Significance
Anchoring nanoscale active sites on a substrate has proven to be an effective way to maximize catalytic performance. Herein, we report a strategy to turn surface defects into Fenton-like catalytic active sites in situ. The Ti-deficit vacancies of the exfoliated Ti3C2MXene were transformed to discrete titanium oxide with multivalent Ti. The combined use of theoretical calculation and experimental characterizations revealed the superior and stable Fenton-like catalytic activity toward degradation of a typical herbicide, atrazine. Our results showcased an elegant and a universal use of MXene family materials as templates and multivalent metal precursors for the in situ formation of Fenton-like catalysts.
Keywords: heterogeneous catalysis, in situ, MXene, multivalence, template synthesis
Abstract
Controllable in situ formation of nanoclusters with discrete active sites is highly desirable in heterogeneous catalysis. Herein, a titanium oxide–based Fenton-like catalyst is constructed using exfoliated Ti3C2 MXene as a template. Theoretical calculations reveal that a redox reaction between the surface Ti-deficit vacancies of the exfoliated Ti3C2 MXene and H2O2 molecules facilitates the in situ conversion of surface defects into titanium oxide nanoclusters anchoring on amorphous carbon (TiOx@C). The presence of mixed-valence Tiδ+ (δ = 0, 2, 3, and 4) within TiOx@C is confirmed by X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure (XAFS) characterizations. The abundant surface defects within TiOx@C effectively promote the generation of reactive oxygen species (ROS) leading to superior and stable Fenton-like catalytic degradation of atrazine, a typical agricultural herbicide. Such an in situ construction of Fenton-like catalysts through defect engineering also applies to other MXene family materials, such as V2C and Nb2C.
Developing effective and reliable Fenton-like heterogeneous catalysts serves an essential role in addressing the ever-growing environmental pollution and water security challenges in both scientific and industrial fields (1, 2). Heterogeneous catalysts with decreasing physical sizes, such as nanodots, atomic clusters, and single-atom catalysts (SACs), are of great interest due to the abundance of catalytic reaction sites (3–6). However, ultrasmall sizes are prone to aggregation due to the exponential increase of surface energy, resulting in blockage of active sites, instability, and poor recyclability (7–9). In this regard, utilizing a substrate to anchor active sites and maximize the dispersion degree has proven to be a promising strategy (10–12). Carbon-based two-dimensional nanomaterials, including graphene (13), carbon nitride (14), and N, S-doped carbon frameworks (15), showed the ability to trap, spatially confine, or bond metal clusters to reduce aggregation. But most of the formation of catalytic active metal oxides requires the addition of metal precursors (16, 17). Hence, it is appealing to explore substrates that contain a metal ingredient to offer opportunities for the in situ formation of catalytic active sites.
MXenes, an exciting family of two-dimensional transition metal carbides, nitrides, or carbonitrides (18–20), have shown clear advantages in energy and environmental remediation fields. The layered structure of MXene provides an ideal substrate for anchoring catalytic active sites. Double transition metal MXene nanosheets (Mo2TiC2Tx) immobilizing single Pt atoms showed excellent catalytic performance for hydrogen evolution (21). Interestingly, the etching process to remove Al layers from the MAX phase material would occasionally strip away transition metal atoms leaving single vacancies or vacancy cluster defects that are highly oxophilic (22, 23). These defect sites are highly reactive to form oxide clusters through hydrolysis or abstraction of oxygen. As a result, the compositing transition metals of MXene, i.e., Ti, V, and Nb, could become the source materials for the in situ formation of transition metal oxides. These transition metal elements with multivalence are poised to facilitate the reaction with H2O2 for Fenton-like catalysis (24–27).
Here, we propose an in situ construction of Fenton-like catalysts by turning surface defects into catalytic active sites. Exfoliated Ti3C2 MXene was subjected to mild oxidation by H2O2 to create titanium oxide nanoclusters anchoring on a silk-like carbon substrate (TiOx@C). Density functional theory (DFT) calculations revealed that the redox reaction occurred at the Ti-deficit defects of exfoliated Ti3C2 MXene. The multivalence of Ti and surface defects in TiOx@C were fully characterized by X-ray photoelectron spectroscopy (XPS), X-ray absorption fine structure (XAFS), and positron annihilation life spectroscopy (PALS). The effectiveness and stability of the Fenton-like catalytic activity were assessed based on the degradation of atrazine, a typical agriculture herbicide. The universalness of the strategy was also tested on other MXene family materials, including V2C and Nb2C.
Results and Discussion
In Situ Formation of Carbon-Supported Titanium Oxide (TiOx@C) Templated by Exfoliated MXene.
