Abstract
Background
Antibiotic resistance in Gram-negative bacilli poses a serious problem for public health. In hospitals, in addition to high mortality rates, the emergence and spread of resistance to practically all antibiotics restricts therapeutic options against serious and frequent infections.
Objectives
The aim of this work is to present the views of a group of experts on the following aspects regarding resistance to antimicrobial agents in Gram-negative bacilli: 1) the current epidemiology in Spain, 2) how it is related to local clinical practice and 3) new therapies in this area, based on currently available evidence.
Methodology
After reviewing the most noteworthy evidence, the most relevant data on these three aspects were presented at a national meeting to 99 experts in infectious diseases, clinical microbiology, internal medicine, intensive care medicine, anaesthesiology and hospital pharmacy.
Results and conclusions
Subsequent local debates among these experts led to conclusions in this matter, including the opinion that the approval of new antibiotics makes it necessary to train the specialists involved in order to optimise how they use them and improve health outcomes; microbiology laboratories in hospitals must be available throughout a continuous timetable; all antibiotics must be available when needed and it is necessary to learn to use them correctly; and the Antimicrobial Stewardship Programs (ASP) play a key role in quickly allocating the new antibiotics within the guidelines and ensure appropriate use of them.
Keywords: Antimicrobial resistance, Multiresistance, Enterobacterales
Abstract
Contexto
La resistencia a los antibióticos en bacilos gramnegativos representa un grave problema de salud pública. En el hospital, además de unas elevadas tasas de mortalidad, la aparición y propagación de resistencias a la práctica totalidad de los antibióticos limita las opciones terapéuticas frente a infecciones graves y frecuentes.
Objetivos
Este trabajo tiene por objetivo dar a conocer la visión de un grupo de expertos en los siguientes aspectos respecto a la resistencia a agentes antimicrobianos en bacilos gramnegativos: 1) la epidemiología actual en España, 2) su relación con la práctica clínica local y 3) las novedades terapéuticas en este ámbito, fundamentada en la evidencia actualmente disponible.
Metodología
Tras la revisión de la evidencia más destacada, los datos más relevantes de estos 3 aspectos fueron presentados en una reunión nacional ante 99 expertos en enfermedades infecciosas, microbiología clínica, medicina interna, medicina intensiva, anestesiología y farmacia hospitalaria.
Resultados y conclusiones
De debates locales posteriores entre estos expertos se extrajeron conclusiones al respecto entre las que se destacan que la aprobación de nuevos antibióticos hace necesaria la formación de los especialistas implicados para optimizar su uso y mejorar los resultados en salud; los laboratorios de Microbiología de los hospitales deben estar disponibles en horario continuado; todos los antibióticos deben estar disponibles para cuando sean necesarios y se debe aprender a usarlos de forma correcta; y los Programas de Optimización del Uso de Antimicrobianos (PROA) desempeñan una labor clave en ubicar de forma ágil los nuevos antibióticos en las guías y asegurar un uso apropiado de los mismos.
Palabras clave: Resistencia a los antimicrobianos, Multirresistencia, Enterobacterales
INTRODUCTION
Antibiotic resistance in Gram-negative bacilli poses a serious problem for public health. It is estimated that every year around 33,000 people die in Europe as a result of infections caused by multi-resistant (MR) microorganisms [1,2]. Moreover, there is a high economic cost for the health system associated with such infections [3]. In hospitals, in addition to these high mortality rates, the appearance of resistance to practically all antibiotics restricts therapeutic options against serious and frequent infections in our environment [4].
These resistances can come about due to mutation or via the acquisition of genes found in mobile structures (plasmids or transposons). The dissemination of MR strains is largely due to the spread of high-risk clones (HRC) which, under high antibiotic pressure, are capable of being selected and persisting over time [5,6].
Both the World Health Organization (WHO) [7] and the Centers for Disease Control and Prevention (CDC) [8] include Enterobacterales that produce extended-spectrum beta-lactamases (ESBL) and carbapenemases (CPE), together with MR Pseudomonas aeruginosa and Acinetobacter baumannii, as critical priority pathogens as regards antimicrobial resistance, since they often cause infections with high morbidity and mortality in a hospital setting.
ESBLs, which hydrolyse extended-spectrum cephalosporins and aztreonam, are inhibited by clavulanic acid. Infections caused by ESBL-producing Enterobacterales in our environment have shown a growing incidence over the last decade, being found in up to 16% and 9% of intra-abdominal infections caused by Klebsiella pneumoniae and Escherichia coli respectively [9]. In infections related to healthcare in Spain, the percentage of ESBL in the enterobacteria that give rise to bacteremic urinary tract infections is 29% [10]. This rise in resistance has been one of the factors leading to a greater consumption of carbapenems with the resulting selection of CPE. Bacteria that produce carbapenemases, enzymes that lend resistance to carbapenems, have shown a growing incidence in recent years. Carbapenemases have been found in numerous species of enterobacteria, with carbapenemase-producing K. pneumoniae (the so-called KPC carbapenemases) having a significant epidemiological and clinical impact [11]. In Spain, the main carbapenemases are of the OXA-48 and KPC type [12,13].
This situation requires new antibiotics to be introduced and, consequently, training for the specialists involved in order to optimise their use, adapt their use to local epidemiology and stewardship practices to achieve better results for patients. This study aims to review the evidence published, complement it with expert opinions, and raise awareness about the current epidemiology in Spain regarding resistance to antimicrobials in Gram-negative bacilli, how it is related to local clinical practice and new therapies in this area.
METHODOLOGY
This project was carried out between June and December 2021. In the first phase, a scientific committee was formed, made up of three recognised experts in clinical microbiology and infectious diseases, who were in charge of defining the project’s theme-based blocks: 1) the epidemiology of bacterial resistance in Gram-negative bacilli, 2) the impact on clinical practice, and 3) new therapeutic options. For each of these blocks, each expert drew up a bibliographic compilation of the most relevant publications and summarised all of the information in a presentation.
The data from these three thematic blocks was presented in June 2021 at a national meeting broadcast via streaming to an audience of 99 experts in the areas of infectious diseases, clinical microbiology, internal medicine, intensive medicine, anaesthesiology and hospital pharmacy.
Then, in order to identify the different local microbiological peculiarities, those attending the general meeting were divided into 13 groups moderated by key professionals in the diagnosis and management of these infections. The moderators had to be specialists in infectious diseases, clinical micro-biology, intensivists or internists, with specialist engagement or having authored relevant publications in the field. These 13 meetings ensured a geographical diversity among the opinions expressed, coming from Badajoz, Barcelona (2), Bilbao, A Coruña, Madrid (2), Malaga, Murcia, Seville, Tenerife, Valencia and Valladolid.
The scientific committee analysed the results gathered in the 13 meetings and made a summary taking into account, for each of the established blocks, a search and review of available evidence or, in the event that there was no such evidence or it was not conclusive, the opinions given by experts. The information gathered was arranged into the sections shown in Table 1. The final report was validated jointly by the scientific committee and the local moderators.
Table 1.
The information gathered in the local meetings and the most relevant conclusions have been arranged into the following sections.
| EPIDEMIOLOGY |
| There is a relationship between mortality and bacterial resistance, and between resistance and the excessive use of antibiotics, although many other aspects have an influence on the appearance and evolution of resistance. The prevalence of CPE and its various types in Spain is very diverse in terms of its evolution locally and over time. KPCs are an emerging variable distribution problem associated with HRC. Some KPC variants lend resistance to ceftazidime-avibactam but remain susceptible to meropenem-vaborbactam and imipenem-relebactam. |
| CLINICAL PRACTICE |
| In general, MR scoring systems are not considered useful in routine clinical practice. On introducing antibiotic treatment, knowledge of the local epidemiology, individualised risks, clinical record of infections and colonisation, and previous experience are all considered to be of great importance. The perception of the risk of multi-drug resistance is not homogeneous among healthcare professionals, and the unnecessary excess of antibiotic coverage and the lack of timely de-escalation are considered to be problematic. The risk of MR is mistakenly associated with the patient’s severity. There are high error rates in empirical antibiotic therapies that could be improved by introducing new antibiotics: those already available with a broader spectrum, those with a better PK-PD profile, and the use of combinations. It is necessary for the ASP teams to train professionals to estimate the risk of MR infections and other aspects such as how to interpret antibiograms and optimise PK-PD. The opening hours of the Microbiology Service varies greatly, which leads to delays in the results and inequality among patients; it must be universal with a continuous time-table 24 hours a day, 7 days a week (24×7). Clinical professionals are needed who are experts and specialists in infectious diseases, trained and involved in the management of patients with complicated or serious infections, who can make quick, accurate decisions. There is a very heterogeneous antibiotic arsenal available with complex approval in each centre, which gives rise to inequalities in treating patients with serious infections. All of the therapeutic options must be available in a hospital, since they all provide some advantage. Due to the epidemiological situation, the availability of antibiotics against Gram-negatives is of great importance. |
| NEW ASPECTS |
| Some hospitals do not have all of the new antibiotic options. The new options need to be available to avoid excessive use of certain molecules. Beta-lactamase inhibitors improve the efficacy of beta-lactams. Depending on their spectrum of action, the different antibiotics could be recommended for different approaches against CPEs. |
HRC: high-risk clones; CPE: carbapenemase-producing Enterobacterales; KPC: a type of carbapenemase produced by Klebsiella pneumoniae; MR: multi-resistant; PK-PD: pharmacokinetics-pharmacodynamics; ASP: Antimicrobial Stewardship Programs
Below, for each block analysed, the most relevant conclusions from the reviewed publications are presented, followed by the comments from experts to each question asked (given in the local meetings).
RESULTS AND DISCUSSION
1. LOCAL EPIDEMIOLOGY
Background. The prevalence of carbapenem-MR Enterobacterales has been growing in Spain for years, reaching 1.6% in E. coli invasive isolates and 4.4% in K. pneumoniae according to the 2020 ECDC report [14]. Similarly, multi-resistance has been increasing in P. aeruginosa and A. baumannii, reaching values of 12-15% and 50.6%, respectively [15].
