Abstract
Colorectal cancer (CRC) continues to be a significant public health problem worldwide. CRC screening programs have reduced the incidence rates of CRCs but still suffer from the problems of missed lesions and interval cancers. Chemopreventive strategies against CRC would benefit high-risk populations but trials testing synthetic and naturally occurring compounds have not yielded a front runner. Immune mechanisms promoting cancer have been modulated to develop immunotherapy for cancer treatment that has revolutionized cancer management, but could also be applied to cancer interception, that is, cancer immunoprevention. Cancer immunoprevention refers to approaches that can enhance the immune system, either directly or by removing natural breaks such as immune checkpoints, to survey and destroy tumor cells. In this primer, we aim to explain the concepts behind vaccine-based cancer immunoprevention. Multiple cancer vaccines have been tried in advanced cancer populations, but most have failed primarily because of an immunosuppressive environment that accompanies advanced cancers. Preventive vaccines in immunocompetent hosts may have a better clinical response compared with therapeutic vaccines in immunosuppressed hosts. The first randomized controlled trial testing the mucin1 vaccine against CRC in the prevention setting has been successfully completed. For the benefit of the clinician, we briefly discuss important concepts related to the workings of preventive vaccines. Prevention with vaccines is a highly attractive approach because of the potential for highly targeted therapy with minimal side effects that could theoretically provide lifelong protection.
Key Words: cancer immunoprevention, vaccines, colorectal cancers
Colorectal cancer (CRC) is the third most common cancer globally, representing 10% of cancer diagnoses and 9.4% of cancer deaths worldwide.1 Because of its public health importance, multiple cancer prevention societies endorse CRC screening through one of the many established modalities.2,3 If an option such as fecal blood or stool DNA testing is chosen, then the abnormal result is followed by colonoscopy, which is the only modality that can not only detect CRC, but also can remove precancerous lesions to prevent CRC. Although data from ongoing randomized controlled trials of colonoscopy are not available, extrapolation from randomized controlled trials of flexible sigmoidoscopy supports the potential efficacy of adenoma removal in preventing the development of CRC.4,5 However, interval cancers continue to occur because of missed lesions or incomplete resection of existing lesions.5,6 Worthy of additional concern is the documented trend of rising incidence and mortality of CRC in adults below 50 years of age in the United States.7 Therefore, continued efforts to develop novel approaches for CRC prevention are needed.
Chemopreventive strategies using synthetic and naturally occurring compounds have not yielded a front runner for CRC prevention. Aspirin in doses of 75 to 300 mg showed a lot of promise at reducing both incidence and mortality of CRC.8 Higher dose of aspirin at 600 mg was tested in patients with Lynch syndrome as demonstrated in the CAPP2 (Colorectal Adenoma/Carcinoma Prevention Programme 2) trial. In this trial, the investigators randomized patients with Lynch syndrome to receive either 600 milligrams of aspirin per day or placebo and showed ~40% reduction in CRC incidence in those receiving aspirin at 20-year follow up.9 However, recent data from the Aspirin in Reducing Events in the Elderly (ASPREE) trial showed that low-dose (81 mg) aspirin use increased the risk of advanced stage CRC in older adults.10 Thus, the preventive effects of aspirin against CRC may depend on the patient population and the dose. These factors need to be carefully considered in future trials. Immune mechanisms promoting cancer have been modulated to develop immunotherapy for cancer treatment that has revolutionized cancer management,11 but could also be applied to cancer interception, that is, cancer immunoprevention. Cancer immunoprevention refers to approaches that can enhance the immune system, either directly or by removing natural breaks such as immune checkpoints, to survey and destroy tumor cells. In this primer, we aim to explain the concepts behind vaccine-based cancer interception. Vaccines can train the immune system to recognize aberrantly expressed human antigens on epithelial cells with subsequent elimination of these abnormal cells to prevent malignant progression. This approach can work because the molecular pathogenesis of the majority of colorectal tumors is well-established by the adenoma-carcinoma sequence which describes the transformation of the epithelium from normal to dysplastic because of the accruement of multiple tumor-associated mutations.12 Another distinct pathway for colon tumorigenesis is the serrated polyp pathway, which represent ∼10% of all CRC. Although the tumorigenesis pathways are different between adenomatous versus serrated polyps, several of the antigens, for example, mucin1 (MUC1) being targeted by the current vaccines are shared between the 2 forms of polyps.13 Therefore, the current vaccines under investigation targeting the adenomatous pathway may also prevent CRCs arising from the serrated pathway but will need to be formally tested in clinical trials targeting patients with serrated polyps.
