Abstract
Purpose:
Voice therapy is the primary treatment for children presenting with benign morphological vocal fold changes. This study examined the number of voice therapy sessions required to meet treatment goals and identified factors that predicted treatment length for pediatric voice patients.
Method:
An observational cohort design was employed. Data were extracted from the University of Wisconsin–Madison Voice and Swallow Outcome Database. This study examined 62 children who completed a course of voice therapy with a speech-language pathologist (SLP) addressing dysphonia caused by benign vocal fold lesions. Extracted data included patient demographics, auditory-perceptual assessments, acoustic and aerodynamic voice measures, videostroboscopy ratings, and medical comorbidities. Linear regression was used to identify predictors of number of therapy sessions.
Results:
Patients received an average of 7.5 sessions of voice therapy prior to discharge. Baseline auditory-perceptual assessment of dysphonia (p = .032), phonation threshold pressure (PTP, p = .005), Glottal Function Index (GFI) score (p = .006), and glottic closure pattern (p = .023) were significant predictors of number of voice therapy sessions. These measures, as well as hourglass glottic closure, predicted longer intervention duration. The regression model had an overall r 2 of .62.
Conclusions:
Pediatric voice therapy addressing benign vocal fold lesions and/or laryngeal edema required an average of 7.54 sessions before voice outcomes were sufficiently improved for discharge. More severe overall SLP ratings of dysphonia, GFI scores, PTP, or hourglass glottic closure pattern significantly predicted increased number of therapy sessions prior to discharge. Future work should determine what other factors affect treatment duration and how the efficiency of pediatric voice therapy can be maximized.
Pediatric voice disorders impede communication for many children due to fluctuating vocal intensity, voice breaks, and/or distracting vocal quality (Connor et al., 2008; Hoffman et al., 2015). The true prevalence of pediatric dysphonia is unknown as many children with voice disorders go undiagnosed; however, estimates range from 1.4% to 23.9% (Baynes, 1966; Bhattacharyya, 2015; Carding et al., 2006; Johnson et al., 2020; Powell et al., 1989). Voice disorders often restrict social and emotional functioning (Connor et al., 2008); dysphonia influences peer, adolescent, and adult perceptions of children (Lass et al., 1991; Ma & Yu, 2013). In addition, children and adolescents with voice disorders report frustration and limited participation in important events as a result of their voices (Connor et al., 2008). Although potential etiologies of pediatric dysphonia are varied, benign vocal fold lesions tend to be the most common etiology (Akif Kiliç et al., 2004; Gramuglia et al., 2014).
Benign vocal fold lesions or other vocal fold changes (i.e., edema) may require intervention with an otolaryngologist (Fearon, 1980; Healy, 1987; Hron et al., 2019); nevertheless, the primary treatment for these disorders is voice therapy with a speech-language pathologist (SLP; Braden & Verdolini Abbott, 2018; Cohen et al., 2015). Even when surgical interventions are indicated, outcomes are generally thought to be most efficacious when combined with behavioral therapy to promote safe voice use and prevent future injury (Murry & Woodson, 1992). Pediatric voice therapy addressing morphological vocal fold changes can produce gains in objective voice measures (Braden & Thibeault, 2020; Senkal & Ciyiltepe, 2013; Tezcaner et al., 2009; Valadez et al., 2012) and patient-reported quality of life scales (Hartnick et al., 2018; Kollbrunner & Seifert, 2013).
Voice therapy has also been reported to improve auditory-perceptual voice assessments in children (Akın Şenkal & Özer, 2015; Braden & Thibeault, 2020; Hirschberg et al., 1995; Lee & Son, 2005; Mackiewicz-Nartowicz et al., 2014; Mori, 1999; Mumović et al., 2014; Senkal & Ciyiltepe, 2013; Tezcaner et al., 2009; Trani et al., 2007). For example, improvements in perceived voice quality, measured using the Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V; Kempster et al., 2009) or the Grade, Roughness, Breathiness, Asthenia and Strain scale, have been documented following therapy addressing benign vocal fold lesions (Braden & Thibeault, 2020; So, 2018). These improvements are encouraging as they document functional improvements in voice quality that cannot currently be captured with any single objective voice measure (Fujiki & Thibeault, 2021).
As auditory-perceptual assessments can be vulnerable to various sources of bias (Eadie & Kapsner-Smith, 2011; Eadie et al., 2011; Helou et al., 2010; Kreiman & Gerratt, 2000; Kreiman et al., 2007; Wong et al., 2021), it is reassuring that improvements in perceived voice quality have often been accompanied by improvements in acoustic and aerodynamic voice measures (Senkal & Ciyiltepe, 2013; Tezcaner et al., 2009). For example, improvements in perturbation measures, such as jitter, shimmer, and/or noise-to-harmonic ratio (NHR), have been observed following therapy (Feinstein & Abbott, 2021; Glaze, 1996; Lee & Son, 2005; Mumović et al., 2014; Tezcaner et al., 2009; Valadez et al., 2012), indicating decreases in cycle-to-cycle variation in frequency and amplitude (Glaze et al., 1988), as well as decreased noise in the voice signal (Jotz et al., 2002). Additionally, improvements in aerodynamic measures such as phonation threshold pressure (PTP; Braden & Thibeault, 2020) indicate increased laryngeal pliability and decreased vocal fold viscosity following therapy (Fisher & Swank, 1997; Hoffman et al., 2019). These gains have also been associated with decreased vocal fold lesion mass (Deal et al., 1976; Hartnick et al., 2018; Niedzielska et al., 2001) and improvements in self-reported quality of life scales (Hartnick et al., 2018; Kollbrunner & Seifert, 2013; So, 2018), suggesting that patients feel less limited by their voices following treatment.
Even though considerable study supports the effectiveness of voice therapy, important aspects of pediatric voice intervention have rarely been examined (Desjardins et al., 2017; Fujiki et al., 2022). Required dosage of pediatric voice therapy has not been addressed. As such, it is unclear how many sessions of voice therapy are required to produce the favorable voice outcomes previously discussed. The study of intervention intensity in related fields suggests that the number of sessions required to produce desired therapeutic targets is important information for patients (Cherney, 2012) as it is directly related to the monetary costs of treatment. In addition, dose frequency—or the number of therapy sessions in a specific time period—clarifies intervention intensity (Warren et al., 2007), and total intervention time allows patients to understand the number of weeks (months or years) they may require treatment (Baker, 2012). These metrics have not been formally studied with regard to pediatric voice therapy. This is unfortunate as evidence suggests that health outcomes in general improve when patients know what to expect from treatment (Adams, 2010). At present, it is difficult for SLPs to advise children and their families regarding expected voice therapy dose. This, in turn, makes it difficult for patients and their parents to adjust their expectations and allocate the resources necessary to pursue voice intervention.
Additionally, it is not understood how patient demographics, voice measures taken at baseline, or patient/parent perception of vocal impairment might predict the number of sessions required to achieve voice-related treatment outcomes. Given the differences documented in cognitive function (Mabbott et al., 2006; Vygotsky, 1978) and laryngeal anatomy between young children, adolescents, and adults (Hammond et al., 1998, 2000; Jiang et al., 2000; Schweinfurth & Thibeault, 2008), it is possible that patient age may impact the number of sessions required to treat dysphonia. Alternatively, it could be that baseline severity of voice impairment drives the required number of therapy sessions. If this is the case, it would be important to know what types of voice measures best capture the impairments in vocal function that influence treatment duration. It could also be the case that medical comorbidities such as reflux, allergies, articulation delay, or dysphagia impact the number of required therapy sessions. Determining the factors that predict therapy duration is important as it allows clinicians to individualize their predictions for each patient and may promote treatment efficiency.
