Significance
This study sought to assess the impact of microglial autophagic–lysosomal pathway on demyelination. Consequently, various assessments were carried out in an animal model of CNS demyelination. Our results demonstrated that the inhibition of excessive activation of autophagic–lysosomal pathway during the acute demyelination could result in enhanced cellular lipid metabolism and better neurological outcomes. We also confirmed that supplementation with certain lipid metabolites, such as conjugated linoleic acid, could mimic these protective effects, including improving autophagic–lysosomal overactivation and cellular dysfunction in microglia and yielding critical regenerative properties beyond myelin debris clearance. Our study proposes that pharmacological inhibition targeting microglial autophagic–lysosomal overactivation or supplementation with conjugated linoleic acid could be a potential therapeutic strategy in CNS demyelinated disorders.
Keywords: demyelination, autophagic–lysosomal pathway, microglia, conjugated linoleic acid, lipid metabolism
Abstract
Microglia play a critical role in the clearance of myelin debris, thereby ensuring functional recovery from neural injury. Here, using mouse model of demyelination following two-point LPC injection, we show that the microglial autophagic–lysosomal pathway becomes overactivated in response to severe demyelination, leading to lipid droplet accumulation and a dysfunctional and pro-inflammatory microglial state, and finally failed myelin debris clearance and spatial learning deficits. Data from genetic approaches and pharmacological modulations, via microglial Atg5 deficient mice and intraventricular BAF A1 administration, respectively, demonstrate that staged suppression of excessive autophagic–lysosomal activation in microglia, but not sustained inhibition, results in better myelin debris degradation and exerts protective effects against demyelination. Combined multi-omics results in vitro further showed that enhanced lipid metabolism, especially the activation of the linoleic acid pathway, underlies this protective effect. Supplementation with conjugated linoleic acid (CLA), both in vivo and in vitro, could mimic these effects, including attenuating inflammation and restoring microglial pro-regenerative properties, finally resulting in better recovery from demyelination injuries and improved spatial learning function, by activating the peroxisome proliferator-activated receptor (PPAR-γ) pathway. Therefore, we propose that pharmacological inhibition targeting microglial autophagic–lysosomal overactivation or supplementation with CLA could represent a potential therapeutic strategy in demyelinated disorders.
Neuroinflammation results from numerous insults to the central nervous system (CNS) and is the hallmark of multiple sclerosis (MS). It is characterized by microglial overactivation, demyelination, and progressive neurodegeneration (1). Microglia, as brain-resident innate immune cells, have a spectrum of activation states, ranging in function, often dampening and playing a critical role in the context of both myelin injury and repair in MS (2).
The microglial autophagic-lysosomal pathway is known to be responsible for the degradation of engulfed myelin debris (3, 4). Increasing evidence indicates that lysosomes are able to sense the status of cellular metabolism, control the switch between anabolism and catabolism, and serve as a regulatory hub for cellular homeostasis (3). Myelin is rich in lipids and contains high levels of cholesterol and glycosphingolipids (5). During demyelination, it is obvious that the autophagic-lysosomal system of the myelin-engulfed cells encounters a highly increased load of lipids to digest, causing a transient or prolonged lysosomal storage phenotype (6, 7), and pushing the catabolic machinery to their limit (5–8). This lipid overload has been observed to impair autophagic-lysosomal function in various diseases (3, 8), including severe demyelination by reasonable speculation. However, its underlying mechanisms and their association with MS progression or tissue repair remain to be fully elucidated.
During demyelination, the accumulation of lipid droplets (LD) in microglia significantly impacts their function, but in a different way than in the context of classical and alternative activation (9, 10). Increasing evidence suggests that lipid aggregation may affect the autophagic-lysosomal pathway, and impaired autophagic-lysosomal processing could result in further lipid droplet accumulation, thus resulting in a vicious cycle that boosts lipid aggregation and toxicity in microglia (5, 8, 10, 11). A deeper understanding of the molecular and metabolic mechanisms underlying autophagic-lysosomal pathway dysfunction and subsequent microglia-mediated neuroinflammation could help unveil therapeutic targets to treat demyelination.
In the current study, we dissected the autophagic-lysosomal pathway in microglial-degrading myelin debris following two-point lysophosphatidylcholine (LPC) injection into corpus callosum, a commonly used mouse model of CNS demyelination diseases (12), and lined the pathway in lipid droplet accumulation and microglial function. For the first time, we elucidated that the inhibition of excessive activation of autophagy during the acute stage of demyelination could result in enhanced cellular lipid metabolism and better neurological outcomes. We further confirmed that supplementation with certain lipid metabolites, such as conjugated linoleic acid (CLA), could ameliorate autophagic-lysosomal overactivation and cellular dysfunction in microglia and result in critical regenerative effects, beyond the clearance of myelin debris.
Results
Microglia Accumulate LD in Response to a Severe Demyelination Episode.
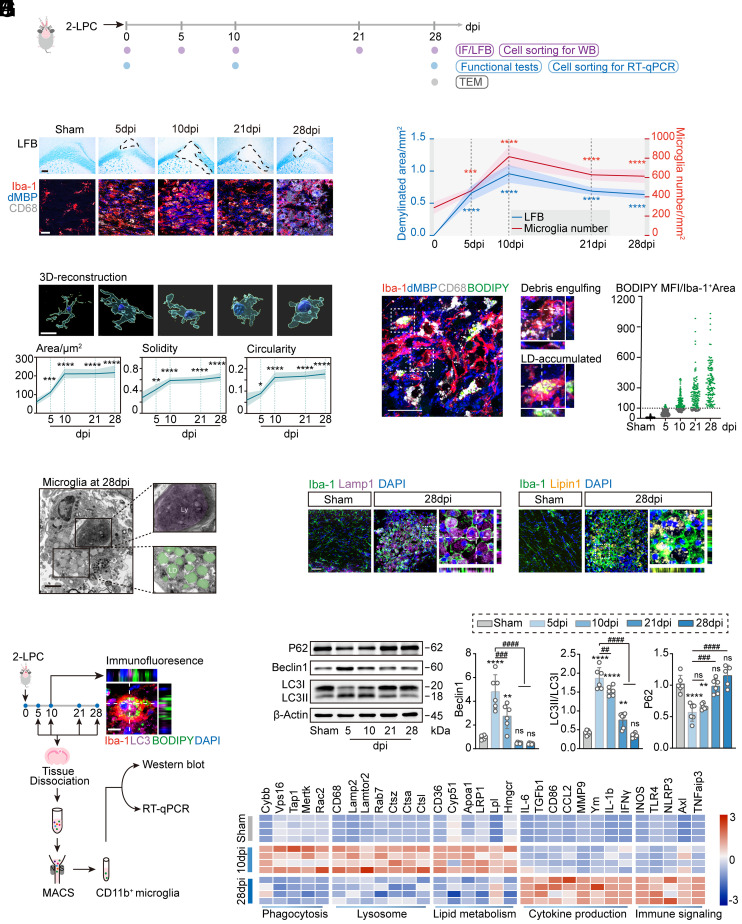
In an attempt to probe the dynamic activation of microglia during severe demyelination, we chose a two-point LPC-primed demyelination mouse model with substantial and sustained demyelinated lesions in the corpus callosum (12) and analyzed the brain sections by histological and immunofluorescence staining (Fig. 1A). Quantification of Luxol fast blue (LFB) staining areas revealed a significant reduction in myelin coverage at 10 d post LPC injection (dpi), which was sustained throughout 28 dpi (Fig. 1 B and C). Co-labeling of degraded myelin basic protein (dMBP)+ myelin debris with the microglia/macrophage marker ionized calcium-binding adaptor 1 (Iba-1) revealed the highest increase in Iba-1+ cells, accompanied by myelin debris peaking at 10 dpi, which remained thereafter throughout demyelinated lesion formation. Three-dimensional (3D) surface rendering revealed the morphological characteristics of Iba-1+ cells, whereby microglia displayed a higher degree of complexity, with increased surface area, solidity, and circularity (Fig. 1D).