Prevention with vaccines is a highly attractive approach because of the potential for highly targeted therapy with minimal side effects that could theoretically provide lifelong protection.14 Well-designed vaccines may prime T cells to recognize tumor antigens on preadenomas to shift immunosurveillance from equilibrium to elimination at the premalignant stage.14,15 Short-term treatment could lead to long-term prevention. The majority of the work using vaccines in the field of CRC has been done in patients with established cancers, many of who have metastatic disease. However, extensive work suggests that administration of vaccines during advanced stages in carcinogenesis has limited effects because of the patients’ inability to mount an effective immune response.14,16,17 Therefore, there has been renewed interest in testing vaccines against CRC in the prevention setting. The impetus for this primer was successful completion of the first randomized controlled trial testing the MUC1 vaccine against CRC in the prevention setting (NCT02134925). For the benefit of the clinician, we briefly discuss important concepts related to the workings of preventive vaccines including mechanisms, efficacy and safety as we approach an era where clinicians may prescribe cancer vaccines for immunoprevention in high-risk patients for CRC interception. Important concepts discussed in this review are defined in the Table 1.
TABLE 1.
Important Concepts Related to Vaccine-Based Immunoprevention
| Term | Definition |
|---|---|
| Elimination | Elimination refers to the process by which the immune system detects and eliminates developing tumor cells |
| Equilibrium | The phase of antitumor immunity characterized by tumor dormancy in which a proportion of variant tumor cells become capable of evading the immune response |
| Escape | The final phase of antitumor immunity characterized by an immunosuppressive microenvironment that fosters uninhibited tumor growth |
| Humoral immunity | Immunity mediated by antibodies against extracellular antigens |
| Cellular immunity | Immunity mediated by T cells against intracellular antigens |
| Tumor-associated antigen (TAA) | A protein that is normally expressed in the human body but is either overexpressed or modified in tumor cells, for instance, hypoglycosylated MUC1 overexpressed in adenomatous polyps and adenocarcinomas |
| Tumor-specific antigen (TSA) | Neoantigen that is not expressed in normal cells but is generated because of somatic mutations within tumor cells,23,24 for instance, frameshift peptides expressed within tumors in the background of inherited gastrointestinal cancer syndromes such as Lynch syndrome |
| Chemoprevention | The use of medications to prevent the development or progression of cancer |
| Immunoprevention | The concept of preventing cancer development using agents that enhance the immune system to survey and destroy tumor cells |
IS VACCINE-BASED CANCER PREVENTION FEASIBLE?
Immunosurveillance, as a theoretical construct, describes a process by which the immune system is able to recognize and destroy cells that have gained oncogenic mutations.15 However, the complexity of the interplay between tumor and host cells is better captured by the conceptual model of immunoediting, which describes three sequential phases of antitumor immunity: elimination, equilibrium, and escape.18 Elimination describes the initial immune response elicited by oncogenic mutations. Equilibrium refers to the subsequent phase characterized by selection pressure in which only cells capable of evading the immune response survive and replicate. Escape refers to the phase resulting from equilibrium and is characterized by an immunosuppressive microenvironment that fosters uninhibited tumor growth.19 With this in mind, we can come to appreciate the powerful concept of immunoprevention: tailoring the host immune response to influence and thus prevent cancer initiation.
CRC develops in a stepwise manner directed by a series of genetic and epigenetic changes, which produce antigens that are detectable by the immune system. Peptide antigens are presented to T cells in HLA class I and II molecules by antigen-presenting cells (APCs), primarily dendritic cells (DCs). A selection of immune effector mechanisms come into play to recognize abnormal cells.20 Natural killer (NK) cells facilitate the innate immune response, eliminating the tumor cells once identified.21 In contrast to the innate immune response, the adaptive immune response involves CD8+ cytotoxic lymphocyte-mediated killing of cancer cells, a process intimately regulated by CD4+ T helper cells and regulatory T cells (Tregs).22 Humoral responses are also deployed when tumor antigens trigger specific antitumor antibody production through mature B cells.23 Innate immunity is nonspecific and short-term, whereas adaptive immunity is specific and durable. It is known that high tumor-infiltrating lymphocyte density is a good prognostic factor in many types of cancer including CRC.24 All in all; the consensus gleaned from both mouse models and human studies provides ample support for the existence of cancer immunosurveillance.
