Significance
Whereas osteoblast- and chondrocyte-derived IGF-1 is critical for skeletal development, the main sources of IGF-1 that regulate maintenance and regeneration of the adult skeleton are poorly understood. In this study, we show that IGF-1 derived from BMSCs promotes osteogenesis and inhibits BM adipogenesis, while IGF-1 derived from MKs/PLTs promotes osteogenesis, contributes to systemic IGF-1 level, and underlies the therapeutic effects of PRP.
Keywords: IGF-1, bone marrow, osteogenesis, adipogenesis, hematopoiesis
Abstract
Insulin-like growth factor I (IGF-1) is a key regulator of tissue growth and development in response to growth hormone stimulation. In the skeletal system, IGF-1 derived from osteoblasts and chondrocytes are essential for normal bone development; however, whether bone marrow (BM)-resident cells provide distinct sources of IGF-1 in the adult skeleton remains elusive. Here, we show that BM stromal cells (BMSCs) and megakaryocytes/platelets (MKs/PLTs) express the highest levels of IGF-1 in adult long bones. Deletion of Igf1 from BMSCs by Lepr-Cre leads to decreased bone formation, impaired bone regeneration, and increased BM adipogenesis. Importantly, reduction of BMSC-derived IGF-1 contributes to fasting-induced marrow fat accumulation. In contrast, deletion of Igf1 from MKs/PLTs by Pf4-Cre leads to reduced bone formation and regeneration without affecting BM adipogenesis. To our surprise, MKs/PLTs are also an important source of systemic IGF-1. Platelet-rich plasma (PRP) from Pf4-Cre; Igf1f/fmice showed compromised osteogenic potential both in vivo and in vitro, suggesting that MK/PLT-derived IGF-1 underlies the therapeutic effects of PRP. Taken together, this study identifies BMSCs and MKs/PLTs as two important sources of IGF-1 that coordinate to maintain and regenerate the adult skeleton, highlighting reciprocal regulation between the hematopoietic and skeletal systems.
The growth hormone (GH)/Insulin-like growth factor I (IGF-1) axis plays a pivotal role in regulating tissue growth and aging (1, 2). Igf1- or Igf1r-deficient mice die perinatally with significantly reduced body size, suggesting that IGF-1 signaling is essential for skeletal development (3, 4). Although liver has been considered as the major source of systemic IGF-1 in adulthood (5), conditional deletion of Igf1 from hepatocytes does not affect linear bone growth or trabecular bone volume, with only minor defects in radial bone growth (6–8). In contrast, conditional deletion of Igf1 from osteoblasts (OBs) or chondrocytes showed severe skeletal defects (9, 10), suggesting that locally derived IGF-1 from the bone and cartilage is more important for skeletal development. Given that germline Cre lines (e.g., Col1a1-Cre or Col2a1-Cre) were used to conditionally delete Igf1 (9, 10), it is unclear whether osteoblasts and chondrocytes remain the major source of IGF-1 in adulthood. Our previous studies demonstrated that leptin receptor (LepR) marks BM stromal cells (BMSCs) that maintain homeostasis and remodeling of the adult skeleton (11, 12). LepR+ BMSCs which arise perinatally can differentiate into osteoblasts and adipocytes under homeostatic condition and give rise to chondrocytes after injuries (11, 12). LepR+ BMSCs also secrete high levels of hematopoietic stem cell (HSCs) maintenance factors such as stem cell factor (SCF) and CXCL12 to orchestrate the HSC niche (13, 14). However, whether these cells are an important source of IGF-1 in the adult skeleton remains unknown.
The role of IGF-1 in regulating bone marrow (BM) adipogenesis has been controversial. Whereas in vitro administration of recombinant IGF-1 has been shown to promote proliferation and adipogenic differentiation by cultured BMSCs (15, 16), in vivo evidence revealed an inverse correlation between BM adipogenesis and systemic IGF-1 level. In mice, aging and calorie restriction significantly increase marrow fat accumulation, which correlates with decreased serum IGF-1 (11, 17, 18). In vivo administration of rosiglitazone, a potent peroxisome proliferator-activated receptor γ agonist, also significantly increases BM adipogenesis and reduces serum IGF-1 (19, 20). Compared to C3H/HeJ strain, C57BL/6J mice show less marrow fat but higher serum IGF-1 level (21). In humans, aging and anorexia lead to significantly increased marrow fat accumulation and decreased systemic IGF-1 level (22, 23). An inverse correlation between vertebral marrow fat and systemic IGF-1 level was also observed in obese premenopausal women (24). However, whether IGF-1 inhibits BM adipogenesis under homeostatic or stress conditions has not been demonstrated by direct genetic evidence. Furthermore, whether locally produced IGF-1 plays an important role in regulating BM adipogenesis remains to be tested.
HSCs give rise to megakaryocytes (MKs) in the BM, which expand and differentiate into platelets (PLTs) in response to thrombopoietin (TPO) to initiate coagulation (25). IGF-1 administration can also stimulate MK differentiation to accelerate PLT recovery independent of TPO (26), suggesting a critical role of IGF-1 in promoting thrombopoiesis. Interestingly, MKs can reciprocally regulate the maintenance of HSCs by secreting TGF-β and CXCL4 (27, 28); however, whether MKs/PLTs secrete osteogenic factors to promote skeletal maintenance and regeneration is poorly understood. Notably, platelet-rich plasma (PRP) has been clinically proven to alleviate osteoarthritis (29), promote fracture healing (30), and repair bone defects (31). Growth factors such as IGF-1, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and TGF-β are highly enriched in PRP (32), but which factor underlies the therapeutic effects of PRP in skeletal regeneration remains elusive. Furthermore, no genetic evidence has been provided to elucidate whether MKs/PLTs are the main source of growth factors enriched in PRP.
In this study, we found that BMSCs and MKs/PLTs are the main sources of IGF-1 in the adult skeleton. IGF-1 derived from BMSCs promotes osteogenesis and inhibits adipogenesis, while IGF-1 derived from MKs/PLTs promotes osteogenesis and underlies the therapeutic effects of PRP.
Results
IGF-1 is Highly Expressed in BMSCs and MKs/PLTs in Adult Long Bones.
To test whether BM-resident cells are important sources of IGF-1, we analyzed the microarray data generated by a previous study of BM HSC niche (13). Interestingly, we found that Igf1 is highly expressed in Scf-GFP+ BMSCs and CD41+ MKs/PLTs as compared to whole BM cells, osteoblasts, and endothelial cells (ECs) (Fig. 1A). We confirmed this result by real-time quantitative PCR (qPCR) in different populations of BM cells after flow cytometry sorting (Fig. 1B). We also analyzed other components of the GH/IGF-1 axis in the microarray data and found that Ghr, Igfbp3-5, and Pappa are highly expressed in BMSCs (SI Appendix, Fig. S1A). To elucidate the expression profile of GH/IGF-1 axis at single-cell resolution, we reanalyzed our recently generated single-cell RNA-sequencing (scRNA-seq) dataset of the skeletal lineage cells in adult mice (33). By integrated analysis of Prrx1-Cre- and Lepr-Cre-traced cells in 8-wk-old long bones (SI Appendix, Fig. S1B), we confirmed that Igf1, Ghr, Igfbp3-5, and Pappa are all highly expressed in BMSC subsets and, to a lesser extent, in downstream osteoprogenitors (Pro/Pre-OB) (Fig. 1C). In stark contrast, terminally differentiated skeletal cells such as OBs and chondrocytes show minimal expression of Igf1 and other components of GH/IGF-1 axis (Fig. 1C).
