Abstract
Objective
Disease-modifying anti-rheumatic drugs (DMARDs) that treat rheumatoid arthritis (RA) may reduce immune responses to COVID-19 vaccination. We compared humoral and cell-mediated immunity before and after a 3rd dose of mRNA COVID vaccine in RA subjects.
Methods
RA patients that received 2 doses of mRNA vaccine enrolled in an observational study in 2021 before receiving a 3rd dose. Subjects self-reported holding or continuing DMARDs. Blood samples were collected pre- and 4 weeks after the 3rd dose. 50 healthy controls provided blood samples. Humoral response was measured with in-house ELISA assays for anti-Spike IgG (anti-S) and anti-receptor binding domain IgG (anti-RBD). T cell activation was measured after stimulation with SARS-CoV-2 peptide. Spearman's correlations assessed the relationship between anti-S, anti-RBD, and frequencies of activated T cells.
Results
Among 60 subjects, mean age was 63 years and 88% were female. 57% of subjects held at least 1 DMARD around the 3rd dose. 43% (anti-S) and 62% (anti-RBD) had a normal humoral response at week 4, defined as ELISA within 1 standard deviation of the healthy control mean. No differences in antibody levels were observed based on holding DMARDs. Median frequency of activated CD4 T cells was significantly greater post- vs. pre-3rd dose. Changes in antibody levels did not correlate with change in frequency of activated CD4 T cells.
Conclusion
Virus-specific IgG levels significantly increased in RA subjects using DMARDs after completing the primary vaccine series, though fewer than two-thirds achieved a humoral response like healthy controls. Humoral and cellular changes were not correlated.
Keywords: Rheumatoid arthritis, COVID-19, Vaccine, Humoral immunity, Cellular immunity, DMARDs, Immunosuppression
Introduction
Immunosuppressed individuals, such as patients with rheumatoid arthritis (RA) taking immunomodulatory disease-modifying anti-rheumatic drugs (DMARDs), are at increased risk for severe COVID-19 disease [1,2]. Two mRNA COVID-19 vaccines (mRNA-1273, manufactured by Moderna and BNT162b2, manufactured by Pfizer) demonstrated excellent efficacy against severe COVID-19 among healthy adults in two large randomized controlled trials (RCTs) [3,4]. In the summer of 2021, the Centers for Disease Control and Prevention (CDC) defined the primary vaccine series for non-immunosuppressed adults as 2 doses of mRNA vaccine separated by 3–4 weeks [5]. The primary vaccine series for moderately or severely immunosuppressed adults was defined as 2 doses of mRNA vaccine separated by 3–4 weeks plus a 3rd dose of mRNA vaccine at least 4 weeks later [5]. Booster doses after the primary vaccine series were recommended for immunosuppressed individuals, healthy adults ages 50 and older, and others at that time; recommendations for boosters have since broadened to include all adults. The American College of Rheumatology recommends interrupting (“holding”) certain DMARDs around the time of COVID-19 vaccination in attempt to improve immunogenicity based on data from a previously published trial of holding versus continuing methotrexate around influenza vaccination [6].
A growing number of reports have demonstrated comparatively lower antibody responses in immunosuppressed persons following COVID-19 vaccination compared to healthy controls [7], [8], [9]. Rituximab, a B-cell depleting therapy, in particular has been associated with reduced humoral response to COVID-19 vaccination [10], [11], [12] and reduced antibody production following influenza vaccination [13], [14], [15]. Cell-mediated immunity following vaccination, which is stimulated by COVID-19 vaccination and may provide protection against infection, was more frequent than serologic response in one cohort of RA patients treated with rituximab [16,17]. While both humoral and cell-mediated immunity are outcomes of interest in vaccination studies, the absolute level of antibodies or T cells conferring protection against SARS-CoV-2 variant infection is incompletely defined.
Herein, we charted humoral and cell-mediated responses before and after a 3rd dose of mRNA COVID-19 vaccine in a cohort of individuals with RA [18], comparing those that held versus continued DMARDs.
Methods
Study design and participants
We enrolled adults aged ≥ 18 years with RA treated at Brigham and Women's Hospital, Boston, US, that had received 2 doses of Moderna or Pfizer mRNA COVID-19 vaccine and had not received a 3rd dose of mRNA vaccine before enrollment that occurred between July and December 2021. Subjects completed study procedures before and after the 3rd dose.
Study procedures
Subjects gave written informed consent prior to the start of any study procedures. Subjects self-reported RA disease activity weekly using a validated patient-reported outcome measure, the RA Disease Activity Index-5 (RADAI-5) [19], from enrollment through week 8 after the 3rd dose. Subjects completed surveys at enrollment and two days after the 3rd dose to indicate use of specific DMARDs, whether they held or continued DMARDs around each dose of vaccine, and vaccine-related side effects after the 3rd dose.
Blood samples were collected before the 3rd dose and 4 weeks after the 3rd dose. Humoral response to vaccination was measured using previously described ELISA assays [20] for anti-Spike IgG (anti-S) and anti-receptor binding domain IgG (anti-RBD, a portion of the SARS-CoV-2 spike protein). Briefly, ELISA plates were coated with 20 ng/well Spike or 20 ng/well RBD at 4 °Celsius overnight. After 2 h of 3% BSA blocking at room temperature, thawed plasma inactivated by 1% Triton were serially diluted and incubated on ELISA plates at 4 °C overnight. Plates were read at 405 nm by Biotek synergy H1 after incubation with alkaline phosphatase conjugated anti-human IgG. The monoclonal antibody (mAb) CR3022 was serially diluted and included as standard on each ELISA plate, and results were reported as ug/mL mAb equivalent to allow normalization across batches. Pre- and post-3rd dose samples from each subject were run in the same batch. 50 healthy controls provided a blood sample 4–8 weeks after a 2nd dose of mRNA vaccine, i.e. after completing the healthy adult primary vaccine series. 14 of these healthy controls also provided a blood sample approximately 6 months after the 2nd dose, and 16 provided blood 4–8 weeks after a 3rd dose of mRNA vaccine (i.e. booster) [20].