CPE were detected in Spain for the first time in 2003 [16]. Their complexity has been increasing until today in terms of the variety of bacteria that produce them and the types of carbapenemases and associated resistance mechanisms. As multi-center studies have shown [12], OXA-48 has shown a clear predominance in recent years (incidence of 71.5%). However, provinces not included in this study such as Córdoba published the dispersion of the KPC-3-producing ST512 K. pneumoniae HRC, with added resistance to colistin, demonstrating its spread to other hospitals [5]. Subsequently, the National Epidemiology Centre reported the presence in Spain of other HRCs such as ST11/KPC-2 and ST 101/KPC-2 that could also acquire resistance to colistin [13]. This trend has been confirmed in the EuSCAPE programme supported by the European Center for Disease Control and Prevention (ECDC) [17].
It is therefore necessary to monitor possible epidemiological changes. As revealed by the iCREST study, carried out in Spain based on urinary tract infection isolates [18], K. pneumoniae was the most frequent CPE. There was not a predominance of OXA-48 in all hospitals, but KPC also had a relevant role. This complexity has been confirmed by studies that have described the co-production of carbapenemases and ESBLs, especially OXA-48 and CTX-M-15 [19], a combination of different types of carbapenemases [19] and the emergence of clones producing NDM-6 even in environments outside the hospital [20]. The diversity of carbapenemases has been growing over time. A recent study in blood culture isolates showed that KPC-producing Enterobacterales could account for more than 25% of all CPEs in our environment [21].
Another aspect that should be monitored is the emergence of HRC with KPC variants, such as ST307, that confer resistance to ceftazidime-avibactam [22,23] and that, at least partially, recover the susceptibility to carbapenems such as meropenem-vaborbactam and imipenem-relebactam. This susceptibility recovery is due to collateral susceptibility to meropenem or imipenem and due to the maintenance of inhibitory activity of vaborbactam and relebactam over these KPC variants [22-26]. Cases of KPC variants with resistance to new antibiotics such as cefiderocol have also been described [27].
Experts’ Opinion
“There is a relationship between mortality and bacterial resistance, and between resistance and the excessive use of antibiotics, although many other aspects have an influence on the appearance of resistance and its evolution.”
The attendees expanded on the proposed statement by adding that “multi-resistance may be related to increased mortality”. Several studies show that the wrong choice of initial treatment (due to resistance to the chosen agent) is related to mortality, regardless of the virulence [28,29] (Table 2). However, in these studies there may be a bias of patients with multiple pathologies and other risk factors with a worse prognosis, common in patients infected by MR bacteria, so it is not easy to weigh up the importance of resistance in the patient’s mortality. Mortality cannot always be unequivocally attributed to an MR microorganism, either due to its virulence or due to the inadequacy of the empirical treatment. Moreover, it is difficult to compare the studies due to the variation in their design or in defining mortality, and this is often due to local epidemiological matters (not only to the species, but to specific strains) [28].
Table 2.
Classical studies reporting a quantitative measure of the association between empirical antibiotic therapy and outcomes in patients with severe hospital-acquired Gram-negative infections [30-52]. Adapted from Marquet 2015 [28]
| Author | Study years | Country | Design | Sites | n | Outcome | Main infection | Severity index scale and significance of the difference |
|---|---|---|---|---|---|---|---|---|
| Kang et al. | 1998-2002 | Korea | R | 2 | 286 | M | BSI Gram (-) | APACHE II: ns |
| Micek et al. | 1997-2002 | USA | R | 1 | 305 | M | BSI P. aeruginosa |
SAPS II: NC |
| Luna et al. | 1999-2003 | Argentina | P | 6 | 76 | M | VAP | APACHE II: ns |
| Kim et al. | 1998-2001 | South Korea | R | 1 | 238 | M | SAB | McCabe’s classification, Jackson: ns |
| Scarsi et al. | 2001-2003 | USA | R | 1 | 884 | M | BSI Gram (-) | Charlson index: ns |
| Marschall et al. | 2006-2007 | USA | P | 1 | 250 | M, LOS | Bacteraemia Gram (-) | Charlson index, McCabe’s classification: ns |
| Rodríguez-Baño et al. | 2003 | Spain | P | 59 | 209 | M | Sepsis | Charlson index: NC |
| Ammerlaan et al. | 2007 | Western Europe | R | 60 | 334 | M | SAB | Modified Charlson index: ns |
| Erbay et al. | 2005-2008 | Turkey | R | 1 | 103 | M | Bacteraemia A. baumannii |
APACHE II: NC |
| Kumar et al. | 1996-2005 | Canada, USA, Saudi Arabia | R | 22 | 5,715 | M | Septicemia | APACHE II: NC |
| Tseng et al. | 2005-2007 | Taiwan | R | 1 | 163 | M | Pneumonia | Charlson index: NC |
| Micek et al. | 2002-2007 | USA | R | 1 | 760 | M | Sepsis Gram (-) | APACHE II, Charlson index: ns |
| Joung et al. | 2000-2006 | Korea | R | 1 | 116 | M | HAP |
APACHE II: ns |
| Shorr et al. | 2002-2007 | USA | R | 1 | 760 | LOS | Sepsis Gram (-) | APACHE II, Charlson index: ns |
| Reisfeld et al. | 2005-2007 | Israel | R | 1 | 378 | M | Bacteraemia Gram (-) | NM |
| Wilke et al. | 2007 | Germany | R | 5 | 221 | M, LOS | VAP, HAP | NM |
| Lye et al. | 2007-2009 | Singapore | R | 2 | 675 | M | Bacteraemia Gram (-) | APACHE II <0.001; Charlson index: ns |
| Tseng et al. | 2007-2008 | Taiwan | R | 1 | 163 | M | VAP | APACHE II, Charlson index, SOFA: NC |
| Chen et al. | 2006-2011 | China | R | 1 | 118 | M | SAB | APACHE II: NC |
| Labelle et al. | 2002-2007 | USA | R | 1 | 436 | M | Septicemia | APACHE II, Charlson index: NC |
| Chen et al. | 2008-2009 | Taiwan | P | 1 | 937 | M, LOS | BSI | MEDS, Charlson index: NC |
| Frakking et al. | 2008-2010 | Netherlands | R | 8 | 232 | M | Bacteraemia ESBL | Pitt bacteraemia score: ns |
| Tumbarello et al. | 2008-2010 | Italy | R | 1 | 110 | M | Pneumonia P. aeruginosa |
SAPS II, SOFA: ns |
APACHE II, Acute Physiology and Chronic Health Evaluation II; BSI, bloodstream infection; ESBL, extended-spectrum beta-lactamase; HAP, hospital-acquired pneumonia; LOS: length of stay; M, mortality; MEDS, mortality in emergency department sepsis; MRSA, methicillin resistant S. aureus; NC, not compared; NM, not mentioned; ns, not significant; P, prospective; R, retrospective; SAB, S. aureus bacteraemia; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; VAP, ventilation-associated pneumonia.
There are also other factors that influence the appearance of resistance. It is accepted that an excessive use, number and duration of antibiotic therapies is associated with its appearance. Nevertheless, other factors can also affect the emergence of resistance, such as the specific microbiota of the niche [53] or the existing policy in each hospital regarding control over antibiotics and infections [54-61].
Hence, it would be suitable to supplement the classic policy of containment in using antibiotics with initiatives generated through the ASP that improve early detection of serious infection, the integration of new microbiological diagnostic techniques into the care procedure, and the optimisation of antibiotic therapy in more compromised situations (individualised empirical therapy for serious infections, and more effective targeted therapy considering all of the options available today for optimal treatment of infections caused by resistant bacteria) [62-64].
“The prevalence of CPE and its various types in Spain is very diverse in terms of its evolution locally and over time.”
The attendees agreed with this statement. The following have recently been described as the most widespread HRC of carbapenemase-producing K. pneumoniae in Spain : ST11, ST147, ST392 and ST15 (mainly associated with OXA-48) and ST258/512 (in all cases KPC producers) [65]. In this study, carried out on 401 isolates, the following prevalence was detected: OXA-48 (75.1%), KPC (10.8%), MBL (13.9%) and GES-6 (0,2%) [65].
“KPCs are an emerging variable distribution problem associated with high risk clones.”
Added to this statement is the possibility that the increase in KPCs compared to other carbapenemases may determine the emergence of resistance to ceftazidime-avibactam, although such resistance is not generalised. This could be associated with the overuse of antibiotics in the situation of COVID-19 and the difficulty due to prescription shortages of ceftolozane-tazobactam, since some hospitals have been using broad-spectrum antibiotics, including associations with carbapenemase inhibitors such as ceftazidime-avibactam [66]. In order to discover the possible existence of ceftazidime-avibactam-resistant KPCs, it is essential for healthcare professionals to have epidemiological data within a reasonable time, so projects such as the Network of Laboratories for the Surveillance of Resistant Microorganisms (RedLabRA) [67], included in the Spanish Action Plan on Antimicrobial Resistance (PRAN), could be of help.
“Some KPC variants lend resistance to ceftazidimeavibactam but remain susceptible to meropenemvaborbactam and imipenem-relebactam.”
Some strains are resistant to ceftazidime-avibactam due to KPC variants, and they recover susceptibility to meropenem and imipenem, maintaining the effect of the inhibitors vaborbactam and relebactam [22,24-26]. Knowing this information can help in selecting antibiotics when the susceptibility of others is compromised.
