Abstract
Free tissue transfer has become the reconstructive modality of choice for replacing composite tissue defects. While the success rate in high-volume centers is reported to be greater than 95%, up to 10% of patients will require revision of their vascular anastomosis secondary to thrombosis or compromise to flow. In the intraoperative setting, immediate revision is successful in the majority of cases. Rarely, the flap cannot be revascularized and a secondary option must be used. In the perioperative setting revision is successful if the patient can be brought back to the operating room in a timely fashion. Revision rates up to 70% are reported. A small number of these patients may then suffer a second episode of compromise where revision is less successful at 30%. In these cases, consideration should be given to secondary reconstruction rather than attempting salvage. Finally, there are a small number of patients whose flaps will fail following discharge from the hospital. These patients can rarely be salvaged and secondary reconstructive options should be explored.
Keywords: free flap failure, salvage, free flap compromise
Free tissue transfer has become the reconstructive modality of choice for composite soft-tissue defects in the head and neck. The ability to replace composite tissue loss with similar tissue has allowed reconstructive surgeons to minimize postoperative morbidity and maximize the rehabilitative prospects for the patient. Soft-tissue replacement allows for three-dimensional reconstruction of the soft tissues of the oral cavity or pharynx with similar supple soft tissue. Bony reconstruction allows for cosmetic and functional rehabilitation with the potential for full oral dental rehabilitation. As free tissue transfer has become more integrated into the armamentarium of the reconstructive surgeon, its success rates have approached 95%. 1 Free tissue transfer success depends on successful revascularization of the transferred tissue. This involves an anastomosis between the flap vasculature and vessels in the head and neck. It is well recognized that there is a low incidence of vascular compromise in the immediate perioperative phase. 2 This usually consists of compromise of the arterial or venous system supplying the tissue that has been transferred. When this happens, it has potential for significant morbidity. Vascular compromise can occur at any time point in the perioperative period. It is most common in the first 72 hours. Immediate recognition with return to the operating room allows for successful revascularization in up to 60% to 70% of cases, with long-term flap survival rates of 95%. 2
This article will discuss the management of vascular compromise at the various stages in the perioperative period. Paradigms have been established that are individualistic among different institutions. No single paradigm has been adapted as a standard. The reader should utilize the system that works best in their institution and in their practice.
Compromised Flow in the Intraoperative Period
Successful revascularization following vascular anastomosis is recognized by an immediate color change to the skin paddle, bleeding from the muscle/skin edges or bone, and a palpable pulse in the artery. While under the microscope, pulsations of the artery and flow from the flap draining out of the veins can be visualized. All of these signs should be looked for immediately following revascularization. In our institution, a Cook-Swartz Doppler is placed on the artery just distal to the anastomosis to monitor for arterial flow. Signs of lack of arterial inflow will be the absence of a pulse in the artery, lack of a sound on the Cook-Swartz Doppler, the flap appearing pale, poor capillary refill, and lack of venous outflow. The adequacy of venous outflow is monitored by direct inspection of the veins as well as the flap. Inadequate venous outflow is demonstrated by more than expected venous oozing from the flap edges, the skin paddle of the flap demonstrating the blue tinge of venous congestion, engorgement of the vein proximal to the anastomosis, and a negative strip test.
During the perioperative phase, the incidence of vascular compromise or potential vascular issues has not been well documented in the literature. In the majority of cases, the issue is immediately addressed and flow is reestablished at the time of surgery. Thus, the prevalence or need to intervene is not well described. In our institution, up to 10% of flaps required some form of re-anastomosis of the vascular structures during the initial procedure. 2 3 In cases where vascular compromise is observed, the issue should be addressed immediately. If the microscope has been moved out of the operating field, it should be brought back in to closely examine the pedicle geometry and any extrinsic factors that may be interfering with the vascular structures. If there are no geometric or anatomic issues with the vascular anastomosis, clinical judgment is utilized to decide whether the anastomosis should be taken down and revised. It is our policy that if we are not 100% confident in the flow, then the anastomosis is revised immediately.
