Abstract
Crop yield gains are needed to keep pace with a growing global population and decreasing resources to produce food. Cultivated emmer wheat is a progenitor of durum wheat and a useful source of genetic variation for trait improvement in durum. Here, we evaluated a recombinant inbred line population derived from a cross between the North Dakota durum wheat variety Divide and the cultivated emmer wheat accession PI 272527 consisting of 219 lines. The population was evaluated in 3 field environments and 2 greenhouse experiments to identify quantitative trait locus associated with 11 yield-related traits that were expressed in a consistent manner over multiple environments. We identified 27 quantitative trait locus expressed in at least 2 field environments, 17 of which were also expressed under greenhouse conditions. Seven quantitative trait locus regions on chromosomes 1B, 2A, 2B, 3A, 3B, 6A, and 7B had pleiotropic effects on multiple yield-related traits. The previously cloned genes Q and FT-B1, which are known to be associated with development and morphology, were found to consistently be associated with multiple traits across environments. PI 272527 contributed beneficial alleles for quantitative trait locus associated with multiple traits, especially for seed morphology quantitative trait locus on chromosomes 1B, 2B, and 6A. Three recombinant inbred lines with increased grain size and weight compared to Divide were identified and demonstrated the potential for improvement of durum wheat through deployment of beneficial alleles from the cultivated emmer parent. The findings from this study provide knowledge regarding stable and robust quantitative trait locus that breeders can use for improving yield in durum wheat.
Keywords: wheat, durum, emmer, yield, QTL, Plant Genetics and Genomics
Introduction
Wheat (Triticum ssp.) is one of the major global food crops, supplying approximately 20% of the calories in the average human’s diet. About 95% of wheat grown is hexaploid common (bread) wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD genomes), and tetraploid durum (macaroni) wheat (Triticum turgidum ssp. durum L., 2n = 4x = 28, AABB genomes) accounts for about 5%. Durum wheat is grown on approximately 16 million hectares worldwide and is used to make pasta and other semolina-based products (Arriagada et al. 2020). Due to the rapidly rising global population, wheat production and yields need to increase by upwards of 50% of current production by 2050 to meet expected demands (IWYP 2020). Rapid increases in wheat yields were observed in the mid to late 1900s during the green revolution (Hedden 2003). In the latter part of the 20th century, the increase in wheat yield per year due to genetic improvements was slight, with changing agronomic practices contributing more to yield gains. Genetic advancements in wheat partially lagged due to the complexity of the wheat genome and the ability of researchers to identify genes underlying wheat yield components (Brinton and Uauy 2019; Taagen et al. 2021).
Grain yield is a complex trait controlled by a multitude of genes and pathways (Cao et al. 2020). Final grain yield is determined by 3 main subcomponents: the number of spikes per unit area, the number of kernels per spike (KPS), and grain weight/size and shape (Gegas et al. 2010; Brinton and Uauy 2019; Cao et al. 2020). Each component can be further split into numerous subcomponents, which adds to the overall complexity of wheat yield. Also, many yield genes are present as homoeologs, which can also influence the phenotypic variation explained by individual loci (Borrill et al. 2019). In addition, the wheat genome is an allopolyploid and is comparably larger in size than most cultivated crop genomes (IWGSC 2018), requiring a larger number of markers to adequately cover the genome.
Recently, multiple reviews have been published on quantitative trait loci (QTLs) present in durum wheat (Arriagada et al. 2020; Colasuonno et al. 2021) along with reviews on yield component traits and pathways in tetraploid and hexaploid wheat (Nadolska-Orczyk et al. 2017; Brinton and Uauy 2019; Gauley and Boden 2019; Cao et al. 2020). However, most research to identify mechanisms governing wheat yield components has been in hexaploid wheat (Cao et al. 2020). In durum wheat, various quality, abiotic, and biotic stress QTL studies have been published, whereas relatively few have been published on mapping yield components (Arriagada et al. 2020; Colasuonno et al. 2021). Among the major yield components, grain morphology has traditionally been understudied due to the difficulty in measuring this trait (Gegas et al. 2010), and the majority of studies that have been conducted involved hexaploid wheat populations (Russo et al. 2014; Sun et al. 2020). Grain yield and grain size/shape components have been reported to be significantly correlated (Gegas et al. 2010; Russo et al. 2014; Sun et al. 2020; Corsi et al. 2021); therefore, breeding for increased grain size may increase grain yield.
Cultivated emmer, T. turgidum ssp. dicoccum (Schrank) Schübl (2n = 4x = 28, AABB), is the direct progenitor of durum wheat and is considered a minor crop globally (Zaharieva et al. 2010; Scott et al. 2019). Cultivated emmer was first domesticated in the Fertile Crescent (Faris 2014; Scott et al. 2019) from wild emmer [T. turgidum ssp. dicoccoides (Körn.) Thell (2n = 4x = 28, AABB)] due to mutations in the Br loci, which resulted in cultivated emmer having a nonbrittle rachis. However, the seed of cultivated emmer remained nonfree threshing and hulled (Faris 2014). Subsequently, durum arose from cultivated emmer through the acquisition of mutations in Q and the tenacious glume (Tg) genes, which resulted in free-threshing plants (Faris et al. 2014; Sharma, Running, et al. 2019).
Historically, genes from cultivated emmer have been deployed for disease resistance and stress tolerance (reviewed by Zaharieva et al. 2010; Mohler et al. 2013; Ellis et al. 2014), and emmer has also been shown to have genes for increased glutenin and decreased amylose content in the grain (Sun et al. 2004; Guzmán et al. 2011). A few studies have been published using bi-parental populations to map spike morphology and agronomic characters. Previously, durum × cultivated emmer populations were grown under greenhouse conditions by Faris et al. (2014) and Sharma, Running, et al. (2019) and under field conditions by Russo et al. (2014). Findings from these studies revealed some yield traits governed by QTLs derived from cultivated emmer, which indicated that cultivated emmer could be a promising resource for improving durum genetic diversity.
In this study, we evaluated a durum × cultivated emmer population derived from Divide × PI 272527, referred to as the DP527 population, under field and greenhouse conditions. Our 2 primary objectives were (1) to identify QTLs associated with 11 yield component traits in the DP527 population expressed in multiple field environments and (2) determine which of the consistent QTLs associated with yield component traits under field environments were also expressed under greenhouse conditions. The identification of consistent QTLs associated with yield components will benefit durum breeding programs in their endeavors to increase wheat yield. Knowing which of the consistent QTLs can also be evaluated under greenhouse conditions can help to expedite introgressions into adapted material, and it can also greatly help to expedite the identification of genes underlying the QTLs.
Materials and methods
Plant materials
The durum × cultivated emmer recombinant inbred line (RIL) population DP527 was evaluated for grain yield components under greenhouse and field conditions in North Dakota, USA. The DP527 population was developed by crossing Divide (PI 642021), a North Dakota hard amber durum variety (Elias and Manthey 2007), with PI 272527, a cultivated emmer accession collected near Pest, Hungary (Fig. 1). The DP527 population consisted of 219 RILs developed using the single-seed descent method to the F7 generation and bulked to produce F7:8 RILs. The DP527 population was originally developed to evaluate resistance to Fusarium head blight.
Fig. 1.

Spike and seed morphology of the durum variety Divide and the cultivated emmer wheat accession PI 272527, the 2 parental lines of the Divide × PI 272527 (DP527) population. a) Mature spikes of Divide (left) and PI 272527 (right). b) Seed of Divide (top) and PI 272527 (bottom).
Phenotyping
The DP527 population and parental lines were evaluated under field conditions in a total of 3 seasons and were grown in a randomized complete block design with 3 replicates each season. Plants were grown in hill plots, with each plot consisting of 10–15 seeds and considered an experimental unit. The 2017 and 2019 plots were grown at the North Dakota State University (NDSU) field site near Prosper, ND (47.002°N, 97.115°W). The 2020 plots were grown at the NDSU agronomy seed farm near Casselton, ND (46.880°N, 97.243°W).
The DP527 population and parental lines were phenotyped for 11 traits including days to heading (DTH), plant height (PHT), total number of spikelets per spike (SPS), KPS, grain weight per spike (GWS), thousand kernel weight (TKW), kernel area (KA), kernel width (KW), kernel length (KL), kernel circularity (KC), and kernel length:width ratio (KLW). DTH was measured as the number of days from planting until 50% of the spikes emerged completely beyond the flag leaf. PHT was measured from the base of the hill plot to the tip of the highest spike (excluding awns) in the plot in centimeters. Eight heads were used for phenotypic evaluations. SPS was counted as the total number of spikelets per head. KPS, GWS, TKW, KA, KW, KL, KC, and KLW data were obtained using a MARVIN grain analyzer (GAT Sensorik GMBH, Neubrandenburg, Germany). KPS and GWS data from the MARVIN were divided by the number of heads in the sample to obtain an average per wheat head. For the 2019 environment, planting occurred in late May, and by early September about one-third of the lines were not mature. Therefore, only DTH, PHT, and SPS were evaluated in the 2019 field season.
The DP527 population and parents were evaluated under greenhouse conditions in 2 greenhouse seasons (2018 and 2019) with 2 replicates per season. Plants were grown in 15-cm diameter pots in a greenhouse with 16-h photoperiod and a temperature of 21°C. All plants were grown in a completely randomized design with 1 plant per pot, which was 1 experimental unit. DTH was measured as the number of days from planting until the emergence of the first spike beyond the flag leaf, and PHT was measured from the base of the plant to the tip of the highest spike in centimeters. Plants were hand harvested and 4 heads per plant were used for the rest of the phenotypic evaluations, which were measured as described for field environments.
