Abstract
Previous views of reactive oxygen species (ROS) depicted them as harmful byproducts of metabolism as uncontrolled levels of ROS can lead to DNA damage and cell death. However, recent studies have shed light into the key role of ROS in the self-renewal or differentiation of the stem cell. The interplay between ROS levels, metabolism, and the downstream redox signaling pathways influence stem cell fate. In this review we will define ROS, explain how they are generated, and how ROS signaling can influence transcription factors, first and foremost forkhead box-O transcription factors, that shape not only the cellular redox state, but also stem cell fate. Now that studies have illustrated the importance of redox homeostasis and the role of redox signaling, understanding the mechanisms behind this interplay will further shed light into stem cell biology.
Keywords: Pluripotent stem cell, Adult stem cell, Oxidative stress, Forkhead box O, Stemness, Reactive oxygen species
1. Introduction
Stem cells are essentially the raw materials of the body as they engender differentiated cells that are the building blocks of tissues and organs [1]. These unspecialized cells are present in embryonic, fetal, and adult phases of mammalian development. Interestingly, all stem cells do not behave in the same manner. Pluripotent stem cells (PSCs) can produce cell types from each of the three embryonic germ layers: the endoderm, mesoderm, and ectoderm [2–4]. The most prominently used PSCs in research are the embryonic stem cells (ESCs) that are derived from the inner cell mass of the mammalian blastocyst. The newest addition to the PSC pool are the induced pluripotent stem cells (iPSCs). iPSCs are not derived from the embryo, but mimic ESCs in many properties [5–7]. Instead, these cells are in vitro engineered by ‘reprogramming’ adult mouse fibroblasts, which represent a fully differentiated cell, to a pluripotent state. This is achieved through co-expression of four genes: Oct-3/4, Sox-2, c-Myc and Kfl4 under ESC culture conditions [5]. Research illustrated that iPSCs were synonymous to ESCs in their morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell markers, ability to differentiate into cells of the three germ layers, and telomerase activity [5,6].
PSCs differ from adult stem cells (ASCs) in that the latter can only differentiate into tissue-specific cells and are derived from adult tissues instead of the embryo but are not able to self-renew indefinitely. However, they are present throughout the life of the organism and produce committed progenitors that promote several functions of terminally differentiated cells thereby exhibiting the capacity to replace dying cells and restore damaged tissues.
No matter ASCs or PSCs, three defining features make stem cells inimitable. These are: (1) their ability to self-renew - a special kind of proliferation, which allows them to regenerate the stem cell pool, (2) their clonality – the fact that all progeny is ascending from a single cell, (3) and their potency – the ability to specialize into different cell types. The balance between self-renewal and differentiation remains a key area of study needed to fully understand the role of stem cells in development, disease, and medicine. This is invigorating because the regenerative nature of stem cells has been tightly linked to reactive oxygen species (ROS) (see Fig. 1) as shall be discussed here in depth.
Fig. 1. ROS levels govern stemness and differentiation.
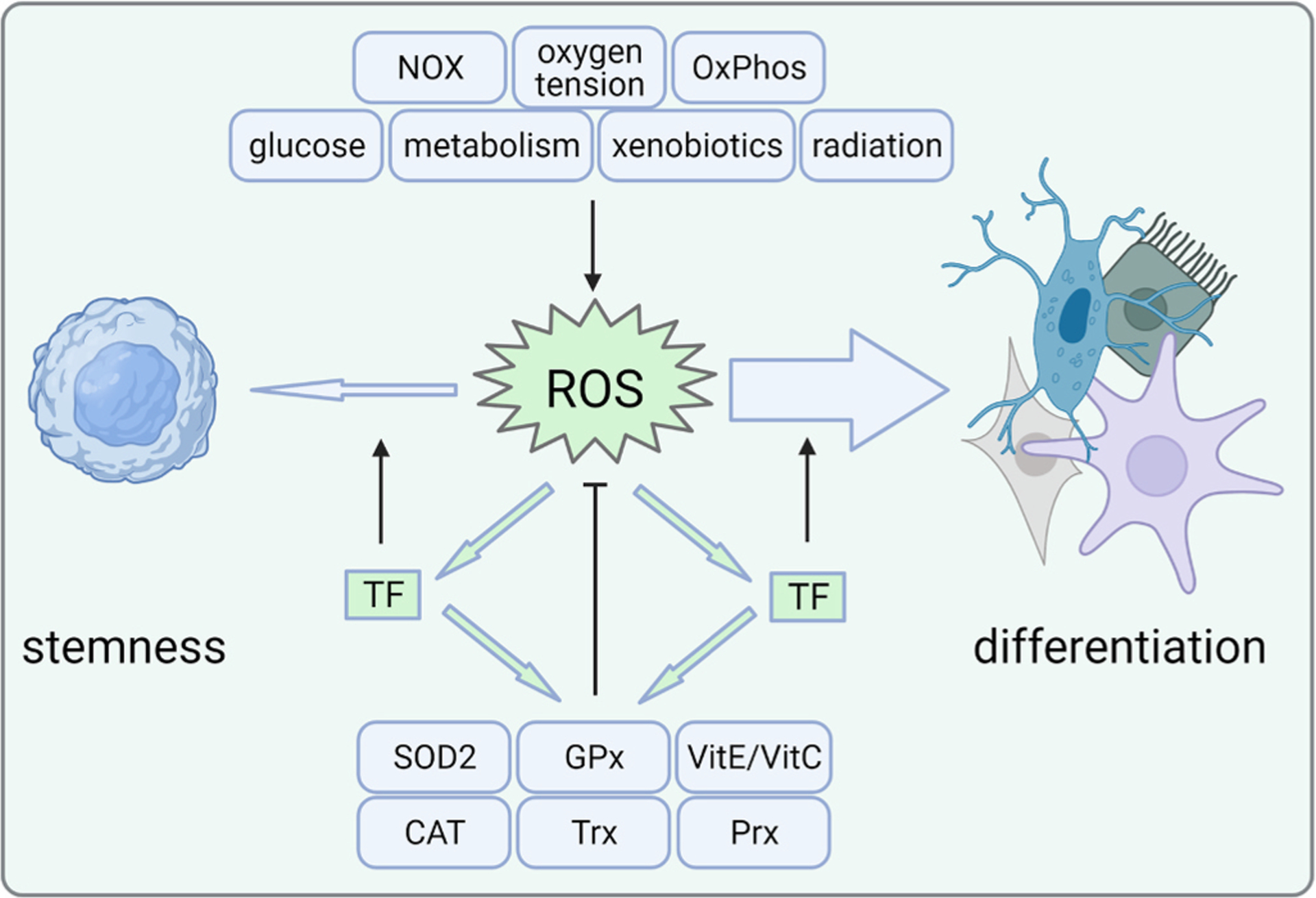
ROS can be generated by several endogenous processes or external insults. In a healthy steady-state, stem cells seem to possess numerous antioxidant mechanisms that balance their redox state including enzymes that act as redox sensors and non-enzymatic antioxidants. When there is an excess production of ROS relative to the cellular antioxidant defense, differentiation is initiated, possibly mediated by redox-sensitive transcription factors. These in turn may act to increase the cellular defense against oxidative stress, however may also modulate the expression of stemness-associated genes. CAT, catalase; GPx, glutathione peroxidase; NOX, NADPH oxidase; OxPhos, oxidative phosphorylation; Prx, periredoxin; ROS, reactive oxygen species; SOD, superoxide dismutase; TF, transcription factor; Trx, thioredoxin; Vit, vitamin. Created with BioRender.com.
2. Molecular control of stemness and differentiation induction
In the body, ASCs reside in so-called niches, in which cell-matrix and cell-cell adhesion factors (i.e. integrins), soluble signals and the orientation of the mitotic spindle determine their stemness state. Quiescent for most of their lifetime, ASCs re-enter the cell cycle in response to a variety of growth factors, chiefly among them WNT signaling [8–11].
However, unlike PSCs that have high cell cycle activity, ASCs are mainly in a quiescent state and spend most of their lifetime removed from the cell cycle in the G0 phase. Quiescence is achieved by high activation of prominent cyclin dependent kinase inhibitors (CKIs), such as p21Cip1, p27Kip1, and p57Kip2, which inhibit the binding of cyclin-dependent kinases to cyclins to block cell cycle progression. Deletion of any of these CKIs in hematopoietic stem cells (HSCs) initially leads to the expected impairment of self-renewal, a loss of quiescence, and high proliferation levels [12–14]. Long-term effects of CKI ablation, however, led to premature stem cell exhaustion [15]. Also, retinoblastoma (Rb) proteins are mostly inactive and as a result, cell division genes are primarily “off”. Cells generated during the first few divisions after exit from the niche act as transit amplifying cells, which have a significant proliferative ability that is utilized to generate the many daughter cells needed for tissue homeostasis or repair. In culture and in the body, ASCs exhibit a limited lifespan and senesce after a pre-determined number of divisions, a state that is molecularly characterized by even higher levels of p21cip1 and p53, accumulation of actin stress fibers and loss of differentiation potential [16].
Pluripotent cells, in turn, are found around the peri-implantation period and represent a transitory state only as their natural tendency in the embryo is to differentiate. In the lab, however, these cells exhibit an unsurpassed self-renewal potential coupled with an ability to differentiate into more than 200 different cell fates. These properties rest on a specialized cell cycle characterized by a shortened G1/S transition, continuously phosphorylated Rb and coordination of gene expression by a triad of transcription factors. Together, this triad composed of NANOG, OCT-4, and SOX-2 co-occupies many chromatin loci and is repressive on differentiation-associated loci and activating on pluripotency associated loci [17–19]. Thus, it is not surprising that the levels of these transcription factors control the exit from pluripotency.
For cells to preserve their pluripotency, they must self-renew not only through extensive proliferation, but also through the inhibition of differentiation [20]. In the culture dish, this is achieved through the addition of Leukemia Inhibitory Factor (LIF) [21,22], a cytokine belonging to the interleukin-6 family that binds to a heterodimeric receptor that is comprised of a LIF-receptor subunit and a glycoprotein 130 (gp130) subunit. Binding of LIF initiates a signal cascade that starts with activation of janus kinase (JAK) and ends with the dimerization of phosphorylated STAT3, which translocates to the nucleus to direct the regulation of a variety of genes required for pluripotency [23,24], making STAT3 a crucial and necessary component of the pluripotency machinery.
Unfortunately, an ‘unwanted’ LIF effect results from the activation of secondary kinase cascades down-stream of the gp130 subunit, which lead to the ultimate activation of extracellular signal-regulated kinase 1/2 (ERK1/2). This secondary pathway is linked to exit from pluripotency [25]. Thus, a dual activation of two separate arms of the LIF pathway marries the desired pluripotency maintenance effect to the inapt initiation of differentiation [24], which seems to explain the sometimes not negligible portion of murine ESCs that prematurely differentiate when cultured with LIF. Additional signaling molecules have since been identified that shift the balance to pluripotency including BMPs and WNTs [26–31]. Identification of a proper batch of serum that contains the correct levels of such additional growth factors helps with keeping murine ESCs in an undifferentiated state.
Also downstream of the LIF receptor is the activation of protein kinase B (AKT), an important survival kinase that participates in active cellular proliferation, an integral aspect of self-renewal. ESCs expressing a myristoylated, active form of AKT maintain pluripotency even in the absence of LIF [30]. While published studies generally investigate AKT without distinguishing between individual isoforms, there is an indication that not all AKT isoforms are equally involved. During iPSC generation, enhancing AKT1 and AKT2 promoted complete reprogramming mainly through increased activation of STAT3 in concert with LIF, and to a lesser extent, through promotion of colony formation. However, blocking AKT1 but not AKT2 expression prohibited cell proliferation and reprogramming [32].
Interestingly, once human ESCs (hESCs) had been derived, it became evident that there were differences between hESCs and murine ESCs (mESCs). At first, these differences were thought to be due to species-specific variation. However, it soon became apparent that human ESCs did not maintain pluripotency in response to LIF [4,33]. Instead, they self-renewed upon addition of basic fibroblast growth factor (bFGF) and TGFβ/Activin A [34–37], culture additives typically used to culture murine epiblast stem cells (EpiSCs) [38,39]. Hence, the hypothesis formed that hESCs rather resembled a post-implantation pluripotent or epiblast-like state, which is nowadays referred to as “primed” pluripotency. On the other hand, mESCs derived from the ICM are referred to as “naïve” [40,41].
Transitory in the embryo, this naïve state can be propagated by combining LIF supplementation with an ERK inhibitor in the absence of serum in culture. While blocking the differentiation into the neuroectodermal lineage [42], ERK inhibition is not free from infelicitous side effects. Since ERK typically activates the ribosomal S6 kinase RSK which in turn catalyzes the phosphorylation and inactivation of the Bcl-2 pro-apoptotic family member protein BAD [43,44], ERK suppression consequently diminishes cell division. The result of ERK inhibition thus is a pluripotent cell that divides slowly. To resolve this issue a second kinase inhibitor is included in the so-called 2i + LIF medium [41], which targets glycogen-synthase kinase 3 beta (GSK-3β) to stabilize beta-catenin [45]. The beta-catenin target cyclin D1 [46,47] then ultimately moves the cell cycle forward. Subsequent work revealed that both murine EpiSCs and hESCs can be reprogrammed to revert into a more naïve state.
Studies pertaining to stem cell pluripotency and differentiation have mainly focused on gene networks and transcription factors, even though it is becoming more apparent that ROS in synchrony with metabolic pathways are also key regulators of stem cell identity to the effect that they not only control the switch between pluripotency and differentiation, but also the transition between naïve and primed states.
3. What are reactive oxygen species?
ROS are a group of highly reactive unstable oxygen containing molecules that readily react with other molecules in the cell. ROS can be demarcated into two different classes including nonradical and free radical ROS, the first class consisting of hydrogen peroxide (H2O2), ozone, peroxynitrite (ONOO-), and hydroxide and the latter of the prevalent superoxide anion (O2 •−), nitric oxide (NO), and HO [48]. In normal physiological conditions, the cell produces healthy levels of ROS. However, unregulated high levels of intracellular ROS can be detrimental and increase oxidative stress [49], a state in which the balance between free radicals, ROS, and endogenous antioxidant defense mechanisms is interrupted [50]. In simplistic terms, it is the disturbance in the equilibrium between oxidant-antioxidant states in which the oxidant condition is favored. Excessive ROS may oxidize nucleic and amino acids, carbohydrates, proteins, and lipids leading to damage to DNA, RNA, and proteins and ultimately senescence or apoptosis [51]. Therefore, the prevailing thought has been that ROS were detrimental as they played a vast role in stress and diseases [52].
