Abstract
RNA viruses are a widespread, biologically diverse group that includes the narnaviridiae, a family of unencapsidated RNA viruses containing a single ORF that encodes an RNA-dependent RNA polymerase. In the yeast Saccharomyces cerevisiae, the 20S and 23S RNA viruses are well-studied members of the narnaviridiae, which are present at low intracellular copy numbers, unless induced by stress or unfavorable growth conditions, and are not known to affect host fitness. In this study, we describe a new S. cerevisiae narnavirus that we designate as N1199. We show that N1199 is uniquely present as a double-stranded RNA at a high level relative to other known members of this family in 1 strain background, YJM1199, and is present as a single-stranded RNA at lower levels in 98 of the remaining 100-genomes strains. Furthermore, we see a strong association between the presence of high level N1199 and host phenotype defects, including greatly reduced sporulation efficiency and growth on multiple carbon sources. Finally, we describe associations between N1199 abundance and host phenotype defects, including autophagy.
Keywords: RNA viruses, narnavirus, autophagy, Ras/PKA, 100-genomes strains, Saccharomyces cerevisiae
Introduction
RNA viruses comprise a large and extremely diversified group. As is the case for many other species, the yeast Saccharomyces cerevisiae is a host for RNA viruses. S. cerevisiae RNA viruses are vertically transmitted, cytoplasmic genetic elements, which can be divided into 2 families—Totiviridiae (L-A and L-BC, which are encapsidated double-stranded RNA viruses of approximately 4,600 bp) and Narnaviridiae (Wickner et al. 2013). Narnaviruses, which have the simplest genome structure of any extant RNA viruses, consist of a single-stranded (ss) monocistronic RNA molecule that encodes an RNA-dependent RNA polymerase (Hillman and Cai 2013). Narnaviruses have been reported in fungal pathogens, such as Magnaporthe oryzae (Lin et al. 2020), Alternaria tenuissima (Nerva et al. 2019), Fusarium sp. (Osaki et al. 2016), and Aspergillus fumigatus (Zoll et al. 2018), water molds (Hacker et al. 2005) as well as in invertebrates, algae, and protozoans (Grybchuk et al. 2018; Waldron et al. 2018; Goertz et al. 2019; Richaud et al. 2019). Two well-known members of this family in budding yeast are 20S and 23S (Wesolowski and Wickner 1984; Wickner 1992) that were subsequently characterized by cloning and sequencing their double-stranded (ds) forms W and T, respectively (Matsumoto and Wickner 1991; Esteban et al. 1992). The 20S- and 23S-encoded RNA-dependent RNA polymerases (p91 and p104, respectively) form complexes with their single-stranded RNA genomes in a 1:1 stoichiometry in the host cytoplasm (Solorzano et al. 2000). 20S and 23S, which have no known phenotypes in host cells, exist in such low copy numbers in vegetatively growing cells as to preclude detection by agarose gel analysis; increased copy numbers are only observed under specific conditions, such as sporulation (Garvik and Haber 1978) and heat shock (Wesolowski and Wickner 1984; Wickner 1992).
Previous surveys of the 100-genomes strains collection revealed extensive natural variation in their nuclear, mitochondrial, and 2-µm genomes (Strope, Kozmin, et al. 2015; Strope, Skelly, et al. 2015; Vijayraghavan et al. 2019). In this study, we identified and characterized a novel narnavirus in S. cerevisiae, designated N1199, that we first discovered in 1 of the 100-genomes strains, YJM1199, as a highly abundant double-stranded RNA molecule. We cloned and sequenced N1199 from YJM1199, analyzed its phylogeny relative to other narnaviruses, determined the presence (and RT-PCR genotypes) of N1199 in the other 100-genomes strains, demonstrated cytoplasmic inheritance, and characterized the effects of high copy number N1199 on host phenotypes. Our work demonstrates that a narnavirus, when present in high copy number, can significantly alter host fitness. Our key findings and their implications are described and discussed below.
Materials and methods
Strains, plasmids, and PCR primers
The S. cerevisiae strains listed in Supplementary Table 1 have been deposited in, and should be requested from, the Fungal Genetics Stock Center <http://www.fgsc.net>. For additional descriptions of the sequenced 100-genomes S. cerevisiae strains, or genetic backgrounds, listed in Supplementary Table 1, see Strope, Kozmin, et al. (2015), Strope, Skelly, et al. (2015), and Vijayraghavan et al. (2019). The PCR (and sequencing? ) primers used in this study are listed in Supplementary Table 2. To construct pGAL-PDE1, the PDE1 ORF was amplified from S288C with oligos SV342 and SV343 (Supplementary Table 2), purified, digested with XhoI and NheI (NEB), and ligated into pLND46 digested with SpeI and SalI (NEB). Ligation mixture was transformed into E.coli, and transformants were screened for correctly ligated plasmids with restriction digests. The plasmids listed in Supplementary Table 2 have been deposited in, and should be requested from, Addgene (http://www.addgene.org/John_McCusker/).
Media and phenotypic analysis
YPD and YPE (1% yeast extract, 2% bacto peptone) contained 2% dextrose and 2% ethanol (added after autoclaving), respectively. Similarly, SD and SE (0.67% yeast nitrogen base) contained 2% dextrose and 2% ethanol (added after autoclaving), respectively. YPD, YPE, SD, and SE plates contained 2% agar. Phenotypic analysis was performed on subsets of strains by 10-fold spot dilutions onto 100 mm diameter plates. Media containing other carbon sources at a 2% final concentration were prepared as above. For autophagy induction, cultures were grown in liquid YPD containing 0.1–250 nM rapamycin (StemCell Tech, Cat# 73362) for 2−3 days, and subsequently streaked to YPD plates to allow recovery. Single colonies from the YPD plate were subsequently analyzed for further assays as described below and in the text. For galactose induction experiments, transformants were grown on YP +2% galactose and 100 μg/mL nourseothricin for 2 days and subsequently plated to YPD. Single colonies from YPD plates were analyzed for further experiments as described in the text. The same schematic was followed for analyzing strains transformed with either the test plasmid or an empty vector.
Virus presence/absence determination by PCR and gel electrophoresis
Total nucleic acids were extracted using a slight modification of the phenol-mediated method described by (Maqueda et al. 2010), as follows. Cells of 5-mL overnight YPD cultures were collected by centrifugation and washed once with 50 mM Na2EDTA (pH 7.5). Cell pellets were then resuspended in 1 mL of 50 mM Tris-H2SO4 (pH 9.3)/1% 2-mercaptoethanol solution and incubated for 15 min at room temperature. Cells were subsequently collected by centrifugation and resuspended in 1 mL of 0.1 M NaCl/10 mM Tris–HCl (pH 7.5)/10 mM Na2EDTA/0.2% sodium dodecyl sulfate solution. 0.7 mL of phenol (pH 8.0) was added, and samples were incubated on a shaking platform for 1 h at room temperature, after which 0.7 mL of the aqueous phase was recovered by 5-min centrifugation. Nucleic acids were precipitated by the addition of 70 µL of 3 M potassium acetate and 0.7 mL of ice-cold isopropanol, followed by incubation for 5 min at room temperature and centrifugation at 14,000 rpm for 10 min. The precipitated nucleic acids were washed with 70% ethanol, dried using a SpeedVac (Eppendorf), dissolved in 70–100 µL of water, and stored at −80°.
