin-10 induces macrophage apoptosis and expression of CD16 (FcgammaRIII) whose engagement blocks the cell death programme and facilitates differentiation. Immunology 2001;102:331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sato S, Hasegawa M, Takehara K.. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci 2001;27:140–6. [DOI] [PubMed] [Google Scholar]
- 29. Boyle JJ, Christou I, Iqbal MB. et al. Solid-phase immunoglobulins IgG and IgM activate macrophages with solid-phase IgM acting via a novel scavenger receptor a pathway. Am J Pathol 2012;181:347–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yeap WH, Wong KL, Shimasaki N. et al. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci Rep 2016;6:34310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hasegawa M, Fujimoto M, Kikuchi K, Takehara K.. Elevated serum tumor necrosis factor-alpha levels in patients with systemic sclerosis: association with pulmonary fibrosis. J Rheumatol 1997;24:663–5. [PubMed] [Google Scholar]
- 32. Beirne P, Pantelidis P, Charles P. et al. Multiplex immune serum biomarker profiling in sarcoidosis and systemic sclerosis. Eur Respir J 2009;34:1376–82. [DOI] [PubMed] [Google Scholar]
- 33. Brembilla NC, Dufour AM, Alvarez M. et al. IL-22 capacitates dermal fibroblast responses to TNF in scleroderma. Ann Rheum Dis 2016;75:1697–705. [DOI] [PubMed] [Google Scholar]
- 34. Kawaguchi Y, McCarthy SA, Watkins SC, Wright TM.. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J Rheumatol 2004;31:1946–54. [PubMed] [Google Scholar]
- 35. Dantas AT, Gonçalves SMC, Pereira MC. et al. Interferons and systemic sclerosis: correlation between interferon gamma and interferon-lambda 1 (IL-29). Autoimmunity 2015;48:429–33. [DOI] [PubMed] [Google Scholar]
- 36. Gasparini G, Cozzani E, Parodi A.. Interleukin-4 and interleukin-13 as possible therapeutic targets in systemic sclerosis. Cytokine 2020;125:154799. [DOI] [PubMed] [Google Scholar]
- 37. Goncalves RSG et al. IL-17 and related cytokines involved in systemic sclerosis: perspectives. Autoimmunity 2018;51:1–9. [DOI] [PubMed] [Google Scholar]
- 38. Xu D, Mu R, Wei X.. The roles of IL-1 family cytokines in the pathogenesis of systemic sclerosis. Front Immunol 2019;10:2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Juhl P, Bay-Jensen A-C, Hesselstrand R, Siebuhr AS, Wuttge DM.. Type III, IV, and VI collagens turnover in systemic sclerosis - a longitudinal study. Sci Rep 2020;10:7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhattacharyya S, Tamaki Z, Wang W. et al. FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci Transl Med 2014;6:232ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kawaguchi Y. Contribution of Interleukin-6 to the pathogenesis of systemic sclerosis. J Sclerod Related Disord 2017;2:S6–S12. [Google Scholar]
- 42. Kawaguchi Y, Hara M, Wright TM.. Endogenous IL-1alpha from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest 1999;103:1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gur C, Wang S-Y, Sheban F. et al. LGR5 expressing skin fibroblasts define a major cellular hub perturbed in scleroderma. Cell 2022;185:1373–88.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabib T, Huang M, Morse N. et al. Myofibroblast transcriptome indicates SFRP2(hi) fibroblast progenitors in systemic sclerosis skin. Nat Commun 2021;12:4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.