Abstract
This review aims to highlight the common pharmacological and nonpharmacological interventions utilized for thromboprophylaxis as well as flap salvage in microsurgery. A literature review was conducted in PubMed/National Center for Biotechnology Information, Scopus, Web of Science, and MEDLINE databases. Articles with a focus on thromboprophylaxis in microsurgical procedures spanning head and neck surgery, breast and extremity microvascular reconstruction, deep venous thrombosis/pulmonary embolus in microvascular surgery, and flap thrombosis and salvage were included in this review. The majority of available evidence supports mechanical venous thromboembolism (VTE) prophylaxis in all patients undergoing microsurgery given the presence of multiple risk factors for VTE within this particular patient population. Based on the literature review, addition of VTE chemoprophylactic agents is beneficial and an algorithmic approach to thromboprophylaxis in microsurgery patients and management of patients with thrombosis based on literature review and senior authors' experience is recommended and outlined.
Keywords: microsurgery, flap, thromboembolism, DVT, salvage, prophylaxis
Venous thromboembolism (VTE) represents a spectrum of pathologic manifestations including asymptomatic or symptomatic acute deep venous thrombosis (DVT), postthrombotic syndrome characterized by valvular incompetence, venous insufficiency and hypertension of the extremity, and pulmonary embolus (PE) with sequelae including chronic pulmonary hypertension, heart failure, and even death. 1 VTE events are common, costly complications of most surgical interventions, yet such events are potentially preventable. The American College of Chest Physicians (CHEST) recently released updated guidelines for VTE management that also includes recommendations for VTE prevention in surgical patients. 2
Different risk assessment models (RAMs) have been introduced to individualize prediction of VTE risk based on presence of various patient-specific factors. The Caprini risk scoring model is a widely applied model for both medical and surgical patients, and this model has been retrospectively validated in multiple studies for surgical patients. More recently, the Caprini-Davison RAM was modified to be more relevant to plastic and reconstructive surgery. This refined model included additional risk factors specific to plastic surgery (e.g., free flap and microsurgical procedures) in addition to other major surgery procedures, while removing nonplastic surgeries and their associated risks (e.g., arthroscopic or laparoscopic surgery procedures). 3 Using this more refined and pertinent model, 90% of VTE events were successfully predicted in a retrospective study on patients who had body contouring procedures. 4 Pannucci et al validated Caprini RAM 2005 version for accurate prediction of symptomatic VTE in plastic and reconstructive surgery patients. In this group's review of the Venous Thromboembolism Prevention Study (VTEPS) database, all categories of plastic and reconstructive surgeries requiring general anesthesia coupled with an overnight stay or longer were included within this study. Those patients having calculated Caprini scores greater than 8 experienced a 20 times higher risk of VTE as compared to patients with calculated Caprini scores of 3 to 4 (11.3%). 5 In patients having Caprini scores greater than 6, VTE risk was not limited to the immediate postoperative course and was evident through extension of up to 60 days after their index surgery. 5 The overall VTE incidence in this review was reported as being 1.7%, with the highest incidence among patients undergoing trunk reconstruction (7.3%), followed by burn reconstruction (6.3%), pressure ulcer surgery (3.1%), head and neck reconstruction (2%), and breast reconstruction (1.3%), respectively. 5 The authors, however, excluded lower extremity trauma free flap reconstruction within their study mainly due to the preoperative administration of anticoagulants in the vast majority of the patients who underwent microvascular reconstructions. Statistical analysis of this patient population demonstrated family history of VTE and central venous access as significant independent risk factors for VTE.
In 2008, the Plastic Surgery Foundation founded the VTEPS study to acknowledge VTE as a top patient safety priority to focus. 6 A total of 3,681 adult patients (2,114 controls and 1,567 patients in the enoxaparin study group) who underwent plastic and reconstructive surgeries across four different tertiary care centers were retrospectively reviewed. These data revealed that postoperative inpatient enoxaparin significantly reduced the rate of symptomatic VTE for up to 60 days in postoperative follow-up, and this reduced rate was especially relevant in those patients having a calculated Caprini score higher than 7 (approximately 50% risk reduction). 6 Increased bleeding risk was not appreciated within this study. 7 8
DVT events may not initially be clinically apparent, and even those DVT events which do not progress to become PEs can still lead to development of venous insufficiency (a particular concern for those patients experiencing injuries to the lower extremity[-ies]). 9 In a retrospective review of 170 patients who underwent free flap reconstruction of the lower extremities, 14% of these patients experienced venous insufficiency and 7% a DVT, respectively. An important finding in this study illustrated that the Caprini score was a valid predictor of venous insufficiency following lower extremity microvascular reconstruction. Furthermore, patients with Caprini scores greater than 7 experienced a three times higher risk of venous insufficiency. 9
In this article, the authors will highlight the common antiplatelet and anticoagulation therapies and nonpharmacological interventions utilized for thromboprophylaxis as well as flap salvage within microsurgery. We will also highlight the basis for and our group's algorithm for thromboprophylaxis as well as the management of microsurgery patients who experience thrombosis events.
Methods
Those full-text, peer-reviewed publications discussing DVT/PE/VTE prophylaxis in microsurgery or free flap-based reconstruction as well as flap thrombosis and salvage were included in our review. PubMed/National Center for Biotechnology Information, Scopus, Web of Science, and MEDLINE database and Google Scholar search engine were used to identify pertinent publications using a combination of keywords including: DVT, thromboprophylaxis, venous thrombosis, pulmonary embolism, microsurgery, free flap, mechanical, pneumatic compression, dangling protocol, anticoagulation, aspirin, heparin, low molecular weight heparin, LMWH, dextran, warfarin, Toradol, ketorolac, oral anticoagulants, fondaparinux, thrombin inhibitors, flap salvage, tPA, plasminogen activator, urokinase, and streptokinase. Approximately 120 articles were selected for review. Each paper is reviewed by two junior and at least one senior author.
Common Pharmacological Agents Utilized for VTE Prophylaxis in Microsurgery Patients
Antiplatelets
Aspirin
Aspirin (ASA) plays an effective role in reducing the rate of arterial thrombosis via the nonselective irreversible inhibition of cyclooxygenase-1 that prevents the production of thromboxane-A2, which ultimately inhibits platelet activation and aggregation. At higher doses, ASA inhibits cyclooxegenase-2 and, subsequently prostacyclin and the inflammatory response. 10 A landmark study in 2012 solidified the role of ASA in VTE prophylaxis. 11 This seminal study noted a significant reduction of recurrent unprovoked VTEs in the cohort of patients who took ASA for 2 years after their long-term anticoagulation regimen (6–18 months) when compared to a cohort of patients who took a placebo after completion of their long-term anticoagulation regimen, without an increased risk of bleeding in the ASA study group. Twenty-eight out of 205 patients (6.6%) in the ASA cohort were found to have a VTE compared to 43/197 (11.2%) in the placebo cohort; hazard ratio, 0.58; 95% confidence interval (CI): 0.36 to 0.93. 11 Within the realm of head and neck microsurgery, the role of ASA in thromboprophylaxis was assessed in a retrospective case series of 390 consecutive free flaps. 12 In this study, 184 patients did not receive anticoagulation, 142 patients received ASA alone, 25 patients received subcutaneous heparin (SQH) or enoxaparin, 23 patients received ASA with prophylactic SQH or low molecular weight heparin (LMWH), and 16 patients received therapeutic heparin drip with a goal partial thromboplastin time (PTT) in the low therapeutic range (i.e., 60–80 seconds). Of note, there were significantly more complications in the group that received ASA compared to the cohort that did not receive thromboprophylaxis without an appreciable difference in bleeding complications or flap failure, and the ASA-treated groups underwent more revision surgeries. 12 Given these mixed reports, more prospective and randomized control trials are needed to determine the risk/benefit profile of ASA in thromboprophylaxis in microsurgery.
Dextran
Intravenous dextran disrupts von Willebrand factor, factor VII, and fibrin networks and increases intravascular volume. However, compared to ASA, a study found that dextran is correlated with higher rates of complications such as anaphylaxis, congestive heart failure, myocardial infarction, pneumonia, pulmonary edema, pleural effusion, and nephrotoxicity, without any added benefit of reducing free flap complications. 13 Specifically, when compared to groups that underwent head and neck reconstruction that were administered ASA (325 mg) for 120 hours postoperatively, the groups that received low-molecular-weight dextran postoperatively for 48 and 128 hours had a 3.9 and 7.2 time greater risk of the aforementioned complications, respectively. 13 Experts in the field have argued that the increased complication rate is not directly linked with dextran, but more associated with perioperative factors such as intraoperative time, presence of kidney disease, amount of volume resuscitation, and urine output, and have reported successful outcomes when using dextran in conjunction with ASA. 14
Ketorolac
Ketorolac (Toradol)—an intravenous drug, which is Food and Drug Administration (FDA)-approved for pain control in perioperative setting—nonselectively inhibits cyclooxygenase-1 and 2, which prevents the conversion of arachidonic acid to prostaglandins (anti-inflammatory and analgesia) and subsequently inhibits platelet activation and aggregation. In addition to its use as an analgesic, microsurgeons have utilized ketorolac as an antiplatelet agent in patients with platelet counts higher than 300,000/µL and who may have a contraindication for ASA or have not received ASA prophylaxis. Ketorolac can increase susceptibility to gastrointestinal tract damage and compromise renal blood perfusion. A comparative study looked at patients who underwent free tissue transfer for lower extremity reconstruction that received ketorolac ( n = 80) and compared them to those who did not ( n = 48). 15 The cohort that did not receive ketorolac had significantly higher rates of vascular-related complications after confounders were adjusted. This study also found that longer periods of ketorolac use led to lower complication rates. 15 A retrospective cohort study looked at the effectiveness of ketorolac for analgesia and the rate of bleeding complications in 138 patients who underwent head and neck free flaps. 16 This study demonstrated that ketorolac does not increase the risk of bleeding or seroma in head and neck free flap patients, but authors also noted that administration of ketorolac was not associated with a reduction in narcotic use during the postoperative period. 16 The role of ketorolac in microsurgery remains to be fully elucidated but it may be an acceptable alternative to ASA in acute settings or intraoperatively to reduce platelet function when desired.
Clotting Cascade Anticoagulants Applicable in Microsurgery
Heparin
Given its rapid onset of action and short half-life, subcutaneous or intravenous heparin has been utilized commonly within the perioperative setting. Heparin potentiates the activity of antithrombin III, while subsequently leading to the inactivation of thrombin and factor Xa. 17 Typically, a 5,000 U subcutaneous bolus is utilized intraoperatively, followed by the continued use of SQH every 8 hours until discharge for low-risk patients (Caprini < 8). 18 Patients with a high-risk profile for thromboembolism (Caprini > 8) may benefit from the initiation of subtherapeutic intravenous heparin at 500 to 1,000 U/h and the subsequent transition to a therapeutic heparin drip prescribed according to weight-based dosing. 18 For patients with higher susceptibilities to thromboembolism or having certain hypercoagulable conditions, close collaboration with a hematologist is recommended to develop the proper perioperative or therapeutic regimen. 19
The development of an evidenced-based strategy for implementing a protocol that reduces the rate of free flap compromise and bleeding complications remains elusive. A systematic review compared heparin thromboprophylaxis regimens to ASA and found no statistically significant difference in the rate of flap loss between the two groups. High doses of heparin or LMWH led to increased rates of flap loss compared to cohorts that utilized low-dose heparin or LMWH for thromboprophylaxis. 20 Despite these studies and findings, heparin remains the most common anticoagulant in many microsurgical centers. Heparin also carries the risk of heparin-induced thrombocytopenia (HIT), for which clinicians must remain vigilant.