Cell-mediated response was assessed in a subset of seropositive RA subjects and 9 healthy controls that had provided a blood sample for ELISA 4–8 weeks after 2nd dose of mRNA vaccine. Cryopreserved PBMCs from RA subjects pre- and post-3rd dose blood draws were thawed and rested overnight. One million PBMCs were incubated (i.e. stimulated) with SARS-CoV-2 peptide pool (Prot_S Complete, Miltenyi Biotec) at a concentration of 1 μg/mL and 2 μg/mL anti-CD28 antibodies for 2 h. At that time, brefeldin A was added to inhibit cytokine release, and the cells were then incubated an additional 4 h. A separate aliquot of PBMCs was incubated with culture medium alone (i.e. unstimulated) to measure baseline activation of T cells. Intracellular flow cytometry staining was performed to assess the frequencies of expression of cytokines (IFNγ, TNF, and IL-2), and surface markers of activation (4–1BB, CD40L, and CD69) among CD4 and CD8 T cells under (a) stimulated and (b) unstimulated conditions. Reagents are listed in Supplementary Table 1. Data were acquired on a BD Fortessa analyzer using FACSDiva software. Compensation and analysis were performed using FlowJo 10.7.1. Gates were set using unstimulated and phorbol 12-myristate 13-acetate and ionomycin (PMA/iono)-stimulated samples as guides (Supplementary Figs. 1 and 2).
Outcomes
The co-primary outcomes for humoral response were fold-change in anti-S and fold-change in anti-RBD. Secondary humoral response outcomes included absolute change (herein referred to as “change”) in anti-S and anti-RBD. A “detectable humoral response” was defined as an antibody level at least twice the average of samples obtained from four individuals before the COVID-19 pandemic. The percentage achieving a “normal humoral response” to the primary vaccine series was defined as the percentage of RA subjects with an ELISA level at week 4 post-3rd dose within one standard deviation of the mean ELISA level in 50 healthy vaccinated controls 4–8 weeks after a 2nd dose (e.g., after completing the primary vaccine series for non-immunosuppressed adults).
The primary outcome for cell-mediated response was the adjusted frequency of activated CD4 T cells (T cells expressing ≥ 2 activation markers: IFNγ, TNF, IL-2, 4–1BB, CD40L, CD69), calculated as the frequency of stimulated CD4 T cells expressing ≥ 2 activation markers minus the frequency of unstimulated CD4 T cells expressing ≥ 2 activation markers. Secondary cell-mediated outcomes included CD8 T cell response categorized as present (>0%) vs. absent (0%) and continuous frequency of activated CD8 T cells.
Statistical analysis
Analyses included subjects that provided blood samples at both study visits to allow for assessment of fold-change and absolute change in virus-specific antibody levels; those providing blood at just one visit were excluded. Raw ELISA values were multiplied by 10 and log-transformed prior to statistical analysis. Fold-change of anti-S and anti-RBD levels were calculated as post-3rd dose divided by pre-3rd dose levels. Change in anti-S and anti-RBD levels were calculated as post-3rd dose minus pre-3rd dose levels. Adjusted frequency of activated T cells (herein referred to as “frequency of activated T cells”) was calculated as the frequency of stimulated CD4 (or CD8) T cells expressing ≥ 2 activation markers minus the frequency of unstimulated CD4 (or CD8) T cells expressing ≥ 2 activation markers.
Continuous data were summarized as median (Q1, Q3) and compared pre- vs. post-3rd dose using Wilcoxon rank sum tests; binary data were compared using Fisher's exact tests. Spearman's correlation coefficients assessed the relationship between anti-S, anti-RBD, and frequencies of activated CD4 T cells. Missing data were not imputed and are reported in footnotes in Tables.
In secondary analyses, we compared subjects (1) holding at least 1 DMARD versus continuing all DMARDs around the 3rd dose and (2) using methotrexate +/- conventional synthetic DMARD (csDMARD) (reference exposure group) in pairwise comparisons versus other DMARD groups: TNF inhibitor (TNFi) +/- csDMARD; JAK inhibitor (JAKi) +/- csDMARD; or other.
Post-hoc power calculations were performed to estimate power to detect differences in humoral response between those holding versus continuing DMARDs (see Supplementary Table 2). The study had 99.7% power to detect a difference of 0.3 in fold-change in anti-S between 34 subjects holding DMARDs and 26 subjects continuing DMARDs, with alpha 0.05 and standard deviation as observed in the study sample.
Analyses were conducted using SAS v9.4, and p < 0.05 was considered significant. The Mass General Brigham Institutional Review Board approved all aspects of this study prior to the start of study procedures.
Results
As shown in Table 1 , RA subjects were mostly females in their early- to mid-sixties. Nearly half were receiving TNFi and nearly a quarter methotrexate. The mean interval between the 2nd and 3rd dose of COVID vaccine was 181 days. Over half of subjects held at least 1 DMARD around the 3rd dose and just under half held at least 1 DMARD around the 1st and/or 2nd dose (Table 4 and Supplementary Table 3). Subject characteristics before the 3rd dose, including RA disease activity, did not significantly differ between those that held vs. continued DMARDs (Table 5).
Table 1.