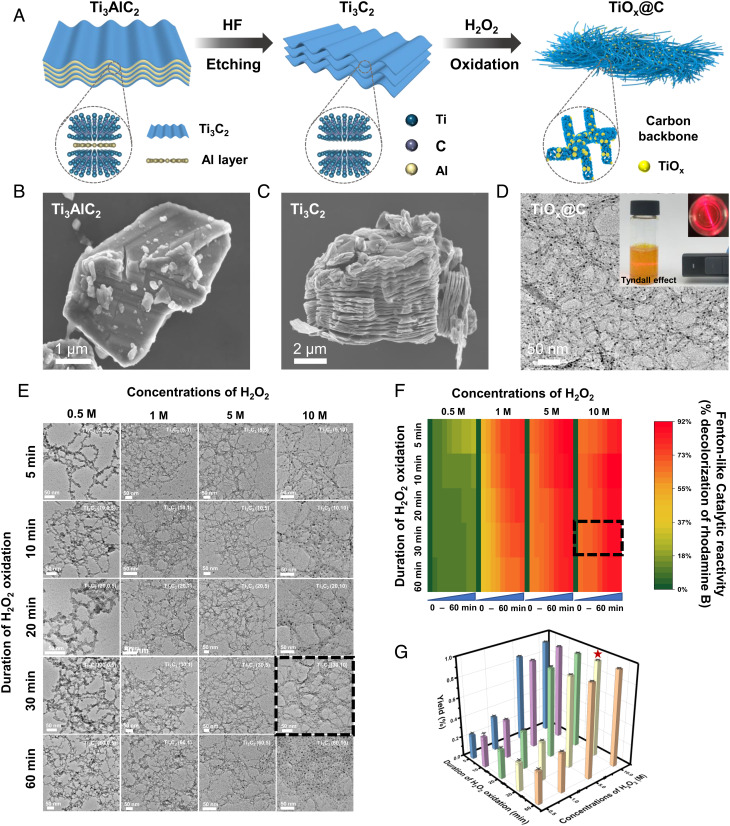
Fig. 1A illustrates the main steps of in situ formation of TiOx@C. Bulk Ti3AlC2 with its Al layer etched away by hydrofluoric acid (HF) was further treated with H2O2. The typical accordion-like structure of Ti3C2 MXene under scanning electron microscopy (SEM) demonstrated a successful etching of the Al layer (Fig. 1 B and C). The final product displayed a silk-like structure in light gray color decorated with darker dots of 2 to 5 nm on a microscopic scale and a uniform and stably-dispersed yellow-orange suspension showing the Tyndall effect (Fig. 1 D, Inset). Elemental analysis (energy-dispersive X-ray spectroscopy mapping) confirmed the absence of the Al element in the etched Ti3C2 MXene (SI Appendix, Fig. S1). electron paramagnetic resonance (EPR) analysis showed a clear signal at g = 1.946 of the exfoliated Ti3C2 MXene, suggesting the presence of single Ti vacancy or vacancy clusters after HF etching (SI Appendix, Fig. S2). The EPR signal was indicative of the existence of Ti3+ defects (28). Based on transmission electron microscopy (TEM) observation, increasing concentrations (0.5 to 10 M) and treatment duration (5 to 60 min) of H2O2 improved the uniformity of the nanoclusters decorated on the silk-like structure. And the overall morphology remained the same after the treatment of 10 M H2O2 for 30 min, suggesting that the H2O2 was fully consumed (Fig. 1E). These characteristics suggested a likely composition of the final product, i.e., titanium oxide nanoclusters decorated on thin carbon layers (TiOx@C). The extent of decolorization of rhodamine B was used to evaluate the Fenton-like catalytic activity of TiOx@C. As shown in Fig. 1F, a higher extent of RhB decolorization was associated with a higher amount of darker dots in the silk-like structure. Moreover, the percent yield of TiOx@C quantified by the weight percentage of the final product vs. the exfoliated Ti3C2 MXene was approximately 97%, showing an almost complete use of the source materials (Fig. 1G). Since HF exfoliation of MXene could lead to the breakage of Ti–Al bonds, resulting in Ti vacancy or vacancy clusters on the MXene substrate, it is reasonable to hypothesize that the resulted defects could play a vital role in the formation of TiOx nanoclusters (23). Since these defects were highly oxophilic, exfoliated MXene was expected to abstract oxygen from chemical compounds such as H2O2 to form oxides (29). And such a reaction would allow the in situ formation of nanoclusters with an intimate interface with the MXene substrate (30).
Fig. 1.
Synthesis and characterizations of TiOx@C catalyst. (A) Schematic description of in situ formation of TiOx@C templated by exfoliated Ti3C2 MXene. SEM images of bulk Ti3AlC2 (B) and Ti3C2 (C) displaying a typical accordion-like structure after HF etching. (D) TEM image of TiOx@C with TiOx nanoclusters (dark dots) decorated on an amorphous carbon backbone (light gray). Inset: photograph of TiOx@C aqueous dispersion showing the typical Tyndall effect. (E) TEM images of TiOx@C fabricated by increasing concentrations of H2O2 (0.5 M, 1 M, 5 M, and 10 M) and duration of oxidation (5 min, 10 min, 20 min, 30 min, and 60 min). (F) Heat map of the Fenton-like catalytic performance of TiOx@C based on decolorization of rhodamine B. Red to green indicates high to low activities. (G) Yield (%) of TiOx@C from oxidation of Ti3C2 by variations of H2O2 concentrations and duration of oxidation.
DFT Calculations Revealed the Key Reaction for Nanocluster Formation.
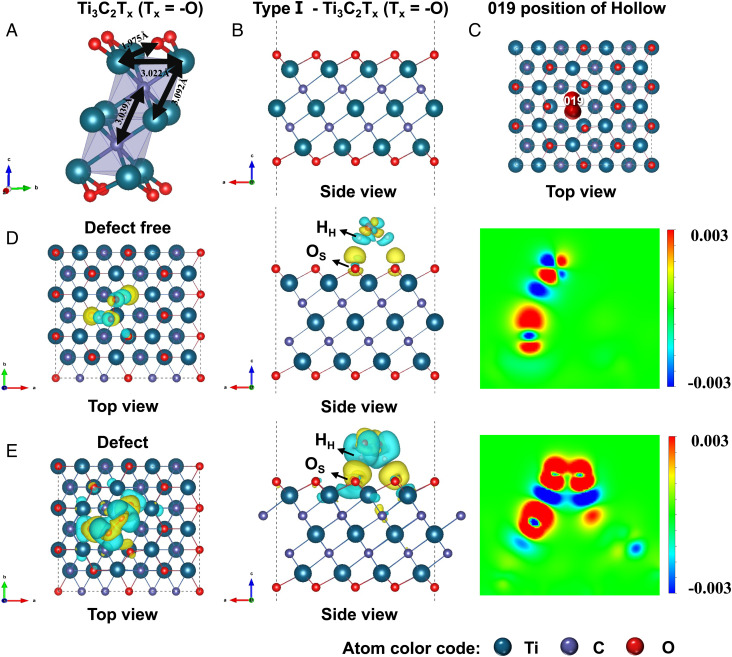
DFT calculations were conducted to validate the hypothesis on the in situ formation of TiOx@C. The free-standing Ti3C2 and two typical Ti3C2Tx (Tx = −O or −OH) monolayer structural forms were constructed (as shown in Fig. 2A and SI Appendix, Figs. S3 and S4) with the optimized geometries (types I, II, and III) delineated in details (SI Appendix, Figs. S5 and S6). Among them, the type I structure of Ti3C2Tx (Tx = −O) unit cells was selected to build up models based on the lowest total energies (Fig. 2B and SI Appendix, Table S1). The likely adsorption sites for H2O2 molecules on the exfoliated MXene were calculated to be twenty-seven adsorption positions (top (T), bridge (B), and hollow (H), nine positions in each case) (SI Appendix, Figs. S7 and S8). As shown in Fig. 2C and SI Appendix, Table S2, the 019 position of hollow (H) was the most likely adsorption site due to the lowest adsorption energy. In contrast to the defect-free MXene (Fig. 2D), the presence of surface Ti-deficit vacancies brought distortion to neighboring atoms and led to a clear electronic delocalization (Fig. 2E). The HH atom in the adsorbed H2O2 molecule showed a strong interaction with the surface O of Ti-deficit MXene (Os). The DFT calculations further strengthened our speculation that the surface Ti-deficit vacancies in the exfoliated Ti3C2 were the key reactive sites for the formation of TiOx nanoclusters.