Fig. 1.
Microglia experienced autophagic-lysosomal activation and LD accumulation as demyelination progressed. (A) Experimental schematic of the two-point LPC injection model. (B) Representative images of LFB staining and corpus callosum staining for Iba-1 (microglia), dMBP (myelin debris), and CD68 (lysosome). (Scale bar, 200 μm for LFB staining and 20 μm for immunofluorescent images.) (C) Quantification of the demyelinated area and the number of Iba-1+ microglia. n = 6 mice per group, n = 20 to 30 cells per mouse. Data are expressed as mean ± SD, ***P < 0.001, and ****P < 0.0001 vs. the sham group, one-way ANOVA with Bonferroni’s post hoc test. (D) 3D reconstructions of Iba-1+ microglia and average surface area, solidity, and circularity of cells. n = 6 mice per group. Data are expressed as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 vs. the sham group, one-way ANOVA with Bonferroni’s post hoc test. (Scale bar, 10 μm.) (E) Representative images of the corpus callosum stained for Iba-1 (microglia), dMBP (myelin debris), CD68 (lysosomes), and BODIPY (LD). The Right panels show z-sections of debris-engulfing and lipid droplet-accumulated microglia. Quantification of BODIPY+ lipid droplet mean fluorescence intensity (MFI) in Iba-1+ microglia. n = 6 mice per group, n = 60 to 70 cells per mouse. (Scale bar, 5 μm.) (F) Electron microscopic images of microglia from the corpus callosum at 28 dpi. (Scale bar, 2 μm.) Ly, lysosome; LD, lipid droplet. (G) Representative images of the corpus callosum stained with Iba-1, Lamp1, and Lipin1 at 28 dpi. (Scale bar, 50 μm.) (H) Experimental design for cell sorting of microglia from lesions. Representative images of the corpus callosum stained for Iba-1, LC3, and BODIPY. (Scale bar, 5 μm.) (I) Representative immunoblots and quantification of P62, Beclin-1, and LC3II/I ratio in sorted microglia from demyelinated lesions. n = 6 mice per group. Data are expressed as mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ns not significant, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. the sham group; #P < 0.05, ##P < 0.01, ####P < 0.0001. (J) Quantitative RT-PCR analysis of sorted microglia from demyelinated lesions induced by two-point LPC injection. Each square represents data obtained from one mouse. n = 4 mice per group. Numerical data with statistics are shown in SI Appendix, Table S1.
Staining of Iba-1+ microglia with BODIPY, a dye that specifically labels neutral lipids and is commonly used to detect LD (9), demonstrated that LDs were primarily found in microglia and were particularly abundant at the later stage of demyelination (10 to 28 dpi) (Fig. 1E). The mean fluorescence intensity of BODIPY+ LD in Iba-1+ microglia gradually increased and was more than 70 times higher at 28 dpi compared with microglia in the sham group (Fig. 1E). We thereafter analyzed the microglial cytoplasmic contents within the corpus callosum at 28 dpi using transmission electron microscopy. Consistently, we identified a large number of LD in microglia, characteristically adjacent to dense lysosomal material (Fig. 1F).
Microglia Undergo Autophagic-Lysosomal Overactivation and Subsequent Cellular Dysfunction.
Interestingly, we observed a transient increase in immunoreactivity for the autophagic marker LC3, lysosome-associated protein LAMP1, and glycolipid enzyme LIPIN1 in Iba-1+ microglia within the demyelinated lesions (Fig. 1 G and H). To assess the induction of autophagy, we isolated CD11b+CD45low microglia from demyelinated lesions at different time points following LPC injection and analyzed the protein expression of several autophagic factors by western blotting (Fig. 1H and SI Appendix, Fig. S1). Demyelination induced a significant increase in the LC3II/I ratio and Beclin-1 expression and a significant decrease in P62 expression in isolated microglia at 5 dpi, all of which gradually returned to normal levels at 28 dpi (Fig. 1I).
To compare the time course of LD accumulation during autophagy induction, we exposed primary microglia to lipopolysaccharide (LPS) to stimulate an inflammatory environment during severe demyelination and assessed the expression of markers related to the autophagic-lysosomal system. Consistent with previous reports (9), LD accumulation was observed in primary microglia following prolonged LPS stimulation (SI Appendix, Fig. S2A). The intensities of autophagic markers (LC3 and Beclin-1) and lysosomal markers (lysotracker and CD68) peaked 24 h post LPS stimulation and returned to normal levels with LD accumulation (SI Appendix, Fig. S2B), suggesting that LD overload might in turn inhibit autophagic-lysosomal function. Our in vivo and in vitro findings pointed to a negative correlation between microglial autophagic-lysosomal activation levels and lipid droplet accumulation.
Cellular dysfunction is considered a hallmark of foamy microglia in various CNS disorders (9). To assess whether LD-deposited microglia demonstrated signs of altered activity, we further analyzed the transcriptome signature of the isolated microglia from demyelinated lesions by qPCR. Of note, genes related to key functions of microglia, including phagocytosis, lysosomes, and lipid metabolism, were significantly suppressed at 28 dpi as compared to microglia at 10 dpi. However, genes related to cytokine production and immune signaling were enhanced in LD-deposited microglia at 28 dpi (Fig. 1J and SI Appendix, Table S1).
Collectively, our data suggest that lipid-rich myelin debris can overwhelm the autophagic-lysosomal system in phagocytes, resulting in the accumulation of foamy microglia with large amounts of LD deposition, impaired function, and pro-inflammatory characteristics.
Staged Regulation of Excessive Activation of Autophagy Attenuates LD Deposition and Neurological Deficits during Demyelination.
