Abstract
Background
In 2021, we showed an increased risk associated with COVID-19 in pregnancy. Since then, the SARS-CoV-2 virus has undergone genetic mutations. We aimed to examine the effects on maternal and perinatal outcomes of COVID-19 during pregnancy, and evaluate vaccine effectiveness, when omicron (B.1.1.529) was the variant of concern.
Methods
INTERCOVID-2022 is a large, prospective, observational study, involving 41 hospitals across 18 countries. Each woman with real-time PCR or rapid test, laboratory-confirmed COVID-19 in pregnancy was compared with two unmatched women without a COVID-19 diagnosis who were recruited concomitantly and consecutively in pregnancy or at delivery. Mother and neonate dyads were followed until hospital discharge. Primary outcomes were maternal morbidity and mortality index (MMMI), severe neonatal morbidity index (SNMI), and severe perinatal morbidity and mortality index (SPMMI). Vaccine effectiveness was estimated, adjusted by maternal risk profile.
Findings
We enrolled 4618 pregnant women from Nov 27, 2021 (the day after WHO declared omicron a variant of concern), to June 30, 2022: 1545 (33%) women had a COVID-19 diagnosis (median gestation 36·7 weeks [IQR 29·0–38·9]) and 3073 (67%) women, with similar demographic characteristics, did not have a COVID-19 diagnosis. Overall, women with a diagnosis had an increased risk for MMMI (relative risk [RR] 1·16 [95% CI 1·03–1·31]) and SPMMI (RR 1·21 [95% CI 1·00–1·46]). Women with a diagnosis, compared with those without a diagnosis, also had increased risks of SNMI (RR 1·23 [95% CI 0·88–1·71]), although the lower bounds of the 95% CI crossed unity. Unvaccinated women with a COVID-19 diagnosis had a greater risk of MMMI (RR 1·36 [95% CI 1·12–1·65]). Severe COVID-19 symptoms in the total sample increased the risk of severe maternal complications (RR 2·51 [95% CI 1·84–3·43]), perinatal complications (RR 1·84 [95% CI 1·02–3·34]), and referral, intensive care unit (ICU) admission, or death (RR 11·83 [95% CI 6·67–20·97]). Severe COVID-19 symptoms in unvaccinated women increased the risk of MMMI (RR 2·88 [95% CI 2·02–4·12]) and referral, ICU admission, or death (RR 20·82 [95% CI 10·44–41·54]). 2886 (63%) of 4618 total participants had at least a single dose of any vaccine, and 2476 (54%) of 4618 had either complete or booster doses. Vaccine effectiveness (all vaccines combined) for severe complications of COVID-19 for all women with a complete regimen was 48% (95% CI 22–65) and 76% (47–89) after a booster dose. For women with a COVID-19 diagnosis, vaccine effectiveness of all vaccines combined for women with a complete regimen was 74% (95% CI 48–87) and 91% (65–98) after a booster dose.
Interpretation
COVID-19 in pregnancy, during the first 6 months of omicron as the variant of concern, was associated with increased risk of severe maternal morbidity and mortality, especially among symptomatic and unvaccinated women. Women with complete or boosted vaccine doses had reduced risk for severe symptoms, complications, and death. Vaccination coverage among pregnant women remains a priority.
Funding
None.
Introduction
In 2021, we reported a consistent association between COVID-19 in pregnancy (mostly the wild-type SARS-CoV-2) and higher rates of adverse maternal and neonatal outcomes when compared with pregnant women without a COVID-19 diagnosis.1 Since our initial report, a living systematic review and meta-analysis of the clinical manifestations, risk factors, and maternal and perinatal outcomes of COVID-19 in pregnancy has been published.2 This review found that pregnant women with COVID-19, compared with those without, are more likely to deliver preterm, have an increased risk of maternal death, and be admitted to the intensive care unit, and that their babies are more likely to be admitted to the neonatal intensive care unit.
Research in context.
Evidence before this study
COVID-19 in pregnancy (mostly SARS-CoV-2wt) is associated with higher rates of adverse maternal and neonatal outcomes when compared with pregnant women without COVID-19. Although the omicron (B.1.1.529) variant is associated with a higher risk of COVID-19, there is little evidence regarding the effect of omicron on pregnancy outcomes and vaccine effectiveness in pregnant women and concomitant pregnant controls.
Added value of this study
In a large, multinational, observational study comparing pregnant women with a laboratory-confirmed COVID-19 diagnosis and pregnant women without such diagnosis during the 6 months after omicron was declared the variant of concern, we have shown that infection was associated with an increased risk for maternal morbidity and severe complications, mostly among symptomatic and unvaccinated women. Women with severe symptoms who had obesity, or who were overweight, were at the highest risk for both outcomes. Vaccination effectively prevented severe symptoms and complications, including maternal death and admission to intensive care, if women were completely vaccinated, with increased effectiveness if women had received a booster dose as well. mRNA vaccines with a booster dose provided the most effective protection against severe COVID-19 and its complications for at least 10 months after the last dose of vaccine, but viral vector vaccines with a booster also provided adequate protection.
Implications of all the available evidence
Vaccinating all pregnant women, especially those who are overweight or have obesity, with a booster dose is a public health priority as it is very difficult to predict who is going to develop severe symptoms and complications of COVID-19.
The wild-type SARS-CoV-2 has also undergone several genetic mutations that have changed the clinical and epidemiological profile of the pandemic. The SARS-CoV-2 variant B.1.1.529 (omicron) was declared a variant of concern by WHO on Nov 26, 2021. Early reports indicated that omicron, although more transmissible, produces milder disease in non-pregnant individuals than previous variants.3 However, some evidence suggests that hospitalisation and mortality rates might be comparable to those of other SARS-CoV-2 variants after adjusting for confounders and vaccination status.4 Additionally, there is evidence of considerable misinformation from social media and other sources regarding the relative risks of COVID-19 and benefits of vaccination.5, 6 All these issues should be considered in the context of an increasing community prevalence of COVID-19 and an increasing number of patients with COVID-19 reported in European countries.7, 8
Hence, the INTERCOVID-2022 Study aimed to: examine the effects on maternal and perinatal outcomes of COVID-19 during pregnancy, as compared with pregnant women without a COVID-19 diagnosis, enrolled during the first 6 months of omicron as the variant of concern; evaluate vaccine effectiveness against COVID-19 diagnosis, severe maternal complications, and hospitalisation or death, as compared with non-vaccinated pregnant women, according to vaccine type and dose; and clarify disease risk and prevention effectiveness to challenge misinformation in the medical community and general public.