Vaccines may help overcome the immunosuppressive environment permissive to cancer growth and shift the host response from that of equilibrium to elimination of tumor cells. The tumor burden, the tumor mutational load, and the immunosuppressive microenvironment dictate the success of this strategy. The discovery of many early lesion-associated tumor antigens cements immunoprevention as a viable pathway for CRC prevention. Concerning vaccines, their success in preventing viral infection-induced cancers will hopefully transfer into immunoprevention of nonviral cancers, like CRC. MUC1 antigen vaccines are already in clinical trial (NCT02134925). Similar ventures using CEA-specific vaccine regimens or therapeutic vaccines with tumor antigens like KRAS and TP53 are being investigated.25,26 Thus, CRC prevention is a fertile ground for cultivating this modality.
With deeper understanding of antitumor immunity has come the concept of immunoprevention, which aims to weaponize the host immune response against tumorigenesis and thus prevent cancer development through the use of medications and/or vaccinations.19 If used in the appropriate population, immunoprevention is a powerful tool capable of revolutionizing the way we practice cancer prevention.
CHOICE OF ANTIGENS TO INCLUDE IN THE CANCER PREVENTION VACCINES
The two types of antigens that can be incorporated into cancer vaccines are tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs). TAA refers to proteins that are normally expressed in the human body but are either overexpressed or changed in some way, such as altered glycosylation, during tumorigenesis.27 TSAs are neoantigens that are not expressed in normal cells but are generated because of somatic mutations within tumor cells.27,28 Both TAAs and TSAs can be recognized by the immune system to generate both humoral and cellular immune response.29 The selection of target antigens (TAAs and TSAs) for vaccine development is a complex process and needs a team science approach with coordinated efforts between cancer biologists, immunologists, and bioinformatics specialists. The antigen should be involved in tumorigenesis and should be sufficiently immunogenic. The pipeline to design a vaccine for clinical trials is illustrated in Figure 1.
FIGURE 1.
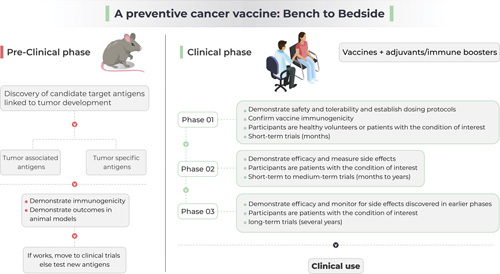
The process of development of a preventive cancer vaccine.
TAAs that are expressed in precursor lesions such as adenomatous polyps and serrated polyps represent significant opportunities as potential targets for immunoprevention. One of the best studied and promising TAAs is MUC1.30 Typically, there is a low-level expression of heavily glycosylated MUC1 on the apical surface of healthy epithelial cells of various organs like the colon. It has been seen, however, that most adenocarcinomas and their premalignant lesions overexpress hypoglycosylated MUC1. A colitis-associated colon cancer model showed that hypoglycosylated MUC1 promotes inflammatory cytokine release, encouraging tumor growth and positive feedback on MUC1 expression.31
TSAs that are generated through frameshift mutations are exciting candidates for vaccines because of tumor-specific expression that could lead to vaccine-based destruction of tumor cells with preservation of normal cells.32 TSA-based vaccines may be more applicable to tumors that occur in the background of inherited gastrointestinal cancer syndromes such as Lynch syndrome. This is discussed later in this article.
The efficacy of TAA-based versus TSA-based vaccines may depend on the tumor stage. For instance, a pioneering study that used sequencing to understand the immunology of the premalignant stage of CRC in patients with Lynch syndrome showed minimal expression of TSAs in the precancer stage.33 More work is needed to understand the clinical usefulness of TAA-based versus TSA-based vaccines for CRC prevention.
CAN VACCINES WORK IN THE PREVENTION SETTING?
