Dear editor,
Recently, Deng et al. reported a significant proportion of patients having persistent olfactory dysfunction (OD) even 2 years after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Moreover, the prevalence of OD evaluated by psychophysical tests was higher than that estimated based on self-reported information. We had a valuable opportunity to carefully read this interesting manuscript and additional published studies to further explore the persistent OD after the coronavirus disease 2019 (COVID-19) infection.
COVID-19 was caused by SARS-CoV-2 infection, and it was listed as a global pandemic by the World Health Organization on March 11, 2020. The virus is mainly transmitted through respiratory droplets and physical contact. Besides respiratory symptoms, the most common symptom is dysfunction of smell and taste.
Post-acute COVID-19 syndrome, also known as long COVID, is a prolonged illness after the acute phase of COVID-19. It is defined as “the collection of symptoms that develop during or following a confirmed or suspected case of COVID-19 and continue for more than 28 days.”2 The OD associated with COVID-19 is usually transient, and it tends to subside spontaneously within a few weeks after the infection, especially in mild-to-moderate patients. However, some patients still complain of persistent OD 6 months after infection. Lechien et al. found the prevalence of OD significantly decreased from 50.8% (baseline) to 18.7% (1 year) and 3.5% (2 years) according to the psychophysical olfactory evaluations.3 Some authors reported significantly higher rates of persistent OD, but only a few of them investigated the prevalence of OD after 6 months or even more than 1 year of infection using objective methods.
Loss of smell can have a serious impact on quality of life as it can negatively affect food enjoyment, nutritional balance, social communication, cognitive skills, and psychological functioning. In addition, it can expose patients and their families to dangerous situations.4 Given the above-mentioned harms, there is an urgent need to develop effective treatments. We sought to explore the prevalence of persistent OD after COVID-19 infection, which is essential to understand the chronic symptoms of long COVID and to guide treatments.
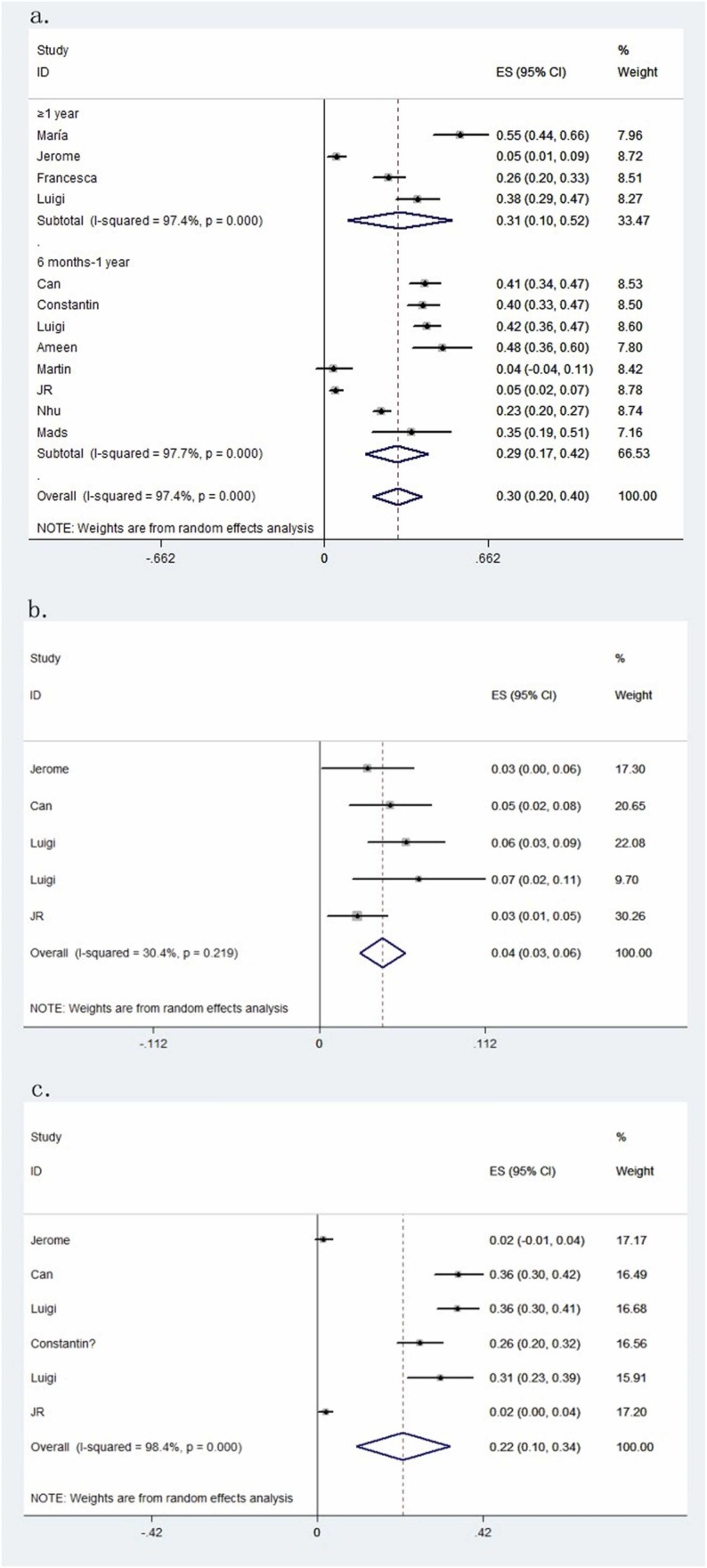