Fig. 1.
IGF-1 is highly expressed in BMSCs and MKs/PLTs. (A and B) Microarray (A) and qPCR (B) analyses of Igf1 expression in 8-wk-old long bones. WBM (Whole BM cells); BMSCs (CD45-Ter119−CD31−Scf-GFP+/LepR+); MKs/PLTs (CD41+); osteoblasts (OBs, CD45-Ter119−CD31−Col2.3-GFP+); endothelial cells (ECs, CD144+); myeloid (CD11b+Gr-1+); erythroid (Ter119+CD71+); B cells (B220+); T cells (CD3+) (n = 3 mice from three independent experiments). The statistical significance was assessed using one-way ANOVA with Dunnett's multiple comparisons test. Data represent mean ± SD (***P < 0.001). (C) Violin plots showing genes related to GH/IGF-1 axis in different cell clusters. Integrated analysis of Prrx-1-Cre- and Lepr-Cre-traced cells in 8-wk-old long bones was shown. (D) Heatmap showing genes related to GH/IGF-1 axis in Lepr-Cre-traced BMSCs under homeostatic and stress conditions. Young adult: 8-wk-old mice; Aging: 12-mo-old mice; Rosiglitazone diet: 10-wk-old mice on 20 g/kg rosiglitazone-containing chow for 5 wk; Irradiation: 8-wk-old sub-lethally irradiated (5 Gy) mice; Fracture: 8-wk-old fractured mice. (E) Pseudotime analysis within Lepr-Cre-traced osteogenic lineage cells under homeostatic and stress conditions. (F) Dynamic expression of genes related to GH/IGF-1 axis in osteogenic lineage cells along the differentiation trajectory.
Next, we performed integrated analysis of Lepr-Cre-traced cells in adult long bones under homeostatic and stress conditions (SI Appendix, Fig. S1C) (33). During aging, LepR+ BMSCs undergo increased adipogenesis at the expense of osteogenesis (11). Consistent with a recent study (17), we found that GH/IGF-1 axis is down-regulated in aged LepR+ BMSCs as compared to young adults (Fig. 1D). Within the osteogenic lineage cells including Pro/Pre-OB and mature OBs (SI Appendix, Fig. S1D), pseudotime analysis showed consistently down-regulated GH/IGF-1 axis as BMSCs undergo osteogenic differentiation (Fig. 1 E and F), suggesting that IGF-1 signaling is more active in upstream BMSCs and osteoprogenitor cells as opposed to mature OBs in the adult skeleton. Taken together, these data indicated that BMSCs and MKs/PLTs are the main sources of IGF-1 in adult long bones, and that IGF-1 signaling could finetune the balance of osteogenesis and adipogenesis under homeostatic and stress conditions.
BMSC-Derived IGF-1 Promotes Osteogenesis and Skeletal Repair.
To test whether BMSC-derived IGF-1 regulates osteogenesis, we deleted Igf1 from BMSCs by crossing Lepr-Cre with Igf1-flox allele (Lepr-Cre; Igf1f/f) (11). qPCR analysis showed that Igf1 level was decreased by ~80% in Lepr-Cre; Igf1f/f BMSCs as compared to littermate controls (Igf1f/f) (SI Appendix, Fig. S2A). Igf1 expression in other tissues including brain, heart, kidney, visceral fat, liver, and skeletal muscle was not affected (6, 9, 10) (SI Appendix, Fig. S2B). In contrast to global deletion of Igf1 (3, 4), Lepr-Cre; Igf1f/f mice developed normally, with similar body weight as compared to littermate controls (SI Appendix, Fig. S2 C and D).
Next, we analyzed the bone parameters in 12-wk-old Lepr-Cre; Igf1f/f mice and littermate controls. MicroCT analyses in the distal femur metaphysis showed significantly decreased trabecular bone volume fraction, trabecular number, and significantly increased trabecular spacing in Lepr-Cre; Igf1f/f mice as compared to littermate controls (Fig. 2 A–F). MicroCT analyses of cortical bones in the mid-diaphysis showed significantly decreased total area, bone area, and polar moment of inertia (pMOI) in Lepr-Cre; Igf1f/f mice as compared to littermate controls (Fig. 2 G–L), suggesting slender bones with inferior mechanical property. Calcein double labeling showed significantly decreased mineral apposition rate (MAR) and bone formation rate (BFR) in trabecular bones (Fig. 2 M–O). MAR was also significantly decreased in cortical bones (Fig. 2 P and Q). Histomorphometry analyses on femur sections revealed significantly decreased number of OBs but not osteoclasts (OCs) in Lepr-Cre; Igf1f/f mice as compared to littermate controls (Fig. 2 R, S, U, and V). Consistent with this, enzyme-linked immunosorbent assay (ELISA) showed significantly decreased bone formation marker P1NP in the plasma (Fig. 2T), while the urinary bone resorption marker DPD remained unchanged (Fig. 2W). Primary BMSCs from Lepr-Cre; Igf1f/f mice showed significantly reduced in vitro osteogenic differentiation as compared to littermate controls (Fig. 2 X and Y), suggesting that IGF-1 promotes osteogenesis in an autocrine manner. MicroCT analysis of 12-mo-old mice showed more profound loss of the trabecular bones in Lepr-Cre; Igf1f/f mice as compared to littermate controls (SI Appendix, Fig. S2 E–P). Notably, Lepr-Cre is a germline Cre allele that also deletes in pre-OBs and mature OBs downstream of BMSCs (11). To test whether pre-OBs/OBs are an important source of IGF-1 to maintain the adult skeleton, we conditionally deleted Igf1 from pre-OBs/OBs in 8-wk-old mice by Osx-CreER (34) and found no significant changes of the body weight or bone parameters (except for a marginal decrease of cortical BMD) in 12-wk-old Osx-CreER; Igf1f/f mice as compared to littermate controls (SI Appendix, Fig. S3 A–O). Together, these data demonstrated that BMSC-derived IGF-1 promotes bone formation to maintain the homeostasis of adult long bones.
Fig. 2.
Deletion of Igf1 from BMSCs impairs bone maintenance by reducing bone formation. (A) Representative microCT images of trabecular bone in the distal femur metaphysis of 12-wk-old male Lepr-Cre; Igf1f/f mice and littermate controls. (B–F) MicroCT analysis of trabecular bone volume ratio (B), trabecular number (C), trabecular thickness (D), trabecular spacing (E), and trabecular bone mineral density (F) (n = 11 mice per genotype from at least three independent experiments). (G) Representative microCT images of cortical bone in the middle femur diaphysis of 12-wk-old male Lepr-Cre; Igf1f/f mice and littermate controls. (H–L) MicroCT analysis of cortical total area (H), bone area (I), cortical thickness (J), pMOI (K), and cortical bone mineral density (L) (n = 11 mice per genotype from at least three independent experiments). (M–Q) Calcein double labeling in trabecular and cortical bones. Representative trabecular (M) and cortical (O) images were shown, with quantifications of MAR (N and Q), BFR (O) (n = 8 mice per genotype from three independent experiments). (R and S) Alkaline phosphatase (ALP) staining of femur section and quantification of osteoblast number /bone surface from 12-wk-old male Lepr-Cre; Igf1f/f mice and littermate controls (n = 6 mice per genotype from three independent experiments). (T) ELISA measurement of plasma P1NP level (n = 7 mice per genotype from two independent experiments). (U and V) Tartrate-resistant acid phosphatase (TRAP) staining of femur section and quantification of osteoclast number /bone surface from 12-wk-old male Lepr-Cre; Igf1f/f mice and littermate controls (n = 6 mice per genotype from three independent experiments). (W) ELISA measurement of plasma urinary DPD levels. DPD level was normalized to urinary creatinine (n = 7 mice per genotype from two independent experiments). (X and Y) In vitro differentiation of BMSCs. Primary BMSCs were cultured from Lepr-Cre; Igf1f/f mice and littermate controls and subjected to osteogenic differentiation for 14 d. Alizarin red staining (S) and qPCR analysis of Col1a1 (T) were shown (n = 3 mice per genotype from three independent experiments). The statistical significance was assessed using two-tailed unpaired Student’s t test. Data represent mean ± SD (*P < 0.05, **P < 0.01).