Multiple cancer vaccines have been tried in advanced cancer populations, but most have failed.14,28,34,35 The 2 main reasons these vaccines have failed are: 1) low levels of circulating immune cells17 (animal studies have shown that higher levels of circulating CD8+ cells are needed to cause tumor regression36); and 2) immunosuppressive tumor microenvironment in advanced cancers that can suppress immune response to cancer vaccines.14 It has been suggested that past exposure to tumor antigens may be critical in the development of a tumor-specific response because cancer prevention vaccines are expected to potentiate pre-existing tumor immunity.27 Experimental support for the feasibility of cancer preventive vaccines comes from an elegant study of cytokine-expressing whole tumor cell-based therapy.37 The investigators engineered a whole cell CT26 colon cancer vaccine to secrete a complex of interleukin (IL)-15 and IL-15Ralpha to boost vaccine-based immunity and then tested immunization of mice both in the prevention and the treatment setting. Although the immunization in the treatment setting delayed tumor growth in a syngeneic mouse model, immunization in the prevention setting was much more effective by preventing 80% of the tumors for clone 1 vaccine and 100% of the tumors for clone 2 vaccine compared with 0% in the control group. This approach provided immunity for up to 3 months with generation of durable immune responses. In another study comparing the effects of vaccination for prevention versus treatment, mice were injected with recMASH2 [Human achaete scute homolog 2 (HASH2) and its murine ortholog MASH2] combined with a proprietary AS15 immune enhancer.38 In the prevention cohort of mice (adapted from APCmin mice: in this model, mice develop spontaneous colorectal tumors), when mice were vaccinated before tumor establishment, the number of colonic microadenomas were significantly reduced. However, in the treatment setting, when mice with established tumors were studied, there was no significant difference. Thus, vaccines may work better in the prevention setting compared with the treatment setting.
WHAT KIND OF IMMUNITY (HUMORAL VS. CELLULAR) MAKES THE CANCER PREVENTION VACCINES EFFECTIVE?
Humoral immunity refers to B-cell-mediated immunity whereas cellular immunity refers to T-cell-mediated immunity (through helper CD4+ T cells and cytotoxic CD8+ T cells). The relative benefit of humoral versus cell-mediated immunity depends on the location of the tumor antigen. For intracellular proteins, circulating antibodies may not play a direct role in tumor cell killing. For instance, a study that targeted a tumor antigen MAGE-A3 (with a stimulant) showed that depletion of B cells did not affect the antitumor effect, thus raising doubts about the importance of humoral immunity. However, immune complexes formed between the circulating antibodies and the tumor antigens could be taken up by the APCs such as DCs and then cross-presented to T cells to promote immunity.39 Thus, humoral immunity could enhance overall tumor immunity.
The relative importance of CD4+ and CD8+ T cells is not entirely clear for antitumor immune effects against CRC. Histologic studies showed that increased infiltration of CRC tumors by memory CD8+ T lymphocytes was predictive of reduced risk of recurrence and improved survival.40–42 Multiple studies suggest that cytotoxic CD8+ T cells may be the primary cells responsible for tumor elimination.40,43 However, CD4+ T cells appear to be necessary to generate adequate immune responses to tumor antigens.44 Using genomewide approaches, investigators showed that CD4+ T cells help optimize the activity of cytotoxic T cells by creating a specific pool of effector memory T cells with antigen recall properties.45 Although CD8+ T cells are the main effector cells for tumor eradication, CD4+ helper T cells are needed to maintain optimal protective immunity and to generate memory CD8+ cells.46 On the basis of the above data, the most effective vaccines will be the ones that lead to both CD4+ and CD8+ T-cell responses. That being said, the overall mechanisms for vaccine-induced antitumor immunity are complex, making it critical to measure clinically relevant endpoints in studies of cancer prevention vaccines. The ideal clinically relevant endpoint would be the CRC incidence, however, the costs of such a study will be prohibitive. Therefore, previous trials (NCT02134925) have included adenoma recurrence rate as a surrogate endpoint to test the vaccine efficacy but in an enriched population such as patients with advanced adenomas.