For this reason, PubMed, Web of Science, Embase, and Cochrane Library databases were extensively searched for all compliant studies published from January 1, 2020, to December 27, 2022. The inclusion criteria were as follows: (1) adult patients with COVID-19 confirmed by reverse transcriptase–polymerase chain reaction; (2) peer-reviewed original studies in English; (3) OD after COVID-19 persisting for more than 6 months after infection; and (4) normal cognitive function before COVID-19 and no severe nasal injuries. Twelve studies report persistent OD after COVID-19 infection. General information about the included studies is summarized in Supplementary Material ( Table 1). We focused on recording the number of patients with COVID-19 having OD for more than 6 months; especially those in which the dysfunction persisted for more than 1 year were of more concern to us. The results of 12 studies listed in Fig. 1 showed among patients with COVID-19, OD lasted for more than 6 months in about 30% of patients (95% CI, 0.20–0.40; P < 0.01). The heterogeneity (I 2) was 97.4% and the Begg's test P-value was 0.631, indicating no publication bias. To further explore the persistence of olfactory impairment, we categorized the duration into 6 months to 1 year and more than 1 year. Similar rates were found for those in which the dysfunction lasted more than 1 year. The estimated prevalence rate was 31% (95% CI, 0.10–0.52; P < 0.01). OD can be subdivided into anosmia and hyposmia. In five studies, patients had anosmia 6 months after being infected with COVID-19, and the proportion was 4% (95% CI, 0.03–0.06; P < 0.01). Also, in six studies, the rate of patients having hyposmia was 22% (95% CI, 0.10–0.34; P < 0.01).
Table 1.
The basic information of the included literature. OD: olfactory dysfunction. N: no data.
| Author | Country | Age | Duration | Total No. of persistent OD | Total No. of OD |
|---|---|---|---|---|---|
| María | Spain | 46.8 ± 13.9 | 12 months | 43 | 78 |
| Jerome | Multicenter | 45.0 ± 12.0 | 24 months | 6 (4 anosmia, 2 hyposmia) | 123 |
| Can | France | 50.4 ± 18.9 | 9.5 months | 93 (11 anosmia, 82 hyposmia) | 229 |
| Constantin | Germany | 49.0 ± 14.4 | 8 months | 85 (55 hyposmia) | 212 |
| Luigi | Italy | 38.4 ± 12.5 | 253.4 ± 70.5 days | 127 (18 anosmia, 109 hyposmia) | 306 |
| Francesca | Italy | N | 12 months | 46 | 176 |
| Luigi | Multicenter | 39.9 ± 0.5 | 419.8 days | 45 (8 anosmia, 37 hyposmia) | 119 |
| Ameen | Israel | 37.5 (19–74) | 229 days | 31 | 65 |
| Martin | Germany | 45 ± 2.06 | 201 days | 1 | 26 |
| JR | Multicenter | 44.5 ± 16.4 | 6 months | 11 (6 anosmia, 5 hyposmia) | 233 |
| Nhu | France | 45 | 6 months | 136 | 584 |
| Mads | Multicenter | 43.3 | 6 months | 12 | 34 |
Fig. 1.
(a) The pooled prevalence of persistent olfactory dysfunction after 6 months of infection in COVID-19 can be divided into 6 months to 1 year and more than 1 year. (b) The pooled prevalence of persistent anosmia after 6 months of infection in COVID-19. (c) The pooled prevalence of persistent hyposmia after 6 months of infection in COVID-19.
