Abstract
Real-time monitoring of serum hepatitis B virus (HBV) levels is essential for the management of patients with chronic HBV infection in clinical practice, including monitoring the resistance of anti-HBV nucleotide analog or the detection of HBV reactivation. In this context, serum HBV deoxyribonucleic acid (DNA) quantification should be rapidly measured. A rapid HBV DNA quantification assay was established on the Fully Automated Genetic Analyzer, μTASWako g1. The assay performs automated sample preparation and DNA extraction, followed by the amplification and detection of quantitative polymerase chain reaction (PCR) combined with capillary electrophoresis (qPCR-CE) on integrated microfluidic chip. This study aimed to evaluate the analytical and clinical performance of HBV DNA assay on the μTASWako g1 platform in human serum and EDTA-plasma. The HBV DNA assay has a linear quantitative range from 20 to 108 IU/mL of HBV DNA with standard deviation (SD) of ≤0.14 log10 IU/mL. The limits of detection of the assay were 4.18 for the serum and 4.35 for EDTA-plasma. The HBV assay demonstrated the equivalent performance in both human serum and EDTA-plasma matrices. The HBV genotypes A to H were detected with an accuracy of ±0.34 log10 IU/mL. In quantification range, the HBV DNA assay was correlated with Roche cobas AmpliPrep/cobas TaqMan Ver2.0 (CAP/CTM v2) (r = 0.964). The mean difference (μTASWako g1–CAP/CTM v2) of the reported HBV DNA was −0.01 log10 IU/mL. Overall, the sensitivity, accuracy, and precision of the μTASWako g1 HBV assay were comparable to the existing commercial HBV DNA assay, and the assay can be completed within 110 min. This evaluation suggests that the HBV DNA assay on the μTASWako g1 is potentially applied for alternative method of the HBV viral load test, in particular with the advantage of the HBV DNA result availability within 2 h, improving the HBV infection management.
Introduction
Hepatitis B virus (HBV) specifically infects the hepatocytes and causes severe liver diseases [1, 2]. Chronic HBV infection was associated with significant risks for the occurrence of cirrhosis and hepatocellular carcinoma, the major causes of death associated with HBV infection [3–5]. The World Health Organization (WHO) reported that approximately 257 million people were chronically infected with HBV in 2015 [3]. Serum HBV DNA measurement is a pivotal test for the management of patients with HBV, including the assessment on the risk for liver flare and, in particular, monitoring the efficacy of antiviral therapy [6].
In addition to chronic infection, HBV is potentially reactivated in some patients. HBV reactivation occurs in patients who undergo chemotherapy or immunosuppressive therapies and often causes life-threatening liver failure. Reactivation occurs not only in HBsAg-seropositive patients but also in those with resolved HBV infection who are seronegative for HBsAg but seropositive for antibody against hepatitis B core antigen (anti-HBc) and/or antibody against HBsAg (anti-HBs) [7–11]. To prevent HBV reactivation-related hepatitis, HBV DNA monitoring-guided preemptive antiviral therapy using anti-HBV nucleos(t)ide analogs (NAs) is recommended by several guidelines for patients with resolved HBV infection [12–14]. However, the duration required for HBV DNA measurements, including both qualitative and quantitative, hinders agile and effective preventive strategies in real clinical practice, and HBV DNA measurement technology with rapidly available results.
The HBV DNA measurement in human serum and plasma utilizes quantitative real-time polymerase chain reaction (qPCR)- or TMA-based assay incorporated in commercial systems, such as cobas AmpliPrep/cobas TaqMan HBV Test ver.2.0 (CAP/CTM v2) (Roche Diagnostics, Indianapolis, IN, USA), RealTime HBV assay (Abbott Laboratories, Des Plaines, IL, USA), and Aptima HBV Quant assay (Hologic Inc., San Diego, CA, USA) [15, 16]. These systems are generally designed to be high-throughput tests for batch testing of multiple specimens within a run. Therefore, the turnround time varies and may require until a few days from the time of blood collection to obtain results.
In contrast, HBV RNA testing using μTASWako g1 analyzer can analyze a single sample or up to four samples in parallel with a single run, facilitating a rapid turnover. The analyzer conducts automated sample preparation and nucleic acid extraction, followed by amplification and detection of target amplicons by qPCR combined with capillary electrophoresis (CE) on integrated microfluidic chip [17, 18]. The total assay time on μTASWako g1 is within 45 min for Mycobacterium tuberculosis DNA qualitative assay, within 70 min for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA qualitative assay. In this study, we established an HBV DNA quantification assay by taking advantage of the capability and usability of the μTASWako g1 platform. This assay can complete the HBV DNA quantification within 110 min. We evaluated the analytical and clinical performance of HBV DNA assay on the μTASWako g1 in human serum and EDTA-plasma to assess whether the assay can be applied as a rapid alternative method for HBV DNA measurement.