As of late, however, the balance amongst oxidant and antioxidant species has been suggested to be beneficial to several cellular processes such as gene expression, protein translation, cellular signaling, and coordinating cellular activity in response to nutrients [53–55]. In stem cells, it is becoming more apparent that ROS homeostasis and changes in the redox state influence self-renewal and cell fate. Many studies on this topic suggest that the amount of ROS present will determine the cellular destiny. For instance, low levels of intracellular ROS seem beneficial to perpetuate stemness and self-renewal. As such, low NO concentrations seem to increase expression of pluripotent markers Nanog, Oct4, and Sox 2 and maintain stemness [56]. Mechanistically, this impediment to differentiation seemed dependent on activation of beta-catenin and PI3K/AKT, which is additionally consistent with the reinforcement of proliferation and self-renewal executed by these molecules.
Conversely, when provided with high concentrations of ROS, differentiation of hESCs into bi-potent mesendodermal cell lineages is promoted [57]. Similarly, high levels of NO induce differentiation as evident through the detection of early differentiation markers and repression of Nanog [58,59]. Indeed, as ESCs exit from pluripotency naturally they sharply increase the levels of endogenously generated ROS [60,61], a process that favors the generation of cardiomyocytes and osteoblasts [61–64]. Since this increase in ROS is accompanied by the expression of the pan-mesodermal T-Brachyury and supplementation with an NO donor can enhance T-Bra mRNA as well as the number of T-BRA positive cells [61], it is quite possible that NO augmentation shortly after pluripotency exit can support the generation of other mesodermally-derived cell types. While a concomitant up-regulation of beta-catenin nuclear activity is noted in this scenario also, this time it is elicited by high NO concentrations not low concentrations as in undifferentiated pluripotent cells. This evokes the notion of a reversed mechanistic response to ROS in pluripotent versus differentiating cells.
In point of fact, ROS and NO are implicated also in later differentiation events. For instance, increased levels of ROS augmented the expression of neuronal differentiation markers in NT2 cells, a pluripotent human embryonal carcinoma cell line, and human adipose tissue-derived multipotent stem cells demonstrating how increased levels of ROS may push differentiation towards a neural phenotype [65,66]. Our own group was able to demonstrate how exogenous NO augmented calcification when supplemented at the apt time during osteogenic maturation [64].
While it is becoming increasingly clear that ROS affect self-renewal as well as differentiation capacity of stem cells, the molecular underpinnings of such phenotypic responses remain under investigation. Understanding how oxidative stress affects numerous stem cells is imperative not only to better control stem cells in a dish, but also since oxidative dysregulation is attributed to disease states resulting from stem cells dysfunction, such as neurodegenerative, cardiovascular, and inflammatory diseases and cancer [67–72]. To further shed light on the molecules and pathways potentially implicated, we will next review sources of ROS and how cells typically balance their oxidant with their antioxidant capacity.
4. Energy metabolism as a source of ROS
Three major sources of ROS production come from the endoplasmic reticulum, the membrane-bound NADPH oxidase, and the mitochondria electron-transport chain, whereby the latter represent the principal source of ROS production amounting to approximately 90% of all intracellular ROS [55,73]. Hypoxanthine/xanthine oxidase, lipoxygenase and cyclooxygenase and gamma-glutamyl transpeptidase epitomize further sources of ROS [60]. For a breakdown of intracellular locations of antioxidant systems and details of chemical reactions the reader is referred to a review by Kurutas [74].
Given that the majority of ROS are generated during mitochondrial aerobic energy production, it comes to no surprise that the preferential utilization of glycolysis over mitochondrial oxidative metabolism by stem cells may represent a mechanism to preserve genomic integrity by limiting ROS-mediated damage of nuclear and mitochondrial DNA as well as reducing oxidation of proteins and lipids [75]. In addition, PSCs are known to have immature mitochondria with lower levels of mtDNA and mitochondrial mass [76] again supporting the notion that the metabolic preference of the pluripotent stem cell is to glycolysis. It needs to be pointed out that glycolysis occurs in the presence of oxygen and is followed by lactate production, which prevents glucose from entering the TCA cycle [77,78]. Shared with cancer cells, this is known as the ‘Warburg effect’ and allows for the support of ATP generation without accumulation of high ROS levels supporting rapid cell growth and preventing differentiation (reviewed in Ref. [79]).
As pluripotent cells begin to differentiate, both in the embryo and in vitro, the mitochondria mature, increase in numbers, and a metabolic switch from glycolysis to oxidative phosphorylation (OxPhos) occurs [80–83]. Consequently, all somatic cells rely more heavily on OxPhos. Should these fully differentiated cells accumulate oxidative damage, the repercussions are insignificant as they have a limited lifespan and are shed periodically to be replaced by new cells.
During the conversion of somatic cells to iPSCs the reverse switch is observed and with interesting timing: the up-regulation of glycolysis precedes the activation of pluripotency markers [84–86]. While Shyh-Chang and colleagues [86] speculate that the timing of glycolysis reactivation suggests that glycolysis may not be specific to a pluripotent but rather to a rapidly proliferating cell, it is also possible that glycolysis is specifically selected as it may control pluripotency through mandating low ROS levels. This possibility seems pertinent given that ROS governs multiple transcription factors that not only play a role in ROS removal but have also recently been implicated in the governance of pluripotency.
Intriguingly however, not all pluripotent cells exhibit the same metabolic pattern. As such, the naïve ground-state pluripotency state distinguishes itself from the primed state through an alternate metabolic phenotype: metabolic flux in naïve ESCs is bivalent with both glycolysis and OxPhos being utilized for ATP generation, and mitochondrial respiration is reduced in primed pluripotency [87–89], as has also been proposed to occur in utero [88]. This may potentially explain the genomic instability often associated with the naïve state and the inability to stably culture naïve cells for prolonged periods of time [90].
Although ASCs represent a more committed population than PSCs, they also rely primarily on glycolysis. This state contributes to the protection of the cells from oxidative stress damage to promote lifelong renewal. For example, the most characterized ASC, hematopoietic stem cells, have low numbers of mitochondria, lower levels of oxygen consumption and intracellular ATP levels [91]. Specifically, as a variety of ASCs reside in hypoxic niches, the reliance on anaerobic glycolysis rather than aerobic glycolysis to generate ATP is commonly seen [91–93]. Similar to the benefits of the PSC reliance on aerobic glycolysis is the minimal production of ROS needed to maintain ASCs in their quiescent state [94]. A deviance from the norm are the more recently identified Lgr5+ columnar base cells in the crypt of the small intestine, which in contrast to the long known stem cells at position +4, mainly use OxPhos [92,95]. Their OxPhos is fueled by pyruvate that is provided by the adjacent Paneth cells and generates mitochondrial ROS. The differential reliance on OxPhos may potentially be explained by the difference in function between position +4 intestinal stem cells and Lgr5+ CBCs, whereby the latter is rapidly dividing and participating in tissue homeostasis, and the former is highly quiescent and represents a population of ‘reserve’ stem cells that may divide only when the actively dividing CBCs are challenged, for example due to stress or injury [96–99]. It remains to be seen if such additional rapidly cycling stem cells also exist in other tissues and whether their reliance is more on OxPhos than on glycolysis.
Due to the relationship between metabolic flux and ROS formation laid out above, it is indisputable that variations to the concentration of glucose in the extracellular milieu influence stemness and differentiation. In vivo such milieu changes occur for example in diabetic patients and impact vascular and multiorgan function as well as bone micro-architecture [100–104]. Interestingly, newer studies have put forth the idea that the long-term side-effects of high blood glucose are mediated by stressed ASCs. For example, the long-term changes in the diabetic cellular microenvironment incite intrinsic dysfunction of the bone marrow stem cell niche ultimately resulting in mesenchymal stem cell (MSC) failure to mobilize and differentiate into osteoblasts [105]. This fits well with the in vitro evidence suggesting that hyperglycemia promotes MSC aging characterized by up-regulation of p53, actin stress fibers and loss of osteogenic differentiation potential [16].
In mESCs, culture in hyperglycemia elicited greater O2•−levels along with H2O2 than culture in normoglycemia, however, over time these levels declined back to baseline [106]. This may point to the efficient ability of PSCs to counteract their levels of oxidative stress. Nonetheless, the initial activation of c-jun N-terminal kinase (JNK) downstream of glucose-induced oxidative stress was sufficient to increase the expression of p21cip and p27kip and with that dampen the proliferative rate of the cells [106], despite the greater availability of nutrients.
Mechanistically, glucose stress may coax the mitochondrial electron transport chain into overproduction of O2•− due to increased diversion of pyruvate into the mitochondria (reviewed in Ref. [102] (compare Fig. 2). This scenario is often observed in differentiated cells [107], which consequently would demand the action of an antioxidant mechanism that can defeat mitochondrial ROS, for instance superoxide dismutase 2 (SOD2), also known as manganese-dependent superoxide dismutase (MnSOD). Alternatively, plasma membrane-associated NADPH oxidases have also been described to couple glucose stress and O2•− formation [108,109]. It may be possible that the latter mechanism is primarily activated in the response of mESCs to hyperglycemia as these cells make little use of OxPhos (Fig. 2). This remains speculative since not much research has been done on the topic. In point of fact, a recent paper suggests that deficiency in p38 MAPK in mESCs increases NOX2 levels which is accompanied by a rise in Fgf 5, an mRNA that associates with a primed pluripotency state [110]. However, p38 MAPK inhibition is exploited to return primed hESCs to a naïve state [111], which is either out of line with our argumentation or suggests that p38 holds a role in naïve cells that is independent of NOX expression or activity.
Fig. 2. Energy metabolism and stem cell fate.
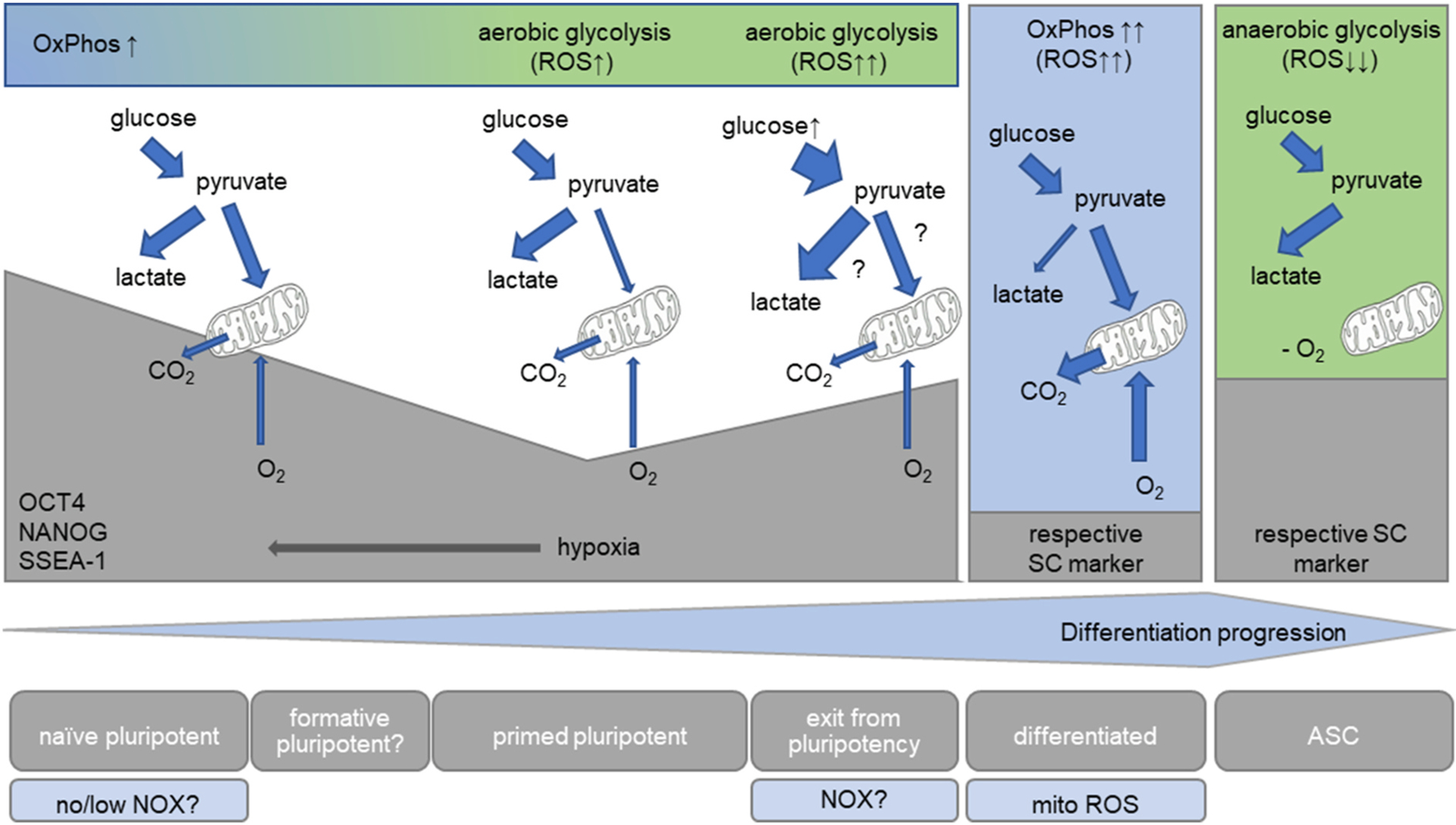
Since they are located in hypoxic niches, ASCs conduct glycolysis in the absence of oxygen, which seems to contribute to their self-renewal and potency. In contrast, differentiated cells primarily use OxPhos, a process which generates mitochondrial ROS (by means outlined in the main text), suggesting that an increase in ROS may contribute to the loss of stemness and could be necessary for differentiation. Primed PSCs, in turn, have oxygen at their disposal, but still prefer glycolysis, which, compared to differentiated cells, keeps ROS levels low. This contrasts with naïve pluripotent cells: both mitochondrial respiration and glycolysis generate ATP. Due to the lower numbers of mitochondria found in PSCs compared to differentiated cells, ROS may originate from NADPH oxidases. In serum + LIF cultured murine ESCs, which represent a mixed population of primed and naïve pluripotent cells, glucose stress increases the available pyruvate, which either may be converted into lactate or fed into the mitochondria. The glucose-induced increase in O2•− may thus either result from NOXs or might be mitochondria-mediated. Similar to ASCs, an increase in ROS seems associated with a deviation form the stem cell state. For details see the text. ASC, adult stem cell; NOX, NADPH oxidase; OxPhos, oxidative phosphorylation; ROS, reactive oxygen species; SC, stem cell.
This leaves the following question to be answered: Why are naïve cells pluripotent and differentiating cells committing to a destined cell fate if they both use OxPhos? First, as mentioned above, naïve cells generate ATP both through OxPhos and aerobic glycolysis, while differentiating cells use mostly OxPhos. Secondly, differentiating cells contain a greater number of mitochondria and thus their capacity for respiration and generation of mitochondrial ROS seems higher, again exposing a potential role for oxidative stress in differentiation.
