Abstract
Background/Aims
The usefulness of ultrasonography (US) in diseases of the gastrointestinal tract has been reported recently. This prospective study aimed to determine the features of US findings in immune-mediated colitis (IMC), an adverse event induced by immune checkpoint inhibitor, and examine the correlation between US findings, colonoscopy (CS) findings, and severity of colitis.
Methods
We studied patients examined using CS and US upon suspicion of IMC in Hokkaido University Hospital between April 2018 and February 2021. Endoscopic findings of IMC were assessed using the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). The severity of US findings in IMC was evaluated using US grade, which is the ultrasonographic grading scale in ulcerative colitis. Bowel wall thickness and the intensity of the color Doppler signal were also analyzed. Severity of colitis was evaluated using Common Terminology Criteria for Adverse Events (CTCAE) grade version 5.
Results
Fourteen patients with IMC were enrolled. The US findings were bowel wall thickening, loss of stratification, ulceration and increased blood flow signal. The US grade was moderately correlated with the UCEIS (r=0.687, P=0.009) and CTCAE grade (r=0.628, P=0.035). Bowel wall thickness and UCEIS (r=0.628, P=0.020), as well as color Doppler signal grade and CTCAE grade (r=0.724, P=0.008), were significantly correlated.
Conclusions
US findings in IMC were mainly similar to those of ulcerative colitis, but there were some findings that were characteristic only of IMC. Significant correlation was found between US findings, CS findings, and severity of colitis. Hence, US could be useful for the evaluation of IMC.
Keywords: Transabdominal ultrasonography, Colonoscopy, Immune-mediated colitis, Immune checkpoint inhibitor, Immune-related adverse events
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have proven to be effective against various carcinomas, and their range of indications is continuously expanding [1-9]. ICIs exhibit anti-tumor effects by activating immune responses against tumor cells. However, their activity might also cause immune dysfunction, leading to side effects such as autoimmune diseases or autoinflammatory diseases; the latter are called “immune-related adverse events” (irAEs) [10]. Among irAE-related studies, patients diagnosed with immune-mediated colitis (IMC) based on imaging data and abdominal symptoms accounted for 5% to 16% of those treated with anti-CTLA-4 antibodies and 1% to 2% of those treated with anti-programmed cell death receptor 1 (PD-1)/anti-PD-ligand 1 (PD-L1) antibodies [11]. IMC can occur at any time, i.e., in some cases, IMC develops a few days after initiating treatment, while in others, enteritis develops several months after completing ICI therapy [12]. Therefore, proper evaluation and treatment of diarrhea and enteritis is essential after initiating ICI therapy [13]. To diagnose IMC, tests such as stool culture, Clostridium toxin test, serum cytomegalovirus antigen test are performed to rule out infectious enteritis, and patients also undergo colonoscopy (CS) [14-16]. CS findings in IMC are often similar to those found in inflammatory bowel disease, particularly in ulcerative colitis (UC). Sometimes, the following findings can also be observed: patch or diffuse erythema, loss of vascular pattern, granular or edematous mucosa, exudates, aphtha, and ulceration [11,17-21]. Further, in most cases of IMC, lesions can be found in the left colon [22,23]. The treatment may consist of discontinuation of medication or the use of antidiarrheal drugs or the use of steroids and anti-tumor necrosis factor α antibodies such as infliximab [24,25]. Meanwhile, cases of relapse also frequently occur after induction of remission, and enteritis has been reported to relapse in approximately 30% of patients upon resuming ICI treatment [26]. It has been suggested that the Ulcerative Colitis Endoscopic Index of Severity (UCEIS), a method to evaluate endoscopic severity in UC, may more accurately reflect the severity of IMC than the Common Terminology Criteria for Adverse Events (CTCAE) grade [27-29]. The diagnosis of IMC is established based on CS; however, because of invasiveness and pretreatment requirements, CS cannot be used for frequent monitoring [30,31]. In contrast, transabdominal ultrasonography (US) is noninvasive, does not require pretreatment, and can be performed regardless of the patient’s general physical condition [32-35]. In addition, the usefulness of US in diseases of the gastrointestinal tract, such as UC and Crohn’s disease (CD), has been reported recently [36-45]. With an increase in the indications for ICIs, the incidence of IMC may also increase, and modalities allowing for evaluation of the disease condition through less invasive methods will be needed. Therefore, our study aimed to elucidate the features of US findings in IMC and determine the usefulness of US in IMC.