Low Molecular Weight Heparin
LMWH has a similar mechanism of action to heparin but with less effect on thrombin and a longer half-life. 21 LMWH is typically the first-line therapy used for long-term anticoagulation in high-risk patients and patients without impaired kidney function.
In a systematic review of risks and benefits of different pharmacological options for VTE prophylaxis in head and neck microsurgery, LMWH is recommended as the first-line option compared to unfractionated heparin due to better risk profile and potential of decreased bleeding complications in this patient population. 22
Fondaparinux
Fondaparinux is a synthetic factor Xa inhibitor with a similar mechanism of action to heparin and LMWH. 23 It has a longer half-life (17–21 hours) than either heparin or LMWH and is given once daily. 24 There exist reports in the literature of fondaparinux being utilized in microvascular surgery as a second-line choice for thromboprophylaxis for patients with HIT. 25 However, it is not FDA-approved to be utilized in this context, and direct thrombin inhibitors are preferred in the acute setting of HIT. 26 27
Warfarin
Warfarin is an oral anticoagulant that inhibits vitamin K epoxidase in the liver, subsequently reducing the generation of vitamin K-dependent clotting factors (II, V, VII, and X) and anticoagulants (proteins C and S). 28 Warfarin is typically held in the perioperative setting and reinitiated later in patients that are clinically required to be anticoagulated with warfarin. It is typically not utilized in the armamentarium for acute perioperative phase thromboprophylaxis in microvascular surgery.
Direct Oral Anticoagulants
The direct oral anticoagulants (DOACs) currently available in the United States include: apixaban, dabigatran, rivaroxaban, betrixaban, and edoxaban. These oral anticoagulation agents are direct and reversible inhibitors of factor Xa and prothrombinase. 29 Andexanet alfa is an FDA-approved antidote for the reversal of factor Xa inhibitors. Andexanet is a modified recombinant derivative of factor Xa that acts as a decoy receptor with high affinity for factor Xa inhibitors. 30 Prothrombin complex concentrates (PCCs) are other options to reverse laboratory measures and bleeding from factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban. PCCs are a mixture of factors II, IX, and X. Some versions also include factor VII. 30 Dabigatran is a direct and reversible inhibitor of thrombin. 31 Idarucizumab is used as an antidote for dabigatran toxicity. Idarucizumab is a specific monoclonal antibody fragment that binds dabigatran. The usual dose is 5 g for life-threatening bleeding or need for emergency surgery while taking dabigatran. 30
These agents are not typical first- or second-line agents in the anticoagulation treatment for the common microsurgical patient, but they may be more applicable in certain hypercoagulable states or in vascular and cardiac patients who require microsurgical procedures. Patients previously on DOACs typically stop and resume these agents as outlined by their care team. Consulting with the hematology team is indicated when treating patients on these agents especially when patients have a known hypercoagulable state.
Direct Thrombin Inhibitors
Direct thrombin inhibitors (DTIs) act by directly binding to thrombin, reducing its activity. The FDA has approved the following intravenous DTIs, which are primarily used in the management of HIT: argatroban, lepirudin, bivalirudin, and desirudin. Dabigatran is an oral DTI utilized in the outpatient treatment of VTE. A case report described a successful head and neck microvascular reconstruction with the utilization of argatroban as an intraluminal irrigation solution. 32 More studies are needed to elucidate these agents' definite role in microsurgical thromboprophylaxis. Close collaboration with hematology is recommended if the use of DTIs is required in the perioperative period.
Table 1 summarizes the most common antiplatelet and anticoagulant agents applicable in microsurgery.
Table 1. Common pharmacological agents utilized for VTE prophylaxis in microsurgery patients.
| Drug | Category | Mechanism | Route | Dose | Microsurgical uses |
|---|---|---|---|---|---|
| Aspirin (ASA) | Antiplatelet | The nonselective irreversible inhibition of COX-1 and 2 to reduce the pain, inflammatory response, and platelet aggregation | PO, PR | 81 to 325 mg | Perioperative thromboprophylaxis |
| Dextran | Antiplatelet | Disrupts von Willebrand factor, factor VII, and fibrin networks and increases intravascular volume | IV | 20 mL/h for up to 120 hours | Usage has declined in recent years due to high-risk side effect profile. Used in conjunction with ASA in patients with limited comorbidities |
| Ketorolac | Antiplatelet (off-label) | The nonselective inhibition of COX-1 and 2 reduces pain, inflammatory response, and platelet aggregation | IV | 15 mg Q6H for up to 5 days. Dosage should not exceed 60 mg/day | Decreases opioid burden and thromboprophylaxis in patients with platelet > 300, 000/µL |
| Heparin | Anticoagulant | Potentiates the activity of antithrombin III subsequently leading to the inactivation of thrombin and factor Xa | SQ, IV | Various depending on indication | Thromboprophylaxis |
| Low-molecular-weight heparin | Anticoagulant | Similar mechanism of action to heparin (above) but with less effect on thrombin and a longer half-life | SQ | 0.5 mg/kg based on TBW once or twice daily | First-line therapy used for long-term anticoagulation in high-risk patients and patients without impaired kidney function |
| Fondaparinux | Anticoagulant | Synthetic factor Xa inhibitor with a similar mechanism of action to heparin and low molecular weight heparin | SQ | 7.5 mg (patients with body weight ≥ 50, ≤ 100 kg) once daily | Second-line choice for thromboprophylaxis in patients with heparin-induced thrombocytopenia |
| Warfarin | Anticoagulant | Inhibits vitamin K epoxidase in the liver, subsequently reducing the generation of vitamin K-dependent clotting factors (II, V, VII, and X) and anticoagulants (proteins C and S) | PO | Various–dependent on INR and indication | Not typically used in the perioperative setting but restarted in patients who used prior to procedure; not used in armamentarium for thromboprophylaxis |
| DOAC (Apixaban, rivaroxaban, betrixaban, and edoxaban) | Anticoagulant | Direct and reversible inhibitor of factor Xa and prothrombinase | PO | Various–depending on specific DOAC used | These agents are not typical first- or second-line agents in the anticoagulation treatment for the common microsurgical patient, but they may be more applicable in certain hypercoagulable states or seen in vascular and cardiac patients may require microsurgical procedures |
| DTI (Argatroban) | Anticoagulant | Direct inhibition of thrombin | IV | Various depending on indication | Intraoperative and postoperative thromboprophylaxis in patients with HIT |
Abbreviations: ASA, acetylsalicylic acid; DOAC, direct oral anticoagulant; DTI, direct thrombin inhibitor; HIT, heparin-induced thrombocytopenia; INR, international normalized ratio; IV, intravenous; PO, per ostium; PR, per rectum; SQ, subcutaneous; TBW, total body weight.
Mechanical VTE Prophylaxis Used in Microsurgery
Mechanical measures for VTE prophylaxis include graduated compression stockings, intermittent pneumatic compression (IPC) devices, foot pumps, and early monitored ambulation.
In a comprehensive review of all trials including either pharmacological or mechanical intervention for thromboprophylaxis and VTE outcome evaluation, Roderick et al in a meta-analysis reported that mechanical compression methods reduced the risk of DVT by approximately 67% when used as monotherapy and 53% when added as an adjunctive therapy to a pharmacological agent. 33
The American College of Chest Physicians Evidence-Based Clinical Practice guidelines recommended the following: (1) early ambulation in very low-risk patient (< 0.5%) after general or abdominal-pelvic surgeries, (2) mechanical prophylaxis, preferably IPC for patients at low risk for VTE (∼1.5%), (3) either mechanical or pharmacological VTE prophylaxis in patients at moderate risk for VTE (∼3%), and (4) mechanical as well as pharmacological prophylaxis in patients with higher risk for VTE. 34 For patients with cancer or any additional risk for VTE, the standard approach is chemoprophylaxis in conjunction with mechanical VTE prophylaxis measures. In the absence of a clear contraindication (such as severe peripheral arterial disease), patients undergoing a surgical procedure would be expected to derive a net benefit from a mechanical method of VTE prophylaxis, irrespective of their absolute risk of VTE.
Data on postoperative mobilization in patients undergoing microvascular reconstruction is limited; however, the expert consensus is that early monitored mobilization should be a key component of thromboprophylactic protocol. In patients undergoing lower extremity procedures that limit their ambulation, a lower extremity progressive dangling protocol is recommended. Dangling protocols vary by surgeons and institutions. A systematic review of different protocols conducted by Lee et al in 2021 showed considerable variation in dangling protocol initiation, time, and frequency in lower extremity flap patients. Patient's comorbidities and characteristics of different types of flaps are factors that influence protocol modification. 35 In our institution the progressive dangling protocol starts at an average of postoperative day 7, with dangling the lower extremity for 5 minutes three times a day. Dangles advance 5 minutes each day until postoperative day 14 after which the duration of dangle time doubles each week thereafter until 6 weeks when there is no limitation to dangle time or duration. If at any time the lower extremity becomes swollen or discolored, it should be immediately elevated, and retrogress a day in the protocol. In patients with a history of a takeback for VTE or thrombosis event, our group typically delays the dangling protocol initiation for an additional 24 to 48 hours.
VTE Prevention in Microsurgery Patients
Despite benefits demonstrated in multiple studies, chemoprophylaxis is sometimes deferred, particularly in the microsurgical setting, due to misconception about increased risk of bleeding. Microsurgery procedures inherently consist of components that increase patients' perioperative morbidity. Prolonged operative time, multiple comorbidities including cancer, severe trauma, prolonged immobility, chemotherapy, or radiation, and advanced age, all may contribute to the additive risks of perioperative VTE.
Current data about VTE risk and prevention strategies are varied based on different microsurgical procedures. Here, we review some of the most common reconstructive microsurgical procedures and provide recommendations based on the available evidence.
Breast Microvascular Reconstruction
Patients who undergo free flap reconstruction of breast cancer usually present with several risk factors for VTE. Prolonged general anesthesia time, especially pertinent in immediate reconstruction after mastectomy, a history of cancer, an advanced age, a higher body mass index (BMI) as a prerequisite for autologous reconstruction, central indwelling port, and cancer-related therapies (including chemotherapy or radiotherapy) all constitute important additional risk factors for VTE within the breast microsurgical patient population. The presence of multiple risk factors in such patients necessitates individualized risk assessment for VTE. Models such as Caprini are validated for risk stratification in these patients and endorsed by the major surgical societies.