5. Prevention of oxidative damage
5.1. Maintaining a proper niche environment
The first line of defense to prevent oxidative damage is to avert contact with harmful external stimuli that may enter the cell and wreak intracellular havoc. ASCs achieve this by living in niches characterized by low oxygen tension (1%–8% O2). This necessitates the use of anaerobic glycolysis as a mechanism for energy production resulting in the blockage of OxPhos, suppression of ROS generation, and the prevention of stress damage and differentiation [53,112–115] (for an illustration see Fig. 2). As the pre-implantation blastocyst develops under low oxygen conditions at approximating at 2–9% O2 [116], it becomes important to mimic this microenvironment also when culturing PSCs in vitro. A variety of studies illustrate the significant benefit in terms of cell proliferation using low oxygen tensions [117,118]. As such, primed hESCs appear to grow more efficiently under low oxygen concentrations when compared to room air, even long-term [119,120] (Fig. 2). Oxygen conditions equivalent to what is found in the early embryo not only help to maintain pluripotency and reduce spontaneous differentiation in ESCs [119,121,122], but also to maintain two active X chromosomes, to preserve methylation status and improve chromosome stability [123–125]. Thus, low oxygen conditions have become mainstream for naïve stem cell generation and maintenance because of their stabilizing conditions [87,111,126,127].
5.2. Cell-endogenous defense mechanisms
A point of principle then is how cells may be capable of returning to a beneficial redox status in cases where cell-internal oxidative stress cannot be prevented. To return to the appropriate homeostatic balance stem cells will recruit antioxidants, activate their redox sensors and kinases, and ultimately shuttle transcription factors involved with such changes. Antioxidants may be endogenous or consumed with the diet, but share the common feature of being readily absorbable, capable of eliminating ROS and the free radicals generated from them, as well as function well in both aqueous and lipid (i.e. membrane) domains. Generally, there are two different antioxidant systems that multicellular organisms have developed: enzymatic or non-enzymatic, which work together to protect the cells against radical damage.
Those classified as non-enzymatic mechanism include the antioxidants Vitamin E and C, thiol (glutathione, thioredoxin and lipoic acid), melatonin, carotenoids, and natural flavonoids [74] and are often the substrates for the enzymatic reactions as they have the capability to cycle between oxidized and reduced states. Pretreatment with vitamin E in MSCs leads to increased survival in oxidative stress conditions due to increased scavenging of ROS [128]. In addition, vitamin E partially rescued an adipogenic differentiation defect otherwise noted in high levels of ROS [129]. In mESCs, addition of vitamin E and glutathione ethyl ester can squelch the rise in O2•− registered in response to hyperglycemia and prevent the decline in cell proliferation [106]. The addition of ascorbic acid (vitamin C) into the normoxic culture of MSCs, which would otherwise induce senescence, promoted MSC proliferation [130]. Due to its reducing power, it is sometimes selected as culture additive specifically to mimic hypoxia or also with hypoxia, for example in naïve pluripotency media [89,111]. Not only does ascorbic acid exert a favorable effect on stemness but may be included into differentiation media: it enhances cardiomyocyte production and osteoblast yield from ESCs and iPSCs [6,131–134].
The major enzymatic mitochondrial defender against is SOD2. Situated in the mitochondrial matrix it functions to protect mitochondrial targets against produced on the cytosolic side of the inner mitochondrial membrane [135,136]. It converts the highly chemically reactive with its unpaired electron to hydrogen peroxide (H2O2) [53, 137]. Due to the high concentrations of mitochondrial superoxide dismutase, the mitochondrial levels are kept at low steady state levels and only H2O2, which can permeate the mitochondrial membrane [138], may escape to the cytoplasm.
H2O2 can assume many fates, but one can be the conversion into highly toxic hydroxyl radical through the attainment of an electron [53]. If this is not regulated in a proper homeostatic manner, this could be problematic in stem cells. Luckily, cells have adopted numerous alternative ways to ameliorate unwanted H2O2. One of them is the conversion of hydrogen peroxide to harmless water and oxygen through catalase [139]. In mESCs, attainment of redox balance after glucose-induced oxidative stress is initiated by SOD2 and catalase [106], yet is accompanied by a reduction of cells in the S-phase of the cell cycle. In this context, when nutrient availability is higher than physiological, dampened proliferation seems the preferred and healthier state over a superproliferative outcome that potentially may become uncontrolled and turn into cancer (Fig. 3). In contrast, when nutrient availability is at the appropriate steady-state level, inhibition of cell proliferation through overexpression of SOD2 and catalase [140,141] might seem contraindicated, spurring the notion that ROS are needed as “life signals” that preserve cellular division [60].
Fig. 3. Outcome of SOD and Catalase enhancement depends on cellular redox state.
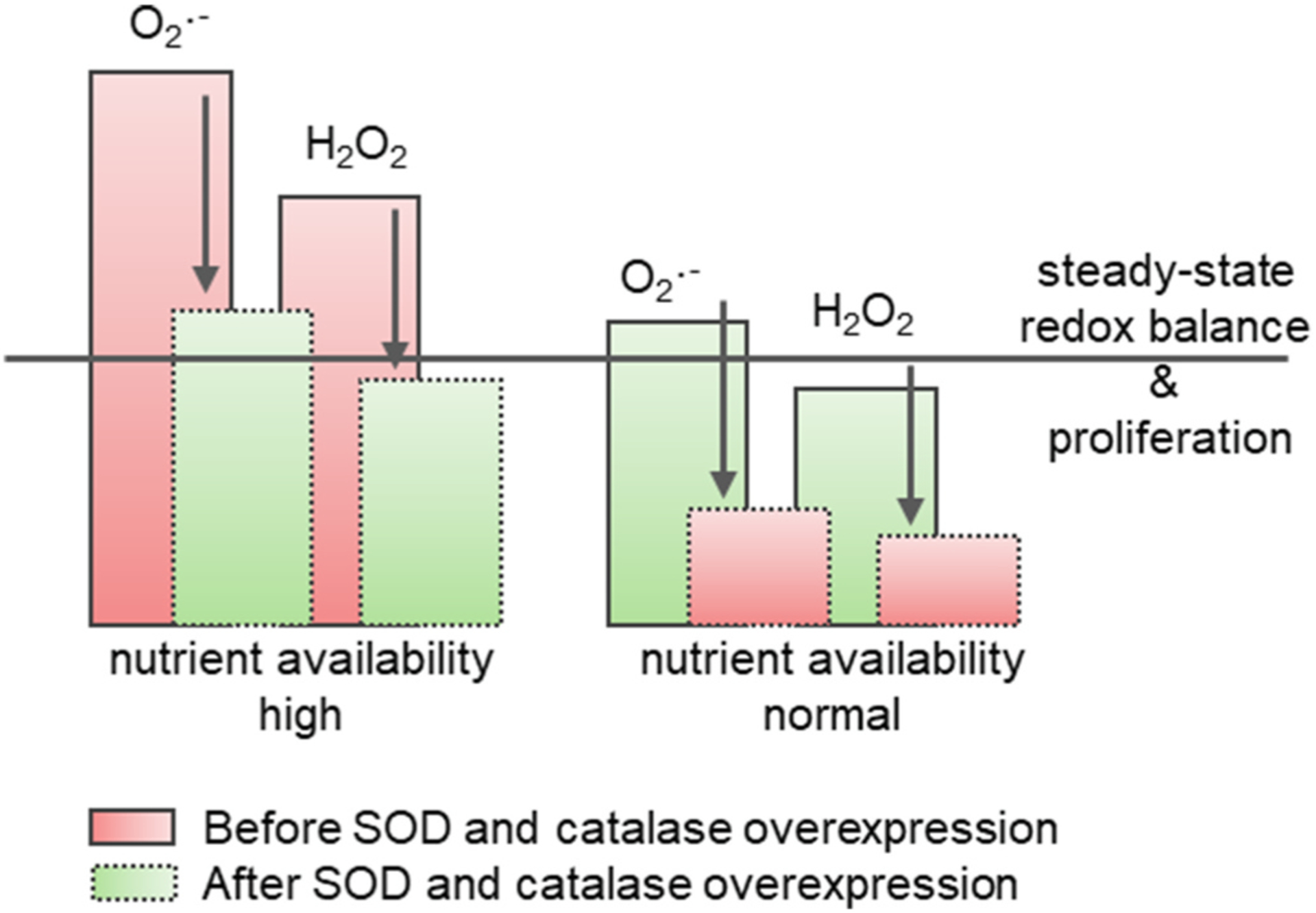
In cells with high steady-state levels of ROS, either due to a specific cellular metabolism or trigger-induced, overexpression of Sod and catalase rescues redox-balance and reduces proliferation to healthy levels. In contrast, the reduction of proliferation stemming from their overexpression in cells with healthy redox-state negatively impacts survival.
H2O2 is also reduced by the glutathione peroxidase family of proteins (GPx) to water and lipid alcohols, respectively, with participation of reduced glutathione (GSH) which has been identified as an election donor. GSH, in turn, can be regenerated from its oxidized disulfide form (GSSG) by glutathione reductase (GR) using reduced nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor. In PSCs glutathione reductase is increased throughout ESC differentiation [142]. However, it is not clear whether the increase in GR expression precedes differentiation initiation, or whether GR activation is a byproduct of the switch to OxPhos.
Given its mechanism of action, GPx classifies as a “redox sensor”, a molecule which reads ROS levels. Such sensors possess highly conserved free cysteine (Cys) residues in which the functional thiol groups (RSH) are the targets of ROS that reversibly oxidize them to disulfides. In contrast to typical Cys residues, Cys targets of reversible modification routinely possess a low-pKa sulphahydryl group which supports susceptibility to oxidation [143]. They are recycled back to their original thiol states either through enzymatic or non-enzymatic dethiolation [144,145]. Classically, the level of Cys oxidation controls protein folding, as well as multimerization. For a detailed review of disulfide bond creation and biology the reader is referred to Ref. [146].
In addition to the GPx cycle, thioredoxin (Trx) is another classic redox sensor and part of the Trx system, which is comprised of enzymatic and non-enzymatic antioxidants. Trx also acts to reduce H202 and oxidize proteins by reducing oxidized cysteines and cleaving disulfide bonds. In ESCs Trx was able to restore OCT4 DNA binding activity by reducing the cysteines in its DNA binding domain, indicating that these antioxidants play a role in maintaining stem cell pluripotency and in early embryonic development by maintaining proper OCT4 activity [147]. In addition, thioredoxin interacting protein (TXNIP) maintained HSCs and ROS levels by increasing p53 activity [148]. Further amplifying this point is that Txnip knockout mice showed a decrease in antioxidant enzyme expression elevating ROS levels and reducing HSC number.
Peroxiredoxin (Prx) can be identified as a thiol-specific antioxidant that is reduced to thioredoxin (Trx) after the reduction of peroxide forming oxidized Prx and water [149] and thus also acts as a modulator of the redox balance [150]. Prx has been linked to preservation of ESC stemness during neurogenesis by the suppression of ROS-sensitive signaling [151] again underlining the notion that increased ROS purport differentiation events. Like Prx 1 regulation in ESCs, where levels drop with differentiation, the levels of another Prx isoform, Prx6, also decrease during osteogenic differentiation of human dental pulp stem cells [150]. Whether there is an ASC-specific Prx isoform that increases with differentiation as seen with Prx 2 in ESCs or Prx6 exerts a seemingly contrasting effect in ASCs still needs to be explored.
6. Transcription factors as redox sensors
The most prevalent of the redox sensors that contain modifiable cysteines are the antioxidant enzymes discussed above [152,153]. However, and importantly for the topic of this review, transcription factors, which have the ability to set new gene expression programs in motion that alter cell fate, may also contain redox-responsive cysteines and form disulfide double bonds. In the classical understanding of redox regulation, the oxidation of these cysteines should exchange the thiols to create disulfide bonds, moderating their dissociation from DNA. However, this is not always observed in stem cells. Instead, the actual ability of the transcription factors to bind DNA seems dependent on additional regulation through phosphorylation, availability of secondary redox sensors that modulate their oxidative state, recruitment of histone modifiers and co-activators as well as overall cellular approach to generate energy. As such, H2O2 has been identified as the main ROS mediator of cellular signaling. It has the capacity to inhibit tyrosine phosphatases through oxidation of cysteine residues in their catalytic domain, which in turn activates tyrosine kinases and downstream signaling [154]. The de facto response thus is highly cell-context -specific and seems to vary between ASCs, PSCs and fully differentiated cells. As such, many transcription factors, including HIF-1α, Nrf2, STAT-3, p53, and NFκB are upregulated both by the hypoxic niche in ASCs and in response to ROS in PSCs [86,155–166]. The individual circumstances that might explain the transactivation state of these transcription factors seem to depend on their function as transcriptional repressors or activators, and the consequence of this regulation might be activation or repression of gene expression programs. To complicate matters further, these proteins cannot only be positively or negatively regulated by oxidative stress but act as redox sensors to inhibit ROS levels.
7. Governance of stemness is linked to forkhead box-O transcription factors
Transcription factors of the Forkhead box-O (FOXO) family have initially been reported to get activated in response to oxidative stress. They are the focus of this review since more recent reports also implicate them in development, differentiation, and maintenance of the stem cell state [167]. Intriguingly, their regulation through cysteine modification has not been fully elucidated for all family members, yet they participate in the resetting of the redox-state through transcriptional activation of antioxidant enzymes that lower ROS levels.
FOXO3a was the first of the FoxO family members to be implicated in the regulation of oxidative stress, followed by FOXO4 [168,169]. In humans, FOXO3a and FOXO4 have five cysteines, FOXO1 has seven, and FOXO6, identified as a neural-specific FOXO [170], has ten. Two cysteines in the four FOXOs are conserved: one is within a small, conserved region in the transactivation domain near the C-terminus. This cysteine is Cys 477 in FOXO4 which represents the main site for disulfide formation with acetyltransferase p300/cAMP-response element binding protein (CREB)-binding protein (CBP) [159,171]. The binding of these two cofactors with FOXO is necessary for downstream FOXO transcriptional regulation as p300 and CBP act to weaken histone DNA interactions for target gene expression [172]. In contrast, FOXO3a transcriptional activity is repressed by deacetylation with NAD-dependent deacetylase Sirt1 (Sirtuin 1) [173]. Of note, Sirt6 is a FOXO transcriptional target [174], representing an epigenetic feed-forward loop.