Materials and methods
HBV primers
Specific amplification of 227-bp HBV DNA is achieved using a pair of primers targeting a conserved region in S gene of HBV among all genotypes (forward primer: 5’-TAMRA-AGACTCGTGGTGGACTTC; reverse primer: GACAAACGGGCAACATAC). The 5’-end of the forward primer was labeled using a fluorescent dye, carboxytetramethylrhodamine (TAMRA), which allowed the HBV amplicon to be labeled for fluorescence detection through electrophoresis.
WHO international standard and HBV-positive control
The 4th WHO international standard of HBV DNA for NAT (10/266) was obtained from the National Institute for Biological Standards and Control. The HBV-positive control was 3288-bp of the linear DNA fragment amplified by PCR. The construction of HBV-positive control consisted of a sequence from pCR2.1-TOPO and 227-bp of the HBV target sequence derived from HBV serotype adw (#45020D, American Type Culture Collection, VA, USA). The HBV target sequence is amplifiable by the set of HBV primer for the assay. The HBV-positive control concentration (unit: IU/mL) was determined based on the 4th International WHO Standard for HBV DNA.
Internal control (IC) for the HBV assay
IC is 3426-bp of the linear DNA fragment amplified by PCR, which contains 365-bp of the IC target sequence. Both ends of the IC target sequence are composed of HBV target sequence for primers (5’ end of the IC target is HBV forward primer sequence and 3’ end of the IC target is HBV reverse primer sequence), which allows co-amplification of both HBV and IC target using one set of the HBV primer in the PCR. IC (approximately 1200 copies/assay) was added to the sample at the beginning of the sample preparation to monitor the assay process, including DNA extraction from specimens and PCR amplification and detection. The amount of IC added into the assay was optimized to reproducibly detect the entire quantitative range of the assay without interfering HBV target amplification. The IC concentration (unit: copies/mL) was determined based on the concentration measured by absorbance at 260 nm.
HBV genotype samples
The 1st international reference panel for HBV genotypes for nucleic acid amplification technique-based assays (5086/08) was obtained from Paul-Ehrlich-Institut. The HBV genotype H-positive plasma specimen (Product#203589) was obtained from SeraCare Life Sciences, Inc (Milford, MA, USA).
HBV-negative serum and EDTA-plasma
A total of 160 individual HBV-negative serum and 60 individual HBV-negative EDTA-plasma specimens were obtained from BIOIVT (Hicksville, NY, USA). Pooled HBV-negative serum and EDTA-plasma specimens were obtained from BIOIVT (Hicksville, NY, USA).
Clinical samples from HBV-infected individuals
The assay was examined using 207 clinical serum samples from patients with chronic HBV infection obtained at Ogaki Municipal Hospital, Japan. HBV infection had been confirmed by HBV antigens and HBV DNA testing for all patients. The HBV DNA levels of 207 samples had been measured using cobas AmpliPrep/cobas TaqMan HBV Test ver 2.0, ranging from negative (target not detected), <20 IU/mL (positive and not quantifiable) to 1.7 × 108 IU/mL. Among the 207 samples, 50 were obtained from patients during treatment with nucleoside analogs for anti-HBV therapy. The HBV DNA negative, which comprised a total of 16 samples, were obtained from patients undergoing nucleoside analog treatment. Samples were collected from 97 males and 110 females, with a median age of 58 years. Patient background is shown in S1 Table. The study protocol on clinical serum samples complied with the Helsinki Declaration and was approved by the institutional review board of Ogaki Municipal Hospital. Written informed consent was waived due to the retrospective use of the stored serum samples. Clinical samples were stored at −80°C and kept frozen during transport with dry ice before testing.
Automated DNA extraction and sample preparation for qPCR
IC was added to 1 mL of the serum or EDTA-plasma sample. For nucleic acids, the sample was treated with proteinase K and guanidine hydrochloride through incubation at an elevated temperature for 5 min. The released nucleic acids were captured using grass fiber membrane filter assembled in a column with ethanol. Subsequently, the column was washed with a wash buffer containing guanidine hydrochloride, ethanol, and then washed with a wash buffer containing ethanol. The captured DNA was eluted with Tris buffer solution. The eluted DNA solution was mixed with the KOD buffer, dNTPs, KOD exo (-) DNA polymerase (TOYOBO, Osaka, Japan), and HBV primers. A 25 uL of reaction mix was added to the PCR chamber of the microfluidic chip and took approximately 54 min to finish the process from DNA extraction to sample preparation for quantitative polymerase chain reaction (qPCR).
On-chip quantitative PCR using real-time detection by electrophoresis
A previous study demonstrated experiments of quantitative PCR combined capillary electrophoresis (CE) for real-time detection of target amplicons. Details of instrument setup, the design of an integrated microfluidic chip for qPCR-CE, and the detection methods were described in a previous report [18]. During PCR, air pressure (138 kPa) was used to the PCR chamber and channels through a manifold covering the chamber and reagent wells to suppress evaporation of the reaction mix. Initial denaturation was performed for 30 s at 97°C. Seven short PCR cycles (3.7 s at 99°C, 10.3 s at 64°C, and 14 s at 73.5°C) were then performed. Afterward, 33 PCR cycles with a longer extension (70 s at 73.5°C) was performed with one CE injection from the PCR chamber for each subsequent cycle to analyze the accumulated target amplicons and took 56 min to complete the entire qPCR-CE step.