PCR analysis of sequenced RNA viruses was performed as follows. 15 µL aliquots of total nucleic acids samples were incubated for 2 min at 98° and then placed on ice. cDNA synthesis was performed using the Maxima first-strand cDNA synthesis kit (Thermo), in accordance with the manufacturer's protocol, using 5 µL aliquots of nucleic acid samples as a template. PCR reactions were performed with OneTaq DNA polymerase (NEB) in accordance with the manufacturer's protocol. The primers used to detect the presence and, where present, their PCR genotypes, are listed in Supplementary Table 2.
For gel electrophoresis, 5 μL aliquots of total nucleic acid samples (to detect abundant RNA viruses) or RT-PCR-derived nucleic acid samples (to detect PCR products of sequenced RNA viruses) were mixed with 1 × gel-loading buffer and loaded on 1.3% agarose gels prestained with GreenGlo SAFE DNA dye (Denville) and/or ethidium bromide (Bio-Rad) according to the manufacturer's instructions. Electrophoresis was carried out in 1 × TAE buffer at room temperature at a constant voltage of 6 V/cm for 45–60 min. Gels were subsequently imaged under UV light using the Alpha Innotech Red gel documentation system.
Nuclease treatments
Nucleic acid samples were treated with DNaseI, RNaseIf, RNaseH, or ShortCut RNaseIII (all from NEB) at 37°C and samples were analyzed on 1.2% TAE-agarose gel stained with ethidium bromide. For subsequent analyses, treated samples were column purified using standard PCR purification kits (Qiagen), reverse transcribed with Maxima first-strand cDNA synthesis kit (Thermo), and subjected to PCR to test for the presence/absence of various genomic and viral elements, as described above. PCR samples were analyzed on 2% TBE (Tris-borate EDTA) gels at 4–6 V/cm for 75–90 min in prechilled 0.5 × TBE buffer.
Phylogenetic analysis of the N1199 RNA-dependent RNA polymerase
Sequences were aligned with MUSCLE (v3.8.31) configured for the highest accuracy (MUSCLE with default settings). The phylogenetic tree was reconstructed using the maximum likelihood method implemented in the PhyML program (v3.1/3.0 aLRT). The WAG substitution model was selected assuming an estimated proportion of invariant sites (of 0.002) and 4 gamma-distributed rate categories to account for rate heterogeneity across sites. The gamma shape parameter was estimated directly from the data (gamma = 1.211). Reliability for internal branch was assessed using the aLRT test (SH-Like). Graphical representation and edition of the phylogenetic tree were performed with TreeDyn (v198.3) (Guindon and Gascuel 2003; Edgar 2004; Anisimova and Gascuel 2006; Chevenet et al. 2006; Dereeper et al. 2008, 2010).
Cloning and sequence determination of N1199 from YJM1199
Full-length sequence of the N1199 dsRNA from YJM1199 was determined by various modifications to the previously described FLAC (full-length amplification of cDNA) method (Maan et al. 2007; Nomikou et al. 2009; Potgieter et al. 2009; Vepstaite-Monstavice et al. 2018; Sanchez et al. 2019). The 2.6 kb dsRNA from YJM1199 was gel-extracted, purified, and ligated to the PC3-T7 loop primer (5′ P-GGATCCCGGGAATTCGGTAATACGACTCACTAT ATTTTTATAGTGAGTCGTATTA-3′). Approximately 1 μg of the dsRNA was ligated to 250 ng PC3-T7-loop primer in the presence of 20% PEG-6000 (VWR), 0.01% BSA, 1% DMSO, 20U RiboLock RNase inhibitor (Thermo), 5U T4 RNA Ligase (Thermo), 0.5 μL T4 DNA Ligase (NEB) and 2 μL each of T4 RNA and DNA ligase buffers in a total volume of 40 µL. The ligation reaction was carried out at 37° for 40 min, followed by incubation at 4°C.
The oligo-ligated dsRNA was subsequently purified using the NucleoSpin Gel and PCR Clean-up kit (Machery-Nagel) and denatured at 99° for 3 min in the presence of 1 M betaine (Sigma) and 3% DMSO. cDNA synthesis was carried out using ∼ 300 ng denatured oligo-ligated RNA with M-MuLV reverse transcriptase (RNaseH minus; NEB) for 5 min at 25° followed by 60 min at 42° and 20 min at 65°. RNA removal was performed by adding 5U RNaseH (NEB) directly to the cDNA reaction, followed by incubation at 37° for 25 min and 95° for 5 min. The primary cDNA strands were annealed without further purification by incubating the above mix at 95° for 4 min, and by a gradual lowering of temperature to 80° at the rate of 1°/min, 50° at the rate of 3°/min and finally to 4° at 0.1°/min.
PCR amplification of the cDNA was carried out using Phusion DNA polymerase with PC2 primer (5′-CCGAATTCCCGGGATCC-3′) per the manufacturer's protocol, with a single additional 2 min incubation step at 72° immediately following initial denaturation. PCR products were analyzed on a 1%TAE-agarose gel. The 2.6 kb PCR amplicon from the previous step was phosphorylated with polynucleotide kinase and blunt-ligated into pAG25 (Goldstein and McCusker 1999) linearized with EcoRV and dephosphorylated with CIP, to yield plasmids pSV35 and pSV36. Clones were analyzed by restriction digests and sequencing. All enzymes were obtained from NEB unless noted otherwise. The full sequence of the cloned cDNA was determined by primer walking and aligning overlapping sequences in silico using the SnapGene software (from GSL Biotech; available at snapgene.com). DNA and protein sequence homologies were determined using NCBI Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Cell fractionation experiments
Cell fractionation was performed according to Keogh et al. (2006). Cells from 50 mL overnight YPD cultures of YJM1199 were collected by centrifugation, washed with ddH2O, and subsequently with PSB (20 mM Tris-Cl pH 7.4, 2 mM EDTA, 100 mM NaCl, 10 mM β-mercaptoethanol) and SB (1 M sorbitol, 20 mM Tris-Cl pH 7.4). Cells were treated with 10 mg/mL Zymolyase 20T in 1 mL SB at 30°C with rotation until spheroplasts were observed. Spheroplasts were harvested at 2000×g for 5 min at 4°C, washed twice with SB, and resuspended in 500μL EBX (20 mM Tris-Cl, pH 7.4, 100 mM NaCl, 0.25% Triton X-100, 15 mM β-mercaptoethanol and 20 mM phenylmethylsulfonyl fluoride). To lyse the plasma membrane, the suspension was treated with 0.5% Triton X-100 and incubated on ice with gentle mixing for 10 min, after which a small aliquot of the lysate was removed for gel analysis and the remainder layered over 1 mL NIB (20 mM Tris-Cl pH 7.4, 100 mM NaCl, 1.2 M sucrose, 15 mM β-mercaptoethanol, and 20 mM phenylmethylsulfonyl fluoride) and centrifuged at 12,000×g for 15 min at 4°C. The upper layer containing the cytoplasmic fraction was stored for gel analysis, while the pellet containing the nuclear fraction was resuspended in 500μL EBX and treated with 1% Triton X-100 to lyse the nuclear membrane. After 10 min of incubation on ice, the sample was spun down as previously to yield a supernatant containing the nucleoplasm fraction and a chromatin-containing pellet. The pellet was washed with EBX 3 times and resuspended in 50 μL Tris pH 8.0. Each fraction was phenol:chloroform extracted to remove proteins and lipids followed by nucleic acid precipitation with 3 M potassium acetate and isopropanol. All fractions were analyzed on 1.3% TAE-agarose gels as described earlier.