Perhaps one of the problems with detecting KPC variants is that most centres do not use automated susceptibility testing sys tems with new antibiotics such as ceftazidime-avibactam or ceftolozane-tazobactam, but only with isolates in which the presence of carbapenemases is suspected. If such isolates regain susceptibility to meropenem or imipenem, it is not known whether somehow the detection of these KPC variants is being overlooked [26]. Some of the systems commonly used in the laboratory can also fail and other types of tools such as molecular ones must be used to detect them. Given that it is possible that some KPC variants are not being detected, it may be useful to use differential chromogenic plates adapted to their profile in studying the carriers.
To end this block, the experts pointed out the differences between large and small hospitals, which makes it difficult to follow common guidelines. Each of them must continuously evaluate their local epidemiological situation and establish specific protocols to respond to it in the right time and place [68,69].
2. CLINICAL PRACTICE
Background. Infections associated with MR Gram- negative bacteria are significantly associated with higher costs, longer hospital stays and higher mortality, as revealed in a recent systematic review and meta-analysis [70]. Other studies concentrating on CPE consider that susceptibility to carbapenems is a protective factor against the outcome of an infection and its long-term sequelae [71].
There are different solutions in clinical practice to tackle this problem. Firstly, there is prevention, avoiding colonisation or infection by keeping risk factors under control (which may depend on the microorganism, the host, the route of transmission or the selective pressure of antibiotics), or the application of ASP measures for good use of antimicrobials [62-64]. To do this, it is essential to have well-established nosocomial infection prevention programmes. If, despite this, a CPE infection occurs, it is essential to make an early diagnosis and identify possible MR risk factors in the patient, such as immunosuppression, admissions to the Intensive Care Unit (ICU), exposure to antimicrobials, history of surgery, mechanical ventilation, or catheters [72], and to confirm this using the available micro-biological techniques. The risk factors will differ depending on whether the risk of CPE infection is considered compared to an absence of infection or an infection by bacteria susceptible to carbapenems (with risk factors mainly related to interventionism) [73]; or else compared only to infection by bacteria susceptible to carbapenems, with risk factors such as length of stay, previous admission, kidney failure, neurological disease, dialysis or exposure to quinolones and glycopeptides, in addition to considering possible reservoirs in the environs such as fibrescopes or endoscopes [74].
One of the scoring systems described to assess CPE risk factors is the score from Giannella et al [75], aimed at determining the weight of different risk factors regarding acquisition of bacteraemia through carbapenemase-producing K. pneumoniae. It sets out a score of 7 as the cut-off point for considering a high risk of infection by this microorganism in previously colonised patients. A subsequent study [76] related this score with the INCREMENT score [77], which relates risk factors for mortality in patients with CPE bacteraemia. Based on this relationship, a strategy for action was set up, whether the patient is an asymptomatic carrier (Giannella score <7), or if patients with possible bacteraemia require empirical treatment with a lower or higher spectrum depending on the risk established by the two scoring systems. A combined treatment was set up against KPC in K. pneumoniae if both scores were high [76].
For early diagnosis, various studies emphasise that, although such diagnosis is fast, it is still essential that a team of experts from ASP should be responsible for conveying the information to the clinical prescriber to consequently optimise management of the patient. This is the only way to have an influence on their survival [78,79].
Finally, it is important to underline the importance of treating the infection well, either through non-antibiotic treatment (support and control of the focus) or antibiotics, taking into account the CPEs present in the hospital, the patient’s risk factors and the drugs available [80]. Good treatment goes beyond administering an antibiotic. In addition to controlling the focus, the emergence of new types of resistance must be considered [81,82], which is related, among other factors, to the inoculum effect [83]. In this vein, the drugs’ pharmacokinetic-pharmacodynamic (PK-PD) parameters are part of the optimisation of the treatment and the prevention of resistance [84,85]. It is essential to generate new evidence related to the PK-PD optimisation of new drugs, such as imipenem-relebactam and meropenem-vaborbactam.
Experts’ Opinion
“In general, multi-resistance score systems are not considered useful in routine clinical practice.”
Repeatedly, the experts in the regional discussions considered the existing scores to be generally of little use in routine practice due to their complexity, lack of sensitivity, lack of discrimination and difficulty to extrapolate them, as they have been designed for specific populations. Most prescribers are not familiar with them due to their complexity or the low dissemination they obtained. Moreover, these scores, which group and summarise risk factors known to specialists, in some cases reinforce the introduction of broad-spectrum antibiotic therapy, which is difficult to de-escalate later. In any case, in the opinion of the experts, common sense should prevail, with good knowledge of the local epidemiology and an individualised assessment of the patient’s risk, their treatments and previous colonisations.
On the other hand, the severity of the infection should be stressed as a key factor in decision-making, especially in vulnerable patients. The presence of multi-resistance risk factors in any case increases the pressure or need to make the right choice of empirical antibiotic therapy. This places great importance on the speciality of Microbiology in terms of the need to optimise early diagnosis and improve the sensitivity of current diagnostic methods to favour targeted treatment over empirical treatment, so it is necessary for the Microbiology laboratory to be open 24 hours (see below) [86]. As far as possible, the resulting information should always be placed in the hands of an infectious disease expert.
“On introducing antibiotic treatment, knowledge of the local epidemiology, individualised risks, clinical history of infections and colonisation, and previous experience are all considered to be of greater importance.”
It is essential for experts in the area of infectious diseases to be able to know the patient’s colonisation status, as well as to establish the positive and negative predictive value of such data. Carrier status screening may need to be carried out in units other than the ICU and the like, depending on the local epidemiology (screening the entire hospital would overload the Microbiology laboratory). Based on clinical experience, the carrier status appears to be of greatest value at the time infection occurs.
It should also be noted that colonisation cannot be equated with infection. It is essential to know the patient’s type and degree of colonisation and the presence or absence of HRCs circulating in the hospital. This requires monitoring and caution when planning treatment based on the patient’s colonisation. Carrier status as a risk factor for infection varies from one microorganism to another, and it is sometimes difficult to establish its involvement in the risk of infection. This knowledge must be in expert hands.
It is also useful to evaluate colonisation as an epidemiological parameter, since this will help to establish the strategies in empirical treatment guides for the ASP teams on a local scale. Experts highlight the value of the “Zero Resistance Project” [87] and the monitoring of EPC colonisation incidence density carried out by the Microbiology laboratories, the ASP teams and the nosocomial infection control teams [62-64].
“The perception of the risk of multi-drug resistance is not homogeneous among healthcare professionals, and the unnecessary excess of antibiotic coverage and the lack of timely de-escalation are considered to be problematic.”
The risk of multi-drug resistance may be influenced by the prevalence of MR microorganisms. Other specialists should be trained, emphasising the ASP’s role and the optimised use of antibiotics. It is necessary to foster “new” initiatives through the ASP with a greater ability to directly improve the prognosis of serious infections. This justifies the need to have microbiologists and experts in infectious diseases available in hospitals on a continuous timetable.
“The risk of multi-resistance is mistakenly associated with the patient’s severity.”
In this regard, the fact that non-expert clinicians link MR infection to a serious infection has been posed. This misconception often leads to the unnecessary use of broad-spectrum antibiotic therapies. This fact once again reinforces the need for experts in designing empirical treatment policies and their transferal into routine clinical practice.
“Empirical antibiotic therapies could be improved by different strategies such as including new antibiotics with a better antimicrobial activity or optimising the use of those already available (those with a broader spectrum or a better PK-PD profile). Additionally, the use of more adequate antibiotic combinations is also very useful.”
There was agreement on this matter, according to the first statement from the first block: “There is a relationship between mortality and bacterial resistance, and between resistance and the excessive use of antibiotics, though many other aspects have an influence on its appearance and evolution.”
“It is necessary for the ASP teams to train professionals in estimating the risk of MR infections and other aspects such as the interpretation of antibiograms and PK-PD optimisation.”
Most of the attendees point out the relevance of the ASP’s role in training. Also, the need of specific specialisation in Microbiology and Infectious Diseases was pointed.
“The Microbiology Service timetable varies a lot, which leads to delays in results and inequality for patients. Clinical professionals are needed, who are experts in infectious diseases, trained and involved in the management of patients with complicated or serious infections, who can make quick, accurate decisions.”
The experts agreed on the great disparity of resources available, in terms of both the Microbiology Service opening hours and the number of specialists in Microbiology and Infectious Diseases per hospital. It is possible that the need for both services can be established based on the hospital’s complexity, the number of beds and extrahospitalary area. It is considered a priority to have a Microbiology Service available 24 hours a day, 7 days a week, as recommended by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and as is happening in many sites due to COVID-19 [86]. For small hospitals where this is not possible, hybrid models have been proposed in which the expert works in one hospital and provides assistance to others. The problem can also be alleviated by using point-of-care (POC) diagnostic systems or ones for use in the patient care setting, which must be backed by advice and assessment from the microbiologist to launch and/or validate it.
Along these lines, there is a need for clinical professionals trained and involved in the management of patients with complicated or serious infections who can take quick, accurate decisions based on individualised risk estimation and microbiological results.
“There is a very heterogeneous antibiotic arsenal available, with complex approval in each centre, which leads to inequalities in treating patients with serious infections. All the therapeutic options must be available in a hospital, since they all provide some advantage.”
There was agreement on these issues, which are addressed in the following block (“Key aspects of new therapies”).
“Due to the epidemiological situation, the availability of antibiotics against Gram-negatives is of greater importance.”
The experts agreed that, on a clinical level, Gram-negative bacilli infections are of greater concern, as well as the therapeutic options available to treat them properly. This can be explained by the greater involvement of Gram-negatives in healthcare-associated infections (HAIs).
3. NEW THERAPIES
Background. Focusing on the new antibiotics against Gram-negative beta-lactamase-producing bacteria, the following lines deal with ceftazidime-avibactam, imipenem- relebactam, meropenem-vaborbactam, ceftolozane-tazobactam and cefiderocol.