Inspection of the vascular anastomosis may reveal flow across the anastomosis but distal vasospasm. Initial measures to break this vasospasm involve mechanical stripping of the vessel. The artery is carefully dissected to separate it from the venous structures. Mechanically gripping it and then stripping it will oftentimes break the vascular spasm. Most surgeons utilize papaverine or lidocaine as a routine measure to prevent vasospasm. 4 5 We routinely use papaverine and inject it into the adventitia of the vascular pedicle. In recalcitrant cases, 4% lidocaine can be injected into the adventitia. These measures will almost certainly break the vasospasm and allow for flow to return to the flap. During a recent papaverine shortage, head and neck microvascular surgeons resorted to using a verapamil–nitroglycerin mixture to prevent and treat vasospasm. Historically, this had been utilized in peripheral limb and cardiac surgery. It was found to be very successful in relieving vasospasm. 4 5
The most common reason for arterial anastomosis revision is due to technical error resulting in a clot formation at the anastomosis site. Inspection under the microscope of the excised anastomotic site may reveal intimal damage that requires resection of the artery to normal intima. Occasionally, significant portions of the artery will need to be resected to find normal intima. Vein grafts may need to be considered. This is unusual. In regard to venous anastomosis, the adaptation of the venous coupling device has led to a common cause of venous obstruction from twisting of the pedicle with a kink at the anastomosis. It is most easily managed by excising the old anastomosis and revising it.
The utilization of postoperative anticoagulation, commonly heparin, in the setting of a revised vascular anastomosis is discussed but not well described in the literature. As mentioned in the beginning of the section, each surgeon has their own paradigm for management of intraoperative thrombosis. Because of the retrospective nature of reports, it is challenging to obtain a denominator or baseline for how often this problem occurs. It is also difficult to quantify how frequently one institutes antithrombotic therapy or the rationale for why one does not. The generalized consensus concerning the use of anticoagulation is dependent on whether the surgeon felt the arterial thrombosis was due to merely a technical issue versus an intrinsic vascular issue. Usually, if we are successful with our first re-anastomosis attempt, we do not use anticoagulation. When the artery has to be revised three or more times, our policy has been to heparinize the patient using a stroke protocol for 5 days.
Curry and coworkers looked at a series of 2,482 free flaps and evaluated outcomes in 6% of patients that had intraoperative revisions. The long-term failure rate was 19%. This was statistically worse than those that had no intra- or postoperative compromise. Furthermore, more than 20% of patients that underwent intraoperative revision eventually required a postoperative revision for vascular compromise. It would seem to indicate that intraoperative issues predict postoperative issues. 2 Our protocol for heparinization in these patients is to provide a bolus of 5,000 units upfront and then start a heparin drip to maintain the partial thromboplastin time between 50 and 80 seconds. This is maintained for 5 days, at which point an empiric decision is made to either anticoagulate the patient through oral medication for 3 months or discontinue the heparin and no further intervention is needed. The decision is based on how much the surgeon feels technical issues played in the vascular compromise .
Another area that is not well described is a paradigm for what to do with the flap that cannot be salvaged or transferred intraoperatively. Thomas et al looked at anterolateral thigh free flaps that could not be transferred successfully during the primary procedure. Due to vascular abnormalities and intraoperative technical issues, up to 2.5% of flaps required a second reconstructive option to be utilized. They described a paradigm for management of the anterolateral thigh flap that could not be successfully harvested and transferred. 6 In general, one should attempt to replace the flap with a similar composite flap at the same setting.
There exists a small number of flaps that require multiple attempts at revascularization. Anecdotally, many surgeons will reflect on the experience of not being able to revascularize a flap after many attempts. The decision regarding when to abandon the flap depends on surgical judgment. This issue was described and discussed at a panel of the American Head and Neck Society several years ago. Expert opinion of the microvascular reconstructive panelists, with more than an 8,000-flap experience, was that each surgeon should develop an individual paradigm for when one stops and accepts that the flap cannot be transferred successfully. The expert opinion on this panel varied between four and seven attempts at re-anastomosis before the flap was abandoned and a secondary reconstructive modality was undertaken.
