Abstract
Background and Purpose
Hyperglycemia has been associated with unfavorable outcome of acute ischemic stroke, but this association has not been verified in patients with endovascular thrombectomy treatment. This study aimed to assess the impact of stress hyperglycemia ratio on early neurological deterioration and favorable outcome after thrombectomy in patients with acute ischemic stroke.
Methods
Stroke patients with endovascular thrombectomy in two comprehensive centers were enrolled. Early neurological deterioration was defined as ≥4 points increase of National Institutes of Health Stroke Scale (NIHSS) at 24 hours after endovascular procedure. Favorable outcome was defined as modified Rankin Scale (mRS) score of 0-2 at 90 days of stroke onset. Multivariate regression analysis was used to identify the predictors for early neurological deterioration and favorable outcome.
Results
Among the 559 enrolled, 74 (13.2%) patients developed early neurological deterioration. The predictors for early neurological deterioration were high stress hyperglycemia ratio at baseline (OR =5.77; 95% CI, 1.878-17.742; P =0.002), symptomatic intracranial hemorrhage (OR =4.90; 95% CI, 2.439-9.835; P <0.001) and high NIHSS score after 24 hours (OR =1.11; 95% CI, 1.071-1.151; P <0.001). The predictors for favorable outcome were stress hyperglycemia ratio (OR =0.196, 95% CI, 0.077-0.502; P =0.001), age (OR =0.942, 95% CI, 0.909-0.977; P =0.001), NIHSS score 24 hours after onset (OR =0.757, 95% CI =0.693-0.827; P <0.001), groin puncture to recanalization time (OR =0.987, 95% CI, 0.975-0.998; P =0.025), poor collateral status before treatment (ASITN/SIR grade 0-3, OR =62.017, 95% CI, 25.920-148.382; P <0.001), successful recanalization (mTICI 2b or 3, OR =7.415, 95% CI, 1.942-28.313; P =0.001).
Conclusion
High stress hyperglycemia ratio may be related to early neurological deterioration and decreased likelihood of favourable outcomes after endovascular thrombectomy in patients with acute ischemic stroke.
Keywords: acute ischemic stroke, early neurological deterioration, endovascular thrombectomy, large artery occlusion, stress hyperglycemia ratio
Introduction
Endovascular thrombectomy has been involving as the first-line treatment for acute ischemic stroke caused by large artery occlusion (1–3). However, mechanical recanalization not always necessarily resulted in favorable outcome even when patients were treated within 6 hours of stroke onset (4, 5). Exploring the possible factors associated with early neurological deterioration (END), a strong predictor for functional outcomes, is of vital importance for continuously improving the efficacy of endovascular thrombectomy in stroke patients (6, 7).
Hyperglycemia was associated with END in patients with acute ischemic stroke (8–10). Hyperglycemia could destruct blood-brain barrier, aggravate ischemic lesion, increase risk of hemorrhage transformation after cerebral infarction, and reduce duration of ischemic penumbra existence (11–14). Glycated hemoglobin (HbA1c) is more stable than blood glucose level in patients with acute ischemic stroke, and stress hyperglycemia ratio, defined as the stress fasting glycemia/HbA1c ratio (SHR), may be more feasible for evaluating the functional outcome. Some studies observed that stroke patients with high SHR had decreased likelihood of favorable functional outcome and increased likelihood of recurrence and intracranial hemorrhage after recanalization treatment (15–17); others missed these phenomena (18). Therefore, the relationship between SHR and END or functional outcome after endovascular recanalization treatment in patients with acute ischemic stroke is far from determined. This study aimed to investigate the effects of SHR on END and functional outcome in patients with acute ischemic stroke and treated with endovascular thrombectomy.
Methods
Study population
Stroke patients with endovascular thrombectomy in two comprehensive centers were screened for eligibility during November 1, 2018 and May 31, 2022. Local ethic review board approved the study protocol. Due to its retrospective nature, patient consent was waived.
Patients were treated with endovascular thrombectomy if they: 1) aged 18 years or old; 2) had ischemic stroke caused by large artery occlusion in anterior or posterior circulation; 3) pre-stroke mRS score ≤2; and 4) had arterial sheath being placed in 6 hours of stroke onset or met the DAWN or DEFUSE criteria (19, 20). Patients were not treated with endovascular thrombectomy if they: 1) had a life expectancy <12 months; 2) had severe cardiopulmonary failure; 3) had a platelet count of <55×1000/mm3; or 4) had anemia (hemoglobin <100 g/l) or other conditions which may affect HbA1c measurement.