Determination of N1199hi frequency and loss rates
Seven independent colonies of YJM1199 N1199hi grown on YPD plates were scraped off and resuspended in water, generating 7 independent cell suspensions. Appropriately diluted aliquots of each suspension were plated on selective maltose-containing plates (to determine the number of Mal+ cells in a colony) and control dextrose-containing plates (to determine a total number of viable cells in a colony) and incubated for 2–3 days at 30°C. N1199 loss frequencies (f) were calculated as a ratio of Mal+ cells in a colony to the number of total viable cells in a colony. Using median f value and median total number of viable cells in a colony (Nt), N1199 loss rate per cell per generation (m) was calculated by Drake's equation: m = f/ln(Nt•µ) (Drake 1991). 95% nonparametric confidence limits for median f value was calculated per (Altman 1991) and transformed to rate confidence limits using Drake's equation.
Curing of the mitochondrial genome from YJM1199
YJM1199 cells were passaged twice in YPD containing ethidium bromide (first in 25 mg/L for 6 h, then in 10 mg/L for 12 h), plated to YPD, and then tested for petite phenotype on YPEG (2% ethanol, 2% glycerol). Petites were confirmed as ρ0 by DAPI staining (Williamson and Fennell 1979).
GFP-Atg8 fluorescence microscopy
The plasmid pSV58 was generated by subcloning a 2.6 kb NsiI-EcoRI fragment from GFP-ATG8(416)/GFP-AUT7(416) (Addgene plasmid #49425) into a pAG36 backbone linearized with NsiI-EcoRI digestion create an episomal vector with a NAT marker expressing GFP-Atg8 under the control of the endogenous Atg8 promoter. Transformants were selected on YPD containing 100 μg/mL nourseothricin. The assay was performed as described in Torggler et al. (2017) with modifications. Cultures of YSV914 and YSV918 were grown overnight in YPD were subcultured at a density of roughly 1.5•107 cells/mL to SD, SD-N (lacking a nitrogen source), and SD + 100 nM rapamycin media and incubated with shaking at 30°C for 48 h. All cultures contained 100 μg/mL nourseothricin throughout the experiment for plasmid maintenance. 20 μg/mL phenylmethylsulfonyl fluoride (PMSF, Sigma-Millipore) was added to cultures to prevent vacuolar degradation and increase the intensity of the GFP signal. Since ammonium sulfate can interfere with aminoglycoside antibiotics, L-glutamate (monosodium salt) was instead used as a nitrogen source in SD cultures (Cheng et al. 2000). For fluorescence microscopy, an aliquot of the cultures was spun down at 3000 × g for 5 min, washed in dH2O once, and resuspended in water. 5 μL of the cell suspension was applied to poly-L-lysine coated glass slides, sealed with a coverslip, and imaged with a Zeiss AxioObserver inverted fluorescence microscope with a mounted CCD camera using a 63 × oil-immersion objective using the appropriate DIC and fluorescence filters. Images were pseudo-colored to highlight the GFP signal.
Statistical methods
Graphical data analysis was performed using GraphPad Prism version 8 for macOS, GraphPad software (La Jolla, CA, www.graphpad.com). Data were plotted as the mean and standard deviation of 3 independent replicates. Statistical significance was analyzed by 2-way ANOVA correcting for multiple comparisons using statistical hypothesis testing (Sidak test). Multiplicity-adjusted P-values were reported for each comparison.
Results and discussion
An abundant, predominantly double-stranded RNA in YJM1199
By RT-PCR with multiple primer pairs, the homothallic diploid strain YJM1199 was found to be L-A0, L-BC+, 20S+, and 23S + . By agarose gel electrophoresis, 2 prominent RNA species were observed in YJM1199—a 4.6 kb band, consistent with L-BC, and a highly abundant 2.6 kb band of unknown identity (Fig. 1). Among the 100-genomes strains, a highly abundant 2.6 kb gel band was observed only in YJM1199 (referred to as N1199hi throughout the text).
Fig. 1.
Nucleic acid analysis of N1199. a) Nucleic acid samples treated with the above enzymes per manufacturer's recommendations and run on a 1.3% TAE-agarose gel. rRNA species are indicated. b) PCR products for the indicated species were analyzed on a 3% TBE-agarose gel. Primers used for PCRs: L-BC–L-BC F2 + R2, 20S–RW307 + RW308, 23S–23S F2 + R2, N1199− SV231 + 232 (Supplementary Table 2).
Purified nucleic acid from YJM1199 was subjected to various nuclease treatments (Fig. 1). Treatment with DNaseI did not change the abundance of the 4.6 kb or 2.6 kb bands, consistent with both species being RNA molecules (Fig. 2). To rule out RNA–DNA hybrids, we also tested with RNaseH, which specifically hydrolyzes RNA strands in RNA–DNA hybrids and observed no change in either RNA species. In contrast, RNaseIII treatment eliminated all RNA species. To test whether the 4.6 and 2.6 kb RNA species were single- or double-stranded, we treated samples with RNaseIf, which is specific for ssRNA, and found that both the putative 4.6 kb L-BC band and the 2.6 kb band were unaffected. In contrast, known ssRNA species including the ribosomal RNAs, as well as the single-stranded narnaviruses 20S and 23S, were depleted by RNaseIf treatment, as confirmed by agarose gel and RT-PCR analyses, respectively (Fig. 1a, b). We concluded that the highly abundant 2.6 kb species in YPD-grown YJM1199 is dsRNA.
Fig. 2.
Nuclease treatment and PCR analysis of YJM1199 and YJM682. a) Nucleic acid samples treated with DNaseI (D), RNaseIf (I), or ShortCut RNaseIII (III). U-untreated sample. 25S and 18S rRNA species are single-stranded RNA (ssRNA) species commonly observed in gel analysis of nucleic acids from S. cerevisiae strains. gDNA-genomic DNA. b) Samples from (a) were reverse transcribed and analyzed for the presence/absence of the following nucleic acid species: L-BC, 20S narnavirus, 23S, N1199, and MAT locus (as a metric for the presence of intact genomic DNA). No RT control indicates PCRs carried out on control samples reverse transcribed without the addition of the reverse transcriptase enzyme. Primer pairs that produced comparable amplification of the given species for the 2 parental strains were chosen for PCR analyses and were as follows: L-BC–L-BC F2 + R2, 20S–RW307 + RW308, 23S–23S F2 + R2, N1199– SV231 + 232, Mat locus–SV155 + SV156 + SV157 (Supplementary Table 2). MW-DNA molecular weight marker. PCR samples were analyzed on 2% TBE gels. RNaseIII = ShortCut RNaseIII.