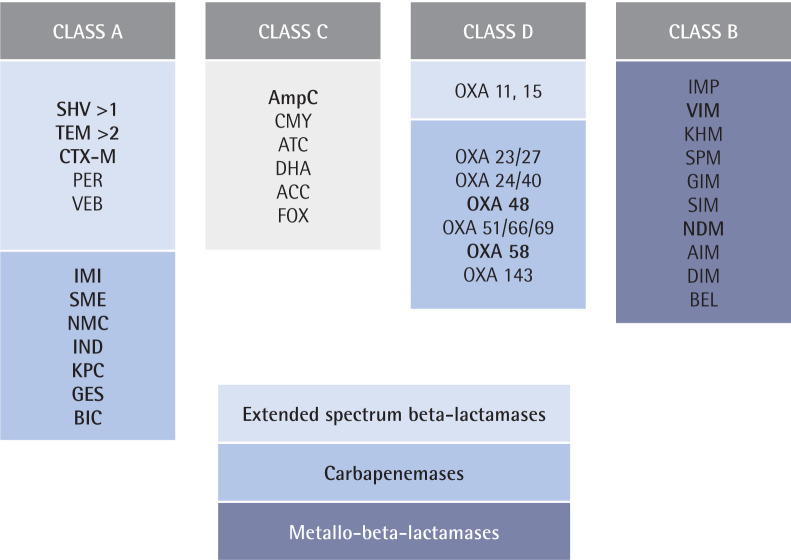
Beta-lactamases are divided into 4 classes based on their structure [88], although within each class they can have different functionalities (Figure 1). Metallo-beta-lactamases are the most difficult to treat due to the lack of specific inhibitors on the market at the present time.
Figure 1.
Beta-lactamase classification. Adapted from Ambler et al [88]. Class A: serine-beta-lactamases; Class B: metallo-beta-lactamases; Class C: chromosomal or plasmid AmpC; and Class D: OXA-beta-lactamases. Main beta-lactamases regarding epidemiology are included.
Ceftazidime-avibactam. Avibactam is an inhibitor for ESBL, both plasmid and chromosomal AmpC enzymes and most of the serine-dependent carbapenemases, including KPCs and OXA-48 enzymes. It is not active against metallo-beta-lactamases. There are seven clinical trials with ceftazidime-avibactam: RECLAIM 1/2 [89] and RECLAIM-3 [90] in complicated intra-abdominal infections, RECAPTURE 1/2 in lower urinary tract infections [91], REPRISE in infections caused by microorganisms resistant to ceftazidime [92] and REPROVE in nosocomial pneumonia [93,94].
There are also various observational studies with ceftazi-dime-avibactam in infections caused by KPC, with success rates of 60-85% and a resistance emergence of 8% [95]. The observational series with OXA-48 also give success rates of 60-80%, and resistance rates of up to 9% have been reported in comparative studies [94]. Adverse effects (AEs) include acute renal failure or infections due to Clostridioides difficile. In studies on bacteraemia due to KPC, ceftazidime-avibactam demonstrates it is superior to the control treatment in terms of survival [94,96]. It is worth noting that in bacteria that cause infections subjected to successive cycles of ceftazidime-avibactam, genetic changes such as transpositions, insertions and deletions are observed that foster the appearance of resistance to this association [97].
Ceftazidime-avibactam’s safety profile is consistent with that of ceftazidime monotherapy and similar to other injectable cephalosporins [91]. The most commonly reported AEs in the seven phase II and III clinical trials were a positive direct Coombs test, nausea, and diarrhoea. In the phase III trials, the frequencies of serious AEs leading to discontinuation of treatment or death among recipients of ceftazidime-avibactam were generally low and similar to those of the comparators. Renal insufficiency may affect clearance of the drug and thus lead to increased exposure, as a result of which neurological effects such as tremor, myoclonus, nonconvulsive status epilepticus, seizure, encephalopathy, and coma have all been reported occasionally [98].
Imipenem-relebactam. Imipenem-relebactam is a KPC-type carbapenemase inhibitor with no inhibitory activity against OXA and good activity against P. aeruginosa MDR [99]. Both components are poor substrates for efflux pumps, giving it a competitive advantage over P. aeruginosa [100,101].
In complicated urinary tract infections, imipenem-rele-bactam was non-inferior to the imipenem placebo [102], and likewise in complicated intra-abdominal infection [103] and nosocomial pneumonia (RESTORE-IMI 2 trial) [104]. It is worth highlighting the study that compared it to colistin-imipenem in infections caused by imipenem-resistant bacteria (RESTORE-IMI 1 trial) [105], resulting in similar efficacy and lower mortality figures with imipenem-relebactam.
Imipenem-relebactam’s safety profile is similar to that of other carbapenems. The AEs may include seizures, confusional states, and myoclonus due to imipenem. The ones most frequently reported in the two phase II clinical trials were diarrhoea, nausea, vomiting, headache, increased alanine aminotransferase and increased aspartate aminotransferase. In the RESTORE-IMI 1 trial, the AEs included lower creatinine clearance, hyperglycaemia, infusion site erythema, and pyrexia. In the RESTORE-IMI 2 trial, the most common AEs were diarrhoea, increased alanine aminotransferase and increased aspartate aminotransferase [106].
Meropenem-vaborbactam. As for meropenem-vaborbactam is a non-beta-lactam inhibitor of class A serine beta-lactamases, including KPC, and class C cephalosporinases. It acts by forming a covalent bridge with beta-lactamases and is stable against beta-lactamase-mediated hydrolysis. Vaborbactam does not inhibit class B enzymes (metallo-beta-lactamases) or class D (OXA) carbapenemases. It also has no antibacterial activity per se [107]. It is the first inhibitor derived from cyclic boronic acid that has been approved by the FDA, in 2017, in combination with meropenem [108]. On the other hand, it has a lower capacity for selecting resistant mutants. No specific mutations have been described in KPC that affect its inhibition profile, but resistance due to overexpression of the blaKPC gene has been described, as well as resistance due to mutations in porins and as a possible effect of mutations in efflux pumps [99].
Its two components share the same pharmacokinetics. Its renal elimination is very extensive (80-90%) and it binds to proteins at 33%, so the dose must be administered every 8 hours. It shows excellent lung penetration (ratios vs. plasma of 0.63 and 0.53 for the two components respectively) [109].
Furthermore, the binding of vaborbactam to the boron atom rapidly inactivates the enzyme, so it inhibits KPC very powerfully without being hydrolysed (unlike avibactam) and with a reversible bond [110]. As a result, meropenem-vaborbactam demonstrates pharmacokinetics that are dependent on the area under the curve (AUC) rather than on time [111]. In addition, its proportion and dosage have been designed to prevent resistant mutants from being selected [111]. In contrast, avibactam forms irreversible bonds and hydrolyses, so it is necessary to maintain high concentrations (≥8 mg/l) between doses. This is difficult to achieve in alveolar epithelial fluid for a long time, given that the mean peak is 5-6 mg/l after a 500 mg dose of avibactam [112,113].
Meropenem-vaborbactam is indicated for complicated urinary infections, including pyelonephritis; complicated intra-abdominal infection; and nosocomial pneumonia, including the kind associated with mechanical ventilation [107], a bacteraemia co-occurring or suspected to be associated with any of the infections mentioned above.
Meropenem-vaborbactam is also indicated for the treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options [107]. It demonstrated superiority in terms of efficacy and lower mortality compared to the best available therapy in the phase III TANGO II clinical trial, specifically designed to evaluate the efficacy and safety of meropenem-vaborbactam in patients with suspected or confirmed CPE infection [114,115]. Based on the available data, meropenem-vaborbactam monotherapy is associated with higher clinical cure rates and lower rates of AEs, especially with regard to nephrotoxicity, when compared to older combination therapies [115]. There is also real-life data published about meropenem-vaborbactam, with clinical success rates of 65-70% in critical patients [116-118].
In the pivotal studies on meropenem-vaborbactam, the most common AEs were headache, diarrhoea, infusion-site phlebitis, and nausea [107]. In the TANGO II clinical trial, the most frequently reported AEs were diarrhoea, anaemia and hypokalaemia [119].
Ceftolozane/tazobactam. Tazobactam is an inhibitor of several molecular class A beta-lactamases, including the enzymes CTX-M, SHV and TEM but not carbapenemases [119]. It is very active against P. aeruginosa, including multidrug resistant rods. Ceftolozane/tazobactam has obtained its approval by regulatory agencies based on a series of three clinical trials, the phase III ASPECT trials. It demonstrated non-inferiority compared to active comparators in hospitalised patients. Then, ceftolozane-tazobactam obtained an indication for complicated intra-abdominal infections, complicated urinary tract infections, acute pyelonephritis, and hospital-acquired pneumonia [120].
The AEs most frequently associated with ceftolozane-tazobactam are those associated with any other cephalosporin, such as nausea, vomiting and diarrhoea. In the phase III ASPECT clinical trials, a similar frequency of AEs was observed between ceftolozane-tazobactam and those treated with comparators [121].
Cefiderocol. Cefiderocol is a cephalosporin siderophore that uses the iron transport system to increase its periplasmic penetration. It is stable against many class A, B, C and D beta-lactamases, and very active against P. aeruginosa. It is one of the future molecules with the greatest antimicrobial spectrum [122,123].
In the phase II trial, APEKS-cUTI was non-inferior to imipenem in urinary tract infections [124]. In the APEKS-NP trial it demonstrated clinical and microbiological curing of nosocomial pneumonia, with mortality not inferior to meropenem in extended perfusion [125]. The CREDIBLE-CR study is noteworthy, where cefiderocol was compared with the best available treatment for pneumonia and urinary tract infections caused by carbapenemase-resistant Enterobacterales [126]. In this study, similar cure rates between the two treatments were obtained, but very favourable ones for cefiderocol in the case of metallo-beta-lactamases (75% vs. 29%). In contrast, mortality was higher with cefiderocol in infections caused by A. baumannii (49% vs. 18%).