Recanalization treatment and baseline assessment
All thrombectomy procedures were performed with Solitaire (Covidien, Irvine, CA) and Catalyst6 devices (Stryker, Kalamazoo, MI) alone or in combination. Successful recanalization was defined as grade 2b-3 in modified thrombolysis in cerebral infarction (mTICI). Collateral circulation was evaluated using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral vessel grading system (ASITN/SIR), and categorized into grade 0 or 1, 2, and 3 or 4. The door to groin puncture time (DPT), groin puncture to final recanalization time (PRT), number of retriever passes, intravenous thrombolysis, and rescue treatment (including angioplasty, stenting, intra-artery thrombolysis) were recorded. Possible pre-procedure infarction were quantified using the Alberta Stroke Program Early CT Score (ASPECTS) or ASPECTS for posterior circulation (pc-ASPECTS) on non-contrast CT. CT was performed immediately and 24 hours after the endovascular procedures to detect possible intracranial hemorrhage. An extra CT scan was arranged whenever as the novel symptoms indicated.
Follow-up assessment
Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) score. Stress hyperglycemia ratio was defined as the stress fasting glycemia/HbA1c ratio (SHR). END was defined as an increase of 24-hour NIHSS score of ≥4 points after endovascular procedure (21). Favorable outcome was defined as a mRS score of 0-2 at 90 days of stroke onset. Symptomatic intracranial hemorrhage (sICH) was defined and classified according to the European Cooperative Acute Stroke Study (ECASS-III) criteria (22). Malignant brain edema was defined and classified according to the SITS-MOST (Safe Implementation of Thrombolysis in Stroke-Monitoring Study) protocol, and grade 3 was defined as malignant edema (23, 24).
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and analyzed with χ2 or Fisher’s exact test. Quantitative variables were expressed as medians and interquartile ranges (IQRs), and were analyzed with Mann-Whitney U test. Receiver Operating Characteristic Curve (ROC) was constructed to explore the cutoff value of SHR for predicting favorable outcome. Multivariable logistical regression model was used to assess the potential factors associated with favorable outcome. Parameters with P <0.05 in univariate analysis entered in multivariate analysis. The covariates included in the multivariable logistical regression were mTICI score (2b or 3), MCE, sICH, NIHSS 24 hours after procedure, Pre-procedure ASPCET score, PTR, Pre-procedure ASITN/SIR score, homocysteine, retriever passes >3 times, lymphocyte, HbA1c, fasting blood glucose, glycosylated hemoglobin, SHR. Model 1 and Model 2 were diabetic group and non-diabetic group, respectively. Model 3 included fasting blood glucose and glycosylated hemoglobin as confounders, and model 4 excluded fasting blood glucose and glycosylated hemoglobin as confounders. P value of <0.05 was considered as statistically significant. Statistical analyses were performed using SPSS 25.0 (IBM, Armonk, NY).
Results
A total of 559 stroke patients were enrolled. The median (IQR) age was 70 (63–77) years, and NIHSS score after 24 hours of thrombectomy was 12 (6-19). There were 357 (63.9%) male patients. Among the enrolled, 74 (13.2%) patients developed END. There were 69 (12.3%) patients occurred sICH in 24 hours, and 81 patients (14.5%) died in 90 days. Favorable outcome was obtained in 284 (50.8%) patients. There were 190 (34.0%) patients had high SHR. The ROC curve showed that the optimal cutoff value of SHR for predicting favorable outcome was 1.07, the sensitivity was 84.7%, the specificity was 52.5%, and the Youden index was 0.372 ( Figure 1 ).
Figure 1.