Methods
Study design and participants
This was a prospective, observational, cohort study involving 41 hospitals in 18 countries (Argentina, Brazil, Egypt, France, Indonesia, Israel, Italy, Japan, Mexico, Nigeria, North Macedonia, Pakistan, Spain, Switzerland, Türkiye, the UK, Uruguay, and the USA). Participating hospitals are part of the Oxford Maternal and Perinatal Health Institute worldwide network of research institutions that provide routine care to several thousand women and newborn babies every year following standardised protocols.9 These hospitals were not selected to represent the underlying populations, rather to enable us to enrol the maximum number of diagnosed and concomitant non-diagnosed pregnant women in the shortest possible time.
We enrolled, at any time during pregnancy or delivery, women with a documented laboratory-confirmed diagnosis of COVID-19 (real-time PCR or rapid test) who delivered between Nov 27, 2021, and June 30, 2022, at the participating hospitals. Live and stillborn singleton and multiple births, and newborn babies with congenital anomalies, were included. Mothers and their live newborn babies were followed up until hospital discharge. After each woman with a COVID-19 diagnosis was enrolled, to minimise risk of bias, two unmatched women without a COVID-19 diagnosis, as representative of the pregnant population at each study site, were enrolled concomitantly and consecutively, at delivery or at the same level of care (if identified antenatally). If a woman without a COVID-19 diagnosis did not agree to participate, the next woman was approached until two women without a COVID-19 diagnosis were enrolled per woman with a diagnosis. If a woman without a diagnosis reported, or had, a documented COVID-19 diagnosis before the index pregnancy, she was considered without a diagnosis for the risk analyses but considered immunologically exposed for the vaccine effectiveness analyses.
The Oxford Tropical Research Ethics Committee and all local ethics committees approved the study, which did not interfere with clinical management. Informed consent (oral or written) was obtained from study participants according to local requirements, except for cases in which a local committee granted a waiver or exemption. We adhered to the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol, including the laboratory tests used, has been previously published.1
Procedures
We obtained ecological information on the predominant variant during the study period from official reports from catchment areas of each participating hospital. During the study period, universal screening for pregnant women at any hospital admission, including delivery, was implemented in 28 (68%) of the 41 hospitals, representing 3615 (78%) of the 4618 pregnant women enrolled. The other 1003 (22%) women were tested if they were symptomatic or if they were asymptomatic but had had direct contact with cases or family members of cases, or were health-care providers, schoolteachers, front-line public workers, or patients at high risk, according to local protocols. If women were test-positive but asymptomatic, they were analysed under the asymptomatic strata.
For consistency, we used the same procedures, documentation, and data management system as in our previous COVID-19 study.1 Pregnancy, delivery, and neonatal information was obtained from the medical records. Gestational age estimation was on the basis of fetal crown-rump length (<14 weeks' gestation) against the international INTERGROWTH-21st standard,10 or the best obstetric estimate (all clinical and ultrasonography data available at the time of delivery). Neonates' weight, length, and head circumference at birth were assessed against the international INTERGROWTH-21st standards.11
Vaccination history was obtained from the medical records, vaccination registries, primary care records, maternal vaccination cards, maternal oral report, or any other documentation or registration system. If none of these methods provided evidence of vaccination, women were considered unvaccinated.
For stratified, a priori determinate analyses, we documented the type of vaccine, number of doses, and time between the last dose received and the first (post-vaccination) COVID-19 positive laboratory test. We categorised women as boosted if they received three doses of any vaccine or two doses of Ad26.COV2.S (Janssen and Johnson & Johnson) vaccine; completely vaccinated if they received two doses of any vaccine or one dose of Ad26.COV2.S; partially vaccinated if they received one dose of any vaccine other than Ad26.COV2.S, or if they indicated they were vaccinated but did not provide further information; and unvaccinated if they received no doses or vaccination status was not reported. We grouped vaccinated women according to the type of vaccine administered: mRNA (mRNA-1273 [Moderna] or BNT162b2 [Pfizer-BioNTech]), inactivated virus (Ad5-nCoV [Cansino Biologics], Coronovac [SinoVac], BBV152 Covaxin [Bharat Biotech], or BBIBP-CorV [Sinopharm]), and viral vector (ChAdOx1-S [AstraZeneca], AZD1222 [Covishield], Ad26.COV2.S [Janssen and Johnson & Johnson], or Sputnik [Gamaleya Institute]). If vaccine type was missing, we imputed data on the basis of the vaccine offered to pregnant women in the hospital's catchment area at the time of the study.
Outcomes
The analytical strategy was based on two sets of comparisons: between women exposed and women not exposed to COVID-19, and between women exposed and women not exposed to vaccination. The primary outcomes were the same as in our previous report:1 maternal morbidity and mortality index (MMMI), including at least one complication during pregnancy (including vaginal bleeding, pregnancy-induced hypertension, pre-eclampsia, eclampsia, haemolysis, elevated liver enzymes, and low platelet count [HELLP] syndrome), preterm birth, or infection requiring antibiotics, or maternal death, admission to an intensive care unit (ICU), or referral to higher dependency care; severe neonatal morbidity index (SNMI), including at least one morbidity (including bronchopulmonary dysplasia, hypoxic-ischaemic encephalopathy, sepsis, anaemia requiring transfusion, patent ductus arteriosus, intraventricular haemorrhage, necrotising enterocolitis, and retinopathy of prematurity); and severe perinatal morbidity and mortality index (SPMMI), including any of the morbidities listed in the SNMI, intrauterine or neonatal death, or a neonatal ICU stay of 7 days or longer. Secondary outcomes were each component of the above indices considered separately and included analysis of factors associated with neonatal SARS-CoV-2 positivity.
We compared women with and without a diagnosis according to vaccination status (all women and unvaccinated) and COVID-19 symptoms (asymptomatic, any related symptom, moderate symptoms, and severe symptoms). We evaluated vaccine effectiveness against laboratory-confirmed COVID-19 diagnosis and moderate or severe symptomatic COVID-19 or complications (referral, ICU admission, or death). We also used a composite variable of disease severity and maternal complications for the vaccine effectiveness analyses, which included the presence of COVID-19 severe symptoms or maternal referral, ICU admission, or death.
Statistical analysis
Sample size estimates were calculated using specialised software12 with a study design consisting of two women without a diagnosis for each woman with a diagnosis for 80% power with a type 1 error of 0·05 (95% CI). The required sample size is based on the proportion of women without a diagnosis that had an outcome, and the estimated relative risk (RR) was calculated using Poisson regression models. We hypothesised that the omicron variant would have half the risk of SARS-COV-2 and used 50% of the RR observed for each outcome among asymptomatic pregnant women with COVID-19 from our previous report.1 The number of women diagnosed with COVID-19 that should be enrolled for MMMI was 536, for SNMI was 1041, for SPMMI was 468, and for pre-eclampsia (often reported as associated with COVID-19 diagnosis) was 1400. Hence, a pragmatic sample size of 1500 women diagnosed with COVID-19 was the target to provide adequate power for our primary outcomes of interest.