Characteristics of study subjects.
| All subjects (N = 60) |
Subset of subjects analyzed by flow cytometry (N = 26) | |
|---|---|---|
| Age, mean (SD) | 62.7 (12.0) | 62.2 (13.8) |
| Female, n (%) | 53 (88%) | 21 (81%) |
| White, n (%) | 56 (93%) | 23 (88%) |
| Seropositive rheumatoid arthritis, n (%) | 52 (87%) | 26 (100%) |
| RADAI-5 pre-3rd dose, mean (SD) | 3.2 (1.8) ⁎⁎ | 3.0 (1.7) |
| Brand of initial 2 dose vaccine series, n (%) | ||
| Pfizer | 35 (58%) | 12 (46%) |
| Moderna | 25 (42%) | 14 (54%) |
| Brand of 3rd dose, n (%) | ||
| Pfizer | 34 (57%) | 12 (46%) |
| Moderna | 26 (43%) | 14 (54%) |
| COVID-19 infection before 3rd dose, n (%) | 2 (3%) | 0 |
| Days from completing initial 2 dose vaccine series to 3rd dose, mean (SD) | 180.8 (46.4) | 193.8 (48.4) |
| DMARDs at the time of 3rd dose, n (%) | ||
| Methotrexate +/- csDMARD | 14 (23%) | 7 (27%) |
| TNFi +/- csDMARD | 26 (43%) | 12 (46%) |
| JAKi +/- csDMARD | 11 (18%) | 7 (27%) |
| Other* | 9 (15%) | 0 |
| Held DMARD(s) around 1st and/or 2nd doses, n (%) | 25 (42%) | 14 (54%) |
| Held DMARD(s) around 3rd dose, n (%) | 34 (57%) | 14 (54%) |
Abbreviations: RADAI-5, Rheumatoid Arthritis Disease Activity Index-5. DMARD, disease modifying anti-rheumatic drug. csDMARD, conventional synthetic DMARD.
Other DMARDs: IL-6 inhibitors, abatacept, tacrolimus, hydroxychloroquine.
Among 58 subjects with RADAI-5 data available pre-3rd dose.
Table 4.
Duration of Holding DMARDs Around the 3rd COVID-19 Vaccine Dose among 34 Subjects that Held At Least 1 DMARD.
| Held All DMARDs (n = 22) | < 1 week | 1 week | 2 weeks | > 2 weeks | Total |
|---|---|---|---|---|---|
| MTX monotherapy | 1 | 1 | 1 | 3 | 6 |
| TNFi monotherapy | – | 3 | 1 | 1 | 5 |
| TNFi + csDMARD | – | 2 | 1 | 4 | 7 |
| JAKi + csDMARD | 1 | – | – | – | 1 |
| Abatacept only | – | 1 | – | 1 | 2 |
| Other | 1 | – | – | – | 1 |
| Total | 3 | 7 | 3 | 9 | 22 |
| Held at least 1 but not all DMARDs (n = 12) | < 1 week | 1 week | 2 weeks | > 2 weeks | Total |
| MTX + csDMARD | – | – | – | 2 | 2 |
| TNFi + csDMARD | – | 3 | 1 | 1 | 5 |
| JAKi + csDMARD | – | 1 | 2 | 2 | 5 |
| Total | – | 4 | 3 | 5 | 12 |
Abbreviations: DMARD, disease modifying anti-rheumatic drug. MTX, methotrexate. TNFi, tumor necrosis factor inhibitor. JAKi, Janus kinase inhibitor. csDMARD, conventional synthetic disease modifying anti-rheumatic drug.
Table 5.
Baseline characteristics among subjects that continued all DMARDs vs. held at least 1 DMARD around the 3rd dose of COVID-19 vaccine.
| Continued all DMARDs (n = 26) |
Held at least 1 DMARD (n = 34) |
P value | |
|---|---|---|---|
| Age, mean (SD) | 63.4 (12.2) | 62.1 (12.1) | 0.68 |
| Female, n (%) | 23 (88%) | 30 (88%) | 1.00 |
| White, n (%) | 25 (96%) | 31 (91%) | 0.75 |
| Seropositive rheumatoid arthritis, n (%) | 24 (92%) | 28 (82%) | 0.45 |
| RADAI-5 pre-3rd dose, mean (SD) | 3.0 (1.6) | 3.3 (2.0)⁎⁎ | 0.58 |
| Brand of initial 2 dose vaccine series, n (%) | |||
| Pfizer | 12 (46%) | 23 (68%) | 0.09 |
| Moderna | 14 (54%) | 11 (32%) | |
| Brand of 3rd dose, n (%) | |||
| Pfizer | 13 (50%) | 21 (62%) | 0.36 |
| Moderna | 13 (50%) | 13 (38%) | |
| COVID-19 infection before 3rd dose, n (%) | 0 (0%) | 2 (6%) | 0.50 |
| Mean (SD) days from completing initial 2 dose vaccine series to 3rd dose | 185.6 (46.2) | 177.1 (46.9) | 0.49 |
| DMARDs at the time of 3rd dose, n (%) | |||
| Methotrexate +/- csDMARD | 6 (23%) | 8 (24%) | 0.42 |
| TNFi +/- csDMARD | 9 (35%) | 17 (50%) | |
| JAKi +/- csDMARD | 5 (19%) | 6 (18%) | |
| Other* | 6 (23%) | 3 (9%) | |
| Held DMARD(s) around 1st/2nd doses, n (%) | 7 (27%) | 18 (53%) | 0.04 |
Abbreviations: DMARD, disease modifying anti-rheumatic drug. RADAI-5, RA Disease Activity Index-5. csDMARD, conventional synthetic DMARD. TNFi, tumor necrosis factor inhibitor. JAKi, Janus kinase inhibitor.