The FOXO targets important for ROS regulation are the ROS scavengers catalase, MnSOD, and Prx [106,175]. Upon irradiation or serum starvation, FOXOs can also promote expression of genes involved in DNA repair and cell cycle arrest such as DNA damage-inducible gene 45α (GADD45α), cyclin D2, and the cell cycle inhibitors p21cip1 and p27kip1, glucose metabolism (Glc6p, Pepck) and apoptosis (Bim, FasL) [106,176–181]. The decision which set of target genes to activate seems dependent on the type of insult, but also on the severity of the insult and what kind of co-factor binding is fortified. In conditions of weak oxidative stress, FOXO3a has been found to be captured by p53, suppressing FOXO3a activity and leading to low levels of pro-apoptotic genes [182]. In turn, intermolecular interaction with p53 does not seem to affect cell cycle genes cyclin G2 and p27kip1. Extensive oxidative stress disrupts the binding and restores the individual transactivation capacity of both transcription factors and - since expression of their pro-apoptotic gene targets is enhanced - seems to increase their affinity to such loci.
The other conserved cysteine, in turn, is likely implicated in regulation through AKT, since it is situated next to the threonine close to the N-terminus that is phosphorylated by AKT and part of the AKT consensus site [182]. Consequent to phosphorylation by AKT, controlled by the insulin signaling pathway and P13K, FOXOs are unbound from DNA and exported from the nucleus [175]. Additionally, phosphorylation of FOXO3a by the serum- and glucocorticoid-regulated kinase SGK [183] also causes cytoplasmic translocation [184]. Cytoplasmic FOXO proteins are then ubiquitinated and degraded in the proteasome [185]. In contrast, c-jun N-terminal kinase (JNK) phosphorylates FOXO4 at a distinct position to induce nuclear translocation [169,173]. FOXO3a also has such a potential JNK phosphorylation site in the N-terminus, which may promote nuclear translocation. In fact, our group was able to confirm that FOXO3a nuclear translocation in murine ESCs in response to glucose as shown in Fig. 4 is indeed mediated by JNK [106]. None of the other cysteines in the FOXOs are conserved between any of two family members, posing possibilities for differential regulation of FOXO family members through cysteine oxidation, which might help to explain some seemingly controversial observations in stem cells.
Fig. 4. Cellular redox state and FOXO activity status seem differentially regulated in PSCs versus ASCs.
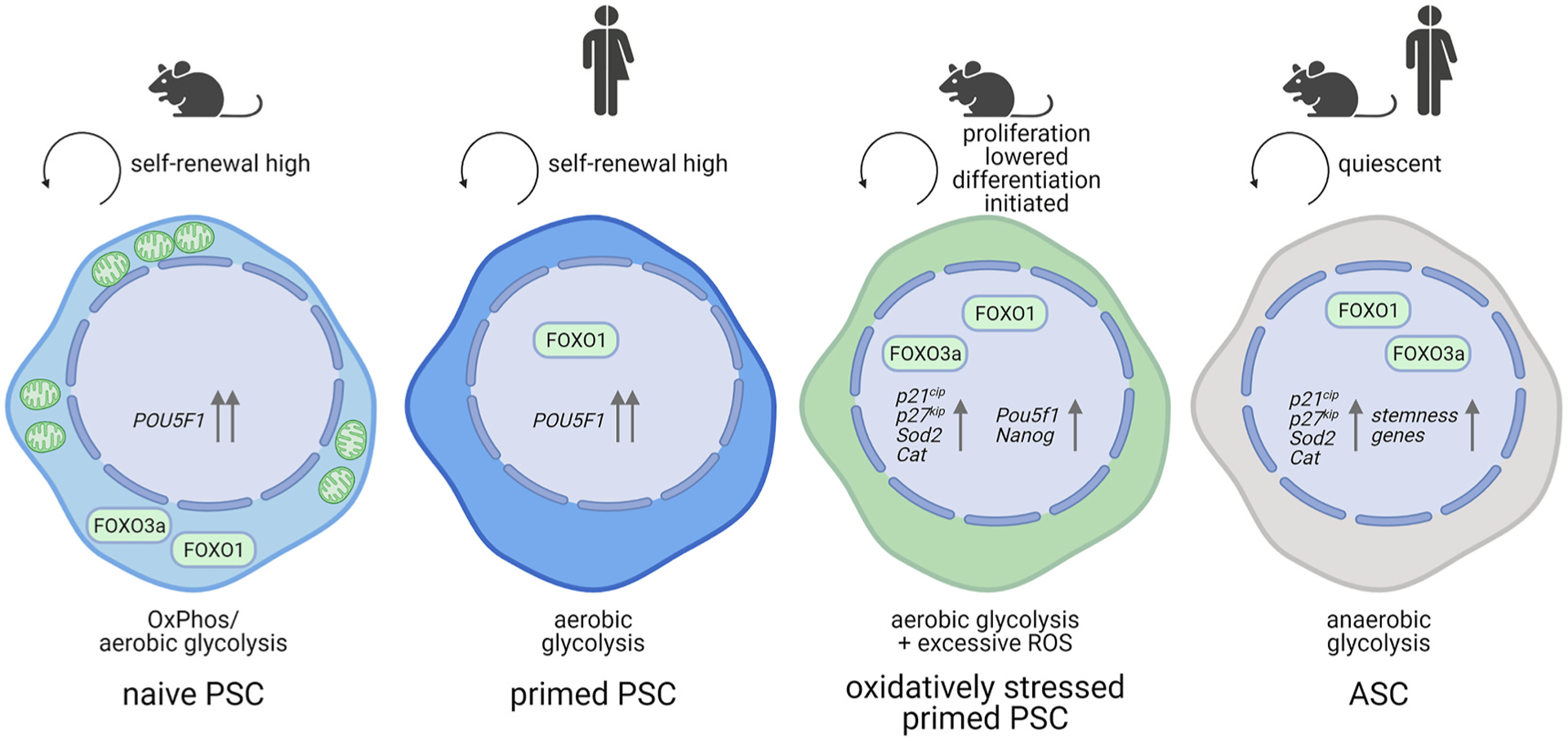
Quiescent ASCs exhibit high FOXO1 and FOXO3a activity [190,192]. In turn, FOXO3a and FOXO1 are mostly cytoplasmic in murine ESCs selected for naiveté, but both shuttle to the nucleus when oxidatively stressed [106]. In primed hESCs, however, nuclear FOXO1, not FOXO3a, was shown to transactivate the OCT4 gene, POU5F1. ASC, adult stem cell; OxPhos, oxidative phosphorylation; PSC, pluripotent stem cell. Created with BioRender.com.
Among the FOXOs, FOXO1, FOXO3a, and FOXO4a have been shown to play a role in stem cell maintenance [181], with most studies focusing on the first two. FOXO1 and FOXO3 knockdown lead to differentiation of intestinal stem cells into goblet and paneth cells [186]. Similarly, HSCs deficient in all three FOXOs display uncontrolled ROS levels caused by decreased SOD1/3 and activation of p53/p21cip1/waf1 leading to changes in HSC cell cycling, exit from quiescence, and eventually into arrest [187–189]. In neural stem cells (NSCs), FOXO1 knockdown increases neurogenic differentiation while constitutive activity inhibits differentiation [190]. Similarly, FoxO3 activity was also higher in NSCs undergoing self-renewal rather than differentiation. Additionally, ablation of FoxO3 in mutant mice led to a decrease in the NSC population. Interestingly, without FoxO3, the NSCs potentially lose capability to re-enter quiescence after they divide. This could result in an increase in progenitors and the exhaustion of the NSC pool in vivo [191]. Lastly, isolation of NSCs from FoxO3−/− mutant mice illustrated the downregulation of genes involved in oxidative stress resistance (Selenbp1), and in glucose metabolism and transport (Pdk1, Slc2a3) [191]. All these studies conclusively proof that the role of FOXOs is to maintain ASC stemness (Fig. 4).
However, the control of cell cycle genes or maintenance of the redox balance does not seem to exhaustively describe FOXO action. In an elegant study, Webb and colleagues conducted a ChiP-seq analysis in NSCs, which for the first time identified an additional cluster of FOXO3a controlled loci that demarcate a neural phenotype and confirmed the expression of the associated genes in activated NSCs and primary mouse neural progenitor cells [192]. Furthermore, binding of FOXO3 and Achaete-scute homolog 1 (ASCL1), a proneural transcription factor activated in response to Notch inhibition, was seen at enhancers of genes involving Wnt and Notch signaling, which both have roles in NSC maintenance and homeostasis [193–195]. Their results thus suggest another alternative mechanism for modulation of FOXO3a action by a secondary transcription factor. While the fact that FOXO3a shares loci with ASCL1 and inhibits expression of a group of ASCL1 neurogenic targets could be interpreted to mean that FOXO3a is repressive on those loci, the authors instead conclude that FOXO3a affects ASCL dependent transcription rather than binding to such target genes [192]. The repressive nature of FOXOs, potentially dependent on co-factors, thus remains likely, but needs to be experimentally proven.
Interestingly, FOXO1 and FOXO3a seem to behave differently from each other in PSCs, which consequently portends that one of them is oppositely regulated in PSCs than in ASCs (Fig. 4). This seems to be FOXO1. First, FOXO1 is highly expressed in undifferentiated primed H1 hESCs, while FOXO3a expression was negligible [160]. Secondly, FOXO1 knockdown did not significantly regulate proliferation or apoptosis and did not provoke changes in expression of FOXO targets involved in antioxidant or stress response. However, FOXO1 did regulate pluripotent gene expression [160]. Specifically, ChIP revealed that endogenous FOXO1 bound to sequences within the regulatory regions of OCT4 and SOX2. However, the cells were not stressed with any insult that would increase oxidation to activate FOXO, therefore the conclusion that in undifferentiated hESCs FOXOs do not function as redox regulators cannot be ultimately drawn.
Indeed, both FOXO3a and FOXO1, while expressed at the mRNA and protein level, are mostly cytoplasmic in mESCs [106] (Fig. 4). In response to glucose-induced oxidative stress, mESCs shuttle both FOXO3a and FOXO1 into the nucleus (Fig. 4). In the nucleus, FOXO3a and FOXO1 bind to the transcriptional co-activator beta-catenin (CTNNB1), which is also enriched in the nucleus upon glucose stimulation. The more abundant FOXO3a/CTNNB1 complex then stimulates p21cip1, p27kip1 and Sod2 transcription [106] explaining the ability of the cells to recuperate from oxidative stress. Our so far unpublished results further suggest that glucose-induced oxidative stress comes with a down-regulation of Oct4 and Nanog mRNA and acquisition of a more committed, potentially primed, state (unpublished observation). Thus, we speculate that in the antioxidant process nuclear FOXO3a may enhance its occupancy at pluripotency-associated promoters and contribute to their repression, but this hypothesis needs to be experimentally confirmed.
It is quite possible that the differential activity and localization of FOXOs observed in hESCs and mESCs hinges on the distinct pluripotent states these cells represent, which in turn are controlled by distinguishable redox states and divergent signals. Especially given that only FOXO3a, but not FOXO1 seems to bear cysteines within the DNA binding domain that are modulated in dependence of redox state [170], the differential contribution of either transcription factor to the glucose-dependent phenotype will also not surprise.
7.1. Upstream FOXO regulators with roles in stemness
7.1.1. AKT and JNK
AKT, as the negative regulator of FOXO, can also undergo redox modification. During oxidative stress, a disulfide bond between Cys 297 and Cys 311 can enhance dephosphorylation which will lead to loss of AKT activity [196]. Upstream of AKT is phosphatase and tensin homolog (PTEN), which - depending on the redox state of the cell - can stimulate AKT activity to negatively regulate FOXOs. The catalytic cysteines within the active site of PTEN can undergo oxidation upon exposure to ROS and this modification can potentiate AKT activity [197,198].
As mentioned earlier, JNK is the main kinase that mediates FOXO nuclear shuttling in response to ROS. JNK achieves FOXO nuclear entry through phosphorylation of FOXO3 at Ser 574 and FOXO1 at Thr447 and Thr451 [199,200]. JNK also phosphorylates the same threonines on FOXO4, which disrupts the interaction with 14–3–3 proteins allowing for FOXO nuclear entry [169,201,202]. Activation of FOXO by JNK can lead to upregulation of antioxidant genes needed to control ROS levels as well as of those involved in apoptotic signaling. Aside from FOXOs JNK can phosphorylate BCL-2 to prevent premature senescence in both tumor and normal cells [203]. In response to DNA damage, JNK phosphorylates p53 and also stabilizes p73 leading to the expression of Bax and Puma pro-apoptotic molecules [204,205].
As further piece of evidence supporting the hypothesis that FOXO3a activation would induce differentiation, JNK activity has been shown to control exit from pluripotency. However, this might entail proliferation separately from differentiation inhibition. Current evidence suggests it might be the JNK2 isoenzyme that negatively controls proliferation: Wild-type and Jnk1−/− mESCs showed similar proliferation rates whereas Jnk2−/− and Jnk1−/− Jnk2−/− mESCs proliferated more rapidly than the wild-type [206]. Indeed, this is in line with our findings that causally relate ROS to JNK activation, subsequent FOXO1/3a nuclear localization, p21cip1/p27kip1 transcriptional activation and decelerated proliferation [106]. Despite not having distinguished between different JNK isoforms, but considering the studies of our colleagues, it seems feasible to speculate then that this effect was mediated by JNK2.
The relationship between JNK activation and stem cell proliferation seems less clear in ASCs and one can find examples for positive and negative regulation. For example, JNK inhibition by JNK–IN–8, which inhibits all three isoenzymes, increased self-renewal of human HSCs [207], whereas gut specific JNK1 activation stimulated the cell cycle in intestinal stem cells and differentiation [208]. Such divergent response is not easily reconciled as both effects were moderated by c-JUN phosphorylation [207,208].
As these examples exemplify, JNK function can be through FOXO or other transcription factors, most relevant such of the AP-1 transcription factor family. Intriguingly, the selection of co-factors seems to also dictate the end outcome of JNK activation regarding stem cell differentiation: Jnk1−/− and Jnk1−/− Jnk2−/−, but not Jnk2−/− mESCs display elevated Sox 17 and Hnf1 gene expression, both of which are crucial for differentiation of definitive and visceral endoderm, respectively [206]. Likewise, when treated with JNK inhibitor II (SP600125) and JNK inhibitor III (inhibits JNK/c-Jun), hESCs displayed decreased Oct4 and Nanog expression [209], suggesting that JNK signaling has a role in maintaining the capacity to differentiate.