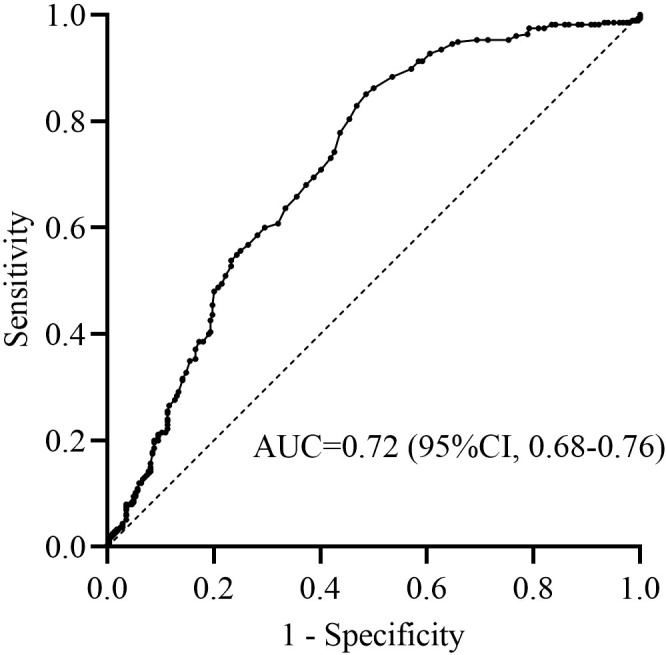
Sensitivity and specificity of SHR in predicting favorable outcome.
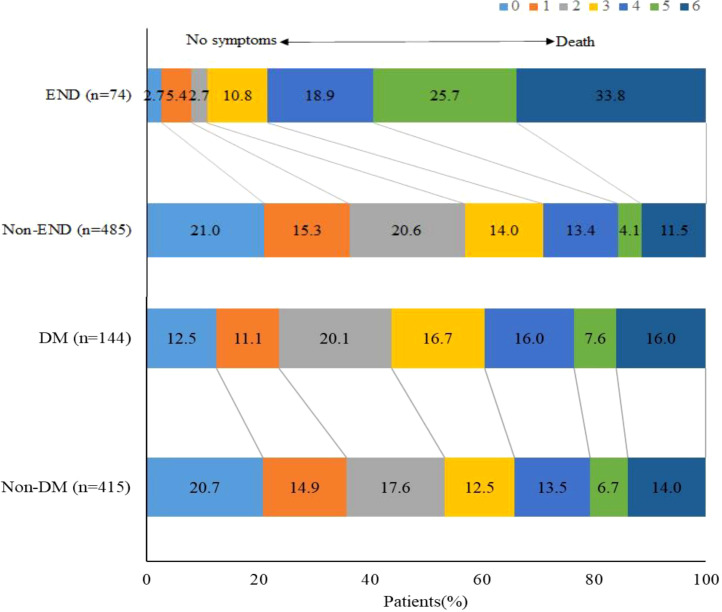
Compared with patients without END, those with END had higher median NIHSS scores after 24 hours (29 vs. 11, P <0.001), higher homocysteine (16.4 vs. 14.6, P =0.029), higher fasting glucose levels (8.7 vs. 6.9, P <0.001), higher glycated hemoglobin (6.4 vs. 5.9, P =0036), higher SHR (1.4 vs. 1.1, P <0.001), lower pre-procedure ASPECTS score (8 vs. 9, P =0.001), lower pre-procedure ASITN grade (1 vs. 3, P <0.001), longer PRT (80 vs. 67, P =0.014), higher proportion of multiple retriever passes (16.2% vs. 8.2%, P =0.028), lower proportion of successful recanalization (83.8% vs. 91.3%, P =0.040), and higher proportion of malignant brain edema (28.4% vs. 15.5%, P =0.006). The proportion of sICH was higher (48.6% vs. 6.8%, P <0.001) in patient with END than that in patient without. Proportion of favorable outcome was lower (10.8% vs. 56.9%, P <0.001), and mortality (33.8% vs. 11.5%, P <0.001) was higher in patients with END. Moreover, subgroup analysis showed that proportion of favorable outcome was lower in patients with diabetes mellitus. ( Table 1 , Figure 2 ).
Table 1.
Baseline characteristics and clinical outcomes according to END.