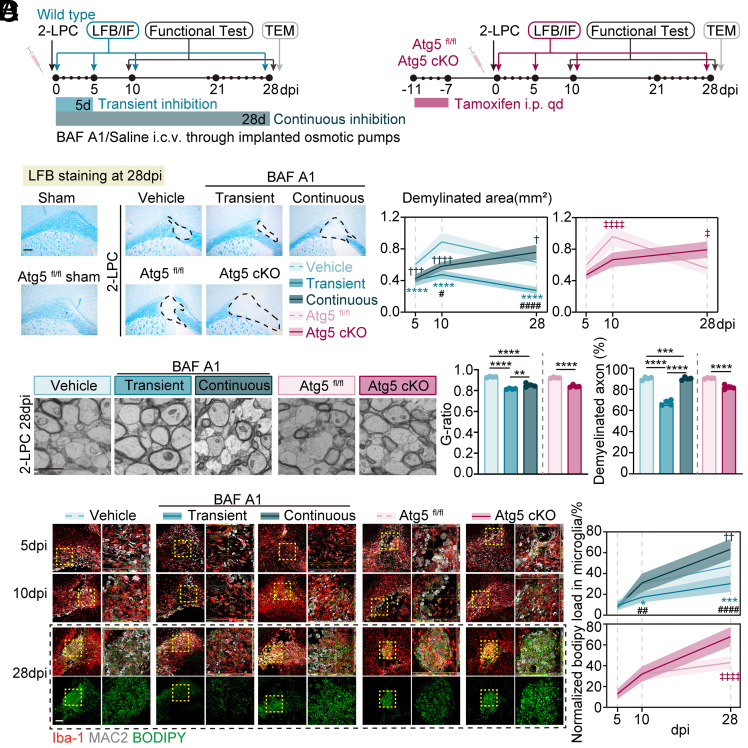
Excessive and abnormal autophagy activation may be the key process mediated by lipid substrates entering lysosomes and eventually overwhelming the degradation capacity of phagocytes. Hence, we hypothesized that the inhibition of microglial autophagy might be protective against demyelination. Three different strategies were adopted: 1) microglia-specific Atg5 deficient mice presented sustained lower autophagy levels in microglia and were directly compared with their littermate controls after LPC injection, 2) Bafilomycin A1 (BAF A1), a specific and potent V-ATPases inhibitor that could effectively inhibit classical autophagic-lysosomal pathway, was intracerebroventricularly administered in the first 5 d, demonstrating suppressed autophagy activation only at the early stage, and 3) BAF A1 was continuously intracerebroventricularly administered for 28 d, suggesting continuous inhibition of autophagy. The BAF A1-treated mice were compared with the vehicle group after LPC injection (Fig. 2A and SI Appendix, Fig. S3A). As expected, both genetic and pharmacological inhibition of autophagy led to fewer demyelinated areas by LFB at 5 to 10 dpi. However, sustained inhibition in microglial Atg5 deficient mice and wild-type mice with continuous administration of BAF A1 exhibited the more severe demyelination at 28 dpi as compared with their controls, whereas staged inhibition via BAF A1 administration (for 5 d) led to the smallest demyelinated lesions at 28 dpi among the five groups, as assessed by LFB staining, and the lowest g-ratio and percentage of demyelinated axons by transmission electron microscopy (Fig. 2 B and C).
Fig. 2.
Staged inhibition of autophagy activation led to less lipid droplet accumulation and smaller demyelinated lesions. (A) Schematic representing the experimental strategy in microglia-specific Atg5 deficient mice and wild-type mice receiving BAF A1. For transient and continuous inhibition of autophagy, micro-osmotic pumps were applied for the intracerebroventricular administration of BAF A1 (4 nmol/kg/d) for 5 d and 28 d, respectively. (B) Representative images of LFB staining and quantification of demyelinated area. n = 6 mice per group. (Scale bar, 200 μm.) Data are expressed as mean ± SD, two-way ANOVA with Bonferroni’s post hoc test; ****P < 0.0001 represented transient inhibition group vs. vehicle group, ††††P < 0.0001 represented continuous inhibition group vs. vehicle group; #P < 0.05 and ####P < 0.0001 represented continuous inhibition group vs. transient inhibition group. ‡P < 0.05 and ‡‡‡‡P < 0.0001 for Atg5 fl/fl vs. Atg5 cKO group. (C) Electron microscope images of corpus callosum at 28 dpi and quantification of g-ratios, demyelinated axon numbers. n = 4 mice per group. Data are expressed as mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; **P < 0.01, ***P < 0.001, ****P < 0.0001. (Scale bar, 1 μm.) (D) Representative images of corpus callosum stained for Iba-1+ microglia, BODIPY+ LD, Mac2+ phagocytosis, quantification of BODIPY+ lipid droplet area normalized to lesion area. n = 6 mice per group. Data are expressed as mean ± SD, two-way ANOVA with Bonferroni’s post hoc test; *P < 0.05, and ****P < 0.0001 represented vehicle vs. BAF A1 group; ###P < 0.001 and ####P < 0.0001 represented Atg5 fl/fl vs. Atg5 cKO group. (Scale bar, 100 μm (Left) and 20 μm (Right).)
Consistent with the dynamic changes in demyelinated lesions, both genetic and pharmacological inhibition of autophagy improved spatial cognitive function at 10 dpi, as manifested by a reduction in the latency to find the hidden platform (improved spatial learning) in the Morris water maze (SI Appendix, Fig. S3B). The improvement of spatial cognitive function in Atg5 deficient mice and mice with continuous BAF A1 treatment was abolished at 28 dpi, but was still profound in transient BAF A1-treated mice when compared with control mice (SI Appendix, Fig. S3C). There was no significant difference in swimming speed and time spent in the goal quadrant among the different groups, as reflected in similar swimming skills and memory retention across the groups.
In parallel, microglial BODIPY+ LD was deposited less in the transient BAF A1-treated group at 28 dpi, but more pronounced in Atg5 deficient mice and mice with continuous BAF A1 treatment (Fig. 2D).
These data suggest that staged suppression of excessive autophagic activation, but not sustained inhibition of microglial autophagy, leads to improved myelin debris degradation and demonstrates protective effects against demyelination.
Inhibition of Microglial Autophagy Attenuates Neuroinflammation and Restores Microglial Regenerative Properties.
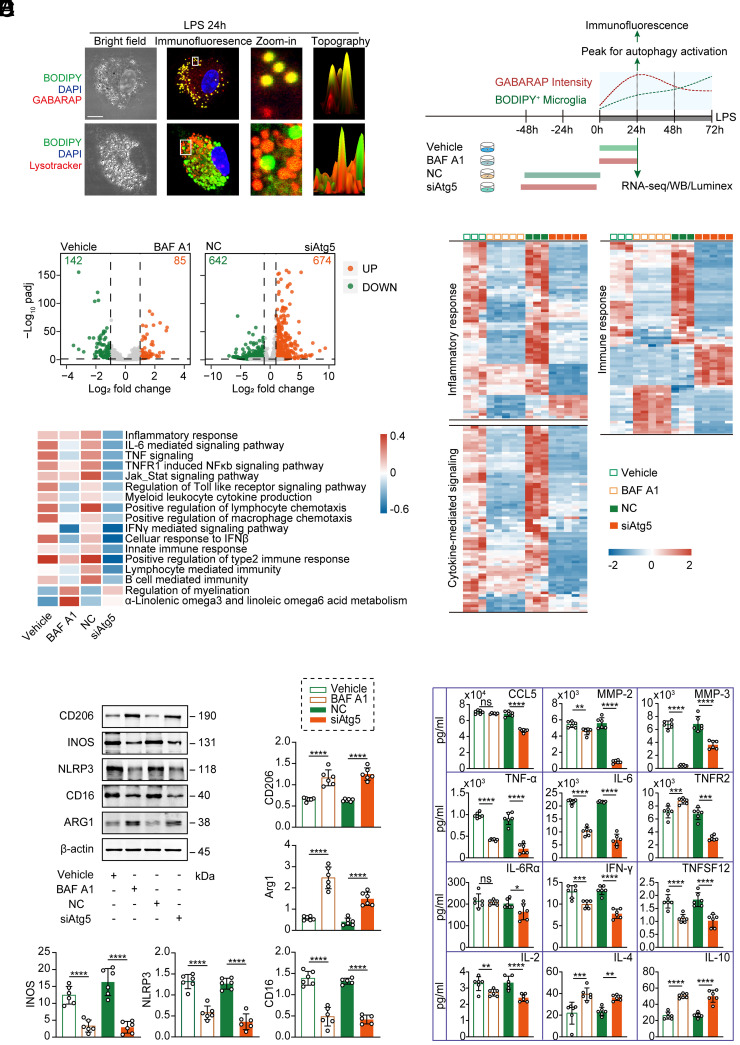
To assess the transcriptional phenotype of microglia under genetic or pharmacological modulation of autophagy, primary microglia stimulated with LPS were exposed to BAF A1 or vehicle, or transfected with siControl (non-targeted control, NC) or siAtg5, and then analyzed by RNA sequencing (RNA-seq) (Fig. 3A). We identified 227 and 1,316 differentially expressed genes in different states of microglia treated with or without BAF A1 and transfected with or without siAtg5, respectively (Fig. 3B). Unsupervised cluster analysis segregated BAF A1-treated or Atg5-deleted cells from control microglia and revealed prominent differences in their transcriptomes. Genome-wide transcriptional profiles of the enriched BPs (“inflammatory response,” “immune response,” and “cytokine-mediated signaling”) revealed that genetic or pharmacological inhibition of autophagy led to suppressed pro-inflammatory activation in LPS-stimulated microglia (Fig. 3C and SI Appendix, Table S2).