We described baseline characteristics (number and percentage or mean and SD) comparing women with and without a diagnosis, and comparing vaccinated with unvaccinated women. We used Poisson regression models with a log link function and robust SEs expressed as RR (95% CI) to assess our main morbidity indices (MMMI, SNMI, and SPMMI) as binary outcomes, as well as pregnancy complications and specific perinatal or neonatal events using women without a diagnosis as the reference group. In further subanalyses we assessed associations with number of days in ICU using negative binomial models with robust SEs (expressed as an incidence rate ratio and 95% CI). We set statistical significance at p<0·05.
We adjusted all models for maternal age, country, and a binary variable (yes or no) for any pre-existing maternal morbidities (including diabetes, thyroid, and other endocrine disorders; cardiac disease; hypertension; chronic respiratory disease; kidney disease; or tuberculosis). Models with preterm birth in the index pregnancy as the outcome were also adjusted for previous preterm birth.
We defined moderate COVID-19 symptoms as fever, chest pain, or shortness of breath without a severe symptom; and severe symptoms as admission to hospital during pregnancy for respiratory disease or respiratory tract infection requiring antibiotic or antiviral treatment.
In sensitivity analyses for risk, we also adjusted for maternal educational level and maternal work outside the home. We calculated RR (95% CI) for the strata and outcomes above, adjusting for maternal age, pre-existing morbidities, and being overweight or having obesity (BMI >25 kg/km2). We also did sensitivity analyses excluding women who delivered during the study period but were diagnosed before Jan 1, 2022.
Vaccine effectiveness was defined as the proportionate reduction in COVID-19 diagnoses among vaccinated relative to unvaccinated women (1–RR; 95% CI).
As the raw data from our non-randomised, observational design increased the risk of selection bias due to the behaviour and risk profile of the women that accepted vaccination, we evaluated vaccine effectiveness adjusting RR (95% CI) for maternal age, overweight or obesity, country, and pre-existing morbidities. In sensitivity analyses, we also adjusted the models for vaccine effectiveness by maternal educational level and working outside the home.
To evaluate vaccine effectiveness over time, we plotted Kaplan-Meier curves with the percentage of women with the composite outcome of severe symptomatic COVID-19, referral or ICU admission, or death from the time of their last vaccine dose according to vaccination status (partial, complete, mRNA vaccine with mRNA booster, and other vaccine with mRNA booster) and type of vaccine (mRNA, viral vector, or inactivated virus).
Role of the funding source
There was no funding source for this study.
Results
We enrolled 4618 pregnant women between Nov 27, 2021, and June 30, 2022, of whom 1545 (33%) were diagnosed with COVID-19: 1236 (80%) by RT-PCR and 309 (20%) by rapid tests. The remaining 3073 (67%) women without a diagnosis, and without any positive test during the index pregnancy, were concomitantly and consecutively enrolled at the same level of care; 2229 (73%) of these women had at least one negative COVID-19 test during pregnancy (appendix p 8). The mean gestational age at COVID-19 diagnosis was 33·1 weeks (SD 8·7); the median was 36·7 weeks (IQR 29·6–38·9). The mean interval between the first positive test and delivery was 5·5 weeks (SD 8·4). Among the women who delivered during the study period, 190 (12%) of 1545 women were diagnosed with COVID-19 before Nov 27, 2021.
Ecological information during the study period from official reports showed that the percentage of individuals diagnosed with omicron in all regions increased dramatically during December, 2021; by January, 2022, more than 80% of the cases, in all regions studied, were estimated by local authorities to be due to the omicron variant.
Women with and without a diagnosis had similar demographic characteristics and obstetric or gynaecological histories. However, there were more smokers (116 [8%] of 1545 vs 177 [6%] of 3073) and people who were overweight or with obesity (758 [49%] of 1545 vs 1390 [47%] of 3073) women with a diagnosis, who also had slightly fewer pre-existing morbidities (276 [18%] of 1545 vs 614 [20%] of 3073), than women without a diagnosis (appendix p 2).
Women with a diagnosis, compared with those without a diagnosis had increased risks of MMMI (RR 1·16 [95% CI 1·03–1·31]) and SPMMI (RR 1·21 [95% CI 1·00–1·46]). Regarding the secondary endpoints, women with a diagnosis also were at increased risk for maternal referral to higher care or ICU admission or death (RR 1·50 [95% CI 1·01–2·21]). Women with a diagnosis, compared with those without a diagnosis, also had increased risks of SNMI (RR 1·23 [95% CI 0·88–1·71]), preterm birth (RR 1·12 [95% CI 0·97–1·30]), and pre-eclampsia, eclampsia, and HELLP syndrome (RR 1·25 [95% CI 0·97–1·62]), although the lower bounds of the 95% CI crossed unity (table 1 ).
Table 1.
Maternal and perinatal outcomes among women with and without a COVID-19 diagnosis, according to symptoms and vaccination status
|
MMMI |
SNMI |
SPMMI |
Preterm birth (<37 weeks' gestation) |
Pre-eclampsia or eclampsia or HELLP syndrome |
Maternal referral to higher care or ICU admission, or death |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | RR (95% CI) | N | RR (95% CI) | N | RR (95% CI) | N | RR (95% CI) | N | RR (95% CI) | N | RR (95% CI) | |||
| All pregnant women | 857 | .. | 138 | .. | 402 | .. | 612 | .. | 218 | .. | 95 | .. | ||
| Without a COVID-19 diagnosis | 543 | 1 (ref) | 86 | 1 (ref) | 252 | 1 (ref) | 393 | 1 (ref) | 135 | 1 (ref) | 55 | 1 (ref) | ||
| With a COVID-19 diagnosis | 314 | 1·16 (1·03–1·31)* | 52 | 1·23 (0·88–1·71) | 150 | 1·21 (1·00–1·46)* | 219 | 1·12 (0·97–1·30) | 83 | 1·25 (0·97–1·62) | 40 | 1·50 (1·01–2·21)* | ||
| Asymptomatic | 108 | 0·95 (0·79–1·14) | 28 | 1·53 (1·01–2·32)* | 59 | 1·14 (0·87–1·50) | 85 | 1·02 (0·82–1·27) | 33 | 1·11 (0·77–1·61) | 11 | 0·85 (0·45–1·62) | ||