Genotyping and linkage mapping
DNA of the DP527 population along with the parental lines was extracted using the methods described in Sharma, Running, et al. (2019). The DP527 population along with the parental lines was genotyped using the iSelect 90k wheat SNP array (Wang et al. 2014). The genotyping assay was carried out using Illumina’s Infinium assay following the manufacture’s protocols. SNP clustering and genotyping calling were performed using Illumina’s GenomeStudio software v.2011.1. The genotype callings were manually inspected to correct cluster shifts due to copy number differences and to ensure call accuracy for every SNP.
The Q gene functional marker Xfcp650(Q), a simple sequence repeat (SSR) marker designed by Simons et al. (2006), was used to map the Q gene on chromosome 5A. The primer sequences for the FCP650 set are 5′-GCACTAGCTAATTCAGTGGTTAGATTTGCTCA-3′ and 5′-ATTCAGTGGTAGCAACAGTTTCAGTAAGCTGG-3′, and an annealing temperature of 65°C was used.
Linkage maps were assembled using MapDisto 1.7.7.0.1.1 (Lorieux 2012). Markers were first organized into groups using the “find groups” command with a minimum LOD = 3.0 and a maximum theta of 0.30. The “order” sequence command was used to establish the initial order of markers within a linkage group. Subsequent interrogation of the sequence using the “check inversions,” “ripple order,” and “drop locus” commands was conducted to determine the best map. Map distances were calculated using the Kosambi mapping function (Kosambi 1943).
Statistical analysis and QTL mapping
Statistical analysis was performed using the PROC GLM procedure in SAS 9.4 (SAS institute, Cary, NC, USA). Fisher’s least significant difference (LSD) test was used to determine significant differences among the RILs, with α = 0.05. For each field season, Bartlett’s Chi-squared test for homogeneity of error variances (Snedecor and Cochran 1989) was used to determine if replicates within the same environment could be combined (Supplementary Tables 1 and 2). For those traits that were not normally distributed, Levene’s test (Levene 1960) was used instead. For the greenhouse data, the same homogeneity tests were run with the 4 replicates across the 2 environments together to determine if the 2 greenhouse experiments could be combined for each trait. If the 2 greenhouse experiments could not be combined, the homogeneity tests were run on the 2 replicates of each experiment separately to determine if those could be combined for further analysis. For those that could be combined, the scores of each replicate for those traits were used to calculate the overall mean across all environments, which was used in further analyses and QTL mapping. For field data, TKW 2020 replicates were not homogeneous using both Bartlett’s and Levene’s, and DTH and GWS could not be combined across GH seasons; therefore, each environment for these traits was analyzed separately. Trait mean, maximum, minimum, and correlations were calculated in R v4.0.3, with Pearson correlation coefficients calculated using the R command cor (R Development Core Team) and plotted using R/corrplot (Wei and Simko 2017).
QTL analysis was performed using R/qtl (Broman et al. 2003). For simple interval mapping, significant QTLs were identified using the function “scanone” with the extended Haley–Knott method (Haley and Knott 1992). An LOD significance threshold was determined using a permutation test with 1,000 interactions. “Scantwo” with the extended Haley–Knott method was used to identify QTL × QTL interactions and an LOD significance threshold was determined using a permutation test with 1,000 interactions. Multiple QTL mapping was performed using the stepwiseqtl command (Manichaikul et al. 2009) using method=imp (Sen and Churchill 2001). A forward/backward search method was used, with a maximum of 12 QTLs allowed. The initial model was given based on the “scanone” QTL results. An approximate Bayesian credible interval was calculated using “Bayesint” with a probability of 0.99 (Broman et al. 2003). QTL names include the trait abbreviation followed by “fcu,” the laboratory designation for J. Faris.
Markers that were significantly associated with each QTL were subjected to BLASTn searches against Svevo RefSeq Rel. 1.0 pseudomolecules (Maccaferri et al. 2019), Zavitan WEWSeq v2.0 pseudomolecules (Zhu et al. 2019), and the Chinese Spring IWGSC RefSeq v2.1 genome assembly (Zhu et al. 2021) using the Graingenes website (https://wheat.pw.usda.gov/GG3/) to obtain the physical positions for comparing QTLs between environments, along with identifying previously reported genes that may reside within each QTL region. The sequence for reported genes was obtained from Genbank or the gene cloning papers, which are cited in Supplementary File 2.
Results
Map construction
The DP527 population map was constructed using the 90K iSelect polymorphic SNP results and the SSR marker Xfcp650(Q) (Table 1). The final map consisted of 10,486 markers and was assembled into 14 linkage groups representing each of the 14 durum wheat chromosomes (Supplementary File 1). The average number of markers per chromosome was 749, with a range of 466 markers on chromosome 5A to 1,188 markers on chromosome 1B (Table 1). The average chromosome map length was 174.31 cM, with chromosome 6A being the shortest at 129.23 cM in length and chromosome 5A was the longest at 226.73 cM. Density ranged from 2.06 to 6.79 markers/cM among linkage groups, with a genome-wide density of 4.30 markers/cM. The B genome had a higher marker density than the A genome. A total of 3,519 (33.56%) markers had segregation ratios that significantly (P < 0.05) deviated from the expected 1:1 ratio. The percentage of markers with distorted segregations varied, ranging from 0.19 on chromosome 4B to 79.04 on chromosome 1B.
Table 1.
Chromosome assignment and distribution of markers, length of chromosome linkage groups, and marker density of maps generated in the Divide × PI 272527 (DP527) recombinant inbred population.
| Chromosome | SSR | SNP | Total markers | Loci | Length (cM) | Markers (cM) | % distorted markers |
|---|---|---|---|---|---|---|---|
| 1A | 0 | 658 | 658 | 137 | 163.68 | 4.02 | 27.20 |
| 1B | 0 | 1,188 | 1,188 | 181 | 166.03 | 7.16 | 79.04 |
| 2A | 0 | 699 | 699 | 120 | 187.95 | 3.72 | 46.49 |
| 2B | 0 | 1,107 | 1,107 | 220 | 188.00 | 5.89 | 56.37 |
| 3A | 0 | 637 | 637 | 163 | 196.38 | 3.24 | 7.38 |
| 3B | 0 | 923 | 923 | 215 | 169.11 | 5.46 | 17.01 |
| 4A | 0 | 554 | 554 | 126 | 173.57 | 3.19 | 55.78 |
| 4B | 0 | 523 | 523 | 118 | 141.72 | 3.69 | 0.19 |
| 5A | 1 | 465 | 466 | 144 | 226.73 | 2.06 | 41.20 |
| 5B | 0 | 771 | 771 | 173 | 192.16 | 4.01 | 9.60 |
| 6A | 0 | 621 | 621 | 133 | 129.23 | 4.81 | 43.00 |
| 6B | 0 | 964 | 964 | 170 | 141.94 | 6.79 | 17.22 |
| 7A | 0 | 680 | 680 | 176 | 195.50 | 3.48 | 7.94 |
| 7B | 0 | 695 | 695 | 137 | 168.29 | 4.13 | 26.62 |
| A genome | 1 | 4,314 | 4,315 | 999 | 1,273.04 | 3.39 | 31.82 |
| B genome | 0 | 6,171 | 6,171 | 1,214 | 1,167.25 | 5.29 | 34.78 |
| Total | 1 | 10,485 | 10,486 | 2,213 | 2,440.29 | 4.30 | 33.56 |
Trait evaluations
Under greenhouse conditions, we observed no significant differences in KPS, GWS, TKW, and KW between Divide and PI 272527; however, we did observe significant differences among the RILs, indicating that different genes were controlling these traits (Table 2). PI 272527 had increased DTH, PHT, SPS, KA, KL, KC, and KLW compared to Divide. Transgressive segregation was observed for all other traits as well (Supplementary Fig. 1), indicating that numerous genes were segregating for each trait.
Table 2.
Parental and population means, ranges, and least significant differences (LSD) at the 0.05 level of probability (P < 0.05) for the Divide × PI 272527 (DP527) population grown under greenhouse conditions.
| Traita | Mean | Population range | LSD (0.05) | ||
|---|---|---|---|---|---|
| Divide | PI 272527 | Population | |||
| DTH18b | 62.50 | 87.00 | 70.31 | 54.50–95.50 | 9.85 |
| DTH19b | 52.00 | 82.50 | 59.63 | 39.00–83.00 | 14.06 |
| PHT | 87.25 | 152.5 | 112.98 | 56.25–154.00 | 27.40 |
| SPS | 17.06 | 31.00 | 22.89 | 14.75–28.56 | 4.25 |
| KPS | 33.38 | 33.25 | 30.19 | 4.19–45.81 | 12.67 |
| GWS18b | 2.25 | 1.78 | 1.74 | 0.10–2.57 | 0.75 |
| GWS19b | 0.71 | 1.20 | 1.00 | 0.06–1.96 | 0.71 |
| TKW | 40.89 | 44.53 | 43.90 | 27.01–60.46 | 11.85 |
| KA | 18.07 | 21.16 | 19.49 | 15.22–23.82 | 2.96 |
| KW | 3.39 | 3.44 | 2.31 | 1.49–3.80 | 0.31 |
| KL | 7.32 | 9.01 | 8.05 | 6.87–9.55 | 0.66 |
| KC | 1.46 | 1.72 | 1.56 | 1.40–1.76 | 0.07 |
| KLW | 2.18 | 2.64 | 2.40 | 2.03–2.82 | 0.14 |
Trait abbreviations are: days to heading (DTH), plant height (PHT), spikelets per spike (SPS), kernels per spike (KPS), grain weight per spike (GWS), thousand kernel weight (TKW), kernel area (KA), kernel width (KW), kernel length (KL), kernel circularity (KC), and kernel length:width ratio (KLW). PHT was measured in cm, GWS and TKW in g, KA in mm2, and KW and KL in mm.
Replicates across environments were not statistically homogeneous and therefore each environment was analyzed separately.