Managing Episodes of Initial Flap Failure in the Postoperative Setting
The first episode of free flap failure is recognized through clinical examination of the flap. Venous congestion is evidenced through a dark purple hue to the flap skin, increased flap warmth and turgor, and drawing dark blood on pinprick, as seen in Fig. 1 . Arterial compromise clinically presents as a pale color to the flap, cool flap temperature, and lack of bleeding on pinprick. These clinical signs are evident during the end stages of flap failure. The beginning stages of flap compromise and decreased vascular inflow are often clinically confounding, clouding the algorithm for management. 6 The use of implantable arterial and venous Doppler is an adjunct to clinical flap monitoring and can alert surgeons of flap failure from intravascular thrombosis prior to clinical signs becoming evident. 3
Fig. 1.
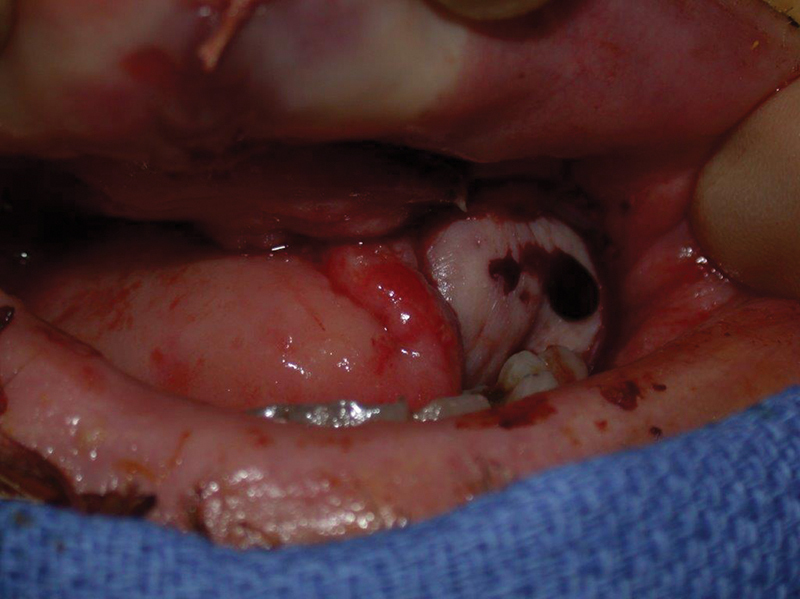
This flap demonstrates the classic signs of venous congestion. A dark blue tinge of the tissue with dark blood on skin prick.
When vascular compromise is recognized, a set window of time exists to resolve intravascular issues and salvage flap viability. 7 Considering that multiple resources need to be mobilized such as operating room personnel and technical perspective, it is important to establish a meaningful dialogue with the operating room front staff to facilitate return of these patients on an emergent/immediate basis. Ahmad et al established a 5-hour window where salvage rates began to deteriorate rapidly once that window was passed. 8
Upon return to the operating room, the initial procedural steps involve opening the incision overlying the flap pedicle and examining the pedicle for kinking, edema, and twisting. Technical issues with the geometry of the vascular pedicle may involve reinsetting or redraping of the vessels. The inset of the flap is examined to confirm that it is not overly tight, resulting in generalized venous congestion. If the pedicle is tunneled through soft tissue to reach vessels, there needs to be confirmation that the pedicle is not strangulated. There may be inadequate flow through the vascular anastomosis due to size mismatch or thrombus development. If these findings are present, they should be resolved, and then measures are instituted to ensure that something similar does not occur again ( Fig. 2 ).
Fig. 2.