Within 50 days after COVID-19 diagnosis, acute symptoms such as diarrhea, stomach pain, and cold-like symptoms develop. However, what make people suffer more physically and mentally are the persistent cardiopulmonary symptoms, musculoskeletal symptoms, sensory symptoms (taste loss or olfactory disturbance), and general symptoms within 90–150 days after diagnosis.5 It is estimated that as many as 1.6 million people in the United States have chronic OD due to COVID-19.6 Chronic olfactory disorders may lead to eating behavior disorders, depression, and a general decrease in quality of life. However, little is known about the long-term course of OD associated with COVID-19. Previously, it was thought that SARS-CoV-2 caused anosmia by affecting the olfactory epithelium, the peripheral organ for olfaction that lines the olfactory cleft of the nasal cavity. The olfactory epithelium houses the primary olfactory sensory neurons responsible for detecting odors. Transient gene expression changes in olfactory sensory neurons, alterations in the characteristics of the mucus layer surrounding their cilia, and inflammation are thought to cause acute anosmia in animal models of SARS-CoV-2 infection.7 Recent findings indicate that acute SARS-CoV-2 infection drives a proinflammatory reprogramming that is thought to induce long-term alterations in the function of other immune cells. T cell-mediated inflammation persists in the olfactory epithelium long after SARS-CoV-2 has been eliminated from the tissue, suggesting a mechanism for long-term post-COVID-19 smell loss.8
These mechanisms suggest therapeutic strategies. The persistent COVID-19-related chemical sensory dysfunction, a harmful sequela, needs to be treated physically and psychologically. For example, oral and intranasal corticosteroids, olfactory training, and oral vitamin–mineral supplementation are all feasible treatments. The olfactory scores indicate that the effect of rehabilitation combined with drugs may not be significantly different from that of single olfactory training therapy. However, there is an improvement in olfactory function after olfactory training.9 Therefore, olfactory training cannot be ignored as an easy-to-administer, low-cost treatment with negligible side effects.
In conclusion, one-third of the patients still have OD 6 months after the first COVID-19 infection. Even after recovery from COVID-19, persistent symptoms still deserve our attention. In the future, large-scale long-term follow-ups of patients with OD may provide continuous insights into the etiology and treatment of OD.
Funding
This study was supported by Hangzhou Science and Technology Bureau Fund (No. 20191203B96; No. 20191203B105); Youth Fund of Zhejiang Academy of Medical Sciences (No. 2019Y009); Medical and Technology Project of Zhejiang Province (No. 2021KY890); Clinical Research Fund of Zhejiang Medical Association (No. 2020ZYC-A13); Zhejiang Traditional Chinese Medicine Scientific Research Fund Project (No. 2022ZB280); Zhejiang Kangenbei Hospital Management Soft Science Research Project (No. 2022ZHA-KEB316). The funders have no role in the data collection, data analysis, preparation of manuscript and decision to submission.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgment
The work was supported by the Key medical disciplines of Hangzhou.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jinf.2023.01.041.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Deng Y.K., Shi K.T., Liu Z., Zeng M. Persistent olfactory dysfunction 2 years after onset of COVID-19. J Infect. 2023;86(2):154–225. doi: 10.1016/j.jinf.2022.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfadda A.A., Rafiullah M., Alkhowaiter M., Alotaibi N., Alzahrani M., Binkhamis K., et al. Clinical and biochemical characteristics of people experiencing post-coronavirus disease 2019-related symptoms: a prospective follow-up investigation. Front Med. 2022;9 doi: 10.3389/fmed.2022.1067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien J.R., Vaira L.A., Saussez S. Prevalence and 24-month recovery of olfactory dysfunction in COVID-19 patients: a multicentre prospective study. J. Intern Med. 2023;293(1):82–90. doi: 10.1111/joim.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaylacı A., Azak E., Önal A., Aktürk D.R., Karadenizli A. Effects of classical olfactory training in patients with COVID-19-related persistent loss of smell. Eur Arch Otorhinolaryngol. 2022:1–7. doi: 10.1007/s00405-022-07570-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballering A.V., van Zon S.K.R., Olde Hartman T.C., Rosmalen J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutani D.L., Ross A.G., Lehman A.Y., Shindler K.S. Resolution of COVID-19 induced anosmia following treatment with ST266. Otolaryngol Case Rep. 2022;25 doi: 10.1016/j.xocr.2022.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zazhytska M., Kodra A., Hoagland D.A., Frere J., Fullard J.F., Shayya H., et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185(6):1052–1064. doi: 10.1016/j.cell.2022.01.024. e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay J.B., Brann D.H., Abi Hachem R., Jang D.W., Oliva A.D., Ko T., et al. Persistent post-COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci Transl Med. 2022;14(676) doi: 10.1126/scitranslmed.add0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asvapoositkul V., Samuthpongtorn J., Aeumjaturapat S., Snidvongs K., Chusakul S., Seresirikachorn K., et al. Therapeutic options of post-COVID-19 related olfactory dysfunction: a systematic review and meta-analysis. Rhinology. 2023;61(1):2–11. doi: 10.4193/Rhin22.221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material