Data acquisition and analysis
An amplification curve for each amplicon was generated by plotting the peak height (relative fluorescence unit [RFU]) versus the number of PCR cycles. The quantitative cycle (Cq) values were determined based on the intersection between the amplification curve and threshold line on the plots. Cq values were converted to log10 IU/mL based on predetermined calibration curve traceable to the 4th WHO International Standard for HBV DNA. The analysis was conducted using a proprietary software developed using the μTASWako g1 analyzer.
Statistical analysis
Probit analysis for Limit of detection (LOD) test was performed using Minitab software. Person’s correlation coefficients and Passing–Bablok regression analysis were performed using Analyze-it software to confirm the correlation in clinical samples. Bland–Altman analysis was performed to compare methods between μTASWako g1 and CAP/CTM v2 for data obtained from clinical samples.
Results
Real-time detection of HBV target and IC amplification by CE
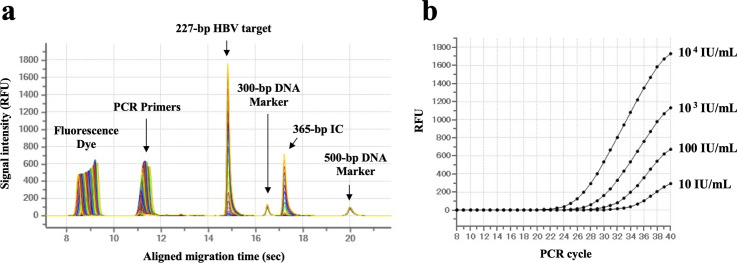
To detect HBV amplification signal by CE using the μTASWako g1 analyzer, the 4th WHO international standard for HBV DNA samples was tested. Fig 1A shows overlay of electropherograms at the PCR cycles from 8th to 40th. The graph exhibits the grown amplicon signals of the HBV target (227-bp) and IC (365-bp). The peak alignment using 300-bp and 500-bp of DNA markers was performed to detect HBV and IC peaks at a specific migration time in electropherograms. A growth curve of HBV was generated by plotting the peak height of the amplicon against the number of PCR cycles. Fig 1B shows the composite growth curve tested with 10 to 104 IU/mL of the WHO international standard for HBV.
Fig 1. Real-time detection of HBV target using the μTASWako g1 analyzer.
(a) Overlay of electropherograms in the PCR-CE for 104 IU/mL of the 4th WHO International Standard for HBV. Merged images of 33 electropherograms from 8th to 40th PCR cycle. (b) HBV amplification growth curves. RFU stands for relative fluorescence unit.
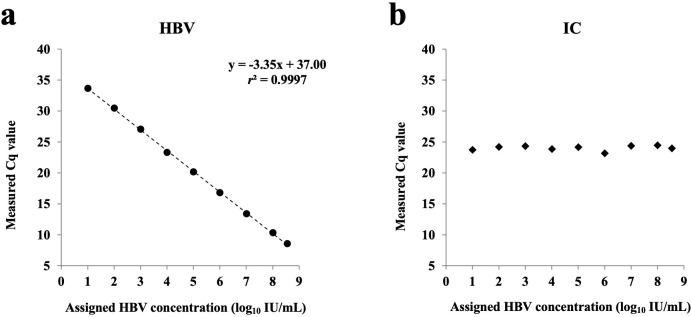
To assess the linear dynamic range of the HBV assay using qPCR-CE, nine serial dilutions of the HBV-positive control, which is traceable to the 4th WHO International standard, were tested using the μTAS g1 analyzer. In Fig 2A, the HBV Cq plot demonstrated the linear response of HBV Cq values across the range from 10 to 3.5 × 108 IU/mL of the HBV DNA concentration. The PCR efficiency of HBV target amplification was 98.8%. IC Cq plot is shown in Fig 2B when 10 to 3.5 × 108 IU/mL of the HBV-positive controls tested. The IC Cq was stably detected within a range (24.5 ± 1), regardless of the variation of HBV concentration in samples. Data indicated that IC was detected as an analyte for monitoring the assay process.
Fig 2. HBV Cq and IC Cq values plot.
3.5 ×108, 108, 107, 106, 105, 104, 103, 102, and 10 IU/mL of the HBV-positive control in the serum were tested using the μTASWako g1 Analyzer. One replicate for each concentration. PCR efficiency of HBV target amplification was 98.8%.
Analytical performance evaluation
Sensitivity and specificity
LOD, defined as the HBV concentration detected with a probability of 95%, was assessed by seven dilutions using the 4th WHO International standard. Dilutions (1.25, 2.5, 5, 7.5, 10, 20, and 50 IU/mL) were prepared in HBV-negative human serum and EDTA-plasma samples, and each dilution was tested in 63 replicates. Probit analysis was performed to determine LOD and 95% confidence interval (CI). Results in Table 1 demonstrate that the HBV assay effectively detected HBV DNA at a concentration of 4.18 IU/mL (95% CI, 3.34–5.92) for 1 mL of the serum and 4.35 IU/mL (95% CI, 3.52–5.99) for 1 mL of EDTA-plasma samples. Results indicated no significant difference in the analytical sensitivity of the HBV assay between serum and EDTA-plasma matrices.