AEs associated with cefiderocol are also consistent with those of other cephalosporins and similar to those of the comparators in clinical trials. The most frequent ones were diarrhoea, administration site reactions, constipation, skin rash, candidiasis, cough, elevations in liver tests, headache, hypokalaemia, nausea and vomiting in patients with complicated urinary tract infections, and elevations in liver function tests, hypokalaemia, diarrhoea, hypomagnesemia, and atrial fibrillation in patients with nosocomial pneumonia [127].
Finally, among the possible new molecules designed to treat infections caused by beta-lactamases-producing Gram-negative bacteria there are: aztreonam-avibactam, eravacycline, and plazomicin [128].
Experts’ Opinion
“Some hospitals do not have all of the new antibiotic options.”
All antibiotics must be available when needed and their correct use must be learned. Once they are approved by the regulatory agencies (EMA, AEMPS), there should be no delay in including them in the centres’ therapeutic guidelines or in giving access to them due to screening by pharmacy boards and hospitals. It is the ASP teams that must work to include the new antibiotics and set out the antimicrobial strategies in the guidelines and protocols of the sites and of the affected specialties. Equally, there should be greater involvement demanded at the institutional level from healthcare heads and authorities in the search for solutions to improve the prognosis of serious infections.
“The new options need to be available to avoid excessive use of certain molecules.”
The importance of diversified use of antibiotics was mentioned, as well as the need for new antibiotics due to the appearance of resistance.
“Beta-lactamase inhibitors improve the efficacy of beta-lactams.”
The experts agreed with this statement, which has diverse evidence to back it up [94,96,114,126].
“Depending on their spectrum of action, the different antibiotics could be recommended for different approaches against CPEs.”
The importance of diversified use of antibiotics was mentioned, as well as the need to have new antibiotics given the emergence of resistance. Table 3 gives the spectrum of action of ceftolozane-tazobactam, ceftazidime-avibactam, imipenem-relebactam, meropenem-vaborbactam, and cefiderocol.
Table 3.
Spectrum of action of commonly-used antibiotics against multi-resistant Gram-negative bacilli. Expected susceptibility: green >80%; yellow 30-80%; red <30%. Adapted from Tamma 2019 [128,129]
| Antibiotics | Enterobacterales | P. aeruginosa | A. baumannii | S. maltophilia | ||
|---|---|---|---|---|---|---|
| KPC | NDM | OXA-48 | ||||
| Ceftolozane/tazobactam | ||||||
| Ceftazidime-avibactam | ||||||
| Imipenem-relebactam | ||||||
| Meropenem-vaborbactam | ||||||
| Cefiderocol | ||||||
KPC: Carbapenemase-producing Klebsiella pneumoniae; NDM: metallo-betalactamase; OXA: oxacillinase.
CONCLUSIONS
The arrival of new antibiotics makes it necessary to train professionals involved in studying them in the laboratory and in prescribing them, in order to optimise their use and improve patient health outcomes.
This work reviews the most notable aspects of the evidence published and adds to it with expert opinions, placing special emphasis on local peculiarities. The current epidemiology in Spain of antimicrobial resistance in Gram-negative bacilli is described, as well as its relationship with local clinical practice and new aspects in this sphere.
Microbiology laboratories must be available as much as possible on a continuous timetable (24 ×7) in hospitals, and have the necessary techniques to be able to discriminate the different types of carbapenemases and the sufficient means to study CPE carriers. Also, the need of specialist in infectious diseases to adress the treatment of complex multi-drug resistant bacteria.
All antibiotics must be available when necessary and their correct use must be learned. The ASP’s work continues to be essential in incorporate the new antibiotics and guaranteee an approrioate use of them. Equally, there should be greater involvement demanded in the institutional sphere from health-care heads and authorities in the search for solutions to improve the prognosis of serious infections, from the prevention phase to diagnosis and optimal treatment. This involvement is a matter of equality and is directly related to improving health outcomes.
ACKNOWLEDGEMENTS
The authors would like to thank the attendees at the different meetings for their participation, their experience and contribution to the creation of this article. Thanks also to Menarini, for their collaboration in the carrying out the project.
FUNDING
This project has been funded by Menarini. The sponsor played no part in designing, implementing, interpreting or writing the document.
CONFLICTS OF INTEREST
Pilar Retamar-Gentil has taken part in training programmes organised by Menarini and MSD, and have participated in advisory board for Shionogi.
Rafael Cantón has taken part in training programmes organised by Menarini, MSD, Pfizer and Shionogi and in research studies funded by MSD, Shionogi and Venatorx.
Juan Pablo Horcajada has taken part in training programmes organised by Menarini, MSD and Pfizer.
Vicente Abril has received fees for educational activities and consulting work from Pfizer, Shionogi, Menarini, MSD and Angelini Pharma.
José Barberán has taken part in training programmes organised by MSD, Pfizer, Angelini and Shionogi.
Andrés Canut Blasco has taken part in training programmes organised by MSD and Pfizer.
Nieves Larrosa has taken part in training programmes organised by Menarini, MSD, Pfizer and Shionogi.
Jaime Lora-Tamayo has received fees for the following activities: aid to attend congresses: Pfizer, Novartis, Angelini Pharma, Advanz, Menarini; payment for conferences: Menarini, Novartis, Advanz; aid for research projects: Correvio; Advisory Boards: Pfizer, Debiopharm, Correvio, TenNor Therapeutics.
Carlos Martín has taken part in training programmes financed by MSD and Pfizer.
Juan Pasquau has taken part in training, consultancy and research activities financed by Pfizer, MSD, Angelini, Astellas, Advanz Pharma, Shionogi and Menarini.
Pedro Rascado has taken part in training programmes sponsored by Pfizer, MSD, Shionogi, Menarini.
Óscar Sanz has taken part in training programmes organised by Pfizer, MSD and Menarini, has participated as a speaker for Pfizer and as a consultant for Shionogi and has participated in research studies funded by Pfizer.
The following authors state they have no conflicts of interest: Carlos Dueñas Gutiérrez, Carolina García-Vidal; Genoveva Yagüe, and Francisco Javier Martínez Marcos.
References
- 1.ECDC Surveillance Atlas-Antimicrobial resistance. 2019. [cited 14 July 2022]. Available at: https://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=4
- 2.Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantón R, Huarte R, Morata L, Trillo-Mata JL, Muñoz R, González Jet al. Determining the burden of infectious diseases caused by carbapenem-resistant gram-negative bacteria in Spain. Enferm Infecc Microbiol Clin (Engl Ed). 2021;39(4):179-83. doi: 10.1016/j.eimc.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Garbajosa P, Cantón R. [Epidemiology of multi-drug resistant gramnegative bacilli]. Rev Esp Quimioter. 2016;29 Suppl 1:21-5. PMid: . [PubMed] [Google Scholar]
- 5.López-Cerero L, Egea P, Gracia-Ahufinger I, González-Padilla M, Rodríguez-López F, Rodríguez-Baño Jet al. Characterisation of the first ongoing outbreak due to KPC-3-producing Klebsiella pneumoniae (ST512) in Spain. Int J Antimicrob Agents. 2014;44(6):538-40. doi: 10.1016/j.ijantimicag.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin Microbiol Rev. 2019;32(4):e00031-19. doi: 10.1128/CMR.00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venter H. Reversing resistance to counter antimicrobial resistance in the World Health Organisation’s critical priority of most dangerous pathogens. Biosci Rep. 2019; 29(4):1-12. doi: 10.1042/BSR20180474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) . Healthcare Facilities: Information about CRE. Antibiotic resistance threats in the United States, 2013. [cited 14 July 2022]. See: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
- 9.Cantón R, Loza E, Aznar J, Barrón-Adúriz R, Calvo J, Castillo FJet al. Antimicrobial susceptibility trends and evolution of isolates with extended spectrum β-lactamases among Gram-negative organisms recovered during the SMART study in Spain (2011-2015). Rev Esp Quimioter. 2018. 31(2):136-145. SMART-Spain Working Group. PMid: . [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Zorrilla S, Becerra-Aparicio F, López Montesinos I, Ruiz de Gopegui E, Grau I, Pintado Vet al. ; REIPI/GEIRAS-GEMARA SEIMC ITUBRAS-2 Group . A Large Multicenter Prospective Study of Community-Onset Healthcare Associated Bacteremic Urinary Tract Infections in the Era of Multidrug Resistance: Even Worse than Hospital Acquired Infections? Infect Dis Ther. 2021;10(4):2677-99. doi: 10.1007/s40121-021-00537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojo V, Vázquez P, Reyes S, Puente L, Cervero M. [Risk factors and clinical evolution of carbapenemase-producing Klebsiella pneumoniae infections in a university hospital in Spain. Case-control study]. Rev Esp Quimioter. 2018;31(5):427-34. PMid: . [PMC free article] [PubMed] [Google Scholar]
- 12.Oteo J, Ortega A, Bartolomé R, Bou G, Conejo C, Fernández-Martínez Met al. Prospective multicenter study of carbapenemase-producing Enterobacteriaceae from 83 hospitals in Spain reveals high in vitro susceptibility to colistin and meropenem. Antimicrob Agents Chemother. 2015;59(6):3406-12. doi: 10.1128/AAC.00086-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oteo J, Pérez-Vázquez M, Bautista V, Ortega A, Zamarrón P, Saez Det al. ; Spanish Antibiotic Resistance Surveillance Program Collaborating Group . J Antimicrob Chemother. 2016;71(12):3392-9. doi: 10.1093/jac/dkw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control . Facility Guidance for Control of Carbapenem-resistant Enterobacteriaceae (CRE). ECDC, 2015. [cited 14 July 2022]. Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf
- 15.European Centre for Disease Prevention and Control . Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2019. Stockholm: ECDC, 2020. [cited 14 July 2022]. See: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019
- 16.Tórtola MT, Lavilla S, Miró E, González JJ, Larrosa N, Sabaté Met al. First detection of a carbapenem-hydrolyzing metalloenzyme in two Enterobacteriaceae isolates in Spain. Antimicrob Agents Chemother. 2005;49:3492–4. doi: 10.1128/AAC.49.8.3492-3494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević ATet al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017; 17:153-63. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 18.García-Castillo M, García-Fernández S, Gómez-Gil R, Pitart C, Oviaño M, Gracia-Ahufinger Iet al. ; iCREST Study Group . Activity of ceftazidime-avibactam against carbapenemase-producing Enterobacteriaceae from urine specimens obtained during the infection-carbapenem resistance evaluation surveillance trial (iCREST) in Spain. Int J Antimicrob Agents. 2018;51(3):511-5. doi: 10.1016/j.ijantimicag.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-García M, Pérez-Viso B, Turrientes MC, Díaz-Agero C, López-Fresneña N, Bonten Met al. J Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. Antimicrob Chemother. 2018;73:3039–43. doi: 10.1093/jac/dky284. [DOI] [PubMed] [Google Scholar]
- 20.Xanthopoulou K, Urrutikoetxea-Gutiérrez M, Vidal-Garcia M, Diaz de Tuesta Del Arco JL, Sánchez-Urtaza S, Wille Jet al. First Report of New Delhi Metallo-beta-Lactamase-6 (NDM-6) in a Clinical Acinetobacter baumannii Isolate From Northern Spain. Front Microbiol. 2020;11:589253. doi: 10.3389/fmicb.2020.589253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aracil B, Cañada JE, García Cobos S, Pérez Vázquez MD, Oteo J y Red EARS-Net España . Resistencia a carbapenémicos y colistina en aislados invasivos de Escherichia coli y Klebsiella pneumoniae: resultados nacionales de la Red EARS-Net (2009-2019) XXIV Congreso Nacional SEIMC, 5-11 junio 2021. Available at: https://intranet.pacifico-meetings.com/amsysweb/faces/publicacionOnlineSEIMCLIBRO.xhtml?id=667 [Google Scholar]
- 22.Hernández-García M, Castillo-Polo JA, Cordero DG, Pérez-Viso B, García-Castillo M, Saez de la Fuente Jet al. Impact of Ceftazidime-Avibactam Treatment in the Emergence of Novel KPC Variants in the ST307-Klebsiella pneumoniae High-Risk Clone and Consequences for Their Routine Detection. J Clin Microbiol. 2022;60(3):e0224521. doi: 10.1128/jcm.02245-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano Á, Guzmán-Puche J, García-Gutiérrez M, Castón JJ, Gracia-Ahufinger I, Pérez-Nadales Eet al. Use of carbapenems in the combined treatment of emerging ceftazidime/avibactam-resistant and carbapenem-susceptible KPC-producing Klebsiella pneumoniae infections: Report of a case and review of the literature. J Glob Antimicrob Resist. 2020;22:9-12. doi: 10.1016/j.jgar.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Dandan Yin, Renru Han, Yan Guo, Yonggui Zheng, Shi Wuet al. Emergence and Recovery of Ceftazidime-avibactam Resistance in blaKPC-33-Harboring Klebsiella pneumoniae Sequence Type 11 Isolates in China. Clin Infect Dis. 2020;71(Suppl 4):S436-S439. doi: 10.1093/cid/ciaa1521. [DOI] [PubMed] [Google Scholar]
- 25.Niu S, Chavda KD, Wei J, Zou C, Marshall SH, Dhawan P. A Ceftazi-dime-Avibactam-Resistant and Carbapenem-Susceptible Klebsiella pneumoniae Strain Harboring blaKPC-14 Isolated in New York City. mSphere 2020;5(4):e00775-20. doi: 10.1128/mSphere.00775-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-García M, García-Castillo M, Ruiz-Garbajosa P, Bou G, Siller-Ruiz M, Pitart C, Gracia-Ahufinger Iet al. In Vitro Activity of Cefepime-Taniborbactam against Carbapenemase-Producing Enterobacterales and Pseudomonas aeruginosa Isolates Recovered in Spain. Antimicrob Agents Chemother. 2022;66(3):e0216121. doi: 10.1128/aac.02161-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo-Polo JA, Morosini MI, Pérez-Viso B, Hernández-García M, Soriano C, De Pablo Ret al. Descripción de un brote de Klebsiella pneumoniae productora de KPC-62 y CTX-M-15 resistente a cefiderocol y ceftazidima-avibactam en un hospital universitario en Madrid. Sesión: SO-08. Epidemiología de la resistencia a los antimicrobianos. XXIV Congreso Nacional SEIMC, 5-11 junio 2021. Available at: https://intranet.pacifico-meetings.com/amsysweb/faces/publicacionOnlineSEIMCLIBRO.xhtml?id=667
- 28.Marquet K, Liesenborg A, Bergs J, Vleugels A, Claes Net al. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Review Crit Care. 2015;19(1):63. doi: 10.1186/s13054-015-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadri SS, Lai YL, Warner S, Strich JR, Babiker A, Ricotta EE, et al. ; forming the National Insititutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI) . Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241-51. doi: 10.1016/S1473-3099(20)30477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang C, Kim S, Park WB, Lee K, Kim H, Kim Eet al. Bloodstream infections caused by antibiotic-resistant Gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–6. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RMet al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54:1742–8. doi: 10.1128/AAC.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luna CM, Aruj P, Niederman MS, Garzon J, Violi D, Prignoni A, Ríos Fet al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia’. Eur Respir. 2006;27:158–64. doi: 10.1183/09031936.06.00049105. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Park W, Lee K, Kang C, Bang J, Kim Het al. Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicriobial Chemother. 2004;54:489–97. doi: 10.1093/jac/dkh366. [DOI] [PubMed] [Google Scholar]
- 34.Scarsi KK, Feinglass JM, Scheetz MH, Postelnick MJ, Bolon MK, Noskin GA. Impact of inactive empiric antimicrobial therapy on inpatient mortality and length of stay. Antimicrob Agents Chem-other. 2006;50:3355–60. doi: 10.1128/AAC.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marschall J, Agniel D, Fraser VJ, Doherty J, Warren DK. Gram-negative bacteraemia in non-ICU patients: factors associated with inadequate antibiotic therapy and impact on outcomes. J Antimicriobial Chemother. 2008;61:1376–83. doi: 10.1093/jac/dkn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez-Baño J, Millán AB, Domínguez MA, Borraz C, González MP, Almirante Bet al. Impact of inappropriate empirical therapy for sepsis due to health care-associated methicillin-resistant Staphylococcus aureus. J Infect. 2009;58:131–7. doi: 10.1016/j.jinf.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli Met al. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteraemia in 9 Western European countries. Clin Infect Dis. 2009;49:997–1005. doi: 10.1086/605555. [DOI] [PubMed] [Google Scholar]
- 38.Erbay A, Idil A, Gözel MG, Mumcuoğlu I, Balaban N. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents. 2009;34:575–9. doi: 10.1016/j.ijantimicag.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parillo Jet al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–48. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 40.Tseng C-C, Fang W-F, Huang K-T, Chang P-W, Tu M-L, Shiang Y-Pet al. Risk factors for mortality in patients with nosocomial Stenotrophomonas maltophilia pneumonia. Infect Control Hosp Epidemiol. 2009;30:1193–202. doi: 10.1086/648455. [DOI] [PubMed] [Google Scholar]
- 41.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chem-other. 2005;49:1306–11. Doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joung M, Kwon K, Kang C, Cheong H, Rhee J, Jung Det al. Impact of inappropriate antimicrobial therapy on outcome in patients with hospital-acquired pneumonia caused by Acinetobacter baumannii. J Infect. 2010;61:212–8. doi: 10.1016/j.jinf.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Shorr AF, Micek ST, Pharm D, Kollef MH. Inappropriate therapy for methicillin-resistant Staphylococcus aureus: resource utilization and cost implications. Crit Care Med. 2008;36:2335–40. doi: 10.1097/CCM.0b013e31818103ea. [DOI] [PubMed] [Google Scholar]
- 44.Reisfeld S, Paul M, Gottesman BS, Shitrit P, Leibovici L, Chowers M. The effect of empiric antibiotic therapy on mortality in debilitated patients with dementia. Eur J Clin Microbiol Infect Dis. 2011;30:813–8. doi: 10.1007/s10096-011-1161-x. [DOI] [PubMed] [Google Scholar]
- 45.Wilke MH, Grube R, Bodmann KF. Guideline-adherent initial intravenous antibiotic therapy for hospital-acquired, ventilator-associated pneumonia is clinically superior, saves lives and is cheaper than non-guideline-adherent therapy. Eur J Med Res. 2011;16:315–23. doi: 10.1186/2047-783x-16-7-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lye DC, Earnest A, Ling ML, Lee T, Yong H, Fisher DAet al. The impact of multidrug resistance in healthcare-associated and nosocomial Gramnegative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect. 2012;18:502–8. doi: 10.1111/j.1469-0691.2011.03606.x. [DOI] [PubMed] [Google Scholar]
- 47.Tseng C-C, Liu S-F, Wang C-C, Tu M-L, Chung Y-H, Lin M-Cet al. Impact of clinical severity index, infective pathogens, and initial empiric antibiotic use on hospital mortality in patients with ventilator-associated pneumonia. Am J Infect Control. 2012;40:648–52. doi: 10.1016/j.ajic.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Chen R, Yan Z, Feng D, Luo Y, Wang L, Shen D. Nosocomial bloodstream infection in patients caused by factors for hospital mortality. Chin Med J. 2012;125:226–9. PMid: . [PubMed] [Google Scholar]
- 49.Labelle A, Juang P, Reichley R, Micek S, Hoffmann J, Hoban Aet al. The determinants of hospital mortality among patients with septic shock receiving appropriate initial antibiotic treatment. Crit Care Med. 2012;40:2016–21. doi: 10.1097/CCM.0b013e318250aa72. [DOI] [PubMed] [Google Scholar]
