Abstract
Unraveling cell–cell interaction is fundamental to understanding many biological processes. To date, genetic tools for labeling neighboring cells in mammals are not available. Here, we developed a labeling strategy based on the Cre-induced intercellular labeling protein (CILP). Cre-expressing donor cells release a lipid-soluble and membrane-permeable fluorescent protein that is then taken up by recipient cells, enabling fluorescent labeling of neighboring cells. Using CILP, we specifically labeled endothelial cells surrounding a special population of hepatocytes in adult mice and revealed their distinct gene signatures. Our results highlight the potential of CILP as a platform to reveal cell–cell interactions and communications in vivo.
Keywords: genetic tool, intercellular interactions, membrane-permeable protein, liver zonation, niche
Cell–cell interactions are essential to direct cellular behaviors during many physiological and pathological processes (1, 2). Several methods have been reported recently to dissect cell interactions. PIC-seq and ProximID were developed to sort and analyze adjacent cells that are attached to each other (3–5). Based on physical contact, synthetic Notch receptors were also used to label and manipulate the interacting cells (6). Additionally, the Sortase A transpeptidase and FucoID were applied to monitor interactions between T and dendritic cells (7). Furthermore, cell-penetrating fluorescent protein has also been used to label tumor microenvironment in mice by cell transplantation (8). Recently, trans-Tango, TRACT, and BAcTracer have been reported for transsynaptic labeling in drosophila (9–11), providing a flexible platform for neuronal circuit analysis. Despite its importance, a genetic tool that defines and labels neighboring cells of a specific cell type in mammals has yet to be developed.
Results
In principle, lipid-soluble protein (sLP) secreted by donor cells can enter and mark the surrounding recipient cells with a genetic reporter containing a secretary peptide(s) and a transactivator of transcription kappa peptide (8, 12, 13) (Fig. 1 A and B). To label neighboring cells of defined cell types in vivo, we developed a method termed CILP by generating two knock-in mouse lines, R26-sLP-mCh-GFP and R26-sLP-mCh (Fig. 1B). Therefore, these genetic tools allowed specific sLP-mCherry expression driven under the control of cell type-specific Cre.
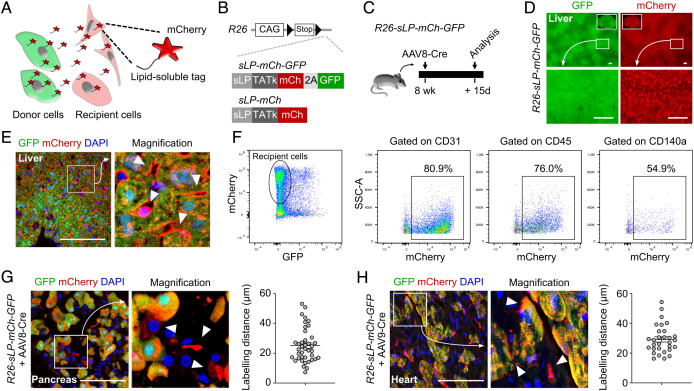
Fig. 1.
Generation of CILP for labeling of neighboring cells in vivo. (A) Cartoon showing the working principle of CILP. (B) Schematic showing generation of R26-sLP-mCh-GFP and R26-sLP-mCh mice lines. (C) Schematic showing the experimental design. (D) Whole-mount fluorescence images of livers from R26-sLP-mCh-GFP after AAV8-Cre treatment. (E) Immunostaining for GFP and mCherry on liver sections from R26-sLP-mCh-GFP after AAV8-Cre treatment. Arrowheads, GFP–mCherry+ cells. (F) FACS analysis of liver samples from R26-sLP-mCh-GFP after AAV8-Cre treatment. (G and H) Immunostaining for GFP and mCherry on sections of pancreas (G) or heart (H) samples from R26-sLP-mCh-GFP 3 wk after AAV-Cre treatment. Arrowheads, GFP–mCherry+ cells. Right panels showing the quantification of labeling distance. n = 40 cells for the pancreas, and n = 30 cells for the heart. Data are representative of 3 mouse samples. (Scale bars, 100 μm.)
For proof-of-concept, we designed liver hepatocytes as donor cells to express sLP-mCherry and GFP and tested whether the nonhepatocytes could serve as recipient cells to be labeled by mCherry. We injected AAV8-TBG-Cre (AAV8-Cre), which specifically targets mouse hepatocytes (14), to R26-sLP-mCh-GFP mice and collected their livers for analyses (Fig. 1C). Whole-mount analysis revealed that the livers exhibited robust GFP and mCherry expression after AAV8-Cre injection (Fig. 1D). It is remarkable to observe that, compared with GFP signal, mCherry was detected in nonhepatocytes as tiny speckles on the liver surface (Fig. 1D). In addition to the GFP+mCherry+ hepatocytes, GFP–mCherry+ cells were observed in the liver samples, such as endothelial cells, immune cells, and fibroblasts (Fig. 1 E and F), indicating the transfer of mCherry protein from GFP+mCherry+ donor hepatocytes to other nonhepatocyte cell lineages. Furthermore, we could also detect the GFP–mCherry+ recipient cells near the GFP+mCherry+ donor acinar cells or near the GFP+mCherry+ donor cardiomyocytes (Fig. 1 G and H). These examples suggested that CILP could be applied in multiple organ systems.