Other DMARDs: IL-6 inhibitors, abatacept, tacrolimus, hydroxychloroquine.
Among 32 subjects that held at least 1 DMARD and had RADAI-5 data available pre-3rd dose.
Humoral response
Before the 3rd dose, 95% of RA subjects had a detectable anti-S and anti-RBD humoral response; 98.3% had detectable anti-S and anti-RBD 4 weeks after the 3rd dose. Forty-three percent of RA subjects had a normal anti-S humoral response and 62% had a normal anti-RBD humoral response 4 weeks after completing the primary vaccine series, i.e. after the 3rd dose (Table 3). The percent achieving a normal humoral response did not significantly differ between those that held vs. continued DMARDs (see Table 3). The interval between the 2nd dose and 3rd dose was significantly longer in those that did not achieve a normal anti-S response vs. those achieving a normal anti-S response; other features did not significantly differ by achievement of a normal humoral response (Supplementary Table 4).
Table 3.
Percentage of RA subjects achieving a normal humoral response* 4 weeks after a 3rd dose of mRNA COVID-19 vaccine.
| All RA subjects (n = 60) | Held DMARDs around 3rd dose (n = 34) | Continued DMARDs around 3rd dose (n = 26) | p value+ | |
|---|---|---|---|---|
| Anti-S | 43.3% | 41.2% | 46.2% | 0.70 |
| Anti-RBD | 61.7% | 70.6% | 50.0% | 0.10 |
Anti-S, anti-Spike. Anti-RBD, anti-receptor binding domain. DMARD, disease modifying anti-rheumatic drug.
Normal humoral response was defined as ELISA level at week 4 post-3rd dose within one standard deviation of the mean ELISA level in 50 healthy vaccinated controls 4–8 weeks after a 2nd dose (e.g., after completing the primary vaccine series for non-immunosuppressed adults).
p values comparing subjects that held vs. continued DMARDs.
The fold-increase in anti-S was similar in those holding and continuing DMARDs (p = 0.56 for anti-S, p = 0.76 for anti-RBD) (Figs. 1 B & 1C; 2 B & 2C). Absolute change in anti-S and change in anti-RBD also did not statistically differ between subjects that held vs. continued DMARDs (p = 0.78 for anti-S, p = 0.57 for anti-RBD).
Fig. 1.
Log-transformed anti-Spike ELISA levels pre- and post-3rd dose. Horizontal line represents the detectable humoral response threshold (>2x the average response in historical pre-COVID era healthy controls). Median (Q1, Q3) post- vs. pre-3rd dose fold-change (green) and absolute change (blue) are indicated on each panel. P values comparing absolute change are from Wilcoxon signed rank sum test (* indicates p < 0.01, ** indicates p < 0.0001). Top panels: (A) all RA subjects (n = 60) and healthy controls (HC) that provided blood (i) 4–8 weeks after 2nd dose of mRNA vaccine (n = 50, serving as a contrast against RA subjects 4 weeks after 3rd dose), (ii) 6 months after 2nd dose (n = 14), (iii) 4–8 weeks after 3rd dose (n = 16, no comparable timepoint collected for RA subjects); (B) RA subjects that held at least 1 DMARD around the 3rd dose; (C) those that continued all DMARDs around the 3rd dose. Bottom panels present data by DMARD (not stratified by holding or continuing DMARDs): (D) methotrexate +/- conventional synthetic (cs)DMARDs; (E) TNFi +/- csDMARDs; (F) JAKi +/- csDMARDs; (G) other DMARDs (IL-6 inhibitors, abatacept, tacrolimus, hydroxychloroquine).
Fig. 2.
Log-transformed anti-RBD ELISA levels pre- and post-3rd dose. Horizontal line represents the detectable humoral response threshold (>2x the average response in historical pre-COVID era healthy controls). Median (Q1, Q3) post- vs. pre-3rd dose fold-change (green) and absolute change (blue) are indicated on each panel. P values comparing absolute change are from Wilcoxon signed rank sum test (* indicates p < 0.01, ** indicates p < 0.0001). Top panels: (A) all RA subjects (n = 60) and healthy controls (HC) that provided blood (i) 4–8 weeks after 2nd dose of mRNA vaccine (n = 50, serving as a contrast against RA subjects 4 weeks after 3rd dose), (ii) 6 months after 2nd dose (n = 14), (iii) 4–8 weeks after 3rd dose (n = 16, no comparable timepoint collected for RA subjects); (B) RA subjects that held at least 1 DMARD around the 3rd dose; (C) those that continued all DMARDs around the 3rd dose. Bottom panels present data by DMARD (not stratified by holding or continuing DMARDs): (D) methotrexate +/- conventional synthetic (cs)DMARDs; (E) TNFi +/- csDMARDs; (F) JAKi +/- csDMARDs; (G) other DMARDs (IL-6 inhibitors, abatacept, tacrolimus, hydroxychloroquine).
Comparing subjects using methotrexate vs. other DMARD groups, fold-change in anti-S and anti-RBD and change in anti-S and anti-RBD did not significantly differ in pairwise comparisons (p > 0.05 for each pairwise comparison).
Among 14 healthy controls, anti-S increased 1.47-fold (1.31, 1.61) and anti-RBD increased 1.60-fold (1.44, 2.12) when comparing samples obtained 6 months after the 2nd dose to samples collected 1 month after the 3rd dose (Figs. 1A and 2A). Fold-change and absolute change in anti-S and anti-RBD were not significantly different 1 month after completing the primary vaccine series for RA subjects (i.e., 3rd dose) and healthy controls (i.e., 2nd dose) (Figs. 1A and 2A).