- 50.Chen H-C, Lin W-L, Lin C-C, Hsieh W-H, Hsieh C-H, Wu M-Het al. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J Antimicrob Chemother. 2013;68:947–53. doi: 10.1093/jac/dks475. [DOI] [PubMed] [Google Scholar]
- 51.Frakking FNJ, Rottier WC, Dorigo-Zetsma JW, van Hattem JM, van Hees BC, Kluytmans JAJWet al. Appropriateness of empirical treatment and outcome in bacteraemia caused by extended-spectrumβ-lactamase-producing bacteria. Antimicrob Agents Chem-other. 2013;57:3092–9. doi: 10.1128/AAC.01523-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia Ret al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013;39:682–92. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 53.Bengtsson-Palme J, Kristiansson E, Larsson DGJ. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol Rev. 2018;42(1):fux053. doi: 10.1093/femsre/fux053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-Medrano F, Juan SR, Lizasoain M Catalán M, Ferrari JM, Chaves Fet al. A non-compulsory stewardship programme for the management of antifungals in a university-affiliated hospital. Clin Microbiol Infect. 2013;19:56-61. doi: 10.1111/j.1469-0691.2012.03891.x. [DOI] [PubMed] [Google Scholar]
- 55.Cisneros JM, Neth O, Gil-Navarro MV, Lepe JA, Jiménez-Parrilla F, Cordero Eet al. Global impact of an educational antimicrobial stewardship programme on prescribing practice in a tertiary hospital centre. Clin Microbiol Infect. 2014;20:82-8. doi: 10.1111/1469-0691.12191. [DOI] [PubMed] [Google Scholar]
- 56.Valerio M, Muñoz P, Rodriguez CG, Caliz B, Padilla B, Fernández-Cruz Aet al. Antifungal stewardship in a tertiary-care institution: a bedside intervention. Clin Microbiol Infect. 2015;21:492. e1-9. doi: 10.1016/j.cmi.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Del Arco A, Tortajada B, de la Torre J, Olalla J, Prada JL, Fernández Fet al. The impact of an antimicrobial stewardship programme on the use of antimicrobials and the evolution of drug resistance. Eur J Clin Microbiol Infect Dis. 2015;34:247-51. doi: 10.1007/s10096-014-2225-5. [DOI] [PubMed] [Google Scholar]
- 58.Güerri-Fernández R, Villar-García J, Herrera-Fernández S, TrenchsRodríguez M, Fernández-Morato J, Moro Let al. An antimicrobial stewardship program reduces antimicrobial therapy duration and hospital stay in surgical wards. Rev Esp Quimioter. 2016;29:119-21. PMid: . [PubMed] [Google Scholar]
- 59.Molina J, Peñalva G, Gil-Navarro MV, Praena J, Lepe JA, Pérez-Moreno MAet al. Long-term impact of an educational antimicrobial stewardship program on hospital-acquired candidemia and multi-drug-resistant bloodstream infections: a quasi-experimental study of interrupted time-series analysis. Clin Infect Dis. 2017;65:1992-9. doi: 10.1093/cid/cix692. [DOI] [PubMed] [Google Scholar]
- 60.Álvarez-Lerma F, Grau S, Echeverría-Esnal D, Martínez-Alonso M, Gracia-Arnillas MP, Horcajada JPet al. A before-and-after study of the effectiveness of an antimicrobial stewardship program in critical care. Antimicrob Agents Chemother. 2018;62(4):e01825-17. doi: 10.1128/AAC.01825-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esteve-Palau E, Grau S, Herrera S, Sorlí L, Montero M, Segura Cet al. Impact of an antimicrobial stewardship program on urinary tract infections caused by extended-spectrum ć-lactamase-producing Escherichia coli. Rev Esp Quimioter. 2018;31:110-7. PMid: . [PMC free article] [PubMed] [Google Scholar]
- 62.Tacconelli E, Mazzaferri F, De Smet AM, Bragantini D, Eggimann P, Huttner BDet al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin Microbiol Infect. 2019;25(7):807-17. doi: 10.1016/j.cmi.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention (CDC) . Healthcare Facilities: Information about CRE, 2019. [cited 14 July 2022]. Available at: https://www.cdc.gov/hai/organisms/cre/cre-facilities.html
- 64.Junta de Andalucía. Consejería de Salud y Familias. Programa PIRASOA, 2020. [cited 14 July 2022]. Available at: http://pirasoa.iavante.es
- 65.Vázquez-Ucha JC, Seoane-Estévez A, Rodiño-Janeiro BK, González-Bardanca M, Conde-Pérez K, Martínez-Guitián Met al. ; GEMARA-SEIMC/REIPI.Enterobacterales Study Group . Activity of imipenem/relebactam against a Spanish nationwide collection of carbapenemase-producing Enterobacterales. J Antimicrob Chem-other. 2021;76(6):1498-510. doi: 10.1093/jac/dkab043. [DOI] [PubMed] [Google Scholar]
- 66.Semicyuc. Registro ENVIN [cited 14 July 2022]. Available at: https://semicyuc.org/envin/
- 67.Ministerio de Ciencia e Innovación . Red de Laboratorios para la Vigilancia de los Microorganismos Resistentes (RedLabRA). 2021. [cited 14 July 2022]. Available at: https://www.isciii.es/QueHacemos/Servicios/DiagnosticoMicrobiol%C3%B3gicoyProgramasVigilancia/Paginas/RedLabRA.aspx
- 68.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJet al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodríguez-Baño J, Paño-Pardo JR, Alvarez-Rocha L, Asensio A, Calbo E, Cercenado Eet al. [Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document]. Enferm Infecc Microbiol Clin. 2012;30(1):22.e1-22.e23. Spanish. doi: 10.1016/j.eimc.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 70.Serra-Burriel M, Keys M, Campillo-Artero C, Agodi A, Barchitta M, Gikas Aet al. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS One. 2020;15(1):e0227139. doi: 10.1371/journal.pone.0227139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Budhram DR, Mac S, Bielecki JM, Patel SN, Sander B. Health outcomes attributable to carbapenemase-producing Enterobacteriaceae infections: A systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2020;41(1):37-43. doi: 10.1017/ice.2019.282. [DOI] [PubMed] [Google Scholar]
- 72.Liu P, Li X, Luo M, Xu X, Su K, Chen Set al. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microb Drug Resist. 2018;24(2):190-8. doi: 10.1089/mdr.2017.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu WM, Yuan Z, Zhou HY. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9(1):23. doi: 10.1186/s13756-020-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Predic M, Delano JP, Tremblay E, Iovine N, Brown S, Prins Cet al. Evaluation of patient risk factors for infection with carbapenem-resistant Enterobacteriaceae. Am J Infect Control. 2020;48(9):1028-31. doi: 10.1016/j.ajic.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 75.Giannella M, Trecarichi EM, De Rosa FG, Del Bono V, Bassetti M, Lewis REet al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect. 2014;20(12):1357-62. doi: 10.1111/1469-0691.12747. [DOI] [PubMed] [Google Scholar]
- 76.Cano A, Gutiérrez-Gutiérrez B, Machuca I, Gracia-Ahufinger I, Pérez-Nadales E, Causse Met al. Risks of Infection and Mortality Among Patients Colonized With Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: Validation of Scores and Proposal for Management. Clin Infect Dis. 2018;66(8):1204-10. doi: 10.1093/cid/cix991. [DOI] [PubMed] [Google Scholar]
- 77.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JRet al. ; REIPI/ESGBIS/INCREMENT Investigators . Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17(7):726-34. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 78.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KLet al. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-analysis. Review Clin Infect Dis. 2017;64(1):15-23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 79.Jeon YD, Seong H, Kim D, Ahn MY, Jung IY, Jeong SJet al. Impact of matrix-assisted laser desorption/ionization time of flight mass spectrometric evaluation on the clinical outcomes of patients with bacteraemia and fungemia in clinical settings lacking an antimicrobial stewardship program: a pre-post quasi experimental study. BMC Infect Dis. 2018;18(1):385. doi: 10.1186/s12879-018-3299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2):e00079-17. doi: 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hobson CA, Cointe A, Jacquier H, Choudhury A, Magnan M, Courroux Cet al. Cross-resistance to cefiderocol and ceftazi-dime-avibactam in KPC beta-lactamase mutants and the inoculum effect. Clin Microbiol Infect. 2021;27(8):1172.e7-1172.e10. doi: 10.1016/j.cmi.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 82.Gijón Cordero D, Castillo-Polo JA, Ruiz-Garbajosa P, Canton R. Antibacterial spectrum of cefiderocol. Rev Esp Quimiter. 2022;35(Suppl.2):20-27. doi: 10.37201/req/s02.03.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim T, Lee SC, Bae M, Sung H, Kim MN, Jung Jet al. In vitro activities and inoculum effects of ceftazidime-avibactam and aztreonam-avibactam against carbapenem-resistant Enterobacterales Isolates from South Korea. Antibiotics (Basel). 2020;9(12):912. doi: 10.3390/antibiotics9120912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gudiol C, Cuervo G, Carratalà J. Optimizing therapy of bloodstream infection due to extended-spectrum β-lactamase-producing Enterobacteriaceae. Curr Opin Crit Care. 2019;25(5):438-448. doi: 10.1097/MCC.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 85.Valero A, Rodríguez-Gascón A, Isla A, Barrasa H, Del Barrio-Tofiño E, Oliver A, et al. Pseudomonas aeruginosa susceptibility in Spain: antimicrobial activity and resistance suppression evaluation by PK/ PD Analysis. Pharmaceutics. 2021;13(11):1899. doi: 10.3390/pharmaceutics13111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bou G, Calbo E, Crespo M, Cantón R, Álvarez de Luna FF, Rodriguez JGet al. Justification for 24/7 clinical microbiology services. Enferm Infecc Microbiol Clin (Engl Ed). 2022. 40(1):1-4. doi: 10.1016/j.eimce.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 87.Ministerio de Sanidad, SeMicyuc. Proyecto Resistencia Zero. [cited 14 July 2022]. Available at: https://seguridaddelpaciente.es/es/practicas-seguras/seguridad-pacientes-criticos/proyecto-resistencia-zero