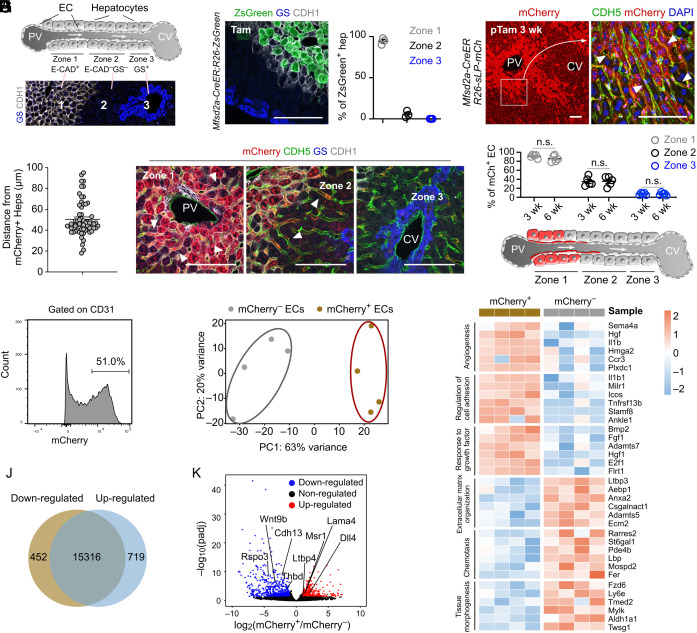
The liver lobules could be divided into three metabolic zones with distinct properties and molecular markers (14) (Fig. 2A). Mfsd2a is a periportal zonation marker (Fig. 2B), and Mfsd2a+ hepatocytes can expand and regenerate during injury (15), suggesting the essential role of these subpopulations in the liver. To illustrate the interaction between a subpopulation of hepatocytes and their specific neighboring ECs, we utilized CILP to label ECs that were in the proximity of Mfsd2a+ hepatocytes. We generated Mfsd2a-CreER;R26-sLP-mCh mice in which sLP-mCherry expression was induced after tamoxifen treatment. We found that hepatocytes near the periportal vein expressed mCherry, leading to subsequent labeling of surrounding nonparenchymal cells that included CDH5+ ECs (Fig. 2C). The diffusion distance of sLP-mCherry was ~50 µm, which was about 2 to 3 hepatocytes layers in thickness (Fig. 2D). Quantification showed that >90% of ECs in zone 1 and ~30% of ECs in zone 2 were mCherry+, while ECs in zone 3 were rarely labeled (Fig. 2 E and F). For ECs in each zone, there is no significant difference in their labeling efficiency between samples collected at 3 and 6 wk after tamoxifen induction (Fig. 2F), suggesting the minimal sequential transfer of sLP-mCherry to second-recipient neighboring cells. These data demonstrated that a subset of EC population in the liver (such as ECs in zone 1) could be targeted by CILP, which could be used to study the potential communication with their neighboring hepatocytes (Fig. 2G).
Fig. 2.
Identification and characterization of neighboring ECs of Mfsd2a+ hepatocytes. (A) Cartoon showing 3 liver zones from the PV (portal vein) to the CV (central vein). The Lower panel shows liver sections immunostaining with E-cadherin (CDH1) and glutamine synthetase (GS). (B) Immunostaining for CDH1 and GS on liver sections from Mfsd2a-CreER;R26-ZsGreen at 2d after tamoxifen treatment. The Right panel shows the quantification of the percentage of ZsGreen+ hepatocytes from each zone. n = 3. (C) Immunostaining on liver sections from Mfsd2a-CreER;R26-sLP-mCh at 3 wk after tamoxifen treatment. Arrowheads, mCherry+CDH5+ ECs. (D) Quantification of labeling distance of sLP-mCherry for endothelial cells in livers from (C). n = 60 ECs. (E) Immunostaining on liver sections from Mfsd2a-CreER;R26-sLP-mCh at 3 wk after tamoxifen treatment. Arrowheads, mCherry+CDH5+ ECs. (F) Quantification of the mCherry+ ECs for each zone at 3 or 6 wk after tamoxifen treatment. n = 5. (G) Cartoon showing the ECs labeled by sLP-mCherry in Mfsd2a-CreER;R26-sLP-mCh mice. (H) FACS analysis of the percentage of mCherry+ ECs. (I) Principal component analysis of endothelial cell signatures. n = 4. (J) Venn diagram of differentially expressed genes between mCherry+ and mCherry– ECs. (K) Volcano plot of differentially expressed genes between mCherry+ and mCherry– ECs. (L) Heatmaps of top differentially expressed genes according to Gene Ontology classes. Data are represented as mean ± SEM. n.s., not significant (P > 0.05). (Scale bars, 100 μm.)