Table 1. Limit of detection in the serum and EDTA-plasma using the 4th WHO international standard for HBV.
| WHO std. concentration IU/mL | No. of replicates | Serum | EDTA-Plasma | ||||
|---|---|---|---|---|---|---|---|
| No. HBV Positive | Detection rate % | LOD* (95% CI) IU/mL | No. HBV Positive | Detection rate % | LOD* (95% CI) IU/mL | ||
| 50.00 | 63 | 63 | 100 | 4.18 (3.34–5.92) | 63 | 100 | 4.35 (3.52–5.99) |
| 20.00 | 63 | 63 | 100 | 63 | 100 | ||
| 10.00 | 63 | 63 | 100 | 63 | 100 | ||
| 7.50 | 63 | 63 | 100 | 62 | 98 | ||
| 5.00 | 63 | 61 | 97 | 61 | 97 | ||
| 2.50 | 63 | 50 | 79 | 52 | 83 | ||
| 1.25 | 63 | 39 | 62 | 33 | 52 | ||
| 0 | 63 | 0 | 0 | 0 | 0 | ||
* LOD, defined as the HBV concentration detected with a probability of 95%, was determined using Probit analysis.
To verify the specificity of the HBV assay, 160 individual HBV-negative serum and 60 individual HBV-negative EDTA-plasma samples were analyzed using the μTASWako g1 HBV assay. No HBV target peak was observed in all samples, and data analysis revealed nonspecific signals at baseline in electropherograms obtained from all samples, which were significantly lower than the detection threshold (30 RFU) in the assay.
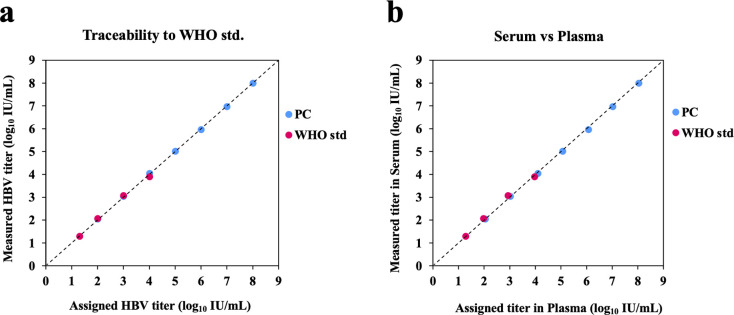
Traceability to the WHO standard and matrix equivalency
HBV-positive control and 4th WHO international standard were prepared using the HBV-negative serum and EDTA-plasma samples. The concentration range tested for positive control was from 102 to 108 IU/mL, and the WHO standard was from 20 to 104 IU/mL. Each dilution sample was tested in three replicates using the μTASWako g1 analyzer. In Fig 3A, the positive control and WHO standard demonstrated co-linear dilution performance across the linear dynamic range. The measured HBV titers of positive controls and the WHO standard were similar to the expected value (or assigned value) with deviation of ≤0.09 log10 IU/mL. The mean difference of the measured HBV titer between the serum and EDTA-plasma samples was ≤0.05 log IU/mL for positive control and the WHO standard. Fig 3B demonstrates the performance equivalency of the HBV assay between serum and EDTA-plasma matrices.
Fig 3. Traceability to the WHO international standard and matrix equivalency between serum and plasma on μTASWako g1 analyzer.
Dilutions of the positive control (PC) and WHO international standard (WHO std) were tested in three replicates for each concentration. The dots represent the mean log10 transformed titer. The dashed line represents the equality line.
Precision (Inter-assay precision)
To assess the assay precision, three dilutions of the positive control and two dilutions of the WHO standard were tested in 21 replicates using the μTASWako g1. Results are shown in Table 2. In the concentration range tested for positive control from 102 to 107 IU/mL, SD of the measured HBV titers were <0.10 log10 IU/mL. In 10 or 20 IU/mL of the WHO standard tested, SD of the measured HBV titers was <0.20 log10 IU/mL. Deviation of the mean titer from the assigned titer was larger (−0.09 log10 IU/mL) when 10 IU/mL of the WHO standard tested.
Table 2. Precision of μTASWako g1 analyzer.
| Assigned HBV DNA concentration | Source Material | No. of replicates | Precision of μTASWako g1 analyzer | |||
|---|---|---|---|---|---|---|
| IU/mL | log10 IU/mL | Mean | SD | CV (%) | ||
| (log10 IU/mL) | (log10 IU/mL) | |||||
| 10,000,000 | 7.00 | Positive control | 21 | 6.98 | 0.05 | 0.73 |
| 10,000 | 4.00 | Positive control | 21 | 4.00 | 0.06 | 1.50 |
| 100 | 2.00 | Positive control | 21 | 1.98 | 0.09 | 4.34 |
| 20 | 1.30 | WHO standard | 21 | 1.28 | 0.14 | 11.00 |
| 10 | 1.00 | WHO standard | 21 | 0.91 | 0.19 | 20.92 |
Note: CV stands for coefficient of variation.