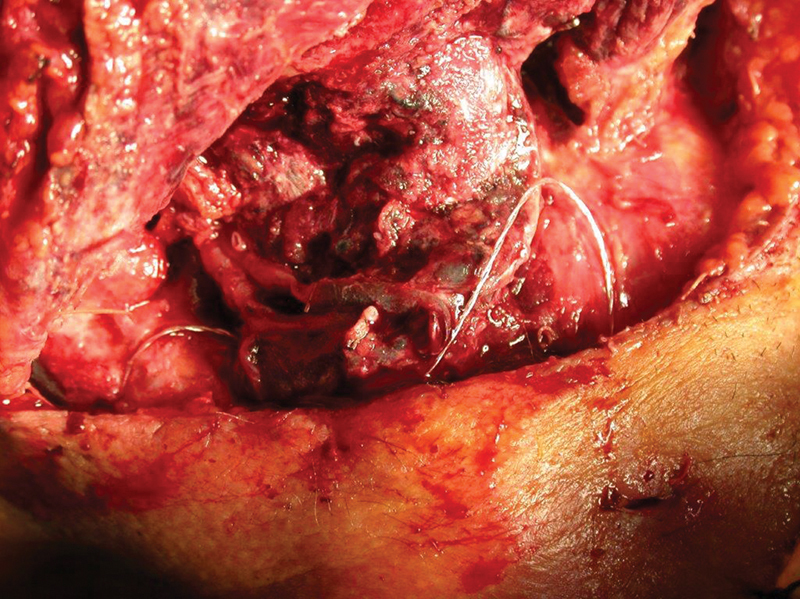
The venous pedicle exhibits no flow and a vascular clot.
When vessel geometry has contributed to kinking of the vascular pedicle, the pedicle needs to be reoriented to allow for better flow. Most often, thrombosis of the artery will have occurred ( Fig. 3 ). In this situation, the arterial anastomosis should be divided and examined further. Manual evacuation of the clot may be performed with microsurgery instruments. For distal or extensive clots, a Fogarty catheter may be passed, inflated, and withdrawn to remove the thrombus. The proximal and distal portions of the vessels may need to be trimmed until the intima is visualized to be normal and undamaged. Occasionally, vein grafting will be required as the pedicle can be significantly shortened in the process of obtaining good intima for re-anastomosis. In certain situations, the surgeon may not want to reuse the same recipient artery based on the previous thrombosis and appearance of the vessel after pedicle division. Other cervical arteries can be inspected to decide whether to reuse the same artery or utilize a different vessel. Factors that contribute to the decision-making process include artery size, geometry and vessel orientation, and whether the surgeon determines the artery is still viable after thrombus removal. Once the donor and recipient arteries have been cleaned, they are re-anastomosed. The flap pedicle is irrigated with a heparinized solution until there is adequate flow through the intact arterial system with the solution exiting the vein.
Fig. 3.
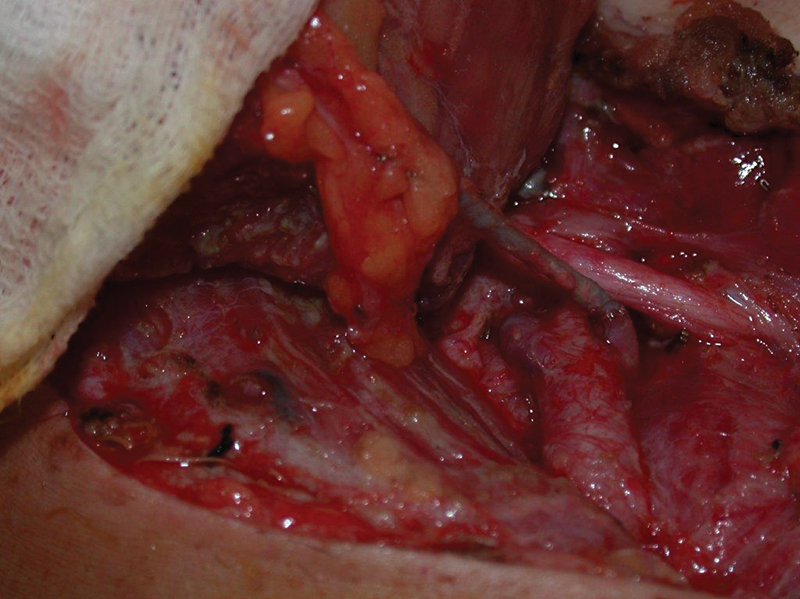
The artery demonstrates a clot at the anastomotic site that proceeds distally down the flap pedicle.