While this has not been experimentally investigated, JNK may further dictate stem cell state through its regulation of metabolism. Challenging HeLa cells with high concentrations of pyruvate increased the activity of JNK1, but not JNK2, via enhanced ROS production. The ROS-JNK1 axis then activated the ribosomal kinase p70S6K, which normally represses glycogen synthase kinase-3β. The result is increased activity of glycogen synthase, which converts glucose to glycogen, effectively sending the oversupply of nutrients into storage rather than into the mitochondria [210]. In cortical neurons, activation of JNK3 represses pyruvate metabolism in mitochondria and promotes pyruvate conversion to lactate in the cytosol [211]. Interestingly, JNK may also act to promote glycolysis in conditions of severe stress that require life and death decisions through the activation of phosphofructokinase-1, a key enzyme that catalyzes glycolysis, via phosphorylation of Bad (a BH3-only pro-apoptotic Bcl-2 family protein) [212]. In all noted examples glucose or glucose by-products are diverted from the mitochondria to promote glycolysis. Thus, it is conceivable that activation of JNK in PSCs could promote a shift from naïve pluripotency to primed pluripotency, since the latter cells increase their glycolytic rate over the former.
7.1.2. AMPK and mTOR complexes
Another link between energy production, stemness and FOXO activation is through AMP-dependent protein kinase (AMPK). AMPK is a key regulator of energy metabolism; its activity is regulated by a plethora of physiological conditions, many anti-diabetic drugs as well as lack of glucose availability. Under glucose starvation, with low levels of ATP, AMPK is activated to decrease anabolism and increase catabolism [213, 214]. While AMPK acts as the main sensor of AMP concentrations [2015], it can also be positively regulated by ROS levels. Exposure to H2O2 can result in the oxidation or S-glutathionylation of the cysteine residues in the α- and β-subunits of AMPK, which leads to an increase in kinase activity [216,217]. This then, based on the argumentation above, would suggest that activated AMPK would be detrimental to stemness and induce differentiation.
However, multiple examples shall be listed in support of the opposite. In HSCs, some level of AMPK is active, which can lead to FOXO activation to maintain the stem cell at low ROS levels and promote self-renewal [218,219]. Under glucose deprivation, AMPK can inhibit the activity of mammalian target of rapamycin (mTOR), which positively regulates protein synthesis [218,220], a cellular process that is in high demand as cells change their identity during differentiation [221]. The resulting decrease in protein and ribosome synthesis reduces ROS levels [220,222]. Indeed, the opposite activation of mTOR led to enhanced mitochondrial biogenesis, which together with ROS accumulation, caused a loss of HSC quiescence [218,219]. Specifically, increased ROS strengthen the interaction between mTOR and regulatory-associated protein of mTOR (Raptor), which complex together in mTOR complex 1 (mTORC1), in consequence of which ROS levels and oxidative stress increase further [218,219]. This feed-forward loop may be contributing to the reinforcement of the exit from self-renewal and preventing a reversion back to the stem cell state once differentiation has begun.
A similar phenotype is found upon deletion of TSC1 (tuberous sclerosis 1), which normally represses mTORC1 signaling. Due to increased mitochondrial biogenesis proliferation of long-term mouse HSCs increased, which eventually compromised hematopoiesis [218]. Excessive mTOR signaling has also been shown to cause adult epidermal stem cell exhaustion and progressive hair loss in mice [223]. Similarly, loss of LKB1 (serine/threonine protein kinase 11), which is upstream of AMPK in differentiated somatic cells [224], results in a domino effect in which AMPK activity, quiescence, and long-term repopulating HSC pools are lost, ultimately impairing hematopoiesis [187,225,226]. Together these examples manifest that some level of AMPK activation and inhibition of mTORC1 may benefit ASC stemness.
However, relatively little is known about whether AMPK hardwires nutrient supply, survival and differentiation in PSCs. Since mTORC1-mediated protein translation needs to be tightly controlled to prevent exit from pluripotency, one might speculate AMPK activation to be beneficial also for PSC self-renewal. Indeed, AMPK activators such as AICAR and metformin increase mRNA expression of pluripotency markers and decrease mRNA expression of differentiation markers in murine ESCs [227], an observation that we have confirmed in our laboratory (unpublished). Pursuant to the notion that AMPK activation might correlate to a less committed naïve pluripotent state, another study found that AMPK may enhance OxPhos and mitochondria biosynthesis [228,229]. However, crosstalk between metabolism, ROS, and cell fate is something that needs to be further clarified. For example, AMPK can directly and indirectly post-translationally modify histone tails to regulate the activity of histone deacetylases and histone acetyl transferases by controlling substrate availability [229], making clear that it is not only the network of redox-sensitive kinases and transcription factors that may control stem cell state in response to oxidative stress, but that additional layers of epigenetic regulation are at play that have not been considered here.
AMPK activation is often coupled with inhibition of AKT phosphorylation [230]. This inverse relationship between the two spawns from the differential activation of a secondary mTOR complex, mTOR complex 2 (mTORC2), in which mTOR associates with Rictor, and which is inversely regulated than mTORC1. In fact, down-regulation of mTORC1 induces a feedback regulation to enhance PI3K signaling, thus indirectly activating mTORC2 [231]. AMPK may therefore exert its effects on proliferation/differentiation through its inverse relationship with AKT/FOXO in an mTORC1 mediated fashion. However, this is not in line with our findings that report higher AKT activity levels in mESCs cultured in physiological glucose concentrations [106], which would suggest a positive correlation between AMPK and AKT. Indeed, such positive relationship does exist in somatic cells in conditions of energetic stress, where AMPK has been shown to associate directly with mTORC2, phosphorylating mTOR at Ser1261 and increasing activity of the complex toward AKT [232]. The hallmark of mTORC2 activation is increased phosphorylation of AKT at Ser 473. It is quite possible that PSCs lend themselves to this stress mechanism to control stemness and differentiation. The latter scenario would also explain how AMPK activation and FOXO nuclear exclusion could co-exist in PSCs, while AMPK is described to directly enhance FOXO3 transcriptional activity by recruiting CBP and p300 in other cells [167,215].
In conclusion, we would like to mention another interesting thought that may pertain to FOXO regulation and could potentially extend to all redox-sensitive transcription factors. Due to their nuclear localization and nuclear exit signals, they have the ability to shuttle in and out of the nucleus. While some are simply non-active when in the cytoplasm, others may translocate to many diverse cellular compartments and fulfill separate roles depending on their location. For example, mitochondrial p53 participates in apoptosis induction [233], while in the cytoplasm it is susceptible to degradation [234] and binds to DNA in the nucleus. The concentrations of some redox regulators however, i.e. glutathione and Trx, are greater in the nucleus than in the cytoplasm [235–237], especially in times of stress [238,239]. Entry of the redox-sensitive FOXOs into the nucleus, therefore, may enhance their reduction and therewith DNA-binding ability, ultimately fine-tuning their response to ROS. Similarly, redox-sensitive cysteine residues may directly be involved in nuclear export, as has been shown for Cys 199 in human Nrf2 [240].
8. Conclusion
It is becoming increasingly apparent that the mechanism behind stem cell renewal is not solely reliant on the nucleus but also the crosstalk between the mitochondria and the nucleus, with redox sensors, redox-sensitive transcription factors and kinases relaying the message. Therefore, connecting metabolic pathways, ROS levels, and stem cell maintenance remains an important area to study. The exact mechanism of ROS mediated stem cell renewal clearly seems oppositely regulated in adult stem cells versus pluripotent stem cells, with even additional differences between naïve and primed PSCs. Full network analyses of the possibly involved molecules introduced above, coupled with a thorough investigation of the metabolic cell state as well as the redox state of the involved proteins would help shed light on the fascinating interplay between redox homeostasis and stem cell fate. Many times, neither their complete absence nor their full-on activation seemed beneficial to stemness. What also did become clear is that the induction of differentiation, loss of stemness or alteration of proliferative capacity repeatedly seems to be an undesired byproduct of the cell’s fight against oxidative stress and its attempt to return to a balanced redox state. It will be interesting to see if some stem cells will ultimately cycle between reduced and oxidized states, in cases where the initial trigger persists, and the kinetics of the differentiation event do not outpace the redox-cycling.
Acknowledgements
We apologize to the many authors whose wonderful work we could not cite here due to space constrains. NzN is funded by an RO1 from the National Institute of Dental and Craniofacial Research, grant no RO1DE025330. None of the authors have any conflict of interest to declare.
Footnotes
Declaration of interest
None.
References
- [1].Kolios G, Moodley Y, Introduction to stem cells and regenerative medicine, Respiration 85 (2013) 3–10. [DOI] [PubMed] [Google Scholar]
- [2].Robinton DA, Daley GQ, The promise of induced pluripotent stem cells in research and therapy, Nature 481 (7381) (2012) 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Martin GR, Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells, Proc. Natl. Acad. Sci. U.S.A 78 (12) (1981) 7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM, Embryonic stem cell lines derived from human blastocysts, Science 282 (5391) (1998) 1145. [DOI] [PubMed] [Google Scholar]
- [5].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell 126 (4) (2006) 663–676. [DOI] [PubMed] [Google Scholar]
- [6].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell 131 (5) (2007) 861–872. [DOI] [PubMed] [Google Scholar]
- [7].Hochedlinger K, Plath K, Epigenetic reprogramming and induced pluripotency, Development (Camb.) 136 (4) (2009) 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H, Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4, Nat. Genet 19 (4) (1998) 379–383. [DOI] [PubMed] [Google Scholar]
- [9].Kuhnert F, Davis CR, Wang H-T, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ, Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1, Proc. Natl. Acad. Sci. U.S.A 101 (1) (2004) 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luis TC, Weerkamp F, Naber BAE, Baert MRM, de Haas EFE, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJM, Staal FJT, Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation, Blood 113 (3) (2009) 546–554. [DOI] [PubMed] [Google Scholar]
- [11].Bengoa-Vergniory N, Kypta RM, Canonical and noncanonical Wnt signaling in neural stem/progenitor cells, Cell. Mol. Life Sci 72 (21) (2015) 4157–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT, Hematopoietic stem cell quiescence maintained by p21cip 1/waf1, Science 287 (5459) (2000) 1804–1808. [DOI] [PubMed] [Google Scholar]
- [13].Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y, Nakayama K, Nakayama KI, P57 is required for quiescence and maintenance of adult hematopoietic stem cells, Cell Stem Cell 9 (3) (2011) 262–271. [DOI] [PubMed] [Google Scholar]
- [14].Zou P, Yoshihara H, Hosokawa K, Tai I, Shinmyozu K, Tsukahara F, Maru Y, Nakayama K, Nakayama KI, Suda T, P57(Kip 2) and p27(Kip 1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70, Cell Stem Cell 9 (3) (2011) 247–261. [DOI] [PubMed] [Google Scholar]
- [15].Kippin TE, Martens DJ, van der Kooy D, p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity, Gene Dev. 19 (6) (2005) 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stolzing A, Coleman N, Scutt A, Glucose-induced replicative senescence in mesenchymal stem cells, Rejuvenation Res. 9 (1) (2006) 31–35. [DOI] [PubMed] [Google Scholar]
- [17].Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S, The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells, Cell 113 (5) (2003) 631–642. [DOI] [PubMed] [Google Scholar]
- [18].Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA, Core transcriptional regulatory circuitry in human embryonic stem cells, Cell 122 (6) (2005) 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kallas A, Pook M, Trei A, Maimets T, SOX2 is regulated differently from NANOG and OCT4 in human embryonic stem cells during early differentiation initiated with sodium butyrate, Stem Cell. Int 2014 (2014) 298163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Niwa H, How is pluripotency determined and maintained? Development (Camb.) 134 (4) (2007) 635–646. [DOI] [PubMed] [Google Scholar]
- [21].Suda Y, Suzuki M, Ikawa Y, Aizawa S, Mouse embryonic stem cells exhibit indefinite proliferative potential, J. Cell. Physiol 133 (1) (1987) 197–201. [DOI] [PubMed] [Google Scholar]
- [22].Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM, Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells, Nature 336 (6200) (1988) 684–687. [DOI] [PubMed] [Google Scholar]
- [23].Niwa H, Burdon T, Chambers I, Smith A, Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3, Gene Dev. 12 (13) (1998) 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T, STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells, EMBO J. 18 (15) (1999) 4261–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huh S, Song HR, Jeong GR, Jang H, Seo NH, Lee JH, Yi JY, Lee B, Choi HW, Do JT, Kim JS, Lee SH, Jung JW, Lee T, Shim J, Han MK, Lee TH, Suppression of the ERK-SRF axis facilitates somatic cell reprogramming, Exp. Mol. Med 50 (2) (2018) e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ying QL, Nichols J, Chambers I, Smith A, BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3, Cell 115 (3) (2003) 281–292. [DOI] [PubMed] [Google Scholar]
- [27].Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH, Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor, Nat. Med 10 (1) (2004) 55–63. [DOI] [PubMed] [Google Scholar]
- [28].Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H, Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells, Biochem. Biophys. Res. Commun 343 (1) (2006) 159–166. [DOI] [PubMed] [Google Scholar]