VACCINES AGAINST SINGLE VERSUS MULTIPLE EPITOPES
Earlier, we discussed that cancer prevention vaccines need to target either TAAs or TSAs. Multivalent vaccines can lead to a diversified portfolio of antigen-specific T cells with improved tumor control. This can also protect against the effects of tumor immunoediting with selection of tumor cells resistant to vaccination because of downregulation of one or more of the tumor antigens. Support for a multiantigenic approach comes from a study in a mouse model of breast cancer where, after the development of preinvasive lesions, mice were immunized with a multivalent peptide vaccine against 3 antigens: neu, insulin-like growth factor-binding protein 2 and insulin-like growth factor receptor-I.47 The multiantigen vaccine was more effective than the individual vaccines preventing palpable tumors in 65% of mice.47 A problem with using a multivalent vaccine is antigenic competition between the individual antigens.48 However, studies suggest that this may be more of a theoretic concern. In an innovative study, the investigators used a synthetic DNA vector that contained 3 cancer-specific epitopes in tandem. The multivalent DNA vaccine resulted in T-cell responses to all 3 tumor antigens and inhibited tumor growth of MC38 colon cancer cell line in combination with PD-1 blockade.49 Another approach that has been used to promote immunity against multiple tumor antigens is introduction of inactivated whole cancer cell vaccines to introduce a variety of TAAs. A fundamental problem with this approach, however, has been that the precise mechanism of action remains unclear which makes it difficult to further improve the technology. A multivalent vaccine is appealing because it may be more effective by generating a broad repertoire of antigen-specific T cells against multiple antigens such that even if the cancer cells lose one of the antigens, immunity against other antigens can still prevent tumor growth. HLA polymorphism is an important issue that can affect antigen recognition. As discussed earlier, processed tumor antigens (TAAs or TSAs) are presented within the HLA molecules for recognition by the effector T cells. Thus, HLA expression on the host tumor cells may determine vaccine efficacy50 and needs to be considered as a variable to interpret vaccine failures. Further research is needed.
CANCER PREVENTION VACCINES NEED AN IMMUNE ADJUVANT TO BOOST THE IMMUNE RESPONSE
Extensive literature suggests that vaccines with tumor antigens alone may not be sufficient but indeed need an immunoadjuvant to maximize immunogenicity and generate high numbers of circulating immune cells.17,51 Various agents have been tried to maximize vaccine(s)’s immunogenicity.51 A toll-like receptor-3 agonist has been used in the previous MUC1 peptide vaccine trial and was well-tolerated.52 Interleukin-12 had high liver and bone marrow toxicity in early phase cancer clinical trials.53 An investigational IL-15 superagonist, is a newer immune adjuvant that based on administration to over 600 participants in 25 completed and ongoing studies has an acceptable safety profile.54 One of the biggest advantages of adding IL-15 superagonist is its promotion of NK cells to infiltrate into tumors. This is important because depletion of NK cells in mice has been shown to impair tumor-specific T-cell immunity during cancer cell vaccination.55 In addition, activated NK cells kill immature DC responses but spare mature DC responses to guarantee successful T-cell priming.56,57 In addition, NK cells can directly kill tumor cells through activating receptor engagement or antibody-dependent cell-mediated cytotoxicity.
Immune checkpoint inhibitors can be powerful adjuvants to promote immune responses to cancer vaccines. In a study that tested a synthetic multivalent DNA vaccine encoding three cancer-specific epitopes in tandem, T-cell responses were seen to all 3 tumor antigens and inhibited tumor growth of the MC38 colon cancer cell line.49 Strikingly, this inhibition of tumor growth only occurred when the vaccine was administered in combination with PD-1 blockade.49 This may be particularly relevant because exhausted CD8+ T cells express a high level of immune inhibitory molecules such as PD-1, LAG-3, and TIM3.58 Thus, immune checkpoint inhibitors could boost vaccine efficacy by changing these exhausted T cells into activated T cells but have the potential for autoimmune effects11 and need further demonstration of safety in combination with vaccines.
CLINICAL DATA: ARE THE CANCER PREVENTION VACCINES SAFE AND EFFICACIOUS?