Genotype accuracy
To evaluate whether the assay covers HBV genotypes (A, B, C, D, E, F, G, and H), the lower concentration of the 1st WHO International Reference Panel and HBV-positive patient plasma genotype H was tested using the μTASWako g1. Table 3 shows the accuracy of the HBV assay for all genotypes A to H. Measured HBV titers were detected within the accuracy of ±0.34 log10 IU/mL for all genotypes. The absolute mean difference between the measured and assigned titers was ≤0.12 log10 IU/mL. Based on these results, the assay can quantify the HBV DNA with an accuracy of ±0.50 log10 IU/mL for HBV genotypes A to H.
Table 3. Accuracy for HBV genotypes on μTASWako g1 analyzer.
| Sample | Genotype | Assigned HBV titer | No. of replicates | Measured HBV titer by μTASWako g1 | |
|---|---|---|---|---|---|
| Mean (±SD) | Difference* | ||||
| log10 IU/mL | log10 IU/mL | log10 IU/mL | |||
| WHO Panel #1 | A | 1.70 | 3 | 1.75 (±0.15) | 0.05 |
| WHO Panel #2 | A | 1.70 | 3 | 1.82 (±0.24) | 0.12 |
| WHO Panel #3 | A | 1.70 | 3 | 1.58 (±0.09) | -0.12 |
| WHO Panel #4 | B | 1.70 | 3 | 1.68 (±0.01) | -0.01 |
| WHO Panel #5 | B | 1.66 | 3 | 1.78 (±0.12) | 0.12 |
| WHO Panel #6 | B | 1.70 | 3 | 1.81 (±0.06) | 0.11 |
| WHO Panel #7 | C | 1.70 | 3 | 1.82 (±0.10) | 0.12 |
| WHO Panel #8 | C | 1.70 | 3 | 1.68 (±0.02) | -0.02 |
| WHO Panel #9 | C | 1.70 | 3 | 1.67 (±0.03) | -0.02 |
| WHO Panel #10 | D | 1.70 | 3 | 1.76 (±0.12) | 0.06 |
| WHO Panel #11 | D | 1.70 | 3 | 1.64 (±0.02) | -0.06 |
| WHO Panel #12 | D | 1.70 | 3 | 1.69 (±0.03) | -0.01 |
| WHO Panel #13 | E | 1.70 | 3 | 1.60 (±0.14) | -0.10 |
| WHO Panel #14 | F | 1.70 | 3 | 1.68 (±0.14) | -0.02 |
| WHO Panel #15 | G | 1.70 | 3 | 1.79 (±0.02) | 0.09 |
| SeraCare #203589 | H | 1.60 | 3 | 1.50 (±0.11) | -0.10 |
Note: 1st WHO International Reference Panel for HBV genotypes (WHO Panel) from #1 to #15 were corresponding to genotype A, B, C, D, E, F, and G. Genotype H sample was obtained from SeraCare.
* Difference of HBV titer in log10 IU/mL between the mean measured titer and the assigned titer.
Correlation with CAP/CTM v2 in clinical samples
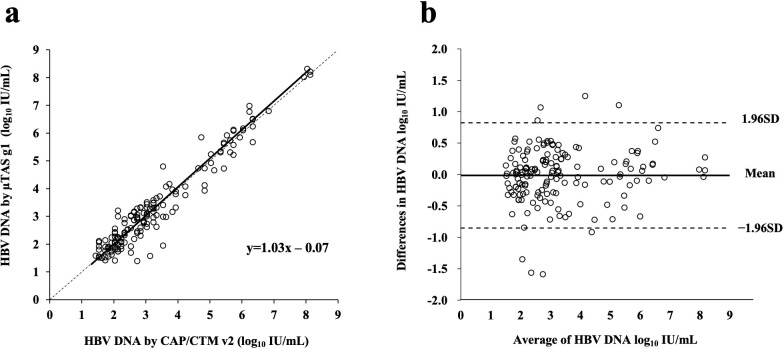
The performance of the μTASWako g1 HBV assay was assessed against the widely used assay, Roche CAP/CTM v2, by testing 207 clinical samples (serum) obtained from HBV-infected patients. Qualitative results from the two assays are categorized in Table 4. The concordance rate of qualitative results was 90.3%. To assess the relationship between HBV DNA levels between the two methods, the Passing–Bablock regression analysis was performed in 157 clinical samples, which reached over the linear dynamic range (20 to 108 IU/mL, 1.3 to 8.0 log10 IU/mL). Fig 4A demonstrates that the measured HBV log10 titers between the two methods are highly correlated across the entire dynamic range (y = 1.03 × −0.07). The correlation coefficiency (r) was 0.964 (95% CI: 0.959–0.968). To investigate the measurement bias between the two methods, Bland–Altman analysis was performed. The difference in the measured log10 titers between CAP/CTM v2 and μTASWako g1 is shown in Fig 4B. The mean difference in log10 tier was −0.01 (95% CI: −0.085 to 0.053). The upper and lower limits of agreement were 0.82 and −0.85, respectively. In nine (5.7%) samples, the difference between the two methods were >1.96 times the SD. In 151 (96.2%) samples, the difference between the two methods was ≤1.0 log10 IU/mL.