- [29].Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A, Inhibition of glycogen synthase kinase-3 alleviates Tcf 3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation, Nat. Cell Biol 13 (7) (2011) 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T, Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells, Oncogene 25 (19) (2006) 2697–2707. [DOI] [PubMed] [Google Scholar]
- [31].Ye S, Zhang D, Cheng F, Wilson D, Mackay J, He K, Ban Q, Lv F, Huang S, Liu D, Ying QL, Wnt/β-catenin and LIF-Stat3 signaling pathways converge on Sp5 to promote mouse embryonic stem cell self-renewal, J. Cell Sci 129 (2) (2016) 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tang Y, Jiang Z, Luo Y, Zhao X, Wang L, Norris C, Tian XC, Differential effects of Akt isoforms on somatic cell reprogramming, J. Cell Sci 127 (18) (2014) 3998–4008. [DOI] [PubMed] [Google Scholar]
- [33].Dahéron L, Opitz SL, Zaehres H, Lensch MW, Andrews PW, Itskovitz-Eldor J, Daley GQ, LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells, Stem Cells (Dayton) 22 (5) (2004) 770–778. [DOI] [PubMed] [Google Scholar]
- [34].Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA, Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells, Nat. Methods 2 (3) (2005) 185–190. [DOI] [PubMed] [Google Scholar]
- [35].Vallier L, Alexander M, Pedersen RA, Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells, J. Cell Sci 118 (Pt 19) (2005) 4495–4509. [DOI] [PubMed] [Google Scholar]
- [36].Akopian V, Andrews PW, Beil S, Benvenisty N, Brehm J, Christie M, Ford A, Fox V, Gokhale PJ, Healy L, Holm F, Hovatta O, Knowles BB, Ludwig TE, McKay RDG, Miyazaki T, Nakatsuji N, Oh SKW, Pera MF, Suemori H, Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cellular & Developmental Biology, Animal 46 (3–4) (2010) 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JMC, Thomson JA, Chemically defined conditions for human iPSC derivation and culture, Nat. Methods 8 (5) (2011) 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L, Derivation of pluripotent epiblast stem cells from mammalian embryos, Nature 448 (7150) (2007) 191–195. [DOI] [PubMed] [Google Scholar]
- [39].Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG, New cell lines from mouse epiblast share defining features with human embryonic stem cells, Nature 448 (7150) (2007) 196–199. [DOI] [PubMed] [Google Scholar]
- [40].Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A, The ground state of embryonic stem cell self-renewal, Nature 453 (7194) (2008) 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nichols J, Smith A, Naive and primed pluripotent states, Cell Stem Cell 4 (6) (2009) 487–492. [DOI] [PubMed] [Google Scholar]
- [42].Yu Y, Wang X, Zhang X, Zhai Y, Lu X, Ma H, Zhu K, Zhao T, Jiao J, Zhao ZA, Li L, ERK inhibition promotes neuroectodermal precursor commitment by blocking self-renewal and primitive streak formation of the epiblast, Stem Cell Res. Ther 9 (1) (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME, Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms, Science 286 (5443) (1999) 1358–1362. [DOI] [PubMed] [Google Scholar]
- [44].Ballif BA, Blenis J, Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals, Cell Growth Differ. : Mole. Biol. J. Am. Assoc. Canc. Res 12 (8) (2001) 397–408. [PubMed] [Google Scholar]
- [45].Huelsken J, Behrens J, The Wnt signalling pathway, J. Cell Sci 115 (Pt 21) (2002) 3977–3978. [DOI] [PubMed] [Google Scholar]
- [46].Tetsu O, McCormick F, Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells, Nature 398 (6726) (1999) 422–426. [DOI] [PubMed] [Google Scholar]
- [47].Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A, The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway, Proc. Natl. Acad. Sci. U.S.A 96 (10) (1999) 5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shi X, Zhang Y, Zheng J, Pan J, Reactive oxygen species in cancer stem cells, Antioxidants Redox Signal. 16 (11) (2012) 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schieber M, Chandel NS, ROS function in redox signaling and oxidative stress, Curr. Biol 24 (10) (2014) R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dayem AA, Choi HY, Kim JH, Cho SG, Role of oxidative stress in stem, cancer, and cancer stem cells, Cancers 2 (2) (2010) 859–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Seifried HE, Anderson DE, Fisher EI, Milner JA, A review of the interaction among dietary antioxidants and reactive oxygen species, J. Nutr. Biochem 18 (9) (2007) 567–579. [DOI] [PubMed] [Google Scholar]
- [52].Ogasawara MA, Zhang H, Redox regulation and its emerging roles in stem cells and stem-like cancer cells, Antioxidants Redox Signal. 11 (5) (2009) 1107–1122. [DOI] [PubMed] [Google Scholar]
- [53].Bigarella CL, Liang R, Ghaffari S, Stem cells and the impact of ROS signaling, Development 141 (22) (2014) 4206–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Holmström KM, Finkel T, Cellular mechanisms and physiological consequences of redox-dependent signalling, Nat. Rev. Mol. Cell Biol 15 (6) (2014) 411–421. [DOI] [PubMed] [Google Scholar]
- [55].Liang R, Ghaffari S, Stem cells, redox signaling, and stem cell aging, Antioxidants Redox Signal. 20 (12) (2014) 1902–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tapia-Limonchi R, Cahuana GM, Caballano-Infantes E, Salguero-Aranda C, Beltran-Povea A, Hitos AB, Hmadcha A, Martin F, Soria B, Bedoya FJ, Tejedo JR, Nitric oxide prevents mouse embryonic stem cell differentiation through regulation of gene expression, cell signaling, and control of cell proliferation, J. Cell. Biochem 117 (9) (2016) 2078–2088. [DOI] [PubMed] [Google Scholar]
- [57].Ji AR, Ku SY, Cho MS, Kim YY, Kim YJ, Oh SK, Kim SH, Moon SY, Choi YM, Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage, Exp. Mol. Med 42 (3) (2010) 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mora-Castilla S, Tejedo JR, Hmadcha A, Cahuana GM, Martín F, Soria B, Bedoya FJ, Nitric oxide repression of Nanog promotes mouse embryonic stem cell differentiation, Cell Death Differ. 17 (6) (2010) 1025–1033. [DOI] [PubMed] [Google Scholar]
- [59].Beltran-Povea A, Caballano-Infantes E, Salguero-Aranda C, Martín F, Soria B, Bedoya FJ, Tejedo JR, Cahuana GM, Role of nitric oxide in the maintenance of pluripotency and regulation of the hypoxia response in stem cells, World J. Stem Cell 7 (3) (2015) 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sauer H, Wartenberg M, Hescheler J, Reactive oxygen species as intracellular messengers during cell growth and differentiation, Cell. Physiol. Biochem 11 (4) (2001) 173–186. [DOI] [PubMed] [Google Scholar]
- [61].Ding H, Keller KC, Martinez IK, Geransar RM, zur Nieden KO, Nishikawa SG, Rancourt DE, zur Nieden NI, NO-β-catenin crosstalk modulates primitive streak formation prior to embryonic stem cell osteogenic differentiation, J. Cell Sci 125 (Pt 22) (2012) 5564–5577. [DOI] [PubMed] [Google Scholar]
- [62].Mujoo K, Sharin VG, Bryan NS, Krumenacker JS, Sloan C, Parveen S, Nikonoff LE, Kots AY, Murad F, Role of nitric oxide signaling components in differentiation of embryonic stem cells into myocardial cells, Proc. Natl. Acad. Sci. U. S. A 105 (48) (2008) 18924–18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Padmasekar M, Sharifpanah F, Finkensieper A, Wartenberg M, Sauer H, Stimulation of cardiomyogenesis of embryonic stem cells by nitric oxide downstream of AMP-activated protein kinase and mTOR signaling pathways, Stem Cell. Dev 20 (12) (2011) 2163–2175. [DOI] [PubMed] [Google Scholar]
- [64].Ehnes DD, Geransar RM, Rancourt DE, zur Nieden NI, Exogenous nitric oxide enhances calcification in embryonic stem cell-derived osteogenic cultures, Differentiation 89 (3–4) (2015) 97–103. [DOI] [PubMed] [Google Scholar]
- [65].Hu Q, Khanna P, Ee Wong BS, Lin Heng ZS, Subhramanyam CS, Thanga LZ, Sing Tan SW, Baeg GH, Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation, Oncotarget 9 (3) (2017) 4223–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Domenis R, Bergamin N, Gianfranceschi G, Vascotto C, Romanello M, Rigo S, Vagnarelli G, Faggiani M, Parodi P, Kelley MR, Beltrami CA, Cesselli D, Tell G, Beltrami AP, The redox function of APE1 is involved in the differentiation process of stem cells toward a neuronal cell fate, PloS One 9 (2) (2014), e89232, 10.1371/journal.pone.0089232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Battin EE, Brumaghim JL, Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms, Cell Biochem. Biophys 55 (1) (2009) 1–23. [DOI] [PubMed] [Google Scholar]
- [68].Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M, Free radicals, metals and antioxidants in oxidative stress-induced cancer, Chem. Biol. Interact 160 (1) (2006) 1–40. [DOI] [PubMed] [Google Scholar]
- [69].Cadet J, Sage E, Douki T, Ultraviolet radiation-mediated damage to cellular DNA, Mutat. Res 571 (1–2) (2005) 3–17. [DOI] [PubMed] [Google Scholar]
- [70].Lloyd DR, Phillips DH, Carmichael PL, Generation of putative intrastrand cross-links and strand breaks in DNA by transition metal ion-mediated oxygen radical attack, Chem. Res. Toxicol 10 (4) (1997) 393–400. [DOI] [PubMed] [Google Scholar]
- [71].De Flora S, Izzotti A, Mutagenesis and cardiovascular diseases Molecular mechanisms, risk factors, and protective factors, Mutat. Res 621 (1–2) (2007) 5–17. [DOI] [PubMed] [Google Scholar]
- [72].Brewer GJ, Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease, Exp. Biol. Med 232 (2) (2007) 323–335. [PubMed] [Google Scholar]
- [73].Balaban RS, Nemoto S, Finkel T, Mitochondria, oxidants, and aging, Cell 120 (4) (2005) 483–495. [DOI] [PubMed] [Google Scholar]
- [74].Kurutas EB, The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state, Nutr. J 15 (1) (2016) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Perales-Clemente E, Folmes CD, Terzic A, Metabolic regulation of redox status in stem cells, Antioxidants Redox Signal. 21 (11) (2014) 1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lees JG, Rathjen J, Sheedy JR, Gardner DK, Harvey AJ, Distinct profiles of human embryonic stem cell metabolism and mitochondria identified by oxygen, Reproduction 150 (4) (2015) 367–382. [DOI] [PubMed] [Google Scholar]
- [77].Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR, Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state, Cell Stem Cell 19 (4) (2016) 476–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CA, Ramalho-Santos J, Van Houten B, Schatten G, Energy metabolism in human pluripotent stem cells and their differentiated counterparts, PloS One 6 (6) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Satoorian TB, zur Nieden NI, Metabolism in cancer cells and pluripotent stem cells, in: Hayat MA (Ed.), Stem Cells and Cancer Stem Cells, Volume 9: Therapeutic Applications in Disease and Injury, Springer; Netherlands, 2013, pp. 83–92. [Google Scholar]
- [80].Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park DJ, Park KS, Lee HK, Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells, Biochem. Biophys. Res. Commun 348 (4) (2006) 1472–1478. [DOI] [PubMed] [Google Scholar]
- [81].Sart S, Agathos SN, Li Y, Process engineering of stem cell metabolism for large scale expansion and differentiation in bioreactors, Biochem. Eng. J 84 (2014) 74–82. [Google Scholar]
- [82].St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP, The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells, Clon Stem Cell 7 (3) (2005) 141–153. [DOI] [PubMed] [Google Scholar]
- [83].Tsogtbaatar E, Landin C, Minter-Dykhouse K, Folmes CDL, Energy metabolism regulates stem cell pluripotency, Frontiers in Cell and Developmental Biology 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Folmes CDL, Dzeja PP, Nelson TJ, Terzic A, Metabolic plasticity in stem cell homeostasis and differentiation, Cell Stem Cell 11 (5) (2012) 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hansson J, Rafiee MR, Reiland S, Polo JM, Gehring J, Okawa S, Huber W, Hochedlinger K, Krijgsveld J, Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency, Cell Rep. 2 (6) (2012) 1579–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shyh-Chang N, Daley GQ, Cantley LC, Stem cell metabolism in tissue development and aging, Development (Camb.) 140 (12) (2013) 2535–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W, Reik W, Bertone P, Smith A, Resetting transcription factor control circuitry toward ground-state pluripotency in human, Cell 158 (6) (2014) 1254–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, Ruohola-Baker H, HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition, EMBO J. 31 (9) (2012) 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Guo G, von Meyenn F, Santos F, Chen Y, Reik W, Bertone P, Smith A, Nichols J, Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass, Stem Cell Reports 6 (4) (2016) 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Di Stefano B, Ueda M, Sabri S, Brumbaugh J, Huebner AJ, Sahakyan A, Clement K, Clowers KJ, Erickson AR, Shioda K, Gygi SP, Gu H, Shioda T, Meissner A, Takashima Y, Plath K, Hochedlinger K, Reduced MEK inhibition preserves genomic stability in naive human embryonic stem cells, Nat. Methods 15 (9) (2018) 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Simsek T, Kocabas F, Zheng J, DeBerardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA, The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche, Cell Stem Cell 7 (3) (2010) 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Stringari C, Edwards RA, Pate KT, Waterman ML, Donovan PJ, Gratton E, Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH, Sci. Rep 2 (1) (2012) 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Candelario KM, Shuttleworth CW, Cunningham LA, Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1 alpha expression, J. Neurochem 125 (3) (2013) 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shyh-Chang N, Ng H-H, The metabolic programming of stem cells, Gene Dev. 31 (4) (2017) 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM, Interplay between metabolic identities in the intestinal crypt supports stem cell function, Nature 543 (7645) (2017) 424–427. [DOI] [PubMed] [Google Scholar]
- [96].Li L, Clevers H, Coexistence of quiescent and active adult stem cells in mammals, Science 327 (5965) (2010) 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. , Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration, Nature 528 (2015) 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Richmond CA, Shah MS, Deary LT, Trotier DC, Thomas H, Ambruzs DM, Jiang L, Whiles BB, Rickner HD, Montgomery RK, et al. , Dormant intestinal stem cells are regulated by PTEN and nutritional Status, Cell Rep. 13 (2015) 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Beumer J, Clevers H, Regulation and plasticity of intestinal stem cells during homeostasis and regeneration, Development 143 (20) (2016) 3639–3649. [DOI] [PubMed] [Google Scholar]
- [100].Yu T, Robotham JL, Yoon Y, Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology, Proc. Natl. Acad. Sci. U.S.A 103 (8) (2006) 2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Baynes JW, Thorpe SR, Role of oxidative stress in diabetic complications: a new perspective on an old paradigm, Diabetes 48 (1) (1999) 1–9. [DOI] [PubMed] [Google Scholar]