Under field conditions, Divide had increased KPS compared to PI 272527, whereas PI 272527 had increased DTH, PHT, SPS, TKW, KA, KL, KC, and KLW (Table 3). As with the data collected under greenhouse environments, transgressive segregation was observed for the field environments as well (Supplementary Fig. 2).
Table 3.
Parental and population means, ranges, and LSD at the 0.05 level of probability (P < 0.05) for the Divide × PI 272527 (DP527) population grown under field conditions.
| Traita | Yearb | Mean | Population range | LSD (0.05) | ||
|---|---|---|---|---|---|---|
| Divide | PI 272527 | Population | ||||
| DTH | 2017 | 57.00 | 69.67 | 62.56 | 55.33–71.67 | 3.38 |
| DTH | 2019 | 63.33 | 94.50 | 65.98 | 49.33–91.00 | 6.86 |
| DTH | 2020 | 53.00 | 71.33 | 57.09 | 47.33–75.00 | 4.33 |
| PHT | 2017 | 90.67 | 129.00 | 110.36 | 73.33–150.33 | 7.41 |
| PHT | 2019 | 119.33 | 144.00 | 122.32 | 88.00–157.33 | 14.83 |
| PHT | 2020 | 82.00 | 138.00 | 105.88 | 70.00–138.00 | 11.23 |
| SPS | 2017 | 15.38 | 24.04 | 19.04 | 13.50–25.83 | 2.63 |
| SPS | 2019 | 18.92 | 24.13 | 21.31 | 16.71–25.63 | 2.02 |
| SPS | 2020 | 18.71 | 25.23 | 22.22 | 17.25–27.29 | 2.09 |
| KPS | 2017 | 40.67 | 34.63 | 33.44 | 15.92–52.75 | 6.01 |
| KPS | 2020 | 39.08 | 23.56 | 31.02 | 15.67–43.54 | 5.95 |
| GWS | 2017 | 1.77 | 2.00 | 1.70 | 0.73–2.49 | 0.32 |
| GWS | 2020 | 1.36 | 1.02 | 1.20 | 0.58–1.83 | 0.34 |
| TKW | 2017 | 45.31 | 59.72 | 51.12 | 40.23–70.36 | 5.87 |
| TKW | 2020 | 34.82 | 43.52 | 38.39 | 13.28–53.47 | 7.60 |
| KA | 2017 | 18.65 | 24.06 | 21.94 | 18.30–25.40 | 1.16 |
| KA | 2020 | 17.54 | 19.83 | 19.11 | 15.65–22.70 | 1.59 |
| KW | 2017 | 3.24 | 3.48 | 3.45 | 3.00–3.80 | 0.12 |
| KW | 2020 | 3.17 | 3.26 | 3.23 | 2.85–3.76 | 0.17 |
| KL | 2017 | 7.82 | 9.82 | 8.79 | 7.70–9.90 | 0.22 |
| KL | 2020 | 7.61 | 8.66 | 8.27 | 7.36–9.44 | 0.33 |
| KC | 2017 | 1.54 | 1.75 | 1.61 | 1.50–1.80 | 0.05 |
| KC | 2020 | 1.57 | 1.70 | 1.65 | 1.45–1.85 | 0.06 |
| KLW | 2017 | 2.43 | 2.83 | 2.57 | 2.20–3.00 | 0.08 |
| KLW | 2020 | 2.42 | 2.67 | 2.58 | 2.10–3.04 | 0.10 |
Trait abbreviations are: days to heading (DTH), plant height (PHT), spikelets per spike (SPS), kernels per spike (KPS), grain weight per spike (GWS), thousand kernel weight (TKW), kernel area (KA), kernel width (KW), kernel length (KL), kernel circularity (KC), and kernel length:width ratio (KLW). PHT was measured in cm, GWS and TKW in g, KA in mm2, and KW and KL in mm.
Each field environment (year) was analyzed separately.
Correlations
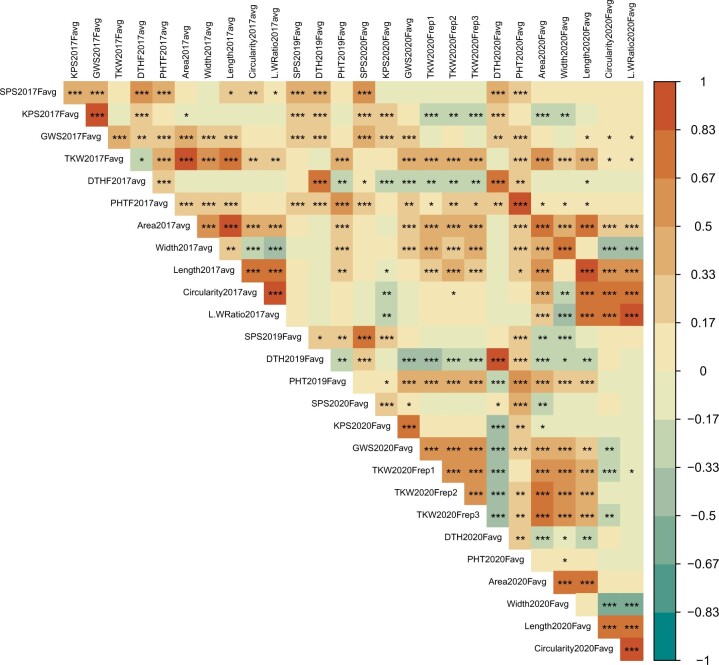
Detailed correlation results are presented in Supplementary Tables 3–6 and Figs. 2 and 3. We observed the same general trend under greenhouse and field conditions, in which traits that had a positive correlation with one another under 1 environment in most cases had a positive correlation in the other environment. As the number of SPS increased, so did the number of KPS and GWS. KPS and GWS were also positively correlated with one another. As kernel weight increased, measured as TKW, so did the overall spike weight, measured as GWS. The kernel size traits KA and KW were positively correlated with the kernel weight traits GWS and TKW. Additional positive correlations we observed were KA with KW and KC with KLW.
Fig. 2.
Pearson correlation coefficients between the 11 traits measured in the Divide × PI 272527 (DP527) population grown under greenhouse conditions. Trait abbreviations are: days to heading (DTH), plant height (PHT), spikelets per spike (SPS), kernels per spike (KPS), grain weight per spike (GWS), thousand kernel weight (TKW), kernel area (KA), kernel width (KW), kernel length (KL), kernel circularity (KC), and kernel length:width ratio (KLW). For DTH and GWS, the replicates across environments were not statistically homogeneous but replicates in each environment were homogenous and therefore were combined within that environment for analysis. Along the right is a color scale for the correlation values. Blocks that are tan to blue have a negative correlation, with dark blue being a correlation close to −1. Blocks that are tan to orange have a positive correlation, with orange being a correlation close to 1. Significance values are denoted as *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 3.
Pearson correlation coefficients between the 11 traits measured in the Divide × PI 272527 (DP527) population grown under field conditions in 2017, 2019, and 2020. Trait abbreviations are: days to heading (DTH), plant height (PHT), spikelets per spike (SPS), kernels per spike (KPS), grain weight per spike (GWS), thousand kernel weight (TKW), kernel area (KA), kernel width (KW), kernel length (KL), kernel circularity (KC), and kernel length:width ratio (KLW). For TKW 2020, the replicates were not combined because they were not statistically homogeneous. For 2019, only the traits SPS, DTH, and PHT were evaluated. Along the right is a color scale for the correlation values. Blocks that are tan to blue have a negative correlation, with blue being a correlation close to −1. Blocks that are tan to orange have a positive correlation, with orange being a correlation close to 1. Significance values are denoted as *P < 0.05, **P < 0.01, ***P < 0.001.
Some correlation trends differed between greenhouse and field experiments. Under field conditions, as the number of KPS increased, this often resulted in smaller KA, but the correlation with individual kernel weight (TKW) was generally neutral (Supplementary Tables 4 and 6 and Fig. 3). In addition, KW generally had a negative correlation with the kernel dimension traits KC and KLW. These correlations differed under greenhouse conditions where KPS was positively correlated with KW, and TKW was positively correlated with KA and KW (Supplementary Table 3 and Fig. 2). KC and KLW had no correlation with GWS, TKW, and KW under greenhouse conditions, but a positive correlation was observed between KC and KLW with KA.
It is interesting to note that, under both greenhouse and field conditions, PH was positively correlated with most of the yield traits evaluated (Supplementary Tables 3, 4, and 6 and Figs. 2 and 3). Also, with the exception of SPS, DTH tended to be negatively correlated with the yield traits. Therefore, the taller, early heading plants tended to have better yield.
QTL analysis under greenhouse and field conditions
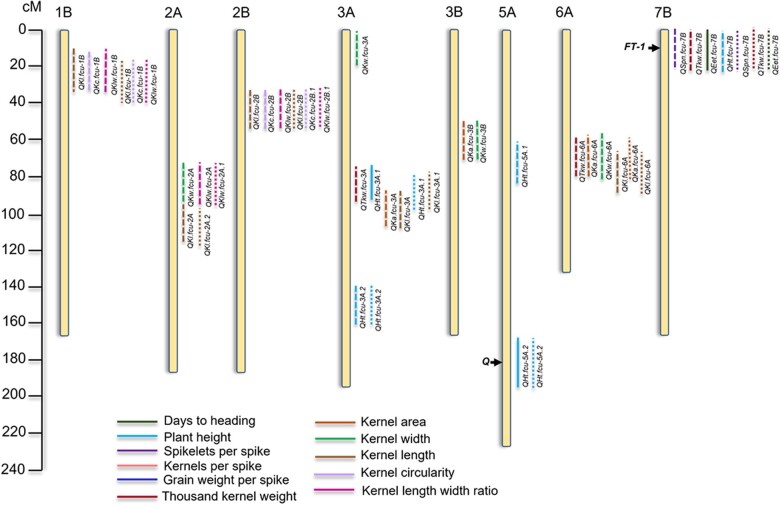
In the greenhouse experiments, we identified a total of 67 significant QTLs and QTL×QTL interactions (Supplementary File 2). The main purpose for evaluating yield traits under greenhouse conditions was to identify field-expressed QTLs that were also expressed under greenhouse conditions. Therefore, only QTLs identified under greenhouse conditions that were also identified under 2 or more field environments are considered below and presented in Table 4 and Fig. 4. Of the 67 total QTLs identified under greenhouse conditions, 17 were also identified under 2 or more field environments.