In a large review of 36,000 patients who underwent autologous breast reconstruction, the overall rate of VTE was reported as 0.13%. 31 This study included pedicle and free flap reconstructions, with the highest rate of VTE (0.26%) being observed within the pedicle transverse rectus abdominis myocutaneous (TRAM) flap cases. Twenty-three percent of the patients in this study underwent free deep inferior epigastric artery perforator (DIEP) flap reconstruction with a 0.19% VTE incidence, while 18% of patients had a free TRAM flap reconstruction with an incidence of VTE of 0.08%. The authors reported advanced age (> 65), obesity, chronic lung disease, history of chemotherapy, and immediate versus delayed reconstruction as independent predictors of in-hospital VTE within this study. 36
Multiple studies reported higher incidence of VTE in free flap-based breast reconstruction. A retrospective study on 354 consecutive free flap cases for breast reconstruction that was performed in a single institution reported VTE events in 1.2%. 37 Another recent study reported VTE incidence after abdominally based microsurgical breast reconstruction in 701 patients to be 2.1% (0.57% DVT, and 1.6% PE). 38 In this review all patients received mechanical and chemical VTE prophylaxis (unfractionated heparin or LMWH) during their hospital stay and discharged on ASA 325 mg daily for 30 days. All diagnosed cases of VTE were in the first 30 days after surgery. In another study on more than 400 DIEP flaps for breast reconstruction, symptomatic PE incidence was reported in 4% of patients, all of whom received LMWH in addition to mechanical prophylaxis as part of VTE prevention protocol. High BMI, operation duration, and BRCA mutation were identified as predictors of PE occurrence in these patients. 39
It is estimated that approximately 50% of VTE events remain undiagnosed. In a prospective cohort study on 118 women who underwent free abdominally based breast reconstruction bilateral lower extremity duplex ultrasound screening was performed before hospital discharge for objective evaluation of asymptomatic DVT. Interestingly, 3.4% of these patients showed evidence of distal lower extremity DVT in 5 days postop period compared to a control group of similar cohort with no symptomatic VTE event and no screening. 40 Patients in both groups received dalteparin (a LMWH) as part of the standard VTE prophylaxis protocol. Patients with asymptomatic DVT who identified in this study received anticoagulation therapy for 6 months if primary cancer was cleared or indefinitely while being on cancer chemoradiation treatment.
Studies suggest that a minority of breast reconstructive surgeons adhere to guidelines established by American College of Chest Physicians for VTE prevention in surgical patients given the established high risk of hematoma in breast surgery. Liao et al in a retrospective cohort study of 679 consecutive pedicled and free TRAM cases found lower rate of clinically detected thromboembolic events (0.8 vs. 1.4 overall and 1.6% in free TRAM) in patients who received chemoprophylaxis with heparin with no significant increase in hematoma rate. 41
In review of literature no consensus for the type of chemoprophylactic regimen in breast microsurgery reconstruction patients has been established. Pannucci et al compared 40 mg enoxaparin (Lovenox) daily with twice a day starting 6 to 8 hours after surgery and showed zero incidence of VTE in the twice daily group versus 5.3% in the daily group. However, the patients who received twice daily enoxaparin experienced a higher rate of clinically significant hematoma (6.8% vs. 3.2% in once daily group). 42 The authors measured anti-factor Xa level to quantify enoxaparin anticoagulative efficacy. With twice daily regimen at least 90% of patients received adequate anticoagulation, however, 27% were overtreated. The authors then performed a pharmacokinetics study and recommended weight-based administration of enoxaparin to avoid inadequate or excessive anticoagulation. 43 An ongoing double-blind randomized control trial designed to compare 40 mg twice daily dose with weight-based 0.5 mg/kg twice daily in the plastic surgery patient population to answer questions about optimal chemoprophylaxis dose is underway. 44 Others described regimens for chemoprophylaxis in breast reconstruction patients including SQH 5,000 units every 12 or 8 hours, or LMWH treatments, most commonly enoxaparin 40 mg daily or twice a day. 41 42 Lemaine et al used dalteparin (a LMWH) as part of triple thromboprophylaxis regimen with sequential compression devices before the induction of anesthesia and early ambulation in 225 abdominally based free flap breast reconstruction patients. 40 None of their patients had symptomatic VTE events, however, silent DVT was identified in 3.4% and total bleeding risk was 5.3%. Antiplatelet agents have not been widely used in free flap-based breast reconstruction patients. Enajat et al evaluated 592 autologous breast reconstructions who received nadroparin, a LMWH, 0.6 mL daily with or without 40 mg daily acetylsalicylic acid and showed that addition of ASA did not improve microvascular thrombosis; however, hematoma occurred more often in the ASA group (9.2% vs. 4.7%). 45
In review of literature about VTE prophylaxis in breast microsurgical reconstruction, most studies suggest some type of mechanical VTE prophylaxis using sequential compression device before anesthesia induction and early ambulation postoperatively; however, no consensus for the type of chemoprophylactic regimen has been established. Most commonly described regimen includes SQH 5,000 units every 8 hours, or LMWH, most commonly enoxaparin 40 mg daily or 30 mg twice a day.
Upper and Lower Extremity Microvascular Reconstruction
Incidence of flap failure is reported to be significantly higher in lower extremity reconstruction when compared to free flap in other anatomic areas such as breast or head and neck. A critical factor for increased free flap failure in the lower extremity patient is believed to be secondary to venous thrombosis and insufficiency. The most common cause of extremity defects requiring microsurgical reconstruction is trauma. In the context of trauma, VTE risk is increased significantly by prothrombotic hypercoagulable states. 46 47 In major trauma with injury severity scores (ISS) greater than 30, incidences of in-hospital lower extremity DVTs have been reported to be as high as 5 to 15%. Reconstructive surgeons are often consulted to perform complex soft tissue coverage procedures within extremities having open fractures and/or exposed vital structures. In patients with lower extremity trauma, approximately 24% of tibia fractures present with open injuries requiring soft tissue coverage or flap reconstruction. In these patients, a 6% flap failure has been reported, most commonly due to microanastomotic venous thrombosis. 48 In this context, lower extremity reconstruction represents a unique challenge to reconstructive surgeons as these patients often present with open fractures in a multitrauma context with increased risk of VTE and the potential risk of bleeding from their acquired injuries. Furthermore, chemoprophylaxis within this population may be contraindicated due to presence of concomitant brain injury or severe intra-abdominal organ injuries.
In a recent retrospective cohort study on 165 lower extremity free flap reconstructions for trauma, Bendon and Crick reported a 19.4% rate of occult DVT identified in preoperative screening. They also found 7 further DVT cases intraoperatively. Multilevel limb injury, injury at or above the knee, and bilateral lower extremity injuries were independent predictors of DVT in this study. Preoperative or intraoperative identification of DVT prompted a change in microsurgical plan in the patients to use a superficial venous system in most of the cases and vein bypass graft in one case. In this cohort 12 patients with DVT were treated with therapeutic dose of LMWH, 2 patients received prophylactic LMWH plus ASA, and 4 patients were not treated for DVT with anticoagulants and instead had prophylactic inferior vena cava filters (IVCFs) placed. Flap failure rate was 4.9% (25% of them had DVT). 49
With presence of such a high incidence of occult DVT in extremity trauma patients, the questions become should preoperative screening for DVT be part of routine protocols in this patient population, and whether presence of DVT should preclude free flap reconstruction options. In a retrospective study on 137 upper and lower extremity flap procedures including 61 free flaps performed by the senior author, it was demonstrated that despite a high incidence of preoperative DVT (16.1% in total and 22.4% in upper extremity flap group), flap-based reconstruction was deemed safe and successful with low complication rate when managed judiciously. 50
The 9th edition of American College of Chest Physicians' Guideline for Prevention of VTE in Orthopedic Surgery Patients published in 2012 provides a comprehensive recommendation for VTE prevention in orthopaedic surgery patients that can be applied to extremity microsurgical reconstruction patients given many common risk factors shared between these two patient populations. 51 Based on these evidence-based guidelines, all patients undergoing major orthopaedic surgery should receive at least one type of chemoprophylaxis (LMWH, fondaparinux, dabigatran, apixaban, rivaroxaban, low-dose unfractionated heparin, adjusted dose vitamin K antagonist, and/or ASA) for a minimum of 10 to 14 days, and up to 35 days if they have higher personal risk of thromboembolism. Use of LMWH is preferred given its predictable bioavailability and relatively short half-life. Placement of IPC device is recommended in addition to chemoprophylaxis during the hospital stay. In patients with elevated risk of bleeding, mechanical prophylaxis is recommended over chemoprophylaxis. The guidelines recommend against IVCF as a prophylactic measure over no prophylaxis in patients with contraindications for other types of prophylaxis given the low-quality evidence for benefits and increased adverse events related to IVCF placement. 51
The Eastern Association for the Surgery of Trauma practice management guideline for VTE prevention in trauma patients is another relevant practical guideline that can be used in patients with lower extremity microsurgery. 52 Based on this guideline insertion of prophylactic IVCF is recommended in very high-risk trauma patients who cannot receive anticoagulation because of increased bleeding risk and have injury patterns rendering them immobilized for prolonged periods including Glasgow Coma Scale score less than 8, paraplegia or quadriplegia, and/or complex pelvic fracture with associated long bone fractures. However, the meta-analysis of studies found no class I evidence to support prophylactic IVCF in trauma patients without established DVT or PE. Based on this meta-analysis spinal cord injury, ISS and blood transfusion were identified as independent risk factors for VTE in trauma patients. Use of low-dose heparin alone showed little proven efficacy in high-risk trauma patients. In the setting of vascular surgery in lower extremity, evidence suggests superiority of LMWH to unfractionated heparin for prevention of arterial thrombosis. 53
Geoghegan et al investigated the ability of VTE RAMs in predicting microvascular thrombosis following lower extremity free tissue transfer. 54 This study included 58 adult patients with lower extremity open fractures and associated soft tissue injury requiring free flap reconstruction. All patients in this study received mechanical prophylaxis in the form of Flowtron boots on the contralateral limb and chemoprophylaxis (enoxaparin 20 mg daily and ASA 75 mg) perioperatively. Authors utilized three different risk stratification tools for VTE including Caprini, Department of Health, and Padua. All patients in this study were deemed high risk for VTE based on all three RAMs. Symptomatic VTE within the 90 days postoperative period was reported as 3.5%. Microanastomotic venous thrombosis was reported in 7% of the cases, while flap hematoma was reported in 8.6% of cases. This study failed to show a significant difference in RAM scales between VTE and non-VTE group, and suggested that these RAM assessment systems were unable to accurately predict the risk of VTE and microvascular venous thrombosis in patients with lower extremity reconstruction; however, the retrospective nature of the study and small number of patients in the VTE group should be considered as limitations of this study.