Fig. 3.
Inhibition of microglial autophagy attenuated inflammation and exhibited pro-regenerative properties in vitro. (A) Time course of autophagy activation and lipid droplet accumulation in LPS-stimulated primary microglia with different treatment paradigms. Representative images of primary microglia (treated with LPS for 24 h) staining for BODIPY (green), GABARAP/Lysotracker (red), and DAPI (blue) (Left). Schematic paradigm (Right). (Scale bar, 10 μm.) (B) Volcano plot of LPS-stimulated microglia with or without BAF A1 treatment/Atg5 siRNA transfection (|log2FC| > 1, FDR < 0.05). (C) Heatmaps depicting transcriptional profiles of selected BPs (“inflammatory responses,” “cytokine-mediated signaling,” and “immune response”). Numerical data with statistics are shown in SI Appendix, Table S2. (D) Heatmap showing the differences in biological processes by GSVA enrichment scores across the different groups. (E) Representative immunoblots and quantification of the classic microglial markers CD206, iNOS, NLRP3, CD16, Arg-1, and β-actin. n = 6 independent replicates. Data are expressed as the mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ****P < 0.0001. (F) Quantification of pro-inflammatory and anti-inflammatory cytokines and chemokines using the Luminex assay. n = 6 independent replicates. Data are expressed as the mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
A gene set variation analysis (GSVA) of differentially expressed genes revealed that the downregulation of several key pro-inflammatory signaling pathways in microglia, including “inflammatory response” and “immune response,” was associated with autophagy inhibition, while genes related to “regulation of myelination” were up-regulated (Fig. 3D). In line with transcriptional findings, protein expression levels of characteristic pro-inflammatory markers (iNOS, NLRP3, and CD16) and several secretory pro-inflammatory factors decreased under autophagy inhibition, whereas alternative activation markers (CD206 and Arg-1) and several anti-inflammatory cytokines were significantly elevated in BAF A1-treated or Atg5-deleted cells as compared to control cells (Fig. 3 E and F).
Together, these findings reveal that LPS triggers autophagy overactivation in microglia, and inhibition of autophagy shows a transcriptional signature of anti-inflammation, facilitating microglial phenotypic transition toward pro-regeneration.
Fatty Acid Metabolism Enhancement Underlies BAF A1 Neuroprotective Effects against Demyelination.
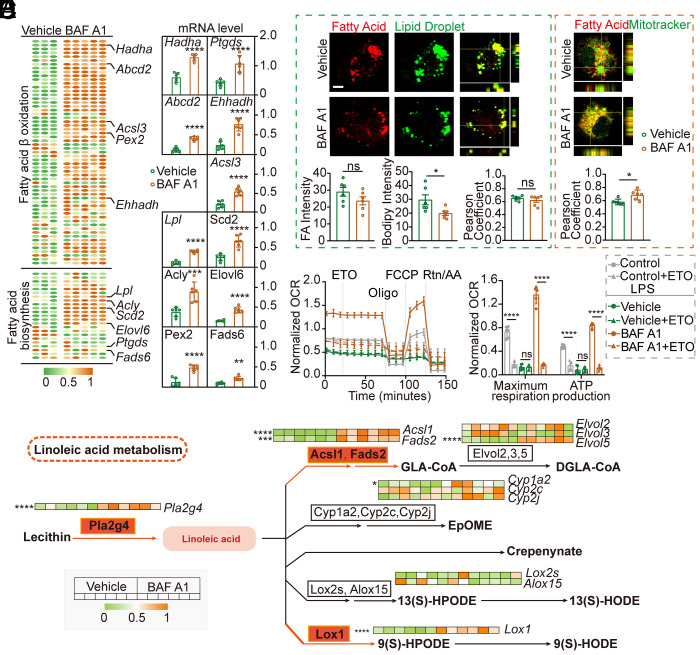
Given that metabolic reprogramming underlies microglia reactive phenotypes, (13) we investigated whether lipid metabolism alteration was one of the key mechanisms in LD accumulation changes and microglial phenotypic switches upon autophagy inhibition. To mechanistically probe this regulatory system, the microglial transcriptional profile of fatty acid metabolism was observed during BAF A1 treatment. Notably, transcriptional levels of genes related to fatty acid biosynthesis and β-oxidation were significantly elevated in BAF A1-treated microglia compared to those in vehicle cells (Fig. 4 A and B and SI Appendix, Table S3). Furthermore, BAF A1 treatment in LPS-stimulated microglia resulted in a decrease in fatty acid and lipid droplet accumulation, and an increase in fatty acid colocalization with mitochondria (Fig. 4C). To elucidate the dependence of fatty acid β-oxidation on metabolic activity, we performed seahorse extracellular flux analysis to generate real-time metabolic measurements of these cells (Fig. 4 D and E). BAF A1-treated microglia displayed a significant reduction in maximal respiration when treated with etomoxir (ETO) and a significantly lowered Oxygen consumption rate (OCR) at baseline. In contrast, vehicle-treated cells maintained equal levels of OCR, implying that fatty acids represent an important substrate for oxidation in BAF A1-treated microglia, but not in vehicle-treated microglia (Fig. 4 D and E).
Fig. 4.
BAF A1 administration enhanced anabolic and catabolic metabolism of fatty acid in LPS-stimulated microglia. (A) Heatmap depicting the transcriptional profiles of fatty acid biosynthesis and β-oxidation pathways. Numerical data with statistics are shown in SI Appendix, Table S3. (B) Quantification of selective genes by qPCR analysis. n = 6 independent replicates. Data are expressed as mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; **P < 0.01, ****P < 0.0001 vs. vehicle group. (C) Representative images of LD or mitochondria co-stained with fatty acids (FA) in LPS-stimulated microglia with or without BAF A1 treatment. Quantification of the intensity of LD and FA per cell and the colocalization of LD with FA and mitochondria with FA are shown as Pearson coefficients. n = 6 independent replicates. Data are expressed as mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ns not significant, *P < 0.05, vs. vehicle group. (Scale bar, 10 μm.) (D) OCR per well measured over time using a Seahorse XFe24 analyzer, n = 5 to 6 independent replicates. Data are expressed as the mean ± SD. (E) Summary data displaying maximal respiration and adenosine triphosphate (ATP) production per well with pre-injection of ETO relative to vehicle controls in LPS-stimulated microglia with or without BAF A1 treatment. Maximal respiration = (maximum rate measurement after carbonyl cyanide-p-trifluoromethox- yphenyl-hydrazon (FCCP) injection) – (minimum rate measurement after rotenone/antimycin A injection); ATP production = (last rate measurement before oligomycin injection) – (minimum rate measurement after oligomycin injection). n = 5 to 6 independent replicates. Data are expressed as the mean ± SD, unpaired t test; ****P < 0.0001. (F) Network analysis combining mass spectrometry and RNA-seq data highlights the differences between LPS-stimulated microglia with or without BAF A1 treatment. Enzyme-encoding mRNAs and metabolites down-regulated or up-regulated in BAF A1-treated cells are indicated by green or red nodes and connectors, respectively. Heatmap depicting transcriptional changes in genes related to selective enzymes. n = 6 independent replicates, one-way ANOVA with Bonferroni’s post hoc test; *P < 0.05, ***P < 0.001, ****P < 0.0001. Numerical data with statistics are shown in SI Appendix, Table S5.