Occasionally, the entire venous pedicle will be clotted, and other measures must be utilized to remove and restore flow. The true incidence of this is relatively small and not well described. The distal vascular pedicle can be identified and divided to attempt irrigation for clot removal. When the arterial anastomosis is intact and there is adequate flow to the flap, a clot-dissolving agent can be injected into the artery to dissolve distal clot in the flap. Successful revascularization outcomes can be predicted when irrigation through a divided artery demonstrates good venous outflow or there is good venous outflow with an intact arterial anastomosis. When venous outflow is weak or not present, flap survival is poor and consideration of a secondary reconstructive method should be considered.
The value of intraoperative and postoperative heparin is not well defined in the surgical literature. There exists a heavy bias as to when patients are anticoagulated and thus no clear guidelines can be established. The individualized use of anticoagulation is surgeon and institution driven.
Management of Second Episodes of Flap Failure
A second episode of flap failure may occur in the postoperative period, with equivocal rates of arterial, venous, or combined intravascular thrombosis. Slijepcevic et al demonstrated a 30% salvage rate if a second episode of vascular compromise occurred and an isolated arterial thrombus was present. 1 However, venous congestion in the setting of a second episode of compromise portends a worse prognosis, and the risks and benefits of pursuing flap salvage must be considered on a case-by-case basis. A flap with a history of prior arterial or identifiable pedicle thrombus may have a better chance of survival following salvage attempts for second episodes of flap failure.
If the flap does not survive a second episode of failure with operative salvage attempt, there should be a discussion with the patient regarding the assortment of reconstructive options ranging from simple to complex to optimize rehabilitation of form and function of the defect. Healthy and younger patients may elect for a second free flap from a new donor site to pursue an optimal reconstruction method. Patients unable to undergo a second free flap may choose from smaller and less complex reconstructions like a skin graft versus a large locoregional flap reconstruction. The ultimate decision on reconstruction after free flap failure depends on the patient's functional and aesthetic goals as well as their overall disease prognosis. Patients who developed an unprovoked intravascular thrombus with no identifiable surgical error may benefit from hematologic work-up prior to pursuing another free flap.
Management of Third Episodes of Flap Failure
To date, there are no available data regarding the optimal practice management of the third episodes of flap compromise. During the third episode of free flap compromise, a thorough and complete assessment of all potential causes of flap failure will guide surgeons in choosing the best secondary reconstruction. Flaps with underlying anatomic issues including inadequate skin perforators, poor vascular outflow, or soft-tissue detachment from the vascular pedicle may have failed from technical errors. A new flap is likely to survive and provide a good reconstructive method in these cases. Free flap reconstruction may still be the optimal option for maximal aesthetic and functional outcomes. 9 Patients, however, may be unwilling or unable to undergo another free flap surgery, especially with unknown or likely poor outcomes following three revision attempts. Therefore, a detailed discussion with the patient regarding the potential success of a new free flap along with alternative reconstructive options is necessary.
Three episodes of free flap compromise may represent a chronic underlying issue that is unlikely to be resolved with further surgery. If the flap shows no signs of underlying anatomic issues, a work-up for underlying coagulopathies is needed. Patients with known coagulopathies pursuing free flap reconstruction are recommended to have adequate anticoagulation during and after flap reconstruction. 10 If possible, defect reconstruction with local or locoregional flaps is recommended for patients with known coagulopathies. Secondary reconstructions utilizing the reconstructive quiver include skin grafts, local advancement flaps, and locoregional rotational flaps to provide adequate functional rehabilitation of defects.