In review of literature related to extremity microvascular reconstruction, it seems that there is a high incidence of occult DVT in these patients particularly in the trauma setting, that places this patient population at a higher risk for VTE. Preoperative screening for DVT is recommended to be part of routine protocols in this patient population. All patients undergoing microvascular reconstruction of extremity should receive mechanical VTE prophylaxis such as IPC in unaffected lower extremity, and at least one type of chemoprophylaxis for a minimum of 10 to 14 days, and up to 35 days if they have higher personal risk of thromboembolism. Use of LMWH is preferred given its predictable bioavailability and relatively short half-life.
Head and Neck Microvascular Reconstruction
Microvascular reconstruction of the head and neck region is most commonly cancer related. These patients are at higher risk of VTE development typically due to advanced age, tobacco and alcohol use, decreased pulmonary function, decreased mobility in mechanical ventilation-dependent cases, and the prothrombotic state of cancer. In a prospective study of 100 consecutive patients who underwent surgery for head and neck cancer reconstruction without receiving any VTE chemoprophylaxis, DVT screening on postoperative day 2 or 3 demonstrated 13% VTE incidence with 5% asymptomatic DVT. 55 Retrospective studies reported lower rates of symptomatic VTE after microsurgical reconstruction ranging between 0.3 and 6%. 56 57 In a large retrospective study on 1,061 head and neck free flap procedures between 2006 and 2020, Crippen et al reported 3.8% of patients had a history of VTE that was significantly related to advanced age, chemotherapy, and comorbidities such as stroke. The only independent predictor of flap thrombosis identified was the history of DVT.
The Enhanced Recovery After Surgery published practical recommendations for preoperative care in head and neck free flap patients in 2017, demonstrating that no specific chemoprophylactic management is proven to reduce free flap microvascular thrombosis or improve outcome in these patients. 58 However, many studies have performed risk stratification assessments and recommended VTE chemoprophylaxis in high-risk patients. 59 VTE chemoprophylaxis in head and neck microvascular surgery patients is widely debated as bleeding complication can lead to possible adverse sequela including airway compromise. In a meta-analysis of VTE in otolaryngology-head and neck surgery including free flap reconstruction, Moubayed et al reported that addition of chemoprophylaxis did not decrease VTE rate, however, increased bleeding complications (odds ratio [OR]: 3.8). 60 On the other hand, Cevik et al, in a review of 306 studies and meta-analysis of nine studies of head and neck microsurgical patients showed that anticoagulation lowers the risk of VTE in this patient group, but also increases the bleeding risk, necessitating risk stratification using the Caprini RAM to make decisions. 61 Based on their meta-analysis, LMWH appeared to be superior to heparin when was given twice daily but equal to heparin three times daily with similar complication profile in cancer patients. 61
Chien et al in a retrospective study on 216 patients who underwent head and neck microvascular reconstruction showed that combination of SQH (5,000 U twice daily) and ASA (325 mg orally daily) does not have significant effect on hematoma rate (5.6% vs. 5.3%). 62
In a prospective study Ambani et al studied 90-day postoperative VTE and bleeding events in 78 patients who underwent free tissue reconstruction following tumor resection for head and neck or breast cancer. In this study patients received standard fixed enoxaparin dosing at 30 mg twice daily in head and neck and 40 mg daily in breast reconstruction group. Anti-factor Xa was measured in all patients with prophylactic target of 0.2 to 0.5 IU/mL. They showed that only 33% of patients achieved the target anti-Xa level and all VTE events (5%) and bleeding events (8%) occurred in subprophylactic anti-Xa group. Total body weight was negative predictor for anti-Xa level, therefore authors recommended weight-based enoxaparin dosing for VTE prophylaxis. 63 In another retrospective review of 153 patients who underwent head and neck free flap reconstruction following cancer ablative surgery anti-Xa level were measured in 47 cases, and of these, only 22 (47%) were within the prophylactic range (0.2 IU/mL or more) despite receiving prophylactic dose of LMWH (dalteparin 5,000 IU daily). Among those 47 cases, there were 6 flap complications and 4 out of 6 had subprophylactic anti-Xa level. 64
A systematic review of literature on VTE prophylaxis in head and neck microsurgery by Abraham et al demonstrated routine use of prophylactic dose of SQH or LMWH in combination with mechanical methods as an effective VTE prophylaxis protocol in head and neck microsurgery. 22 LMWH is preferred in more vulnerable population due to decreased risk of bleeding and higher safety profile. The authors also concluded that use of ASA alone does not increase flap survival but may increase bleeding complication. 22
Overall, it appears that most head and neck microsurgeons have consensus on use of VTE chemoprophylaxis in addition to mechanical measures. LMWH is a preferred agent with lower increase in hematoma rate and better safety profile.
Duration of the Postoperative Chemoprophylaxis
United Kingdom's Million Women study demonstrated that in middle-aged women, VTE risk remains substantially high up to 90 days after inpatient surgery. 65 The Enoxaparin and Cancer (ENOXACAN II) study, a randomized controlled trial of 253 versus 248 patients with intra-abdominal or pelvic cancer who underwent surgery and received 40 mg enoxaparin daily versus placebo for VTE prevention, showed significant VTE risk reduction with 28-day postoperative enoxaparin as compared to 7 days of enoxaparin during a 90-day follow-up. 66
The American College of Chest Physician's guideline for prevention of VTE in orthopaedic surgery patients recommended to receive at list one type of chemoprophylaxis for a minimum of 10 to 14 days, and up to 35 days if they have higher personal risk of thromboembolism. 51
Rau et al retrospectively reviewed 65 consecutive patients who underwent lower extremity free flap harvest for head and neck reconstruction and compared them to 37 patients who underwent similar procedures but were prospectively studied for postoperative asymptomatic DVT at 1 and 4 weeks after surgery. All patients in this study received chemoprophylaxis with unfractionated heparin 5,000 units three times per day and ASA 81 mg daily preoperatively, which was restarted 6 hours after surgery and discontinued at the day of discharge. Hematomas were reported in 3.9% of these cases. Rate of symptomatic DVT in both groups were similar (3.1% in the retrospective group and 2.7 in prospective group). Note that 8.1% of patients were found to have acute asymptomatic DVT after screening at 1 week, and 16.7% of patients had evidence of new acute DVT in duplex ultrasound screening at 4 weeks. The authors of this study recommend extending the duration of chemoprophylaxis to 4 weeks after microsurgery involving the lower extremity. 67
Management of Microsurgical Patients with VTE
Preoperative Management of Microsurgical Patient who has VTE: Free Flap or Not?
Studies have shown successful free flap reconstruction and high flap survival rate in the presence of perioperative VTE when it is performed with appropriate considerations. Microsurgeons usually encounter this challenging situation in two typical scenarios. One is acute DVT as a result of trauma in extremities, and to less extent in other body areas requiring free tissue transfer. The other common scenario is older patients with comorbidities and cancer history in need of oncologic tissue reconstruction with newly diagnosed VTE or history of previous VTE.
Similar approaches including recommended therapeutic anticoagulation in these two patient populations have shown different outcomes. In a retrospective study of 201 lower extremities below knee traumatic reconstruction with free or local flaps (82 free flaps), 9 patients (6.3%) diagnosed with preoperative symptomatic DVT underwent flap reconstruction. In this cohort 8 DVT events occurred in ipsilateral limb to the flap reconstruction. All patients diagnosed with DVT were placed on therapeutic anticoagulation doses of either subcutaneous dalteparin, enoxaparin or oral warfarin, while one patient received an IVCF alone due to anticoagulation contraindications. Anticoagulation therapy continued preoperatively and the day of surgery. Flap success rate in the VTE group was reported to be 100% in this study. The only difference in outcomes between the VTE and non-VTE groups was significantly increased recipient site hematoma (OR: 25) specifically in the free flap patients (40.0% vs. 2.6%, p = 0.02). 68 In these patients careful preoperative and intraoperative decisions must be taken. For example, in the latter study, intraoperatively the recipient veins were carefully evaluated first for signs of obstructive thrombosis such as flow absence or backflow. In such circumstances, superficial venous system was utilized alone or in combination with deep veins.
Valerio et al reported significantly higher perioperative VTE (26%) in young military patients with warfare-related trauma as compared to civilian trauma patients. Despite such a high incidence of VTE in this unique patient population with high-velocity blast trauma (mean ISS 29), extremity salvage with free tissue transfer was performed with a high success rate. In this patient cohort 173 flaps (99 pedicle and 74 free flaps) were reviewed. The free flap group had an even higher rate of preoperative VTE (33%) than the pedicle flap procedure group. These patients were placed on anticoagulation regimens consisting of therapeutic LMWH (1 mg/kg twice daily), or PTT titrated heparin drip. Fifty percent of patients with DVT also received IVCF. All patients received ASA 325 mg the day before, and therapeutic LMWH the morning of procedure. Therapeutic anticoagulation resumed the evening of the procedure (6 hours postop) or the next day in the morning. Authors described specific intraoperative steps to assure adequate venous drainage, including dissection of the venous outflow first, and utilizing both the deep and superficial venous systems with two veins often used for outflow, in recipient limbs with known DVT diagnosis. Unsurprisingly, patients with VTE on full-dose anticoagulation experienced increased incidence of hematoma (20% vs. 5%) with comparable nonhemorrhagic events between the VTE and non-VTE group. 50
In contrast to free flap trauma reconstruction, cancer patients requiring microvascular reconstruction have shown higher rate of flap failure. 69 In a case review of two patients with free flap reconstruction following thigh sarcoma resection with simultaneous DVT in the same limb, Murray et al reported flap failure in one patient due to venous congestion resulting in thrombosis of pedicle. In the second case use of superficial venous system for salvage resulted in flap survival. 69
In patients with diagnosed VTE or with high suspicions for VTE undergoing microvascular surgeries collaboration with hematologist to develop appropriate anticoagulation regimen is necessary. Preoperative venogram for operative planning is strongly suggested. Anticoagulation with 1 mg/kg LMWH twice a day or PTT-adjusted heparin infusion should be started preoperatively in patients with evidence of DVT. Fondaparinux or argatroban have been used in patients with HIT. Preoperative IVCF placement should be considered in patients with contraindications to anticoagulation. In trauma or cancer patients with platelet count higher than 300,000/µL preoperative ASA 325 mg is recommended. 70 All patients should receive mechanical thromboprophylaxis throughout their hospital stay ( Fig. 1 ).
Fig. 1.