The abundant dsRNA in YJM1199 is a novel narnavirus, designated N1199, that is widespread within the 100-genomes strains as a low abundance ssRNA that varies in sequence
We utilized a modified FLAC cloning methodology (Potgieter et al. 2002, 2009; Maan et al. 2004, 2007) (Supplementary Fig. 1) to isolate, reverse transcribe and clone the abundant dsRNA in YJM1199; we then sequenced the clone end-to-end with flanking and nested primers. In doing so, we identified a single 2589 bp ORF encoding a putative RNA-dependent RNA polymerase (RdRP). A protein BLAST search with the N1199 putative RdRP-encoding ORF revealed 38% similarity between N1199 and 20S (95% query coverage) and 31% similarity between N1199 and 23S (54% query coverage). Phylogenetic analysis of the N1199-encoded putative RdRP showed its proximity to that of the 20S narnavirus, the recently identified I-329 narnavirus (Mardanov et al. 2020), as well as to that of a Rhizopus sp. narnavirus (Supplementary Fig. 2).
Based on the N1199 sequence information, we designed multiple primer pairs to perform an RT-PCR analysis of N1199 in the 100-genomes strains (Supplementary Tables 2 and 3). As an initial test, a side-by-side comparison of YJM1199 with YJM682 revealed that N1199 could be successfully amplified from both strains, despite the lack of a prominent 2.6 kb gel band in YJM682 (Fig. 2). Nuclease treatments of RNA from the 2 strains showed equivalent effects on N1199, except for RNaseIf treatment where a marked depletion was noted in YJM682, suggesting that N1199 can exist at a low level as a predominantly single-stranded species in other strains, like the 20S and 23S narnaviruses.
As stated above, in contrast to the parental YJM1199 that has a high level of N1199 (N1199hi), none of the 99 remaining 100-genome strains exhibited a high abundance 2.6 kb gel band. Therefore, we used RT-PCR with 5 primer pairs (see Supplementary Table 2 for primer sequences and N1199 sequence coordinates) to test for the presence of N1199 in these strains. While there was strain-specific variation in the level of amplification with some primer pairs, we detected N1199 amplicons with at least 1 of the 5 primer pairs in all but 1 strain (Supplementary Table 3), consistent with 98 of the 100-genomes strains carrying N1199 at a low level (N1199lo); that is, N1199lo is not visible via total nucleic acid gel but is detectable by RT-PCR while N11990 is detectable by neither total nucleic acid gel nor by RT-PCR. BLAST analysis of the original genome sequences of the 100-genome strains showed no DNA sequences with similarity to N1199; thus, widespread genomic N1199 cDNA(s) were excluded as a source of N1199 PCR products. Based on the presence/absence of amplicons from the 5 primer pairs, which is hypothesized to be due to N1199 sequence variation, there were 8 RT-PCR N1199 genotypes (Supplementary Table 3).
N1199lo is cytoplasmically inherited and N1199hi is not affected by treatments that cause the loss of amyloid prions or mitochondrial DNA but is lost with SKI1 overexpression
We were unable to test the cytoplasmic inheritance of N1199hi because all YJM1199 N1199hi segregants were N11990 (see below). Instead, we tested the inheritance of N1199lo by crossing YSV698 (isogenic with YJM682 (L-A0 L-BC+ 20S+ 23S+ N1199lo)) with YSV706 (isogenic with YJM1463 (L-A+ L-BC+ 20S0 23S0 N11990)), sporulating the resulting diploid, and analyzing spore clones from independent tetrads (Supplementary Fig. 3). Using RT-PCR, in addition to 4:0 inheritance of L-A, 20S and 23S, we observed 4:0 inheritance for N1199lo, consistent with the cytoplasmic transmission that is typical of yeast viruses. We confirmed the cytoplasmic localization with cell fractionation of YJM1199 N1199hi samples, whereby N1199 RNA was exclusively present in the cytoplasmic fraction but not in the nuclear fraction (Supplementary Fig. 4). To assess the possibility that N1199 is a mitochondrially localized narnavirus that requires mitochondrially encoded gene products for its replication (Hillman and Cai 2013), we made ρ0 derivatives of YJM1199 and found that these still contained high levels of N1199 (Supplementary Fig. 5a).
Like RNA viruses, prions are cytoplasmically inherited (Wickner 1994, 1996; Nakayashiki et al. 2005; Wickner et al. 2013). Thus, we tested the hypothesis that N1199hi maintenance or abundance is affected by the presence of amyloid prions. Treatment of yeast strains with the chaotropic salt guanidine hydrocholoride (Gdn-HCl) results in the rapid elimination of [PSI + ] (Tuite et al. 1981; Eaglestone et al. 2000) and other amyloid prions (Wickner et al. 2015). However, N1199 levels remained high in YJM1199 after treatment with 5 mM Gdn-HCl (Supplementary Fig. 5b). Given the effects of N1199hi on carbon source utilization, in particular glycerol, that are described below, we also tested for the presence of the [GAR + ] prion that affects carbon source utilization (Brown and Lindquist 2009; Jarosz et al. 2014). However, neither N1199hi nor N11990 YJM1199 background strains exhibited the ability to grow on glycerol in the presence of glucosamine, a [GAR + ] phenotype (Supplementary Fig. 5c).
SKI/XRN1 is an exosome-associated RNA exonuclease, the overexpression of which previously has been shown to cure S. cerevisiae strains of L-A; curing of 20S was only seen when its 5′-stem structure was weakened (Esteban et al. 2008). Upon galactose-mediated overexpression of SKI1, while there was no effect on L-BC by gel, we consistently observed loss of N1199hi in multiple isolates, as assessed by gel analysis, often accompanied by the co-curing of 20S and 23S, as assessed by RT-PCR (Supplementary Fig. 6a). In contrast, when strains carrying an empty vector were similarly induced with galactose, we did not observe a difference in N1199 gel abundance (Supplementary Fig. 7).
N1199hi is associated with sporulation and multiple carbon source utilization defects
We previously noted poor sporulation efficiency for YJM1199 under multiple conditions (Strope, Skelly, et al. 2015). Interestingly, we observed a complete loss of N1199 (N11990) in all YJM1199 spore clones, both by agarose gel analysis and RT-PCR (Supplementary Fig. 6b), a counter-intuitive result for a yeast RNA virus. Furthermore, while there was no loss of L-BC, the loss of N1199 was accompanied by a concomitant loss of 20S and 23S narnaviruses from the spore clones (Supplementary Fig. 6). The poor sporulation of the parental N1199hi YJM1199 was alleviated in the HO N11990 derivatives (Fig. 3a).
Fig. 3.
Phenotypic comparisons of isogenic YJM1199 background N1199+ vs N11990 strains. a) Comparison of sporulation efficiencies of YJM1199 derivatives containing (top, N1199hi) or lacking (bottom, N11990) N1199; arrows indicate asci. MW-DNA molecular weight marker. Quantification of sporulation efficiency of N1199hi vs N11990 isolates of YJM1199 shown with an asterisk denoting a significant difference in means. b) Spot dilutions on media containing different carbon sources. Top lane is the parental YJM1199 containing high-abundance N1199 (N1199hi). The next four lanes are spore clones from a single tetrad of sporulated YJM1199, each of which has lost N1199 (N11990, YSV 765–768, Supplementary Table 1). c) Plate assay comparing the growth of YJM1199 on media containing dextrose (left) or maltose (right) as the sole carbon source. Approximately 1,000 cells were plated on each plate and growth was recorded after 2–3 days. d) Representative gel showing RNA samples from colonies isolated from growth on maltose. Image has been cropped to only display a subset of samples (1–3, YSV790–792, Supplementary Table 1).