- [102].Brownlee M, Biochemistry and molecular cell biology of diabetic complications, Nature 414 (6865) (2001) 813–820. [DOI] [PubMed] [Google Scholar]
- [103].Green K, Brand MD, Murphy MP, Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes, Diabetes 53 (Suppl 1) (2004) S110–S118. [DOI] [PubMed] [Google Scholar]
- [104].Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M, Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage, Nature 404 (6779) (2000) 787–790. [DOI] [PubMed] [Google Scholar]
- [105].van de Vyver M, Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: implications for autologous cell therapy, Stem Cell. Dev 26 (14) (2017) 1042–1053. [DOI] [PubMed] [Google Scholar]
- [106].McClelland Descalzo DL, Satoorian TS, Walker LM, Sparks NR, Pulyanina PY, zur Nieden NI, Glucose-induced oxidative stress reduces proliferation in embryonic stem cells via FOXO3A/β-catenin-dependent transcription of p21(cip 1), Stem Cell Reports 7 (1) (2016) 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yu T, Robotham JL, Yoon Y, Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology, Proc. Natl. Acad. Sci. U. S. A 103 (8) (2006. Feb 21) 2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Xia L, Wang H, Munk S, Kwan J, Goldberg HJ, Fantus IG, Whiteside CI, High glucose activates PKC-zeta and NADPH oxidase through autocrine TGF-beta 1 signaling in mesangial cells, Am. J. Physiol. Ren. Physiol 295 (6) (2008. Dec) F1705–F1714. [DOI] [PubMed] [Google Scholar]
- [109].Balteau M, Tajeddine N, de Meester C, Ginion A, Des Rosiers C, Brady NR, Sommereyns C, Horman S, Vanoverschelde JL, Gailly P, Hue L, Bertrand L, Beauloye C, NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1, Cardiovasc. Res 92 (2) (2011. Nov 1) 237–246. [DOI] [PubMed] [Google Scholar]
- [110].Binó L, Veselá I, Papežíková I, Procházková J, Vašíček O, Štefková K, Kučera J, Hanáčková M, Kubala L, Pacherník J, The depletion of p38alpha kinase upregulates NADPH oxidase 2/NOX2/gp91 expression and the production of superoxide in mouse embryonic stem cells, Arch. Biochem. Biophys 671 (2019. Aug 15) 18–26. [DOI] [PubMed] [Google Scholar]
- [111].Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, Rais Y, Shipony Z, Mukamel Z, Krupalnik V, Zerbib M, Geula S, Caspi I, Schneir D, Shwartz T, Hanna JH, Derivation of novel human ground state naive pluripotent stem cells, Nature 504 (7479) (2013) 282–286. [DOI] [PubMed] [Google Scholar]
- [112].Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A, Oxygen in stem cell biology: a critical component of the stem cell niche, Cell stem cell 7 (2) (2010) 150–161. [DOI] [PubMed] [Google Scholar]
- [113].Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T, Regulation of the HIF-1α level is essential for hematopoietic stem cells, Cell Stem Cell 7 (3) (2010) 391–402. [DOI] [PubMed] [Google Scholar]
- [114].Suda T, Takubo K, Semenza GL, Metabolic regulation of hematopoietic stem cells in the hypoxic niche, Cell Stem Cell 9 (4) (2011) 298–310. [DOI] [PubMed] [Google Scholar]
- [115].Fujisawa K, Takami T, Okada S, Hara K, Matsumoto T, Yamamoto N, Yamasaki T, Sakaida I, Analysis of metabolomic changes in mesenchymal stem cells on treatment with desferrioxamine as a hypoxia mimetic compared with hypoxic conditions, Stem Cells (Dayton) 36 (8) (2018) 1226–1236. [DOI] [PubMed] [Google Scholar]
- [116].Rodesch F, Simon P, Donner C, Jauniaux E, Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy, Obstet. Gynecol 80 (2) (1992) 283–285. [PubMed] [Google Scholar]
- [117].Ivanović Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V, Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent, Transfusion 40 (12) (2000) 1482–1488. [DOI] [PubMed] [Google Scholar]
- [118].Studer L, Csete M, Lee S-H, Kabbani N, Walikonis J, Wold B, McKay R, Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen, J. Neurosci 20 (19) (2000) 7377–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ezashi T, Das P, Roberts RM, Low O2 tensions and the prevention of differentiation of hES cells, Proc. Natl. Acad. Sci. U.S.A 102 (13) (2005) 4783–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Prasad SM, Czepiel M, Cetinkaya C, Smigielska K, Weli SC, Lysdahl H, Gabrielsen A, Petersen K, Ehlers N, Fink T, Minger SL, Zachar V, Continuous hypoxic culturing maintains activation of Notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation, Cell Prolif 42 (1) (2009) 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Barbosa HSC, Fernandes TG, Dias TP, Diogo MM, Cabral JMS, New insights into the mechanisms of embryonic stem cell self-renewal under hypoxia: a multifactorial analysis approach, PloS One 7 (6) (2012), e38963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zachar V, Prasad SM, Weli SC, Gabrielsen A, Petersen K, Petersen MB, Fink T, The effect of human embryonic stem cells (hESCs) long-term normoxic and hypoxic cultures on the maintenance of pluripotency, In Vitro Cell. Dev. Biol 46 (3) (2010) 276–283. [DOI] [PubMed] [Google Scholar]
- [123].Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, Alagappan R, Frampton GM, Xu P, Muffat J, Santagata S, Powers D, Barrett CB, Young RA, Lee JT, Jaenisch R, Mitalipova M, Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations, Cell 141 (5) (2010) 872–883. [DOI] [PubMed] [Google Scholar]
- [124].Rebuzzini P, Zuccotti M, Redi CA, Garagna S, Chromosomal abnormalities in embryonic and somatic stem cells, Cytogenet. Genome Res 147 (1) (2015) 1–9, 10.1159/000441645. [DOI] [PubMed] [Google Scholar]
- [125].Xie P, Sun Y, Ouyang Q, Hu L, Tan Y, Zhou X, Xiong B, Zhang Q, Yuan D, Pan Y, Liu T, Liang P, Lu G, Lin G, Physiological oxygen prevents frequent silencing of the DLK1-DIO3 cluster during human embryonic stem cells culture, Stem Cell. 32 (2) (2014) 391–401. [DOI] [PubMed] [Google Scholar]
- [126].Theunissen TW, Friedli M, He Y, Planet E, O’Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M, Duc J, Cohen MA, Wert KJ, Castanon R, Zhang Z, Huang Y, Nery JR, Drotar J, Lungjangwa T, Jaenisch R, Molecular criteria for defining the naive human pluripotent state, Cell Stem Cell 19 (4) (2016) 502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, Tesar PJ, Okada J, Margaretha L, Sperber H, Choi M, Blau CA, Treuting PM, Hawkins RD, Cirulli V, Ruohola-Baker H, Derivation of naïve human embryonic stem cells, Proc. Natl. Acad. Sci. U.S.A 111 (12) (2014) 4484–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bhatti FU, Mehmood A, Latief N, Zahra S, Cho H, Khan SN, Riazuddin S, Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis, Osteoarthritis Cartilage 25 (2) (2017) 321–331. [DOI] [PubMed] [Google Scholar]
- [129].Sriram S, Yuan C, Chakraborty S, Tay W, Park M, Shabbir A, Toh S-A, Han W, Sugii S, Oxidative stress mediates depot-specific functional differences of human adipose-derived stem cells, Stem Cell Res. Ther 10 (1) (2019) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Fujisawa K, Hara K, Takami T, Okada S, Matsumoto T, Yamamoto N, Sakaida I, Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells, Stem Cell Res. Ther 9 (1) (2018) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cao N, Liu Z, Chen Z, Wang J, Chen T, Zhao X, Ma Y, Qin L, Kang J, Wei B, Wang L, Jin Y, Yang HT, Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells, Cell Res. 22 (1) (2012) 219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Puig-Sanvicens VA, Semino CE, zur Nieden NI, Cardiac differentiation potential of human induced pluripotent stem cells in a 3D self-assembling peptide scaffold, Differentiation 90 (4–5) (2015) 101–110. [DOI] [PubMed] [Google Scholar]
- [133].zur Nieden NI, Kempka G, Ahr HJ, In vitro differentiation of embryonic stem cells into mineralized osteoblasts, Differentiation 71 (1) (2003) 18–27. [DOI] [PubMed] [Google Scholar]
- [134].Sparks N, Martinez I, Soto CH, zur Nieden NI, Low osteogenic yield in human pluripotent stem cells associates with differential neural crest promoter methylation, Stem Cell. 36 (3) (2018) 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Han D, Williams E, Cadenas E, Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space, Biochem. J 353 (Pt 2) (2001) 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Starkov AA, The role of mitochondria in reactive oxygen species metabolism and signaling, Ann. N. Y. Acad. Sci 1147 (2008) 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Dröge W, Free radicals in the physiological control of cell function, Physiol. Rev 82 (1) (2002) 47–95. [DOI] [PubMed] [Google Scholar]
- [138].Chance B, Sies H, Boveris A, Hydroperoxide metabolism in mammalian organs, Physiol. Rev 59 (3) (1979) 527–605. [DOI] [PubMed] [Google Scholar]
- [139].Michiels C, Raes M, Toussaint O, Remacle J, Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress, Free Radical Biol. Med 17 (3) (1994) 235–248. [DOI] [PubMed] [Google Scholar]
- [140].Brown MR, Miller FJ Jr., Li WG, Ellingson AN, Mozena JD, Chatterjee P, Engelhardt JF, Zwacka RM, Oberley LW, Fang X, Spector AA, Weintraub NL, Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells, Circ. Res 85 (6) (1999) 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zhong W, Oberley LW, Oberley TD, Yan T, Domann FE, St Clair DK, Inhibition of cell growth and sensitization to oxidative damage by overexpression of manganese superoxide dismutase in rat glioma cells, Cell Growth Differ. 7 (9) (1996) 1175–1186. [PubMed] [Google Scholar]
- [142].Solari C, Petrone MV, Toro A, Vazquez Echegaray C, Cosentino MS, Waisman A, Francia M, Barañao L, Miriuka S, Guberman A, The pluripotency transcription factor Nanog represses glutathione reductase gene expression in mouse embryonic stem cells, BMC Res. Notes 12 (1) (2019) 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Meng TC, Fukada T, Tonks NK, Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo, Mol. Cell 9 (2) (2002) 387–399. [DOI] [PubMed] [Google Scholar]
- [144].Ghezzi P, Bonetto V, Fratelli M, Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation, Antioxidants Redox Signal. 7 (7–8) (2005) 964–972. [DOI] [PubMed] [Google Scholar]
- [145].Gallogly MM, Mieyal JJ, Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress, Curr. Opin. Pharmacol 7 (4) (2007) 381–391. [DOI] [PubMed] [Google Scholar]
- [146].Sousa SF, Neves R, Waheed SO, Fernandes PA, Ramos MJ, Structural and mechanistic aspects of S-S bonds in the thioredoxin-like family of proteins, Biol. Chem 400 (5) (2019) 575–587. [DOI] [PubMed] [Google Scholar]
- [147].Guo Y, Einhorn L, Kelley M, Hirota K, Yodoi J, Reinbold R, Scholer H, Ramsey H, Hromas R, Redox regulation of the embryonic stem cell transcription factor Oct-4 by thioredoxin, Stem Cell. 22 (3) (2004) 259–264. [DOI] [PubMed] [Google Scholar]
- [148].Jung H, Kim MJ, Kim DO, Kim WS, Yoon S-J, Park Y-J, Yoon SR, Kim T-D, Suh H-W, Yun S, Min J-K, Lee HG, Lee YH, Na H-J, Lee DC, Kim H-C, Choi I, TXNIP maintains the hematopoietic cell pool by switching the function of p53 under oxidative stress, Cell Metabol. 18 (1) (2013) 75–85. [DOI] [PubMed] [Google Scholar]
- [149].Rhee SG, Kang SW, Chang TS, Jeong W, Kim K, Peroxiredoxin, a novel family of peroxidases, IUBMB Life 52 (1–2) (2001) 35–41. [DOI] [PubMed] [Google Scholar]
- [150].Park Y-H, Kim HS, Lee D-S, Kim S-U, Peroxiredoxin VI regulates the epithelial-mesenchymal transition in colorectal cancer, Faseb. J 33 (S1) (2019) lb273–lb273. [Google Scholar]
- [151].Kim SU, Park YH, Kim JM, Sun HN, Song IS, Huang SM, Lee SH, Chae JI, Hong S, Sik Choi S, Choi SC, Lee TH, Kang SW, Rhee SG, Chang KT, Lee SH, Yu DY, Lee DS, Dominant role of peroxiredoxin/JNK axis in stemness regulation during neurogenesis from embryonic stem cells, Stem Cell. 32 (4) (2014) 998–1011. [DOI] [PubMed] [Google Scholar]
- [152].Laurent TC, Moore EC, Reichard P, Enzymatic synthesis of deoxyribonucleotides. 4. Isolation and characterization of thioredoxin hydrogen donor from E. coli B, J. Biol. Chem 239 (1964) 3436–3444. [PubMed] [Google Scholar]
- [153].Holmgren A, Thioredoxin, Annu. Rev. Biochem 54 (1985) 237–271. [DOI] [PubMed] [Google Scholar]
- [154].Tonks NK, Redox redux: revisiting PTPs and the control of cell signaling, Cell 121 (5) (2005) 667–670. [DOI] [PubMed] [Google Scholar]
- [155].Angkeow P, Deshpande SS, Qi B, Liu Y-X, Park YC, Jeon BH, Ozaki M, Irani K, Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis, Cell Death Differ. 9 (7) (2002) 717–725. [DOI] [PubMed] [Google Scholar]
- [156].Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, Stojkovic M, Lako M, The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis, Hum. Mol. Genet 15 (11) (2006) 1894–1913. [DOI] [PubMed] [Google Scholar]
- [157].Kang HB, Kim YE, Kwon HJ, Sok DE, Lee Y, Enhancement of NF-kappaB expression and activity upon differentiation of human embryonic stem cell line SNUhES3, Stem Cell. Dev 16 (4) (2007) 615–623. [DOI] [PubMed] [Google Scholar]
- [158].Kim YE, Kang HB, Park JA, Nam KH, Kwon HJ, Lee Y, Upregulation of NF-kappaB upon differentiation of mouse embryonic stem cells, BMB reports 41 (10) (2008) 705–709. [DOI] [PubMed] [Google Scholar]
- [159].Dansen TB, Smits LMM, van Triest MH, de Keizer PLJ, van Leenen D, Koerkamp MG, Szypowska A, Meppelink A, Brenkman AB, Yodoi J, Holstege FCP, Burgering BMT, Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity, Nat. Chem. Biol 5 (9) (2009) 664–672. [DOI] [PubMed] [Google Scholar]
- [160].Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmelé P, Kennedy M, Sellers R, Landthaler M, Tuschl T, Chi NW, Lemischka I, Keller G, Ghaffari S, FOXO1 is an essential regulator of pluripotency in human embryonic stem cells, Nat. Cell Biol 13 (9) (2011) 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Cardoso AA, Jiang Y, Luo M, Reed AM, Shahda S, He Y, Maitra A, Kelley MR, Fishel ML, APE1/Ref-1 regulates STAT3 transcriptional activity and APE1/ref-1–STAT3 dual-targeting effectively inhibits pancreatic cancer cell survival, PloS One 7 (10) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Tsai JJ, Dudakov JA, Takahashi K, Shieh J-H, Velardi E, Holland AM, Singer NV, West ML, Smith OM, Young LF, Shono Y, Ghosh A, Hanash AM, Tran HT, Moore MAS, van den Brink MRM, Nrf2 regulates haematopoietic stem cell function, Nat. Cell Biol 15 (3) (2013) 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Jang J, Wang Y, Kim H-S, Lalli MA, Kosik KS, Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells, Stem Cells (Dayton) 32 (10) (2014) 2616–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Yao Y, Lu Y, Chen W, Jiang Y, Cheng T, Ma Y, Lu L, Dai W, Cobalt and nickel stabilize stem cell transcription factor OCT4 through modulating its sumoylation and ubiquitination, PloS One 9 (1) (2014), e86620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hawkins KE, Joy S, Delhove JMKM, Kotiadis VN, Fernandez E, Fitzpatrick LM, Whiteford JR, King PJ, Bolanos JP, Duchen MR, Waddington SN, McKay TR, NRF2 orchestrates the metabolic shift during induced pluripotent stem cell reprogramming, Cell Rep. 14 (8) (2016) 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Dai X, Yan X, Wintergerst KA, Cai L, Keller BB, Tan Y, Nrf2: redox and metabolic regulator of stem cell state and function, Trends Mol. Med 26 (2) (2020) 185–200. [DOI] [PubMed] [Google Scholar]