| Variable | END | P value | |
|---|---|---|---|
| with without n=74 n=485 | |||
| Age, y, median (IQR) | 69 (61-76) | 70 (63-77) | 0.696 |
| Male, n (%) | 49 (66.2) | 308 (63.5) | 0.651 |
| NIHSS at baseline, median (IQR) | 17 (10-20) | 15 (11-19) | 0.943 |
| Hypertension, n (%) | 53 (71.6) | 333 (68.7) | 0.608 |
| Diabetes, n (%) | 24 (32.4) | 120 (24.7) | 0.159 |
| CHD, n (%) | 5 (6.8) | 61 (12.6) | 0.148 |
| Smoking, n (%) | 30 (40.5) | 179 (36.9) | 0.547 |
| Alcohol Drinking, n (%) | 15 (20.3) | 98 (20.2) | 0.990 |
| IV rTPA, n (%) | 33 (44.6) | 197 (40.6) | 0.517 |
| Lymphocyte (1000/mm3) | 1.3 (1.0-1.8) | 1.1 (0.8-1.6) | 0.011 |
| HbA1c (%) | 6.4 (5.7-7.1) | 5.9 (5.5-6.8) | 0.036 |
| HCY (mmol/l) | 16.4(13.3-20.2) | 14.6(12.1-18.7) | 0.029 |
| Pre-procedure ASPCET score | 8 (7-9) | 9 (8-9) | 0.001 |
| Pre-procedure ASITN/SIR score | 1 (1-2) | 3 (2-3) | <0.001 |
| retriever passes >3 times, n (%) | 12 (16.2) | 40 (8.2) | 0.028 |
| TOAST, n (%) | |||
| Large artery atherosclerosis | 36 (48.6) | 235 (48.5) | 0.148 |
| Cardioembolism | 26 (35.1) | 205 (42.3) | |
| Other | 12 (16.2) | 45 (9.3) | |
| Puncture-to-recanalization time, min, median (IQR) | 80 (55-105) | 67 (49-95) | 0.014 |
| mTICI score 2b or 3, n (%) | 62 (83.8) | 443 (91.3) | 0.040 |
| NIHSS 24 hours after procedure, median (IQR) | 29 (17-35) | 11 (5-17) | <0.001 |
| sICH, n (%) | 36 (48.6) | 33 (6.8) | <0.001 |
| MCE, n (%) | 21 (28.4) | 75 (15.5) | 0.006 |
| Favorable outcome, n (%) | 8 (10.8) | 276 (56.9) | <0.001 |
| Mortality, n (%) | 25 (33.8) | 56 (11.5) | <0.001 |
NIHSS, National Institutes of Health Stroke Scale; CHD, coronary atherosclerotic heart disease; IV, intravenous thrombolysis; HbA1c, glycated hemoglobin; HCY, homocysteine; ASPECT, Alberta Stroke Program Early CT Score; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; TOAST, Trial of Org 10172 in Acute stroke treatment; mTICI, modified Thrombolysis in Cerebral Infarction; sICH, symptomatic intracranial hemorrhage; MCE, malignant cerebral edema.
Figure 2.
Functional outcomes according to END and DM. Distribution of modified Rankin Scale scores at 90 days.
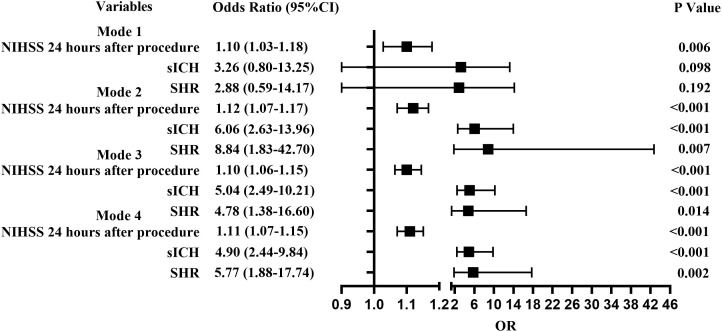
In Model 3 with unadjusted fasting blood glucose and glycosylated hemoglobin being adjusted, SHR (OR =4.78; 95% CI, 1.38-16.60; P =0.014), sICH (OR =5.04; 95% CI, 2.49-10.21; P <0.001), and NIHSS score at 24 hours (OR =1.10; 95% CI, 1.06-1.15; P <0.001) were related to END. In Model 4 with fasting blood glucose and glycosylated hemoglobin being adjusted, SHR (OR =5.77; 95% CI, 1.88-17.74; P =0.002), sICH (OR =4.90; 95% CI, 2.44-9.84; P <0.001) and NIHSS score at 24 hours (OR =1.11; 95% CI, 1.07-1.15; P <0.001) were related to END ( Figure 3 ).
Figure 3.
Forest plots for predictors of END. Model 1, Diabetic group; Model 2, Non-diabetic group; Model 3, Including fasting blood glucose and glycosylated hemoglobin as confounders; Model 4, Excluding fasting blood glucose and glycosylated hemoglobin as confounders.