Table 4.
QTLs associated with the traits evaluated in the Divide × PI 272527 (DP527) recombinant inbred population grown under greenhouse conditions that were also observed in 2 or more field environments.
| Trait | QTL | Chromosome | Position (cM) | Peak marker | LOD | R 2 × 100 | Donor parent for beneficial allele | Putative gene |
|---|---|---|---|---|---|---|---|---|
| SPS | QSpn.fcu-7B | 7B | 10.57 | IWB3164 | 14.20 | 19.58 | PI 272527 | FT-B1 |
| TKW | QTkw.fcu-7B | 7B | 12.49 | IWB11170 | 3.92 | 7.30 | Divide | FT-B1 |
| DTH | QEet.fcu-7B | 7B | 10.57–12.49 | IWB3164-IWB11170 | 15.25–20.65 | 23.08–27.37 | Divide | FT-B1 |
| PHT | QHt.fcu-3A.1 | 3A | 89.34 | IWB65564 | 20.25 | 22.20 | Divide | |
| PHT | QHt.fcu-3A.2 | 3A | 150.09 | IWB16621 | 6.69 | 6.31 | Divide | |
| PHT | QHt.fcu-5A.2 | 5A | 178.53 | Xfcp650 | 22.53 | 25.36 | Divide | Q |
| KA | QKa.fcu-6A | 6A | 68.75 | IWB8079 | 5.23 | 8.42 | PI 272527 | |
| KL | QKl.fcu-1B | 1B | 29.85 | IWB27824 | 5.02 | 4.37 | PI 272527 | |
| KL | QKl.fcu-2A | 2A | 110.72 | IWB72154 | 10.86 | 10.06 | PI 272527 | |
| KL | QKl.fcu-2B | 2B | 43.66 | IWB2317 | 12.47 | 11.76 | PI 272527 | |
| KL | QKl.fcu-3A | 3A | 87.47 | IWB74967 | 4.99 | 4.34 | PI 272527 | |
| KL | QKl.fcu-6A | 6A | 72.56 | IWB7281 | 7.51 | 6.70 | PI 272527 | |
| KC | QKc.fcu-1B | 1B | 31.95 | IWB7813 | 10.20 | 10.55 | PI 272527 | |
| KC | QKc.fcu-2B | 2B | 45.76 | IWB8355 | 7.16 | 7.16 | PI 272527 | |
| KLW | QKlw.fcu-1B | 1B | 30.55 | IWB60559 | 10.47 | 13.20 | PI 272527 | |
| KLW | QKlw.fcu-2A | 2A | 83.82 | IWB62849 | 13.79 | 18.03 | Divide | |
| KLW | QKlw.fcu-2B | 2B | 45.76 | IWB8355 | 7.24 | 8.81 | PI 272527 |
Fig. 4.
Illustration of the chromosomal locations of the QTLs associated with the eleven traits evaluated in the Divide × PI 272527 (DP527) recombinant inbred line population under field and greenhouse conditions. Under field conditions, only those QTLs that were present in 2 or more are shown and for those identified under greenhouse conditions, only those which were also present in the field are illustrated. A total of 27 QTLs are shown, which were identified under field conditions. Those observed in 2 environments are illustrated with dashed lines and those in 3 with solid lines. A total of 17 QTLs associated with greenhouse conditions are illustrated with dotted lines. The known positions of the Q and FT-1 loci are indicated in black. Chromosomes 1A, 4A, 4B, 5B, 6B, and 7A are not shown because no QTL consistently expressed over multiple environments were detected on them.
A total of 108 QTLs were identified among the 3 field environments. Within individual field seasons, 54, 10, and 44 QTLs were identified in 2017, 2019, and 2020, respectively (Supplementary File 2). Fewer QTLs were observed in the 2019 field season because only traits SPS, DTH, and PHT were measured. A total of 24 QTLs were observed in 2 field environments, and 3 were observed in all 3 field environments (Table 5, Fig. 4, and Supplementary File 2). QTLs that were observed in all field environments for which the trait was evaluated and also observed under greenhouse conditions were considered stable QTLs. A total of 11 genomic regions were associated with the 27 QTLs that were observed in multiple field environments. Of these 27 QTLs, 17 were associated with kernel dimension traits. No QTLs for KPS or GWS and no QTL × QTL interactions were observed across multiple environments and therefore will not be discussed.
Table 5.
QTLs associated with the traits evaluated in the Divide × PI 272527 (DP527) recombinant inbred population grown under field conditions and were present in 2 or more environments.
| Trait | QTL | Number of environments | Chromosome | Position (cM) | Peak marker | LOD | R 2 × 100 | Donor parent for beneficial allele | Putative gene |
|---|---|---|---|---|---|---|---|---|---|
| SPS | QSpn.fcu-7B | 3 | 7B | 10.57 | IWB3164 | 4.62–5.19 | 8.37–9.34 | PI 272527 | FT-B1 |
| TKW | QTkw.fcu-3A | 2 | 3A | 78.16–87.47 | IWB49380-IWB74967 | 4.35–7.33 | 7.51–13.49 | PI 272527 | |
| TKW | QTkw.fcu-6A | 2 | 6A | 65.60–71.15 | IWB30925-IWB9600 | 3.52–4.73 | 7.24–7.75 | PI 272527 | GRF4 |
| TKW | QTkw.fcu-7B | 2 | 7B | 4.87–12.49 | IWB70086-IWB11170 | 4.39–6.29 | 7.58–11.45 | Divide | FT-B1 |
| DTH | QEet.fcu-7B | 3 | 7B | 10.57 | IWB3164 | 21.32–34.93 | 23.79–49.64 | Divide | FT-B1 |
| PHT | QHt.fcu-3A.1 | 3 | 3A | 88.88–89.34 | IWB2704-IWB65564 | 10.30–23.43 | 18.30–26.09 | Divide | |
| PHT | QHt.fcu-3A.2 | 2 | 3A | 141.22–144.39 | IWB64845-IWB41859 | 3.71–6.14 | 3.38–4.85 | Divide | |
| PHT | QHt.fcu-5A.1 | 2 | 5A | 75.45–81.01 | IWB66908-IWB6959 | 4.76–7.36 | 4.39–5.90 | Divide | |
| PHT | QHt.fcu-5A.2 | 3 | 5A | 178.53 | Xfcp650 | 5.04–11.25 | 8.44–10.68 | Divide | Q |
| PHT | QHt.fcu-7B | 2 | 7B | 10.57 | IWB3164 | 3.68–7.73 | 3.36–6.22 | Divide | FT-B1 |
| KA | QKa.fcu-3A | 2 | 3A | 87.47–88.88 | IWB74967-IWB2704 | 7.24–8.44 | 9.91–10.33 | PI 272527 | |
| KA | QKa.fcu-3B | 2 | 3B | 60.67–61.84 | IWB76128-IWB13416 | 4.24–4.58 | 4.95–6.08 | Divide | |
| KA | QKa.fcu-6A | 2 | 6A | 65.84–66.55 | IWB33567-IWB81565 | 7.51–11.88 | 10.30–15.10 | PI 272527 | |
| KW | QKw.fcu-2A | 2 | 2A | 83.82 | IWB62849 | 3.81–6.11 | 4.29–8.52 | PI 272527 | |
| KW | QKw.fcu-3A | 2 | 3A | 8.20–8.89 | IWB63094-IWB46311 | 3.66–4.12 | 4.11–5.61 | PI 272527 | |
| KW | QKw.fcu-3B | 2 | 3B | 60.67 | IWB76128 | 9.62–11.86 | 13.92–14.58 | PI 272527 | |
| KW | QKw.fcu-6A | 2 | 6A | 63.95 | IWB66345 | 5.68–6.44 | 6.52–9.01 | PI 272527 | |
| KL | QKl.fcu-1B | 2 | 1B | 23.01–24.93 | IWB72705-IWB21571 | 6.03–9.58 | 7.72–10.21 | PI 272527 | |
| KL | QKl.fcu-2A | 2 | 2A | 102.54–110.72 | IWB35671-IWB72154 | 4.54–5.58 | 4.57–5.76 | PI 272527 | |
| KL | QKl.fcu-2B | 2 | 2B | 44.83–45.52 | IWB77048-IWB76653 | 7.39–12.42 | 9.59–13.65 | PI 272527 | |
| KL | QKl.fcu-3A | 2 | 3A | 80.23–87.47 | IWB38468-IWB74967 | 6.70–7.27 | 7.55–8.64 | PI 272527 | |
| KL | QKl.fcu-6A | 2 | 6A | 72.33–75.17 | IWB6286-IWB78960 | 5.61–11.41 | 7.15–12.40 | PI 272527 | |
| KC | QKc.fcu-1B | 2 | 1B | 24.93–27.77 | IWB21571-IWB16228 | 8.22–10.17 | 9.50–16.93 | PI 272527 | |
| KC | QKc.fcu-2B | 2 | 2B | 45.29 | IWB70916 | 5.02–10.11 | 7.90–11.93 | PI 272527 | |
| KLW | QKlw.fcu-1B | 2 | 1B | 24.93–30.55 | IWB21571-IWB60559 | 7.81–10.36 | 9.27–13.48 | PI 272527 | |
| KLW | QKlw.fcu-2A | 2 | 2A | 83.82 | IWB62849 | 7.24–11.45 | 9.09–14.15 | Divide | |
| KLW | QKlw.fcu-2B | 2 | 2B | 44.83–45.52 | IWB77048-IWB76653 | 7.88–9.56 | 9.98–11.58 | PI 272527 |
Only 1 QTL for SPS was observed in multiple field seasons. QSpn.fcu-7B, which mapped on chromosome 7B, was observed in all 3 field seasons and also under greenhouse conditions making it a stable QTL (Tables 4 and 5 and Fig. 4). QSpn.fcu-7B was within the genomic region known to harbor the Flowering Locus T1 (FT-B1) gene, which plays a critical role in the regulation of flowering (Yan et al. 2006). QSpn.fcu-7B explained up to 19.58% and 9.34% of the variation in SPS under greenhouse and field conditions, respectively, and PI 272527 alleles at QSpn.fcu-7B led to increased SPS.