We previously observed growth defects for parental YJM1199 N1199hi on different carbon sources (Strope, Skelly, et al. 2015). Thus, we compared the carbon source utilization phenotypes of isogenic N1199hi and N11990 YJM1199 derivatives by plating 10-fold dilutions of cells on media containing different carbon sources. The parental YJM1199 N1199hi exhibited poor growth on media containing disaccharides (maltose, melibiose, cellobiose), trisaccharides (raffinose, melezitose), pentose sugars (xylose, arabinose), and glycerol. In contrast, isogenic N11990 derivatives showed markedly improved growth (Fig. 3b, Supplementary 8). Compared to YPD, cultures plated on YP with nonpreferred carbon sources had drastically reduced CFUs (Fig. 3b, c, Supplementary Fig. 9). When tested by gel analysis, 3 Mal+ derivatives of YJM1199 (YSV790–792; Supplementary Table 1) were no longer N1199hi (Fig. 3d). When 4 Mal+ derivatives with no N1199 band visible on a gel were tested by PCR, all were N1199lo, with 2 being 20S+ 23S0 and 2 being 20S0 23S+ (Supplementary Fig. 8b). Using fluctuation analysis (Drake 1991), we estimated the rate of loss of N1199hi per generation to be 7.2 × 10−4 (Supplementary Table 4).
N1199hi is associated with mis-regulated Ras/PKA signaling and autophagy defects
Previous studies in S. cerevisiae have shown that elevated Ras/PKA signaling is linked to increased catabolite repression of maltose genes and consequently severely reduced growth on maltose (Wanke et al. 1997). Similarly, elevated PKA signaling is conducive for vegetative growth whereas low levels are necessary for entry into meiosis (Jungbluth et al. 2012).
To test whether the growth phenotypes seen in YJM1199 are connected to the Ras/PKA pathway, we overexpressed PDE1, which encodes a low-affinity cAMP phosphodiesterase, in the YJM1199 background (pSV55, Supplementary Table 2). Overexpression of PDE1 has been shown to decrease the intracellular levels of cAMP in yeast (Wera et al. 1997; Ma et al. 1999). YJM1199 isolates overexpressing PDE1 were indeed able to grow normally on media containing maltose (Fig. 4a). Upon testing, we found that PDE1 overexpression cured N1199 and resulted in similar phenotypes as observed with other N1199-curing experiments (Fig. 4, Supplementary Fig. 10a).
Fig. 4.
Effects of rapamycin and Pde1 overexpression on N1199. a) Galactose-regulated PDE1 was overexpressed in YJM1199 under Gal control and 2 independent colonies were analyzed (1,2). Empty vec. indicates vector control with galactose induction. Un-uninduced PDE1. N1199-cured isolates were tested phenotypically.1-YSV860, 2-YSV861 (Supplementary Table 1). b) YJM1199 cultures were incubated with 0–100 nM rapamycin for 4 days, allowed to recover on YPD, and checked for the presence/absence of N1199. Strains cured of N1199 at 5 different concentrations were tested phenotypically (1,199 Rapa 0.1–100nM-treated, YSV869-873, respectively).
We hypothesized that the hypothetical Ras/PKA-N1199 level association may affect autophagy. Various studies have demonstrated that increased Ras signaling essentially blocks autophagy in yeast (Abeliovich and Klionsky 2001; Natarajan et al. 2001; Huang and Klionsky 2002; Budovskaya et al. 2004; Stephan et al. 2009). Thus, we asked if autophagy induction affects the copy number and/or maintenance of N1199hi. To test this hypothesis, we used subcytostatic concentrations of the TOR inhibitor Rapamycin, which has been shown to induce autophagy in yeast strains even under nutrient-rich conditions (Abeliovich and Klionsky 2001; Lu et al. 2016; Noda 2017). To test the effects of rapamycin on N1199, cultures of YJM1199 (N1199hi 20S+ 23S+) were grown to stationary phase in minimal media containing 0–250 nM rapamycin, followed by outgrowth on YPD. The resulting isolates were subsequently analyzed to monitor the effects on N1199 RNA abundance. We found that even subnanomolar concentrations of rapamycin-induced N1199 loss, with all the N1199-cured cultures having phenotypes like those produced in other experiments (Fig. 4b, Supplementary Fig. 10b). Like our previous experiments, in addition to the loss of N1199, we also were unable to detect 20S and 23S by RT-PCR in the rapamycin-treated strains, consistent with co-curing of all 3 narnaviruses (Supplementary Fig. 10b).
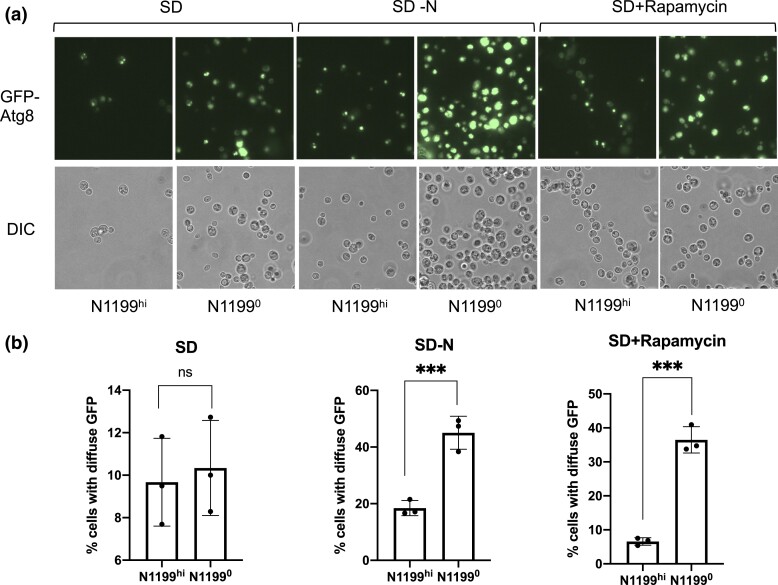
We extended our analysis of the autophagy defect in N1199hi YJM1199 by monitoring the localization and vacuolar processing of Atg8, a ubiquitin-like protein with a regulatory role in autophagosome formation and the cytoplasm-to-vacuole targeting (CVT) pathway (Yorimitsu and Klionsky 2005; Geng and Klionsky 2008; Xie et al. 2008). Induction of autophagy results in the delivery of cytoplasmic Atg8 to the vacuole, where it is degraded by vacuolar proteases (Kirisako et al. 1999; Huang et al. 2000). Using a N-terminal GFP tagged Atg8, bulk autophagy can be quickly and easily monitored via fluorescence microscopy by measuring the change in the intensity of GFP signal within cells (Suzuki et al. 2001). In nutrient-rich conditions, both N1199hi and N11990 strains had low levels of autophagy (SD, P-value 0.7220). In contrast, under conditions of nitrogen starvation, N1199hi cells displayed poor enrichment of the GFP signal compared to N11990 isolates (SD-N, P-value 0.002), with substantial vacuolar fragmentation (Fig. 5). A similar effect was seen when autophagy induction was monitored after rapamycin treatment, whereby there was a substantial loss of GFP signal in YJM1199 N1199hi cells compared to N11990 cells (Fig. 5, SD + Rapamycin, P-value 0.0002). Similar to previous reports (Torggler et al. 2017), rapamycin treatment induced autophagy in the N11990 cells without any associated vacuolar fragmentation, resulting in a more intense but punctate GFP signal (Fig. 5). Due to the low resolution of the above assays, we cannot sufficiently determine trafficking between different autophagosomal compartments. Nevertheless, our results are broadly consistent with the interaction between autophagy and the level of N1199. However, the directionality of the autophagy-N1199 level interaction—autophagy affects the level of N1199 or vice versa—remains to be determined.