To directly highlight the impact of BAF A1 administration on microglial metabolism, we performed mass spectrometry to quantify cellular metabolites and RNA-seq/qPCR to quantify message RNA (mRNA) levels of metabolic enzymes. Analysis of metabolite data alone, or in combination with RNA-seq/qPCR data, revealed widespread differences across the two groups in various metabolites, especially in the linoleic acid pathway (Fig. 4F and SI Appendix, Fig. S4 and Table S4). As compared to vehicle-treated cells, BAF A1-treated microglia under the same conditions exhibited a marked increase in linoleic acid, which could best differ between the two groups among all differentially expressed metabolites (SI Appendix, Fig. S4 C and D). Unbiased network analysis combining metabolic and RNA-seq/qPCR data highlighted the enhancement of metabolites and enzymes (both anabolism and catabolism) involved in the linoleic acid pathway in BAF A1-treated microglia (Fig. 4F and SI Appendix, Table S5).
These observations imply that enhanced fatty acid metabolism underlies the BAF A1-induced microglial phenotypic transition toward a pro-regenerative signature, and linoleic acid might serve as a key mediator involved in BAF A1 myelin-protective effects.
CLA Administration Restores Microglial Glycolipid Metabolism and Pro-Regenerative Properties in LPS-Stimulated Phagocytes In Vitro.
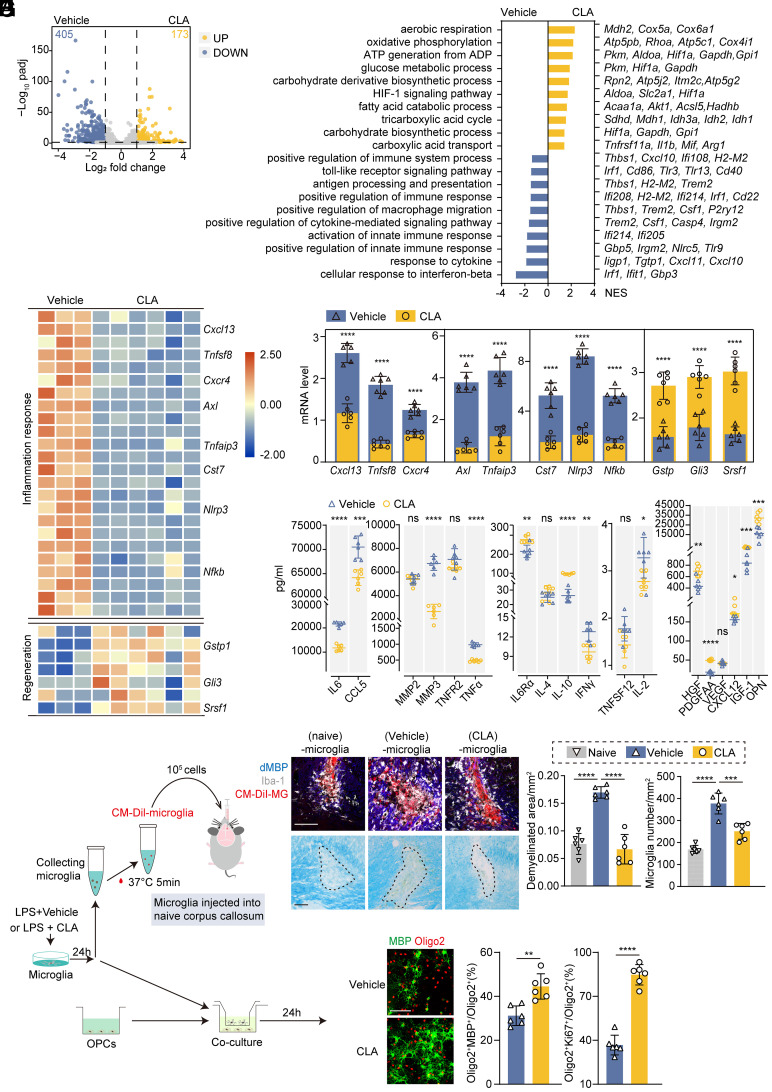
CLA denotes a group of linoleic acid isomers, with biological activities of which documented to have athero-protective (14), anti-cancer (15), and anti-inflammatory effects (16) in various experimental models when administered in an 80:20 blend of its two most abundant isomers, c9,t11-CLA and t10,c12-CLA. Given that direct autophagy inhibitors are difficult to translate into a clinical context, we asked whether the CLA blends could replicate the effects of BAF A1. Primary microglia exposed to LPS were treated with CLA or vehicle, and their transcriptional profiles were analyzed by RNA-seq (SI Appendix, Fig. S5A). As expected, CLA treatment increased multiple glycolipid metabolism pathways in microglia, especially mitochondrial oxidation (“oxidative phosphorylation,” etc.) (Fig. 5B and SI Appendix, Fig. S5 and Table S6). CLA treatment down-regulated the LPS-stimulated expression of pro-inflammatory genes (Fig. 5 B and D and SI Appendix, Table S7), which dampened the secretion of pro-inflammatory cytokines and chemokines (Fig. 5E).
Fig. 5.
CLA administration ameliorated LPS-stimulated microglial inflammation and facilitated OPCs regeneration. (A) Volcano plot of LPS-stimulated microglia with or without CLA treatment showing 173 down-regulated (blue) and 405 up-regulated (yellow) DEGs (|log2FC| > 1, FDR < 0.05). (B) Top up-regulated and down-regulated pathways with featured genes in LPS-stimulated microglia with (Right) and without (Left) CLA. (C and D) Heatmap showing genes linked to inflammation and pro-regenerative properties under LPS stimulation with or without CLA treatment (C) and individual dot plots of selected genes measured by qPCR (D). n = 6 independent replicates. Data are expressed as mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ****P < 0.0001 vs. the vehicle group. Numerical data with statistics are shown in SI Appendix, Table S7. (E) Quantification of secretory cytokines and chemokines as measured using Luminex. n = 6 independent replicates. Data are expressed as mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ns not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. the vehicle group. (F) Schematic depicting LPS-stimulated microglia with or without CLA treatment injected into the naïve corpus callosum and co-cultured with OPCs. (G) Representative images of the corpus callosum stained with dMBP, Iba-1, CM-Dil-microglia, and LFB. Quantification of the demyelinated areas. n = 6 mice per group. One-way ANOVA with Bonferroni’s post hoc test; ***P < 0.001, ****P < 0.0001. (Scale bar, 100 μm.) (H) Representative images of MBP (mature oligodendrocytes) and Olig2 (oligodendrocytes). MBP+ Olig2+ and Ki67+ Olig2+ cells were quantified as percentages of the total Oligo2+ cells. n = 6 independent replicates. Data are expressed as the mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; **P < 0.01, ****P < 0.0001. (Scale bar, 100 μm.)