This study investigated the number of voice therapy sessions and total treatment duration required to meet treatment goals in pediatric voice patients. Additional factors impacting the number of voice therapy sessions required were also considered. It was hypothesized that increased severity of dysphonia as measured by baseline CAPE-V score, perturbation measures, PTP, and patient perception of vocal impairment as measured by the Glottal Function Index (GFI) would increase the number of voice therapy sessions required for discharge from treatment.
Materials and Methods
Study Design
This study employed an observational cohort design with data prospectively extracted from the University of Wisconsin–Madison Voice and Swallow Clinics Outcome Database. The Wisconsin–Madison School of Medicine and Public Health and Institutional Review Board oversees this database and its usage. Parents provide written consent for all patients under the age of 18 years prior to database inclusion. Additionally, written assent is obtained from all adolescents between the ages of 15 and 18 years. Following consent, patient demographics and clinical outcomes are prospectively collected for the database as patients receive health care in the pediatric voice and swallow clinic of a large tertiary pediatric hospital. At the time of this study, 623 pediatric patients of varying diagnoses were included in this database; 62 met criteria for the current study.
Inclusion criteria for children and adolescents who presented to the clinic with dysphonia between April 2009 and June 2021 included (a) being under the age of 18 years, (b) having underwent a voice evaluation with an SLP and a pediatric otolaryngologist including laryngeal imaging, (c) being diagnosed with benign vocal fold lesions or laryngeal edema (significant enough to alter vocal fold edge contour), (d) having completed a course of voice therapy with an SLP, and (e) being successfully discharged from voice therapy. Laryngeal imaging was performed on all patients using pediatric rigid or flexible distal chip scopes using halogen light. Videostroboscopy was performed for the 37 children who could tolerate the exam.
Treatment and Discharge
Voice therapy consisted of a combination of semi-occluded vocal tract exercises (SOVTE), resonant voice exercises, and education regarding vocal hygiene. Although all the patients received a combination of these three techniques, the individual needs of each child were the primary consideration in intervention. Thus, therapy was tailored to individual patient needs as dictated by best practice. Patients and their parents all endorsed good understanding of vocal hygiene concepts as explained by the treating clinician. Individual considerations included resonant voice techniques to teach children to safely increase vocal volume as well as specialized voice use patterns for singing, acting, or athletics. Therapy sessions were 50 min in duration, and patients were assigned homework to practice the voice exercises facilitated in therapy.
Discharge criteria for pediatric voice patients are individualized depending on patient goals and vocal needs. First, patients reported that their vocal function (i.e., vocal intensity, fundamental frequency [f o] range, vocal quality, vocal effort, and vocal endurance) met their daily needs. Additionally, they demonstrated proficiency with assigned voice exercises (SOVTE, resonant voice, or both) and demonstrated use of these techniques to elicit efficient vocal production. Exercise proficiency was rated by the treating clinician, and the percentage of accurate productions was tracked between sessions. Patients also demonstrated understanding of vocal hygiene recommendations. All patients included in this study presented with improvements in voice quality as measured by the CAPE-V (as performed by the treating clinician) and indicated that their voice met their daily needs before discharge. For young patients, this was often determined by parent report.
Potential Predictors of Therapy
The number of therapy sessions required prior to discharge was calculated. The following additional data points were also extracted.
Patient demographics: age and sex
Frequency dose: Therapy schedule was collected. Therapy schedules were coded as either weekly (1 week apart) or those with longer gaps between sessions (2 weeks or more). No patients completed more than one session in a calendar week. In addition, total intervention duration (number of weeks in therapy) was calculated.
Auditory-perceptual voice assessment: CAPE-V scores
Laryngeal imaging findings: Patient diagnosis was determined by the otolaryngologist, SLP ratings of vocal fold oscillation including mucosal wave, amplitude (normal, mild, moderate, or severely decreased), glottic closure (complete, posterior gap, anterior gap, hourglass, or incomplete), and lateral and anterior–posterior compression (none, mild, moderate, or severe). Glottic closure pattern was determined using closure categories previously described in the literature (Poburka et al., 2017; Poburka & Patel, 2021). Glottic closure pattern, mucosal wave, and amplitude data were available for the 37 children who tolerated a full stroboscopic exam.
Acoustic voice measures: Measures included jitter, shimmer, NHR, maximum phonation time (MPT), f o, and maximum and minimal f o of vocal range. Acoustic measures were calculated on sustained vowels and were collected and analyzed using the Computerized Speech Lab by KayPENTAX (Model 4500).
Aerodynamic voice measures: Measures included PTP, subglottal pressure, laryngeal airway resistance, and transglottal airflow. Aerodynamic measures were collected and analyzed using the Phonatory Aerodynamic System (Model 6600) from KayPENTAX.
Patient-reported scales: GFI, a four-question assessment of vocal effort, fatigue, pain, and quality, was completed by patients or their parents (for young children). Scores range from 0 to 20, with scores over 4 indicating a possible voice disorder (Bach et al., 2005). Reflux Severity Index (RSI) scores, a nine-question survey assessing reflux symptoms, was collected (Belafsky et al., 2002).
Medical history: articulation disorder (yes or no), patient reported reflux (yes or no), behavioral health diagnosis (yes or no), allergies (yes or no), and snoring (yes or no)
Statistical Analysis
All statistical analyses were performed using SPSS (Version 28, 2021). Linear regression was utilized to identify predictors of voice therapy duration. All variables mentioned above were included in a correlation matrix to determine which measures should be included in the statistical model (see Table 1). Variables significantly correlated with number of therapy sessions were included in the regression model. Number of therapy sessions was regressed on overall CAPE-V rating, jitter, NHR, PTP, age, GFI score, and glottic closure pattern (complete, posterior gap, anterior gap, hourglass, or incomplete). This analysis was used to predict the total number of therapy sessions. Post hoc analyses were performed using Pearson correlations or t tests with Bonferroni corrections to protect against Type 1 error. Alpha was set at .05 to determine significance.
Table 1.
Correlations used to determine factors for linear regression.
| Factor | Pearson correlation | p value | Factor | Pearson correlation | p value |
|---|---|---|---|---|---|
| Age | −.364 | .005 a | Fundamental frequency | −.016 | .910 |
| CAPE-V Overall | .721 | < .001 a | Glottal vibratory characteristics b | .280 | .067 |
| Glottal Function Index | .665 | < .001 a | Laryngeal airway resistance | .099 | .492 |
| Glottic closure pattern b | .262 | .039 a | Lowest vocal intensity | .207 | .478 |
| Jitter | .426 | .001 a | Maximum phonation time | −.089 | .523 |
| Noise-to-harmonic ratio | .273 | .023 a | Minimum f o | .149 | .297 |
| Phonation threshold pressure | .753 | < .001 a | Maximum f o | −.182 | .205 |
| Allergies | .159 | .204 | Reflux Severity Index | .043 | .750 |
| Articulation and/or language delay | −.077 | .550 | Reflux (patient report) | −.170 | .187 |
| Behavioral health | .105 | .358 | Sex | −.47 | .714 |
| CAPE-V Rough | .228 | .064 | Shimmer | .214 | .131 |
| CAPE-V Breathiness | .130 | .343 | Snoring | .104 | .658 |
| CAPE-V Pitch | −.009 | .946 | Subglottal pressure | .047 | .744 |
| CAPE-V Loudness | .278 | .060 | Therapy schedule | .031 | .809 |
| Dysphagia | .118 | .348 | Transglottal airflow | .103 | .479 |
Note. Bolded font indicates that factor was included in linear regression. CAPE-V = Consensus Auditory-Perceptual Evaluation of Voice.f o = fundamental frequency.
Statistical significance at p < .05.
N = 37.