Fig. 5.
Measurement of autophagy by GFP-Atg8 localization. Fluorescence microscopy of cells from YJM1199 (N1199hi) and an isogenic N1199-cured strain (N11990, YSV860, Supplementary Table 1) under various treatments. Graph is presented as mean values with standard deviation, and * indicates statistical significance based on unpaired 2-tailed t-tests. P-values <0.05 were deemed statistically significant (marked by ** or ***) for shown comparisons. SD-N – SD media without a nitrogen source.
In summary, in contrast to N1199, 20S (26 strains) and 23S (14 strains) are found less frequently in the 100-genomes strains (manuscript in preparation). There are 4 competing hypotheses for the near-universal frequency of N1199 vs the much lower frequencies of 20S and 23S. First, N1199lo may have invaded S. cerevisiae before 20S and 23S. Second, the N1199lo loss rate may be less than that of 20S and 23S. Third, while all 3 narnaviruses are cytoplasmically inherited, N1199lo inheritance may be more efficient than that of 20S and 23S. Finally, while neither 20S, 23S nor N1199lo are known to affect host fitness, potential deleterious effects on host fitness of N1199lo may be less than that of 20S and 23S; conversely, potential beneficial effects on host fitness of N1199lo may be greater than that of 20S and 23S.
Relative to 20S and 23S, N1199 has both similarities and differences. First, N1199hi loss in YJM1199 is frequently accompanied by loss of 20S and/or 23S, which implies that host pathways required for maintenance of all 3 narnaviruses overlap, at least in part; autophagy is likely 1 such pathway. Second, 20S and 23S are present in low levels in YPD-grown cultures and the same is true for N1199 in 98 of the 100-genomes strains. However, in YPD-grown parental YJM1199, N1199 copy number is high while 20S and 23S copy numbers are low, which implies different mitotic growth copy number regulation of N1199 vs 20S and 23S, in at least 1 genetic background. Finally, while we did not examine N1199 copy number in sporulation media, 20S and 23S copy numbers increase in sporulation media. For example, a previous study showed that 20S, when present in strains, accumulated to high levels during sporulation; notably, the 20S0 strain Y55 did not accumulate a narnavirus-sized species during sporulation (Garvik and Haber 1978). In this context, the 20S0 strain Y55 is isogenic with YJM627 (N1199lo 20S0 23S0) (Supplementary Table 3), 1 of the 100-genomes strains (Strope, Skelly, et al. 2015). Thus, in contrast to 20S and 23S, in at least 1 genetic background, N1199 does not accumulate to high levels during sporulation. This result implies different sporulation copy number regulation of N1199 vs 20S and 23S, which may in part explain why N1199, despite its ubiquity, has not been previously described.
Due to sequence quality near the ends of N1199, we were not confident in our ability to construct an N1199 launching vector. In addition, due to the sporulation defect of HO YJM1199 N1199hi and all HO YJM1199 segregants being N11990, we were not able to introduce N1199hi into N11990 strains. Thus, we were not able to determine whether the level of N1199 in the parental YJM1199 N1199hi was due to host genome sequences or N1199 genome sequences, or a combination of both. That said, the YJM1199 N1199hi vs YJM1199 N11990 phenotypes, such as the sporulation and carbon source utilization defects of YJM1199 N1199hi, clearly show that N1199hi is deleterious to both N1199 itself and to the YJM1199 host. Finally, as exemplified by these YJM1199 N1199hi results, RNA virus-dependent phenotypes, if not known or considered, have 2 implications. First, undetected RNA viruses may confound genotype association analyses. Second, undetected loss of RNA virus(es), which may occur during growth on different carbon sources (such as maltose) or drug treatments (such as rapamycin), may confound phenotypic analyses.
Supplementary Material
Acknowledgments
The authors thank Joseph Heitman, Blake Billmyre, Sheng Sun, Guiseppe Ianiri, and Vikas Yadav for their helpful comments and suggestions. We are grateful to the Cöers Laboratory (Duke) for the use of their fluorescence microscope and the Valdivia Lab (Duke) for reagents and the use of LiCOR for imaging northern blots.
Contributor Information
Sriram Vijayraghavan, Department of Molecular Genetics and Microbiology, Duke University Medical Center, 561 Research Drive 3020, Jones Bldg. Room 239, Durham, NC 27710, USA.
Stanislav G Kozmin, Department of Molecular Genetics and Microbiology, Duke University Medical Center, 561 Research Drive 3020, Jones Bldg. Room 239, Durham, NC 27710, USA.
Wen Xi, Department of Molecular Genetics and Microbiology, Duke University Medical Center, 561 Research Drive 3020, Jones Bldg. Room 239, Durham, NC 27710, USA.
John H McCusker, Department of Molecular Genetics and Microbiology, Duke University Medical Center, 561 Research Drive 3020, Jones Bldg. Room 239, Durham, NC 27710, USA.
Data availability
The N1199 sequence and plasmids described in this work have been deposited in NCBI (Accession number OP764685) and Addgene (http://www.addgene.org/John_McCusker/), respectively.
Supplemental material available at G3 online.
Funding
The research was supported by funding from NIH R01GM118936.