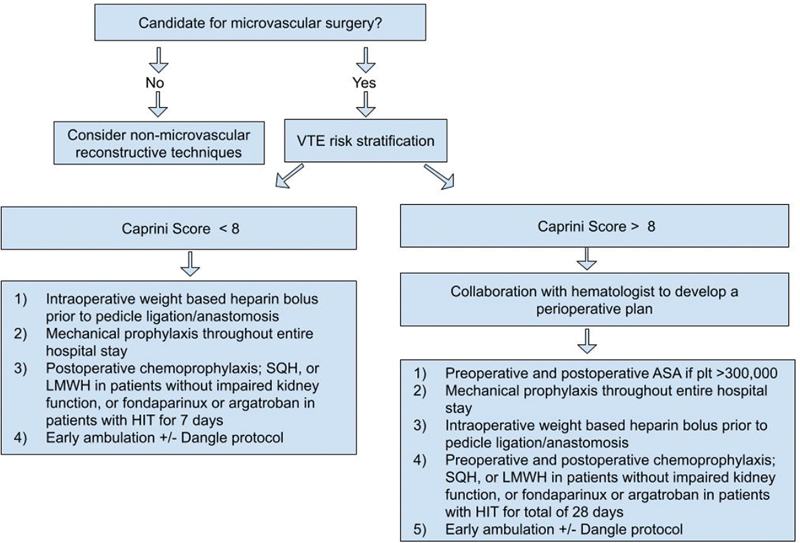
Algorithmic approach for thromboprophylaxis in microvascular surgery. VTE, venothromboemolism; SQH, subcutaneous heparin; LMWH, low-molecular weight heparin; HIT, heparin-induced thrombocytopenia; ASA, acetylsalicylic acid, aspirin; plt, platelets.
Intraoperative Management of VTE or Microvascular Thrombosis
Microsurgery entails necessary operative steps that inherently exacerbate Virchow's triad (i.e., hypercoagulability, venous stasis, and injury to the vessel wall). Therefore, most microsurgeons use at least one intraoperative anticoagulant in patients with a high risk of thrombosis. The most commonly used regimen includes systematic administration of heparin in combination with preoperative ASA. In patients with diagnosed VTE in addition to preoperative therapeutic anticoagulation, intraoperative weight-based bolus of systemic heparin administered prior to the flap pedicle ligation and repeated before microanastomosis. Heparin bolus is also administered in addition to antiplatelet if there is any evidence of acute thrombosis (white clot) encountered during microsurgical anastomosis.
The flow of the elected recipient vein in the extremity free flap can be assessed intraoperatively with color flow Doppler prior to harvest of flap. Performing recipient vein dissection first is recommended to evaluate presence of flow insufficiency, venous hypertension, significant backflow, or any sign of venous obstruction or valvular incompetence. In such circumstances alternative venous options including connecting a second deep and/or a superficial vein should be considered. We recommend considering a venous graft if there is no suitable local recipient vein available.
Flap Salvage
Venous thrombosis is a more common cause of flap loss as compared to arterial thrombosis, mainly due to a low flow system prone to stasis and collapse with external pressure or kinking. When reexplored, however, flap salvage rate is higher in venous thrombosis as compared to arterial thrombosis. Success of flap takeback due to microvascular compromise is variable in different institutions but has improved over time as microsurgical techniques evolve. Salvage rates in published microvascular literature are reported to be 30 to 70%.
Successful flap salvage depends on multiple factors, such as mean time from primary surgery to reexploration, time from flap compromise to reexploration, use of vein graft, 71 72 history of thrombophilia, preoperative platelet count, and microvascular surgeon's experience. 70
In a retrospective review of 2,260 free flaps, Mirzabeigi et al found 47 flap takeback for delayed compromise. Half of the flaps were salvaged successfully. Lowest rate of salvage was related to high preoperative platelet count (> 300,000/µL), time to takeback, and thrombophilia. Experienced surgeons (> 5 years in practice) had lower takeback rate. 70
In a review of 1,193 free flaps that were performed at the Memorial Sloan Kettering Cancer Center between 1991 and 2002, 6% of flaps required reexploration, mostly due to pedicle thrombosis (53%) and hematoma (30%). In the venous thrombosis group, salvage rate was significantly higher than arterial thrombosis (71% vs. 40%). This study showed shorter time to return to operating room after flap compromise as the main predictor of successful salvage. In this study use of a vein graft did not have significant effect on takeback or salvage. Head and neck free flaps showed delayed compromise (mean 5 days) compared to other types of free flaps. Authors recommended longer postoperative flap monitoring and VTE prophylaxis (at least 5 days) for these patients. 72
Kroll et al reviewed 990 consecutive free flaps for head and neck, breast, and extremity reconstruction and showed 80% of pedicle venous thrombosis occurs within two postoperative days, and 90% of arterial thrombosis occurs within first 24 hours after surgery. Based on their findings they recommended 4 days of close postoperative flap monitoring. 73 Nelson et al in a review of 1,277 breast free flap reconstructions over a 17-year period found late flap thrombosis in 10 patients after postop day 3. Three patients presented after postoperative day 5. These patients received medical treatment with anticoagulation (heparin drip) only, without flap salvage attempt through surgery. Two flaps were partially salvaged in the medical treatment group. In patients who underwent operative management 86% of flaps were salvaged. 74 In this study all patients who had evidence of venous thrombosis and underwent salvage procedure received venous grafts. The senior author of this study recommended thrombolysis using 250,000 units of urokinase administration through an arteriotomy proximal to the anastomosis after reestablishing arterial and venous anastomosis. Authors preferred urokinase due to its lower systemic bleeding complications as compared to tissue plasminogen activator (tPA). Heparin drip was immediately started after surgery in these patients. 74
Outpatient flap salvage rate in patients who present later than 5 days after surgery in multiple studies is reported to be extremely low (less than 6%). 75 Trussler et al reported two cases of free flap salvage with application of catheter-directed thrombolysis (CDT). One case presented with arterial thrombosis on postoperative day 12 who received CDT with continuous infusion of urokinase 1 mL/min in combination with heparin 500 u/h for 24 hours with successful salvage. This patient was discharged on warfarin. The other patient presented on postoperative day 6 with venous thrombosis and failed prior operative salvage attempts. He received CDT with alteplase and Retavase for 24 hours. This patient was discharged on ASA 81 mg daily. 76 The other patient presented on postoperative day 6 with venous thrombosis and failed prior operative salvage attempts. He received CDT with alteplase and Retavase for 24 hours. This patient was discharged on ASA 81 mg daily. 76
In case of heparin allergy or HIT, alternative anticoagulants may be used intraoperatively. Shuck et al reported a case of breast free flap reconstruction with history of allergy to heparin and multiple incidences of intraoperative arterial thrombosis. The authors used argatroban infusion (a DTI) intraoperatively and repeated anastomosis 40 minutes after the start of infusion with successful flap survival. 77
Bui et al recommended an algorithm for flap reexploration based on a retrospective review of 1,193 free flaps, emphasizing immediate reexploration as the most crucial step. 72
When to Use Chemical Thrombolysis
There are three common thrombolytic agents used in microvascular salvage attempts: streptokinase, urokinase, and recombinant tPA (r-tPA). Streptokinase is an enzyme produced by a Streptococcus species that enhances conversion of plasminogen to plasmin. It has high rates of antigenicity and anaphylactic reactions have been reported in 0.1% of patients, limiting its clinical use. Urokinase is obtained from human fetal kidney cells. It directly converts plasminogen to plasmin and has a shorter half-life than streptokinase. It has less systemic complications compared to streptokinase but is more expensive compared to the other agents. tPA is produced by vascular endothelial cells and is a direct and potent activator of plasminogen. It has the least systemic complications compared to other thrombolytic enzymes.
Microvascular thrombosis or perianastomotic thrombosis accounts for 10 to 15% of flap failure. The most common site of thrombosis is at the anastomosis site, particularly on the venous side due to slower blood flow. Implementation of thrombolytics in ischemic flap salvage was first described by Puckett in 1983 when he administered streptokinase into the ischemic epigastric flap in a rat model and reported significant improvement in flap survival. 78 Other groups also studied the effect of streptokinase in comparison to urokinase and tPA in animal models. 79 80 First human use of thrombolytics for free flap salvage was performed by Lipton and Jupiter in 1987. 81 They reported a case of osteomyocutaneous fibular flap that was reexplored due to venous congestion 40 hours postoperatively. Intra-arterial injection of 10,000 units streptokinase every 10 minutes for 1 hour resulted in successful salvage of the flap.
In a large case review of 590 free tissue transfers, Panchapakesan et al reported 12% flap reexploration due to impending failure based on clinical findings. In their review approximately half of the cases showed evidence of microvascular thrombosis. Indications for use of thrombolysis included failure to establish venous outflow after establishing arterial inflow. The authors recommended an algorithmic approach for flap salvage. First step in this algorithm is rule out of any external cause of flow obstruction. Then microvascular thrombosis should be evaluated and revised. In case of inflow issues mechanical thrombectomy is applied first utilizing Jewlers thrombectomy, number-3 Fogarty catheter, and heparin solution irrigation. In this study 55% of the thrombosed flaps responded to mechanical thrombectomy only. Failure to reestablish venous flow with this technique was the indication for thrombolysis with either streptokinase or urokinase. Thrombolysis was performed using 250,000 units of enzyme in 50 mL of saline which infused locally to the flap through an arteriotomy or anastomosis site for 30 minutes while venous anastomosis was taken down to prevent systemic distribution. Using this algorithm authors reported 54% overall salvage rate and 30% salvage rate after thrombolysis. 71 Other indications for use of thrombolytic agents include suspected intraflap microvascular thrombosis based on poor venous return despite good arterial inflow, intraoperative findings such as extensive acute thrombosis of pedicle vessels that cannot be removed by mechanical thrombectomy, and the vessel segment containing the clot that cannot be debrided.
Authors' Free Flap Salvage Algorithm
Based on review of current literature and senior author's experience, an algorithmic approach to salvage compromised flap is recommended in this article ( Fig. 2 ). Immediate reexploration is the first and the most crucial step in this algorithm. In patients with platelet count greater than 300,000/µL preoperative ASA 325 mg is recommended. Intraoperatively, if no thrombosis is identified in the pedicle, external compression, kinks, and vasospasm should be ruled out. Use of warm saline, lidocaine, or papaverine can help to decrease vasospasm. Arterial pedicle adventitia should be removed to eliminate sympathetic vasoconstriction via sympathectomy. If there is any evidence of flap congestion without obvious thrombosis, a second venous anastomosis should be considered to improve venous outflow, even if a long vein graft is necessary to achieve tension-free and thrombosis-free healthy end-to-end anastomosis. Vein grafting, when performed in an appropriate setting for congestion-related salvage, significantly improves salvage rates. In case of intraoperative pedicle thrombosis, systemic heparin bolus 3,000 to 5,000 units based on patient's BMI is administered. The microanastomosis should be revised. In the presence of white clot (fresh platelet plug) use of systemic intravenous Toradol (30 mg intravenously) for its antiplatelet effect is recommended. Mechanical thrombectomy is performed next. If arterial inflow is established without adequate venous outflow, microthrombosis within the flap microvasculature and capillary system is suspected. Use of thrombolysis at this step is recommended. Two milligrams of r-tPA in 2.2 mL normal saline is injected into the arterial anastomosis while the venous outflow is clamped. The thrombolytic agent requires to sit inside the flap for 10 to 30 minutes for optimal function. Then, the venous clamp is released and tPA is washed out using saline flush while the pedicle is still disconnected from recipient veins. If red clot (established old clot) was encountered, heparin bolus with or without antiplatelet is administered following mechanical thrombectomy and finally thrombolysis with tPA ( Fig. 2 ).
Fig. 2.