Fig. 2.
DFT calculations. (A) Optimized geometries of the Ti3C2Tx (Tx = −O) monolayer structural forms. (B) Side views of type I—Ti3C2Tx (Tx = −O). (C) Top view of adsorption behaviors between type I—Ti3C2Tx (Tx = −O) and H2O2 molecule at the lowest energy 019 hollow adsorption site. The bonding charge density of the Ti3C2Tx (Tx = −O) at the defect-free system (D) and Ti-defected system (E). Red and blue colors indicate electron accumulation and depletion, respectively. The color scale is in the units of 0.001 e bohr−3. Atom color code: titanium (teal), carbon (indigo blue), and oxygen (red).
Physicochemical Characterizations and Defect Analyses of TiOx@C.
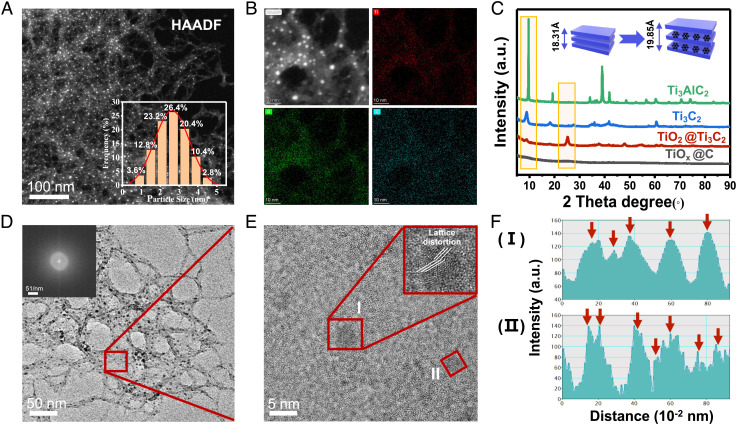
High-angle annular dark-field microscopy (HAADF–STEM) revealed an average size of 2.66 nm for the TiOx nanoclusters (Fig. 3A) and a uniform distribution of Ti and O in the carbon substrate (Fig. 3B). TiOx@C showed excellent dispersibility with a hydrodynamic diameter of 187.43 ± 11.54 nm and a negative surface charge of −27.57 ± 0.42 mV (SI Appendix, Table S3). In the XRD spectra of Ti3AlC2 and Ti3C2 (Fig. 3C), a gradual widening of the 2θ peak at 9.5° indicated increasing interlayer spacing among the exfoliated MXene. The disappearance of the peak after the formation of TiOx@C suggested that the layered structure no longer existed. And the TiOx@C did not show any clear indication of crystallinity, which was consistent with the morphology observed under TEM (Figs. 1F and 3D). This was in clear contrast to loading anatase TiO2 on exfoliated T3C2 MXene (SI Appendix, Fig. S9), where a clear lattice structure of anatase TiO2 (a lattice fringe of 0.35 nm) could be obtained. This result indicated that the in situ formation of TiOx was likely not in the form of typical TiO2 but as TiOx nanoclusters embedded in an amorphous structure. Consistently, the selected area electron diffraction (SAED) patterns of TiOx@C under TEM had no discrete diffraction rings against the clear and continuous crystalline lattice of Ti3AlC2 and Ti3C2 (SI Appendix, Fig. S10). More importantly, lattice distortion defects were prominent throughout the TiOx@C as revealed by high-resolution TEM (HRTEM) (Fig. 3E). The inverse fast Fourier transform (IFFT) profile of two representative areas (I) and (II) showed typical distortion patterns in the TiOx@C (Fig. 3F).
Fig. 3.
Structural and defect characterizations of TiOx@C catalyst. (A) HAADF–STEM image of TiOx@C confirming the presence of TiOx nanoclusters (bright spots) and the corresponding size distribution (Inset). (B) Energy-dispersive X-ray elemental mapping of TiOx@C, suggesting that titanium and oxygen were uniformly distributed in the carbon backbone. (C) XRD spectra of Ti3AlC2, Ti3C2, TiO2@Ti3C2, and TiOx@C. (D) HRTEM image of TiOx@C and the corresponding electron diffraction pattern image (Inset). The red box highlights the area shown in E. (E) HRTEM image with lattice distortion highlighted (areas I and II). (F) Intensity profile of areas I and II in (E).