Communicating editor: B. Andrews
Literature Cited
- Abeliovich H, Klionsky DJ. Autophagy in yeast: mechanistic insights and physiological function. Microbiol Mol Biol Rev. 2001;65(3):463–479. doi: 10.1128/MMBR.65.3.463-479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG. Practical Statistics for Medical Research. New York: Chapman and Hall/CRC; 1991. [Google Scholar]
- Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol. 2006;55(4):539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- Brown JC, Lindquist S. A heritable switch in carbon source utilization driven by an unusual yeast prion. Genes Dev. 2009;23(19):2320–2332. doi: 10.1101/gad.1839109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya YV, Stephan JS, Reggiori F, Klionsky DJ, Herman PK. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J Biol Chem. 2004;279(20):20663–20671. doi: 10.1074/jbc.M400272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TH, Chang CR, Joy P, Yablok S, Gartenberg MR. Controlling gene expression in yeast by inducible site-specific recombination. Nucleic Acids Res. 2000;28(24):E108. doi: 10.1093/nar/28.24.e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevenet F, Brun C, Banuls AL, Jacq B, Christen R. Treedyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinform. 2006;7(1):439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Audic S, Claverie JM, Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol. 2010;10(1):8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard J-F, Guindon S, Lefort V, Lescot M, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(Web Server):W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JW. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci U S A. 1991;88(16):7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaglestone SS, Ruddock LW, Cox BS, Tuite MF. Guanidine hydrochloride blocks a critical step in the propagation of the prion-like determinant [PSI(+)] of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2000;97(1):240–244. doi: 10.1073/pnas.97.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004;5(1):113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban LM, Rodriguez-Cousino N, Esteban R. Double-stranded RNA (dsRNA) sequence reveals that T and W dsRNAs form a new RNA family in Saccharomyces cerevisiae. Identification of 23 S RNA as the single-stranded form of T dsRNA. J Biol Chem. 1992;267(15):10874–10881. doi: 10.1016/S0021-9258(19)50099-0. [DOI] [PubMed] [Google Scholar]
- Esteban R, Vega L, Fujimura T. 20S RNA narnavirus defies the antiviral activity of SKI1/XRN1 in Saccharomyces cerevisiae. J Biol Chem. 2008;283(38):25812–25820. doi: 10.1074/jbc.M804400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvik B, Haber JE. New cytoplasmic genetic element that controls 20S RNA synthesis during sporulation in yeast. J Bacteriol. 1978;134(1):261–269. doi: 10.1128/jb.134.1.261-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 2008;9(9):859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goertz GP, Miesen P, Overheul GJ, van Rij RP, van Oers MM, Pijlman G. Mosquito small RNA responses to West Nile and insect-specific virus infections in Aedes and Culex mosquito cells. Viruses. 2019;11(3):271. doi: 10.3390/v11030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15(14):1541–1553. doi:. [DOI] [PubMed] [Google Scholar]
- Grybchuk D, Akopyants NS, Kostygov AY, Konovalovas A, Lye LF, Dobson DE, Zangger H, Fasel N, Butenko A, Frolov AO, et al. Viral discovery and diversity in trypanosomatid protozoa with a focus on relatives of the human parasite Leishmania. Proc Natl Acad Sci U S A. 2018;115(3):E506–E515. doi: 10.1073/pnas.1717806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52(5):696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hacker CV, Brasier CM, Buck KW. A double-stranded RNA from a Phytophthora species is related to the plant endornaviruses and contains a putative UDP glycosyltransferase gene. J Gen Virol. 2005;86(5):1561–1570. doi: 10.1099/vir.0.80808-0. [DOI] [PubMed] [Google Scholar]
- Hillman BI, Cai G. The family narnaviridae: simplest of RNA viruses. Adv Virus Res. 2013;86:149–176. doi: 10.1016/B978-0-12-394315-6.00006-4. [DOI] [PubMed] [Google Scholar]
- Huang WP, Klionsky DJ. Autophagy in yeast: a review of the molecular machinery. Cell Struct Funct. 2002;27(6):409–420. doi: 10.1247/csf.27.409. [DOI] [PubMed] [Google Scholar]
- Huang WP, Scott SV, Kim J, Klionsky DJ. The itinerary of a vesicle component, Aut7p/Cvt5p, terminates in the yeast vacuole via the autophagy/CVT pathways. J Biol Chem. 2000;275(8):5845–5851. doi: 10.1074/jbc.275.8.5845. [DOI] [PubMed] [Google Scholar]
- Jarosz DF, Lancaster AK, Brown JCS, Lindquist S. An evolutionarily conserved prion-like element converts wild fungi from metabolic specialists to generalists. Cell. 2014;158(5):1072–1082. doi: 10.1016/j.cell.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth M, Mosch HU, Taxis C. Acetate regulation of spore formation is under the control of the Ras/cyclic AMP/protein kinase A pathway and carbon dioxide in Saccharomyces cerevisiae. Eukaryotic Cell. 2012;11(8):1021–1032. doi: 10.1128/EC.05240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, Onishi M, Datta N, Galicia S, Emili A, et al. A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature. 2006;439(7075):497–501. doi: 10.1038/nature04384. [DOI] [PubMed] [Google Scholar]
- Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147(2):435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhou J, Zhou X, Shuai S, Zhou R, An H, Fang S, Zhang S, Deng Q. A novel narnavirus from the plant-pathogenic fungus Magnaporthe oryzae. Arch Virol. 2020;165(5):1235–1240. doi: 10.1007/s00705-020-04586-7. [DOI] [PubMed] [Google Scholar]
- Lu YJ, Swamy KB, Leu JY. Experimental evolution reveals interplay between Sch9 and polyploid stability in yeast. PLoS Genet. 2016;12(11):e1006409. doi: 10.1371/journal.pgen.1006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P, Wera S, Van Dijck P, Thevelein JM. The PDE1-encoded low-affinity phosphodiesterase in the yeast Saccharomyces cerevisiae has a specific function in controlling agonist-induced cAMP signaling. Mol Biol Cell. 1999;10(1):91–104. doi: 10.1091/mbc.10.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan S, Maan NS, Samuel AR, O'Hara R, Meyer AJ, Rao S, Mertens PPC. Completion of the sequence analysis and comparisons of genome segment 2 (encoding outer capsid protein VP2) from representative isolates of the 24 bluetongue virus serotypes. Vet Ital. 2004;40:484–488. doi: 10.1016/j.jviromet.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Maan S, Rao S, Maan NS, Anthony SJ, Attoui H, Samuel AR, Mertens PPC. Rapid cDNA synthesis and sequencing techniques for the genetic study of bluetongue and other dsRNA viruses. J Virol Methods. 2007;143(2):132–139. doi: 10.1016/j.jviromet.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Maqueda M, Zamora E, Rodriguez-Cousino N, Ramirez M. Wine yeast molecular typing using a simplified method for simultaneously extracting mtDNA, nuclear DNA and virus dsRNA. Food Microbiol. 2010;27(2):205–209. doi: 10.1016/j.fm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Mardanov AV, Beletsky AV, Tanashchuk TN, Kishkovskaya SA, Ravin NV. A novel narnavirus from a Saccharomyces cerevisiae flor strain. Arch Virol. 2020;165(3):789–791. doi: 10.1007/s00705-020-04539-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Wickner RB. Yeast 20 S RNA replicon. Replication intermediates and encoded putative RNA polymerase. J Biol Chem. 1991;266(19):12779–12783. doi: 10.1016/S0021-9258(18)98967-2. [DOI] [PubMed] [Google Scholar]