Cell-mediated response
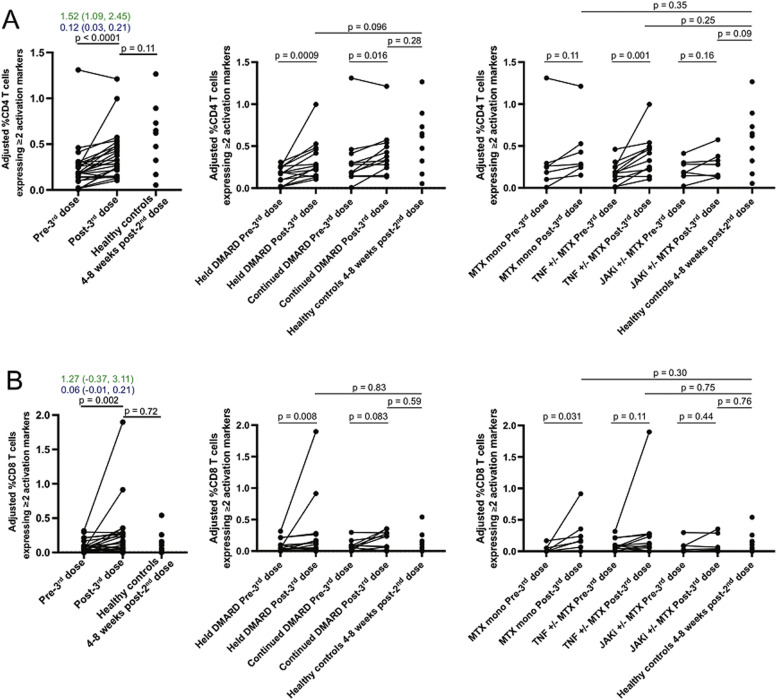
Frequencies of SARS-CoV-2-activated CD4 T cells significantly increased after the 3rd dose (Fig. 3 A). A significant increase was observed both in subjects that held DMARDs (p<0.01) and those that continued DMARDs (p = 0.02). (Fig. 3A and Table 2 ) In subgroups by DMARD use, frequency of activated CD4 T cells significantly increased in those using TNFi +/- methotrexate (p = 0.001) but not in those using methotrexate monotherapy or JAKi +/- methotrexate.
Fig. 3.
SARS-CoV-2-activated CD4 and CD8 T cell responses. Peripheral blood mononuclear cells were stimulated with SARS-CoV-2 spike protein peptides, and activation of CD4 T cells (A) and CD8 T cells (B) was assessed by flow cytometry. To account for differences in baseline T cell activation among individuals, the graphs depict frequency of CD4 or CD8 T cells expressing at least two activation markers (among TNF, IFNγ, IL-2, 4–1BB, CD40L, and CD69) after stimulation with SARS-CoV-2 spike protein peptides minus the frequency of CD4 or CD8 T cells expressing at least two activation markers among unstimulated cells. Fold-change in percentage of CD4 or CD8 T cells post- vs. pre-3rd dose is shown in green; absolute change is shown in blue. Differences between pre- and post-3rd dose responses were assessed using the Wilcoxon signed rank sum test, while comparisons with heathy controls were performed using a Mann-Whitney test.
Table 2.
Adjusted frequency* of CD4 T cells expressing ≥ 2 activation markers⁎⁎ after stimulation with SARS-CoV-2 peptide pool.
| Study sample | Pre-3rd dose median (Q1, Q3) |
Post-3rd dose median (Q1, Q3) |
p value+ |
|---|---|---|---|
| All RA subjects providing PBMCs, n = 26 | 0.19 (0.14, 0.28) | 0.30 (0.22, 0.48) | <0.0001 |
| Held vs. continued DMARD around the time of 3rd dose | |||
| Held at least 1 DMARD, n = 14 | 0.18 (0.09, 0.25) | 0.26 (0.15, 0.46) | <0.001 |
| Continued all DMARDs, n = 12 | 0.23 (0.16, 0.36) | 0.35 (0.26, 0.52) | 0.02 |
| DMARD group | |||
| Methotrexate monotherapy, n = 7 | 0.25 (0.10, 0.29) | 0.28 (0.24, 0.53) | 0.11 |
| TNFi +/- methotrexate, n = 12 | 0.18 (0.12, 0.25) | 0.37 (0.22, 0.49) | 0.001 |
| JAKi +/- methotrexate, n = 7 | 0.19 (0.14, 0.30) | 0.28 (0.13, 0.38) | 0.16 |
| Post-2nd dose++ | |||
| Healthy controls, n = 9 | n/a | 0.62 (0.32, 0.73) | n/a |
Calculated as (% of CD4 T cells expressing activation markers after stimulation with peptide pool) minus (% of CD4 T cells expressing ≥ 2 activation markers after culture in media without stimulus).
Activation markers: IFNγ, TNF, IL-2, 4–1BB, CD40L, CD69.
p values comparing pre- vs. post-3rd dose values using Wilcoxon signed rank sum test.
Healthy controls provided a blood sample 4–8 weeks post-2nd dose, e.g. after completing the primary vaccination series for non-immunosuppressed adults (serving as a contrast against RA subjects 4 weeks after 3rd dose).
A detectable CD8 T cell response (>0%) post-3rd dose was present in approximately 80% of subjects that held or continued DMARDs (p = 1.00) (Supplementary Table 6). Frequencies of activated CD8 T cells significantly increased among all subjects (p = 0.002), those that held DMARDs (p = 0.04), and those using methotrexate monotherapy (p = 0.03) (Fig. 3B and Supplementary Table 5).