Results
Data from 62 children were included in this study (M age = 8.06 years, SD = 3.99; 20 females, 42 males). Distribution of ages is presented in Figure 1D. Forty-six children were diagnosed with benign vocal fold lesions (M age = 7.86 years, SD = 3.5), and 16 children were diagnosed with laryngeal edema of presumed behavioral etiology based on history and appearance (M age = 8.58 years, SD = 4.1). Average number of voice therapy sessions prior to discharge was 7.54 (SD = 4.3). Number of sessions ranged from two to 24, although 72% of patients required between three and 10 sessions. No significant difference in number of sessions was observed between diagnostic groups (p = .113). Dose frequency was weekly for 30 patients and was once every 2 weeks (or more) for 32 patients. Average total intervention duration was 7.2 weeks for individuals receiving weekly therapy and 16 weeks for individuals with two or more weeks between sessions. Dose frequency, or therapy schedule, was not correlated with number of sessions (p = .809).
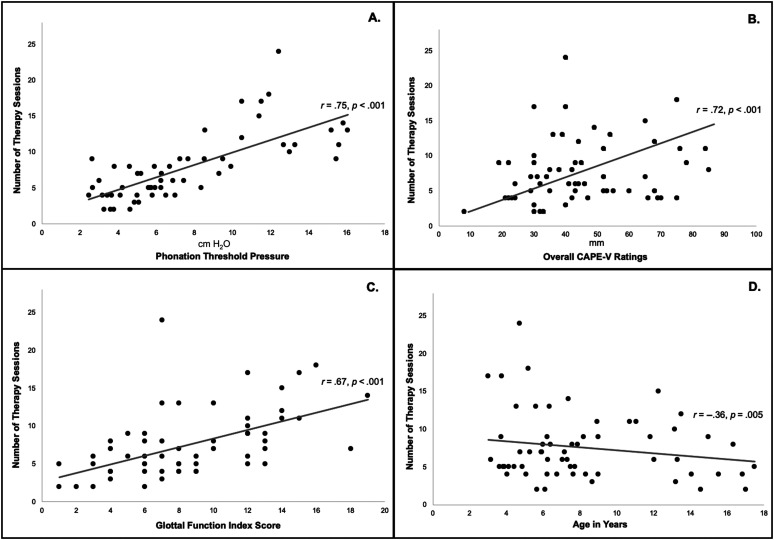
Figure 1.
Correlation of (A) phonation threshold pressure, (B) overall Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V) ratings, (C) Glottal Function Index Scores, and (D) age with number of therapy sessions required prior to discharge.
The regression model had an overall r 2 value of .62 suggesting that the model explained 62% of the variation in the number of sessions required. Statistical summary is presented in Table 2. Baseline overall CAPE-V rating (β = .058, t = 1.92, p = .032) was a significant predictor of number of voice therapy sessions. Overall CAPE-V rating was significantly correlated with the number of sessions required (r = .72, p < .001) as patients judged to be more dysphonic required more therapy. On average, patients who underwent ≤ 6 sessions had significantly lower CAPE-V ratings (M = 37.04, SD = 14.30) than those who underwent ≥ 7 sessions (M = 46.73, SD = 19.89; p = .06). Distribution of CAPE-V ratings and number of therapy sessions is presented in Figure 1.
Table 2.
Statistical summary for linear regression.
| Factor | Coefficient | Standard error | t | p value | CI (2.5%) | CI (97.5%) |
|---|---|---|---|---|---|---|
| Overall CAPE-V rating | .058 | 0.030 | 1.92 | .032* | −0.003 | 0.119 |
| Jitter | .125 | 0.321 | 0.389 | .699 | −0.524 | 0.744 |
| Noise-to-harmonic ratio | .066 | 8.55 | 0.008 | .994 | −17.2 | 17.3 |
| Phonation threshold pressure | .418 | 0.140 | 2.99 | .005* | 0.136 | 0.701 |
| Age | −.157 | 0.111 | −1.41 | .165 | −0.381 | 0.067 |
| Glottal Function Index | .347 | 0.120 | 2.89 | .006* | 0.105 | 0.590 |
| Glottic closure pattern | .734 | 0.312 | 2.35 | .023* | 0.104 | 1.36 |
Note. CI = confidence interval; CAPE-V = Consensus Auditory-Perceptual Evaluation of Voice.
Statistical significance at p < .05.
PTP at baseline was a significant predictor of number of therapy sessions (β = .418, t = 2.99, p = .005). PTP was significantly correlated with number of sessions (r = .75, p < .001) as higher PTP values were associated with more sessions of therapy. On average, patients who underwent ≤ 6 sessions had significantly lower PTP values (M = 4.81, SD = 1.45) than those who underwent ≥ 7 sessions (M = 9.38, SD = 3.9; p < .001). Distribution of PTP and number of therapy sessions is presented in Figure 1.
Baseline GFI score was a significant predictor of number of therapy sessions (β = .347, t = 2.89, p = .006). GFI scores were correlated with the number of sessions required prior to discharge (r = .67, p < .001), as patients with higher scores required more therapy. Seventy-nine percent of patients scored above a 4 on the GFI. On average, patients who underwent ≤ 6 sessions had significantly lower GFI scores (M = 6.31, SD = 3.01) than those who underwent ≥ 7 sessions (M = 11.27, SD = 4.02; p < .01). Distribution of GFI scores and number of therapy sessions is presented in Figure 1.
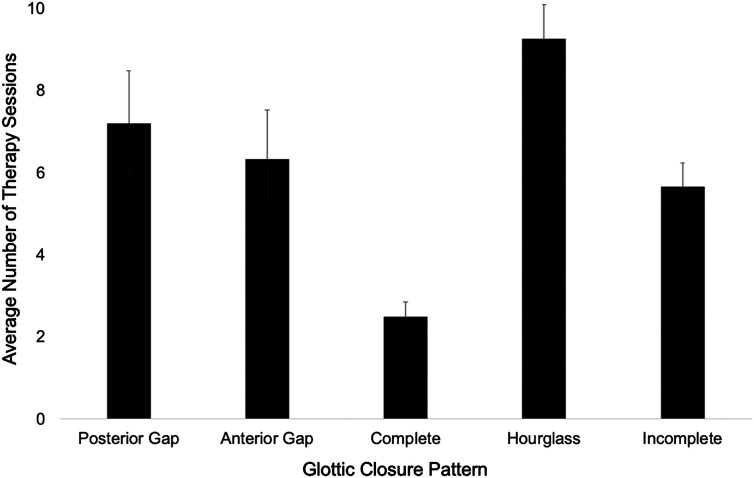
Finally, glottic closure pattern at baseline significantly predicted number of therapy sessions (β = .734, t = 2.35, p = .023). Sixty percent of patients who required ≥ 7 sessions presented with hourglass glottic closure. The predominant glottic closure pattern observed in patients who required ≤ 6 sessions of therapy was either complete or posterior gap closure (61%). Additionally, individuals with hourglass glottic closure required an average of eight therapy sessions, and individuals with other glottic closure types had an average of six therapy sessions. Average number of therapy sessions for patients with each glottic closure pattern is presented in Figure 2. No other stroboscopy parameters were correlated with number of therapy sessions required.
Figure 2.
Means and standard errors for number of therapy sessions across glottic closure patterns.
Although age was not a significant predictor of the number of voice therapy sessions required before discharge (β = −.157, t = −1.46, p = .151), there was a significant negative correlation between age and number of sessions (r = −.354, p = .005), indicating that, on average, older children required fewer sessions of therapy before discharge. Children under the age of 6 years required an average of eight sessions, whereas children older than 6 years required between six and seven sessions. Sex was not significantly correlated with number of sessions.
Two acoustic measures were significantly correlated with—but did not significantly predict—the number of therapy sessions required prior to discharge. Baseline jitter (r = .42, p = .001) and NHR (r = .27, p = .025) were significantly correlated with number of sessions. MPT, f o range, f o, laryngeal airway resistance, subglottal pressure, and transglottal airflow were not significantly correlated with number of sessions required before discharge.