As previously mentioned, the majority of vaccines have been tested in patients with established cancers, many of whom have metastatic disease. In this environment of immunosuppression, the vaccines have not proven to be efficacious.14 The first study to test the feasibility, more specifically, the safety and immunogenicity, of a preventive vaccine against a nonviral TAA MUC1 in patients without established CRC was performed in patients (N=40) with advanced adenomas as a single nonrandomized cohort design.59 All participants received a peptide vaccine against MUC1 along with an adjuvant, a TLR-3 agonist, after the colon was cleared of all polyps including advanced adenomas. The vaccine and the adjuvant were administered at 0, 2, and 10 weeks followed by a booster at 52 weeks. High levels of MUC1 immunoglobulin G were seen in ~44% of the patients. Of those who developed an immunogenic response to the vaccine, 75% responded to the booster, suggesting that the vaccine led to long-term memory responses which would be needed for long-term prevention.59 This is in stark contrast to the testing of vaccines in patients with established cancers, where the vaccines must be repeatedly administered to maintain immunogenicity. The primary reason for the vaccine’s failure to induce immunity in this study was the presence of circulating myeloid-derived suppressive cells, which can suppress adaptive immunity.60 Most of the adverse events were self-limiting injection site reactions and flu-like symptoms. The vaccine did not induce any new autoimmune diseases. The randomized controlled trial testing this MUC1 peptide vaccine in patients with advanced adenomas has now been completed (NCT02134925). Results are eagerly awaited. Multiple other studies have evaluated the safety of cancer vaccines. In a large study that looked at 200 phase I clinical trials that enrolled 4942 patients, grade 3 or higher adverse events occurred at a low rate of 1.25 and 2 per 1000 vaccine administrations.61
VACCINES IN INHERITED GASTROINTESTINAL CANCER SYNDROMES
A vaccine for clinical use is not yet available in patients with familial adenomatous polyposis, but may be feasible. Using the well-established APCmin mouse model of familial adenomatous polyposis, investigators targeted ERBB3 that is required for polyp formation.62 By using a synthetic ERBB3 peptide to vaccinate the mice, they significantly reduced the number of polyps by 100 days of age after 3 injections with the development of both humoral and cellular immunity. Sera from immunized mice inhibited the activity of ERBB3 in an in-vitro cell-based ERBB3 activation assay. Remarkably, the offsprings of vaccinated females also had significantly reduced polyp formation. The mode of transmission of this passive immunity was through milk, suggesting it was antibody mediated. The immunogenicity rate was 63%.62 We may be closer to a vaccine in patients with Lynch syndrome. Phase 2 trials are testing a multivalent vaccine targeting three TAAs MUC1, CEA, and brachyury in patients with Lynch syndrome (NCT05419011). Another vaccine that will target TSA in patients with Lynch syndrome is also ready to be tested in a Phase 1 trial (NCT05078866). TSA may be particularly relevant to the Lynch population because DNA mismatch repair deficiency causes frameshift mutations to generate TSA and vaccines targeting TSAs were immunogenic and safe in this population.32 The relative efficacy of the 2 vaccines (against TAAs vs. TSAs) remains to be determined but it is possible that both vaccines may work synergistically for prevention of CRC in Lynch syndrome.27 As discussed earlier, Lynch polyps may not express these TSAs,33 although the TSA-based vaccine could still target alternate pathways of cancer development besides classic adenomas in patients with Lynch syndrome.
CHEMOPREVENTION BOOSTING IMMUNOPREVENTION
An important strategy in the fight against CRC involves harnessing the power of the immune system through immunomodulation with medications or natural substances. Many known chemopreventive agents63 have been shown to accomplish their antitumor effects at least in part through immunomodulation. The efficacy of chemoprevention through immunomodulation serves as a precedent for the development of novel immunomodulatory and immunopreventive agents. Nonsteroidal anti-inflammatory agents (NSAIDs), for example, have reliably demonstrated an association with a reduced risk of CRC.64 Though the mechanism of tumor prevention is not entirely understood, NSAIDs have been shown to stimulate an antitumor immune response through a variety of mechanisms that contribute to this effect.24,65 One such mechanism is through reversal of the PGE2-induced immunosuppressive microenvironment that otherwise would facilitate evasion of the host antitumor immune response.66 NSAIDs inhibit COX-1 and COX-2, which stimulates production of PGE2 to induce an immunosuppressive microenvironment through its inhibition of DC entry and activity within the tumor.67 As a parallel to NSAID induced inhibition of COX-1/2, genetic ablation of PGE2 in mouse models has been shown to effectively reverse the immunosuppression and thus render the tumor cells susceptible to host immunosurveillance.66 A multicenter phase I clinical trial studying the role of naproxen for CRC prevention in patients with Lynch syndrome demonstrated that naproxen actually acts as an immunostimulant in the colorectal mucosa by activating T cells and DCs.68 In an elegant study, naproxen significantly improved overall survival in a mouse model of Lynch syndrome when these mice were vaccinated with a vaccine containing 4 frameshift peptide neoantigens.32 This suggests the potential for naproxen and other NSAIDs to be used as an adjunct to vaccination to improve efficacy for CRC prevention.