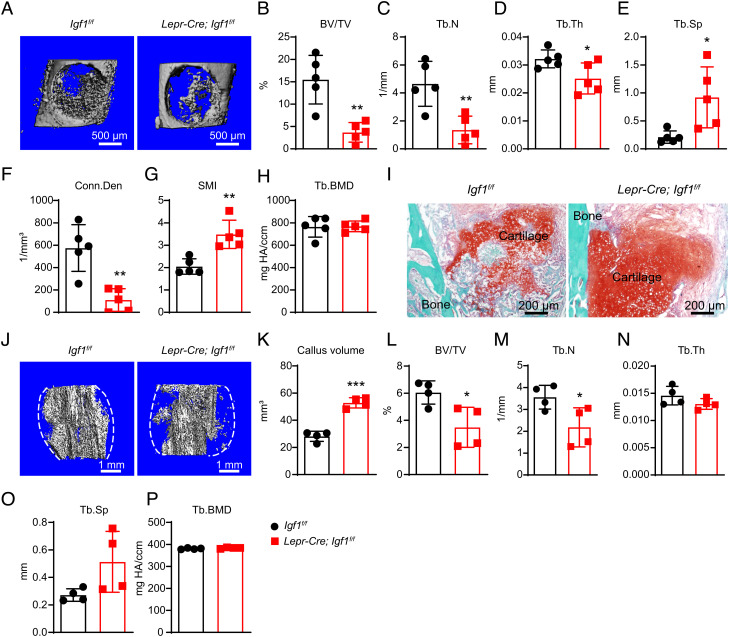
To test whether BMSC-derived IGF-1 is required for skeletal repair, we performed bone drilling in the mid-diaphyseal cortical bones of Lepr-Cre; Igf1f/f and control femurs. Previous studies showed that the drilled cortical bone is mainly repaired by intramembranous ossification (35). One week after the surgery, microCT analysis showed significantly decreased trabecular bone volume fraction, trabecular number, trabecular thickness, connectivity density, and significantly increased trabecular spacing and structure model index (SMI) (Fig. 3 A–H) in Lepr-Cre; Igf1f/f mice as compared to littermate controls. We also performed mid-diaphyseal femur fracture, which is mainly repaired by endochondral ossification (36). Two weeks after fracture, Safranin O staining showed significantly delayed cartilage resorption in Lepr-Cre; Igf1f/f as compared to littermate control mice (Fig. 3I), suggesting delayed fracture healing. Consistent with this, microCT analysis showed significantly increased callus volume and significantly decreased trabecular bone volume fraction and trabecular number in Lepr-Cre; Igf1f/f as compared to littermate control mice (Fig. 3 J–P). Therefore, BMSC-derived IGF-1 is required for skeletal repair by promoting both intramembranous and endochondral ossification.
Fig. 3.
Deletion of Igf1 from BMSCs impairs bone regeneration after injuries. (A) Representative microCT images of the middle femur diaphysis of 12-wk-old male Lepr-Cre; Igf1f/f mice and littermate controls 7 d after bone drilling. (B–H) MicroCT analysis of trabecular bone volume ratio (B), trabecular number (C), trabecular thickness (D), trabecular spacing (E), connectivity density (F), SMI (G), and trabecular bone mineral density (H) in drilled bones (n = 5 mice per genotype from three independent experiments). (I) Representative safranin O/Fast Green images of the bone callus 14 d after mid-diaphyseal femur fracture. (J–P) MicroCT analysis of the bone callus 14 d after mid-diaphyseal femur fracture. Representative images (J), callus volume (K), trabecular bone volume ratio (L), trabecular number (M), trabecular thickness (N), trabecular spacing (O), and trabecular bone mineral density (P) were shown (n = 5 mice per genotype from three independent experiments). The statistical significance was assessed using two-tailed unpaired Student’s t test. Data represent mean ± SD (*P < 0.05, **P < 0.01).
BMSC-Derived IGF-1 Inhibits BM Adipogenesis under Homeostatic and Stress Conditions.
To test whether BMSC-derived IGF-1 regulates BM adipogenesis under homeostatic condition, we performed immunostaining of the adipocyte marker Perilipin on femur sections of 12-wk-old Lepr-Cre; Igf1f/f and control mice (Fig. 4A). The number of BM adipocytes was significantly increased in the distal femur metaphysis of Lepr-Cre; Igf1f/f as compared to control mice (Fig. 4 A and B). ELISA analysis showed that plasma IGF-1 level was comparable between Lepr-Cre; Igf1f/f and control mice, suggesting that conditional deletion of Igf1 from BMSCs does not affect systemic IGF-1, and that locally produced IGF-1 from BMSCs inhibits BM adipogenesis (Fig. 4C).
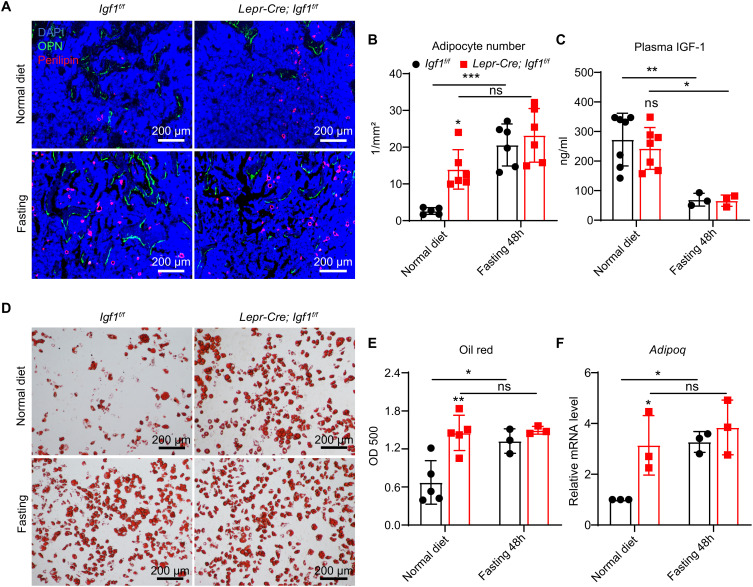
Fig. 4.