Baseline RSI was not significantly correlated with number of therapy sessions. Twenty-four percent of patients scored over 14 on the RSI, and 35% of patients reported a history of reflux. Twenty-nine percent of patients reported allergies to environmental or seasonal triggers, and 4% of patients reported a comorbid behavioral health diagnosis (general anxiety disorder). Additionally, 17% of patients presented with an articulation delay, and 16% of patients presented with snoring. No significant correlations between these factors and number of required therapy sessions were observed.
Discussion
Voice therapy is the primary treatment for children with behaviorally induced vocal fold changes such as benign lesions or laryngeal edema. Although evidence suggests that pediatric voice therapy is effective, therapy dosage has rarely been examined in the pediatric population. This is unfortunate as it prevents SLPs from preparing children and their families for the course of treatment. Additionally, evidence suggests that health outcomes improve when patients know what to expect from intervention (Adams, 2010). As such, this study investigated the number of voice therapy sessions required to meet treatment goals in pediatric voice patients. On average, children required 7.54 sessions of voice therapy before vocal function was sufficiently improved for discharge, per clinical assessment. Frequency dose (weekly or once every 2 weeks or longer) did not affect the number of sessions required for discharge, but less frequent sessions more than doubled total intervention duration. Additional factors impacting the number of voice therapy sessions included baseline overall CAPE-V rating, GFI score, PTP, and glottic closure pattern at baseline. The regression model had an overall r 2 of .62, suggesting that the factors examined in this study accounted for a fair amount of the variance in the number of voice therapy sessions required.
It is not surprising that baseline overall CAPE-V scores predicted the number of therapy sessions required before discharge. Auditory-perceptual voice evaluations assess voice quality from a functional perspective and correlate with patient-reported outcome measures in adults (Fujiki & Thibeault, in press). Present findings suggest that a similar relationship exists between CAPE-V ratings and the voice goals that govern pediatric voice therapy—whether these goals be patient or parent driven (or potentially a mix of the two). In addition, ratings of overall voice quality tend to be better correlated with objective voice measures and patient perception when compared with any one voice quality parameter (roughness, breathiness, strain, etc.; Fujiki & Thibeault, 2021, in press). These findings also add to the body of work suggesting that auditory-perceptual voice assessments are related to voice outcomes (Braden & Thibeault, 2020; Fujiki & Thibeault, 2021; Tezcaner et al., 2009). It is hypothesized that perceived severity of dysphonia drives treatment duration as SLPs specializing in the evaluation of pediatric voice likely encourage patients to continue voice therapy when they perceive patients remain dysphonic. Further work is needed, however, to determine if this is the case, as it is also possible that treatment duration impacts auditory-perceptual assessment—since clinicians may rate voices differently when they are more familiar with them or have heard them over an extended treatment period.
GFI scores significantly predicted the number of voice therapy sessions needed. This may indicate that the GFI reflects patient (or parent) perception of voice disorder severity. As such, children, or parents, less impacted by dysphonia presumably score lower on this scale, whereas those more concerned about their vocal function score higher. For young children, it is often caregivers who complete this measure and are primarily responsible for voice treatment adherence. This is not the first work to examine this measure in children, however, as research indicates that the GFI is a predictor of the presence of laryngeal lesions in children (Cohen et al., 2007) and can be employed reliably to screen for voice disorders in this population (Pribuisiene et al., 2019; Ulozaite-Staniene et al., 2019). These findings support the clinical utility of the GFI, which is an efficient tool (only four questions), for capturing patient perception of voice disorders (Bach et al., 2005). This tool may be particularly useful for children who may have difficulty completing longer questionnaires.
PTP was a significant predictor of the number of voice therapy sessions required prior to discharge. This was expected considering that PTP is a measure of the lung pressure required to initiate and sustain vocal fold oscillation (Titze, 1988) and has been shown to reflect laryngeal viscoelastic properties and pliability (Plexico et al., 2011). Additionally, PTP is sensitive to changes in vocal effort and dehydration (Chang & Karnell, 2004; Fujiki et al., 2021; Sivasankar & Erickson, 2008; Tanner et al., 2010). PTP is also the aerodynamic voice measure most correlated with auditory-perceptual voice assessments in children (Fujiki & Thibeault, 2021), suggesting it may be the aerodynamic measure that best correlates with overall voice function. In addition, evidence suggests that PTP is sensitive to vocal fold changes that result in increased laryngeal stiffness and thickness, such as benign lesions or edema (Zhuang et al., 2013). Although PTP can be challenging to collect in children, evidence suggests that it can be reliably done (Hoffman et al., 2019). Our findings suggest that collecting PTP is worth the effort, as it reflects voice function.
The last significant predictor of the number of therapy sessions required before discharge was glottic closure pattern. On average, children with hourglass glottic closure required more sessions of therapy. This might be expected, as glottic closure is correlated with vocal fold lesion mass severity and location (Martinez-Paredes et al., in press). In addition, glottic insufficiency reduces vocal capacity (Schneider & Bigenzahn, 2003) and diminishes voice quality in children (Nuss et al., 2010). Overall, hourglass glottic closure is associated with vocal fold nodules and often results in more severe glottic insufficiency than posterior or anterior gap closure (Banjara et al., 2012). As such, it is likely that children with hourglass glottic closure also presented with larger laryngeal lesions and more severe glottic insufficiency. It was surprising that glottic closure was the only videostroboscopy parameter that predicted the number of therapy sessions. It is possible that the scale used to measure parameters like mucosal wave and vocal fold amplitude (normal, mild, moderate, severe) was not sufficiently sensitive to capture this relationship. Additionally, clinical observation suggests that mucosal wave and/or vocal fold amplitude can be difficult to quantify in children as they often do not tolerate stroboscopic exams and may struggle to vocalize at habitual intensity with the scope in place.
There were some significant correlations observed between number of sessions required and other factors such as age, jitter, and NHR. R values for these correlations were in low range, however, indicating that in the current study, these relationships were weak. Overall, more severe perturbation measures at baseline were correlated with more therapy sessions. This is likely because perturbation measures are at least partially correlated with auditory-perceptual voice assessments (Fujiki & Thibeault, 2021). It may be that this relationship was not stronger because perturbation measures do not effectively measure severely dysphonic or aperiodic voices (Ludlow et al., 1987). Regarding age, younger children spent more sessions working with clinicians on voice exercises. This reflects clinical experience suggesting that young children often need multiple sessions to learn voice exercises and even more sessions to generalize new patterns to conversational voice use. It may also be the case, however, that motivational factors influence pediatric patients differently. It is likely that older children and adolescents experience more intense emotional side effects from voice disorders (Connor et al., 2008), have more specific voice goals, and hold higher standards for their vocal quality. In contrast, young children may be less aware of dysphonia (Connor et al., 2008).
It is interesting that dose frequency did not affect the number of voice therapy sessions required. This suggests that both weekly sessions and sessions every other week produced positive voice outcomes. This finding supports past work in adults indicating that both weekly and intensive therapy schedules produced comparable outcomes (Fu et al., 2015; Meerschman et al., 2019; Patel et al., 2011; Wenke et al., in press). This may be because clinicians chose the therapy schedule that best met the needs of each child and their family. Both models could have benefits, as more frequent therapy might allow for better short-term retention of skills, but longer periods between sessions could allow for more practice time and more time for laryngeal lesions to heal if patients adhere to hygiene recommendations. It follows, of course, that sessions every other week led to longer total intervention duration. This is not necessarily a drawback, however, as total time in intervention did not increase treatment cost. Best practice would dictate that SLPs take individual patient circumstances into account and choose the therapy schedule that best suits their needs.