When patients were stratified as with and without diabetes mellitus (DM) and adjusted for major confounding factors, multivariant analysis detected that sICH (OR =6.06; 95% CI, 2.63-13.96; P <0.001), 24-hour NIHSS score (OR =1.12; 95% CI, 1.07-1.17; P <0.001), and high SHR (OR =8.84; 95% CI, 1.83-42.70; P =0.007) could influence the development of END in patients without diabetes mellitus. High NIHSS score after 24 hours (OR =1.10; 95% CI, 1.03-1.18; P =0.006) could influence the development of END in patients with diabetes mellitus ( Figure 3 ).
Multivariant analysis detected that SHR (OR =0.20, 95% CI, 0.08-0.50; P =0.001), age (OR =0.94, 95% CI, 0.91-0.98; P =0.001), baseline NIHSS score (OR =1.15, 95% CI, 1.05-1.25; P =0.002), NIHSS score after 24 hours (OR =0.76, 95% CI, 0.69-0.83; P <0.001), PRT (OR =0.99, 95% CI, 0.98-1.00; P =0.025), pre-procedure ASITN grade (OR =62.02, 95% CI, 25.92-148.38; P <0.001) and successful recanalization (OR =7.42, 95% CI, 1.94-28.31; P =0.001) were associated with favorable outcome ( Table 2 ).
Table 2.
Multivariable analysis for favorable outcome.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| SHR | 0.20 | 0.08-0.50 | 0.001 |
| age | 0.94 | 0.91-0.98 | 0.001 |
| NIHSS at baseline | 1.15 | 1.05-1.25 | 0.002 |
| NIHSS 24 hours after procedure | 0.76 | 0.69-0.83 | <0.001 |
| PRT | 0.99 | 0.98-1.00 | 0.025 |
| Pre-procedure ASITN/SIR | 62.02 | 25.92-148.38 | <0.001 |
| mTICI, 2b-3 | 7.42 | 1.94-28.31 | 0.001 |
SHR, stress hyperglycemia ratio; NIHSS, National Institutes of Health Stroke Scale; PRT, puncture-to-recanalization time; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; mTICI, modified Thrombolysis in Cerebral Infarction.
Discussion
This study observed that stroke patients with high SHR had increased incidence of END and decreased likelihood of favorable outcome after endovascular treatment.
SHR was determined as a better quantitative indicator for stress hyperglycemia than blood glucose level when evaluating the outcomes of critical illness (25). High SHR has been associated with increased risk of END and poor outcome in patients with intravenous thrombolysis. But no study on relationship between SHR and END has been reported in patients with endovascular thrombectomy (26). The underlying mechanism for SHR influencing END may be multifactorial. First, increased lactate productions may deteriorate ischemic condition, and disrupt neuron metabolism in penumbra areas (27). Second, stress hyperglycemia could aggravate hemorrhagic transformation after ischemic stroke by inducing mitochondrial dysfunction and endothelial cell apoptosis (28). Third, stress hyperglycemia may have adverse effects on collateral circulation (29). Fourth, the prothrombotic effect of stress hyperglycemia could result in thrombus extension and blood-brain barrier destruction (30).
Previous study confirmed that patients with high SHR had an increased risk of symptomatic intracranial hemorrhage and mortality after endovascular thrombectomy (15). This study associated SHR and unfavorable outcome in patients treated with endovascular thrombectomy. Several explanations may account for the association between SHR and unfavorable outcome after endovascular thrombectomy. First, acute stress response may lead to enhance inflammation reaction, which in turn leads to increased hepatic glycogenolysis, insulin resistance, cell endothelial injury, platelet aggregation, and mitochondrial dysfunction (11, 31). Second, stress hyperglycemia may directly damage ischemic brain tissue through lactic acid accumulation and intra-cellular acidosis, and aggravate ischemic injury (32). Third, stress hyperglycemia could generate reperfusion injury via oxidative stress and inflammatory process with increased expression of endothelial adhesion molecules and monomeric C-reactive protein (33). Fourth, stress hyperglycemia may disrupt blood-brain barrier and promote hemorrhagic transformation (34).
Several limitations of this study should be address when interpreting the results. END was defined as NIHSS score increase within 24 hours after endovascular procedures, but this condition could occur a few days later. We did not monitor dynamics changes of SHR. The effects of antidiabetic agents and anesthesia were not assessed.