Deletion of Igf1 from BMSCs promotes BM adipogenesis that mimics fasting-induced marrow fat accumulation. (A) Representative immunofluorescent images of femur sections in 12-wk-old Lepr-Cre; Igf1f/f and control mice with or without 48-h fasting. Perilipin (red, adipocyte), osteopontin (green, bone), and DAPI (blue, nucleus) staining were shown. (B) Quantification of the number of BM adipocytes. The average number of adipocytes per image area at 4× magnification were quantified (n = 5 to 6 mice per group from two independent experiments). (C) ELISA measurement of plasma IGF-1 level in 12-wk-old Lepr-Cre; Igf1f/f and control mice with or without 48-h fasting (n = 3 to 7 mice per group from three independent experiments). (D and E) In vitro adipogenic differentiation. Primary BMSCs were cultured from mice treated as in (A), and subjected to in vitro adipogenic differentiation. Oil red O staining (D) was performed 7 d after differentiation, and quantified by optical density (OD) measurement at 500 nm (E) (n = 3 to 5 mice per group from two independent experiments). (F) qPCR of Adipoq in primary BMSCs treated as in (D) (n = 3 mice per group from two independent experiments). The statistical significance was assessed using two-way ANOVA with Tukey's multiple comparisons test. Data represent mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001).
To test whether BMSC-derived IGF-1 regulates BM adipogenesis under stress condition, we performed 48-h fasting in 12-wk-old Lepr-Cre; Igf1f/f and control mice to induce marrow fat accumulation. Fasting significantly increased the number of BM adipocytes (Fig. 4 A and B) and decreased plasma IGF-1 level in control mice (Fig. 4C). Notably, fasting did not significantly increase the number of BM adipocytes in Lepr-Cre; Igf1f/f mice, although their plasma IGF-1 level was dramatically decreased to a level comparable with the control mice (Fig. 4 A–C), suggesting that reduction of BMSC-derived IGF-1 contributes to fasting-induced marrow fat accumulation. We also cultured primary BMSCs from these mice and performed in vitro adipogenic differentiation. Consistent with the in vivo data, Oil red O staining and qPCR analyses showed that both Igf1 deficiency and fasting significantly increased adipogenic differentiation by control BMSCs (Fig. 4 D–F). In contrast, fasting did not further increase adipogenic differentiation by Lepr-Cre; Igf1f/f BMSCs (Fig. 4 D–F).
To test whether IGF-1 is sufficient to inhibit marrow fat accumulation after fasting, we administered daily subcutaneous injections of recombinant mouse IGF-1 (rIGF-1, 100 μg/kg body weight) or vehicles in wild-type mice for 2 d, starting from the onset of 48-h fasting. As compared to mice fed ad libitum, fasting significantly decreased plasma IGF-1 level, which was restored by subcutaneous rIGF-1 injection (SI Appendix, Fig. S4A). Strikingly, rIGF-1 administration significantly inhibited marrow fat accumulation induced by fasting (SI Appendix, Fig. S4 B and C). Adipogenic differentiation of primary BMSCs cultured from these mice showed similar results (SI Appendix, Fig. S4 D–F). Taken together, these data suggested that BMSC-derived IGF-1 inhibits BM adipogenesis under homeostatic and stress conditions.
BMSC-Derived IGF-1 Does Not Regulate Hematopoiesis.
Since LepR+ BMSCs are also key components of the HSC niche (13, 14), we tested whether BMSC-derived IGF-1 regulates hematopoiesis. Complete blood counts were comparable between Lepr-Cre; Igf1f/f mice and littermate controls (SI Appendix, Fig. S5 A–C). Lepr-Cre; Igf1f/f mice showed normal BM and spleen cellularity, as well as normal frequencies of HSCs, multipotent progenitors, restricted progenitors (GMPs, CMPs, MEPs and CLPs), and mature blood cells (SI Appendix, Fig. S5 D–K). Lepr-Cre; Igf1f/f and control mice also gave comparable level of long-term multilineage reconstitution after competitive BM transplantation into lethally irradiated mice (SI Appendix, Fig. S5 L–O), suggesting that BMSC-derived IGF-1 is dispensable for HSC maintenance and steady-state hematopoiesis.
MK/PLT-Derived IGF-1 Promotes Osteogenesis and Skeletal Repair.
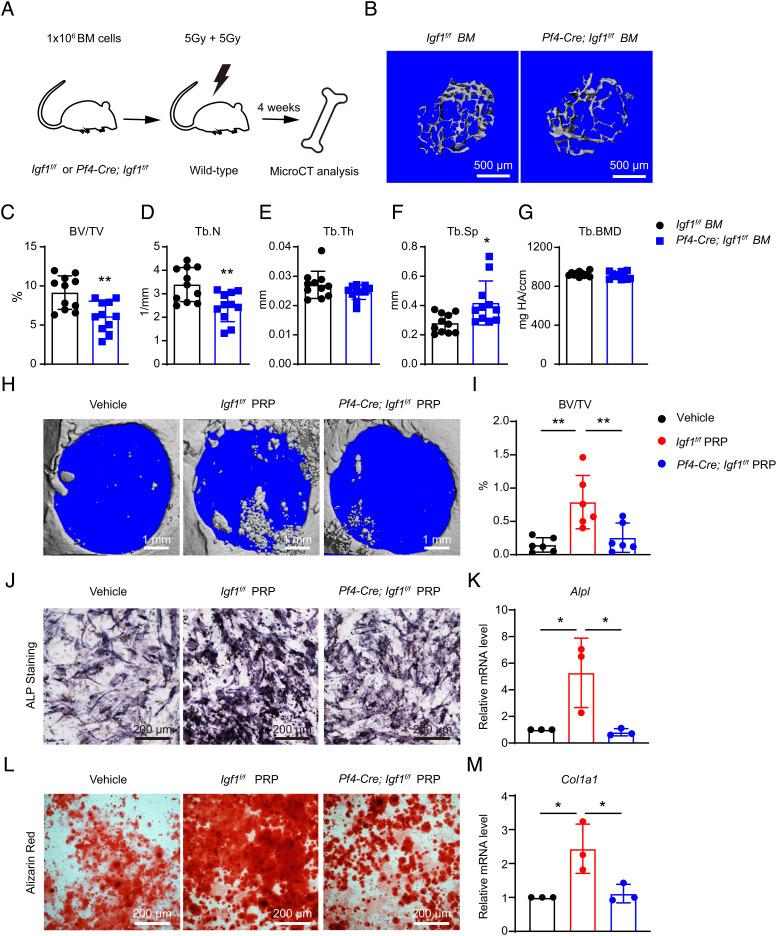
To test the functional role of MK/PLT-derived IGF-1, we deleted Igf1 from MKs/PLTs by generating Pf4-Cre; Igf1f/f mice (37). qPCR analysis in flow cytometrically sorted CD41+ MKs/PLTs from the BM showed ~80% reduction of Igf1 level (SI Appendix, Fig. S6A). Similar to Lepr-Cre; Igf1f/f mice, Pf4-Cre; Igf1f/f mice showed comparable body weight as littermate controls (SI Appendix, Fig. S6 B and C), with no significant developmental defects.