No clear correlation between RSI scores or reported history of reflux and the number of required therapy sessions was observed. Reflux has been at least partially implicated in the etiology of pediatric dysphonia (Block & Brodsky, 2007; Saniasiaya & Kulasegarah, 2020; Wertz et al., 2020); however, this relationship is not well understood (Kavookjian et al., 2020). It is debatable how accurately RSI measures actual laryngopharyngeal reflux in adults (Vance et al., in press), and this tool has never been validated for use in children, who may be less likely to identify symptoms such as globus, dysphonia, or dysphagia. Additionally, it may be that children were managed for reflux if indicated, as they had all seen an otolaryngologist at their initial voice evaluation. No other medical comorbidities such as anxiety, allergies, or articulation delays were significantly correlated with the number of required therapy sessions. This is likely because these factors—although important—do not directly impact laryngeal function in most children. Future studies should, however, examine these factors in larger sample sizes.
Although the findings of this study contribute to our understanding of the duration and intensity of successful intervention in pediatric voice disorders, it should be noted that the regression model explained only 62% of the variance in the number of voice therapy sessions required. Although this is a fairly high r 2 for this type of research question, it is clear that there are important factors outside the scope of this study. Many factors that impact therapeutic success are difficult to measure. These include patient personality, personal voice goals, family dynamics, and clinician–patient rapport. Additionally, factors such as motor, cognitive, and vocal fold tissue development, or an individual's kinesthetic awareness, may impact therapeutic success. Also, it is difficult to determine how factors such as insurance reimbursement may influence clinician discharge decisions. As such, future study should develop effective methods of examining these factors and determining their impact on pediatric voice therapy. Additionally, as multiple factors in the linear regression model examined vocal function, some overlap did, by nature, exist between factors. Correlations between factors were determined to be weak; however, caution should be exercised when examining the role of any one factor from the current analysis in isolation. In addition, although the current study population was larger than that often examined in children, it will also be important to replicate this study in larger numbers and at other medical centers, as these data came from one location. Lastly, it should be remembered that the population examined in this study successfully completed a course of voice therapy. As such, future work is needed to determine factors that impact therapy dropouts and those who choose not to pursue behavioral voice therapy at all.
Conclusions
Pediatric voice therapy addressing benign vocal fold lesions and laryngeal edema required an average of 7.54 sessions before voice outcomes were sufficiently improved for discharge due to successful completion of therapy. As such, eight voice therapy sessions is likely a reasonable benchmark estimate for clinicians to provide to patients and their parents. Almost all patients required ≤ 10 sessions, so this could be a more conservative estimate for patient families who may be concerned if therapy continues longer than expected. Overall CAPE-V ratings, GFI score, PTP, and glottic closure pattern were significant predictors of the number of therapy sessions required. Dose frequency of voice therapy was not correlated with the total number of required sessions but did impact total intervention duration (as increased time between sessions increased total treatment duration). As such, for children with mild baseline CAPE-V ratings, PTP values, GFI scores, or more complete glottic closure, six sessions of therapy could be a more appropriate estimate. Conversely, children with more severely impacted baseline values on these measures may require longer courses of treatment. Additionally, as clinicians must choose from a battery of voice measures when evaluating pediatric voices, the measures documented in this study to be predictive of therapy duration may be particularly valuable. Future work should examine if other voice measures such as cepstral peak prominence (Esen Aydinli et al., 2019) or the pediatric voice-related quality of life scale (Boseley et al., 2006) might predict the number of sessions required before discharge. Future study should also determine what additional factors affect the course of treatment and how the efficiency of voice therapy can be maximized.
Data Availability Statement
No data are available.
Acknowledgments
This work was funded by National Institutes of Health NIDCDT32-DC009401 (University of Wisconsin–Madison).
Funding Statement
This work was funded by National Institutes of Health NIDCDT32-DC009401 (University of Wisconsin–Madison).
References
- Adams, R. J. (2010). Improving health outcomes with better patient understanding and education. Risk Management and Healthcare Policy, 3, 61–72. https://doi.org/10.2147/RMHP.S7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akif Kiliç, M. , Okur, E. , Yildirim, I. , & Güzelsoy, S. (2004). The prevalence of vocal fold nodules in school age children. International Journal of Pediatric Otorhinolaryngology, 68(4), 409–412. https://doi.org/10.1016/j.ijporl.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Akın Şenkal, Ö. , & Özer, C. (2015). Hoarseness in school-aged children and effectiveness of voice therapy in international classification of functioning framework. Journal of Voice, 29(5), 618–623. https://doi.org/10.1016/j.jvoice.2014.10.018 [DOI] [PubMed] [Google Scholar]
- Bach, K. K. , Belafsky, P. C. , Wasylik, K. , Postma, G. N. , & Koufman, J. A. (2005). Validity and Reliability of the Glottal Function Index. Archives of Otolaryngology—Head & Neck Surgery, 131(11), 961–964. https://doi.org/10.1001/archotol.131.11.961 [DOI] [PubMed] [Google Scholar]
- Baker, E. (2012). Optimal intervention intensity. International Journal of Speech-Language Pathology, 14(5), 401–409. https://doi.org/10.3109/17549507.2012.700323 [DOI] [PubMed] [Google Scholar]
- Banjara, H. , Mungutwar, V. , Singh, D. , Gupta, A. , & Singh, S. (2012). Demographic and videostroboscopic assessment of vocal pathologies. Indian Journal of Otolaryngology and Head & Neck Surgery, 64(2), 150–157. https://doi.org/10.1007/s12070-011-0451-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes, R. A. (1966). An incidence study of chronic hoarseness among children. Journal of Speech and Hearing Disorders, 31(2), 172–176. https://doi.org/10.1044/jshd.3102.172 [DOI] [PubMed] [Google Scholar]
- Belafsky, P. C. , Postma, G. N. , & Koufman, J. A. (2002). Validity and reliability of the reflux symptom index (RSI). Journal of Voice, 16(2), 274–277. https://doi.org/10.1016/S0892-1997(02)00097-8 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya, N. (2015). The prevalence of pediatric voice and swallowing problems in the United States. The Laryngoscope, 125(3), 746–750. https://doi.org/10.1002/lary.24931 [DOI] [PubMed] [Google Scholar]