PALS was conducted to determine the types and relative quantities of the defects in TiOx@C. By detecting the time intervals of positron trapped, the position and types of defects can be distinguished. Meanwhile, the ratio of relative intensities indicated the contents of the respective detects (31, 32). Table 1 summarizes two positron lifetime components (τ1 and τ2) that corresponded to two typical defects with relative intensities I1 and I2 for TiOx@C compared with Ti3AlC2, Ti3C2, and TiO2@Ti3C2. The shorter lifetime τ1 was ascribed to the defects in the bulk, while the longer lifetime τ2 was ascribed to the defects on the surface or subsurface of the materials (32, 33). Among them, τ1 for TiOx@C (113.0 ps) was the smallest among all samples. This is likely due to the ultrathin structure of TiOx@C rendering little to no bulk structure. For the surface defects, τ2 for TiOx@C (371.0 ps) was close to that of Ti3C2 (374.0 ps), indicating that the defects within these materials were likely on the surface or subsurface. More importantly, the ratio of relative intensities (I2 vs. I1) provided information on the relative concentrations of the defects within the materials. The I2 vs. I1 value of TiOx@C was the highest (I2/I1 = 6.536) among all samples, indicating the highest amount of surface defects. Meanwhile, the I2 vs. I1 value of Ti3C2 (I2/I1 = 1.977) was about 2.5 times higher than that of Ti3AlC2 (I2/I1 = 0.798), reconfirming that the HF exfoliation process did indeed create surface defects.
Table 1.
Positron lifetime parameters of Ti3AlC2, Ti3C2, TiO2@Ti3C2, and TiOx@C
| Sample name | τ1 (ps) | I1 (%) | τ2 (ps) | I2 (%) | I2/I1 |
|---|---|---|---|---|---|
| TiOx@C | 113.0 | 13.27 | 371.0 | 86.73 | 6.536 |
| Ti3AlC2 | 156.0 | 55.62 | 315.0 | 44.38 | 0.798 |
| Ti3C2 | 145.0 | 33.59 | 374.0 | 66.41 | 1.977 |
| TiO2@Ti3C2 | 158.0 | 27.01 | 359.0 | 72.99 | 2.702 |
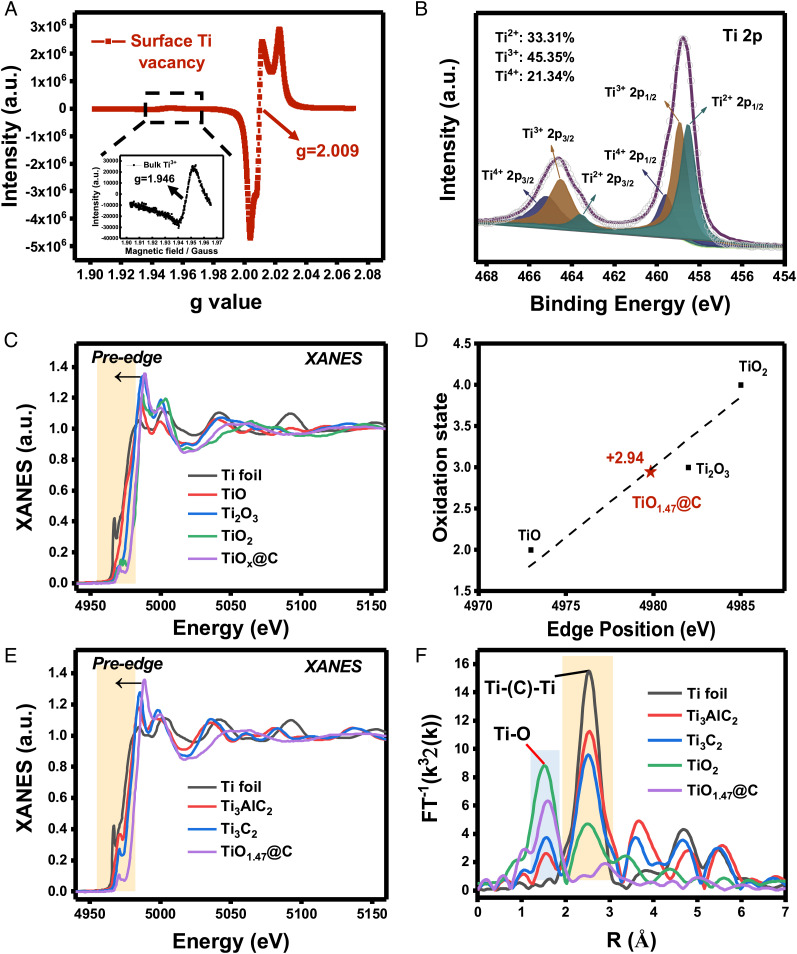
A strong EPR signal at g = 2.009 was detected in TiOx@C (Fig. 4A). This signal did not match with the reported EPR signal of Ti3+ defects (g = 1.940 to 1.990) (34, 35), O2−(g = 2.020) (36), or oxygen vacancies (Vo) (g = 2.003) (37), suggesting that the surface Ti vacancy of TiOx@C was likely situated in a new coordination environment due to the oxidation of H2O2. Meanwhile, a relatively weak EPR signal at g = 1.946 was still present (Fig. 4 A, Inset), indicating that the Ti3+ defects previously detected in the exfoliated T3C2 were still available in the TiOx@C.
Fig. 4.
Chemical state and atomic local structure of TiOx@C catalyst. (A) EPR spectra (77K) of TiOx@C showing a clear surface Ti vacancy signal (g = 2.009) and a significantly weaker bulk Ti3+ signal (g = 1.946, Inset). (B) High-resolution XPS spectrum of Ti 2p. The percentage of valences of Ti element was calculated as Ti2+: 33.31%, Ti3+: 45.35%, and Ti4+: 21.34%. (C) Normalized Ti K-edge XANES spectra of Ti foil, TiO, Ti2O3, TiO2, and TiOx@C. (D) Estimation of the titanium oxidation state in TiOx@C. According to the XANES spectra of Ti from the edge position of references to TiO, Ti2O3, and TiO2, Ti was calculated to be in an average of 2.94+ oxidation state in TiOx@C, with x = 1.47. (E) Normalized Ti K-edge XANES spectra of Ti foil, Ti3AlC2, Ti3C2, and TiO1.47@C, respectively. (F) The k3-weighted FT spectra from Ti K-edge EXAFS.
Chemical State and Local Atomic Structure of TiOx@C.