- [167].Eijkelenboom A, Burgering BMT, FOXOs: signalling integrators for homeostasis maintenance, Nat. Rev. Mol. Cell Biol 14 (2) (2013) 83–97. [DOI] [PubMed] [Google Scholar]
- [168].Kops GJPL, Dansen TB, Polderman PE, Saarloos I, Wirtz KWA, Coffer PJ, Huang T-T, Bos JL, Medema RH, Burgering BMT, Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress, Nature 419 (6904) (2002) 316–321. [DOI] [PubMed] [Google Scholar]
- [169].Essers MAG, Weijzen S, de Vries-Smits AMM, Saarloos I, de Ruiter ND, Bos JL, Burgering BMT, FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK, EMBO J. 23 (24) (2004) 4802–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].de Keizer PL, Burgering BM, Dansen TB, Forkhead box o as a sensor, mediator, and regulator of redox signaling, Antioxidants Redox Signal. 14 (6) (2011) 1093–1106. [DOI] [PubMed] [Google Scholar]
- [171].van der Horst A, Burgering BMT, Stressing the role of FoxO proteins in lifespan and disease, Nat. Rev. Mol. Cell Biol 8 (6) (2007) 440–450. [DOI] [PubMed] [Google Scholar]
- [172].van der Heide LP, Smidt MP, Regulation of FoxO activity by CBP/p300-mediated acetylation, Trends Biochem. Sci 30 (2) (2005) 81–86. [DOI] [PubMed] [Google Scholar]
- [173].Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME, Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase, Science 303 (5666) (2004) 2011–2015. [DOI] [PubMed] [Google Scholar]
- [174].Zhang Y, Nie L, Xu K, Fu Y, Zhong J, Gu K, Zhang L, SIRT6, a novel direct transcriptional target of FoxO3a, mediates colon cancer therapy, Theranostics 9 (8) (2019) 2380–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Dansen TB, Forkhead box O transcription factors: key players in redox signaling, Antioxidants Redox Signal. 14 (4) (2011) 559–561. [DOI] [PubMed] [Google Scholar]
- [176].Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH, The forkhead transcription factor FoxO regulates transcription of p27Kip 1 and Bim in response to IL-2, J. Immunol 168 (10) (2002) 5024–5031. [DOI] [PubMed] [Google Scholar]
- [177].Burgering BM, Kops GJ, Cell cycle and death control: long live Forkheads, Trends Biochem. Sci 27 (7) (2002) 352–360. [DOI] [PubMed] [Google Scholar]
- [178].Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N, FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress, J. Biol. Chem 277 (30) (2002) 26729–26732. [DOI] [PubMed] [Google Scholar]
- [179].Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR, A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR, Canc. Cell 2 (1) (2002) 81–91. [DOI] [PubMed] [Google Scholar]
- [180].Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R, Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor, Proc. Natl. Acad. Sci. U.S.A 100 (11) (2003) 6523–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Storz P, Forkhead homeobox type O transcription factors in the responses to oxidative stress, Antioxidants Redox Signal. 14 (4) (2011) 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Miyaguchi Y, Tsuchiya K, Sakamoto K, P53 negatively regulates the transcriptional activity of FOXO3a under oxidative stress, Cell Biol. Int 33 (8) (2009) 853–860. [DOI] [PubMed] [Google Scholar]
- [183].Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME, Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a), Mol. Cell Biol 21 (3) (2001) 952–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC, IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a, Cell 117 (2) (2004) 225–237. [DOI] [PubMed] [Google Scholar]
- [185].Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A, Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation, Proc. Natl. Acad. Sci. U.S.A 100 (20) (2003) 11285–11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ludikhuize MC, Meerlo M, Gallego MP, Julià MB, Nguyen NTB, Brombacher EC, Paik JH, Burgering BMT, Colman MJR, Mitochondria Define Intestinal Stem Cell Differentiation Downstream of a FOXO/Notch axis, BioRxiv, 2019, p. 777391. [Google Scholar]
- [187].Tothova Z, Gilliland DG, FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system, Cell Stem Cell 1 (2) (2007) 140–152. [DOI] [PubMed] [Google Scholar]
- [188].Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, Suda T, FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging, Blood 112 (12) (2008) 4485–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, Ghaffari S, Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells, J. Biol. Chem 283 (37) (2008) 25692–25705. [DOI] [PubMed] [Google Scholar]
- [190].Kim DY, Hwang I, Muller FL, Paik JH, Functional regulation of FoxO1 in neural stem cell differentiation, Cell Death Differ. 22 (12) (2015) 2034–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A, FoxO3 regulates neural stem cell homeostasis, Cell stem cell 5 (5) (2009) 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Webb AE, Pollina EA, Vierbuchen T, Urbán N, Ucar D, Leeman DS, Martynoga B, Sewak M, Rando TA, Guillemot F, Wernig M, Brunet A, FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis, Cell Rep. 4 (3) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Chenn A, Walsh CA, Regulation of cerebral cortical size by control of cell cycle exit in neural precursors, Science 297 (5580) (2002) 365–369. [DOI] [PubMed] [Google Scholar]
- [194].Zechner D, Fujita Y, Hülsken J, Müller T, Walther I, Taketo MM, Crenshaw EB 3rd, Birchmeier W, Birchmeier C, β-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system, Dev. Biol 258 (2) (2003) 406–418. [DOI] [PubMed] [Google Scholar]
- [195].Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D, Notch signaling is required to maintain all neural stem cell populations—irrespective of spatial or temporal niche, Dev. Neurosci 28 (1–2) (2006) 34–48. [DOI] [PubMed] [Google Scholar]
- [196].Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T, Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of akt, J. Biol. Chem 278 (50) (2003) 50226–50233. [DOI] [PubMed] [Google Scholar]
- [197].Kwon J, Lee S-R, Yang K-S, Ahn Y, Kim YJ, Stadtman ER, Rhee SG, Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors, Proc. Natl. Acad. Sci. U.S.A 101 (47) (2004) 16419–16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Lee S-R, Yang K-S, Kwon J, Lee C, Jeong W, Rhee SG, Reversible inactivation of the tumor suppressor PTEN by H2O2, J. Biol. Chem 277 (23) (2002) 20336–20342. [DOI] [PubMed] [Google Scholar]
- [199].Wang X, Chen WR, Xing D, A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation, J. Cell. Physiol 227 (3) (2012) 1168–1178. [DOI] [PubMed] [Google Scholar]
- [200].Shen B, Chao L, Chao J, Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress, Am. J. Physiol. Heart Circ. Physiol 298 (3) (2010) H1048–H1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG, Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation-and 14-3-3-dependent and -independent mechanisms, Biochem. J 378 (Pt 3) (2004) 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, Tzivion G, Bimodal regulation of FoxO3 by AKT and 14-3-3, Biochim. Biophys. Acta 1813 (8) (2011) 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lee JJ, Lee JH, Ko YG, Hong SI, Lee JS, Prevention of premature senescence requires JNK regulation of Bcl-2 and reactive oxygen species, Oncogene 29 (4) (2010) 561–575. [DOI] [PubMed] [Google Scholar]
- [204].Gong X, Wang M, Tashiro S, Onodera S, Ikejima T, Involvement of JNK-initiated p53 accumulation and phosphorylation of p53 in pseudolaric acid B induced cell death, Exp. Mol. Med 38 (4) (2006) 428–434. [DOI] [PubMed] [Google Scholar]
- [205].Jones EV, Dickman MJ, Whitmarsh AJ, Regulation of p73-mediated apoptosis by c-Jun N-terminal kinase, Biochem. J 405 (3) (2007) 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Xu P, Davis RJ, C-Jun NH2-terminal kinase is required for lineage-specific differentiation but not stem cell self-renewal, Mol. Cell Biol 30 (6) (2010) 1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Xiao X, Lai W, Xie H, Liu Y, Guo W, Liu Y, Li Y, Li Y, Zhang J, Chen W, Shi M, Shang L, Yin M, Wang C, Deng H, Targeting JNK pathway promotes human hematopoietic stem cell expansion, Cell Discov 5 (2019) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, Behrens A, JNK signalling modulates intestinal homeostasis and tumourigenesis in mice, EMBO J. 28 (13) (2009) 1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Brill LM, Xiong W, Lee K-B, Ficarro SB, Crain A, Xu Y, Terskikh A, Snyder EY, Ding S, Phosphoproteomic analysis of human embryonic stem cells, Cell Stem Cell 5 (2) (2009) 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T, Role for mitochondrial oxidants as regulators of cellular metabolism, Mol. Cell Biol 20 (19) (2000) 7311–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zhou Q, Lam PY, Han D, Cadenas E, c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons, J. Neurochem 104 (2) (2008) 325–335. [DOI] [PubMed] [Google Scholar]
- [212].Deng H, Yu F, Chen J, Zhao Y, Xiang J, Lin A, Phosphorylation of Bad at Thr-201 by JNK1 promotes glycolysis through activation of phosphofructokinase-1, J. Biol. Chem 283 (30) (2008) 20754–20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Hardie DG, Ross FA, Hawley SA, AMPK: a nutrient and energy sensor that maintains energy homeostasis, Nat. Rev. Mol. Cell Biol 13 (4) (2012) 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Hardie DG, AMPK: positive and negative regulation, and its role in whole-body energy homeostasis, Curr. Opin. Cell Biol 33 (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [215].Cardaci S, Filomeni G, Ciriolo MR, Redox implications of AMPK-mediated signal transduction beyond energetic clues, J. Cell Sci 125 (9) (2012) 2115–2125. [DOI] [PubMed] [Google Scholar]
- [216].Filomeni G, De Zio D, Cecconi F, Oxidative stress and autophagy: the clash between damage and metabolic needs, Cell Death Differ. 22 (3) (2015) 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E, Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase, J. Biol. Chem 285 (43) (2010) 33154–33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Chen C, Liu Y, Liu R, Ikenoue T, Guan K-L, Liu Y, Zheng P, TSC–mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species, J. Exp. Med 205 (10) (2008) 2397–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Sarbassov DD, Sabatini DM, Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex, J. Biol. Chem 280 (47) (2005) 39505–39509. [DOI] [PubMed] [Google Scholar]
- [220].Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, Zhang S, Huang Q, Shi M, ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway, Mol. Canc 16 (1) (2017) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Easley CA 4th, Ben-Yehudah A, Redinger CJ, Oliver SL, Varum ST, Eisinger VM, Carlisle DL, Donovan PJ, Schatten GP, mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells, Cell. Reprogr 12 (3) (2010) 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Albert V, Hall MN, MTOR signaling in cellular and organismal energetics, Curr. Opin. Cell Biol 33 (2015) 55–66. [DOI] [PubMed] [Google Scholar]
- [223].Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS, MTOR mediates wnt-induced epidermal stem cell exhaustion and aging, Cell Stem Cell 5 (3) (2009) 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC, The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress, Proc. Natl. Acad. Sci. U. S. A 101 (10) (2004) 3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, DePinho RA, LKB1 regulates quiescence and metabolic homeostasis of hematopoietic stem cells, Nature 468 (7324) (2010) 701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, Tzatsos A, Ozsolak F, Milos P, Ferrari F, Park PJ, Shirihai OS, Scadden DT, Bardeesy N, The Lkb1 metabolic sensor maintains haematopoietic stem cell survival, Nature 468 (7324) (2010) 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Alba G, Martínez R, Postigo-Corrales F, López S, Santa-María C, Jiménez J, Cahuana GM, Soria B, Bedoya FJ, Tejedo JR, AICAR stimulates the pluripotency transcriptional complex in embryonic stem cells mediated by PI3K, GSK3β, and β-catenin, ACS Omega 5 (32) (2020) 20270–20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Chaube B, Malvi P, Singh SVN, Viollet B, Bhat MK, AMPK maintains energy homeostasis and survival in cancer cells via regulating p38/PGC-1α-mediated mitochondrial biogenesis, Cell Death Discovery 1 (2015) 15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Gongol B, Sari I, Bryant T, Rosete G, Marin T, AMPK: an epigenetic landscape modulator, Int. J. Mol. Sci 19 (10) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Priebe A, Tan L, Wahl H, Kueck A, He G, Kwok R, Opipari A, Liu JR, Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells, Gynecol. Oncol 122 (2) (2011) 389–395. [DOI] [PubMed] [Google Scholar]
- [231].Saxton RA, Sabatini DM, mTOR signaling in growth, metabolism, and disease, Cell 169 (2) (2017) 361–371. [DOI] [PubMed] [Google Scholar]
- [232].Kazyken D, Magnuson B, Bodur C, Acosta-Jaquez HA, Zhang D, Tong X, Barnes TM, Steinl GK, Patterson NE, Altheim CH, Sharma N, Inoki K, Cartee GD, Bridges D, Yin L, Riddle SM, Fingar DC, AMPK directly activates mTORC2 to promote cell survival during acute energetic stress, Sci. Signal 12 (585) (2019), eaav3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE, SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization, Cell Stem Cell 2 (3) (2008) 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Solozobova V, Rolletschek A, Blattner C, Nuclear accumulation and activation of p53 in embryonic stem cells after DNA damage, BMC Cell Biol. 10 (2009) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Bellomo G, Vairetti M, Stivala L, Mirabelli F, Richelmi P, Orrenius S, Demonstration of nuclear compartmentalization of glutathione in hepatocytes, Proc. Natl. Acad. Sci. U.S.A 89 (10) (1992) 4412–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Xanthoudakis S, Curran T, Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity, EMBO J. 11 (2) (1992) 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Pallardó FV, Markovic J, García JL, Viña J, Role of nuclear glutathione as a key regulator of cell proliferation, Mol. Aspect. Med 30 (1–2) (2009) 77–85. [DOI] [PubMed] [Google Scholar]
- [238].Britten RA, Green JA, Broughton C, Browning PG, White R, Warenius HM, The relationship between nuclear glutathione levels and resistance to melphalan in human ovarian tumour cells, Biochem. Pharmacol 41 (4) (1991) 647–649. [DOI] [PubMed] [Google Scholar]
- [239].Byrne BM, Welsh J, Altered thioredoxin subcellular localization and redox status in MCF-7 cells following 1,25-dihydroxyvitamin D3 treatment, J. Steroid Biochem. Mol. Biol 97 (1–2) (2005) 57–64. [DOI] [PubMed] [Google Scholar]
- [240].Li W, Yu SW, Kong AN, Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain, J. Biol. Chem 281 (37) (2006) 27251–27263. [DOI] [PubMed] [Google Scholar]