| Any symptoms | 206 | 1·32 (1·15–1·52)* | 24 | 0·99 (0·64–1·55) | 91 | 1·26 (1·01–1·59)* | 134 | 1·20 (1·00–1·44)* | 50 | 1·36 (1·00–1·85)* | 29 | 2·11 (1·39–3·20)* | ||
| Moderate symptoms† | 102 | 1·34 (1·12–1·61)* | 9 | 0·75 (0·38–1·48) | 44 | 1·24 (0·92–1·68) | 70 | 1·26 (1·00–1·58) | 23 | 1·37 (0·90–2·09) | 11 | 1·63 (0·89–2·98) | ||
| Severe symptoms‡ | 27 | 2·51 (1·84–3·43)* | 2 | 1·47 (0·37–5·80) | 11 | 1·84 (1·02–3·34)* | 13 | 1·54 (0·93–2·57) | 4 | 2·48 (0·95–6·48)* | 14 | 11·83 (6·67–20·97)* | ||
| Unvaccinated pregnant women | 331 | .. | 40 | .. | 160 | .. | 259 | .. | 79 | .. | 55 | .. | ||
| Without a COVID-19 diagnosis | 186 | 1 (ref) | 24 | 1 (ref) | 95 | 1 (ref) | 155 | 1 (ref) | 42 | 1 (ref) | 20 | 1 (ref) | ||
| With a COVID-19 diagnosis | 145 | 1·36 (1·12–1·65)* | 16 | 1·25 (0·68–2·30) | 65 | 1·23 (0·91–1·65) | 104 | 1·20 (0·96–1·49) | 37 | 1·48 (0·97–2·27) | 35 | 3·33 (1·99–5·59)* | ||
| Asymptomatic | 57 | 1·12 (0·86–1·46) | 9 | 1·47 (0·71–3·05) | 25 | 1·00 (0·65–1·54) | 42 | 1·04 (0·76–1·43) | 18 | 1·44 (0·83–2·49) | 10 | 1·82 (0·84–3·96) | ||
| Any symptoms | 88 | 1·58 (1·27–1·97)* | 7 | 1·05 (0·47–2·32) | 40 | 1·44 (1·01–2·04)* | 62 | 1·33 (1·02–1·74)* | 19 | 1·53 (0·89–2·62) | 25 | 4·90 (2·93–8·18)* | ||
| Moderate symptoms† | 41 | 1·45 (1·09–1·95)* | 4 | 1·17 (0·45–3·07) | 19 | 1·35 (0·86–2·12) | 33 | 1·39 (1·00–1·93) | 7 | 1·08 (0·49–2·38) | 9 | 3·46 (1·71–6·98)* | ||
| Severe symptoms‡ | 22 | 2·88 (2·02–4·12)* | 1 | 1·48 (0·18–11·95) | 8 | 1·69 (0·82–3·45) | 10 | 1·51 (0·84–2·72) | 3 | 3·56 (1·03–12·33)* | 13 | 20·82 (10·44–41·54)* | ||
Models adjusted for maternal age, presence or absence of any pre-existing medical condition, and country. Models for preterm birth additionally adjusted for past history of preterm birth. MMMI=Maternal Morbidity and Mortality Index. SNMI=Severe Neonatal Morbidity Index. SPMMI=Severe Perinatal Morbidity and Mortality Index. HELLP=haemolysis, elevated liver enzymes, and low platelet count. ICU=intensive care unit. RR=relative risk.
p<0·05.
Moderate COVID-19 symptoms are defined as fever, chest pain, or shortness of breath, and not showing any severe symptoms.
Severe COVID-19 symptoms are defined as having been admitted to hospital during pregnancy for either respiratory disease or respiratory tract infection requiring antibiotic or antiviral treatment.
The risks of the individual components of the morbidity and mortality indices are shown in the appendix (pp 5–6). Overall, there was a marginal, significant increase in the risk of pre-eclampsia and eclampsia; a significant increase of infections requiring antibiotics and number of days in ICU; and an increase in the risk of maternal mortality but with a very wide CI (RR 3·00 [95% CI 0·50–17·93]). Similar patterns were observed in unvaccinated women: pre-eclampsia RR 1·54 (95% CI 1·00–2·37) and maternal mortality RR 5·26 (95% CI 0·55–50·51). There was also an increased risk for maternal infections requiring antibiotics, referral or ICU admission, and fetal distress (appendix p 5). All other secondary outcomes were non-siginficant.
The risk of MMMI significantly increased in women with a diagnosis and the presence of any symptoms (RR 1·32 [95% CI 1·15–1·52]) or moderate symptoms (RR 1·34 [95% CI 1·12–1·61]) compared with women without a diagnosis. Compared with women without a diagnosis, the presence of severe symptoms in women with a diagnosis was associated with increased risks of MMMI (RR 2·51 [95% CI 1·84–3·43]), SPMMI (RR 1·84 [95% CI 1·02–3·34]), pre-eclampsia, eclampsia, or HELLP syndrome (RR 2·48 [95% CI 0·95–6·48]), and a substantial, significant increase in the risk of referral, ICU admission, or death (RR 11·83 [95% CI 6·67–20·97]).
In the subgroup of unvaccinated women (n=1732), women with a diagnosis were at an overall higher, significant risk for MMMI and maternal referral to higher care or ICU admission, or death, compared with women without a diagnosis (table 1). Unvaccinated, diagnosed women with symptoms were at an increased risk of MMMI and maternal referral to higher care or ICU admission, or death. Unvaccinated women with severe symptoms were at significantly increased risks of MMMI; pre-eclampsia, eclampsia, or HELLP syndrome; and referral to higher care or ICU admission, or death (table 1).
In sensitivity analyses adjusting for maternal education (data missing in 584 [13%] of 4618 women) and work outside the home (data missing in 347 [8%] of 4618 women), the estimated RRs for COVID-19-associated maternal and neonatal morbidities were very similar to our main results. There were 327 women that either reported or had a documented COVID-19 diagnosis before the index pregnancy (112 [7%] of 1545 in the diagnosed group; 215 [7%] of 3073 in the non-diagnosed group). Adjusted analyses for a COVID-19 diagnosis before the index pregnancy did not change the observed results for maternal and neonatal outcomes.
In further sensitivity analyses, being overweight or having obesity increased the effects of COVID-19 on MMMI; SPMMI; pre-eclampsia, eclampsia, or HELLP syndrome; and referral to higher care, ICU admission, or death in the total population, and more severely so among unvaccinated women (appendix p 2).
570 (36%) of 1577 neonates born to women with a diagnosis were tested for SARS-CoV-2 because they manifested clinical signs of COVID-19; of these, 70 (12%) tested positive. We compared the risk for severe neonatal complications in test-positive versus test-negative neonates born to women with a diagnosis using test-negative neonates as the reference group. The risks were not increased for SNMI, SPMMI, or a neonatal ICU stay of 7 days or longer among the test-positive compared with the test-negative neonates.
None of the factors possibly related with neonatal SARS-CoV-2 positivity (eg, gestational age at delivery, caesarean delivery, neonatal ICU stay 7 days or longer, or exclusive breastfeeding at discharge) were found to be associated. Among test-positive women with test-positive neonates, the caesarean section rate was 42% (29 of 69), similar to test-positive women with test-negative neonates (215 [44%] of 593) and higher than the rate in the women without a diagnosis (1202 [39%] of 3102).