As the evidence of the existence of multivalence-Ti became clearer, XPS was conducted to show the presence of multipeaks of Ti2+ (33.31%), Ti3+ (45.35%), and Ti4+ (21.34%) in the Ti 2p spectrum (Fig. 4B). The local structure at the atomic level was further revealed by X-ray absorption near-edge structure (XANES). As shown in Fig. 4C and SI Appendix, Table S4, the near-edge absorption energy of TiOx@C was situated among standard samples (i.e. Ti foil, TiO, Ti2O3, and TiO2), indicating the positive charge of Tiδ+ was between Ti0 and Ti4+. In addition, the first-order derivative of near-edge absorption energy was analyzed to obtain the energy of the white line peak (E0), which showed a linear relationship with the valence of the Ti element. This allowed the calculation of the titanium element at an average of 2.94+ oxidation state in TiOx@C, resulting in x = 1.47 (Fig. 4D) (38–40). TiO1.47@C was used from here on for the ease of expression. The Ti K-edge XANES analysis (Fig. 4E) showed that the edge energy of TiO1.47@C was higher than that of Ti3AlC2 and Ti3C2. And the Ti K-edge extended x-ray absorption fine structure (EXAFS) spectra (SI Appendix, Fig. S11) demonstrated that the k3χ(k) oscillation displayed a noticeable difference in the range of 2 to 14 Å−1 in comparison with pure Ti foil, Ti3AlC2, and Ti3C2, implying a different local atomic arrangement around Ti. More importantly, the Fourier transform (FT) spectra displayed two peaks in the range of 1.5 to 3.0 Å corresponding to the Ti-O and Ti-C-Ti bonds (Fig. 4F). The first-shell (Ti-O) scattering area of TiO1.47@C increased significantly against Ti3AlC2 and Ti3C2, proving the formation of the Ti-O bonds. And the second-shell (Ti-C-Ti) scattering showed a right shift with a remarkable amplitude reduction compared with TiO2, Ti3AlC2, and Ti3C2. This was likely a result of collapsed layer structure to form the thin carbon layer (41). The presence of the carbon substrate was important to maintain TiO1.47@C at a relative steady state or metastable state. And the thin layer of amorphous carbon was not likely to interfere with the electron transfer during the Fenton-like reaction with H2O2 (42, 43).
Fenton-Like Catalytic Performance of TiO1.47@C toward the Degradation of Atrazine.
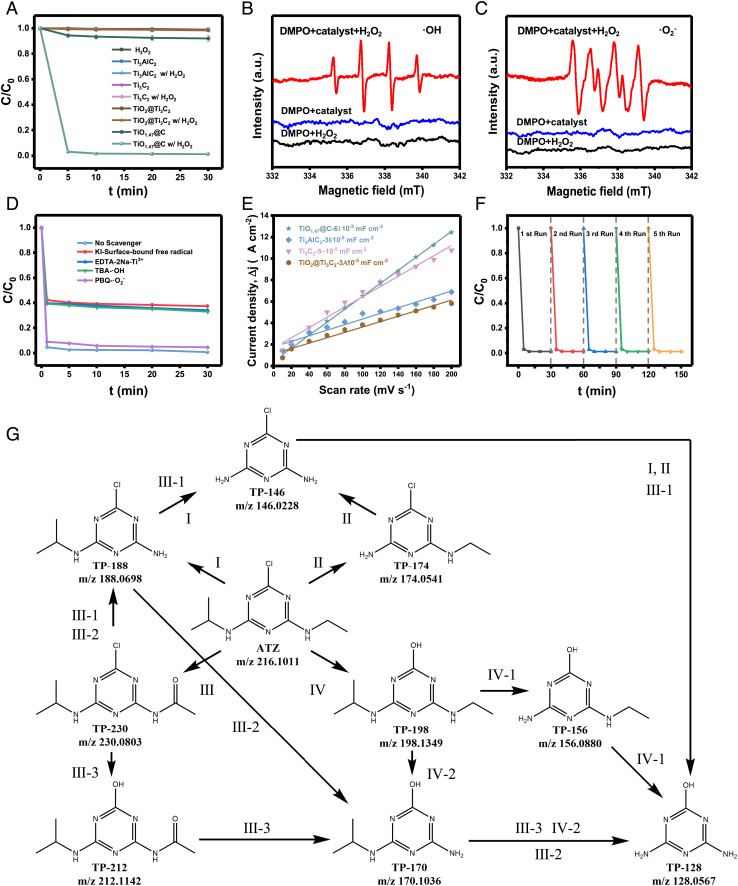
The Fenton-like catalytic performance of TiO1.47@C was evaluated toward the degradation of the typical agriculture herbicide atrazine (ATZ) via activation of H2O2. As shown in Fig. 5A, TiO1.47@C ([Ti] = 0.1 g L−1) showed superior reactivity to activate H2O2 (5 mM) to degrade ATZ (2.5 mg L−1) within 5 min compared with Ti3AlC2, Ti3C2, and TiO2@Ti3C2 under the same conditions. Neither TiO1.47@C nor H2O2 (5 mM) alone showed any degradation. The existence of multivalence-Tiδ+ (δ = 0, 2, 3, and 4) was the key to activating H2O2 for ATZ degradation.
Fig. 5.
Fenton-like catalytic performance and stability assessment of TiO1.47@C. (A) TiO1.47@C showed superior Fenton-like catalytic degradation of atrazine. (B) DMPO spin-trapping EPR spectra of DMPO-·OH (water system). (C) DMPO spin-trapping EPR spectra of DMPO-·O2− (methanol system). (D) Effects of quenching agents (KI, EDTA-2Na, TBA, and PBQ) on the Fenton-like degradation of atrazine. (E) Estimated double-layer capacitances of TiO1.47@C in comparison with Ti3AlC2, Ti3C2, and TiO2@Ti3C2. (F) Stability assessment of the Fenton-like degradation efficiency for five runs. (G) Proposed Fenton-like degradation pathways of atrazine followed by the identification of several intermediates by UHPLC–QTOF–MS. Conditions: [Ti] = 0.1 g L−1, [atrazine] = 2.5 mg L−1, and [H2O2] = 5 mM.