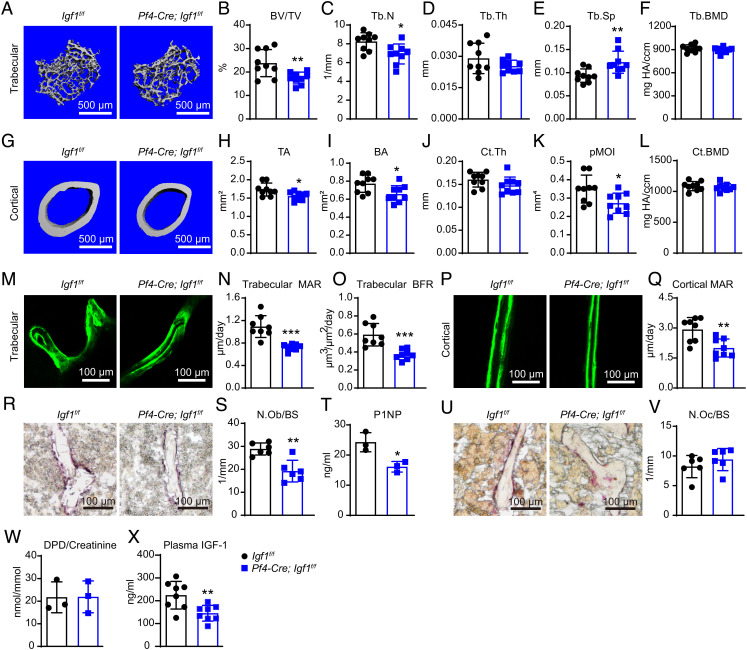
MicroCT analyses in the distal femur metaphysis showed significantly decreased trabecular bone volume fraction, trabecular number, and significantly increased trabecular spacing in Pf4-Cre; Igf1f/f mice as compared to littermate controls (Fig. 5 A–F). MicroCT analyses of the cortical bones in the mid-diaphysis showed significantly decreased total area, bone area, and pMOI in Pf4-Cre; Igf1f/f mice as compared to littermate controls (Fig. 5 G–L). Calcein double labeling showed significantly decreased MAR and BFR in trabecular bones (Fig. 5 M–O). MAR was also significantly decreased in cortical bones (Fig. 5 P and Q). Histomorphometry analyses on femur sections revealed significantly decreased number of OBs but not OCs in Pf4-Cre; Igf1f/f mice as compared to littermate controls (Fig. 5 R, S, U, and V). Consistent with this, ELISA analyses showed significantly decreased P1NP level in the plasma (Fig. 5T), while the urinary DPD level remained unchanged (Fig. 5W), suggesting that the bone loss was caused by decreased bone formation. Since MKs/PLTs can circulate in the peripheral blood, it is likely that deletion of Igf1 from MKs/PLTs might also affect systemic IGF-1 level. Indeed, ELISA analysis showed that plasma IGF-1 level was significantly reduced by ~35% in Pf4-Cre; Igf1f/f mice as compared to littermate controls (Fig. 5X).
Fig. 5.
Deletion of Igf1 from MKs/PLTs impairs bone maintenance by reducing bone formation. (A) Representative microCT images of trabecular bone in the distal femur metaphysis of 12-wk-old male Pf4-Cre; Igf1f/f mice and littermate controls. (B–F) MicroCT analysis of trabecular bone volume ratio (B), trabecular number (C), trabecular thickness (D), trabecular spacing (E), and trabecular bone mineral density (F) (n = 9 mice per genotype from at least three independent experiments). (G) Representative microCT images of cortical bone in the middle femur diaphysis of 12-wk-old male Pf4-Cre; Igf1f/f mice and littermate controls. (H–L) MicroCT analysis of cortical total area (H), bone area (I), cortical thickness (J), pMOI (K), and cortical bone mineral density (L) (n = 9 mice per genotype from at least three independent experiments). (M–Q) Calcein double labeling in trabecular and cortical bones. Representative trabecular (M) and cortical (P) images were shown, with quantifications of MAR (N and Q) and BFR (O) (n = 8 mice per genotype from three independent experiments). (R and S) ALP staining of femur section and quantification of osteoblast number /bone surface from 12-wk-old male Pf4-Cre; Igf1f/f mice and littermate controls (n = 6 mice per genotype from three independent experiments). (T) ELISA measurement of plasma P1NP levels (n = 3 mice per genotype). (U and V) TRAP staining of femur section and quantification of osteoclast number /bone surface from 12-wk-old male Pf4-Cre; Igf1f/f mice and littermate controls (n = 6 mice per genotype from three independent experiments). (W) ELISA measurement of plasma urinary DPD levels. DPD level was normalized to urinary creatinine (n = 3 mice per genotype). (X) ELISA measurement of plasma IGF-1 level (n = 8 mice per genotype). The statistical significance was assessed using two-tailed unpaired Student’s t test. Data represent mean ± SD (*P < 0.05, **P < 0.01).
In contrast to Lepr-Cre; Igf1f/f mice, Pf4-Cre; Igf1f/f mice showed normal BM adipogenesis in vivo (SI Appendix, Fig. S6 D and E), and normal osteogenic and adipogenic differentiation in vitro (SI Appendix, Fig. S6 F–I). Since MKs have been shown to regulate HSC maintenance (27), we also analyzed the hematopoietic parameters in the BM and spleen, but found no significant differences between Pf4-Cre; Igf1f/f and littermate control mice (SI Appendix, Fig. S7 A–O). Therefore, MK/PLT-derived IGF-1 does not regulate BM adipogenesis or steady-state hematopoiesis.
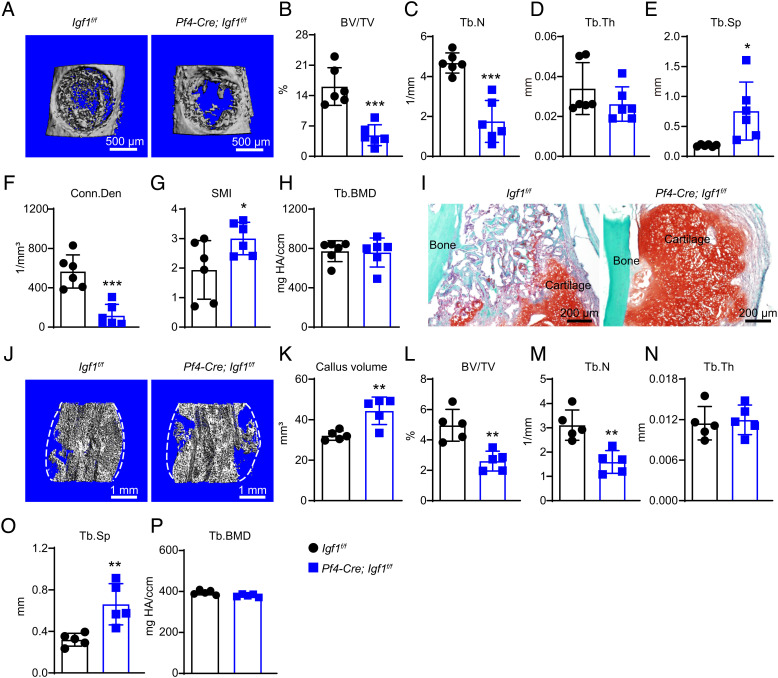
To test whether MK-derived IGF-1 promotes skeletal repair, we performed bone drilling and fracture as described above. One week after bone drilling, microCT analyses at the injury site showed significantly decreased trabecular bone volume, trabecular number, trabecular thickness, connectivity density, and significantly increased trabecular spacing and SMI in Pf4-Cre; Igf1f/f mice as compared to littermate controls (Fig. 6 A–H). Two weeks after mid-diaphyseal femur fracture, Safranin O staining showed significantly delayed cartilage resorption (Fig. 6I), and microCT showed significantly increased callus volume in Pf4-Cre; Igf1f/f as compared to littermate controls (Fig. 6 J and K). Within the bone callus, the trabecular bone volume fraction and trabecular number were significantly decreased, while the trabecular spacing was significantly increased in Pf4-Cre; Igf1f/f mice as compared to littermate controls (Fig. 6 L–P).
Fig. 6.