Overall, 2886 (62%) of 4618 enrolled women had at least a single dose of any vaccine (1897 [66%] mRNA, 554 [19%] viral vector, and 376 [13%] inactivated virus; vaccine type was missing for 59 women and we imputed data on the basis of the vaccine offered to pregnant women in the hospital's catchment area at the time of the study), whilst 1732 (38%) women were unvaccinated. Vaccination status was missing for 19 women, and therefore these women were included in the unvaccinated group. Of the 4618 women, 410 (9%) were partially vaccinated, 1598 (35%) were completely vaccinated, and 878 (19%) had had booster vaccination. Over half of all women (2476 [54%] of 4618) had either complete or booster doses, of which 1668 (67%) were mRNA vaccines. Four women had four doses. 117 (5%) of vaccinated women received a combination of type of vaccines, mostly for booster doses (data not shown).
Vaccinated women tended to have a higher level of education, be more likely to be married or cohabiting, work more outside home, and smoke less than unvaccinated women (appendix p 6). Vaccinated women had more fertility treatments before the index pregnancy, higher rates of nulliparity, and lower rates of overweight or obesity than unvaccinated women. The rate of any pre-existing morbidity was slightly higher in the vaccinated group (578 [20%] of 2886) than in the unvaccinated group (312 [18%] of 1732) mostly due to higher rates of diabetes mellitus, cardiac disease, hypertension, and kidney disease (appendix p 7). Hence, in the vaccine effectiveness analyses, we adjusted for maternal age, overweight or obesity, pre-existing medical conditions, and country, and did sensitivity analyses by maternal education and maternal work outside the home.
The distribution of women immunised with a booster dose was higher in the non-replicative viral vaccine group (122 [34%] of 362) than in the mRNA vaccine group (591 [31%] of 1897) and both were higher than the inactivated virus vaccine group (93 [25%] of 376). Regardless of vaccine type, vaccine effectiveness for a COVID-19 diagnosis, even after a booster dose, was at or below 32% even after a booster dose (table 2 ). Similarly, protection against moderate COVID-19 symptoms was fairly low: the greatest level of protection was in women who received booster doses of mRNA (vaccine effectiveness 54% [95% CI 34–68]).
Table 2.
VE (%) against laboratory-confirmed COVID-19 diagnosis, moderate and severe maternal COVID-19 symptoms, and maternal referral to higher level of care, ICU admission, or death, according to vaccine type and regimen
|
All women: effectiveness against laboratory-confirmed COVID-19 |
All women: effectiveness against moderate COVID-19 symptoms* |
All women: effectiveness against severe COVID-19 symptoms, referral for higher care, ICU admission, or death† |
Women diagnosed with COVID-19: effectiveness against severe symptoms, referral for higher care, ICU admission, or death† |
|||||
|---|---|---|---|---|---|---|---|---|
| N | VE (95% CI) | N | VE (95% CI) | N | VE (95% CI) | N | VE (95% CI) | |
| All vaccines combined | ||||||||
| Unvaccinated | 632 | 0 (ref) | 213 | 0 (ref) | 85 | 0 (ref) | 65 | 0 (ref) |
| Partially vaccinated | 145 | 5% (0–18) | 41 | 26% (0–46) | 13 | 35% (0–64) | 9 | 33% (0–67) |
| Completely vaccinated | 535 | 9% (0–18) | 171 | 20% (1–34)‡ | 36 | 48% (22–65)‡ | 10 | 74% (48–87)‡ |
| Booster vaccination | 233 | 30% (19–39)‡ | 71 | 48% (32–61)‡ | 7 | 76% (47–89)‡ | 2 | 91% (65–98)‡ |
| mRNA vaccine | ||||||||
| Partially vaccinated | 84 | 0 (0–17) | 18 | 32% (0–57) | 6 | 35% (0–72) | 5 | 29% (0–71) |
| Completely vaccinated | 352 | 11% (0–21)‡ | 75 | 41% (22–55)‡ | 18 | 56% (27–74)‡ | 6 | 79% (49–91)‡ |
| Booster vaccination | 152 | 32% (20–42)‡ | 35 | 54% (34–68)‡ | 4 | 81% (47–93)‡ | 1 | 94% (56–99)‡ |
| Non-replicating viral vector vaccine | ||||||||
| Partially vaccinated | 39 | 0 (0–13) | 11 | 0 (0–40) | 2 | 61% (0–90) | 1 | 27% (0–89) |
| Completely vaccinated | 94 | 2% (0–20) | 52 | 0 (0–4) | 6 | 60% (2–83)‡ | 2 | 56% (0–91) |
| Booster vaccination | 56 | 20% (0–38) | 25 | 25% (0–52) | 3 | 49% (0–84) | 1 | 76% (0–96) |
| Inactivated virus vaccine | ||||||||
| Partially vaccinated | 9 | 28% (0–61) | 5 | 4% (0–62) | 2 | 0 (0–70) | 0 | NA |
| Completely vaccinated | 86 | 0 (0–20) | 43 | 0 (0–8) | 12 | 8% (0–52) | 2 | 66% (0–93) |
| Booster vaccination | 24 | 27% (0–51) | 10 | 31% (0–66) | 0 | NA | 0 | NA |
Models adjusted for maternal age, overweight or obesity, presence or absence of any pre-existing medical condition, and country. ICU=intensive care unit. VE=vaccine effectiveness. NA=not applicable due to no cases.
Moderate COVID-19 symptoms are defined as fever, chest pain, or shortness of breath, and not showing any severe symptoms.
Severe COVID-19 symptoms are defined as having been admitted to hospital during pregnancy for either respiratory disease, respiratory tract infection, or requiring antibiotic or antiviral treatment.
p<0·05.
However, vaccine effectiveness increased against severe disease (table 2): 48% (95% CI 22–65) following a complete (two-dose) regimen (n=1598) and 76% (47–89) after a booster (n=878). In stratified analyses according to vaccine type, booster mRNA vaccines (n=591) were highly effective in preventing severe disease (vaccine effectiveness 81% [95% CI 47–94]) after adjusting for maternal age, overweight and obesity, country, and pre-existing morbidities. Vaccine effectiveness for severe disease was 60% (95% CI 2–83) among women completely immunised with viral vector vaccines and 49% (95% CI 0–84) among women who had received viral vector boosters. Among those completely immunised with inactivated virus vaccines, vaccine effectiveness for severe disease was 8% (95% CI 0–52) and there were no cases among those with a booster dose of inactivated viral vaccine (table 2).
We further evaluated vaccine effectiveness against severe COVID-19 symptoms or referral, ICU admission, or death following a laboratory-confirmed COVID-19 diagnosis (table 2). In this sub-sample, vaccine effectiveness increased substantially for all vaccines combined against these severe complications: 74% (95% CI 48–87) following a complete (two-dose) regimen and 91% (65–98) after a booster. In stratified analyses according to vaccine type, booster mRNA vaccines were highly effective in preventing these severe symptoms and complications (vaccine effectiveness 94% [95% CI 56–99]).