Table 4. Qualitative results of μTASWako g1 HBV assay and CAP/CTM v2 assay.
| CAP/CTM v2 assay | ||||
|---|---|---|---|---|
| Virus detected, | Virus detected, | Virus Not | No. of total | |
| ≥20 IU/mL | <20 IU/mL | Detected | ||
| μTASWako g1 HBV assay | ||||
| Virus detected, ≥20 IU/mL | 157 | 3 | 0 | 160 |
| Virus detected, <20 IU/mL | 11 | 16 | 2 | 29 |
| Virus not detected | 0 | 4 | 14 | 18 |
| No. of total | 168 | 23 | 16 | 207 |
Fig 4. Correlation of measurement and limit of agreement between HBV assays on the μTASWako g1 analyzer and CAP/CTM v2.
A total of 157 serum samples from HBV-infected patients were tested with CAP/CTM v2 and μTASWako g1 Analyzer. (a) Passing–Bablok regression fit (Solid line). Correlation coefficiency (r) was 0.964 (95% CI, 0.959–0.968), P < 0.0001. The dashed line represents the equality line. (b) Bland–Altman plot shows the mean difference between CAP/CTM v2 and μTASWako g1 Analyzer was −0.01 (limit of agreement, −0.82 to −0.85 log10 IU/mL). The solid and dashed lines represent the mean difference and mean ±1.96 SD, respectively.
In 30 out of 50 samples, which were either undetectable for HBV DNA or contained <20 IU/mL (<1.3 log10 IU/mL) of HBV DNA levels by CAP/CTM v2 and/or μTASWako g1, these qualitative results were concordant between the two methods. Results of the remaining 20 samples are shown in S2 Table. Of the 16 samples, two samples reported as “target not detected” by CAP/CTM v2 were positive for HBV DNA in the μTASWako g1. Conversely, 4 out of 18 samples reported as “<1.3 log10 IU/mL” by CAP/CTM v2 were negative for HBV DNA in the μTASWako g1.
Discussion
We assessed the potential performance of the HBV DNA quantification assay using the μTASWako g1 analyzer. Results showed that the assay has a high sensitivity, specificity, and reproducibility across the quantification range from 20 to 108 IU/mL. Precision of the μTASWako g1 HBV assay in the qualification range from 100 to 108 IU/mL with SD of 0.02–0.11 log10 IU/mL and coefficient of variation of 0.33%–5.25%. In contrast, SD of the three existing commercial systems, CAP/CTM v2, Abbott RealTime HBV, and Aptima Quant HBV assay were 0.21 to 2.67, 0.44 to 11.25, 0.29 to 5.07, respectively, corresponding with the qualification range [19–22]. Thus, precision of the HBV assay using the μTASWako g1 analyzer was comparable to that of those systems. The LOD of the μTASWako g1 HBV assay (4.18 IU/mL and 4.35 for the serum and EDTA-plasma samples) were almost similar with that of the three existing systems based on the product information sheet (S3 Table).
The μTASWako g1 HBV assay utilizes IC to monitor the DNA purification process to PCR-CE detection. The amount of IC spiked into the samples was predetermined so that IC Cq can be reproducibly detected within a particular range when testing the sample with HBV DNA (up to 3.5 × 108 IU/mL) or without HBV DNA. Deviation of IC Cq from the predetermined range will indicate decreased DNA purification efficiency or the presence of substances that may inhibit the PCR amplification.
In clinical samples obtained from patients with chronic HBV infection, the HBV DNA levels measured using the μTASWako g1 were well correlated with those from CAP/CTM v2. Moreover, the average difference of reported HBV titers between μTASWako g1 and CAP/CTM v2 was relatively smaller when compared with the average difference in previous reports [21, 22]. In the Blant–Altman analysis, four (2.5%) and two (1.3%) samples had difference of >1.0 and 1.5 log10 IU/mL between the μTASWako g1 and CAP/CTM v2. One possible reason for the discrepancy might be the mutation in the PCR amplification region of the HBV genome. However, this study could not assess the sequencing for HBV primer target region due to insufficient remaining sample volume for the analysis after the clinical study. The region targeted by primers of μTASWako g1 HBV assay are S gene, whereas the region targeted by primers/probes of CAP/CTM v2 are preC-C gene. A previous study reported deviation of HBV DNA levels measured by two different PCR assays, amplify different regions of HBV genome, occurring due to a sequence mismatched in the primer or probe in one of the assays [23]. HBV has been known to be a highly variable DNA genome and is divided into genotypes A to J based on the 8% sequence variations in the S gene [24]. We confirmed that the μTASWako g1 HBV assay could measure HBV DNA levels with accuracy of ±0.34 log10 IU/mL for HBV genotypes A to H. Additionally, in silico analysis was performed for primer homology with target region of the S gene for genotypes I and J [25]. The μTASWako g1 primer sequences were perfectly matched to that of the S gene in genotypes I and J (accession no. AB562463 [I1], FJ023669 [I2], AB486012 [J]). The HBV genome mutation was potentially induced by the long-term treatment of nucleoside analogs, such as lamivudine and adefovir [26–28]. Six clinical samples, with difference of >1.5 log10 IU/mL between μTASWako g1 and CAP/CTM v2, were obtained from patients without nucleoside analog treatment. Other mutations in the S gene induced by hepatitis B vaccine were well known [29, 30]. However, several reported mutation points were not associated with the primer regions of the HBV assay. Sequence analysis in the target region may be necessary to identify the reasons of different HBV DNA levels between the methods in clinical samples.