DMPO-trapped EPR further demonstrated the generation of ·OH and ·O2− in the Fenton-like catalytic reaction. Fig. 5 B and C showed clear signals from DMPO-·OH and DMPO-·O2−. The introduction of radical scavengers, i.e., p-benzoquinone (PBQ), tert-butyl alcohol (TBA), and EDTA-2Na, decreased the extent of ATZ degradation by 3.9%, 29.8%, and 30.3%, respectively. The surface-bound free radical quencher, potassium iodide (KI) was also able to significantly decrease the extent of degradation similar to that of TBA and EDTA-2Na (Fig. 5D). These results suggested that the Fenton-like catalytic degradation of ATZ was likely through a surface catalytic process. Consistently, the electrochemically active surface area (ECSA) measurements revealed that TiO1.47@C possessed the highest double-layer capacitance (Cdl) value of 6 × 10−5 mF cm−2, suggesting it had more active surface areas for catalytic reactions (Fig. 5E and SI Appendix, Fig. S12). Moreover, five repeated cycles of Fenton-like reactions showed little changes in the degradation efficiency (Fig. 5F). Neither the crystalline phase nor the morphology showed any changes after five Fenton-like reactions (SI Appendix, Fig. S13). The shelf life of TiO1.47@C was highly desirable as it demonstrated good structural stability beyond 15 mo (SI Appendix, Fig. S14). Interestingly, the solution pH (from 3 to 11) had no interference effect on the degradation efficiency (SI Appendix, Fig. S15). Such stability was consistent with the surface charge measurement, where the zeta potential of TiO1.47@C remained constant within the same pH range (SI Appendix, Table S5). This further confirmed that the interactions between the TiO1.47@C and H2O2 governed the overall catalytic reactivity.
The transformation products (TPs) of ATZ delineated by UHPLC–QTOF–MS also confirmed the oxidation process. Among the nine typical TPs detected, the formation of deethylatrazine (DEA, m/z 188) and deisopropylatrazine (DIA, m/z 174) was due to deethylation and deisopropylation, respectively (SI Appendix, Fig. S16 and Table S6). Similar processes were observed for the transformation from hydroxy atrazine (HA, m/z 198) to deethylhydroxy atrazine (DEHA, m/z 170) and further to deethyldeisopropylhydroxyatrazine (DEIHA, m/z 128). In addition, the oxidation of the amide group in 2-chloro-4-acetamido-6-isopropylamino-1,3,5-triazine (CAIT, m/z 230) might contribute to the formation of partial DEA (m/z 188). The final product DEIHA (m/z 128) was likely formed via successive amide hydrolysis and deisopropylation. The detailed analysis of the seven transformation pathways and three main mechanisms (e.g., dealkylation, alkyl chain oxidation, dechlorination, and hydroxylation) are depicted in Fig. 5G and further explained in SI Appendix. These results confirmed that the degradation of ATZ was mainly through oxidation, in which the dealkylation was likely initialized by the attack of hydroxyl radical (·OH) and further produced carbon-center radicals that led to a cascade of oxidation events (44). And the main TP CAIT (m/z 230) was formed via the oxidation of an intermediate carbinolamine by hydroxyl radical (·OH) (45). In addition, the excellent degradation efficiencies toward different types of pollutants also indicated that the TiO1.47@C/H2O2 system was capable of nonselectively decomposing a variety of organic substances in aqueous systems. Compared with the traditional Fenton system, which showed significant generation of Fe sludge after the Fenton reaction, TiO1.47@C remained highly dispersed after the degradation (SI Appendix, Fig. S17). The excellent dispersibility resulted in some difficulties in recycling and reusing, and embedding these active catalysts onto suitable substrates to form nanocomposites could be a feasible solution (46).
Application of the In Situ Fenton-Like Catalyst Construction Strategy on Other MXene Family Materials.
The universal applicability of the in situ strategy was tested on other MXene family materials, e.g., V2C and Nb2C. SEM (SI Appendix, Figs. S18 A and B and S19 A and B) and TEM (SI Appendix, Figs. S18C and S19C) micrographs showed similar morphological features compared with TiO1.47@C. Both materials showed excellent Fenton-like catalytic performance toward the decolorization of RhB (SI Appendix, Figs. S18D and S19D). It is worth noting that the easiness of exfoliation of MXene correlated with the Fenton-like catalytic performance of the resulting catalysts.
In conclusion, by in situ turning defects into catalytic active sites, we have successfully demonstrated a strategy to turn exfoliated MXene into Fenton-like catalysts. The redox reaction between the Ti-deficit defects after exfoliation and H2O2 resulted in highly dispersed TiO1.47 nanoclusters decorated on a silk-like carbon substrate. The catalysts showed excellent and stable Fenton-like catalytic activity toward the degradation of RhB and atrazine. Theoretical calculations and defect analyses revealed that the Ti-deficit vacancies were the key to the in situ formation of the TiO1.47@C, and the superior Fenton-like catalytic performance came from the abundance of surface defects and the existence of multivalence-Tiδ+ (δ = 0, 2, 3, and 4). Such an approach also applies to other MXene family materials, such as V2C and Nb2C. Our work showcased a strategy to use MXene materials as both substrate and multivalent metal precursor for the in situ formation of heterogeneous Fenton-like catalysts.
Materials and Methods
Synthesis of TiO1.47@C.
Ti3C2 MXene was obtained by a common exfoliation method using HF (40%). Dry powder (100 mg) of the exfoliated Ti3C2 was then dispersed in deionized water by magnetic stirring at room temperature for 10 min. A mild oxidation reaction was conducted by adding H2O2 (0.5 M, 1 M, 5 M, and 10 M) to the Ti3C2 suspension, and the reaction was kept at 0°C under an ice water bath for a series of duration (5 min, 10 min, 20 min, 30 min, and 60 min). The resulted dispersion was centrifuged for 20 min at 8,000 rpm to remove large agglomerates, and the supernatant was collected as the final product. All samples obtained were placed under 4°C for storage.