Vaccine effectiveness for these severe complications among women completely immunised with viral vector vaccines was 56% (95% CI 0–91) and with booster vaccination was 76% (0–96). Among those completely immunised with inactivated virus vaccines, vaccine effectiveness for severe disease was 66% (95% CI 0–93) and there were no cases among those who received a booster dose. The vaccine effectiveness results were very similar after a sensitivity analysis, exploring models with and without maternal education and maternal work outside the home variables, and when we excluded women who delivered during the study period but were diagnosed before Nov 27, 2021, and before Jan 1, 2022.
Importantly, there was no apparent increased risk for vaccine side-effects for the mother, fetus, or neonate, including congenital abnormalities, even among women completely vaccinated during the index pregnancy. In contrast, there was a reduction in the risk of spontaneous and medically indicated preterm birth, most evident among women completely vaccinated during the index pregnancy (appendix p 7).
However, in vaccinated versus unvaccinated women, there were higher rates of infections requiring antibiotics (271 [12%] of 2308 vs 141 [10%] of 1420) and induction of labour (580 [25%] of 2308 vs 287 [20%] of 1420; appendix p 7). This finding is most likely due to the slightly higher proportions of diabetes, tuberculosis, and renal disease in vaccinated versus unvaccinated women (appendix p 6; ie, women at increased risk were considered a priority for vaccination and more often required induction of labour because of underlying disease). We adjusted the results in the appendix (p 3) for pre-existing medical conditions in addition to excluding these women in a sensitivity analysis. The adjusted results were very similar except for spontaneous initiation of labour and medically indicated preterm birth that became significant (p<0·05). When we restricted the population to only COVID-19 diagnosed cases, there was a further reduction in the risk of fetal distress and maternal referral or ICU admission among vaccinated women (appendix p 5). Finally, sensitivity analysis excluding the 327 women that either reported or had documented a COVID-19 diagnosis before the index pregnancy did not change the vaccine effectiveness results.
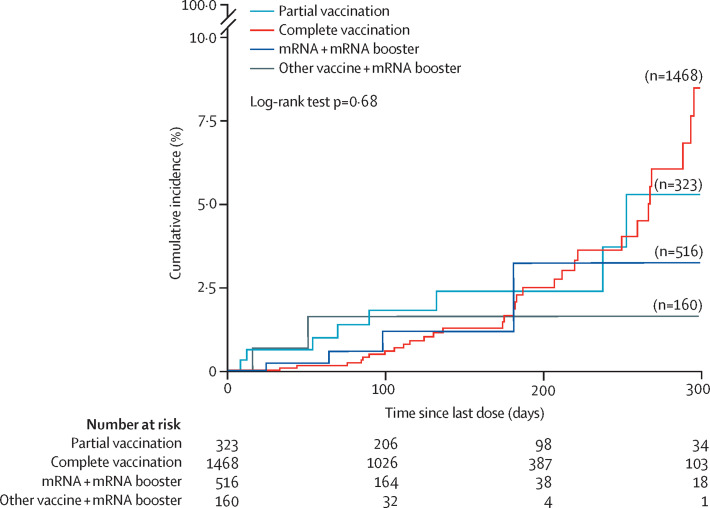
Kaplan-Meier curves showing the risk of women experiencing severe COVID-19 symptoms or referral, ICU admission, or death from the time of their last dose according to partial, complete, and boosted vaccination and stratified for combinations of mRNA and mRNA booster and any other vaccine and mRNA booster show that there was a progressive increase in the cumulative risk for all four groups until approximately 200 days post vaccination (figure 1 ). Thereafter, the cumulative risk in completely and partially vaccinated women increased considerably to more than 5% after 300 days post vaccination; the two different vaccine groups with the mRNA booster dose remained around 3% (figure 1).
Figure 1.
Vaccine effectiveness against severe maternal complications or death by time after vaccination, according to partial, complete, or booster doses during the time of omicron (B.1.1.529) as a variant of concern (all types of vaccines combined)
Sample size differences with Table 1, Table 2 are due to missing information on the date of the last vaccine dose and type of vaccine or booster. Severe maternal complications include severe COVID-19 symptoms or referral to a higher level of care or admission to hospital during pregnancy for either respiratory disease or respiratory tract infection requiring antibiotic or antiviral treatment or intensive care unit admission.
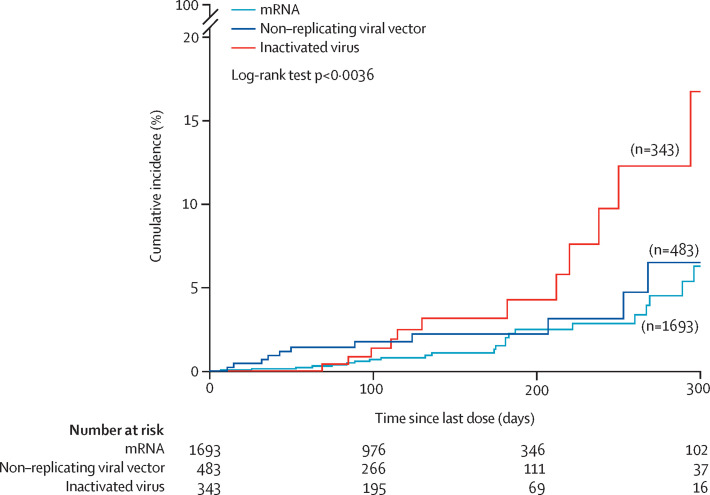
The cumulative risk of severe maternal complications or death was low in the mRNA vaccine and the non-replicating viral vector vaccine groups until day 275, at which time there were slight increases. The cumulative risk in the inactivated virus vaccine group increased after 200 days, reaching a more than 15% cumulative risk after 300 days (figure 2 ).
Figure 2.
Vaccine effectiveness against severe maternal complications or death by time after vaccination, according to type of vaccine during the time of omicron (B.1.1.529) as a variant of concern (all doses combined)
Sample size differences with Table 1, Table 2 are due to missing information on the date of the last vaccine dose and type of vaccine or booster. Severe maternal complications include severe COVID-19 symptoms or referral to a higher level of care or admission to hospital during pregnancy for either respiratory disease or respiratory tract infection requiring antibiotic or antiviral treatment or intensive care unit admission.
Discussion
We have shown, based on a priori stratified analyses, a markedly increased risk from COVID-19 diagnosed in pregnancy of maternal morbidity and mortality, perinatal morbidity and mortality, and maternal referral to a higher level of care or ICU admission among women with moderate or severe COVID-19 symptoms and in women who were overweight or who had obesity early in pregnancy, as compared with pregnant women without a COVID-19 diagnosis. There was a marked increased risk for the same outcomes among symptomatic, unvaccinated women, close to the effect seen in pregnant women before vaccines became available.1 Interestingly, the pattern of association with pre-eclampsia, which we reported early in the pandemic,13 persisted in unvaccinated women with considerable increased risk for women with severe symptoms.