- Block, B. B. , & Brodsky, L. (2007). Hoarseness in children: The role of laryngopharyngeal reflux. International Journal of Pediatric Otorhinolaryngology, 71(9), 1361–1369. https://doi.org/10.1016/j.ijporl.2006.10.029 [DOI] [PubMed] [Google Scholar]
- Boseley, M. E. , Cunningham, M. J. , Volk, M. S. , & Hartnick, C. J. (2006). Validation of the Pediatric Voice-Related Quality-of-Life survey. Archives of Otolaryngology—Head & Neck Surgery, 132(7), 717. https://doi.org/10.1001/archotol.132.7.717 [DOI] [PubMed] [Google Scholar]
- Braden, M. , & Thibeault, S. L. (2020). Outcomes of voice therapy in children with benign vocal fold lesions. International Journal of Pediatric Otorhinolaryngology, 136, 110121. https://doi.org/10.1016/j.ijporl.2020.110121 [DOI] [PubMed] [Google Scholar]
- Braden, M. , & Verdolini Abbott, K. (2018). Advances in pediatric voice therapy. Perspectives of the ASHA Special Interest Groups, 3(3), 68–76. https://doi.org/10.1044/2018_PERS-SIG3-2018-0005 [Google Scholar]
- Carding, P. N. , Roulstone, S. , Northstone, K. , & ALSPAC Study Team. (2006). The prevalence of childhood dysphonia: A cross-sectional study. Journal of Voice, 20(4), 623–630. https://doi.org/10.1016/j.jvoice.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Chang, A. , & Karnell, M. (2004). Perceived phonatory effort and phonation threshold pressure across a prolonged voice loading task: A study of vocal fatigue. Journal of Voice, 18(4), 454–466. https://doi.org/10.1016/j.jvoice.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Cherney, L. R. (2012). Aphasia treatment: Intensity, dose parameters, and script training. International Journal of Speech-Language Pathology, 14(5), 424–431. https://doi.org/10.3109/17549507.2012.686629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. T. , Oestreicher-Kedem, Y. , Fliss, D. M. , & DeRowe, A. (2007). Glottal function index: A predictor of glottal disorders in children. Annals of Otology, Rhinology & Laryngology, 116(2), 81–84. https://doi.org/10.1177/000348940711600201 [DOI] [PubMed] [Google Scholar]
- Cohen, S. M. , Dinan, M. A. , Kim, J. , & Roy, N. (2016). Otolaryngology utilization of speech-language pathology services for voice disorders. The Laryngoscope, 124, 906–912. https://doi.org/10.1002/lary.25574 [DOI] [PubMed] [Google Scholar]
- Connor, N. P. , Cohen, S. B. , . Theis, S. M. , Thibeault, S. L. , Heatley, D. , Bless, D. G. , & Bless, D. M. (2008). Attitudes of children with dysphonia. Journal of Voice, 22(2), 197–209. https://doi.org/10.1016/j.jvoice.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Deal, R. E. , McClain, B. , & Sudderth, J. F. (1976). Identification, evaluation, therapy, and follow-up for children with vocal nodules in a public school setting. Journal of Speech and Hearing Disorders, 41(3), 390–397. https://doi.org/10.1044/jshd.4103.390 [DOI] [PubMed] [Google Scholar]
- Desjardins, M. , Halstead, L. , Cooke, M. , & Bonilha, H. S. (2017). A systematic review of voice therapy: What “effectiveness” really implies. Journal of Voice, 31(3), 392.e13–392.e32. https://doi.org/10.1016/j.jvoice.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Eadie, T. L., & Kapsner-Smith, M. (2011). The effect of listener experience and anchors on judgments of dysphonia. Journal of Speech, Language, and Hearing Research, 54(2), 430–447. https://doi.org/10.1044/1092-4388(2010/09-0205) [DOI] [PubMed] [Google Scholar]
- Eadie, T. , Sroka, A. , Wright, D. R. , & Merati, A. (2011). Does knowledge of medical diagnosis bias auditory-perceptual judgments of dysphonia? Journal of Voice, 25(4), 420–429. https://doi.org/10.1016/j.jvoice.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Esen Aydinli, F. , Özcebe, E. , & İncebay, Ö. (2019). Use of cepstral analysis for differentiating dysphonic from normal voices in children. International Journal of Pediatric Otorhinolaryngology, 116, 107–113. https://doi.org/10.1016/j.ijporl.2018.10.029 [DOI] [PubMed] [Google Scholar]
- Fearon, B. (1980). Laryngeal surgery in the pediatric patient. Annals of Otology, Rhinology & Laryngology, 89(Suppl. 5), 146–149. https://doi.org/10.1177/00034894800890S534 [DOI] [PubMed] [Google Scholar]
- Feinstein, H. , & Abbott, K. V. (2021). Behavioral treatment for benign vocal fold lesions in children: A systematic review. American Journal of Speech-Language Pathology, 30(2), 772–788. https://doi.org/10.1044/2020_AJSLP-20-00304 [DOI] [PubMed] [Google Scholar]
- Fisher, K. , & Swank, P. (1997). Estimating phonation threshold pressure. Journal of Speech, Language, and Hearing Research, 40(5), 1122–1129. https://doi.org/10.1044/jslhr.4005.1122 [DOI] [PubMed] [Google Scholar]
- Fu, S. , Theodoros, D. G. , & Ward, E. C. (2015). Intensive versus traditional voice therapy for vocal nodules: Perceptual, physiological, acoustic and aerodynamic changes. Journal of Voice, 29(2), 260.e31–260.e44. https://doi.org/10.1016/j.jvoice.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Fujiki, R. B. , Fujiki, A. E. , & Thibeault, S. (2022). Factors impacting therapy duration in children and adolescents with Paradoxical Vocal Fold Movement (PVFM). International Journal of Pediatric Otorhinolaryngology, 158, 111182. https://doi.org/10.1016/j.ijporl.2022.111182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki, R. B. , Huber, J. E. , & Sivasankar, M. P. (2021). Mitigating the effects of acute vocal exertion in individuals with vocal fatigue. The Laryngoscope, 131(12), 2732–2739. https://doi.org/10.1002/lary.29627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki, R. B. , & Thibeault, S. L. (2021). The relationship between auditory-perceptual rating scales and objective voice measures in children with voice disorders. American Journal of Speech-Language Pathology, 30(1), 228–238. https://doi.org/10.1044/2020_AJSLP-20-00188 [DOI] [PubMed] [Google Scholar]
- Fujiki, R. B. , & Thibeault, S. L. (in press). Examining relationships between GRBAS ratings and acoustic, aerodynamic and patient-reported voice measures in adults with voice disorders. Journal of Voice. https://doi.org/10.1016/j.jvoice.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze, L. E. (1996). Treatment of voice hyperfunction in the pre-adolescent. Language, Speech, and Hearing Services in Schools, 27(3), 244–250. https://doi.org/10.1044/0161-1461.2703.244 [Google Scholar]
- Glaze, L. E. , Bless, D. M. , Milenkovic, P. , & Susser, R. D. (1988). Acoustic characteristics of children's voice. Journal of Voice, 2(4), 312–319. https://doi.org/10.1016/S0892-1997(88)80023-7 [Google Scholar]
- Gramuglia, A. C. J. , Tavares, E. L. M. , Rodrigues, S. A. , & Martins, R. H. G. (2014). Perceptual and acoustic parameters of vocal nodules in children. International Journal of Pediatric Otorhinolaryngology, 78(2), 312–316. https://doi.org/10.1016/j.ijporl.2013.11.032 [DOI] [PubMed] [Google Scholar]
- Hammond, T. , Gray, S. , Butler, J. , Zhou, R. , & Hammond, E. (1998). Age and gender-related elastin distribution changes in human vocal folds. Otolaryngology—Head & Neck Surgery, 119(4), 314–322. https://doi.org/10.1016/S0194-5998(98)70071-3 [DOI] [PubMed] [Google Scholar]
- Hammond, T. H. , Gray, S. D. , & Butler, J. E. (2000). Age- and gender-related collagen distribution in human vocal folds. Annals of Otology, Rhinology & Laryngology, 109(10), 913–920. https://doi.org/10.1177/000348940010901004 [DOI] [PubMed] [Google Scholar]