For TKW, 3 QTLs were identified under field conditions, designated QTkw.fcu-3A, QTkw.fcu-6A, and QTkw.fcu-7B (Tables 4 and 5 and Fig. 4). QTkw.fcu-7B was observed in both field seasons and the greenhouse making it a stable QTL, whereas QTkw.fcu-3A and QTkw.fcu-6A were observed in both field seasons but not the greenhouse. QTkw.fcu-6A was within the genomic region known to harbor the Growth-Regulating Factor 4 (GRF4-A), which has been shown to be associated with grain weight (Avni et al. 2018) and QTkw.fcu-7B mapped near FT-B1. QTkw.fcu-3A and QTkw.fcu-6A explained as much as 13.49% and 7.75% of the variation in TKW, respectively, and QTkw.fcu-7B explained up to 11.45% and 7.30% of the variation in TKW under field and greenhouse conditions, respectively. PI 272527 contributed the alleles for increased TKW at QTkw.fcu-3A and QTkw.fcu-6A and Divide was the donor parent at QTkw.fcu-7B.
As with SPS and TKW, a QTL within the FT-B1 region on chromosome 7B was associated with DTH (Tables 4 and 5 and Fig. 4). This QTL, designated QEet.fcu-7B, was observed under all 3 field environments and the greenhouse making it a stable QTL, and it explained up to 49.64% of the variation. PI 272527 alleles at QEet.fcu-7B resulted in increased DTH.
A total of 5 consistent QTLs were associated with PHT in the DP527 population. These QTLs, designated QHt.fcu-3A.1, QHt.fcu-3A.2, QHt.fcu-5A.1, QHt.fcu-5A.2, and QHt.fcu-7B were mapped to chromosomes 3A, 5A, and 7B (Table 5 and Fig. 4). Three of these QTLs (QHt.fcu-3A.1, QHt.fcu-3A.2, and QHt.fcu-5A.2) were also observed under greenhouse conditions (Table 4 and Fig. 4). QHt.fcu-3A.1 and QHt.fcu-5A.2 were observed in all 3 field environments making them stable QTLs, whereas the others were observed in only 2 environments. QHt.fcu-5A.2 and QHt.fcu-7B were located near the known genes Q and FT-B1. Under field conditions, QHt.fcu-3A.1, QHt.fcu-3A.2, QHt.fcu-5A.1, QHt.fcu-5A.2, and QHt.fcu-7B explained up to 26.09%, 4.85%, 10.68%, 5.90%, and 6.22% of the variation in PHT, respectively. As for those QTLs under greenhouse conditions, QHt.fcu-3A.1, QHt.fcu-3A.2, and QHt.fcu-5A.2 explained up to 22.20%, 6.31%, and 25.36% of the variation in PHT, respectively. PI 272527 alleles at each QTL led to taller plants.
Three QTLs were identified for KA in both field environments. These QTLs were on chromosomes 3A, 3B, and 6A and designated QKa.fcu-3A, QKa.fcu-3B, and QKa.fcu-6A, respectively (Table 5 and Fig. 4). QKa.fcu-6A was a stable QTL because it was also observed under greenhouse conditions where it explained 8.42% of the variation in KA (Table 4). Under field conditions, QKa.fcu-3A, QKa.fcu-3B, and QKa.fcu-6A explained up to 10.33%, 6.08%, and 15.10% of the variation in KA, respectively. Divide alleles contributed increased KA at QKa.fcu-3B whereas PI 272527 alleles increased KA at QKa.fcu-3A and QKa.fcu-6A.
For KW, 4 QTLs were identified on chromosomes 2A, 3A, 3B, and 6A in both field environments (Table 5 and Fig. 4). However, none of these 4 QTLs were observed under greenhouse conditions and therefore none were considered as stable QTLs. These QTLs, designated QKw.fcu-2A, QKw.fcu-3A, QKw.fcu-3B, and QKw.fcu-6A explained up to 8.52%, 5.61%, 14.58%, and 9.01% of the variation in KW, respectively. PI 272527 alleles at all for loci contributed to increased KW.
Five QTLs were identified for KL, and because all 5 were observed in both field environments as well as under greenhouse conditions, all 5 were considered stable QTLs (Tables 4 and 5 and Fig. 4). Under greenhouse conditions, QKl.fcu-1B, QKl.fcu-2A, QKl.fcu-2B, QKl.fcu-3A, and QKl.fcu-6A explained 4.37%, 10.06%, 11.76%, 4.34%, and 6.70% of the variation in KL, respectively (Table 4). Under field conditions, these QTLs explained up to 10.21%, 5.76%, 13.65%, 8.64%, and 12.40% of the variation in KL, respectively (Table 5). At all 5 QTLs, the presence of PI 272527 alleles led to longer kernels.
Two stable QTLs for KC were identified in both field environments and under greenhouse conditions (Tables 4 and 5 and Fig. 4). The QTL designated QKc.fcu-1B explained up to 16.93% and 10.55%, and the second QTL, designated QKc.fcu-2B, explained up to 11.93% and 7.16% of the variation in KC under field and greenhouse conditions, respectively. PI 272527 contributed alleles for increased KC at both QTLs.
The 3 QTLs that were observed in both field environments for KLW were also identified under greenhouse conditions making them all considered as stable QTLs (Tables 4 and 5 and Fig. 4). Under field conditions, QKlw.fcu-1B, QKlw.fcu-2A, and QKlw.fcu-2B explained as much as 13.48%, 14.15%, and 11.58% of the variation in KLW, respectively, and in the greenhouse, they explained 13.20%, 18.03%, and 8.81% of the variation, respectively. For this trait, Divide alleles contributed increases in KLW at QKlw.fcu-2A, whereas PI 272527 alleles at QKlw.fcu-1B and QKlw.fcu-2B led to increased KLW.
Identification of RILs potentially useful for improvement of yield in durum wheat
Analysis of the data from combined field environments revealed 3 RILs, DP527-022, DP527-064, and DP527-115, that may be beneficial for improvement of grain weight and size in modern durum varieties. Although none of these RILs had a more KPS than the durum parent Divide, all of them had grain weight (GWS and TKW) values equal to or better than Divide and increased KA, KW, and KL compared to Divide (Table 6, Fig. 5, and Supplementary File 3). In addition, all 3 of these RILs had the desired allele at the stable QTLs for TKW and KA listed in Table 5. With the exception of DP527-022, these RILs also had increased KC compared to Divide, and DP527-022 and DP527-064 had better KLW.
Table 6.
Three recombinant inbred lines from the Divide × PI 272527 (DP527) population with as good or better values compared to the cultivated parent Divide for grain number, size, and weight based on field observations making them potentially useful for improving yield in durum wheat breeding programs.
| Traita | Divide | PI 272527 | DP527-022b | DP527-064c | DP527-115c |
|---|---|---|---|---|---|
| SPS | 17.67 | 24.47 | 21.64 | 21.19 | 21.10 |
| KPS | 39.88 | 29.10 | –d | – | – |
| GWS | 1.57 | 1.51 | 1.58 | 1.84 | 1.58 |
| TKW | 40.10 | 51.62 | 53.16 | 51.67 | 61.37 |
| KA | 18.10 | 21.95 | 23.13 | 22.68 | 23.35 |
| KW | 3.21 | 3.37 | 3.55 | 3.42 | 3.73 |
| KL | 7.72 | 9.24 | 9.04 | 9.11 | 8.69 |
| KC | 1.56 | 1.73 | – | 1.63 | 1.56 |
| KLW | 2.43 | 2.75 | 2.58 | 2.68 | – |
Trait abbreviations are: spikelets per spike (SPS), kernels per spike (KPS), grain weight per spike (GWS), thousand kernel weight (TKW), kernel area (KA), kernel width (KW), kernel length (KL), kernel circularity (KC), and kernel length:width ratio (KLW).
Recombinant inbred line has the PI 272527 allele type at marker Xfcp650(Q) and is therefore non free threshing.
Recombinant inbred line has the Divide allele type at marker Xfcp650(Q) and is therefore free threshing.
A dash indicates that the value was not better than the cultivated parent Divide.
Fig. 5.
Seed morphology of PI 272527, Divide, and the 3 recombinant inbred lines which have increased seed size and weight compared to the Divide parent. DP527-022, DP527-064, and DP527-115 have the desired alleles at the 3 TKWs and the 3 stable QTLs for KA that were observed under multiple field seasons.
Discussion
Trait correlations
We observed that PHT was positively correlated with many of the yield components traits, whereas DTH was negatively correlated. Corsi et al. (2021) had similar findings and suggested that alleles for increased PHT may contribute to an increase in grain size traits. However, we cannot infer how this correlation would hold up in a large-scale field setting as observed under farming systems. In addition, although we saw a positive correlation between height and yield, we observed severe lodging in the field (data not shown) that would not be desired in a breeding program.