Physicochemical Characterizations.
Microscopic and spectroscopic techniques were applied for physicochemical characterizations. The surface morphology and microstructure were characterized by SEM (Hitachi S4800, 3 kV) and TEM (JEM-2011, 200 kV). HRTEM and scanning transmission modes (STEM) were performed using TALOS F200X, 200 kV, equipped with an energy-dispersive spectrometer. The crystalline structure of as-prepared samples was characterized based on X-ray diffraction (XRD) patterns using a D-8 advanced X-ray diffractometer (Bruker-AXS) with Cu Kα radiation operated at a voltage of 40 kV and a current of 40 mA at a scanning speed of 5° min−1. The concentration of Ti element of the as-prepared samples was analyzed by an inductively coupled plasma spectrometer (ICP; ICP7700, Agilent). The hydrodynamic size and zeta potential of the as-prepared samples were measured by a Zetasizer Nano ZS instrument (Malvern Instruments). X-ray photoelectron spectrum (XPS) analysis was conducted with a PHI-1600 X-ray photoelectron spectroscope equipped with Al Kα radiation, and the binding energy was calibrated by the C 1 s peak (284.8 eV). EPR spectroscopy measurements were carried out on a Bruker EMX EPR spectrometer at an X-band frequency of 9.363 GHz, sweep width of 500.00 gauss, and center field of 3390.00 gauss, with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the spin-trapping agents for reactive species. The information on various defects was characterized by positron annihilation lifetime spectroscopy (PALS) under an ORTEC-583 fast–fast coincident system using a coincidence spectrometer at room temperature. Further details can be found in SI Appendix.
XAFS Measurements and EXAFS Analysis.
Chemical speciation of Ti was determined by K-edge XANES. XANES spectra of titanium in TiO1.47@C powder were obtained with a Si (111) double crystal monochromator in the transmission model using the beamline of 14W1A in the Shanghai Synchrotron Radiation Facility. Ti foil, TiO, Ti2O3, TiO2, Ti3AlC2, and Ti3C2 were selected as references. The electron beam energy of the storage ring was 3.5 GeV, and the maximum stored current was approximately 210 mA. The acquired EXAFS data were processed according to the standard procedures using the ATHENA module implemented in IFEFFIT software packages. The k3-weighted EXAFS spectra were obtained by subtracting the postedge background from the overall absorption and then normalizing with respect to the edge-jump step. Subsequently, k3-weighted χ(k) data in the k-space ranging from 2 to 14 Å−1 were Fourier transformed to real (R) space using a Hanning window (dk = 1.0 Å−1) to separate the EXAFS contributions from different coordination shells (47, 48).
DFT Calculations.
All calculations were based on the DFT using Vienna ab initio simulation package (VASP). The electron–ion interaction was described by the projected augmented wave (PAW) method. The exchange–correlation functional was described within the generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE) functional. The van der Waals interactions were modeled using the DFT-D3. The Brillouin zone integration was obtained by an 8 × 8 × 1 Monkhorst–Pack k-points mesh, and the energy cutoff was set to 500 eV. The atomic positions were optimized using the conjugate gradient algorithm and the convergence threshold was set at 1 × 10−6 eV atom−1. For all the calculations, the Hellmann–Feynman force on each atom was relaxed to a convergence criterion of 0.01 eV Å−1.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by the National Key Research and Development Program of China (no. 2018YFC1803100), the National Natural Science Foundation of China (no. 21777116), and the Fundamental Research Funds for the Central Universities.
Author contributions
Y.J. and S.L. designed research; Y.J., D.B., S.J., J.C.-F.L., P.Z., J.T., J.P., L.W., K.S.-Y.L., and W.S. performed research; Y.J., D.B., S.J., J.C.-F.L., P.Z., J.T., J.P., L.W., K.S.-Y.L., W.S., and S.L. analyzed data; and Y.J. and S.L. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and SI Appendix.
Supporting Information
References
- 1.Shannon M. A., et al. , Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Li S., Qin Q., Peng C., Sustainable and feasible reagent-free electro-Fenton via sequential dual-cathode electrocatalysis. Proc. Natl. Acad. Sci. U.S.A. 118, e2108573118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiao B., et al. , Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Zhou X., et al. , Identification of Fenton-like active Cu sites by heteroatom modulation of electronic density. Proc. Natl. Acad. Sci. U.S.A. 119, e2119492119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Y., Lu B., Chen S., Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Adv. Mater. 30, 1801995 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Liu G., et al. , MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 9, 810–816 (2017). [DOI] [PubMed] [Google Scholar]
- 7.White R. J., Luque R., Budarin V. L., Clark J. H., Macquarrie D. J., Supported metal nanoparticles on porous materials . Methods and applications. Chem. Soc. Rev. 38, 481–494 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Wu J.-X., et al. , Graphene-like hydrogen-bonded melamine-cyanuric acid supramolecular nanosheets as pseudo-porous catalyst support. Adv. Mater. 33, 2007368 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Yin Y., et al. , Boosting Fenton-like reactions via single atom Fe catalysis. Environ. Sci. Technol. 53, 11391–11400 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., et al. , The simplest construction of single-site catalysts by the synergism of micropore trapping and nitrogen anchoring. Nat. Commun. 10, 1657 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhiman M., Polshettiwar V., Supported single atom and pseudo-single atom of metals as sustainable heterogeneous nanocatalysts. Chemcatchem 10, 881–906 (2018). [Google Scholar]
- 12.Ramalingam V., et al. , Heteroatom-mediated interactions between ruthenium single atoms and an MXene support for efficient hydrogen evolution. Adv. Mater. 31, 1903841 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Li X., et al. , Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 140, 12469–12475 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Li X., et al. , Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution. Adv. Mater. 28, 2427–2431 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Fei H., et al. , General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018). [Google Scholar]