METHODS
1. Study Design and Patients (Inclusion Criteria)
A single-center, prospective, observational study was performed at the Diagnostic Center for Sonography and Department of Gastroenterology, Hokkaido University Hospital. The study was conducted on patients being treated as outpatients or inpatients between April 2018 and February 2021 and those who underwent CS and US at Hokkaido University Hospital for suspicion of IMC at Hokkaido University Hospital. If it was difficult to conduct US and CS on the same day, these were performed within 4 days of each other. Since there are no fixed diagnostic criteria for IMC, the diagnosis was established by the attending gastroenterologist based on clinical, endoscopic and/or histological characteristics.
2. Transabdominal US
US was performed using an Aplio i800/500 scanner (Canon Medical Systems, Otawara, Japan) with a center frequency of 4.75 MHz and a 6-MHz convex probe as well as a 7.5 MHz linear probe by 6 sonographers and 1 gastroenterologist, who were blinded to CS findings, with more than 5 years of experience. When US and CS were performed on the same day, a bowel cleansing agent was prescribed orally for CS before US. When US and CS were performed on different dates, the patients were fasted for at least 8 hours before US. US was performed in the supine position, and patients were repositioned when necessary. All still images and movie clips were analyzed and interpreted in a consensus manner by 2 registered sonographers (M.N. and S.O.) who had 10 or more years of experience each. They were aware of the IMC diagnosis but were blinded to the patients’ clinical information, CS findings, and other sonographers’ US assessments. The large intestine was divided into 7 segments (cecum, ascending colon, right-sided transverse colon, left-sided transverse colon, descending colon, sigmoid colon, and rectum), and the measured value at the thickest part of each segment was adopted as the bowel wall thickness (BWT). Since there has been no reported method to assess blood flow signals in the intestinal wall in IMCs, blood flow signals were assessed by classifying the color Doppler signals (CDS) into 4 grades ranging from 0 to 3, a method that we previously proposed in CD (Supplementary Fig. 1) [37]. Doppler study was performed using a 7.5-MHz linear probe, with color gain adjusted until the disappearance of noise to maximize the sensitivity. The color Doppler frequency was set from 3.3 to 4.5 MHz, with a pulse repetition frequency from 4.7 to 10.1 cm/sec, which was adjusted according to the lesion depth. The wall filter was set between 3 and 4. Similarly, there had been no reported method to assess the severity of US findings in IMC. Therefore, the severity of US findings was evaluated using US grade, which we previously proposed for severity evaluation of UC using US (Supplementary Fig. 2) [36].
3. Colonoscopy
Using a standard endoscope (PCF-Q260AI, PCF-Q260AZI, PCF-PQ260; Olympus, Tokyo, Japan), CS was performed by 5 expert endoscopists, each of whom had performed more than 2,000 colonoscopies. A polyethylene glycol preparation was used as bowel cleansing agent. Depending on the severity, flexible sigmoidoscopy was performed without using bowel cleansing agent to avoid the risk of intestinal perforation. In CS, same way as US, the entire colon was observed and categorized into 7 segments. The severity was scored using the UCEIS, since it has been reported that the UCEIS may correlate with the severity of IMC (Supplementary Table 1) [27-29]. All endoscopic findings were evaluated by 3 experienced gastroenterologists (T.K., S.O., and K.S.), each with more than 6 years of CS experience. They were aware of the IMC diagnosis but were blinded to the patient’s clinical information and US findings.
4. Outcomes
The outcomes are as follows: (1) the features of US findings in IMC were assessed; (2) the correlation between BWT and UCEIS or CTCAE grade of colitis was evaluated; (3) the correlation between CDS grade and UCEIS or CTCAE grade of colitis was evaluated; (4) the correlation between severity according to US and CS findings was evaluated using US grade and UCEIS; (5) the correlations between US grade, disease activity, C-reactive protein (CRP) levels, and serum albumin levels were evaluated. Disease activity was evaluated using CTCAE grade of colitis, version 5.0 (Supplementary Table 2) [46]; or (6) the optimal cutoff values for the sensitivity, specificity, positive predictive value, and negative predictive value of BWT, CDS grade and US grade for UCEIS or CTCAE grade of colitis were evaluated.