Management of Reconstruction Sites Following Flap Failure
The subsequent management of head and neck defects following the failure of a free flap reconstruction varies by individual patients along with the location and size of the surgical defect. For oral cavity defects without bony involvement, local tissue reconstruction may be optimal in a patient that is unwilling or unable to undergo a second free flap. Defects of the tongue may be reconstructed using a facial artery myomucosal flap, while retromolar trigone or buccal defects may be repaired with a palatal island flap or temporoparietal fascial flap. Patients with mandibulectomy defects and a failed fibula flap reconstruction may be candidates for harvest of the contralateral fibula, pending adequate lower extremity vasculature and stable underlying health. 10 Patients with poor health status or with inadequate lower extremity vasculature may be candidates for mandible reconstruction with hardware and a new soft-tissue free flap. Larger defects of the neck including total laryngectomy sites and large soft-tissue defects may necessitate coverage of the great vessels with a pectoralis major flap; however, this flap is associated with a deformity at the flap rotation site above the clavicle. Defects of the posterior neck and scalp may be repaired with a pedicled latissimus dorsi flap, which is tunneled anterior to the humeral joint. The pedicled latissimus dorsi flap can reach as far as the scalp vertex, but caution must be taken to avoid twisting or crushing the vascular pedicle that often contains small-caliber vessels.
Nonviable flaps in critical locations including the skull base, overlying cranial implants, or covering the great vessels of the neck must be removed as soon as possible, and the underlying tissues should be reconstructed with skin grafts, local flaps, or locoregional flaps. The ideal secondary reconstruction is dependent on the location and the goals of reconstruction. The anterior skull base houses critical neurovascular structures and there is a risk for cerebrospinal fluid leaks. Defects along the anterior skull base can be covered with skin grafts, pericranial flaps, and temporoparietal fascial flaps. Cranial vault defects reconstructed with titanium mesh, bone flaps, or alloplastic implants must be covered with adequate soft tissue to prevent implant or bone exposure resulting in infection of the underlying neural tissues. Large local scalp advancement flaps can cover exposed hardware, while the flap donor site is reconstructed with synthetic dermal matrix or a skin graft. When local scalp tissues do not provide adequate coverage, a pedicled latissimus dorsi flap with or without a skin paddle may be utilized to cover extensive scalp defects. Defects in the deep neck space with exposed great vessels are best reconstructed with pectoralis major flaps. The goal of reconstructing a critical location is for immediate tissue coverage to avoid infection or damage to crucial structures that may result in further morbidity. Local or locoregional flaps are second-tier reconstructive options that may be appropriate in a patient with a prior failed free flap.
Management of Flap That Fails Outside the Acute Perioperative Period
For the purposes of this discussion, delayed free flap failures occur after postoperative day (POD) 7. Following free tissue transfer, neovascularization of the tissues occurs and the flap becomes less reliant on the primary vascular pedicle for flap viability. 11 However, there is no clear consensus in the literature regarding the exact timing of when neovascularization is sufficient enough so the flap no longer relies on the vascular pedicle. Animal studies conducted by Oswald et al demonstrated that occlusion of the vascular pedicle in microvascular free flaps in the rat model as early as POD 5 resulted in survival of the flap. 12 Previously published work has demonstrated free flap survival in humans for head and neck reconstruction ranging from as early as POD 6 with a radial forearm free flap to POD 17 days for a jejunal flap. 13 14 The general consensus is that after a period of 7 to 14 days, angiogenesis has provided new channels of blood flow between the free flap and the surrounding recipient tissue. The length of dependence on the primary vascular pedicle will vary between individuals due to variations in prior radiation history, baseline nutritional status, comorbidities such as diabetes mellitus, hypertension, and peripheral vascular disease, and nicotine use. 15
Previously, delayed flap failure has been reported to be rare, only accounting for less than 1% of flap failures. 16 Interestingly, there has been a recent paradigm shift in the timing of free flap failure with more occurring in the delayed period compared with the acute postoperative period. A recent multi-institutional study evaluating complete free flap loss in 133 patients out of 2,916 flaps transferred delineated that 73% had failure between POD 0 and POD 7 and 27% had flap failure after POD 8. 17 The causes for free flap failure were attributed to vascular compromise with arterial insufficiency at around 6.7 days, infection at around 10 days, and wound breakdown at around 15.7 days. 17 A previous publication evaluating 1,530 flaps with a total of 13 delayed flap failures discovered a similar pattern regarding the timing. Free flap failures between POD 7 and POD 14 were due to pedicle compression or compromise, between POD 30 and POD 90 were due to infection, and greater than POD 90 were due to recurrent disease. 16
The vascular compromise resulting in delayed free flap failure is different than failure that occurs in the acute period. Traditionally, arterial free flap failures occurring within the first 24 hours of reconstruction were attributed to technique issues with the arterial anastomosis. 18 19 20 In contrast, failures occurring greater than 24 hours were commonly due to either external compression of the vascular pedicle or poor arterial quality with atherosclerotic plaque and calcification 21 ( Fig. 4 ). Delayed free flap failure due to arterial compromise may be able to be salvaged, but given the gradual and delayed presentation, there is a higher risk of flap failure despite intervention due to the possibility of irreversible tissue damage and free radical formation. When the patient presents with flap compromise after being discharged from hospital, it is almost impossible to salvage the flap. Anecdotally, surgeons have described a flap with acute color changes, presenting to the office or emergency room and urgently being taken back to the operating room for flap salvage. Logistically, this is often very difficult to coordinate and arrange.