- Hartnick, C. , Ballif, C. , De Guzman, V. , Sataloff, R. , Campisi, P. , Kerschner, J. , Shembel, A. , Reda, D. , Shi, H. , Sheryka Zacny, E. , & Bunting, G. (2018). Indirect vs direct voice therapy for children with vocal nodules: A randomized clinical trial. JAMA Otolaryngology—Head & Neck Surgery, 144(2), 156–163. https://doi.org/10.1001/jamaoto.2017.2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, G. B. (1987). Current management of lesions of the pediatric larynx. Annals of Otology, Rhinology & Laryngology, 96(1), 122–123. https://doi.org/10.1177/000348948709600129 [DOI] [PubMed] [Google Scholar]
- Helou, L. B., Solomon, N. P. , Henry, L. R. , Coppit, G. L. , Howard, R. S. , & Stojadinovic, A. (2010). The role of listener experience on Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V) ratings of postthyroidectomy voice. American Journal of Speech-Language Pathology, 19(3), 248–258. https://doi.org/10.1044/1058-0360(2010/09-0012) [DOI] [PubMed] [Google Scholar]
- Hirschberg, J. , Dejonckere, P. H. , Hirano, M. , Mori, K. , Schultz-Coulon, H.-J. , & Vrtička, K. (1995). Voice disorders in children. International Journal of Pediatric Otorhinolaryngology, 32, S109–S125. https://doi.org/10.1016/0165-5876(94)01149-R [DOI] [PubMed] [Google Scholar]
- Hoffman, H. J. , Li, C. M. , Bainbridge, K. E. , Losonczy, K. G. , Chiu, M. S. , & Rice, M. L. (2015). Voice, speech, and language problems in the U.S. pediatric population: The 2012 National Health Interview Survey (NHIS). International Journal of Epidemiology, 44(Suppl. 1), i260. https://doi.org/10.1093/ije/dyv096.489 [Google Scholar]
- Hoffman, M. R. , Scholp, A. J. , Hedberg, C. D. , Lamb, J. R. , Braden, M. N. , McMurray, J. S. , & Jiang, J. J. (2019). Measurement reliability of phonation threshold pressure in pediatric subjects. The Laryngoscope, 129(7), 1520–1526. https://doi.org/10.1002/lary.27418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hron, T. A. , Kavanagh, K. R. , & Murray, N. (2019). Diagnosis and treatment of benign pediatric lesions. Otolaryngologic Clinics of North America, 52(4), 657–668. https://doi.org/10.1016/j.otc.2019.03.010 [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Lin, E. , & Hanson, D. G. (2000). Vocal fold physiology. Otolaryngologic Clinics of North America, 33(4), 699–718. https://doi.org/10.1016/S0030-6665(05)70238-3 [DOI] [PubMed] [Google Scholar]
- Johnson, C. M. , Anderson, D. C. , & Brigger, M. T. (2020). Pediatric dysphonia: A cross-sectional survey of subspecialty and primary care clinics. Journal of Voice, 34(2), 301.e1–301.e5. https://doi.org/10.1016/j.jvoice.2018.08.017 [DOI] [PubMed] [Google Scholar]
- Jotz, G. P. , Cervantes, O. , Abrahão, M. , Settanni, F. A. P. , & de Angelis, E. C. (2002). Noise-to-harmonics ratio as an acoustic measure of voice disorders in boys. Journal of Voice, 16(1), 28–31. https://doi.org/10.1016/S0892-1997(02)00068-1 [DOI] [PubMed] [Google Scholar]
- Kavookjian, H. , Irwin, T. , Garnett, J. D. , & Kraft, S. (2020). The reflux symptom index and symptom overlap in dysphonic patients. The Laryngoscope, 130(11), 2631–2636. https://doi.org/10.1002/lary.28506 [DOI] [PubMed] [Google Scholar]
- Kempster, G. B. , Gerratt, B. R. , Verdolini Abbott, K. , Barkmeier-Kraemer, J. , & Hillman, R. E. (2009). Consensus auditory-perceptual evaluation of voice: Development of a standardized clinical protocol. American Journal of Speech-Language Pathology, 18(2), 124–132. https://doi.org/10.1044/1058-0360(2008/08-0017) [DOI] [PubMed] [Google Scholar]
- Kollbrunner, J. , & Seifert, E. (2013). Functional hoarseness in children: Short-term play therapy with family dynamic counseling as therapy of choice. Journal of Voice, 27(5), 579–588. https://doi.org/10.1016/j.jvoice.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Kreiman, J. , & Gerratt, B. R. (2000). Sources of listener disagreement in voice quality assessment. The Journal of the Acoustical Society of America, 108(4), 1867–1876. https://doi.org/10.1121/1.1289362 [DOI] [PubMed] [Google Scholar]
- Kreiman, J. , Gerratt, B. R. , & Ito, M. (2007). When and why listeners disagree in voice quality assessment tasks. The Journal of the Acoustical Society of America, 122(4), 2354–2364. https://doi.org/10.1121/1.2770547 [DOI] [PubMed] [Google Scholar]
- Lass, N. J. , Ruscello, D. M. , Bradshaw, K. H. , & Blankenship, B. L. (1991). Adolescents' perceptions of normal and voice-disordered children. Journal of Communication Disorders, 24(4), 267–274. https://doi.org/10.1016/0021-9924(91)90002-Z [DOI] [PubMed] [Google Scholar]
- Lee, E.-K. , & Son, Y.-I. (2005). Muscle tension dysphonia in children: Voice characteristics and outcome of voice therapy. International Journal of Pediatric Otorhinolaryngology, 69(7), 911–917. https://doi.org/10.1016/j.ijporl.2005.01.030 [DOI] [PubMed] [Google Scholar]
- Ludlow, C. , Bassich, C. , Connor, N. , Coulter, D. , & Lee, Y. (1987). The validity of using phonatory jitter and shimmer to detect laryngeal pathology. Laryngeal Function in Phonation and Respiration, 492–508. [Google Scholar]
- Ma, E. P.-M. , & Yu, C. H.-Y. (2013). Listeners' attitudes toward children with voice problems. Journal of Speech, Language, and Hearing Research, 56(5), 1409–1415. https://doi.org/10.1044/1092-4388(2013/11-0242) [DOI] [PubMed] [Google Scholar]
- Mabbott, D. J. , Noseworthy, M. , Bouffet, E. , Laughlin, S. , & Rockel, C. (2006). White matter growth as a mechanism of cognitive development in children. NeuroImage, 33(3), 936–946. https://doi.org/10.1016/j.neuroimage.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Mackiewicz-Nartowicz, H. , Sinkiewicz, A. , Bielecka, A. , Owczarzak, H. , Mackiewicz-Milewska, M. , & Winiarski, P. (2014). Long term results of childhood dysphonia treatment. International Journal of Pediatric Otorhinolaryngology, 78(5), 753–755. https://doi.org/10.1016/j.ijporl.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Martinez-Paredes, J. F. , Menton, S. M. , Thompson, C. C. , & Rutt, A. L. (in press). Evaluation of size, laterality, and location of unilateral vocal fold lesions on voice quality. Journal of Voice. https://doi.org/10.1016/j.jvoice.2021.09.013 [DOI] [PubMed] [Google Scholar]
- Meerschman, I. , Claeys, S. , Bettens, K. , Bruneel, L. , D'haeseleer, E. , & Van Lierde, K. (2019). Massed versus spaced practice in vocology: Effect of a short-term intensive voice therapy versus a long-term traditional voice therapy. Journal of Speech, Language, and Hearing Research, 62(3), 611–630. https://doi.org/10.1044/2018_JSLHR-S-18-0013 [DOI] [PubMed] [Google Scholar]
- Mori, K. (1999). Vocal fold nodules in children: Preferable therapy. International Journal of Pediatric Otorhinolaryngology, 49(Suppl. 1), S303–S306. https://doi.org/10.1016/S0165-5876(99)00181-0 [DOI] [PubMed] [Google Scholar]
- Mumović, G. , Veselinović, M. , Arbutina, T. , & Škrbić, R. (2014). Vocal therapy of hyperkinetic dysphonia. Srpski Arhiv za Celokupno Lekarstvo, 142(11–12), 656–662. https://doi.org/10.2298/SARH1412656M [DOI] [PubMed] [Google Scholar]
- Murry, T. , & Woodson, G. (1992). A comparison of three methods for the management of vocal fold nodules. Journal of Voice, 6(3), 271–276. https://doi.org/10.1016/S0892-1997(05)80153-5 [Google Scholar]
- Niedzielska, G. , Glijer, E. , & Niedzielski, A. (2001). Acoustic analysis of voice in children with noduli vocales. International Journal of Pediatric Otorhinolaryngology, 60(2), 119–122. https://doi.org/10.1016/S0165-5876(01)00506-7 [DOI] [PubMed] [Google Scholar]