We next sought to identify whether activated microglia, with or without CLA treatment, would induce different propagation of demyelination (Fig. 5 F and G). LPS-stimulated microglia were injected into the naïve corpus callosum and found to induce demyelinated lesions (Fig. 5G). In contrast, microglia treated with CLA led to smaller lesions and fewer surrounding activated microglia than vehicle-treated cells (Fig. 5G), suggesting that CLA treatment could ameliorate microglia-mediated propagation of focal inflammation and demyelination.
Next, we assessed whether CLA could rescue the impaired regenerative properties of LPS-challenged microglia. Indeed, CLA-treated microglia showed higher expression of factors known to promote oligodendrocyte progenitor cells (OPCs) differentiation (Gstp1, Gli3, and Srsf1) (Fig. 5 C and D and SI Appendix, Table S7) (17–19), and secreted elevated levels of pro-remyelination factors in the supernatants (Fig. 5E). In vitro co-culture experiments were performed to confirm the direct interactions between microglia and OPCs (Fig. 5F). CLA-treated microglia exhibited a greater capacity than vehicle-treated microglia to enhance OPC proliferation and differentiation (Fig. 5H). These data support the hypothesis that, under our experimental conditions in vitro, CLA administration enhanced glycolipid mitochondrial oxidation, dampened inflammation, and restored microglial pro-regenerative properties.
Exogenous CLA Improves Microglia-Mediated Inflammation In Vivo and Promotes OPCs Regeneration.
Given that CLA is passively transported across the blood-brain barrier, incorporated, and metabolized into brain tissue (20), we probed whether oral supplementation with CLA could rescue microglial function and promote OPCs regeneration in a mouse model of demyelination (Fig. 6A). Interestingly, cognitive deficits after demyelination improved in CLA-treated mice, as manifested by a reduction in the latency to find the hidden platform (improved spatial learning) and increased time spent in the goal quadrant after the platform was removed (improved memory retention) in the Morris water maze (Fig. 6B). Consistently, CLA administration significantly reduced demyelinated lesions quantified by LFB staining and the dMBP+ area in the lesions (Fig. 6C and SI Appendix, Fig. S6A). Transmission electron microscopy analysis further pointed to the role of CLA in maintaining white matter integrity after demyelination (Fig. 6D). Myelin sheath defects, including split myelin layers, myelin discontinuity, and myelin detachment, were observed less frequently in CLA-treated mice (Fig. 6D). Moreover, scatterplots of the g-ratio as a function of axon diameter revealed lower g-ratios in CLA-treated mice, highlighting an increase in myelin thickness in CLA-treated mice after demyelination (Fig. 6D).
Fig. 6.
CLA supplementation alleviated LPC induced demyelination and neurological deficits. (A) Schematic depicting the experimental strategy for CLA supplementation in mice with a two-point LPC injection. (B) Cognitive function was evaluated using the Morris water maze. Representative images represent the swim paths. n = 8 mice per group. Data are expressed as mean ± SD, two-way repeated measure ANOVA for acquisition trial analysis, and unpaired t test for probe trial analysis; P = 0.0014, ***P < 0.001, ns, not significant vs. the vehicle group. (C) Representative images and quantification of LFB staining. n = 6 mice per group. Data are expressed as mean ± SD, unpaired t test; ****P < 0.0001 vs. the vehicle group. (Scale bar, 200 μm.) (D) Electron microscopy images of the corpus callosum with or without CLA supplementation at 28 dpi. Asterisks mark demyelinated axons. Quantification of g-ratios. n = 6 mice per group, n = 40 axons per mouse. Data are expressed as mean ± SD, unpaired t test; ****P < 0.0001 vs. the vehicle group. (Scale bar, 2 μm.) (E) Representative images and quantification of the corpus callosum stained for Olig2 (total oligodendrocytes), APC (mature oligodendrocytes), and BrdU (proliferative marker). n = 6 mice per group. Data are expressed as mean ± SD, One-way ANOVA with Bonferroni’s post hoc test; *P < 0.05, **P < 0.01. (Scale bar, 50 μm.) (F) Representative images of the corpus callosum stained for Iba-1 (microglia), Mac2 (phagocytosis), BODIPY (LD), and dMBP (myelin debris). Quantification of the BODIPY+ and dMBP+ areas. n = 6 mice per group. Data are expressed as mean ± SD, unpaired t test; ***P < 0.001 vs. the vehicle group. (Scale bars, 100 μm (Left) and 20 μm (Right).)
We thereafter quantified proliferative and mature oligodendrocytes in LPC-injected mice with or without CLA treatment by immunostaining the brain slices (Fig. 6E). We observed an increase in the number of both Olig2+BrdU+ newly generated oligodendrocytes and Olig2+APC+ mature oligodendrocytes in CLA-treated mice, indicating enhanced OPCs regeneration after CLA treatment. Notably, CLA treatment reduced the density of MAC2+ microglia (Fig. 6F). The percentage of CD206+Iba-1+ microglia was more than twofold higher in CLA-treated mice than in vehicle-treated control mice, and CD16+Iba-1+ microglia were significantly lower in CLA-treated mice, pointing to the potential role of CLA in polarizing microglia toward an anti-inflammatory and reparative phenotype (SI Appendix, Fig. S6B). In addition, we found a significant decrease in autophagy activation, as revealed by the decreased LC3II/LC3I ratio, Beclin-1 level and increased P62 expression in isolated microglia (SI Appendix, Fig. S6C), and a significantly reduced cellular BODIPY+ area (Fig. 6F), indicating attenuated microglial autophagy activation and enhanced lipid metabolism in CLA-treated mice, recapitulating our in vitro findings.
These findings indicate that dietary CLA supplementation is sufficient to partially resolve microglia-mediated inflammation, restore microglial regenerative properties, and, subsequently, rescue neurological deficits during demyelination.
CLA Exhibited Myelin-Protective Effects by Activating the Microglial PPAR-γ Pathway.
In addition to serving as a key unsaturated fatty acid for lipid metabolism in phagocytes, CLA, as a Peroxisome proliferator-activated receptor (PPAR-γ) agonist, was shown to induce the resolution of inflammation in pre-established atherosclerosis models, in part via a PPAR-γ-dependent mechanism (14). Therefore, we investigated whether CLA exerted its myelin-protective effects by activating the microglial PPAR-γ pathway. In our RNA-seq data, the transcriptional profile of the enriched PPAR-γ signaling pathway and selected genes related to this pathway (PPARg, Ppargc1b, Akt1, Akt2, and Rxrb) revealed that CLA-treated microglia were more responsive to the PPAR-γ pathway than vehicle-treated cells (Fig. 7A and SI Appendix, Table S8).
Fig. 7.
CLA supplementation promoted microglial pro-remyelination properties via the PPAR-γ pathway. (A) Heatmap representing genes related to the PPAR-γ pathway under LPS stimulation with or without CLA treatment and individual dot plots of mRNA levels of selected genes. n = 6 independent replicates. Data are expressed as the mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ****P < 0.0001. Numerical data with statistics are shown in SI Appendix, Table S8. (B) Schematic depicting LPS-stimulated CLA-treated microglia treated with vehicle, T0070907 (PPAR-γ inhibitor), NC or Atg5 siRNA. Microglia with different treatments were co-cultured with OPCs for an additional 3 d. (C) Representative immunoblots and quantification of CD206, iNOS, NLRP3, CD16, and Arg-1 in the microglia. n = 6 independent replicates. Data are expressed as the mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; **P < 0.01, ****P < 0.0001. (D) Representative images of MBP (mature oligodendrocytes) and Oligo2 (oligodendrocytes). MBP+ Oligo2+ and Ki67+ Oligo2+ cells were quantified as percentages of the total Olig2+ cells. n = 6 independent replicates. Data are expressed as the mean ± SD, one-way ANOVA with Bonferroni’s post hoc test; ***P < 0.001, ****P < 0.0001. (Scale bar, 100 μm.) (E) Schematic depicting the experimental design for CLA treatment in microglia-specific PPAR-γ-deficient mice (Cx3cr1CreER+/−PPAR-γfl/fl, PPAR-γ cKO) or their littermate controls (PPAR-γfl/fl) and sample collection. (F and G) Representative images of LFB staining and quantification of demyelinated areas by LFB staining. Representative images and quantification of corpus callosum stained for Iba-1 (microglia), dMBP (myelin debris), and CD68 (lysosomes). n = 6 mice per group. Data are expressed as the mean ± SD, unpaired t test; ***P < 0.001, ****P < 0.0001. (Scale bars, 200 μm (F) and 20 μm (G).)