Increased SPS was positively correlated with KPS and GWS. Plants that have more spikelets have increased capacity to produce more kernels, which influences the GWS (Gauley and Boden 2019). However, the number of KPS is often negatively correlated with kernel weight and size due to limitations in nutrient availability and tradeoffs in the size–shape relationship (Sadras 2007; Mangini et al. 2021). Surprisingly, KPS had a significant positive correlation with TKW and KW under greenhouse conditions, but not under field conditions. The potential reasoning behind these different observations may be due to increased water and nutrient availability for plants under greenhouse conditions compared to the field; therefore, the competition for resources was not as intense as under field conditions (Asseng et al. 2020).
Generally, studies to evaluate different yield component interactions in wheat have shown negative correlations between KPS and kernel size/weight (Li and Yang 2017; Brinton and Uauy 2019; Corsi et al. 2021). In this study, KA was the only kernel dimension trait that was negatively associated with KPS under the field environments. KPS had a correlation value near zero for the other kernel dimension traits. These findings were interesting because increasing kernel size in this population does not lead to a significant reduction in the number of KPS, indicating that the genes that control these pathways may be beneficial for breeders who are interested in increasing the number of KPS without significantly reducing kernel size or vice versa. Additional trials under varying environmental conditions are needed to determine if this lack of a negative correlation is due to environmental factors or has high heritability, and the utility of this population for breeding programs.
As expected, GWS and TKW were positively correlated with KA, KW, and KL, i.e. increased kernel size, in all environments. However, KC and KLW were only correlated with increased TKW in the 2017 field environment and there was a negative correlation between KC and KLW in the 2020 field season. These findings are similar to those reported by Corsi et al. (2021), with KA, KW, and KL being positively associated with kernel weight and KLW not being a significant indicator of increased kernel weight. Using a durum panel of 150 lines, Sun et al. (2020) showed that TKW was significantly correlated with KA, KW, KL, and KC, but not KLW, which is also consistent with our findings. Using KA, KW, and KL may be useful for breeders when selecting lines for increased kernel weight, especially in situations in which kernel weight is difficult to accurately measure.
Interestingly, when comparing tradeoffs between the kernel dimension traits, the results varied depending on the environment. Increases in KW and KL consistently resulted in increased KA and therefore larger kernels. These findings were identical to those of Corsi et al. (2021) and Sun et al. (2020). Under field conditions, both studies also showed KW to be negatively correlated with KLW, and KL to be positively correlated with KLW. We observed identical results in 2 other durum × cultivated emmer populations (Peters Haugrud AR and Faris JD, unpublished). KL must have a larger influence on the KLW ratio than KW. In addition, Gegas et al. (2010) found that kernel shape and size are independent traits and most likely under the influence of different pathways and genes. KL and KLW, along with KC, are often described as kernel shape traits, whereas KA and KW are kernel size traits. Further research elucidating the interaction between the different kernel size components and how they interact to determine kernel size is needed at the molecular and physiological level to untangle these relationships.
QTLs associated with multiple traits in multiple environments
We identified a multitrait QTL on the short arm of chromosome 1B involved in the control of multiple kernel dimension traits under greenhouse and field conditions. Within this region, QTLs for KL, KC, and KLW were identified. Interestingly, no multienvironment QTL for KW was observed, although a significant KLW QTL was present. However, there was a QTL at this location for KW in 2020, indicating that KL, KC, and KLW may be less influenced by environmental factors than KW. Russo et al. (2014) identified a QTL near this physical region for multiple kernel dimension traits in a durum × cultivated emmer population. Mangini et al. (2021) also reported a QTL for KL and KW in a region slightly proximal to our QTL. Li et al. (2018) reported a QTL for KPS near this region in hexaploid wheat. Through BLASTn analysis with rice genes known to be associated with yield, the wheat ortholog of the rice gene OsGSN1 was found to be located near this region. OsGSN1 encodes a mitogen-activated protein kinase phosphatase, and reduced expression in rice results in more but smaller grains (Guo et al. 2018). Therefore, the OsGSN1 ortholog in wheat is a strong candidate for the gene underlying these QTLs. PI 272527 may be a useful source for increasing KL, KC, and KLW in durum wheat if breeders are interested in changing kernel shape.
A region on chromosome 2A from 83.82 to 110.72 cM harbored 3 QTLs. A QTL for KW was observed under field conditions, and QTLs for KLW and KL were observed under both greenhouse and field conditions. Corsi et al. (2021) also identified QTLs for KL, KW, and KLW within this region in a European hexaploid MAGIC population. In addition, Mangini et al. (2021) identified a genomic region on chromosome 2A with QTLs for KA, KL, KW, and TKW, that was physically near our QTL cluster on chromosome 2A and located within their region was an auxin response factor (ARF1) gene. QTLs for KPS and TKW, which are often associated with kernel size, have also been identified near this region (McCartney et al. 2005; Würschum et al. 2018). Although the underlying gene(s) within this region for these traits remains unknown, an increase in kernel size is due to cell size and rate of grain filling (Brinton and Uauy 2019). Also, located less than 100 Mb from the marker most closely associated with our QTL is GNI1-A1, a gene associated with kernel weight and size (Golan et al. 2019; Sakuma et al. 2019), along with orthologs of the rice yield-associated genes OsD11 and OsMKKK10 (Tanabe et al. 2005; Guo et al. 2018). In our study, PI 272527 alleles resulted in an increase in KW, whereas at the same peak marker, Divide alleles resulted in increased KLW. It remains unclear if there is a tradeoff occurring at this locus, and if so, the mechanism behind it.
A stable, multitrait QTL on chromosome 2B was associated with KL, KC, and KLW under both greenhouse and field conditions. The increased effects at this locus were contributed by PI 272527 for each trait, indicating that PI 272527 alleles within this region may be useful for breeding for increased kernel size.
A region spanning 40.52 Mb on chromosome 3A was associated with TKW, PHT, KA, and KL. QTLs near this region for yield component traits have been identified by Blanco et al. (2012), Russo et al. (2014), and Sun et al. (2020), and we observed QTLs for PHT and TKW at the same locus in other durum × cultivated emmer populations (Peters Haugrud AR and Faris JD, unpublished). The physiological relationship between these 2 traits remains unclear. Often, either TKW or PHT is associated with DTH; however, we did not observe a DTH QTL in this region. In rice, the yield-associated genes OsGL2 and OsMAPK6, which have orthologs in wheat located near our QTL region on chromosome 3A, control grain weight and size (Heang and Sassa 2012; Guo et al. 2018). These 2 genes may be considered candidates for these traits at this QTL, but further work is needed to elucidate this. PI 272527 alleles at the 3A QTL may be useful for increasing kernel size and weight in durum wheat; however, the same alleles may lead to unwanted increases in PHT.
A QTL on chromosome 3B was associated with KA and KW under field conditions. QTLs in this region associated with TKW have been previously reported (Russo et al. 2014; Li et al. 2018). The gene TaCKX2 is within 35 Mb of our peak marker (Supplementary File 1). TaCKX2 plays a role in the regulation of cytokinin and influences yield-related traits (Zhang et al. 2012; Jablonski et al. 2021). In addition, the rice gene OsBZR1, which is involved in the brassinosteriod pathway and controlling seed number (Qiao et al. 2017), has an ortholog in wheat located near our QTL region. Although introgression of this QTL may increase kernel size, 1 caveat is that Divide alleles within this region resulted in increased KA whereas PI 272527 alleles resulted in wider kernels.
QTLs for TKW, KA, KW, and KL colocated on the long arm of chromosome 6A. Recently, Corsi et al. (2021) reported that same region to be associated with many of these traits across multiple environments in Europe in a MAGIC population, indicating that gene(s) within this region may play an important role in yield across multiple types of germplasm and environments. The presence of the TKW QTL at this locus is mostly likely due to increased kernel size having a pleiotropic effect and influencing kernel weight (Gegas et al. 2010; Brinton and Uauy 2019). Within this QTL region is the previously identified gene TtGRF4 (Avni et al. 2018). TtGRF4 is an ortholog of OsGRF4 from rice, which encodes the transcription factor GROWTH-REGULATING FACTOR, and is negatively regulated by OsmiR396 (Duan et al. 2016; Sun et al. 2016). OsGRF4 is involved in chromatin-remodeling in rice panicles, with mutations in the miR396 cleavage location having larger and longer hulls and grains (Duan et al. 2016; Sun et al. 2016). Studies are still needed to determine the relationship between TtGRF4 and increased TKW; however, it can be hypothesized that the most likely scenario is that mutations in TtGRF4 result in decreased binding of a transcription factor resulting in larger kernels, which would lead to increased grain weight. Interestingly, PI 272527 was the donor parent of the positive allele at this QTL, indicating that potentially the allele of TtGRF4 is not present or is an alternative allele in North Dakota durum germplasm. Breeding this QTL into local germplasm may prove beneficial to breeders for increasing kernel weight.
Finally, QTLs for SPS, TKW, DTH, and PHT cosegregated at a region of chromosome 7B where the wheat gene FT-B1 is located. FT1 is involved in flowering time regulation and the transition from vegetative to reproductive growth (Yan et al. 2006; reviewed by Gauley and Boden 2019). Dixon et al. (2018) found that the different alleles of FT1 perform different under varying environmental conditions, and FT1 plays a role in promoting inflorescence development. Interestingly, QTLs associated with PHT and TKW have not been previously associated with the FT1 locus. Potentially, because FT1 is part of the regulation of the vegetative to reproductive growth transition, an earlier transition to reproductive growth may result in shorter plants. In addition, the donor parent of increased TKW at this locus was Divide, whereas the other QTL had PI 272527 as the positive parent. Potentially, the Divide FT1 allele may result in reduced number of SPS, which may result in fewer kernels but increased grain weight. Further investigation is needed to determine the molecular and physiological mechanisms behind these relationships and if FT1 is indeed the gene underlying these QTLs. If this is the case, although selecting for the PI 272527 allele at FT1 would result in increased number of SPS, it may lead to an increase in DTH and PHT, which are not desirable traits in most situations.