Reassuringly, the vaccine effectiveness of all vaccines combined to prevent severe maternal morbidity and referral, ICU admission, or death was 76% amongst all women given a booster dose. The respective vaccine effectiveness values for mRNA vaccines with a booster dose was 81%. For women with a diagnosis, vaccine effectiveness for the same outcomes, of all vaccines combined, was 74% (95% CI 48–87) for the complete regimen and 91% (65–98) after a booster dose.
Equally reassuring was that viral vector vaccines (the second most commonly used vaccine type) had a vaccine effectiveness against severe complications or death of 60% for all women who were completely vaccinated; for these vaccines with a booster dose, vaccine effectiveness for severe complications was 76% among women with a diagnosis.
In our study, as in the general population, vaccination during pregnancy did not prevent infection with the omicron variant. This finding is because SARS-CoV-2 has developed considerable antigen escape, shown by its high capacity to infect or re-infect people with a degree of immunity.14, 15 However, complete or booster doses of the two widely used vaccine types are highly effective in preventing severe maternal complications and outcomes within 10 months of the last dose.
Our work is the result of a powerful global research strategy. Between Nov 27, 2021 (immediately after WHO recognised omicron as a variant of concern), and June 30, 2022, we compared a large, international cohort of pregnant women diagnosed with COVID-19 with a concomitant, consecutively recruited reference group of pregnant women without a COVID-19 diagnosis. We used the same study sites, research methodology, and analytical strategy as in our reports of the wild-type virus in pregnancy,1, 13, 16, 17 but, as widely recommended, added estimation of vaccine effectiveness according to dosage and type of vaccine. The standardised nature of data collection across study sites and between the first and second data collection periods makes ours a robust set of findings to inform patient care, education, and public health programming.
Limitations of the study include needing to interpret the association between severe COVID-19 symptoms and some outcomes with caution because of the small sample size and wide confidence intervals. The risk profile of vaccinated women suggests some selection bias, not because of the study design per se, rather that pregnant women's eligibility and willingness for vaccination changed during the study period. Once the risks of COVID-19 in pregnancy were acknowledged, pregnant women ceased being considered members of the general population at low risk because of their age and were offered vaccination because they were pregnant. Consequently, we adjusted vaccine effectiveness analyses for possible confounding variables such as medical risk profile, overweight or obesity, maternal age, COVID-19 diagnosis before the index pregnancy, and country, and further evaluated maternal education and work outside the home in sensitivity analyses.
We did not collect viral genotyping data; rather, the association with omicron was based on the time when it was the variant of concern. Hence, as it is possible that other variants could have caused some infections in December, 2021, we did sensitivity analyses excluding women enrolled before Jan 1, 2022, and found no substantive changes in the results.
Some women without a diagnosis could have had COVID-19 during pregnancy but were not tested, which means they could have been cases. However, this bias produces a more conservative estimate (the comparison group is more like the diagnosed group) in favour of the null hypothesis that omicron infection does not increase the risk of complications.
No sub-Saharan sites contributed to our cohort despite our best efforts during the preparatory phase. This is a major limitation of our study and reduces its external validity. Unfortunately, highly motivated collaborators faced enormous logistical problems such as case under-registration given that there was no large-scale testing,18 and due to reduced antenatal coverage that could not be resolved quickly and without research funding.
We avoided using definitions of the clinical severity of COVID-19 partly because pregnancy is a unique physiological state and partly because a recent meta-analysis highlighted considerable heterogeneity in reporting disease severity.19 Instead, we opted to concentrate on substantive clinical decisions such as referral to higher care and admission to hospital or ICU. Additionally, because this study was not funded, we did not collect any post-discharge follow-up data for the mother or newborn baby, which is a major limitation in this field. The millions of pregnant women affected by COVID-19 deserve to know the long-term implications of the disease.
Our living review of the literature relating to the effects of the omicron variant on pregnancy identified nine primary reports up to Oct 10, 2022. Of these, eight compared outcomes during the omicron phase with earlier COVID-19 variant phases (historical controls).18, 20, 21, 22, 23, 24, 25, 26 The last was a multi-hospital study in New York City, NY, USA, done between December, 2021, and February, 2022, and based on a universally tested cohort that compared test-positive with test-negative pregnant women.27 Only preterm birth and severe maternal morbidity were reported, with small numbers of events. As in our report, preterm birth rates were higher in the COVID-19 group than in the group without a diagnosis; the pre-pandemic, generic, severe maternal morbidity measure was similar in both groups as opposed to our maternal morbidity and mortality index that was increased among all strata of women with symptomatic COVID-19.
Across the populations we studied, 1732 (37%) of 4618 women were unvaccinated, and only 1668 (36%) of 4618 women in the total sample were completely vaccinated or boosted with an mRNA vaccine, which was recommended for pregnancy in 2021 globally.28 Therefore, considerably more effort, at all levels of the health-care system, is required to provide pregnant women with the protection they need to confront present and future waves of the pandemic.
Our data also have implications for research because the US Food and Drug Administration's independent vaccine advisers recently recommended COVID-19 booster formulations that target the omicron variants, specifically the BA.4 and BA.5 subvariants.29 In our view, pregnant women must be included in the assessment of modified vaccines and prioritised for vaccination to avoid past shortcomings.
In summary, we have shown that COVID-19 in pregnancy during the 6 months after omicron was declared the variant of concern was associated with an increased risk of maternal morbidity and severe complications mostly among symptomatic and unvaccinated women and there was also an increased risk of pre-eclampsia among women with severe symptoms. As with previous variants, overweight women, or women with obesity, with severe symptoms were at the highest risk for morbidity and severe outcomes; vaccination had a reduced effect in preventing the disease among pregnant women, but it had a strong effect in preventing severe symptoms and complications including ICU admission and death, especially among women that were diagnosed with COVID-19. As it is very difficult to predict who is going to develop severe symptoms and complications with the exception of overweight women or women with obesity, universal vaccination is key during pregnancy. Vaccine effectiveness for severe presentation or complications of COVID-19 requires complete vaccination as a minimum, and preferably a booster as well. mRNA vaccines had the highest vaccine effectiveness for severe presentation or complications, but viral vector vaccines with a booster also provided adequate protection. Lastly, complete or booster mRNA and viral vector vaccines provide adequate protection for severe disease and complications of COVID-19 in pregnancy for at least 10 months after the last dose.
Data sharing
The study protocol and all data collection forms are available to all at https://intergrowth21.tghn.org/intercovid/. The anonymised database is only accessible to designated personnel as part of a data sharing agreement.