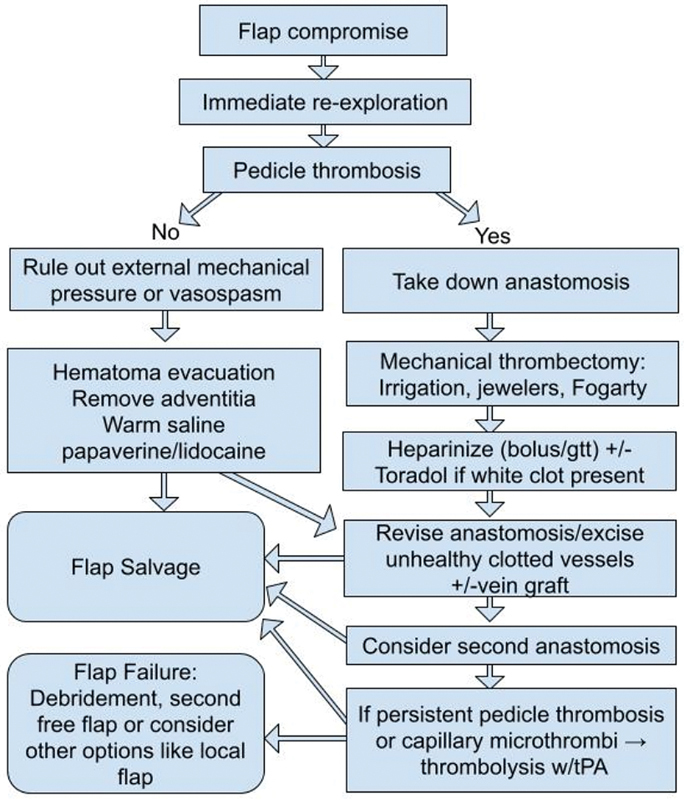
Algorithmic approach for flap salvage. tPA, tissue plasminogen activator.
Perioperative Management of Microsurgical Patients with Known Hereditary or Acquired Hypercoagulable States
The most common hereditary hypercoagulable states include the following: factor V Leiden, prothrombin gene G20210A mutation, protein C deficiency, protein S deficiency, and antithrombin III deficiency and acquired hypercoagulable states include the antiphospholipid antibody syndrome, inflammatory bowel disease, obesity, and cancer. Patients with hereditary and acquired thrombophilias are at higher risk for thrombosis, rendering them more susceptible to free flap failure. 82 A retrospective study analyzed the outcomes for 2,032 free flaps performed at a single institution. From this cohort, 58 free flaps (2.9% of total) consisting of 48 breasts, 6 head and neck, 2 latissimus dorsi, and 2 lower extremity flaps were performed in patients with known hypercoagulable states that comprised of, protein C deficiency, factor V Leiden mutation, hyperhomocysteinemia, prothrombin gene mutation, antiphospholipid antibody syndrome, factor VIII elevation, anticardiolipin antibody syndrome, and essential thrombocytosis. Among this cohort, there was a greater rate of flap failure (15.5% vs. 1.8%, p = 0.0001) and thrombosis (20.7% vs. with known thrombophilia 4.2%, p = 0.0001) among patients with known thrombophilia. 83
No evidence-based antithrombotic protocols have been developed to mitigate the risk for patients with hypercoagulable states. Wang et al looked at four protocols outlined by four different attendings in conjunction with a hematologist that ranged from the implementation of simple DVT prophylaxis with 5,000 units of SQH every 8 hours to the intraoperative and postoperative initiation of a subtherapeutic heparin drip with antiplatelet therapy and there was no statistically significant protocol. 83 In another study, patients with hypercoagulable states who received 2,000 units of intramuscular unfractionated heparin prior to the pedicle anastomosis followed by heparin drip to therapeutic levels (based on activated PTT) were found to have fewer clinical rates of flap loss and thrombotic events. 84 However, patients in this cohort had increased rates of hematoma, bleeding, and transfusion requirements. 84
A cohort study analyzed the effectiveness of thromboprophylaxis in 57 patients with hypercoagulable states—factor V Leiden, prothrombin G20210A, antithrombin II deficiency, protein C and S deficiencies, antiphospholipid antibody testing, reductase, plasminogen activator inhibitor, antiphospholipid antibody testing, hyperhomocysteinemia, and elevated factor VII—that underwent lower extremity reconstruction. Thirty patients were treated with a risk-stratified thromboprophylaxis regimen (ranging from SQH with ASA to the initiation of therapeutic intravenous heparin and ASA) and 27 received SQH for 5 days postoperatively with ASA. 85 Patients with flap loss from both cohorts received a 2-week course of therapeutic LMWH. 85 Patients in the risk-stratified cohort received more frequent administration of intravenous heparin compared to the control cohorts (73% vs. 15%, p < 0.001) and there were lower rates of total (3% vs. 19%, p = 0.06) and partial flap (10% vs. 37%, p = 0.025) loss, postoperative thrombotic events (1.2% vs. 12.3%, p = 0.004), and intraoperative microvascular compromise (86% vs. 25%, p = 0.04) observed in the patients who received the risk-stratified thromboprophylaxis regimen. Salvage rates for postoperative thrombosis were 0% for both cohorts, regardless of the protocol. In these high-risk cohorts, close collaboration with hematologists is recommended. 19
Preoperative Labs
Hemoglobin and Hematocrit
The literature concerning preoperative hemoglobin and its association with free flap failure is mixed. A retrospective cohort study looked at 132 patients with preoperative anemia (mean hemoglobin 11.8 ± 2.4 g/dL); the most profound increase in flap failure rate was observed in patients with a hemoglobin value that was less than 10 g/dL (relative risk 4.76, p = 0.006). 86 Evaluating these findings were reinforced by another study evaluating 483 patients that underwent lower extremity free flap; this study found that preoperative anemia was independently associated with free flap complications consisting of reoperation, readmission, organ space infection, or death (OR = 4.10, CI: 2.00–8.41, p < 0.001). 87 Contrarily, a study that looked at the National Surgery Quality Improvement database found that out of 864 patients that underwent a free tissue transfer, there was no notable difference in flap reoperation rates between the anemic and nonanemic cohorts (3.28% vs. 4.03%, p = 0.0603). 88
Platelets
The determination of preoperative platelet counts may play an important role in the risk stratification of thrombosis in a postsurgical patient. Lower extremity trauma patients with a platelet count greater than 403 × 10 9 units/L had a significantly greater risk of intra- and postoperative thrombosis than patients with normal platelet counts. Additionally, the risk of intraoperative thrombosis was noted in patients with elevated platelet counts who underwent lower extremity reconstruction without a prior history of lower extremity trauma. 89
Prealbumin
Preoperative nutrition plays an important role in optimizing the chances for a successful free tissue transfer. In a retrospective cohort study that analyzed the 1-month free flap survival among 162 patients who underwent a head and neck reconstruction, there was a 76.5% (95% CI, 48.8–90.5%) free flap survival rate observed among patients with preoperative prealbumin levels less than 10 mg/dL and 95.2% survival rate (95% CI, 90.1–97.7%) seen in patients with preoperative prealbumin levels greater than 10 mg/dL ( p = 0.002). Hypo-prealbuminemia was associated with a fourfold increased risk of failure ( p = 0.04) in comparison with those patients with normal prealbumin levels. 90
Postoperative Management of Microsurgery Patient with VTE
Patients with established DVT or PE who are receiving anticoagulation preoperatively should resume anticoagulation the night of surgery or the morning afterward depending on the extent of the procedure and risk of bleeding. Prophylactic or intermediate dose LMWH (e.g., 0.5 mg/kg twice daily) can be considered for 2 to 3 days postoperatively in a patient who is high risk for bleeding prior to resumption of full-dose anticoagulation. Patients on warfarin prior to surgery may need to be “bridged” back to a therapeutic international normalized ratio with LMWH or unfractionated heparin. DOACs can likely be resumed at 24 hours if there is a low bleeding risk and at 48 to 72 hours if the bleeding risk is high.
Patients who sustained a postoperative VTE are typically treated with full-dose systemic anticoagulation for 3 months based on the American Society of Hematology 2018 guidelines for management of VTE, 91 though final decisions may be made in consultation with a hematologist.
Conclusion
Prevention of venous thromboembolic events in microsurgery is an important consideration. Such events, when encountered in microsurgery, can not only potentially threaten the flap reconstruction and outcome, but these events also increase the incidence of complications, the need for additional surgical and medical interventions, as well as the overall cost of care. Furthermore, as reconstructive surgeons, understanding the reason behind thrombotic events is especially critical within takeback situations and/or within the intraoperative setting when acute thrombotic events are being experienced. In such situations, the reconstructive surgeon must remain level-headed, be quick-thinking and responsive, as well as understanding of the pathways for anticoagulation treatment and provision to reverse the hypercoagulable state that is acutely threatening flap viability in an effort to achieve a successful flap salvage. In this article, the authors have outlined algorithms that may be adopted or modified for reconstructive surgeons to apply in their practice or setting to establish best practices in the care of microsurgery patients who are inherently at higher risk and susceptible to thrombotic events.
Footnotes
Conflict of Interest None declared.