- Nuss, R. , Ward, J. , Huang, L. , Volk, M. , & Woodnorth, G. (2010). Correlation of vocal fold nodule size in children and perceptual assessment of voice quality. Annals of Otology, Rhinology & Laryngology, 119(10), 651–655. https://doi.org/10.1177/000348941011901001 [DOI] [PubMed] [Google Scholar]
- Patel, R. R., Bless, D. M. , & Thibeault, S. L. (2011). Boot camp: A novel intensive approach to voice therapy. Journal of Voice, 25(5), 562–569. https://doi.org/10.1016/j.jvoice.2010.01.010 [DOI] [PubMed] [Google Scholar]
- Plexico, L. , Sandage, M. , & Faver, K. (2011). Assessment of phonation threshold pressure: A critical review and clinical implications. American Journal of Speech-Language Pathology, 20(4), 348–366. https://doi.org/10.1044/1058-0360(2011/10-0066) [DOI] [PubMed] [Google Scholar]
- Poburka, B. J. , & Patel, R. (2021). Laryngeal endoscopic imaging: Fundamentals and key concepts for rating selected parameters. Perspectives of the ASHA Special Interest Groups, 6(4), 736–742. https://doi.org/10.1044/2021_PERSP-21-00073 [Google Scholar]
- Poburka, B. J. , Patel, R. R. , & Bless, D. M. (2017). Voice-Vibratory Assessment With Laryngeal Imaging (VALI) Form: Reliability of rating stroboscopy and high-speed videoendoscopy. Journal of Voice, 31(4), 513.e1–513.e14. https://doi.org/10.1016/j.jvoice.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Powell, M. , Filter, M. D. , & Williams, B. (1989). A longitudinal study of the prevalence of voice disorders in children from a rural school division. Journal of Communication Disorders, 22(5), 375–382. https://doi.org/10.1016/0021-9924(89)90012-9 [DOI] [PubMed] [Google Scholar]
- Pribuisiene, R. , Pribuisis, K. , Liutkevicius, V. , Balsevicius, T. , Milasiene, R. , & Uloza, V. (2019). Glottal function index questionnaire for screening of pediatric dysphonia. International Journal of Pediatric Otorhinolaryngology, 123, 97–101. https://doi.org/10.1016/j.ijporl.2019.04.045 [DOI] [PubMed] [Google Scholar]
- Saniasiaya, J. , & Kulasegarah, J. (2020). Dysphonia and reflux in children: A systematic review. International Journal of Pediatric Otorhinolaryngology, 139, 110473. https://doi.org/10.1016/j.ijporl.2020.110473 [DOI] [PubMed] [Google Scholar]
- Schneider, B. , & Bigenzahn, W. (2003). Influence of glottal closure configuration on vocal efficacy in young normal-speaking women. Journal of Voice, 17(4), 468–480. https://doi.org/10.1067/S0892-1997(03)00065-1 [DOI] [PubMed] [Google Scholar]
- Schweinfurth, J. M. , & Thibeault, S. L. (2008). Does hyaluronic acid distribution in the larynx relate to the newborn's capacity for crying? The Laryngoscope, 118(9), 1692–1699. https://doi.org/10.1097/MLG.0b013e3181782754 [DOI] [PubMed] [Google Scholar]
- Senkal, O. A. , & Ciyiltepe, M. (2013). Effects of voice therapy in school-age children. Journal of Voice, 27(6). https://doi.org/10.1016/j.jvoice.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Sivasankar, M. , & Erickson, E. (2008, June 1). Detrimental vocal effects of airway dehydration in males. Annual Symposium of the Voice Foundation. [Google Scholar]
- So, Y. K. (2018). Efficacy of voice therapy for children with vocal nodules. Journal of Clinical Otolaryngology Head & Neck Surgery, 29(2), 229–234. https://doi.org/10.35420/jcohns.2018.29.2.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner, K. , Roy, N. , Merrill, R. M. , Muntz, F. , Houtz, D. R. , Sauder, C. , Elstad, M. , & Wright-Costa, J. (2010). Nebulized isotonic saline versus water following a laryngeal desiccation challenge in classically trained sopranos. Journal of Speech, Language, and Hearing Research, 53(6), 1555–1566. https://doi.org/10.1044/1092-4388(2010/09-0249) [DOI] [PubMed] [Google Scholar]
- Tezcaner, C. Z. , Ozgursoy, S. K. , Sati, I. , & Dursun, G. (2009). Changes after voice therapy in objective and subjective voice measurements of pediatric patients with vocal nodules. European Archives of Oto-Rhino-Laryngology, 266(12), 1923–1927. https://doi.org/10.1007/s00405-009-1008-6 [DOI] [PubMed] [Google Scholar]
- Titze, I. R. (1988). The physics of small-amplitude oscillation of the vocal folds. The Journal of the Acoustical Society of America, 83(4), 1536–1552. https://doi.org/10.1121/1.395910 [DOI] [PubMed] [Google Scholar]
- Trani, M. , Ghidini, A. , Bergamini, G. , & Presutti, L. (2007). Voice therapy in pediatric functional dysphonia: A prospective study. International Journal of Pediatric Otorhinolaryngology, 71(3), 379–384. https://doi.org/10.1016/j.ijporl.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Ulozaite-Staniene, N. , Petrauskas, T. , Šaferis, V. , & Uloza, V. (2019). Exploring the feasibility of the combination of acoustic voice quality index and glottal function index for voice pathology screening. European Archives of Oto-Rhino-Laryngology, 276(6), 1737–1745. https://doi.org/10.1007/s00405-019-05433-5 [DOI] [PubMed] [Google Scholar]
- Valadez, V. , Ysunza, A. , Ocharan-Hernandez, E. , Garrido-Bustamante, N. , Sanchez-Valerio, A. , & Pamplona, M. C. (2012). Voice parameters and videonasolaryngoscopy in children with vocal nodules: A longitudinal study, before and after voice therapy. International Journal of Pediatric Otorhinolaryngology, 76(9), 1361–1365. https://doi.org/10.1016/j.ijporl.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Vance, D. , Alnouri, G. , Shah, P. , O'Connell Ferster, A. P. , Lyons, K. , Ross, J. , & Sataloff, R. T. (in press). The validity and reliability of the reflux finding score. Journal of Voice. https://doi.org/10.1016/j.jvoice.2020.11.008 [DOI] [PubMed] [Google Scholar]
- Vygotsky, L. S. (1978). Mind in society: Development of higher psychological processes. Harvard University Press. [Google Scholar]
- Warren, S. F. , Fey, M. E. , & Yoder, P. J. (2007). Differential treatment intensity research: A missing link to creating optimally effective communication interventions. Mental Retardation and Developmental Disabilities Research Reviews, 13(1), 70–77. https://doi.org/10.1002/mrdd.20139 [DOI] [PubMed] [Google Scholar]
- Wenke, R. , Coman, L. , Walton, C. , Madill, C. , Theodoros, D. , Bishop, C. , Stabler, P. , Lawrie, M. , O'Neill, J. , Gray, H. , & Cardell, E. A. (in press). Effectiveness of intensive voice therapy versus weekly therapy for muscle tension dysphonia: A noninferiority randomised controlled trial with nested focus group. Journal of Voice. https://doi.org/10.1016/j.jvoice.2021.02.011 [DOI] [PubMed] [Google Scholar]
- Wertz, A. , Carroll, L. M. , & Zur, K. B. (2020). Pediatric laryngopharyngeal reflux: Perceptual, acoustic, and laryngeal findings. International Journal of Pediatric Otorhinolaryngology, 133, 109974. https://doi.org/10.1016/j.ijporl.2020.109974 [DOI] [PubMed] [Google Scholar]
- Wong, D. W.-M. , Chan, R. W. , & Wu, C.-H. (2021). Effect of training with anchors on auditory-perceptual evaluation of dysphonia in speech-language pathology students. Journal of Speech, Language, and Hearing Research, 64(4), 1136–1156. https://doi.org/10.1044/2020_JSLHR-20-00214 [DOI] [PubMed] [Google Scholar]
- Zhuang, P. , Swinarska, J. T. , Robieux, C. F. , Hoffman, M. R. , Lin, S. , & Jiang, J. J. (2013). Measurement of phonation threshold power in normal and disordered voice production. Annals of Otology, Rhinology & Laryngology, 122(9), 555–560. https://doi.org/10.1177/000348941312200904 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.