5. Statistical Analysis
The GraphPad Prism 8 software package (GraphPad Software Inc., San Diego, CA, USA) and JMP pro 16 (SAS Institute Inc., Cary, NC, USA) were used for statistical analyses. All variables were expressed as a median (range) or number (%). All reported P-values are two-sided, and P<0.05 was considered statistically significant. P-values were calculated using the chis-quare test for categorical variables. Spearman rank correlation coefficient was used to verify the correlation between US grade, UCEIS, CTCAE grade, BWT, and CDS grade. Receiver operating characteristic (ROC) curves were constructed, and the trapezoidal rule was used to calculate the area under the ROC curve (AUROC). The optimal cutoff points for predicting UCEIS and CTCAE grade of colitis were identified based on the highest Youden index. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated using cutoff values obtained from ROC curves.
6. Ethical Considerations
This study was started after obtaining approval from the Institutional Review Board of Hokkaido University Hospital (IRB No. 017-420). The research protocol was posted on Hokkaido University Hospital’s website, and informed consent was obtained from all patients in compliance with the Declaration of Helsinki.
RESULTS
1. Patient Enrollment
Between April 2018 and February 2021, 528 patients received ICI, 30 of them presented with diarrhea, bloody stools, or abdominal pain during ICI therapy or after discontinuation of ICI. Of these 30 patients, 18 patients underwent CS and US for the suspicion of IMC. Those whose CS or US showed no inflammatory findings as well as those who were diagnosed with other diseases such as infectious enteritis based on tissue biopsy and stool culture were excluded from the study. A total of 14 patients were enrolled who were diagnosed with IMC based on CS findings (Fig. 1).
Fig. 1.
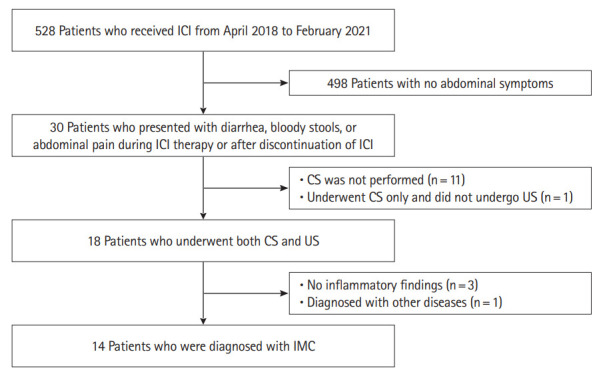
Patient enrollment. Between April 2018 and February 2021, 528 patients received immune checkpoint inhibitor (ICI), 30 of them presented with diarrhea, bloody stools, or abdominal pain during ICI therapy or after discontinuation of ICI. Of these 30 patients, 18 patients underwent colonoscopy (CS) and ultrasonography (US) for the suspicion of immune-mediated colitis (IMC). Those whose CS or US showed no inflammatory findings as well as those who were diagnosed with other diseases such as infectious enteritis based on tissue biopsy and stool culture were excluded from the study. A total of 14 patients were enrolled who were diagnosed with IMC based on CS findings.
2. Patient Characteristics
The enrolled IMC patients had a median age of 64 years (range, 47–83 years) and included 9 males and 5 females. The median body mass index was 21.6 kg/m2 (range, 16.0–25.3 kg/m2). Nine and five patients suffered from IMC induced by anti-PD-1 antibodies (nivolumab in 6 cases, and pembrolizumab in 3 cases), and anti-PD-L1 antibodies (durvalumab in 1 case, atezolizumab in 4 cases), respectively. The median CTCAE grade of colitis was 3 (range, 1–3), the median duration of the period from the initial administration of ICI to the onset of IMC was 124 days (range, 12–459 days). At the onset of IMC, the median serum CRP level was 4.67 mg/dL (range, 0.05–14.90 mg/dL), and the median serum albumin level was 3.3 g/dL (range, 2.5–4.3 g/dL) (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients with Immune-Mediated Colitis
| Characteristics | Value (n = 14) |
|---|---|
| Age (yr) | 64 (47–83) |
| Sex | |
| Male | 9 (64.3) |
| Female | 5 (35.7) |
| Body mass index (kg/m2) | 21.6 (16.0–25.3) |
| Smoking status | |
| Current | 1 (7.1) |
| Former | 8 (57.1) |
| Never | 5 (35.7) |
| Immune checkpoint inhibitor type | |
| PD-1 | 9 (64.3) |
| PD-L1 | 5 (35.7) |
| CTLA-4 | 0 |
| Cancer type | |
| Non-small cell lung carcinoma | 9 (64.3) |
| Pharyngeal carcinoma | 2 (14.3) |
| Renal cell carcinoma | 2 (14.3) |
| Esophageal carcinoma | 1 (7.1) |
| Cancer stage | |
| UICC III | 0 |
| UICC IV | 14 (100) |
| Clinical symptoms | |
| Stool frequency (times/day) | 9 (1–15) |
| Fever | 2 (14.3) |
| Bloody stool | 6 (42.9) |
| CTCAE grade of colitis | |
| 1 | 2 (14.3) |
| 2 | 1 (7.1) |
| 3 | 11 (78.6) |
| 4, 5 | 0 |
| Time to colitis onset (day) | 124 (12–459) |
| Other adverse events | |
| Endocrine | 1 (7.1) |
| Pancreatic | 1 (7.1) |
| Hepatic | 1 (7.1) |
| Serum C-reactive protein (mg/dL) | 4.7 (0.1–14.9) |
| Serum albumin (g/dL) | 3.3 (2.5–4.3) |
Values are presented as median (range) or number (%).