Deletion of Igf1 from MKs/PLTs impairs bone regeneration after injuries. (A) Representative microCT images of the middle femur diaphysis of 12-wk-old male Pf4-Cre; Igf1f/f mice and littermate controls 7 d after bone drilling. (B–H) MicroCT analysis of trabecular bone volume ratio (B), trabecular number (C), trabecular thickness (D), trabecular spacing (E), connectivity density (F), SMI (G), and trabecular bone mineral density (H) in drilled bones (n = 6 mice per genotype from three independent experiments). (I) Representative safranin O/Fast Green images of the bone callus 14 d after mid-diaphyseal femur fracture. (J–P) MicroCT analysis of the bone callus 14 d after mid-diaphyseal femur fracture. Representative images (J), callus volume (K), trabecular bone volume ratio (L), trabecular number (M), trabecular thickness (N), trabecular spacing (O), and trabecular bone mineral density (P) were shown (n = 5 mice per genotype from three independent experiments). The statistical significance was assessed using two-tailed unpaired Student’s t test. Data represent mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001).
Taken together, these results demonstrated that MKs/PLTs are an important source of systemic IGF-1 that promotes osteogenesis and skeletal repair.
MK/PLT-Derived IGF-1 Underlies the Osteogenic Effects of PRP.
A previous study reported that Pf4-Cre also recombines in non-hematopoietic cells such as intestinal epithelial cells (38). To verify that the bone phenotypes were induced by deficiency of hematopoietic cell-derived IGF-1, we transplanted whole BM cells from Pf4-Cre; Igf1f/f and littermate control mice into lethally irradiated wild-type mice (Fig. 7A). Four weeks after transplantation, microCT analysis in the distal femur metaphysis showed significantly decreased trabecular bone volume fraction, trabecular number, and significantly increased trabecular spacing in recipient mice transplanted with Pf4-Cre; Igf1f/f as compared to control BM (Fig. 7 B–G). Thus, we confirmed that hematopoietic cell-derived IGF-1 is critical for osteogenesis.
Fig. 7.
Deletion of Igf1 from MKs/PLTs reduces trabecular bones after BM transplantation and compromises the therapeutic effects of PRP. (A) Schematic illustration of the BM transplantation experiment. One million whole BM cells from Pf4-Cre; Igf1f/f mice or littermate controls were transplanted into lethally irradiated wild-type recipients. The bone phenotypes were analyzed 4 wk after transplantation by microCT. (B) Representative microCT images of the trabecular bone in the distal femur metaphysis. (C–G) MicroCT analysis of trabecular bone volume ratio (C), trabecular number (D), trabecular thickness (E), trabecular spacing (F), and trabecular bone mineral density (G) in recipient mice (n = 11 mice per genotype from two independent experiments). (H and I) In vivo treatment of the calvarial defects by PRP or vehicle control. Critical-size calvarial defects were treated with PRPs from Igf1f/f or Pf4-Cre; Igf1f/fmice, or vehicle control (saline), and analyzed 4 wk after treatment by microCT. Representative microCT images (H) and quantification of trabecular bone volume ratio (I) were shown (n = 6 mice per genotype from two independent experiments). (J–M) In vitro osteogenic differentiation of wild-type BMSCs after PRP or vehicle treatment. PRPs from Igf1f/f or Pf4-Cre; Igf1f/fmice, or vehicle control (PBS), were added to the osteogenic medium (1:50). Alkaline phosphatase (ALP) staining was performed 7 d after differentiation (J), and quantified by qPCR of Alpl (K). Alizarin red staining was performed 14 d after differentiation (L), and quantified by qPCR of Col1a1 (M) (n = 3 independent experiments). The statistical significance was assessed using two-tailed unpaired Student’s t test (C–G), or one-way ANOVA with Tukey's multiple comparisons test (I, K, M). Data represent mean ± SD (*P < 0.05, **P < 0.01).
Pf4-Cre also recombines in a minor population of monocyte/macrophage lineage cells (38), which can further differentiate into OCs on the bone surface to initiate bone resorption (39). By crossing Pf4-Cre with a loxp-STOP-loxp-tdTomato reporter line (Pf4-Cre; tdTomato), we found that Pf4-Cre indeed labels MKs and a subset of TRAP+ OCs on tibia sections (SI Appendix, Fig. S8A). To rule out the possibility that monocyte/OC-derived IGF-1 regulates osteogenesis, we conditionally deleted Igf1 from monocytes by generating LysM-Cre; Igf1f/f mice (40). MicroCT analyses revealed no significant differences of the trabecular and cortical bone parameters in these mice (SI Appendix, Fig. S8 B–M), consistent with the fact that Igf1 mRNA is barely detected in myeloid cells (Fig. 1B).
PRP has been clinically proven to promote skeletal repair (41). Since IGF-1 is a key component of PRP (42), we went on to test the extent to which MK/platelet-derived IGF-1 contributes to the osteogenic effects of PRP. To do this, we created critical size (5 mm diameter) calvarial defects in 12-wk-old wild-type mice, and then locally administered PRP from Pf4-Cre; Igf1f/f mice or littermate controls. Four weeks after the surgery, bone formation was significantly increased in mice treated with PRP from control but not Pf4-Cre; Igf1f/f mice (Fig. 7 H and I). PRP from control mice also promoted osteogenic differentiation by BMSCs, as revealed by significantly increased alkaline phosphatase activity, alizarin red staining, and qPCR analysis (Fig. 7 J–M). In contrast, these osteogenic effects were abolished in PRP from Pf4-Cre; Igf1f/f mice (Fig. 7 J–M). Taken together, these data demonstrated that MK/PLT-derived IGF-1 underlies the osteogenic effects of PRP.
Discussion
Whereas OB- and chondrocyte-derived IGF-1 is known to regulate skeletal development, terminally differentiated OBs and chondrocytes barely express IGF-1 in adult long bones (Fig. 1B). In contrast, genes of the GH/IGF-1 axis are highly expressed in BMSCs, which are down-regulated upon aging or osteogenic differentiation (Fig. 1 D–F). To conditionally delete Igf1 from BMSCs in adult mice, we utilized Lepr-Cre that is not active in BMSCs until perinatal stage (11). Lepr-Cre; Igf1f/f mice showed impaired osteogenesis at steady state and after injuries. We also conditionally deleted Igf1 from pre-OBs/OBs by Osx-CreER but found no significant bone phenotypes except for a marginal decrease of cortical BMD (SI Appendix, Fig. S3). These data suggested that BMSC-derived IGF-1 acts as an autocrine factor to help maintain and regenerate the adult skeleton. It is less likely that systemic changes of IGF-1 contribute to the bone phenotypes, since IGF-1 level was not significantly altered in the plasma or other peripheral tissues isolated from Lepr-Cre; Igf1f/f mice. Although previous studies have used Nes-CreER or Osx-Cre to conditionally delete Igf1 from BM mesenchymal cells (17, 43), only hematopoietic phenotypes were analyzed. Intriguingly, the role of IGF-1 in regulating HSC regeneration and aging has been controversial (17, 44). We did not observe significant hematopoietic defects in Lepr-Cre; Igf1f/f mice (SI Appendix, Fig. S5), probably because we analyzed steady-state hematopoiesis instead of stress hematopoiesis after irradiation and BM transplantation (17). Of note, IGF-1 signaling seems to be more active in BMSCs after irradiation (Fig. 1D), which could explain the discrepancy.