When comparing activated T cell frequencies in 9 healthy controls after a 2nd dose of mRNA vaccine versus 26 RA subjects after a 3rd dose of mRNA vaccine, the frequency of activated CD4 T cells was numerically higher in controls (median 0.62% [interquartile range (IQR) 0.32, 0.73%]) than RA subjects (median 0.30% [IQR 0.22, 0.48%]), though this difference was not statistically significant (p = 0.11) (see Fig. 3A). Frequency of activated CD8 T cells at those time points was numerically similar in controls (median 0.11% [IQR 0.02, 0.16%]) and RA subjects (median 0.12% [IQR 0.02, 0.28%]) (p = 0.72) (Fig. 3B).
Supplementary Table 7 presents correlations between measures of humoral response and T cell-mediated response. Neither fold-change nor change in ELISA level was significantly correlated with the change in frequency of activated CD4 T cells at week 4 post-3rd dose (rho −0.26 to 0.19, p > 0.05 for all correlations). Supplementary Table 8 presents comparisons of immunologic responses by methotrexate dose <20 mg weekly versus ≥20 mg weekly. Absolute change in humoral response, fold-change in humoral response, and frequency of activated CD4 T cells did not significantly differ by methotrexate dose among all methotrexate users or in the subgroup using methotrexate monotherapy.
Discussion
Antibody levels against SARS-CoV-2 spike and receptor binding domain significantly increased in RA subjects using DMARDs 1 month after completing the primary COVID vaccine series (i.e., after 3 doses of mRNA vaccine), though only about half of subjects (43% for anti-S, 62% for anti-RBD) achieved a humoral response within 1 standard deviation of the mean in healthy controls (i.e., 1–2 months after 2 doses of mRNA vaccine) (Table 3 ). Achievement of a normal anti-S humoral response after the 3rd dose was more common with a shorter interval between the 2nd and 3rd doses. The observed increase in antibody levels was similar in RA subjects that held or continued DMARDs around the 3rd dose and did not significantly differ between subjects using methotrexate vs. other DMARDs (Figs. 1 and 2). Frequencies of CD4 T cells activated by SARS-CoV-2 peptide significantly increased after the 3rd dose in subjects that held or continued DMARDs, but they were numerically lower than the frequency of activated CD4 T cells among vaccinated healthy controls. Change in frequency of activated CD8 T cells was statistically significant in those holding DMARDs but not in those continuing DMARDs (Fig. 3). Changes in SARS-CoV-2 antibody levels did not correlate with T cell responses to SARS-CoV-2 peptide pool.
The American College of Rheumatology recommendation to hold certain DMARDs around COVID-19 vaccination drew upon an RCT demonstrating an approximately 20% higher rate of achieving a satisfactory vaccine response (at least a four-fold increase of hemagglutination inhibition titer at week 4) in subjects with RA randomized to hold methotrexate for 2 weeks versus continue methotrexate; data specific to COVID-19 vaccines were not available at the time [21]. A recent RCT among persons with immune-mediated inflammatory disease (Vaccination Response On/Off Methotrexate [VROOM] Study) identified a greater humoral response at week 4 after a 3rd dose of COVID-19 vaccine in those holding methotrexate for 2 weeks vs. continuing methotrexate [22]. The primary outcome in the VROOM Study was geometric mean ratio of anti-S1 RBD antibody titers measured using the Roche Elecsys immunoassay, which was approximately 2-fold greater in subjects randomized to hold vs. continue methotrexate.
Our observational study did not identify statistically significant differences in the fold-change or absolute change in anti-S or anti-RBD among subjects that held versus continued DMARDs. The percentage achieving a normal humoral response for anti-RBD was numerically greater among those holding DMARDs (71%) versus continuing DMARDs (50%), but this did not reach statistical significance (Table 3). In contrast to the VROOM RCT, in which all subjects were using methotrexate, subjects in our study were using a variety of DMARDs and those that held DMARDs did so for different durations (see Table 4 ). Similar to our findings, a prospective observational study of 1647 patients with immune-mediated inflammatory diseases using a range of DMARDs did not identify differences in anti-RBD in those that held vs. continued DMARDs [7]. Our study was underpowered to compare humoral response across subjects that held vs. continued individual DMARDs and in subgroups by methotrexate dose. As this was not an RCT, unmeasured differences in characteristics between subjects that chose to hold vs. continue DMARDs might explain the lack of difference in humoral response, though baseline characteristics were balanced (see Table 5 ).
While holding versus continuing DMARDs was not associated with a difference in change in antibody titers after the 3rd dose, it is striking that only about half of subjects – regardless of DMARD holding or specific DMARDs – achieved a humoral response similar to that of healthy controls after completing the primary vaccine series (Table 3). RA subjects and healthy controls in our study mounted a similar increase (fold-change and absolute change) in humoral response 1 month after completing their respective primary vaccine series (Figs. 1 and 2). The Moderna and Pfizer vaccines induced high levels of neutralizing antibodies in RCTs among non-immunosuppressed adults who had received two doses of vaccine, similar to our healthy control cohort [23], [24], [25]. Our finding that approximately half of immunosuppressed RA patients reach similar titers of anti-S and anti-RBD even after a 3rd dose of vaccine highlights the vulnerability of this population and supports the need for immunization schedules specifically designed to optimize responses of this patient population. Our study also highlights the heterogeneity of antibody response in RA subjects using different DMARDs.