PD-1, programmed cell death receptor 1; PD-L1, PD-ligand 1; UICC, Union for International Cancer Control; CTCAE, Common Terminology Criteria for Adverse Events.
3. Clinical Outcomes
Among the enrolled patients, 11 (78.6%) underwent US and CS on the same day. In patients whom US and CS were performed on the same day, US was performed before CS. Complete CS was performed in 10 individuals (71.4%). Flexible sigmoidoscopy was performed in 4 individuals (28.6%) due to the severity of inflammation or insufficient colon cleansing. On the other hand, the entire large intestine was observed in 11 individuals (78.6%) using US, and values from some of the segments of the large intestine were missing in 3 individuals. Table 2 shows the visualization rate for each segment. The visualization rate for each segment tended to be higher with US than with CS, especially in the right colon. The total visualization rate in all segments were significantly higher in US observations than in those using CS (P=0.027) (Table 2).
Table 2.
Visualization Rate in Each Colonic Segment Examined by Ultrasonography and Colonoscopy
| Colonic segment | Colonoscopy | Ultrasonography | P-valuea |
|---|---|---|---|
| Cecum | 11 (78.6) | 13 (92.9) | 0.280 |
| Ascending colon | 11 (78.6) | 14 (100) | 0.067 |
| Right-sided transverse colon | 11 (78.6) | 14 (100) | 0.067 |
| Left-sided transverse colon | 13 (92.9) | 13 (92.9) | NS |
| Descending colon | 13 (92.9) | 13 (92.9) | NS |
| Sigmoid colon | 14 (100) | 14 (100) | NS |
| Rectum | 14 (100) | 14 (100) | NS |
| All segments | 87 (88.8) | 95 (96.9) | 0.027 |
Values are presented as number (%).
P-values were calculated using the chi-square test for categorical variables.
NS, not significant.
The findings found from US and those from CS are shown in Table 3, as well as their respective proportions. With US, thickened mucosa and submucosa were observed in 14 cases (100%), loss of stratification was found in 8 cases (57.1%), irregular mucosa or hyperechogenic shallow concavity in the mucosa (which suggested ulceration) was found in 2 cases (14.3%), increased blood flow signal detected by color Doppler was found in 13 cases (92.9%) and increased echo levels in the peri-intestinal adipose tissue were found in 3 cases (21.4%). With endoscopy, exudates on the large intestine mucosa were found in 4 cases (28.6%), loss of vascular pattern was found in 12 cases (85.7%), granular or edematous mucosa was found in 11 cases (78.6%), patch or diffuse erythema was found in 9 cases (64.3%), aphthae were found in 11 cases (78.6%), ulceration was found in 3 cases (21.4%), and contact and spontaneous bleeding was found in 6 cases (42.9%) (Table 3).
Table 3.