A fundamental question in skeletal biology concerns the mechanisms that balance osteogenesis and adipogenesis in the BM. Whereas in vitro administration of IGF-1 was shown to promote adipogenic differentiation by BMSCs (15, 16), this could be caused by increased proliferation of BMSCs in response to IGF-1, which leads to enhanced differentiation. Consistent with the in vivo observations that BM adipogenesis negatively correlates with systemic IGF-1 level, destabilizing IGF-1 complexes by Igfals deletion promotes adipogenic differentiation and inhibits osteogenic differentiation by BMSCs (45). In vivo administration of recombinant human IGF-1 in Gh-deficient (dw/dw) rats also decreases BM adipogenesis (46). In this study, we provided definitive genetic evidences that BMSC-derived IGF-1 inhibits BM adipogenesis, and that reduction of BMSC-derived IGF-1 contributes to fasting-induced marrow fat accumulation (Fig. 4). Although not statistically significant, there seems to be a trend toward increased number of BM adipocytes in fasted as compared to ad libitum fed Lepr-Cre; Igf1f/f mice (Fig. 4B). One possible explanation is that residual IGF-1 in BMSCs (~80% deletion efficiency, SI Appendix, Fig. S2A) could be further decreased by fasting. Alternatively, reduction of systemic IGF-1 could also contribute to increased BM adipogenesis after fasting (Fig. 4C). Consistent with the genetic data, in vivo administration of rIGF-1 significantly rescued fasting-induced marrow fat accumulation (SI Appendix, Fig. S4), which strongly supports the inhibitory effect of IGF-1 on BM adipogenesis.
Liver has long been considered as the major source of systemic IGF-1; however, conditional deletion of Igf1 from the liver results in only 75% decrease of serum IGF-1 (6). Here, we found that MKs/PLTs isolated from the BM express high levels of IGF-1. To our surprise, conditional deletion of Igf1 from MKs/PLTs significantly reduced plasma IGF-1 level by 30% (Fig. 5S), which indicates that MKs/PLTs are an important source of systemic IGF-1 in addition to the liver. Whereas conditional deletion of Igf1 from the liver only affects radial bone growth (6–8), Pf4-Cre; Igf1f/f mice showed significant defects in both trabecular and cortical bones, suggesting that MKs/PLT-derived IGF-1 promotes osteogenesis in both endocrine and paracrine manners. In contrast to BMSCs, MKs are sparsely distributed throughout the BM space, which could explain why BM adipogenesis is not affected by IGF-1 deficiency in MKs. Interestingly, Ghr is expressed in BMSCs but not MKs/PLTs (SI Appendix, Fig. S1A), suggesting that MKs/PLT-derived IGF-1 might be independent of GH regulation. Intriguingly, PRPs prepared from Pf4-Cre; Igf1f/f mice failed to stimulate bone formation both in vivo and in vitro, highlighting the essential role of MKs/PLTs-derived IGF-1 in the osteogenic effects of PRPs. Together, these data uncover a mechanism by which hematopoietic cells regulate bone formation and implicate IGF-1 as the key factor of PRP to promote skeletal regeneration.
Materials and Methods
Mice.
Mice used in this study included Igf1-flox (47), Lepr-Cre (48), LysM-Cre (40), Pf4-Cre (37), and Osx-CreER (49). All mice were housed in SPF facilities of the Animal Resource Center at Tongji University. All procedures were approved by the Tongji University Animal Care and Use Committee. For fasting experiments, 12-wk-old male C57BL/6J mice were fasted (only with water supply) for 48 h or fed ad libitum. Recombinant moue IGF-1 (R&D Systems, AF791-SP) was injected daily subcutaneously at a dose of 100 μg/kg for 2 d. Tamoxifen (MCE, HY-13757A) was injected daily intraperitoneally at a dose of 100 mg/kg in 8-wk-old Osx-CreER; Igf1f/f mice and littermate controls for five consecutive days.
Primary BMSC Isolation and Culture.
Enzymatic digestion of BM cells was performed as described previously (50). Intact BM plugs were flushed from the femurs and tibiae with syringe and dissociated with enzymatic digestion solution. Cells were then filtered through 48 μm nylon membrane and counted before seeding into 10-cm dishes. Cultures were maintained at 37 °C with 5% O2 and 5% CO2 to maintain a low oxygen environment. More details can be found in SI Appendix, Materials and Methods.
In Vitro BMSC Differentiation.
Primary BMSCs were cultured for 7 d and passaged into 24-well plates at a cell density of 3 × 104/cm2. After confluence, BMSC medium was changed to adipogenic or osteogenic medium. More details can be found in SI Appendix, Materials and Methods.
MicroCT Analysis.
Femurs and calvaria were dissected and fixed in 70% ethanol at 4 °C overnight and scanned at an isotropic voxel size of 3.5 µm (steady state conditions) or 10 µm (injury models) with peak tube voltage of 70 kV and current of 0.114 mA (µCT 35; SCANCO Medical AG, Bassersdorf, Switzerland). A three-dimensional Gaussian filter (σ = 0.8) with a limited, finite filter support of one was used to suppress noise in the images. More information about threshold and region of interest can be found in SI Appendix, Materials and Methods.
Flow Cytometry.
For primary BMSC sorting, BM plugs from femurs and tibiae were flushed and dissociated as described above. For osteoblast sorting, femurs and tibiae of Col2.3-GFP mice were crushed by mortar and pestle, followed by enzymatic dissociation and antibody staining as described above. For BM EC sorting, mice were retro-orbitally injected with Alexa Fluor 647 conjugated anti-CD144 antibody for 10 min. Whole femurs and tibiae were then crushed, followed by enzymatic dissociation. To analyze hematopoiesis, whole BM cells were flushed and resuspended in staining buffer (Ca2+/Mg2+ free HBSS with 2% fetal bovine serum) into single cells. More details can be found in SI Appendix, Materials and Methods.
Long-Term Competitive Reconstitution Assay.
Eight-week-old recipient mice (CD45.1/CD45.2) were administered a lethal dose of radiation using an X-ray irradiator (RS2000Pro) to deliver two doses of 500 rad (totally 1,000 rad) at least 2 h apart. Next, 500,000 whole BM cells from donor mice (CD45.2) were transplanted along with 500,000 whole BM cells from competitor mice (CD45.1) into lethally irradiated recipients by retro-orbital injection. Tail vein peripheral blood of the recipient mice was collected at week 4/8/12/16 after transplantation. More details can be found in SI Appendix, Materials and Methods.
PRP Preparation.
Peripheral blood (800 to 1,000 μL) was collected from Pf4-Cre; Igf1f/f or control mice with EDTA as anticoagulant, and centrifuged at 300 g, RT for 10 min. The supernatant was centrifuged again at 1,200 g, RT for another 10 min, and the bottom fraction (20% of total volume) was preserved as PRP (51). More details can be found in SI Appendix, Materials and Methods.
Bone Injury Models.
Twelve-week-old mice were used for bone fracture, bone drilling, and calvarial defect model. More details can be found in SI Appendix, Materials and Methods.
ELISA.
Plasma IGF-1 level was measured by Mouse IGF-1 Quantikine ELISA Kit (R&D systems, MG100) according to the manufacturer’s instructions. More details can be found in SI Appendix, Materials and Methods.
Immunofluorescent Staining.
Dissected bones were fixed in 4% paraformaldehyde at 4 °C overnight, decalcified in 10% EDTA (PH = 7) at room temperature for 3 d, and dehydrated in 30% sucrose at 4 °C overnight. Bones were then embedded in optimal cutting temperature compound (OCT) and cryosectioned at 10 μm using the CryoJane tape-transfer system (Leica). More details can be found in SI Appendix, Materials and Methods.
Histological Analysis.