CONCLUSION
The application of immunoprevention strategies in CRC is an exciting, though, thus far, largely an unexplored avenue. The role of the immune system in the life cycle of colonic neoplasms represents a significant source of power in the fight against cancer that has yet to be fully appreciated. Though large strides have been made in this regard, much remains to be understood. Key to the development of efficacious immunoprevention is delineating the specific mechanistic interplay between immunosurveillance and tumor escape tactics. Also of significance is determining the patient population in which the benefits outweigh the potential risks of immunoprevention therapies, especially cancer prevention vaccines. Wholesome understanding of these complexities will create opportunities for targeted, cost-effective strategies with the potential to revolutionize the world of cancer interception.
Footnotes
This work was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA168524 to AB.
M.A.M. is a paid consultant for Design-Zyme, LLC. A.B. has received research support from Freenome Holdings and Stella Diagnostics.
The remaining authors declare that they have nothing to disclose.
Contributor Information
Katy Jackson, Email: kjackson18@kumc.edu.
Sohini Samaddar, Email: sohini.samaddar@gmail.com.
Mary A. Markiewicz, Email: mmarkiewicz@kumc.edu.
Ajay Bansal, Email: abansal@kumc.edu.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Shaukat A, Kahi CJ, Burke CA, et al. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458–479. [DOI] [PubMed] [Google Scholar]
- 3.Helsingen LM, Vandvik PO, Jodal HC, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline. BMJ. 2019;367:l5515. [DOI] [PubMed] [Google Scholar]
- 4.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. [DOI] [PubMed] [Google Scholar]
- 6.Pohl H, Anderson JC, Aguilera-Fish A, et al. Recurrence of colorectal neoplastic polyps after incomplete resection. Ann Intern Med. 2021;174:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. [DOI] [PubMed] [Google Scholar]
- 9.Burn J, Sheth H, Elliott F, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395:1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abril-Rodriguez G, Ribas A. SnapShot: immune checkpoint inhibitors. Cancer Cell. 2017;31:848–848 e841. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz SD, Bertagnolli MM. Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biemer-Huttmann AE, Walsh MD, McGuckin MA, et al. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039–1048. [DOI] [PubMed] [Google Scholar]
- 14.Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18:183–194. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher R, Wang YJ, Schoen RE, et al. Colorectal cancer prevention: immune modulation taking the stage. Biochim Biophys Acta Rev Cancer. 2018;1869:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lollini PL, Cavallo F, Nanni P, et al. The promise of preventive cancer vaccines. Vaccines (Basel). 2015;3:467–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. [DOI] [PubMed] [Google Scholar]
- 19.Roeser JC, Leach SD, McAllister F. Emerging strategies for cancer immunoprevention. Oncogene. 2015;34:6029–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. [DOI] [PubMed] [Google Scholar]
- 21.Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. [DOI] [PubMed] [Google Scholar]
- 22.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Nishihara R, Qian ZR, et al. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor-infiltrating lymphocytes. Gastroenterology. 2016;151:879–892.e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbone DP, Ciernik IF, Kelley MJ, et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol. 2005;23:5099–5107. [DOI] [PubMed] [Google Scholar]
- 26.Greiner JW, Zeytin H, Anver MR, et al. Vaccine-based therapy directed against carcinoembryonic antigen demonstrates antitumor activity on spontaneous intestinal tumors in the absence of autoimmunity. Cancer Res. 2002;62:6944–6951. [PubMed] [Google Scholar]
- 27.Finn OJ. Human tumor antigens yesterday, today, and tomorrow. Cancer Immunol Res. 2017;5:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn BK, Kramer BS. Cancer prevention: lessons learned and future directions. Trends Cancer. 2016;2:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacqueline C, Lee A, Frey N, et al. Inflammation-induced abnormal expression of self-molecules on epithelial cells: targets for tumor immunoprevention. Cancer Immunol Res. 2020;8:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beatty PL, Narayanan S, Gariépy J, et al. Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prev Res (Phila). 2010;3:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gebert J, Gelincik O, Oezcan-Wahlbrink M, et al. Recurrent Frameshift Neoantigen vaccine elicits protective immunity with reduced tumor burden and improved overall survival in a Lynch syndrome mouse model. Gastroenterology. 2021;161:1288–1302 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang K, Taggart MW, Reyes-Uribe L, et al. Immune profiling of premalignant lesions in patients with Lynch syndrome. JAMA Oncol. 2018;4:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thi VAD, Jeon HM, Park SM, et al. Cell-based IL-15:IL-15Rα secreting vaccine as an effective therapy for CT26 colon cancer in mice. Mol Cells. 2019;42:869–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rioux CR, Clapper ML, Cooper HS, et al. Self-antigen MASH2 combined with the AS15 immunostimulant induces tumor protection in colorectal cancer mouse models. PLoS One. 2019;14:e0210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhodapkar KM, Krasovsky J, Williamson B, et al. Antitumor monoclonal antibodies enhance cross-presentation ofcCellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. [DOI] [PubMed] [Google Scholar]