Overall, the assessment revealed that the μTASWako g1 HBV assay has a comparable performance to the existing HBV DNA assay systems. Furthermore, the assay can complete the measurement within 110 min. Therefore, the μTASWako g1 analyzer can provide measurement results to clinicians with minimal delay after blood collection and sample measurement compared to the existing commercial systems. This will be a strong advantage of this system, especially in monitoring patients at risk of HBV reactivation. However, two limitations were identified in the assay using the μTASWako g1 analyzer: (1) the assay throughput is four samples per run and (2) the sample is required for 1 mL of serum or EDTA-plasma sample per test. To enhance the usefulness of the assay for limited sample volume, further improvement of the assay performance may be required.
Conclusions
The HBV assay on the μTASWako g1 analyzer is a sensitive and reproducible assay for HBV DNA quantification, which can provide test results within 2 h. The result also revealed a good correlation with the commercial HBV viral load assay, suggesting that the HBV assay on the μTASWako g1 analyzer potentially becomes an alternative method for HBV viral load test to help manage HBV infection in a timely manner.
Supporting information
Values shown are median (interquartile range).
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors gratefully acknowledge Aurelie Souppe (FUJIFILM Wako Diagnostics USA) for supporting data acquisition and do Jian-Ping Zhang (FUJIFILM Wako Diagnostics USA) for advising on assay design.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
Specific grant numbers; None Initials of authors who received each award; M.W Full names of commercial companies that funded the study or authors; Fujifilm Wako Pure Chemical Corporation Initials of authors who received salary or other funding from commercial companies; M.W and T.K URLs to sponsors’ websites; https://www.fujifilm.com/ffwk/en The funder provided support in the form of salaries for authors M.W and T.K, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020; 182: 104925. doi: 10.1016/j.antiviral.2020.104925 [DOI] [PubMed] [Google Scholar]
- 2.Datta S, Chatterjee S, Veer V, Chakravarty R. Molecular biology of the hepatitis B virus for clinicians, J Clin Exp Hepatol. 2012; 2: 353–65. doi: 10.1016/j.jceh.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global hepatitis report 2017; ISBN 978-92-4-156545–5.
- 4.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma, Cancer. 1988; 61 (10): 1942–56. doi: [DOI] [PubMed] [Google Scholar]
- 5.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination, Epidemiol Rev. 2006; 28: 112–25. doi: 10.1093/epirev/mxj009 [DOI] [PubMed] [Google Scholar]
- 6.Valsamakis A. Molecular testing in the diagnosis and management of chronic hepatitis B, Clin Microbiol Rev. 2007; 20: 426–39. doi: 10.1128/CMR.00009-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med 2001; 344: 68–69. [DOI] [PubMed] [Google Scholar]
- 8.Hui CK, Cheung WWW, Zhang HY, Au WY, Yueng YH, Leung AY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006; 131: 59–68. doi: 10.1053/j.gastro.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 9.Umemura T, Tanaka E, Kiyosawa K, Kumada H. Mortality secondary to fulminant hepatic failure in patients with prior resolution of hepatitis B virus infection in Japan. Clin Infect Dis 2008; 47: e52–e56. doi: 10.1086/590968 [DOI] [PubMed] [Google Scholar]
- 10.Fukushima N, Mizuta T, Tanaka M, Yokoo M, Ide M, Hisatomi T, et al. Retrospective and prospective studies of hepatitis B virus reactivation in malignant lymphoma with occult HBV carrier. Ann Oncol 2009; 20: 2013–2017. doi: 10.1093/annonc/mdp230 [DOI] [PubMed] [Google Scholar]
- 11.Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol 2009; 90: 13–23. doi: 10.1007/s12185-009-0359-5 [DOI] [PubMed] [Google Scholar]
- 12.Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH Guidelines for the management of hepatitis B virus infection. Hepatol Res 2014; 44 (Suppl S1): 1–58. doi: 10.1111/hepr.12269 [DOI] [PubMed] [Google Scholar]