A number of important methodological differences distinguish the present study from other studies of immune responses to COVID-19 vaccination in immunosuppressed populations [17,26,27]. Subjects in our study used a variety of DMARDs and those that held DMARDs did so for different lengths of time (see Table 4). Methods for measuring humoral response differ across studies and include use of commercially available SARS-CoV-2 spike ELISA assays, in-house assays to measure median fluorescence intensity after flow cytometry using SARS-CoV-2 spike and RBD plasmids, and surrogate virus neutralization test [17,[26], [27], [28]]. Because an absolute threshold for serologic protection against COVID-19 remains unknown, we did not attempt to define “seroconversion” or “seroprotection” but rather reported on the fold-change and absolute change of log-transformed antibody levels. Our humoral response metric used a monoclonal antibody positive control, which minimizes between-batch variability and provides a positive standard against which antibody levels can be reliably measured. Given that intra-batch variability for ELISA can vary as much as four-fold, log-transformation is an important measure to minimize variability before data analysis.
We assessed activated T cells by stimulating PBMCs with SARS-CoV-2 peptide pool, similar to a recent study of responses to COVID-19 vaccines in RA patients using rituximab [17]. In the study of rituximab users in which T cell response was not clearly defined, the frequency of CD4 T cell response increased from 53% to 100% and frequency of CD8 T cell response increased from 74% to 100% after the 2nd or 3rd dose of vaccine, respectively [17]. We report CD4 T cell responses using continuous data and defined CD8 T cell responses as present (>0%) versus absent (0%), as our assessment of activated T cells favored CD4 T cell response due to the specific cytokines and cell surface markers measured. T cell responses in studies that performed flow cytometry without stimulating with SARS-CoV-2 peptide pool, or that defined activation by expression of a different set of cytokines, cannot be directly compared against our study due to different methodology [26,29].
Limitations of this study include its observational nature and small sample size within DMARD groups, which may have limited statistical power to detect small between-group differences in humoral response to the 3rd dose. However, post-hoc power calculations demonstrate >99% power to detect a 0.3-fold difference in anti-S response. The definition of “holding DMARDs” reflected real-world practice, in which subjects used different DMARDs and held one or more DMARD for different durations of time. The number of subjects providing PBMCs for T cell assays was limited due to constraints around cost and feasibility, but it was similar to the sample size in a prior study of T cell responses to COVID-19 vaccination in persons with RA [17]. Mean age was 62 years and immune responses in this population may not reflect responses (especially humoral response) in younger people with RA.
These data shed light on the potential for persons with RA to achieve a “normal humoral response” after a 3rd dose of COVID-19 vaccine. Current CDC recommendations for booster doses in immunosuppressed individuals and/or use of preventive monoclonal antibodies are of paramount importance to enhance humoral immunity. Additional studies are needed to determine clinical relevance of quantitative differences in humoral and cell-mediated responses in immunosuppressed patients.
In conclusion, we observed that holding versus continuing DMARDs was not associated with differences in humoral response a month after completing the primary COVID vaccine series. Only about half of RA subjects, regardless of DMARD use, achieved a humoral response similar to that of healthy controls after completing the primary vaccine series. Measures of humoral and cell-mediated immunity were not associated in this study population.
Contributorship statement
SKT and DHS were responsible for conceiving the study, overseeing recruitment, and data interpretation. SKT drafted the first version of the manuscript. JS, JE, and MGW were responsible for subject recruitment, conducting study visits, data entry, and provided comments on the manuscript. KH performed data analysis and provided comments on the manuscript. LC, IA, and KM performed T cell assays and data analyses and provided comments on the manuscript. YC and NW performed ELISA analyses and provided comments on the manuscript. AHJ, DAR, and DRW contributed to study design and provided critical feedback on the manuscript. ModernaTx provided funding for the study and approved the final manuscript, but was not involved with study design, study conduct, data analysis, results interpretation, or drafting the manuscript.
Ethical approval information
This study involves human participants and was approved by an Ethics Committee(s) or Institutional Board(s).
Name of Review Board: Mass General Brigham Institutional Review Board
Protocool # 2021P00763
This study does not involve animal subjects.
Data sharing statement
Requests for access to unpublished de-identified data will be reviewed by the authors and subject to a data use agreement with Brigham and Women's Hospital.
Patient and public involvement
Patients or the public were not included in the design, conduct, reporting, or dissemination plans of our research.
Funding, grant/award information
ModernaTX, Inc, Doris Duke Charitable Foundation, U.S. Department of Health and Human Services, National Institutes of Health, K08 AR072791, K23 AR075070, L30 AR070514, P30 AR070253, P30 AR072577
Key messages
-
•
DMARD type was not associated with humoral response after 3rd COVID-19 vaccine in RA subjects
-
•
Half of RA subjects had a lower humoral response than controls after the primary vaccine series.
-
•
Measures of humoral and cell-mediated immunity were not correlated in RA subjects.
Declaration of Competing Interest
ModernaTx provided support for this investigator-sponsored study (PI: Solomon) with payments made directly to Brigham and Women's Hospital. SKT: research support to Brigham and Women's Hospital from NIH; consulting fees from NGM Biopharmaceuticals, AHJ: research support to Brigham and Women's Hospital from Amgen, DAR: research support to Brigham and Women's Hospital from Doris Duke Charitable Foundation, Jansen, Merck, NIH; scientific advisory board member for Bristol Myers Squibb; patent submitted on Tph cells as a biomarker in autoimmune disease; consulting fees from Jansen, DHS: research support to Brigham and Women's Hospital from ModernaTx, Amgen, Abbvie, CorEvitas, and NIH; royalties from UpToDate for a chapter related to NSAIDs, YC: research support to Brigham and Women's hospital from the NIH, DRW: research support to Brigham and Women's hospital from the NIH, The Massachusetts Consortium on Pathogenesis Readiness, the Bill and Melinda Gates Foundation, the Food Allergy Science Initiative, and the China Evergrande Group.JS, JE, MGW, KH, LC, IA, KEM: none
Acknowledgments
N/A
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.semarthrit.2023.152177.