- 16.Yang J., et al. , Efficient and robust hydrogen evolution: Phosphorus nitride imide nanotubes as supports for anchoring single ruthenium sites. Angew. Chem. Int. Ed. 57, 9495–9500 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y., Qin Y., Yu T., Lin S., Synthesis of sponge-like TiO2 with surface-phase junctions for enhanced visible-light photocatalytic performance. Chin. Chem. Lett. 32, 1823–1826 (2021). [Google Scholar]
- 18.Naguib M., Mochalin V. N., Barsoum M. W., Gogotsi Y., 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 26, 992–1005 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Naguib M., et al. , Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Naguib M., Barsoum M. W., Gogotsi Y., Ten years of progress in the synthesis and development of MXenes. Adv. Mater. 33, 2103393 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., et al. , Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 1, 985–992 (2018). [Google Scholar]
- 22.Sang X., et al. , Atomic defects in monolayer titanium carbide (Ti3C2Tx) MXene. ACS Nano. 10, 9193–9200 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Zhao D., et al. , MXene (Ti3C2) vacancy-confined single-atom catalyst for efficient functionalization of CO2. J. Am. Chem. Soc. 141, 4086–4093 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Li X., et al. , Nonoxidized MXene quantum dots prepared by microexplosion method for cancer catalytic therapy. Adv. Funct. Mater. 30, 2000308 (2020). [Google Scholar]
- 25.Zhang M., Niu Y., Xu Y., Heterogeneous Fenton-like magnetic nanosphere coated with vanadium oxide quantum dots for enhanced organic dyes decolorization. J. Colloid Interface Sci. 579, 269–281 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Pouran S. R., Aziz A. R. A., Daud W. M. A. W., Embong Z., Niobium substituted magnetite as a strong heterogeneous Fenton catalyst for wastewater treatment. Appl. Surf. Sci. 351, 175–187 (2015). [Google Scholar]
- 27.Bokare A. D., Choi W., Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 275, 121–135 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y., Chen C., Wang N., Li Y., Ding H., Stable Ti3+ self-doped anatase-rutile mixed TiO2 with enhanced visible light utilization and durability. J. Phys. Chem. C 120, 6116–6124 (2016). [Google Scholar]
- 29.Cheng X., et al. , A titanium-based photo-Fenton bifunctional catalyst of mp-MXene/TiO2-x nanodots for dramatic enhancement of catalytic efficiency in advanced oxidation processes. Chem. Commun. 54, 11622–11625 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Chen C., et al. , MoS2-on-MXene heterostructures as highly reversible anode materials for lithium-ion batteries. Angew. Chem. Int. Ed. 57, 1846–1850 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Kong M., et al. , Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 133, 16414–16417 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Li C., et al. , Enhanced visible-light-driven photocatalytic hydrogen generation using NiCo2S4/CdS nanocomposites. Chem. Eng. J. 378, 122089 (2019). [Google Scholar]
- 33.Dutta S., et al. , Annealing effect on nano-ZnO powder studied from positron lifetime and optical absorption spectroscopy. J. Appl. Phys. 100, 114328 (2006). [Google Scholar]
- 34.Khomenko V. M., Langer K., Rager H., Fett A., Electronic absorption by Ti3+ ions and electron delocalization in synthetic blue rutile. Phys. Chem. Miner. 25, 338–346 (1998). [Google Scholar]
- 35.Wang S., et al. , Titanium-defected undoped anatase TiO2 with p-type conductivity, room-temperature ferromagnetism, and remarkable photocatalytic performance. J. Am. Chem. Soc. 137, 2975–2983 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Anpo M., et al. , Generation of superoxide ions at oxide surfaces. Top. Catal. 8, 189–198 (1999). [Google Scholar]
- 37.Liu H., et al. , The enhancement of TiO2 photocatalytic activity by hydrogen thermal treatment. Chemosphere 50, 39–46 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Zhao H., et al. , The role of Cu1-O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 5, 818–831 (2022). [Google Scholar]
- 39.Zhang E., et al. , Engineering the local atomic environments of indum single-atom catalysts for efficient electrochemical production of hydrogen peroxide. Angew. Chem. Int. Ed. 61, e202117347 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Zhang F., et al. , High-efficiency electrosynthesis of hydrogen peroxide from oxygen reduction enabled by a tungsten single atom catalyst with unique terdentate N1O2 coordination. Adv. Funct. Mater. 32, 2110224 (2021). [Google Scholar]
- 41.Lotfi R., Naguib M., Yilmaz D. E., Nanda J., van Duin A. C. T., A comparative study on the oxidation of two-dimensional Ti3C2 MXene structures in different environments. J. Mater. Chem. A 6, 12733–12743 (2018). [Google Scholar]
- 42.Guinea F., Charge distribution and screening in layered graphene systems. Phys. Rev. B 75, 235433 (2007). [Google Scholar]
- 43.Chen H., et al. , Measurement of interlayer screening length of layered graphene by plasmonic nanostructure resonances. J. Phys. Chem. C 117, 22211–22217 (2013). [Google Scholar]
- 44.Tauber A., von Sonntag C., Products and kinetics of the OH-radical-induced dealkylation of atrazine. Acta Hydrochim. Hydrobiol. 28, 15–23 (2000). [Google Scholar]
- 45.Nelieu S., Kerhoas L., Einhorn J., Degradation of atrazine into ammeline by combined ozone/hydrogen peroxide treatment in water. Environ. Sci. Technol. 34, 430–437 (2000). [Google Scholar]
- 46.Vaia R. A., Wagner H. D., Framework for nanocomposites. Mater. Today 7, 32–37 (2004). [Google Scholar]
- 47.Ding J., et al. , Single-particle analysis for structure and iron chemistry of atmospheric particulate matter. Anal. Chem. 92, 975–982 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Liu J., et al. , Rapid degradation and high renal clearance of Cu3BiS3 nanodots for efficient cancer diagnosis and photothermal therapy in vivo. ACS Nano. 10, 4587–4598 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and SI Appendix.