Detailed methods for calcein double labeling, safranin O/fast green staining, and ALP and TRAP staining be found in SI Appendix, Materials and Methods.
RNA Extraction and qPCR.
Total RNA was extracted from 104 flow cytometrically sorted primary cells, in vitro differentiated cells or tissue explants using Trizol reagent (Invitrogen). RNA was reverse transcribed into cDNA using 5X All-In-One MasterMix kit (ABM, G492). qPCR was performed using iTaq Universal SYBR Green Supermix (BioRad) on a CFX96 real-time system (BioRad). Primers for qPCR can be found in SI Appendix, Materials and Methods.
Genotyping.
Mice tail tips were lysed using DirectPCR Lysis Reagent (Viagen Biotech, 102-T) according to the manufacturer’s instructions. Primers for PCR can be found in SI Appendix, Materials and Methods.
scRNA-seq Analysis.
scRNA-seq data were downloaded from GEO dataset (GSE138689) and re-analyzed as previously reported (33). More details can be found in SI Appendix, Materials and Methods.
Statistical Analysis.
The statistical significance of differences between two groups was assessed using two-tailed unpaired Student's t test. The statistical significance of differences among more than two groups was assessed using one-way ANOVAs with Tukey’s or Dunnett’s multiple comparisons test, or two-way ANOVAs with Tukey’s or Sidak’s multiple comparisons test for two-factor experimental designs. Detailed statistical methods were specified in the figure legends. All data represent mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Prof. Caiwen Duan for providing the Igf1-flox allele. This work was supported by grants from the National Key R&D Program of China (2022YFA1103200, 2021YFA1100900, 2017YFA0106400), National Natural Science Foundation of China (91749124, 81772389, 82070108), Fundamental Research Funds for the Central Universities (22120190149 and kx0200020173386), and Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai.
Author contributions
J.W. and R.Y. designed research; J.W., Q.Z., Q.P., X.Z., C.L., C.Z., and B.O.Z. performed research; J.W. and D.C. analyzed data; and J.W. and R.Y. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Yakar S., Werner H., Rosen C. J., Insulin-like growth factors: Actions on the skeleton. J. Mol. Endocrinol. 61, T115–T137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Junnila R. K., et al. , The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 9, 366–376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powell-Braxton L., et al. , IGF-I is required for normal embryonic growth in mice. Genes Dev. 7, 2609–2617 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Liu J. P., et al. , Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 (1993). [PubMed] [Google Scholar]
- 5.Ohlsson C., et al. , The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 30, 494–535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakar S., et al. , Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. U.S.A. 96, 7324–7329 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yakar S., et al. , Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J. Bone Miner. Res. 24, 1481–1492 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svensson J., et al. , Liver-derived IGF-I regulates cortical bone mass but is dispensable for the osteogenic response to mechanical loading in female mice. Am. J. Physiol. Endocrinol. Metab. 311, E138–144 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Govoni K. E., et al. , Conditional deletion of insulin-like growth factor-I in collagen type 1α2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 148, 5706–5715 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govoni K. E., et al. , Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol. Genomics 30, 354–362 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Bo O., et al. , Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue R., et al. , Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell 18, 782–796 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Ding L., Saunders T. L., Enikolopov G., Morrison S. J., Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L., Morrison S. J., Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia D., Heersche J. N., Insulin-like growth factor-1 and -2 stimulate osteoprogenitor proliferation and differentiation and adipocyte formation in cell populations derived from adult rat bone. Bone 27, 785–794 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Hu L., et al. , IGF1 promotes adipogenesis by a lineage bias of endogenous adipose stem/progenitor cells. Stem Cells 33, 2483–2495 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young K., et al. , Decline in IGF1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell 28, 1473–1482 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devlin M. J., et al. , Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Miner. Res. 25, 2078–2088 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackert-Bicknell C. L., et al. , Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology 150, 1330–1340 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecka-Czernik B., et al. , Activation of peroxisome proliferator-activated receptor γ (PPARγ) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology 148, 903–911 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen C. J., et al. , Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35, 1046–1058 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Tuljapurkar S. R., et al. , Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J. Anat. 219, 574–581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazeli P. K., et al. , Changes in marrow adipose tissue with short-term changes in weight in premenopausal women with anorexia nervosa. Eur. J. Endocrinol. 180, 189–199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bredella M. A., et al. , Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity 19, 49–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machlus K. R., Italiano J. E. Jr., The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 201, 785–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S., et al. , IGF-1 facilitates thrombopoiesis primarily through Akt activation. Blood 132, 210–222 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Zhao M., et al. , Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 20, 1321–1326 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Bruns I., et al. , Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 20, 1315–1320 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing D., et al. , Intra-articular platelet-rich plasma injections for knee osteoarthritis: An overview of systematic reviews and risk of bias considerations. Int. J. Rheum. Dis. 20, 1612–1630 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Simman R., et al. , Role of platelet-rich plasma in acceleration of bone fracture healing. Ann. Plast. Surg. 61, 337–344 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Roffi A., et al. , Platelet-rich plasma for the treatment of bone defects: From pre-clinical rational to evidence in the clinical practice. A systematic review. Int. Orthop. 41, 221–237 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Qiao J., An N., Ouyang X., Quantification of growth factors in different platelet concentrates. Platelets 28, 774–778 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Mo C., et al. , Single-cell transcriptomics of LepR-positive skeletal cells reveals heterogeneous stress-dependent stem and progenitor pools. EMBO J. 41, e108415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizoguchi T., et al. , Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushita Y., et al. , A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun. 11, 332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X., et al. , Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10, e1004820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiedt R., Schomber T., Hao-Shen H., Skoda R. C., Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 109, 1503–1506 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Pertuy F., et al. , Broader expression of the mouse platelet factor 4-cre transgene beyond the megakaryocyte lineage. J. Thromb. Haemost. 13, 115–125 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Teitelbaum S. L., Bone resorption by osteoclasts. Science. 289, 1504–1508 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Clausen B. E., et al. , Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Fernandes G., Yang S., Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 4, 16036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogino Y., Ayukawa Y., Kukita T., Koyano K., The contribution of platelet-derived growth factor, transforming growth factor-beta1, and insulin-like growth factor-I in platelet-rich plasma to the proliferation of osteoblast-like cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 101, 724–729 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Yu V. W., et al. , Distinctive mesenchymal-parenchymal cell pairings govern B cell differentiation in the bone marrow. Stem Cell Rep. 7, 220–235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng C.-W., et al. , Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14, 810–823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fritton J. C., et al. , The insulin-like growth factor-1 binding protein acid-labile subunit alters mesenchymal stromal cell fate. J. Biol. Chem. 285, 4709–4714 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gevers E. F., Loveridge N., Robinson I. C., Bone marrow adipocytes: A neglected target tissue for growth hormone. Endocrinology 143, 4065–4073 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Liu J. L., et al. , Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: Manipulation using the Cre/loxP system in transgenic mice. Mol. Endocrinol. 12, 1452–1462 (1998). [DOI] [PubMed] [Google Scholar]
- 48.DeFalco J., et al. , Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 291, 2608–2613 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Maes C., et al. , Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suire C., Brouard N., Hirschi K., Simmons P. J., Isolation of the stromal-vascular fraction of mouse bone marrow markedly enhances the yield of clonogenic stromal progenitors. Blood 119, e86–e95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang K., et al. , Optimization of the platelet-rich plasma concentration for mesenchymal stem cell applications. Tissue Eng. Part A 25, 333–351 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.