References
- 1.O'Donnell M, Linkins L A, Kearon C, Julian J, Hirsh J. Reduction of out-of-hospital symptomatic venous thromboembolism by extended thromboprophylaxis with low-molecular-weight heparin following elective hip arthroplasty: a systematic review. Arch Intern Med. 2003;163(11):1362–1366. doi: 10.1001/archinte.163.11.1362. [DOI] [PubMed] [Google Scholar]
- 2.Stevens S M, Woller S C, Kreuziger L B. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(06):e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Caprini J A, Arcelus J I, Reyna J J.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease Semin Hematol 200138(2, Suppl 5):12–19. [DOI] [PubMed] [Google Scholar]
- 4.Hatef D A, Kenkel J M, Nguyen M Q. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122(01):269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 5.Pannucci C J, Bailey S H, Dreszer G. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212(01):105–112. doi: 10.1016/j.jamcollsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkins E G, Pannucci C J, Bailey S H. Preliminary report on the PSEF Venous Thromoboembolism Prevention Study (VTEPS): validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. Plast Reconstr Surg. 2010;126:107–108. [Google Scholar]
- 7.Pannucci C J, Wachtman C F, Dreszer G. The effect of postoperative enoxaparin on risk for reoperative hematoma. Plast Reconstr Surg. 2012;129(01):160–168. doi: 10.1097/PRS.0b013e318236215c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannucci C J, Dreszer G, Wachtman C F. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconstr Surg. 2011;128(05):1093–1103. doi: 10.1097/PRS.0b013e31822b6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S Y, Lee K T, Mun G H. Postoperative venous insufficiency in microsurgical lower extremity reconstruction and deep vein thrombosis potential as assessed by a Caprini risk assessment model. Plast Reconstr Surg. 2015;136(05):1094–1102. doi: 10.1097/PRS.0000000000001701. [DOI] [PubMed] [Google Scholar]
- 10.Vane J R, Botting R M.The mechanism of action of aspirin Thromb Res 2003110(5-6):255–258. [DOI] [PubMed] [Google Scholar]
- 11.WARFASA Investigators . Becattini C, Agnelli G, Schenone A. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959–1967. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 12.Lighthall J G, Cain R, Ghanem T A, Wax M K. Effect of postoperative aspirin on outcomes in microvascular free tissue transfer surgery. Otolaryngol Head Neck Surg. 2013;148(01):40–46. doi: 10.1177/0194599812463320. [DOI] [PubMed] [Google Scholar]
- 13.Disa J J, Polvora V P, Pusic A L, Singh B, Cordeiro P G. Dextran-related complications in head and neck microsurgery: do the benefits outweigh the risks? A prospective randomized analysis. Plast Reconstr Surg. 2003;112(06):1534–1539. doi: 10.1097/01.PRS.0000083378.58757.54. [DOI] [PubMed] [Google Scholar]
- 14.Buntic R F, Brooks D, Buncke H J, Buncke G M.Dextran-related complications in head and neck microsurgery: do the benefits outweigh the risks? Plast Reconstr Surg 2004114041008, author reply 1008–1009 [DOI] [PubMed] [Google Scholar]
- 15.Lee K T, Jeon B J, Lim S Y. The effects of ketorolac on microvascular thrombosis in lower extremity reconstruction. Plast Reconstr Surg. 2012;129(06):1322–1327. doi: 10.1097/PRS.0b013e31824ec33f. [DOI] [PubMed] [Google Scholar]
- 16.Schleiffarth J R, Bayon R, Chang K E, Van Daele D J, Pagedar N A. Ketorolac after free tissue transfer: a comparative effectiveness study. Ann Otol Rhinol Laryngol. 2014;123(06):446–449. doi: 10.1177/0003489414526849. [DOI] [PubMed] [Google Scholar]
- 17.Björk I, Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982;48(03):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- 18.Xipoleas G, Levine E, Silver L, Koch R M, Taub P J. A survey of microvascular protocols for lower-extremity free tissue transfer I: perioperative anticoagulation. Ann Plast Surg. 2007;59(03):311–315. doi: 10.1097/SAP.0b013e31802fc217. [DOI] [PubMed] [Google Scholar]
- 19.Pannucci C J, Kovach S J, Cuker A. Microsurgery and the hypercoagulable state: a hematologist's perspective. Plast Reconstr Surg. 2015;136(04):545e–552e. doi: 10.1097/PRS.0000000000001591. [DOI] [PubMed] [Google Scholar]
- 20.Pan X L, Chen G X, Shao H W, Han C M, Zhang L P, Zhi L Z. Effect of heparin on prevention of flap loss in microsurgical free flap transfer: a meta-analysis. PLoS One. 2014;9(04):e95111. doi: 10.1371/journal.pone.0095111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsh J, Warkentin T E, Raschke R, Granger C, Ohman E M, Dalen J E.Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety Chest 1998114(5, Suppl):489S–510S. [DOI] [PubMed] [Google Scholar]
- 22.Abraham M, Badhey A, Hu S. Thromboprophylaxis in head and neck microvascular reconstruction. Craniomaxillofac Trauma Reconstr. 2018;11(02):85–95. doi: 10.1055/s-0037-1607068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhang M, Tan L, Pan N, Zhang L. The clinical use of fondaparinux: a synthetic heparin pentasaccharide. Prog Mol Biol Transl Sci. 2019;163:41–53. doi: 10.1016/bs.pmbts.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 24.PubChem [Internet] Bethesda (MD)National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5282448, Fondaparinux; (January 10, 2023). Available at:https://pubchem.ncbi.nlm.nih.gov/compound/Fondaparinux [Google Scholar]
- 25.Mehdizade T, Kelahmetoglu O, Gurkan V, Çetin G, Guneren E. Early suspicion of heparin-induced thrombocytopenia for successful free flap salvage: reports of two cases. J Hand Microsurg. 2021;13(03):178–180. doi: 10.1055/s-0040-1713692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linkins L-A. Heparin induced thrombocytopenia. BMJ. 2015;350:g7566. doi: 10.1136/bmj.g7566. [DOI] [PubMed] [Google Scholar]
- 27.Warkentin T E, Pai M, Linkins L A. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130(09):1104–1113. doi: 10.1182/blood-2017-04-778993. [DOI] [PubMed] [Google Scholar]
- 28.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G.Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest 2008133(6, Suppl):160S–198S. [DOI] [PubMed] [Google Scholar]
- 29.Samama M M. The mechanism of action of rivaroxaban–an oral, direct factor Xa inhibitor–compared with other anticoagulants. Thromb Res. 2011;127(06):497–504. doi: 10.1016/j.thromres.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Liss D B, Mullins M E. Antithrombotic and antiplatelet drug toxicity. Crit Care Clin. 2021;37(03):591–604. doi: 10.1016/j.ccc.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Ammollo C T, Semeraro F, Incampo F, Semeraro N, Colucci M. Dabigatran enhances clot susceptibility to fibrinolysis by mechanisms dependent on and independent of thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost. 2010;8(04):790–798. doi: 10.1111/j.1538-7836.2010.03739.x. [DOI] [PubMed] [Google Scholar]
- 32.Macias D, Kwon D I, Walker P C, Peterson N R. Local intraluminal irrigation with argatroban during free flap repair in a patient with heparin-induced thrombocytopenia. Ann Otol Rhinol Laryngol. 2017;126(05):407–410. doi: 10.1177/0003489417693015. [DOI] [PubMed] [Google Scholar]
- 33.Roderick P, Ferris G, Wilson K.Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis Health Technol Assess 2005949iii–iv., ix–x, 1–78 [DOI] [PubMed] [Google Scholar]
- 34.Gould M K, Garcia D A, Wren S M.Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2012141(2, Suppl):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Z H, Ramly E P, Alfonso A R. Dangle protocols in lower extremity reconstruction. J Surg Res. 2021;266:77–87. doi: 10.1016/j.jss.2021.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Masoomi H, Paydar K Z, Wirth G A, Aly A, Kobayashi M R, Evans G R. Predictive risk factors of venous thromboembolism in autologous breast reconstruction surgery. Ann Plast Surg. 2014;72(01):30–33. doi: 10.1097/SAP.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 37.Zhong T, Neinstein R, Massey C. Intravenous fluid infusion rate in microsurgical breast reconstruction: important lessons learned from 354 free flaps. Plast Reconstr Surg. 2011;128(06):1153–1160. doi: 10.1097/PRS.0b013e318221da56. [DOI] [PubMed] [Google Scholar]
- 38.Zarb R M, Ramamurthi A, Doren E L, LoGiudice J A, Hijjawi J B, Adamson K A. Clinical course of venous thromboembolism following abdominally based microsurgical breast reconstruction: a case series. J Plast Reconstr Aesthet Surg. 2021;74(10):2550–2556. doi: 10.1016/j.bjps.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Enajat M, Damen T HC, Geenen A, Timman R, van der Hulst R RWJ, Mureau M AM. Pulmonary embolism after abdominal flap breast reconstruction: prediction and prevention. Plast Reconstr Surg. 2013;131(06):1213–1222. doi: 10.1097/PRS.0b013e31828bd35e. [DOI] [PubMed] [Google Scholar]
- 40.Lemaine V, McCarthy C, Kaplan K. Venous thromboembolism following microsurgical breast reconstruction: an objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. 2011;127(04):1399–1406. doi: 10.1097/PRS.0b013e318208d025. [DOI] [PubMed] [Google Scholar]
- 41.Liao E C, Taghinia A H, Nguyen L P, Yueh J H, May J W, Jr, Orgill D P. Incidence of hematoma complication with heparin venous thrombosis prophylaxis after TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;121(04):1101–1107. doi: 10.1097/01.prs.0000302454.43201.83. [DOI] [PubMed] [Google Scholar]
- 42.Pannucci C J, Fleming K I, Agarwal J, Rockwell W B, Prazak A M, Momeni A. The impact of once- versus twice-daily enoxaparin prophylaxis on risk for venous thromboembolism and clinically relevant bleeding. Plast Reconstr Surg. 2018;142(01):239–249. doi: 10.1097/PRS.0000000000004517. [DOI] [PubMed] [Google Scholar]
- 43.Pannucci C J, Fleming K I, Bertolaccini C. Optimal dosing of prophylactic enoxaparin after surgical procedures: results of the double-blind, randomized, controlled FIxed or Variable Enoxaparin (FIVE) trial. Plast Reconstr Surg. 2021;147(04):947–958. doi: 10.1097/PRS.0000000000007780. [DOI] [PubMed] [Google Scholar]
- 44.Pannucci C J, Fleming K I, Bertolaccini C, Prazak A M, Stoddard G J, Momeni A. Double-blind randomized clinical trial to examine the pharmacokinetic and clinical impacts of fixed dose versus weight-based enoxaparin prophylaxis: a methodologic description of the FIxed or Variable Enoxaparin (FIVE) trial. Plast Reconstr Surg Glob Open. 2019;7(04):e2185. doi: 10.1097/GOX.0000000000002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enajat M, Aziz Mohammadi M, Debeij J, van der Hulst R R, Mureau M A. Effect of acetylsalicylic acid on microvascular thrombosis in autologous breast reconstruction. J Reconstr Microsurg. 2014;30(01):65–70. doi: 10.1055/s-0033-1356553. [DOI] [PubMed] [Google Scholar]
- 46.Adams R C, Hamrick M, Berenguer C, Senkowski C, Ochsner M G.Four years of an aggressive prophylaxis and screening protocol for venous thromboembolism in a large trauma population J Trauma 20086502300–306., discussion 306–308 [DOI] [PubMed] [Google Scholar]