High stress hyperglycemia ratio may be related to early neurological deterioration and decreased likelihood of favorable outcomes after endovascular thrombectomy in patients with acute ischemic stroke.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committees of the Affiliated Wuxi People’s Hospital of Nanjing Medical University and the Affiliated Nanjing Hospital of Nanjing Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZD and HC contributed equally to the conception of the research and drafted the manuscript. LL, HJ, and HG acquired the data. XZ, JZ, and FW analyzed the data. YJ revised the manuscript and made contribution to the revision, GX and DL revised the manuscript and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was supported by Foundation of Wuxi Municipal Health Commission (#M202142); the National Science Foundation of China (81471181 and 81870933), Guangdong Basic and Applied Basic Research Foundation (2021A1515011351), Guangzhou Science and Technology Project (202102010127), and the Opening Lab Program of Guangzhou Medical University (0506308) to YJ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart Association/American stroke association. Stroke (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2. Pierot L, Jayaraman MV, Szikora I, Hirsch JA, Baxter B, Miyachi S, et al. Standards of practice in acute ischemic stroke intervention: International recommendations. J neurointerv Surg (2018) 10:1121–6. doi: 10.1136/neurintsurg-2018-014287 [DOI] [PubMed] [Google Scholar]
- 3. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke organisation (ESO) - European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by stroke alliance for Europe (SAFE). Eur Stroke J (2019) 4:6–12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olivot JM, Heit JJ, Mazighi M, Raposo N, Albucher JF, Rousseau V, et al. What predicts poor outcome after successful thrombectomy in early time window? J neurointerv Surg (2022) 14:1051–5. doi: 10.1136/neurintsurg-2021-017946 [DOI] [PubMed] [Google Scholar]
- 5. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet (2016) 387:1723–31. doi: 10.1016/s0140-6736(16)00163-x [DOI] [PubMed] [Google Scholar]
- 6. van de Graaf RA, Samuels N, Chalos V, Lycklama ANGJ, van Beusekom H, Yoo AJ, et al. Predictors of poor outcome despite successful endovascular treatment for ischemic stroke: Results from the MR CLEAN registry. J neurointerv Surg (2022) 14:660–5. doi: 10.1136/neurintsurg-2021-017726 [DOI] [PubMed] [Google Scholar]
- 7. Zhou T, Yi T, Li T, Zhu L, Li Y, Li Z, et al. Predictors of futile recanalization in patients undergoing endovascular treatment in the DIRECT-MT trial. J neurointerv Surg (2022) 14:752–5. doi: 10.1136/neurintsurg-2021-017765 [DOI] [PubMed] [Google Scholar]
- 8. Bhole R, Nouer SS, Tolley EA, Turk A, Siddiqui AH, Alexandrov AV, et al. Predictors of early neurologic deterioration (END) following stroke thrombectomy. J neurointerv Surg (2022) 18:neurintsurg–2022-018844. doi: 10.1136/neurintsurg-2022-018844 [DOI] [PubMed] [Google Scholar]
- 9. Zhang M, Xing P, Tang J, Shi L, Yang P, Zhang Y, et al. Predictors and outcome of early neurological deterioration after endovascular thrombectomy: A secondary analysis of the DIRECT-MT trial. J neurointerv Surg (2022) 10:neurintsurg–2022-018976. doi: 10.1136/neurintsurg-2022-018976 [DOI] [PubMed] [Google Scholar]
- 10. Huang ZX, Huang Y, Zeng J, Hao H, Petroski GF, Lu H, et al. Admission glucose levels may increase the risk for early neurological deterioration in females with acute ischemic stroke. Front Neurol (2020) 11:548892. doi: 10.3389/fneur.2020.548892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: Pathophysiology and clinical management. Nat Rev Neurol (2010) 6:145–55. doi: 10.1038/nrneurol.2009.231 [DOI] [PubMed] [Google Scholar]
- 12. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heartdisease and stroke statistics-2020 update: A report from the American heart association. Circulation (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 13. Huo X, Liu R, Gao F, Ma N, Mo D, Liao X, et al. Effect of hyperglycemia at presentation on outcomes in acute large artery occlusion patients treated with solitaire stent thrombectomy. Front Neurol (2019) 10:71. doi: 10.3389/fneur.2019.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnold M, Mattle S, Galimanis A, Kappeler L, Fischer U, Jung S, et al. Impact of admission glucose and diabetes on recanalization and outcome after intra-arterial thrombolysis for ischaemic stroke. Int J Stroke (2014) 9:985–91. doi: 10.1111/j.1747-4949.2012.00879.x [DOI] [PubMed] [Google Scholar]