- 88.Ambler RP. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980; 289: 321–31. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 89.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank Det al. Efficacy and safety of ceftazidime-avibactam plus metro-nidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62(11):1380–9. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, Chen Qet al. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intraabdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017;49(5):579–88. doi: 10.1016/j.ijantimicag.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GGet al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63(6):754–62. doi: 10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman Aet al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16(6): 661–73. doi: 10.1016/S1473-3099(16)30004-4. [DOI] [PubMed] [Google Scholar]
- 93.Torres A, Zhong N, Pachl Jet al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3): 285–95. doi: 10.1016/S1473-3099(17)30747-8. [DOI] [PubMed] [Google Scholar]
- 94.Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini Cet al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Observational Study Clin Infect Dis. 2019;68(3):355-64. doi: 10.1093/cid/ciy492. [DOI] [PubMed] [Google Scholar]
- 95.Shields RK, McCreary EK, Marini RV, Kline EG, Jones CE, Hao Bet al. Early Experience With Meropenem-Vaborbactam for Treatment of Carbapenem-resistant Enterobacteriaceae Infections. Clin Infect Dis. 2020;71(3):667-71. doi: 10.1093/cid/ciz1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsolaki V, Mantzarlis K, Mpakalis A, Malli E, Tsimpoukas F, Tsirogianni Aet al. Ceftazidime-Avibactam To Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically Ill Mechanically Ventilated Patients. Antimicrob Agents Chemother. 2020;64(3):e02320-19. doi: 10.1128/AAC.02320-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, Mc-Conville TH. Successive Emergence of Ceftazidime-Avibactam Resistance through Distinct Genomic Adaptations in blaKPC-2-Harboring Klebsiella pneumoniae Sequence Type 307 Isolates. Antimicrob Agents Chemother. 2018;62(3):e02101-17. doi: 10.1128/AAC.02101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shirley M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs. 2018;78(6):675-92. doi: 10.1007/s40265-018-0902-x. [DOI] [PubMed] [Google Scholar]
- 99.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel Met al. Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenemβ-Lactamase Inhibitor Combinations. Drugs. 2018;78(1):65-98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 100.Lob SH, Hackel MA, Kazmierczak KM, Young K, Motyl MR, Karlowsky JA. In Vitro Activity of Imipenem-Relebactam against Gram-Negative ESKAPE Pathogens Isolated by Clinical Laboratories in the United States in 2015 (Results from the SMART Global Surveillance Program). Antimicrob Agents Chemother. 2017;61(6):e02209-16. doi: 10.1128/AAC.02209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 2013;68(10):2286-90. doi: 10.1093/jac/dkt178. [DOI] [PubMed] [Google Scholar]
- 102.Sims M, Mariyanovski V, McLeroth P, Akers W, Lee YC, Brown ML. Prospective, randomized, double-blind, Phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother. 2017;72(9):2616-26. doi: 10.1093/jac/dkx139. [DOI] [PubMed] [Google Scholar]
- 103.Lucasti C, Vasile L, Sandesc D, Venskutonis D, McLeroth P, Lala Met al. Phase 2, Dose-Ranging Study of Relebactam with Imipenem-Cilastatin in Subjects with Complicated Intra-abdominal Infection. Antimicrob Agents Chemother. 2016;60(10):6234-43. doi: 10.1128/AAC.00633-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Titov I, Wunderink RG, Roquilly A, Rodríguez D, David-Wang A, Boucher HWet al. A Randomized, Double-blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults With Hospital-acquired or Ventilator-associated Bacterial Pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis. 2021;73(11):e4539-e4548. doi: 10.1093/cid/ciaa803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Motsch J, Murta de Oliveira C, Stus V, Köksal I, Lyulko O, Boucher HWet al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2020;70(9):1799-808. doi: 10.1093/cid/ciz530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Campanella TA, Gallagher JC. A Clinical Review and Critical Evaluation of Imipenem-Relebactam: Evidence to Date. Infect Drug Resist. 2020;13:4297-4308. doi: 10.2147/IDR.S224228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Specifications for Vaborem. Available at: https://cima.aemps.es/cima/dochtml/ft/1181334001/FT_1181334001.html
- 108.Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DCet al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class a serine carbapenemases. J Med Chem. 2015;58:3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 109.Wenzler E, Gotfried MH, Loutit JS, Durso S, Griffith DC, Dudley MNet al. Meropenem-RPX7009 Concentrations in Plasma, Epithelial Lining Fluid, and Alveolar Macrophages of Healthy Adult Subjects. Antimicrob Agents Chemother. 2015;59(12):7232-9. doi: 10.1128/AAC.01713-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bush K. Game Changers: New β-Lactamase Inhibitor Combinations Targeting Antibiotic Resistance in Gram-Negative Bacteria. ACS Infect Dis. 2018;4(2):84-87. doi: 10.1021/acsinfecdis.7b00243. [DOI] [PubMed] [Google Scholar]
- 111.Ambrose PG, Lomovskaya O, Griffith DC, Dudley MN, VanScoy Bet al. β-Lactamase inhibitors: what you really need to know. Curr Opin Pharmacol. 2017;36:86-93. doi: 10.1016/j.coph.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Réville TFet al. Kinetics of avibactam inhibition against Class A, C, and D β-lactamases. J Biol Chem. 2013;288(39):27960-71. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nicolau DP, Siew L, Armstrong J, Li J, Edeki T, Learoyd Met al. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother. 2015;70(10):2862-9. doi: 10.1093/jac/dkv170. [DOI] [PubMed] [Google Scholar]
- 114.Wunderink RG, Yin Y. Antibiotic Resistance in Community-Acquired Pneumonia Pathogens. Semin Respir Crit Care Med. 2016. Dec;37(6):829-838. doi: 10.1055/s-0036-1593753. [DOI] [PubMed] [Google Scholar]
- 115.Novelli A, Del Giacomo P, Rossolini GM, Tumbarello M. Meropenem/vaborbactam: a next generation β-lactam β-lactamase inhibitor combination. Expert Rev Anti Infect Ther. 2020:1-13. doi: 10.1080/14787210.2020.1756775. [DOI] [PubMed] [Google Scholar]
- 116.Shields RK, M Nguyen H, Chen L, Press EG, Potoski BA, Marini RV. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob Agents Chemother. 2017;61(8):e883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alosaimy S, Jorgensen SCJ, Lagnf AM, Melvin S, Mynatt RP, Carlson TJet al. Real-world Multicenter Analysis of Clinical Outcomes and Safety of Meropenem-Vaborbactam in Patients Treated for Serious Gram-Negative Bacterial Infections. Open Forum Infect Dis. 2020;7(3):ofaa051. doi: 10.1093/ofid/ofaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alosaimy S, Lagnf AM, Morrisette T, Scipione MR, Zhao JJ, Jorgensen SCJet al. Real-world, Multicenter Experience With Meropenem-Vaborbactam for Gram-Negative Bacterial Infections Including Carbapenem-Resistant Enterobacterales and Pseudomonas aeruginosa. Open Forum Infect Dis. 2021;8(8):ofab371. doi: 10.1093/ofid/ofab371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J. Effect and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect Dis Ther. 2018;7(4):439-55. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zerbaxa specifications. [cited 14 July 2022]. Available at: https://cima.aemps.es/cima/dochtml/ft/1151032001/FT_1151032001.html
- 121.Giacobbe DR, Bassetti M, De Rosa FG, Del Bono V, Grossi PA, Menichetti Fet al. Ceftolozane/tazobactam: place in therapy. Expert Rev Anti Infect Ther. 2018;16(4):307-20. doi: 10.1080/14787210.2018.1447381. [DOI] [PubMed] [Google Scholar]
- 122.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DFet al. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Non-susceptible Isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother. 2017;61(9):e00093-17. doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Monogue ML, L M Abbo, R Rosa, J F Camargo, O Martinez, R A Bonomo. In Vitro Discordance with In Vivo Activity: Humanized Exposures of Ceftazidime-Avibactam, Aztreonam, and Tigecycline Alone and in Combination against New Delhi Metallo-ć-Lactamase-Producing Klebsiella pneumoniae in a Murine Lung Infection Model. Antimicrob Agents Chemother. 2017;61(7):e00486-17. doi: 10.1128/AAC.00486-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Portsmouth S, Van Veenhuyzen D, Echols R, Machida M, Arjona JC, Ariyasu Met al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319-28. doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- 125.Wunderink RG, Matsunaga Y, Ariyasu M, Clevenbergh P, Echols R, Kaye KS. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Clinical Trial Lancet Infect Dis. 2021;21(2):213-25. doi: 10.1016/S1473-3099(20)30731-3. [DOI] [PubMed] [Google Scholar]
- 126.Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer Ret al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21(2):226-40. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 127.Syed YY. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections [published correction appears in Drugs. 2021;81(18):2167]. Drugs. 2021;81(13):1559-71. doi: 10.1007/s40265-021-01580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tamma PD, J Hsu A. Defining the Role of Novel β-Lactam Agents That Target Carbapenem-Resistant Gram-Negative Organisms. J Pediatric Infect Dis Soc. 2019;8(3):251-260. doi: 10.1093/jpids/piz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pintado V, Ruiz-Garbajosa P, Aguilera D, Baquero-Artigao F, Bou G, Cantón R, et al. Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Micro-biology (SEIMC) on the diagnosis and antimicrobial treatment of infections due to carbapenem-resistant Gram-negative bacteria. Enf Infec Micrbiol Clin 2023; doi: 10.1016/j.eimc.2022.06.004 [DOI] [PubMed] [Google Scholar]