- 47.Owings J T, Bagley M, Gosselin R, Romac D, Disbrow E.Effect of critical injury on plasma antithrombin activity: low antithrombin levels are associated with thromboembolic complications J Trauma 19964103396–405., discussion 405–406 [DOI] [PubMed] [Google Scholar]
- 48.Xiong L, Gazyakan E, Kremer T. Free flaps for reconstruction of soft tissue defects in lower extremity: a meta-analysis on microsurgical outcome and safety. Microsurgery. 2016;36(06):511–524. doi: 10.1002/micr.30020. [DOI] [PubMed] [Google Scholar]
- 49.Bendon C L, Crick A. Occult deep vein thrombosis in lower limb trauma requiring microsurgical reconstruction-a retrospective cohort study. J Plast Reconstr Aesthet Surg. 2021;74(04):775–784. doi: 10.1016/j.bjps.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Valerio I, Sabino J, Heckert R. Known preoperative deep venous thrombosis and/or pulmonary embolus: to flap or not to flap the severely injured extremity? Plast Reconstr Surg. 2013;132(01):213–220. doi: 10.1097/PRS.0b013e318290fa70. [DOI] [PubMed] [Google Scholar]
- 51.Falck-Ytter Y, Francis C W, Johanson N A.Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2012141(2, Suppl):e278S–e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers F B, Cipolle M D, Velmahos G, Rozycki G, Luchette F A. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002;53(01):142–164. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 53.Winkler M S, Larena-Avellaneda A, Diener H, Kölbel T, Debus E S.Risk-adjusted strategies in the prevention of early arterial thrombosis following lower extremity arterial reconstruction: a comparison of unfractionated versus low molecular weight heparin J Cardiovasc Surg (Torino) 201354(1, Suppl 1):183–192. [PubMed] [Google Scholar]
- 54.Geoghegan L, Super J, Machin M. Are venous thromboembolism risk assessment tools reliable in the stratification of microvascular risk following lower extremity reconstruction? JPRAS Open. 2021;29:45–54. doi: 10.1016/j.jpra.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clayburgh D, Stott W, Kochanowski T. Prospective study of venous thromboembolism in patients with head and neck cancer after surgery: interim analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(02):161–167. doi: 10.1001/jamaoto.2013.1372. [DOI] [PubMed] [Google Scholar]
- 56.Moreano E H, Hutchison J L, McCulloch T M, Graham S M, Funk G F, Hoffman H T. Incidence of deep venous thrombosis and pulmonary embolism in otolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 1998;118(06):777–784. doi: 10.1016/S0194-5998(98)70268-2. [DOI] [PubMed] [Google Scholar]
- 57.Thai L, McCarn K, Stott W. Venous thromboembolism in patients with head and neck cancer after surgery. Head Neck. 2013;35(01):4–9. doi: 10.1002/hed.22920. [DOI] [PubMed] [Google Scholar]
- 58.Dort J C, Farwell D G, Findlay M. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: a consensus review and recommendations from the Enhanced Recovery After Surgery Society. JAMA Otolaryngol Head Neck Surg. 2017;143(03):292–303. doi: 10.1001/jamaoto.2016.2981. [DOI] [PubMed] [Google Scholar]
- 59.Crippen M M, Ganti R S, Xu V, Swendseid B, Tzeng D L, Curry J. Outcomes in head and neck free flap reconstruction among patients with a history of venous thromboembolism. Otolaryngol Head Neck Surg. 2022;166(02):267–273. doi: 10.1177/01945998211011999. [DOI] [PubMed] [Google Scholar]
- 60.Moubayed S P, Eskander A, Mourad M W, Most S P. Systematic review and meta-analysis of venous thromboembolism in otolaryngology-head and neck surgery. Head Neck. 2017;39(06):1249–1258. doi: 10.1002/hed.24758. [DOI] [PubMed] [Google Scholar]
- 61.Cevik J, Middleton R, Ramakrishnan A, Cabalag M. Rationalizing post-operative prophylactic anticoagulation in reconstructive head and neck cancer patients: a review. ANZ J Surg. 2021;91(12):2610–2616. doi: 10.1111/ans.16742. [DOI] [PubMed] [Google Scholar]
- 62.Chien W, Varvares M A, Hadlock T, Cheney M, Deschler D G. Effects of aspirin and low-dose heparin in head and neck reconstruction using microvascular free flaps. Laryngoscope. 2005;115(06):973–976. doi: 10.1097/01.MLG.0000163539.97485.F4. [DOI] [PubMed] [Google Scholar]
- 63.Ambani S W, Bengur F B, Varelas L J. Standard fixed enoxaparin dosing for venous thromboembolism prophylaxis leads to low peak anti-factor Xa levels in both head and neck and breast free flap patients. J Reconstr Microsurg. 2022;38(09):749–756. doi: 10.1055/s-0042-1749340. [DOI] [PubMed] [Google Scholar]
- 64.Eley K A, Parker R J, Watt-Smith S R. Low molecular weight heparin in patients undergoing free tissue transfer following head and neck ablative surgery: review of efficacy and associated complications. Br J Oral Maxillofac Surg. 2013;51(07):610–614. doi: 10.1016/j.bjoms.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 65.Million Women Study collaborators . Sweetland S, Green J, Liu B. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ENOXACAN II Investigators . Bergqvist D, Agnelli G, Cohen A T. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346(13):975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 67.Rau A S, Harry B L, Leem T H, Song J I, Deleyiannis F W. Evidence for extending the duration of chemoprophylaxis following free flap harvest from the lower extremity: prospective screening for deep venous thrombosis. Plast Reconstr Surg. 2016;138(02):500–508. doi: 10.1097/PRS.0000000000002399. [DOI] [PubMed] [Google Scholar]
- 68.Badash I, Burtt K, Leland H.Effects of perioperative venous thromboembolism on outcomes in soft tissue reconstruction of traumatic lower extremity injuries Ann Plast Surg 201982(5S, Suppl 4):S345–S349. [DOI] [PubMed] [Google Scholar]
- 69.Murray D J, Neligan P C, Novak C B, Howley B, Wunder J S, Lipa J E. Free tissue transfer and deep vein thrombosis. J Plast Reconstr Aesthet Surg. 2008;61(06):687–692. doi: 10.1016/j.bjps.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 70.Mirzabeigi M N, Wang T, Kovach S J, Taylor J A, Serletti J M, Wu L C. Free flap take-back following postoperative microvascular compromise: predicting salvage versus failure. Plast Reconstr Surg. 2012;130(03):579–589. doi: 10.1097/PRS.0b013e31825dbfb7. [DOI] [PubMed] [Google Scholar]
- 71.Panchapakesan V, Addison P, Beausang E, Lipa J E, Gilbert R W, Neligan P C. Role of thrombolysis in free-flap salvage. J Reconstr Microsurg. 2003;19(08):523–530. doi: 10.1055/s-2004-815638. [DOI] [PubMed] [Google Scholar]
- 72.Bui D T, Cordeiro P G, Hu Q Y, Disa J J, Pusic A, Mehrara B J. Free flap reexploration: indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg. 2007;119(07):2092–2100. doi: 10.1097/01.prs.0000260598.24376.e1. [DOI] [PubMed] [Google Scholar]
- 73.Kroll S S, Schusterman M A, Reece G P. Timing of pedicle thrombosis and flap loss after free-tissue transfer. Plast Reconstr Surg. 1996;98(07):1230–1233. doi: 10.1097/00006534-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 74.Nelson J A, Kim E M, Eftakhari K. Late venous thrombosis in free flap breast reconstruction: strategies for salvage after this real entity. Plast Reconstr Surg. 2012;129(01):8e–15e. doi: 10.1097/PRS.0b013e3182361f7f. [DOI] [PubMed] [Google Scholar]
- 75.Largo R D, Selber J C, Garvey P B. Outcome analysis of free flap salvage in outpatients presenting with microvascular compromise. Plast Reconstr Surg. 2018;141(01):20e–27e. doi: 10.1097/PRS.0000000000003917. [DOI] [PubMed] [Google Scholar]
- 76.Trussler A P, Watson J P, Crisera C A. Late free-flap salvage with catheter-directed thrombolysis. Microsurgery. 2008;28(04):217–222. doi: 10.1002/micr.20480. [DOI] [PubMed] [Google Scholar]
- 77.Shuck J, Endara M, Davison S P. Management of intraoperative free flap arterial thrombosis with argatroban in the heparin-allergic patient. Plast Reconstr Surg. 2014;134(04):672e. doi: 10.1097/PRS.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 78.Puckett C L, Misholy H, Reinisch J F. The effects of streptokinase on ischemic flaps. J Hand Surg Am. 1983;8(01):101–104. doi: 10.1016/s0363-5023(83)80066-5. [DOI] [PubMed] [Google Scholar]
- 79.Lepore D A, Knight K R, Bhattacharya S. Drug mixture which improves survival of ischemic rabbit epigastric skin flaps. Microsurgery. 1994;15(10):685–692. doi: 10.1002/micr.1920151005. [DOI] [PubMed] [Google Scholar]
- 80.Romano J E, Biel M A. Thrombolysis in microvascular surgery using tissue-type plasminogen activator. Arch Otolaryngol Head Neck Surg. 1989;115(11):1318–1321. doi: 10.1001/archotol.1989.01860350052014. [DOI] [PubMed] [Google Scholar]
- 81.Lipton H A, Jupiter J B. Streptokinase salvage of a free-tissue transfer: case report and review of the literature. Plast Reconstr Surg. 1987;79(06):977–981. doi: 10.1097/00006534-198706000-00022. [DOI] [PubMed] [Google Scholar]
- 82.Biben J A, Atmodiwirjo P. Free flap thrombosis in patients with hypercoagulability: a systematic review. Arch Plast Surg. 2019;46(06):572–579. doi: 10.5999/aps.2019.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang T Y, Serletti J M, Cuker A. Free tissue transfer in the hypercoagulable patient: a review of 58 flaps. Plast Reconstr Surg. 2012;129(02):443–453. doi: 10.1097/PRS.0b013e31823aec4d. [DOI] [PubMed] [Google Scholar]
- 84.Nelson J A, Chung C U, Bauder A R, Wu L C. Prevention of thrombosis in hypercoagulable patients undergoing microsurgery: a novel anticoagulation protocol. J Plast Reconstr Aesthet Surg. 2017;70(03):307–312. doi: 10.1016/j.bjps.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 85.DeFazio M V, Economides J M, Anghel E L, Tefera E A, Evans K K. Lower extremity free tissue transfer in the setting of thrombophilia: analysis of perioperative anticoagulation protocols and predictors of flap failure. J Reconstr Microsurg. 2019;35(04):270–286. doi: 10.1055/s-0038-1675145. [DOI] [PubMed] [Google Scholar]
- 86.Hill J B, Patel A, Del Corral G A. Preoperative anemia predicts thrombosis and free flap failure in microvascular reconstruction. Ann Plast Surg. 2012;69(04):364–367. doi: 10.1097/SAP.0b013e31823ed606. [DOI] [PubMed] [Google Scholar]
- 87.Veith J, Donato D, Holoyda K, Simpson A, Agarwal J. Variables associated with 30-day postoperative complications in lower extremity free flap reconstruction identified in the ACS-NSQIP database. Microsurgery. 2019;39(07):621–628. doi: 10.1002/micr.30502. [DOI] [PubMed] [Google Scholar]
- 88.Mlodinow A S, Ver Halen J P, Rambachan A, Gaido J, Kim J Y. Anemia is not a predictor of free flap failure: a review of NSQIP data. Microsurgery. 2013;33(06):432–438. doi: 10.1002/micr.22107. [DOI] [PubMed] [Google Scholar]
- 89.Cho E H, Bauder A R, Centkowski S. Preoperative platelet count predicts lower extremity free flap thrombosis: a multi-institutional experience. Plast Reconstr Surg. 2017;139(01):220–230. doi: 10.1097/PRS.0000000000002893. [DOI] [PubMed] [Google Scholar]
- 90.Shum J, Markiewicz M R, Park E. Low prealbumin level is a risk factor for microvascular free flap failure. J Oral Maxillofac Surg. 2014;72(01):169–177. doi: 10.1016/j.joms.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 91.Lim W, Le Gal G, Bates S M. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2(22):3226–3256. doi: 10.1182/bloodadvances.2018024828. [DOI] [PMC free article] [PubMed] [Google Scholar]