- 41.Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. [DOI] [PubMed] [Google Scholar]
- 42.Camus M, Tosolini M, Mlecnik B, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–2693. [DOI] [PubMed] [Google Scholar]
- 43.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. [DOI] [PubMed] [Google Scholar]
- 44.Gérard C, Baudson N, Ory T, et al. Tumor mouse model confirms MAGE-A3 cancer immunotherapeutic as an efficient inducer of long-lasting anti-tumoral responses. PLoS One. 2014;9:e94883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahrends T, Busselaar J, Severson TM, et al. CD4(+) T cell help creates memory CD8(+) T cells with innate and help-independent recall capacities. Nat Commun. 2019;10:5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borst J, Ahrends T, Bąbała N, et al. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–647. [DOI] [PubMed] [Google Scholar]
- 47.Disis ML, Gad E, Herendeen DR, et al. A multiantigen vaccine targeting neu, IGFBP-2, and IGF-IR prevents tumor progression in mice with preinvasive breast disease. Cancer Prev Res (Phila). 2013;6:1273–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castle JC, Kreiter S, Diekmann J, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. [DOI] [PubMed] [Google Scholar]
- 49.Tondini E, Arakelian T, Oosterhuis K, et al. A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control. Oncoimmunology. 2019;8:1652539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. [DOI] [PubMed] [Google Scholar]
- 51.Chiossone L, Dumas PY, Vienne M, et al. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671–688. [DOI] [PubMed] [Google Scholar]
- 52.Finn OJ, Boardman L, Cruz-Correa M, et al. Abstract CT236: randomized, double-blind, placebo-controlled trial of preventative MUC1 vaccine in patients with newly diagnosed advanced adenomas: results from one-year booster. Cancer Res. 2019;79:CT236–CT236. [Google Scholar]
- 53.Lasek W, Zagozdzon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother. 2014;63:419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barroso-Sousa R, Ott PA. Transformation of old concepts for a new era of cancer immunotherapy: cytokine therapy and cancer vaccines as combination partners of PD1/PD-L1 inhibitors. Curr Oncol Rep. 2018;21:1. [DOI] [PubMed] [Google Scholar]
- 55.Morandi B, Mortara L, Chiossone L, et al. Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLoS One. 2012;7:e39170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferlazzo G, Moretta L. Dendritic cell editing by natural killer cells. Crit Rev Oncog. 2014;19:67–75. [DOI] [PubMed] [Google Scholar]
- 57.Ferlazzo G, Morandi B. Cross-talks between natural killer cells and distinct subsets of dendritic cells. Front Immunol. 2014;5:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong H, Mittman S, Rodriguez R, et al. Coexpression of inhibitory receptors enriches for activated and functional CD8(+) T cells in murine syngeneic tumor models. Cancer Immunol Res. 2019;7:963–976. [DOI] [PubMed] [Google Scholar]
- 59.Kimura T, McKolanis JR, Dzubinski LA, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila). 2013;6:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rahma OE, Gammoh E, Simon RM, et al. Is the “3+3” dose-escalation phase I clinical trial design suitable for therapeutic cancer vaccine development? A recommendation for alternative design. Clin Cancer Res. 2014;20:4758–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bautz DJ, Sherpa AT, Threadgill DW. Prophylactic vaccination targeting ERBB3 decreases polyp burden in a mouse model of human colorectal cancer. Oncoimmunology. 2017;6:e1255395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158:368–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergman M, Djaldetti M, Salman H, et al. Inflammation and colorectal cancer: does aspirin affect the interaction between cancer and immune cells? Inflammation. 2011;34:22–28. [DOI] [PubMed] [Google Scholar]
- 66.Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D, DuBois RN. The role of prostaglandin E(2) in tumor-associated immunosuppression. Trends Mol Med. 2016;22:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reyes-Uribe L, Wu W, Gelincik O, et al. Naproxen chemoprevention promotes immune activation in Lynch syndrome colorectal mucosa. Gut. 2021;70:555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]