About half of ECs in the livers of Mfsd2a-CreER;R26-sLP-mCh mice were mCherry+ (Fig. 2H). FACS isolation and subsequent RNA sequencing analysis revealed that mCherry+ ECs were clearly distinct from the mCherry– ECs (Fig. 2 I and J). Specifically, the periportal landmark genes including Dll4, Lama4, Msr1, and Ltbp4 (4) were up-regulated, whereas the pericentral landmark genes such as Rspo3, Wnt9b, Cdh13, and Thbd (4) were down-regulated in mCherry+ ECs (Fig. 2K). Further analyses demonstrated that genes involved in angiogenesis, regulation of cell adhesion, and response to growth factor were remarkably up-regulated in mCherry+ ECs, whereas genes associated with extracellular matrix organization, chemotaxis, and tissue morphogenesis were significantly down-regulated in these ECs (Fig. 2L). The above data demonstrated that neighboring ECs of Mfsd2a+ hepatocytes could be identified specifically and isolated for further analysis by CILP.
Discussion
Our study reported an in vivo neighboring cell labeling method CILP that recorded the transfer of the membrane-permeable fluorescent protein from specific donor to recipient cells. The term “neighboring” here means both direct cell contact and a close range of distance (like 2-3 hepatocytes here). Indefinite sequential transfer of sLP-mCherry across cells was less likely, as the sLP-mCherry protein can last only 4 days once it is taken up by recipient cells (8). Additionally, our experimental data showed that there was no significant difference in cell labeling between samples collected at different time points, suggesting that indefinite sequential transfer of sLP-mCherry is not likely to occur in our genetic system. Coupled with Cre mouse lines, we envisioned that targeting neighboring cells in a specific niche by CILP would provide a platform to understand intercellular interactions during many biological conditions in a spatiotemporal manner in mice.
Materials and Methods
All mouse studies were approved by the guidelines of the Institutional Animal Care and Use Committee of the Institute of Biochemistry and Cell Biology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences. The R26-sLP-mCh-GFP and R26-sLP-mCh mouse lines were generated by homologous recombination using CRISPR/Cas9. Immunostaining of tissue sections and flow cytometric analysis were performed as previously described (15). Bulk RNA from ECs was evaluated using the Agilent 2100 Bioanalyzer, and the accession number for the RNA-sequencing data is deposited in NCBI Sequence Read Archive: BioProject ID PRJNA895960. Unpaired two-tailed Student’s t tests were used compare differences between two groups. The null hypothesis was rejected if P was < 0.05.
Acknowledgments
This study was supported by the National Key Research & Development Program of China (2019YFA0110403, 2018YFA0108100, 2018YFA0107900, and 2019YFA0802000), NSF of China (31922032, 82088101, 31730112, and 32050087), Shanghai Pilot Program for Basic Research–Chinese Academy of Science, Shanghai Branch (JCYJ-SHFY-2021-0), China Postdoctoral Science Foundation, and the support from the XPLORER PRIZE. We thank Shanghai Model Organisms Center, Inc., for mouse generation.
Author contributions
S.Z., Q.Z., Z.L., K.L., L.H., K.O.L., and B.Z. designed research; S.Z., Q.Z., Z.L., K.L., L.H., and K.O.L. performed research; S.Z., L.W., and B.Z. analyzed data; and L.W. and B.Z. wrote the paper.
Competing interest
The authors declare no competing interest.
Contributor Information
Lixin Wang, Email: wang.lixin@zs-hospital.sh.cn.
Bin Zhou, Email: zhoubin@sibs.ac.cn.
Data, Materials, and Software Availability
All study data are included in the main text.
References
- 1.Scadden D. T., Nice neighborhood: Emerging concepts of the stem cell niche. Cell 157, 41–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato T., et al. , Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boisset J. C., et al. , Mapping the physical network of cellular interactions. Nat. Methods 15, 547–553 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Halpern K. B., et al. , Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat. Biotechnol. 36, 962–970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giladi A., et al. , Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol. 38, 629–637 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Morsut L., et al. , Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 164, 780–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasqual G., et al. , Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature 553, 496–500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ombrato L., et al. , Metastatic-niche labelling reveals parenchymal cells with stem features. Nature 572, 603–608 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talay M., et al. , Transsynaptic mapping of second-order taste neurons in flies by trans-Tango. Neuron 96, 783–795.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang T. H., et al. , Tracing neuronal circuits in transgenic animals by transneuronal control of transcription (TRACT). Elife 6, e32027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cachero S., et al. , BAcTrace, a tool for retrograde tracing of neuronal circuits in Drosophila. Nat. Methods 17, 1254–1261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flinterman M., et al. , Delivery of therapeutic proteins as secretable TAT fusion products. Mol. Ther. 17, 334–342 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barash S., Wang W., Shi Y., Human secretory signal peptide description by hidden Markov model and generation of a strong artificial signal peptide for secreted protein expression. Biochem. Biophys. Res. Commun. 294, 835–842 (2002). [DOI] [PubMed] [Google Scholar]
- 14.He L., et al. , Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science 371, eabc4346 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Pu W., et al. , Mfsd2a+ hepatocytes repopulate the liver during injury and regeneration. Nat. Commun. 7, 13369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the main text.