Next, we assessed whether the PPAR-γ inhibitor T0070907 or its downregulation by siRNA could diminish the protective effects of CLA in primary microglia challenged with LPS (Fig. 7B). Indeed, T0070907 and siPPAR-γ increased pro-inflammatory protein levels (iNOS, NLRP3, and CD16) and attenuated the expression of anti-inflammatory markers (CD206 and Arg-1) in microglia treated with LPS and CLA (Fig. 7C), suggesting that blocking the PPAR-γ pathway could reverse the role of CLA in polarizing microglia toward an anti-inflammatory phenotype. In vitro co-culture experiments were carried out to further confirm the role of PPAR-γ pathway in CLA pro-remyelination properties. Both T0070907-treated and PPAR-γ-depleted microglia exhibited lower capacity to enhance OPC proliferation and differentiation under CLA treatment (Fig. 7D).
Additionally, microglia-specific PPAR-γ conditional deficient mice (Cx3cr1CreER+/ PPAR-γfl/fl) were bred from PPAR-γfl/fl and Cx3cr1CreER+ mice to evaluate the role of this pathway in CLA-enhanced myelin repair after demyelination in vivo (Fig. 7E). Demyelinated areas detected by LFB staining and dMBP+ immunostaining were both significantly increased in PPAR-γ-deficient mice as compared to their littermate controls when treated with CLA (Fig. 7 F and G).
These in vitro and in vivo data collectively highlight the importance of the microglial PPAR-γ pathway in the resolution of inflammation in CLA-treated microglia and the subsequent promotion of oligodendrogenesis and myelin repair during demyelination.
Discussion
Microglia are the major resident phagocytes that clear the myelin debris generated after demyelination (21). Our data demonstrated that microglial uptake and autophagic-lysosomal processing of myelin debris play more important functions than debris clearance. Excessive activation of microglial autophagy was observed at the acute stage of severe demyelination, and overload of LD in microglia was accompanied by impaired autophagic-lysosomal pathways at a later stage, with failure in myelin repair. Notably, staged regulation of microglial autophagy, but not sustained inhibition, led to a better recovery of demyelinated lesions and neurological functions. Suppression of autophagy could ameliorate microglia-mediated inflammation and restore pro-regenerative properties by activating lipid metabolism in both anabolic and catabolic pathways, enhancing cellular metabolic dependence on mitochondrial oxidation, and increasing cellular CLA aggregation. Exogenous supplementation with CLA suppressed neuroinflammation and facilitated remyelination both in vivo and in vitro, possibly by activating the microglial PPAR-γ pathway.
Autophagy is a fundamental pathway for the degradation of intracellular proteins and organelles and has emerged as a main mechanism for myelin debris clearance in various cell types (4, 22, 23). Consistently, we found that during demyelination, microglia, the main myelin debris engulfed cells, undergo transcriptional and functional changes, including a profoundly activated autophagic pathway. The engulfed myelin debris was then delivered via the autophagic-lysosomal pathway for intracellular degradation. Normally, these autophagic routes end by fusion events with lysosomes, the organelles harboring the actual catabolic machinery (5). However, we found that during severe demyelination, excessive and abnormal autophagic processes lead to excessive lipid substrates entering the lysosome for breakdown, thereby overwhelming the degradation capacity of phagocytes. The overload of lipid flooding in lysosomes could inhibit lysosomal acidification and acid hydrolase activity and trigger permeabilization of the lysosomal membrane (3), consequently impairing lysosomal functions and perturbed cellular homeostasis. Lipid droplet-containing microglia were observed to have severe functional deficits and a pro-inflammatory phenotype during demyelination in the aging brain, (9) while the role of LD in buffering various forms of lipotoxicity has been highlighted in phagocytes in another model of acute demyelination (24). Although the role of LD in demyelination has remained open to debate, lipid droplet-accumulated microglia in our study showed impaired functions, including phagocytosis, autophagic-lysosomal pathway, and enhanced secretion of inflammatory cytokines, representing a dysfunctional and pro-inflammatory microglia state in severely demyelinated lesions. An unbiased single-cell transcriptome analysis would be ideal to further explore the diversity of microglia during myelin debris engulfment and lipid droplet accumulation.
Functionally, the engulfment and autophagy-dependent processing of myelin debris by microglia contribute to two critical but opposite processes closely associated with CNS demyelinating disorders: robust inflammation that results in the propagation of demyelination and microglia-mediated clearance of debris and cholesterol cycling that facilitate remyelination (21). Therefore, it may be possible to reverse the effects of microglia by targeting excessive and abnormal autophagic processes. While the microglial autophagic-lysosomal pathway is known to be responsible for the degradation of engulfed myelin debris (3, 4), it is reported that endosomes might play an amateur role of delivering myelin debris to lysosome (22). Thus, it is reasonable speculation that when autophagy is inhibited, myelin could be degraded via endosomal-lysosomal pathway, although at a lower efficiency. Our data further confirmed that inhibition of autophagy led to fewer demyelinated areas at early stages, while sustained inhibition in microglial Atg5 deficient mice or mice with continuous administration of BAF A1 exhibited more severe demyelination at 28 dpi than that in mice with 5-d BAF A1 administration. These findings suggested that inhibition of autophagy during acute stage could protect white matter against demyelination injuries. However, as Atg5 is involved in early phase of autophagy, and BAF A1, a proton pump, blocks autophagosome-lysosome fusion (25, 26), the different outcome between BAF A1 treatment and Atg5 KO might be, at least partly, due to autophagy inhibition at different phase and different gene expression profile between two treatments. Nevertheless, the comparison of short-term (staged, for the first 5 d) and long-term (continuous, for 28 d) BAF A1 infusion provided strong evidence for the protective effects of stage-dependent autophagy suppression on CNS demyelination. Our genetic and pharmacological data together indicated that while the autophagic-lysosomal pathway is required for engulfed myelin debris degradation, staged inhibition of autophagy during acute severe demyelination could help resolve lysosomal dysfunction by acquired lipid overload, facilitate ordered lipid metabolism, and ameliorate prolonged inflammation and secondary demyelination during the progression of neuroinflammatory disorders.
The renewed interest in the metabolic programs of myeloid cells was fueled by the realization that the modulation of the metabolic state of these immune cells would enable changes in the outcome of immune responses (10). Here, we show the pharmacological inhibition of autophagy in microglia leads to more powerful lipid biosynthesis and β-oxidation. Interestingly, beyond general enhancement, the CLA pathway was particularly modulated following BAF A1 administration, with CLA aggregation among all the lipid metabolites. Therefore, whether CLA supplementation has a similar ability to therapeutically manipulate the metabolic state of microglia holds promise for neuroinflammatory diseases, such as MS, characterized by aberrant immune regulation.