Appendix. Supplementary materials
References
- 1.Sparks J.A., Wallace Z.S., Seet A.M., et al. Associations of baseline use of biologic or targeted synthetic dmards with covid-19 severity in rheumatoid arthritis: results from the covid-19 global rheumatology alliance physician registry. Ann Rheum Dis. 2021;80(9):1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.England B.R., Roul P., Yang Y., et al. Risk of covid-19 in rheumatoid arthritis: a national veterans affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021;73(12):2179–2188. doi: 10.1002/art.41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mrna-1273 sars-cov-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the bnt162b2 mrna covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for disease C. Interim clinical considerations for use of covid-19 vaccines currently approved or authorized in the united states. 16 June 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#primary-series.
- 6.Curtis J.R., Johnson S.R., Anthony D.D., et al. American college of rheumatology guidance for covid-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73(10):e60–e75. doi: 10.1002/art.41928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syversen S.W., Jyssum I., Tveter A.T., et al. Immunogenicity and safety of standard and third-dose sars-cov-2 vaccination in patients receiving immunosuppressive therapy. Arthritis Rheumatol. 2022 doi: 10.1002/art.42153. May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepak P., Kim W., Paley M.A., et al. Effect of immunosuppression on the immunogenicity of mrna vaccines to sars-cov-2: a prospective cohort study. Ann Intern Med. 2021;174(11):1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furer V., Eviatar T., Zisman D., et al. Immunogenicity and safety of the bnt162b2 mrna covid-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 10.Boyarsky B.J., Ruddy J.A., Connolly C.M., et al. Antibody response to a single dose of sars-cov-2 mrna vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(8):1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furer V., Eviatar T., Zisman D., et al. Predictors of immunogenic response to the bnt162b2 mrna covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases treated with rituximab. Vaccines. 2022;10(6) doi: 10.3390/vaccines10060901. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auroux M., Laurent B., Coste B., et al. Serological response to sars-cov-2 vaccination in patients with inflammatory rheumatic disease treated with disease modifying anti-rheumatic drugs: a cohort study and a meta-analysis. Jt Bone Spine. 2022;89(5) doi: 10.1016/j.jbspin.2022.105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oren S., Mandelboim M., Braun-Moscovici Y., et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67(7):937–941. doi: 10.1136/ard.2007.077461. [DOI] [PubMed] [Google Scholar]
- 14.van Assen S., Holvast A., Benne C.A., et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62(1):75–81. doi: 10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- 15.Bingham C.O., 3rd, Looney R.J., Deodhar A., et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 16.Oberhardt V., Luxenburger H., Kemming J., et al. Rapid and stable mobilization of cd8(+) t cells by sars-cov-2 mrna vaccine. Nature. 2021;597(7875):268–273. doi: 10.1038/s41586-021-03841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jyssum I., Kared H., Tran T.T., et al. Humoral and cellular immune responses to two and three doses of sars-cov-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4(3):e177–ee87. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedeschi S.K., Stratton J., Ellrodt J.E., et al. Rheumatoid arthritis disease activity assessed by patient-reported outcomes and flow cytometry before and after an additional dose of covid-19 vaccine. Ann Rheum Dis. 2022;81(7):1045–1048. doi: 10.1136/annrheumdis-2022-222232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson J.K., Zimmerman L., Caplan L., et al. Measures of rheumatoid arthritis disease activity: patient (ptga) and provider (prga) global assessment of disease activity, disease activity score (das) and disease activity score with 28-joint counts (das28), simplified disease activity index (sdai), clinical disease activity index (cdai), patient activity score (pas) and patient activity score-ii (pasii), routine assessment of patient index data (rapid), rheumatoid arthritis disease activity index (radai) and rheumatoid arthritis disease activity index-5 (radai-5), chronic arthritis systemic index (casi), patient-based disease activity score with esr (pdas1) and patient-based disease activity score without esr (pdas2), and mean overall index for rheumatoid arthritis (moi-ra) Arthritis Care Res. 2011;63(11):S14–S36. doi: 10.1002/acr.20621. (Hoboken)Suppl. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Tong P., Whiteman N., et al. Immune recall improves antibody durability and breadth to sars-cov-2 variants. Sci Immunol. 2022:eabp8328. doi: 10.1126/sciimmunol.abp8328. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J.K., Lee Y.J., Shin K., et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77(6):898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abhishek A., Boyton R.J., Peckham N., et al. S2213-2600. 2022. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on covid-19 booster vaccine immunity in adults with inflammatory conditions (vroom study): a randomised, open label, superiority trial; p. 00186. (Lancet Respir Med). Jun 27-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson E.J., Rouphael N.G., Widge A.T., et al. Safety and immunogenicity of sars-cov-2 mrna-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mrna vaccine against sars-cov-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two rna-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberman R.H., Herati R., Simon D., et al. Methotrexate hampers immunogenicity to bnt162b2 mrna covid-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80(10):1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamar N., Abravanel F., Marion O., et al. Three doses of an mrna covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arumahandi de Silva A.N., Frommert L.M., Albach F.N., et al. Pausing methotrexate improves immunogenicity of covid-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis. 2022;81(6):881–888. doi: 10.1136/annrheumdis-2021-221876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahil S.K., Bechman K., Raharja A., et al. Humoral and cellular immunogenicity to a second dose of covid-19 vaccine bnt162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. 2022;4(1):e42–e52. doi: 10.1016/S2665-9913(21)00333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.