Endoscopic and Ultrasonographic Findings of Immune-Mediated Colitis
| Findings | No. (%) |
|---|---|
| Ultrasonographic findings | |
| Thickened mucosa and submucosa | 14 (100) |
| Loss of stratification | 8 (57.1) |
| Irregular mucosa or hyperechogenic shallow concavity in the mucosa, which suggests ulceration | 2 (14.3) |
| Increased blood flow signals | 13 (92.9) |
| Echo level increase of mesenteric adipose tissue | 3 (21.4) |
| Endoscopic findings | |
| Exudates | 4 (28.6) |
| Loss of vascular pattern | 12 (85.7) |
| Granular or edematous mucosa | 11 (78.6) |
| Patch or diffuse erythema | 9 (64.3) |
| Aphtha | 11 (78.6) |
| Ulceration | 3 (21.4) |
| Contact and spontaneous bleeding | 6 (42.9) |
Table 4 shows the BWT, CDS grade, US grade, and UCEIS in each segment of the large intestine. The median BWT, CDS grade, US grade, and UCEIS at the most severely affected site in each patient were 8.4 mm, 2, 3, and 3.5, respectively.
Table 4.
BWT, CDS Grade, US Grade, and UCEIS of Severity of Each Colonic Segment
| Colonic segment | BWT (mm) | CDS grade | US grade | UCEIS |
|---|---|---|---|---|
| Cecum | 3.7 (1.7–9.8) | 2.0 (0–3.0) | 2.0 (1.0–4.0) | 1.0 (0.0–3.0) |
| Ascending colon | 3.9 (1.7–10.1) | 1.5 (0–3.0) | 2.0 (1.0–4.0) | 3.0 (0.0–5.0) |
| Right-sided transverse colon | 5.1 (1.5–10.6) | 2.0 (0–2.0) | 2.0 (1.0–4.0) | 1.0 (0.0–5.0) |
| Left-sided transverse colon | 3.8 (1.5–7.7) | 2.0 (0–3.0) | 2.0 (1.0–4.0) | 1.0 (0.0–5.0) |
| Descending colon | 4.7 (2.0–10.7) | 0.5 (0–3.0) | 2.0 (1.0–4.0) | 1.0 (0.0–5.0) |
| Sigmoid colon | 3.2 (1.9–6.8) | 2.0 (0–2.0) | 2.0 (1.0–4.0) | 3.0 (2.0–5.0) |
| Rectum | 7.9 (5.8–12.5) | 0.0 (0–2.0) | 2.0 (1.0–4.0) | 3.0 (1.0–5.0) |
| Most severe lesions | 8.4 (5.8–12.5) | 2.0 (0–3.0) | 3.0 (1.0–4.0) | 3.5 (2.0–5.0) |
Values are presented as median (range).
BWT, bowel wall thickness; CDS, color Doppler signal; US, ultrasonography; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
The correlations at the most severely affected sites for each score are shown in Table 5 and Fig. 2. A moderate correlation was found between BWT and UCEIS (r=0.628, P=0.020), while no significant correlation was found between BWT and CTCAE. The CDS grade and UCEIS showed no significant correlation. However, the CDS grade and CTCAE were strongly correlated (r=0.724, P=0.008) (Fig. 2). The US grade and UCEIS showed a significant correlation in the sigmoid colon and the rectum, while no significant correlation in the other segments. Moderate correlations were also observed between the US grade and UCEIS (r=0.687, P=0.009), and CTCAE grade (r=0.628, P=0.035), respectively. No significant correlation was found between the US grade and serum CRP levels or serum albumin levels (Table 5). Since it has been reported that the diagnostic accuracy of ultrasound in inflammatory bowel disease is low in the rectum [47], we adopted the values for the most severely affected sites in the colon, excluding the rectum, and analyzed the correlation between endoscopic severity and US findings (Supplementary Fig. 3). As a result, we found a strong correlation between BWT and UCEIS (r=0.787, P=0.002), US grade and UCEIS (r=0.832, P=0.001), and a moderate correlation between CDS grade and UCEIS (r=0.682, P=0.010).
Table 5.
Correlation of Each Score and Serum C-Reactive Protein and Albumin
| Correlations | r | P-value |
|---|---|---|
| BWT and UCEISa | 0.628 | 0.020 |
| BWT and CTCAEa | 0.469 | 0.099 |
| CDS grade and UCEISa | 0.445 | 0.117 |
| CDS grade and CTCAEa | 0.724 | 0.008 |
| US grade and UCEIS | ||
| Cecum | 0.311 | 0.379 |
| Ascending colon | 0.582 | 0.064 |
| Right-sided transverse colon | 0.312 | 0.333 |
| Left-sided transverse colon | 0.270 | 0.386 |
| Descending colon | 0.191 | 0.546 |
| Sigmoid colon | 0.616 | 0.020 |
| Rectum | 0.707 | 0.005 |
| Most severe lesions | 0.687 | 0.009 |
| US grade and CTCAEa | 0.628 | 0.035 |
| US grade and C-reactive proteina | 0.244 | 0.398 |
| US grade and albumina | –0.330 | 0.248 |
Correlations were calculated using Spearman correlation coefficient.