Our data revealed that microglia treated with the CLA blends of the two isomers showed enhanced lipid metabolism and reduced lipid droplet accumulation, which further exhibited anti-inflammatory and pro-regenerative properties during neuroinflammation. These findings are consistent with a previous report that at the sites of atherosclerotic lesions, CLA blends could induce inflammation resolution by regulating foamy cells derived from monocytes/macrophages during plaque progression (27). In a recent small sample size pilot study in patients with MS, dietary CLA supplementation for 6 mo significantly enhanced the anti-inflammatory profiles as well as functional signatures of circulating myeloid cells (28). Our in vivo data further indicated that CLA administration suppressed microglia-mediated propagation of demyelination, induced OPCs regeneration, and improved neurological function. Importantly, dietary CLA supplementation was well tolerated in mice, consistent with studies in humans (28, 29), pointing to excellent therapeutic applications in human demyelination disorders.
Mechanistically, the anti-inflammatory effects of CLA have been proved at least partly linked to its direct activation of the nuclear receptors PPAR family in immune cells (30). Our study, in addition, showed that CLA exerted its protective effects by priming microglia/macrophages toward a pro-resolving phenotype, but failed in microglia-specific PPAR-γ-deficient mice or microglia treated with PPAR-γ inhibitors. Thus, we propose that the microglial PPAR-γ pathway may underlie the effects of CLA-mediated neuroprotection in demyelinated models, whereas other PPAR-γ agonists, such as rosiglitazone and telmisartan, may also exert protective effects against demyelination, warranting further investigation.
In conclusion, our study revealed that the microglial autophagic-lysosomal pathway plays a critical role in the degradation of myelin debris generated in CNS disorders associated with prominent demyelination. We found that staged inhibition of excessive autophagic activation could help efficiently degrade myelin debris in microglia by enhancing lipid metabolism. Mechanistically, we determined that the CLA-PPAR-γ pathway was involved in these protective effects, and CLA supplementation could efficiently enhance lipid metabolism in microglia/macrophages (anti-inflammatory mode of action) and secretion of pro-regenerative cytokines (remyelinating mode of action). Thus, CLA supplementation should be further investigated as a therapeutic strategy for neuroinflammation, particularly in the context of several CNS demyelinated diseases (SI Appendix, Fig. S7).
Materials and Methods
Detailed materials and methods are available in SI Appendix.
Animals.
C57BL/6 J mice (aged 8 to 10 wk, weight 20 to 26 g) and neonatal mice (aged 1 to 3 d) were provided by the experimental animal center. Cx3cr1CreER mice and PPAR-γfl/fl mice were purchased from the Jackson Laboratory (stock number: 021160 and 004584). Atg5fl/fl mice were purchased from Riken BioResource Center (stock number: RBRC02975). All animal studies were conducted in compliance with the regulations and ethics guidelines of the Institute of Animal Care Committee of Tongji Medical College, Huazhong University of Science and Technology, China.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
This work was funded by National Natural Science Foundation of China (Grants: 82071380, 81873743, 81801223) and Tongji Hospital (HUST) Foundation for Excellent Young Scientist (Grant No. 2020YQ06).
Author contributions
C.Q. and D.-S.T. designed research; L.-Q.Z., M.-H.D., Z.-W.H., Y.T., Y.-H.C., M.C., and S.Y. performed research; M.-H.D., Z.C., L.-J.W., and W.W. contributed new reagents/analytic tools; L.-Q.Z. and Y.T. analyzed data.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Chuan Qin, Email: chuanqin@tjh.tjmu.edu.cn.
Dai-Shi Tian, Email: tiands@tjh.tjmu.edu.cn.
Data, Materials, and Software Availability
All the data supporting the conclusions of the current study are presented in the figures and tables. RNA-seq data have been deposited in GEO repository with accession number GSE198559.
Supporting Information
References
- 1.Bogie J. F. J., et al. , Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J. Exp. Med. 217, e20191660 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zrzavy T., et al. , Loss of “homeostatic” microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballabio A., Bonifacino J. S., Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21, 101–118 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Berglund R., et al. , Microglial autophagy-associated phagocytosis is essential for recovery from neuroinflammation. Sci. Immunol. 5, eabb5077 (2020). [DOI] [PubMed] [Google Scholar]
- 5.van Eijk M., Aerts J., The unique phenotype of lipid-laden macrophages. Int. J. Mol. Sci. 22, 4039 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhlmann T., et al. , An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Boven L. A., et al. , Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129, 517–526 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Menzies F. M., et al. , Autophagy and neurodegeneration: Pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Marschallinger J., et al. , Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 23, 194–208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Brok M. H., Raaijmakers T. K., Collado-Camps E., Adema G. J., Lipid droplets as immune modulators in myeloid cells. Trends Immunol. 39, 380–392 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Olzmann J. A., Carvalho P., Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Q., et al. , A stable and easily reproducible model of focal white matter demyelination. J. Neurosci. Methods 307, 230–239 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Artyomov M. N., Van den Bossche J., Immunometabolism in the single-cell era. Cell Metab. 32, 710–725 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruen R., Fitzsimons S., Belton O., miR-155 in the resolution of atherosclerosis. Front Pharmacol. 10, 463 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slowikowski B. K., et al. , The influence of conjugated linoleic acid on the expression of peroxisome proliferator-activated receptor-gamma and selected apoptotic genes in non-small cell lung cancer. Mol. Cell Biochem. 466, 65–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., et al. , Orally administered CLA ameliorates DSS-induced colitis in mice via intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokine and gut microbiota modulation. J. Agric. Food Chem. 67, 13282–13298 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., et al. , Low field magnetic stimulation promotes myelin repair and cognitive recovery in chronic cuprizone mouse model. Clin. Exp. Pharmacol. Physiol. 48, 1090–1102 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Hulse R. P., Drake R. A., Bates D. O., Donaldson L. F., The control of alternative splicing by SRSF1 in myelinated afferents contributes to the development of neuropathic pain. Neurobiol. Dis. 96, 186–200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh S., Huang X., Chiang C., Specific requirements of sonic hedgehog signaling during oligodendrocyte development. Dev. Dyn. 234, 489–496 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Murru E., et al. , Conjugated linoleic acid and brain metabolism: A possible anti-neuroinflammatory role mediated by PPARalpha activation. Front Pharmacol. 11, 587140 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voet S., Prinz M., van Loo G., Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med. 25, 112–123 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Zhou T., et al. , Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 22, 421–435 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez J. A., et al. , Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 210, 153–168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouna G., et al. , TREM2-dependent lipid droplet biogenesis in phagocytes is required for remyelination. J. Exp. Med. 218, e20210227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy J. M. M., Towers C. G., Thorburn A., Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klionsky D. J., et al. , Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 17, 1–382 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toomey S., Harhen B., Roche H. M., Fitzgerald D., Belton O., Profound resolution of early atherosclerosis with conjugated linoleic acid. Atherosclerosis 187, 40–49 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Fleck A. K., et al. , Dietary conjugated linoleic acid links reduced intestinal inflammation to amelioration of CNS autoimmunity. Brain 144, 1152–1166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Hartigh L. J., Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 11, 370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viladomiu M., Hontecillas R., Bassaganya-Riera J., Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 785, 87–95 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All the data supporting the conclusions of the current study are presented in the figures and tables. RNA-seq data have been deposited in GEO repository with accession number GSE198559.