Beneficial QTLs from PI 272527 governing yield-related traits
Genes transferred from cultivated emmer into the durum germplasm pool have traditionally been related to disease and stress tolerance (reviewed in Zaharieva et al. 2010; Sharma, Zhang, et al. 2019). Here, we identified 7 robust multitrait QTL regions on chromosomes 1B, 2A, 2B, 3A, 3B, 6A, and 7B, for which many had the agronomically desired phenotype contributed by PI 272527. As discussed above, some of these may be resources for improving yield component traits in durum wheat. PI 272527 had a greater number of SPS, TKW, and grain size compared to Divide. In 2020, Divide was grown on 20% of the acres in North Dakota (https://www.ag.ndsu.edu/publications/crops/north-dakota-durum-wheat-variety-trial-results-for-2020-and-selection-guide) and is considered to have a high test weight. Therefore, because PI 272527 had higher TKW in this study than Divide, it may be useful for improvement of durum varieties in the USA and potentially other regions of the world. Toward this, we selected 3 RILs with increased grain weight and size compared to the elite durum parent Divide. These lines will be evaluated in additional environments to confirm the robustness of the expression of enhanced grain weight and seed size traits, and they may be used in further crossing schemes to generate new durum wheat germplasm. The markers identified in this work may be converted to KASP markers for marker-assisted selection of the beneficial alleles at the desired QTLs. One caveat we must mention is that although these lines had increased grain weight and size compared to Divide, we did not measure end-use quality traits and we are unsure of how using these lines in a breeding program may influence kernel quality and end processing traits.
Conclusions
Previously, the vast majority of yield component studies in wheat have been under either greenhouse or field conditions. In this study, we evaluated the DP527 population under both field and greenhouse conditions for the same 11 traits. The results presented here illustrate that 17 of the QTLs identified in 2 or more environments were also observed under greenhouse conditions, which makes them more amenable to gene cloning methods and the identification of the underlying genes. QTLs expressed in multiple field environments can be considered consistent, and those that were expressed in all field environments as well as the greenhouse can be considered rather robust and stable. Because the consistent and stable QTLs identified in this study (Table 4) are expressed in greenhouse environments, not only does this allow geneticists the opportunity to conduct phenotyping studies for gene cloning purposes, but also breeders and germplasm developers can take advantage by selecting for these QTLs in the off season under controlled conditions. This would allow for the selection and incorporation of beneficial QTLs for PHT, SPS, TKW, DTH, and various seed morphology traits prior to the subsequent field season, thus allowing more rapid progress in the advancement of germplasm lines.
The genes underlying the consistent/stable QTLs identified in this study are good candidates for implementing into durum breeding programs. In addition, 7 multitrait QTL regions were identified, one of which was associated with the FT-B1 gene region on chromosome 7B indicating strong pleiotropic effects of FT-B1. However, it should be noted that beneficial effects of the FT-B1 region are not all conferred by the same allele, i.e. the Divide allele at this locus led to shorter, earlier heading plants with higher TKW and the PI 272527 allele conferred more SPS. Therefore, careful consideration should be taken when using this QTL for durum improvement. The other 6 multitrait QTLs were associated with TKW and grain morphology traits indicating that the consistent expression of these QTLs provides a good opportunity for improving these traits through conventional breeding. Beneficial alleles at the majority of QTLs associated with these traits were derived from the cultivated emmer parent indicating the potential to use cultivated emmer as a source for the improvement of seed size, shape, and weight characters in durum varieties. Recently, it was shown that using SNPs associated with large-effect or stable QTLs as fixed effects may improve genomic selection models in winter wheat (Lozada et al. 2019; Sarinelli et al. 2019). Therefore, QTLs identified in this study, along with their associated markers, may provide useful tools for improving yield in durum breeding programs to increase grain yield and change kernel morphological traits to meet consumer demands.
Acknowledgments
The authors would like to thank Zengcui Zhang, Megan Overlander, Cayley Steen, Sudeshi Senevirantne, Anges Szabo-Hever, Jyoti Saini Sharma, Aliya Momotaz, Katherine Running, Sapna Sharma, Gurminder Singh, Erika Shay Bauer, and Marissa Condron for assistance with field data collection. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Funding
This project was supported by the Agriculture and Food Research Initiative Competitive Grant 2022-68013-36439 (WheatCAP) from the USDA National Institute of Food and Agriculture.
Contributor Information
Amanda R Peters Haugrud, Department of Plant Sciences, North Dakota State University, Fargo, ND 58102, USA.
Qijun Zhang, Department of Plant Sciences, North Dakota State University, Fargo, ND 58102, USA.
Andrew J Green, Department of Plant Sciences, North Dakota State University, Fargo, ND 58102, USA.
Steven S Xu, USDA-ARS Western Regional Research Center, Albany, CA 94710, USA.
Justin D Faris, Cereal Crops Research Unit, Edward T. Schafer Agricultural Research Center, Agricultural Research Service, United States Department of Agriculture, Fargo, ND 58102, USA.
Data Availability
The data underlying this article are available in the article and in its online supplementary material. Supplemental material is available at figshare: https://doi.org/10.25387/g3.20419041.
Literature cited
- Arriagada O, Marcotuli I, Gadaleta A, Schwember AR.. Molecular mapping and genomics of grain yield in durum wheat: a review. Int J Mol Sci. 2020;21(19):7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseng S, Guarin JR, Raman M, Monje O, Kiss G, Despommier DD, Meggers FM, Gauthier PPG.. Wheat Yield potential in controlled-environment vertical farms. Proc Natl Acad Sci USA. 2020;117(32):19131–19135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni R, Oren L, Shabtay G, Assili S, Pozniak C, Hale I, Ben-David R, Peleg Z, Distelfeld A.. Genome based meta-QTL analysis of grain weight in tetraploid wheat identifies rare alleles of GRF4 associated with larger grains. Genes. 2018;9(12):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco A, Mangini G, Giancaspro A, Giove S, Colasuonno P, Simeone R, Signorile A, De Vita P, Mastrangelo AM, Cattivelli L, et al. Relationships between grain protein content and grain yield components through quantitative trait locus analyses in a recombinant inbred line population derived from two elite durum wheat cultivars. Mol Breeding. 2012;30(1):79–92. [Google Scholar]
- Borrill P, Harrington SA, Uauy C.. Applying the latest advances in genomics and phenomics for trait discovery in polyploid wheat. Plant J. 2019;97(1):56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton J, Uauy C.. A reductionist approach to dissecting grain weight and yield in wheat. J Integr Plant Biol. 2019;61(3):337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA.. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19(7):889–890. [DOI] [PubMed] [Google Scholar]
- Cao S, Xu D, Hanif M, Xia X, He Z.. Genetic architecture underpinning yield component traits in wheat. Theor Appl Genet. 2020;133(6):1811–1823. [DOI] [PubMed] [Google Scholar]
- Colasuonno P, Marcotuli I, Gadaleta A, Soriano JM.. From genetic maps to QTL cloning: an overview for durum wheat. Plants. 2021;10(2):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi B, Obinu L, Zanella CM, Cutrupi S, Day R, Geyer M, Lillemo M, Lin M, Mazza L, Percival-Alwyn L, et al. Identification of eight QTL controlling multiple yield components in a German multi-parental wheat population, including Rht24, WAPO-A1, WAPO-B1 and genetic loci on chromosome 5A and 6A. Theor Appl Genet. 2021;134(5):1435–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LE, Farré A, Finnegan EJ, Orford S, Griffiths S, Boden SA.. Developmental responses of bread wheat to changes in ambient temperature following deletion of a locus that includes FLOWERING LOCUS T1. Plant Cell Environ. 2018;41(7):1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan P, Ni S, Wang J, Zhang B, Xu R, Wang Y, Chen H, Zhu X, Li Y.. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat Plants. 2016;2:15203. [DOI] [PubMed] [Google Scholar]
- Elias EM, Manthey FA.. Registration of ‘Divide’ durum wheat. J Plant Reg. 2007;1(1):7–8. [Google Scholar]
- Ellis JG, Lagudah ES, Spielmeyer W, Dodds PN.. The past, present and future of breeding rust resistant wheat. Front Plant Sci. 2014;5:641. 10.3389/fpls.2014.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris JD. Wheat domestication: key to agricultural revolutions past and future. In: Tuberosa R, Graner A, Frison E, editors. Genomics and Plant Genetic Resources Volume 1. Managing, Sequencing and Mining Genetic Resources. Netherlands: Springer; 2014. p. 439–464. [Google Scholar]
- Faris JD, Zhang Q, Chao S, Zhang Z, Xu SS.. Analysis of agronomic and domestication traits in a durum × cultivated emmer wheat populations using a high-density single nucleotide polymorphism-based linkage map. Theor Appl Genet. 2014;127(11):2333–2348. [DOI] [PubMed] [Google Scholar]
- Gauley A, Boden SA.. Genetic pathways controlling inflorescence architecture and development in wheat and barley. J Integr Plant Biol. 2019;61(3):296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegas VC, Nazari A, Griffiths S, Simmonds J, Fish L, Orford S, Sayers L, Doonan JH, Snape JW.. A genetic framework for grain size and shape variation in wheat. Plant Cell. 2010;22(4):1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan G, Ayalon I, Perry A, Zimran G, Ade-Ajayi T, Mosquna A, Distelfeld A, Peleg Z.. GNI-A1 meditates trade-off between grain number and grain weight in tetraploid wheat. Theor Appl Genet. 2019;132(8):2353–2365. [DOI] [PubMed] [Google Scholar]
- Guo T, Chen K, Dong NQ, Shi CL, Ye WW, Gao JP, Shan JX, Lin HX.. GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell. 2018;30(4):871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán C, Caballero L, Alvarez JB.. Molecular characterization of the Wx-B1 allelic variants identified in cultivated emmer wheat and comparison with those of durum wheat. Mol Breeding. 2011;28(3):403–411. [Google Scholar]
- Haley CS, Knott SA.. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity (Edinb). 1992;69(4):315–324. [DOI] [PubMed] [Google Scholar]
- Heang D, Sassa H.. Antagonistic actions of HLH/bHLH proteins are involved in grain length and weight in rice. PLoS One. 2012;7(2):e31325. 10.1371/journal.pone.0031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P. The genes of the green revolution. Trends Genet. 2003;19(1):5–9. [DOI] [PubMed] [Google Scholar]
- International Wheat Genome Sequencing Consortium . Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:6403. [DOI] [PubMed] [Google Scholar]
- International Wheat Yield Partnership . 2019/20 Annual Report; 2020. https://iwyp.org/wp-content/uploads/sites/34/2020/12/IWYP-Annual-Report-2019-20.pdf.