Declaration of interests
LS has been a consultant for Dilafor and Ferring Pharmaceuticals and has received payment in the past for presentations and educational events from Bayer, GlaxoSmithKline, Ferring Pharmaceuticals, and Sigvaris. BMdT received a research grant from the General Health Direction of Geneva, has participated on an advisory board of Effik and Pierre Favre, and has received medical equipment from Pregnolia, Hologic, and PeriLynx. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study did not receive funding, but the original INTERCOVID network was supported by the COVID-19 Research Response Fund from the University of Oxford (Ref 0009083). ATP is supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the National Institute for Health Research (NIHR) Biomedical Research Centre funding scheme. The funding organisations had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed herein are those of the authors and not necessarily those of the National Health Service, NIHR, Department of Health, or any of the other funders. We are very grateful to the contributing institutions and local researchers involved in the study. The appendix contains their details as well as details of the study committees. We acknowledge Ana Langer, Cesar Victora, and Jimena Villar de Onis for their contributions in the implementation and reporting of the study.
Contributors
JV, SHK, and ATP conceptualised and designed the INTERCOVID-2022 Study. JV, SHK, and ATP prepared the original protocol, with later input from AW and RC. JV, SHK, ATP, AW, and RC coordinated the project with invaluable input from BE, ZAB, and JGT. RBG, GBR, SR, JV, ATP, ML, RC, and AW did the data management and analysis. JGT and ATP did the ongoing literature review and interpretation of published data. The Principal Investigators at each study site implemented the protocol at their respective institutions. GBR, AW, and RC led the quality control of data. JV, SHK, and ATP wrote the manuscript with input from all coauthors. All coauthors read the manuscript and made suggestions on its content. JV, RBG, and ATP take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have seen and approved the final text. All authors had full access to all the data in the study, although not all authors will have accessed the totality of the data; rather, they will have accessed their own site data. All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allotey J, Chatterjee S, Kew T, et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strasser Z, Hadavand A, Murphy S, Estiri H. SARS-CoV-2 omicron variant is as deadly as previous waves after adjusting for vaccinations, demographics, and comorbidities. Research Square. 2022 https://assets.researchsquare.com/files/rs-1601788/v1_covered.pdf?c=1651763984 published online May 2. [Google Scholar]
- 5.Gisondi MA, Barber R, Faust JS, et al. A deadly infodemic: social media and the power of COVID-19 misinformation. J Med Internet Res. 2022;24 doi: 10.2196/35552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha YM, de Moura GA, Desidério GA, de Oliveira CH, Lourenço FD, de Figueiredo Nicolete LD. The impact of fake news on social media and its influence on health during the COVID-19 pandemic: a systematic review. J Public Health (Berl) 2021 doi: 10.1007/s10389-021-01658-z. https://doi.org/10.1007%2Fs10389-021-01658-z published online Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Office for National Statistics Coronavirus (COVID-19) latest insights: infections. Nov 4, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/infections
- 8.WHO WHO coronavirus (COVID-19) dashboard. 2022. https://covid19.who.int
- 9.Nuffield Department of Women's and Reproductive Health Oxford Maternal & Perinatal Health Institute (OMPHI) https://www.wrh.ox.ac.uk/research/omphi
- 10.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2014;44:641–648. doi: 10.1002/uog.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 12.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 13.Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289 e1–289e17. doi: 10.1016/j.ajog.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuenkitmongkol S, Solante R, Burhan E, et al. Expert review on global real-world vaccine effectiveness against SARS-CoV-2. Expert Rev Vaccines. 2022;21:1255–1268. doi: 10.1080/14760584.2022.2092472. [DOI] [PubMed] [Google Scholar]
- 15.Markov PV, Katzourakis A, Stilianakis NI. Antigenic evolution will lead to new SARS-CoV-2 variants with unpredictable severity. Nat Rev Microbiol. 2022;20:251–252. doi: 10.1038/s41579-022-00722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskenazi B, Rauch S, Iurlaro E, et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study. Am J Obstet Gynecol. 2022;227:74 e1–74 16. doi: 10.1016/j.ajog.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliani F, Oros D, Gunier RB, et al. Effects of prenatal exposure to maternal COVID-19 and perinatal care on neonatal outcome: results from the INTERCOVID Multinational Cohort Study. Am J Obstet Gynecol. 2022;227:488 e1–488 17. doi: 10.1016/j.ajog.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabore JW, Karamagi HC, Kipruto HK, et al. COVID-19 in the 47 countries of the WHO African region: a modelling analysis of past trends and future patterns. Lancet Glob Health. 2022;10:e1099–e1114. doi: 10.1016/S2214-109X(22)00233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guérin PJ, McLean ARD, Rashan S, et al. Definitions matter: heterogeneity of COVID-19 disease severity criteria and incomplete reporting compromise meta-analysis. medRxiv. 2021 doi: 10.1101/2021.06.04.21257852. published June 11. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahajan NN, Kesarwani S, Salunke C, et al. Clinical presentation, pregnancy complications, and outcomes of pregnant women with COVID-19 during the omicron-dominant third wave in Mumbai, India. Int J Gynaecol Obstet. 2022 doi: 10.1002/ijgo.14348. published online July 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mndala L, Monk EJM, Phiri D, et al. Comparison of maternal and neonatal outcomes of COVID-19 before and after SARS-CoV-2 omicron emergence in maternity facilities in Malawi (MATSurvey): data from a national maternal surveillance platform. Lancet Glob Health. 2022;10:e1623–e1631. doi: 10.1016/S2214-109X(22)00359-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoji K, Akiyama T, Tsuzuki S, et al. Comparison of the clinical characteristics and outcomes of COVID-19 in children before and after the emergence of Delta variant of concern in Japan. J Infect Chemother. 2022;28:591–594. doi: 10.1016/j.jiac.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1·1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378 doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seasely AR, Blanchard CT, Arora N, et al. Maternal and perinatal outcomes associated with the omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Obstet Gynecol. 2022;140:262–265. doi: 10.1097/AOG.0000000000004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birol Ilter P, Prasad S, Mutlu MA, et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during delta and omicron waves. Ultrasound Obstet Gynecol. 2022;60:96–102. doi: 10.1002/uog.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engjom HM, Ramakrishnan R, Vousden N, et al. Severity of maternal SARS-CoV-2 infection and perinatal outcomes during the omicron variant dominant period: UK Obstetric Surveillance System national cohort study. BMJ. 2022 doi: 10.1136/bmjmed-2022-000190. published online Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulersen M, Alvarez A, Rochelson B, Blitz MJ. Preterm birth and severe maternal morbidity associated with SARS-CoV-2 infection during the omicron wave. Am J Obstet Gynecol MFM. 2022;4 doi: 10.1016/j.ajogmf.2022.100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ACOG COVID-19 vaccine and pregnancy: conversation guide. 2020. http://www.cmsdocs.org/COVID19VaccineconversationguideACOG.pdf
- 29.US Food and Drug Administration (COVID-19) Update: FDA recommends inclusion of omicron BA.4/5 component for COVID-19 vaccine booster doses. June 30, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol and all data collection forms are available to all at https://intergrowth21.tghn.org/intercovid/. The anonymised database is only accessible to designated personnel as part of a data sharing agreement.