Comparison at the most severely affected sites.
BWT, bowel wall thickness; UCEIS, Ulcerative Colitis Endoscopic Index of Severity; CTCAE, Common Terminology Criteria for Adverse Events; CDS, color Doppler signal; US, ultrasonography.
Fig. 2.
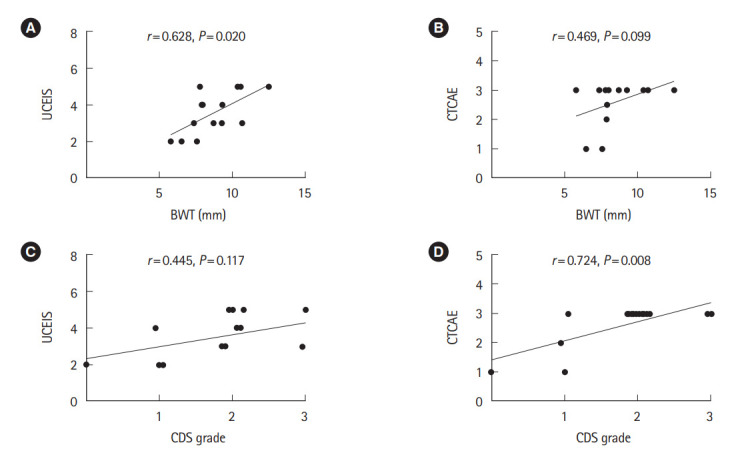
Correlations between BWT, CDS grade, UCEIS, and CTCAE grade of colitis. (A) A moderate correlation was found between BWT and UCEIS, (B) while no significant correlation was found between BWT and CTCAE. (C) The CDS grade and UCEIS showed no significant correlation. (D) However, the CDS grade and CTCAE were strongly correlated. BWT, bowel wall thickness; CDS, color Doppler signal; UCEIS, Ulcerative Colitis Endoscopic Index of Severity; CTCAE, Common Terminology Criteria for Adverse Events.
Table 6 shows performance characteristics of BWT, CDS grade, and US grade for the determination of UCEIS and CTCAE grade of colitis. The optimal cutoff values for the sensitivity, specificity, positive predictive value, and negative predictive value of BWT, CDS grade, and US grade for UCEIS ≥ 5 and CTCAE grade of colitis ≥ 3 are summarized in Table 6. The AUROCs of BWT for the prediction of UCEIS ≥ 5 and CTCAE grade of colitis ≥ 3 were 0.800, and 0.818, respectively. The AUROCs of CDS grade for these grades were 0.675, and 0.970, respectively. The AUROCs of US grade for these grades were 0.900, and 0.909, respectively (Table 6).
Table 6.
Diagnostic Accuracy of BWT, CDS Grade and US Grade for the Diagnosis of UCEIS and CTCAE of Colitis in Immune-Mediated Colitis
| Accuracy | UCEIS ≥ 5 |
CTCAE grade of colitis ≥ 3 |
||||
|---|---|---|---|---|---|---|
| BWT (mm) | CDS grade | US grade | BWT (mm) | CDS grade | US grade | |
| AUROC | 0.800 | 0.675 | 0.900 | 0.818 | 0.970 | 0.909 |
| Cutoff value | 10.4 | 2.0 | 3.0 | 8.0 | 2.0 | 3.0 |
| Sensitivity | 0.750 | 1.000 | 1.000 | 0.727 | 0.909 | 0.727 |
| Specificity | 0.900 | 0.400 | 0.600 | 1.000 | 1.000 | 1.000 |
| PPV | 0.750 | 0.400 | 0.500 | 1.000 | 1.000 | 1.000 |
| NPV | 0.900 | 1.000 | 0.900 | 0.500 | 0.750 | 0.500 |
BWT, bowel wall thickness; CDS, color Doppler signal; US, ultrasonography; UCEIS, Ulcerative Colitis Endoscopic Index of Severity; CTCAE, Common Terminology Criteria for Adverse Events; AUROC, area under receiver the operating curve; PPV, positive predictive value; NPV, negative predictive value.