- 15. Merlino G, Pez S, Gigli GL, Sponza M, Lorenzut S, Surcinelli A, et al. Stress hyperglycemia in patients with acute ischemic stroke due to large vessel occlusion undergoing mechanical thrombectomy. Front Neurol (2021) 12:725002. doi: 10.3389/fneur.2021.725002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Merlino G, Smeralda C, Gigli GL, Lorenzut S, Pez S, Surcinelli A, et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis (2021) 51:789–97. doi: 10.1007/s11239-020-02252-y [DOI] [PubMed] [Google Scholar]
- 17. Shen CL, Xia NG, Wang H, Zhang WL. Association of stress hyperglycemia ratio with acute ischemic stroke outcomes post-thrombolysis. Front Neurol (2021) 12:785428. doi: 10.3389/fneur.2021.785428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osei E, den Hertog HM, Berkhemer OA, Fransen PSS, Roos Y, Beumer D, et al. Admission glucose and effect of intra-arterial treatment in patients with acute ischemic stroke. Stroke (2017) 48:1299–305. doi: 10.1161/STROKEAHA.116.016071 [DOI] [PubMed] [Google Scholar]
- 19. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med (2018) 378:11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 20. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med (2018) 378:708–18. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seners P, Ben Hassen W, Lapergue B, Arquizan C, Heldner MR, Henon H, et al. Prediction of early neurological deterioration in individuals with minor stroke and large vessel occlusion intended for intravenous thrombolysis alone. JAMA Neurol (2021) 78:321–8. doi: 10.1001/jamaneurol.2020.4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic strokee. N Engl J Med (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 23. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): An observational study. Lancet (2007) 369:275–82. doi: 10.1016/s0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 24. Zhang X, Yan S, Zhong W, Yu Y, Lou M. Early nt-probnp (n-terminal probrain natriuretic peptide) elevation predicts malignant edema and death after reperfusion therapy in acute ischemic stroke patients. Stroke (2021) 52:537–42. doi: 10.1161/STROKEAHA.120.029593 [DOI] [PubMed] [Google Scholar]
- 25. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: Introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab (2015) 100:4490–7. doi: 10.1210/jc.2015-2660 [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Cheng Q, Hu T, Wang N, Wei X, Wu T, et al. Impact of stress hyperglycemia on early neurological deterioration in acute ischemic stroke patients treated with intravenous thrombolysis. Front Neurol (2022) 13:870872. doi: 10.3389/fneur.2022.870872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bourcier R, Goyal M, Muir KW, Desal H, Dippel DWJ, Majoie C, et al. Risk factors of unexplained early neurological deterioration after treatment for ischemic stroke due to large vessel occlusion: a post hoc analysis of the HERMES study. J neurointerv Surg (2022), neurintsurg–2021-018214. doi: 10.1136/neurintsurg-2021-018214 [DOI] [PubMed] [Google Scholar]
- 28. Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol (2002) 52:20–8. doi: 10.1002/ana.10241 [DOI] [PubMed] [Google Scholar]
- 29. Wiegers EJA, Mulder M, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, et al. Clinical and imaging determinants of collateral status in patients with acute ischemic stroke in Mr clean trial and registry. Stroke (2020) 51:1493–502. doi: 10.1161/STROKEAHA.119.027483 [DOI] [PubMed] [Google Scholar]
- 30. Lemkes BA, Hermanides J, Devries Jh, Holleman F, Meijers Jc, Hoekstra JB. Hyperglycemia: a prothrombotic factor? J Thromb Haemost (2010) 8:1663–9. doi: 10.1111/j.1538-7836.2010.03910.x [DOI] [PubMed] [Google Scholar]
- 31. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet (2009) 373:1798–807. doi: 10.1016/S0140-6736(09)60553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke (2001) 32:2426–32. doi: 10.1161/hs1001.096194 [DOI] [PubMed] [Google Scholar]
- 33. Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab (2001) 21:1393–400. doi: 10.1097/00004647-200112000-00003 [DOI] [PubMed] [Google Scholar]
- 34. Desilles JP, Syvannarath V, Ollivier V, Journé C, Delbosc S, Ducroux C, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke (2017) 48:1932–40. doi: 10.1161/STROKEAHA.117.017080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.