Fig. 4.

This flap was viable when the patient was discharged. They recall sleeping on that side and waking up with the flap appearing like this. Clearly, it is not salvageable on postoperative day 21.
Delayed flap failure due to an infection results in purulent drainage collecting in the wound, likely leading to vascular compromise ( Fig. 5 ). It is more common in patients with a history of osteoradionecrosis or fistula formation. 16 Prompt identification of a deep neck space abscess with immediate drainage and treatment with intravenous antibiotics may result in salvage of the flap.
Fig. 5.

This patient developed a neck infection requiring washout and irrigation. The flap was nonviable and could not be salvaged on postoperative day 14.
Patients presenting with delayed flap failure months after the immediate postoperative period likely have recurrent disease growing into the flap. Free flap death may occur from tumor growth into the flap vasculature, resulting in ischemia and tissue necrosis. 16 In this situation, there is no option of salvaging the flap.
As microvascular techniques continue to be optimized, there may be more free flap failures occurring after the patient has been discharged from the hospital. Depending on the etiology, the ability to salvage a delayed flap failure may be low but close follow-up and awareness of this complication may result in salvaging reversible causes.
Conclusion
Free tissue transfer is a technically demanding procedure that provides the best rehabilitative potential and healing opportunities for patients who have undergone ablative procedures or require composite tissue reconstruction. There is a recognized intraoperative incidence of vascular compromise that can be repaired in the operating room using a variety of measures. It is well recognized that postoperative monitoring is required and prompt recognition and return to the operating room facilitates long-term survival. Paradigms for management should be individualized to match the surgeon and the institution. Success rates overall remain high.
Footnotes
Conflict of Interest None declared.
References
- 1.Slijepcevic A A, Young G, Shinn J. Success and outcomes following a second salvage attempt for free flap compromise in patients undergoing head and neck reconstruction. JAMA Otolaryngol Head Neck Surg. 2022;148(06):555–560. doi: 10.1001/jamaoto.2022.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart M, Swendseid B, Hammond P. Anastomotic revision in head and neck free flaps. Laryngoscope. 2021;131(05):1035–1041. doi: 10.1002/lary.29094. [DOI] [PubMed] [Google Scholar]
- 3.Wax M K. The role of the implantable Doppler probe in free flap surgery. Laryngoscope. 2014;124 01:S1–S12. doi: 10.1002/lary.24569. [DOI] [PubMed] [Google Scholar]
- 4.Seth R, Badran K W, Cedars E. Vasodilation by verapamil-nitroglycerin solution in microvascular surgery. Otolaryngol Head Neck Surg. 2021;164(01):104–109. doi: 10.1177/0194599820937991. [DOI] [PubMed] [Google Scholar]
- 5.Vargas C R, Iorio M L, Lee B T. A systematic review of topical vasodilators for the treatment of intraoperative vasospasm in reconstructive microsurgery. Plast Reconstr Surg. 2015;136(02):411–422. doi: 10.1097/PRS.0000000000001431. [DOI] [PubMed] [Google Scholar]
- 6.Thomas W W, Calcagno H E, Azzi J. Incidence of inadequate perforators and salvage options for the anterior lateral thigh free flap. Laryngoscope. 2020;130(02):343–346. doi: 10.1002/lary.28176. [DOI] [PubMed] [Google Scholar]