- Jablonski B, Szala K, Przyborowski M, Bajguz A, Chmur M, Gasparis S, Orczyk W, Nadolska-Orczyk A.. TaCKX2.2 genes coordinate expression of other TaCKX family members, regulate phytohormone content and yield-related traits of wheat. Int J Mol Sci. 2021;22(8):4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosambi DD. The estimation of map distances from recombination values. Ann Eugen. 1943;12(1):172–175. [Google Scholar]
- Levene H. Robust tests for equality of variances. In: Olkin I, Hotelling H, editors. Contributions to Probability and Statistics: essays in Honor of Harold Hoteling. Stanford, CA: Stanford University Press; 1960. p. 278–292. [Google Scholar]
- Li W, Yang B.. Translational genomics of grain size regulation in wheat. Theor Appl Genet. 2017;130(9):1765–1771. [DOI] [PubMed] [Google Scholar]
- Li F, Wen W, He Z, Liu J, Jin H, Cao S, Geng H, Yan J, Zhang P, Wan Y, et al. Genome-wide linkage mapping of yield-related traits in three Chinese bread wheat populations using high-density SNP markers. Theor Appl Genet. 2018;131(9):1903–1924. [DOI] [PubMed] [Google Scholar]
- Lorieux M. MapDisto: fast and efficient computation of genetic linkage maps. Mol Breeding. 2012;30(2):1231–1235. [Google Scholar]
- Lozada D, Mason RE, Sarinelli JM, Brown-Guedira G.. Accuracy of genomic selection for grain yield and agronomic traits in soft red winter wheat. BMC Genet. 2019;20(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri M, Harris NS, Twardziok SO, Pasam RK, Gundlach H, Spannagl M, Ormanbekova D, Lux T, Prade VM, Milner SG, et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat Genet. 2019;51(5):885–895. [DOI] [PubMed] [Google Scholar]
- Mangini G, Blanco A, Nigro D, Signorile MA, Simeone R.. Candidate genes and quantitative trait loci for grain yield and seed size in durum wheat. Plants. 2021;10(2):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichaikul A, Moon JY, Sen Ś, Yandell BS, Broman KW.. A model selection approach for the identification of quantitative trait loci in experimental crosses, allowing epistasis. Genetics. 2009;181(3):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CA, Somers DJ, Humphreys DG, Lukow O, Ames N, Noll J, Cloutier S, McCallum BD.. Mapping quantitative trait loci controlling agronomic traits in spring wheat cross RL4452 × ‘AC Domain’. Genome. 2005;48(5):870–883. [DOI] [PubMed] [Google Scholar]
- Mohler V, Bauer C, Schweizer G, Kempf H, Hartl L.. Pm50: a new powdery mildew resistance gene in common wheat derived from cultivated emmer. J Appl Genet. 2013;54(3):259–263. [DOI] [PubMed] [Google Scholar]
- Nadolska-Orczyk A, Rajchel IK, Orczyk W, Gasparis S.. Major genes determining yield-related traits in wheat and barley. Theor Appl Genet. 2017;130(6):1081–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao S, Sun S, Wang L, Wu Z, Li C, Li X, Wang T, Leng L, Tian W, Lu T, et al. The RLA1/SMOS1 transcription factor functions with OsBZR1 to regulate brassinosteriod signaling and rice architecture. Plant Cell. 2017;29(2):292–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MA, Ficco DBM, Laidò G, Marone D, Papa R, Blanco A, Gadaleta A, De Vita P, Mastrangelo AM.. A dense durum wheat × T. dicoccum linkage map based on SNP markers for the study of seed morphology. Mol Breeding. 2014;34(4):1579–1597. [Google Scholar]
- Sadras VO. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crops Res. 2007;100(2–3):125–138. [Google Scholar]
- Sakuma S, Golan G, Guo Z, Ogawa T, Tagiri A, Sugimoto K, Bernhardt N, Brassac J, Mascher M, Hensel G, et al. Unleashing floret fertility in wheat through the mutation of a homeobox gene. Proc Natl Acad Sci U S A. 2019;116(11):5182–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinelli JM, Murphy JP, Tyagi P, Holland JB, Johnson JW, Mergoum M, Mason RE, Babar A, Harrison S, Sutton R, et al. Training population selection and use of fixed effects to optimize genomic predictions in a historical USA winter wheat panel. Theor Appl Genet. 2019;132(4):1247–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MF, Botigué LR, Brace S, Stevens CJ, Mullin VE, Stevenson A, Thomas MG, Fuller DQ, Mott R.. A 3,000-year-old Egyptian emmer wheat genome reveals dispersal and domestication history. Nat Plants. 2019;5(11):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Ś, Churchill GA.. A statistical framework for quantitative trait mapping. Genetics. 2001;159(1):371–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JS, Running KLD, Xu SS, Zhang Q, Peters Haugrud AR, Sharma S, McClean PE, Faris JD.. Genetic analysis of threshability and other spike traits in the evolution of cultivated emmer to fully domesticated durum wheat. Mol Genet Genomics. 2019;294(3):757–771. [DOI] [PubMed] [Google Scholar]
- Sharma JS, Zhang Q, Rouse MN, Klindworth DL, Friesen TL, Long Y, Olivera PD, Jin Y, McClean PE, Xu SS, et al. Mapping a characterization of two stem rust resistance genes derived from cultivated emmer wheat accession PI 193883. Theor Appl Genet. 2019;132(11):3177–3189. [DOI] [PubMed] [Google Scholar]
- Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai YS, Gill BS, Faris JD.. Molecular characterization of the major wheat domestication gene Q. Genetics. 2006;172(1):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG.. Statistical Methods. 8th ed. Ames: Iowa State University Press; 1989. [Google Scholar]
- Sun L, Huang S, Sun G, Zhang Y, Hu X, Nevo E, Peng J, Sun D.. SNP-based association study of kernel architecture in a worldwide collection of durum wheat germplasm. PLoS One. 2020;15(2):e0229159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Yan Y, Jiang Y, Xiao Y, Hu Y, Cai M, Li Y, Hsam SLK, Zeller FJ.. Molecular cloning and comparative analysis of a y-type inactive HMW glutenin subunit gene from cultivated emmer wheat (Triticum dicoccum L.). Hereditas. 2004;141(1):46–54. [DOI] [PubMed] [Google Scholar]
- Sun P, Zhang W, Wang Y, He Q, Shu F, Liu H, Wang J, Wang J, Yuan L, Deng H.. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J Integr Plant Biol. 2016;58(10):836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taagen E, Tanaka J, Gul A, Sorrells ME.. Positional-based cloning ‘fail-safe’ approach is overpowered by wheat chromosome structural variation. Plant Genome. 2021;14(2):e20106. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell. 2005;17(3):776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, et al. ; International Wheat Genome Sequencing Consortium . Characterization of polyploidy wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol J. 2014;12(6):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Simko V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.90); 2017. https://github.com/taiyun/corrplot.
- Würschum T, Leiser WL, Langer SM, Tucker MR, Longin CFH.. Phenotypic and genetic analysis of spike and kernel characteristics in wheat reveals long-term genetic trends of grain yield components. Theor Appl Genet. 2018;131(10):2071–2084. [DOI] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J.. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci U S A. 2006;103(51):19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharieva M, Ayana NG, Hakimi AA, Misra SC, Monneveux P.. Cultivated emmer wheat (Triticum dicoccon Schrank), an old crop with promising future: a review. Genet Resour Crop Evol. 2010;57(6):937–962. [Google Scholar]
- Zhang L, Zhao YL, Gao LF, Zhao GY, Zhou RH, Zhang BS, Jia JZ.. TaCK6-D1, the ortholog of rice OsCKX2, is association with grain weight in hexaploid wheat. New Phytol. 2012;195(3):574–584. [DOI] [PubMed] [Google Scholar]
- Zhu T, Wang L, Rimbert H, Rodriguez JC, Deal KR, De Oliveira R, Choulet F, Keeble‐Gagnère G, Tibbits J, Rogers J, et al. Optical maps refine the bread wheat Triticum aestivum cv. Chinese Spring genome assembly. Plant J. 2021;107(1):303–314. 10.1111/tpj.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Wang L, Rodriguez JC, Deal KR, Avni R, Distelfeld A, McGuire PE, Dvorak J, Luo MC.. Improved genome sequence of wild emmer wheat Zavitan with the aid of optical maps. G3 (Bethesda). 2019;9(3):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material. Supplemental material is available at figshare: https://doi.org/10.25387/g3.20419041.