- Nakayashiki T, Kurtzman CP, Edskes HK, Wickner RB. Yeast prions [URE3] and [PSI + ] are diseases. Proc Natl Acad Sci U S A. 2005;102(30):10575–10580. doi: 10.1073/pnas.0504882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol. 2001;21(13):4347–4368. doi: 10.1128/MCB.21.13.4347-4368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerva L, Turina M, Zanzotto A, Gardiman M, Gaiotti F, Gambino G, Chitarra W. Isolation, molecular characterization and virome analysis of culturable wood fungal endophytes in esca symptomatic and asymptomatic grapevine plants. Environ Microbiol. 2019;21(8):2886–2904. doi: 10.1111/1462-2920.14651. [DOI] [PubMed] [Google Scholar]
- Noda T. Regulation of autophagy through TORC1 and mTORC1. Biomolecules. 2017;7(4):52. doi: 10.3390/biom7030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikou K, Dovas CI, Maan S, Anthony SJ, Samuel AR, Papanastassopoulou M, Maan NS, Mangana O, Mertens PPC. Evolution and phylogenetic analysis of full-length VP3 genes of Eastern Mediterranean bluetongue virus isolates. PLoS One. 2009;4(7):e6437. doi: 10.1371/journal.pone.0006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki H, Sasaki A, Nomiyama K, Tomioka K. Multiple virus infection in a single strain of Fusarium poae shown by deep sequencing. Virus Genes. 2016;52(6):835–847. doi: 10.1007/s11262-016-1379-x. [DOI] [PubMed] [Google Scholar]
- Potgieter AC, Page NA, Liebenberg J, Wright IM, Landt O, van Dijk AA. Improved strategies for sequence-independent amplification and sequencing of viral double-stranded RNA genomes. J Gen Virol. 2009;90(6):1423–1432. doi: 10.1099/vir.0.009381-0. [DOI] [PubMed] [Google Scholar]
- Potgieter AC, Steele AD, van Dijk AA. Cloning of complete genome sets of six dsRNA viruses using an improved cloning method for large dsRNA genes. J Gen Virol. 2002;83(9):2215–2223. doi: 10.1099/0022-1317-83-9-2215. [DOI] [PubMed] [Google Scholar]
- Richaud A, Frezal L, Tahan S, Jiang H, Blatter JA, Zhao G, Kaur T, Wang D, Félix M-A. Vertical transmission in Caenorhabditis nematodes of RNA molecules encoding a viral RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 2019;116(49):24738–24747. doi: 10.1073/pnas.1903903116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MR, Payen C, Cheong F, Hovde BT, Bissonnette S, Arkin AP, Skerker JM, Brem RB, Caudy AA, Dunham MJ. Transposon insertional mutagenesis in Saccharomyces uvarum reveals trans-acting effects influencing species-dependent essential genes. Genome Res. 2019;29(3):396–406. doi: 10.1101/gr.232330.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano A, Rodriguez-Cousino N, Esteban R, Fujimura T. Persistent yeast single-stranded RNA viruses exist in vivo as genomic RNA.RNA polymerase complexes in 1:1 stoichiometry. J Biol Chem. 2000;275(34):26428–26435. doi: 10.1074/jbc.M002281200. [DOI] [PubMed] [Google Scholar]
- Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci U S A. 2009;106(40):17049–17054. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope PK, Kozmin SG, Skelly DA, Magwene PM, Dietrich FS, et al. 2µ plasmid in Saccharomyces species and in Saccharomyces cerevisiae. FEMS Yeast Res. 2015;129(1):e110. doi: 10.1093/femsyr/fov090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope PK, Skelly DA, Kozmin SG, Mahadevan G, Stone EA, Magwene PM, Dietrich FS, McCusker JH. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res. 2015;25(5):762–774. doi: 10.1101/gr.185538.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, et al. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20(21):5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torggler R, Papinski D, Kraft C. Assays to monitor autophagy in Saccharomyces cerevisiae. Cells. 2017;6(3):23. doi: 10.3390/cells6030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite MF, Mundy CR, Cox BS. Agents that cause a high frequency of genetic change from [psi + ] to [psi-] in Saccharomyces cerevisiae. Genetics. 1981;98(4):691–711. doi: 10.1093/genetics/98.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vepstaite-Monstavice I, Luksa J, Konovalovas A, Ezerskyte D, Staneviciene R, Strazdaitė-Žielienė Ž, Serva S, Servienė E. Saccharomyces paradoxus K66 killer system evidences expanded assortment of helper and satellite viruses. Viruses. 2018;10(10):E564. doi: 10.3390/v10100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Kozmin SG, Strope PK, Skelly DA, Lin Z, Kennell J, Magwene PM, Dietrich FS, McCusker JH. Mitochondrial genome variation affects multiple respiration and non-respiration phenotypes in Saccharomyces cerevisiae. Genetics. 2019;211(2):773–786. doi: 10.1534/genetics.118.301546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron FM, Stone GN, Obbard DJ. Metagenomic sequencing suggests a diversity of RNA interference-like responses to viruses across multicellular eukaryotes. PLoS Genet. 2018;14(7):e1007533. doi: 10.1371/journal.pgen.1007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke V, Vavassori M, Thevelein JM, Tortora P, Vanoni M. Regulation of maltose utilization in Saccharomyces cerevisiae by genes of the RAS/protein kinase A pathway. FEBS Lett. 1997;402(2–3):251–255. doi: 10.1016/S0014-5793(97)00009-4. [DOI] [PubMed] [Google Scholar]
- Wera S, Ma P, Thevelein JM. Glucose exerts opposite effects on mRNA versus protein and activity levels of Pde1, the low-affinity cAMP phosphodiesterase from budding yeast, Saccharomyces cerevisiae. FEBS Lett. 1997;420(2–3):147–150. doi: 10.1016/S0014-5793(97)01508-1. [DOI] [PubMed] [Google Scholar]
- Wesolowski M, Wickner RB. Two new double-stranded RNA molecules showing non-Mendelian inheritance and heat inducibility in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:181–187. doi: 10.1128/mcb.4.1.181-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB. Double-stranded and single-stranded RNA viruses of Saccharomyces cerevisiae. Annu Rev Microbiol. 1992;46(1):347–375. doi: 10.1146/annurev.mi.46.100192.002023. [DOI] [PubMed] [Google Scholar]
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264(5158):566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- Wickner RB. Prions and RNA viruses of Saccharomyces cerevisiae. Annu Rev Genet. 1996;30(1):109–139. doi: 10.1146/annurev.genet.30.1.109. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Fujimura T, Esteban R. Viruses and prions of Saccharomyces cerevisiae. Adv Virus Res. 2013;86:1–36. doi: 10.1016/B978-0-12-394315-6.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB, Shewmaker FP, Bateman DA, Edskes HK, Gorkovskiy A, Dayani Y, Bezsonov EE. Yeast prions: structure, biology, and prion-handling systems. Microbiol Mol Biol Rev. 2015;79(1):1–17. doi: 10.1128/MMBR.00041-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DH, Fennell DJ. Visualization of yeast mitochondrial DNA with the fluorescent stain “DAPI”. Methods Enzymol. 1979;56:728–733. doi: 10.1016/0076-6879(79)56065-0. [DOI] [PubMed] [Google Scholar]
- Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19(8):3290–3298. doi: 10.1091/mbc.e07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(S2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoll J, Verweij PE, Melchers WJG. Discovery and characterization of novel Aspergillus fumigatus mycoviruses. PLoS One. 2018;13(7):e0200511. doi: 10.1371/journal.pone.0200511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The N1199 sequence and plasmids described in this work have been deposited in NCBI (Accession number OP764685) and Addgene (http://www.addgene.org/John_McCusker/), respectively.
Supplemental material available at G3 online.