- 13.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016; 10: 1–98. doi: 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67: 370–398. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 15.Allice T, Cerutti F, Pittaluga F, Varetto S, Gabella S, Marzano A, et al. COBAS AmpliPrep-COBAS TaqMan Hepatitis B Virus (HBV) Test: a Novel Automated Real-Time PCR Assay for Quantification of HBV DNA in Plasma. J Clin Microbiol. 2007; 45: 828–34. doi: 10.1128/JCM.00914-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goedel S, Rullkoetter M, Weisshaar S, Mietag C, Leying H, Boehl F. Hepatitis B virus (HBV) genotype determination by the COBAS AmpliPrep/COBAS TaqMan HBV Test, v2.0 in serum and plasma matrices. J Clin Virol. 2009; 45: 232–6. doi: 10.1016/j.jcv.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 17.Chan SDH, Toyoda H, Sanjeeviraman J, Souppe A, Iwamoto M, Wu W, et al. Fully automated rapid quantification of Hepatitis C Virus RNA in human plasma and serum by integrated on-chip RT-qPCR and capillary electrophoresis. Sci Rep. 2020; 10: 7379. doi: 10.1038/s41598-020-64169-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Li C, Li Z, Chan SD, Eto D, Wu W, et al. On-chip quantitative PCR using integrated real-time detection by capillary electrophoresis. Electrophoresis. 2016; 37: 545–52. doi: 10.1002/elps.201500298 [DOI] [PubMed] [Google Scholar]
- 19.Chevaliez S, Bouvier-Alias M, Laperche S, Hézode C, Pawlotsky JM. Performance of version 2.0 of the Cobas AmpliPrep/Cobas TaqMan real-time PCR assay for hepatitis B virus DNA quantification. J Clin Microbiol. 2010; 48: 3641–7. doi: 10.1128/JCM.01306-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevaliez S, Dauvillier C, Dubernet F, Poveda JD, Laperche S, Hézode C, et al. The New Aptima HBV Quant Real-Time TMA Assay Accurately Quantifies Hepatitis B Virus DNA from Genotypes A to F. J Clin Microbiol. 2017; 55: 1211–1219. doi: 10.1128/JCM.02219-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schønning K, Johansen K, Nielsen LG, Weis N, Westh H. Analytical performance of the Hologic Aptima HBV Quant Assay and the COBAS Ampliprep/COBAS TaqMan HBV test v2.0 for the quantification of HBV DNA in plasma samples. J Clin Virol. 2018; 104: 83–88. doi: 10.1016/j.jcv.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Yeh ML, Huang CF, Hsieh MY, Huang JF, Dai CY, Yu ML, et al. Comparison of the Abbott RealTime HBV assay with the Roche Cobas AmpliPrep/Cobas TaqMan HBV assay for HBV DNA detection and quantification. J Clin Virol. 2014; 60: 206–14. doi: 10.1016/j.jcv.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Chang L, Jia T, Guo F, Zhang L, Ji H, et al. Real-time PCR assays for hepatitis B virus DNA quantification may require two different targets. Virol J. 2017; 14: 94. doi: 10.1186/s12985-017-0759-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology. 2014; 57 (3–4): 141–50. doi: 10.1159/000360947 [DOI] [PubMed] [Google Scholar]
- 25.McNaughton AL, Revill PA, Littlejohn M, Matthews PC, Ansari MA. Analysis of genomic-length HBV sequences to determine genotype and subgenotype reference sequences. J Gen Virol. 2020; 101: 271–283. doi: 10.1099/jgv.0.001387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibayama T, Masuda G, Ajisawa A, Hiruma K, Tsuda F, Nishizawa T, et al. Characterization of seven genotypes (A to E, G and H) of hepatitis B virus recovered from Japanese patients infected with human immunodeficiency virus type 1. J Med Virol. 2005; 76: 24–32. doi: 10.1002/jmv.20319 [DOI] [PubMed] [Google Scholar]
- 27.Tipples GA, Ma MM, Fischer KP, Bain VG, Kneteman NM, Tyrrell DL. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996; 24: 714–7. doi: 10.1002/hep.510240340 [DOI] [PubMed] [Google Scholar]
- 28.Lindh M, Hannoun C, Malmström S, Lindberg J, Norkrans G. Lamivudine Resistance of Hepatitis B Virus Masked by Coemergence of Mutations in Probe Region of the COBAS AMPLICOR Assay. J Clin Microbiol. 2006; 44: 2587–2589. doi: 10.1128/JCM.00265-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto H, Yano K, Nozaki Y, Matsui A, Miyazaki H, Yamamoto K, et al. Mutations within the S gene of hepatitis B virus transmitted from mothers to babies immunized with hepatitis B immune globulin and vaccine. Pediatr Res. 1992; 32: 264–8. doi: 10.1203/00006450-199209000-00002 [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto K, Horikita M, Tsuda F, Itoh K, Akahane Y, Yotsumoto S, et al. Naturally occurring escape mutants of hepatitis B virus with various mutations in the S gene in carriers seropositive for antibody to hepatitis B surface antigen. J Virol. 1994; 68: 2671–6. doi: 10.1128/JVI.68.4.2671-2676.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]