- 7.Chen K T, Mardini S, Chuang D C. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg. 2007;120(01):187–195. doi: 10.1097/01.prs.0000264077.07779.50. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad F I, Gerecci D, Gonzalez J D, Peck J J, Wax M K. The role of postoperative hematoma on free flap compromise. Laryngoscope. 2015;125(08):1811–1815. doi: 10.1002/lary.25285. [DOI] [PubMed] [Google Scholar]
- 9.Bender-Heine A, Sweeny L, Curry J M. Management of the acute loss of a free flap to the head and neck—a multi‐institutional review. Laryngoscope. 2021;131(03):518–524. doi: 10.1002/lary.28886. [DOI] [PubMed] [Google Scholar]
- 10.Ghaheri B A, Kim J H, Wax M K. Second osteocutaneous fibular free flaps for head and neck defects. Laryngoscope. 2005;115(06):983–986. doi: 10.1097/01.MLG.0000163106.14939.DC. [DOI] [PubMed] [Google Scholar]
- 11.Khoo C T, Bailey B N. The behaviour o free muscle and musculocutaneous flaps after early loss of axial blood supply. Br J Plast Surg. 1982;35(01):43–46. doi: 10.1016/0007-1226(82)90082-0. [DOI] [PubMed] [Google Scholar]
- 12.Oswald P, Tilgner A, Schumann D. The influence of postoperative vessel occlusion on the viability of free microvascular skin-fat flaps and island flaps in rats. J Reconstr Microsurg. 1988;4(05):403–407. doi: 10.1055/s-2007-1006951. [DOI] [PubMed] [Google Scholar]
- 13.Burns A, Avery B S, Edge C J. Survival of microvascular free flaps in head and neck surgery after early interruption of the vascular pedicle. Br J Oral Maxillofac Surg. 2005;43(05):426–427. doi: 10.1016/j.bjoms.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Chen H C, Tan B K, Cheng M H, Chang C H, Tang Y B. Behavior of free jejunal flaps after early disruption of blood supply. Ann Thorac Surg. 2002;73(03):987–989. doi: 10.1016/s0003-4975(01)03015-6. [DOI] [PubMed] [Google Scholar]
- 15.Salgado C J, Smith A, Kim S. Effects of late loss of arterial inflow on free flap survival. J Reconstr Microsurg. 2002;18(07):579–584. doi: 10.1055/s-2002-35095. [DOI] [PubMed] [Google Scholar]
- 16.Wax M K, Rosenthal E. Etiology of late free flap failures occurring after hospital discharge. Laryngoscope. 2007;117(11):1961–1963. doi: 10.1097/MLG.0b013e31812e017a. [DOI] [PubMed] [Google Scholar]
- 17.Sweeny L, Topf M, Wax M K. Shift in the timing of microvascular free tissue transfer failures in head and neck reconstruction. Laryngoscope. 2020;130(02):347–353. doi: 10.1002/lary.28177. [DOI] [PubMed] [Google Scholar]
- 18.Nakatsuka T, Harii K, Asato H.Analytic review of 2372 free flap transfers for head and neck reconstruction following cancer resection J Reconstr Microsurg 20031906363–368., discussion 369 [DOI] [PubMed] [Google Scholar]
- 19.Suh J D, Sercarz J A, Abemayor E. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 2004;130(08):962–966. doi: 10.1001/archotol.130.8.962. [DOI] [PubMed] [Google Scholar]
- 20.Corbitt C, Skoracki R J, Yu P, Hanasono M M. Free flap failure in head and neck reconstruction. Head Neck. 2014;36(10):1440–1445. doi: 10.1002/hed.23471. [DOI] [PubMed] [Google Scholar]
- 21.Yu P, Chang D W, Miller M J, Reece G, Robb G L. Analysis of 49 cases of flap compromise in 1310 free flaps for head and neck reconstruction. Head Neck. 2009;31(01):45–51. doi: 10.1002/hed.20927. [DOI] [PubMed] [Google Scholar]