4. Case Presentation
Fig. 3 shows CS findings and US findings before and after treatment in a patient who had developed IMC as a result of an anti-PD-L1 antibody therapy (durvalumab). Her condition was resistant to steroids and anti-tumor necrosis factor α antibodies, and remission was induced using vedolizumab. At the onset of colitis, US showed the intestinal wall thickness and partially indistinct stratification, and color Doppler showed increased blood flow signals (Fig. 3A and B). CS showed a circumferential erosion from the cecum to the rectum, and there was exudates and spontaneous bleeding (Fig. 3C). After vedolizumab therapy, US showed improvement of the bowel wall thickening, and the stratified structure became clearly visible, and color Doppler showed no blood flow signals (Fig. 3D and E). Also, CS showed disappearance of the erosion and exudates, and the mucosal vascular pattern became visible (Fig. 3F).
Fig. 3.
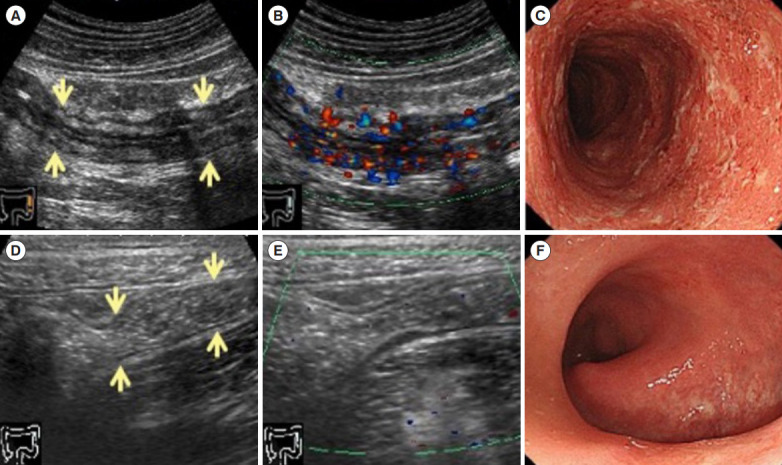
Ultrasonography and colonoscopy findings of immune-mediated colitis (IMC) in a patient who had anti-PD-L1 antibody therapy (durvalumab). IMC was resistant to steroids and anti-tumor necrosis factor α antibodies. The remission was inducted using vedolizumab. At the onset of colitis, ultrasonography (US) shows a thickened bowel wall, the stratified structure is partially indistinct (arrows) (A), and color Doppler shows an increased blood flow signal (B). Colonoscopy (CS) shows a circular erosion from the cecum to the rectum, and there are exudates and spontaneous bleeding (C). After administering vedolizumab, US shows the bowel wall thickening improvement, and the stratified structure becomes clearly visible (arrows) (D), color Doppler shows no blood flow signal (E). CS findings improves that the erosion and exudates are disappeared, and the mucosal vascular pattern becomes also visible (F). PD-L1, programmed cell death receptor ligand 1.
DISCUSSION
US findings of IMC were thickening of the mucosal and submucosal layer, loss of stratification, irregular mucosa or hyperechogenic shallow concavity in the mucosal lumen which suggests ulceration, as well as color Doppler images revealed an increased blood flow signals in the intestinal wall. There were correlations between the severity of BWT and UCEIS, and the CDS grade and the CTCAE grade of colitis. Also, US grades and UCEIS, and CTCAE showed significant correlations.
The diagnosis of IMC is basically established based on CS findings and the gold standard to evaluate IMC is CS. However, CS has several disadvantages. Due to limitations in terms of cost, inconvenience, and invasiveness, CS is not suitable for frequent monitoring. Further, in patients with severe colitis, the procedure poses risks of intestinal perforation and aggravation of disease activity [30,31]. On the other hand, US offers advantages such as no radiation exposure, noninvasiveness, low cost, can be performed the procedure regardless of the patient’s condition, and ability to perform real-time scanning [32-35]. In recent years, the development of US equipments and testing technology has popularized the use of US in the field of gastrointestinal diseases. US has been found useful in CD, UC, and graft-versus-host disease enterocolitis, and its findings correlated with those of endoscopy [36-45]. To the best of our knowledge, there has been only 1 case report in which intestinal US was used in IMC [48]. Therefore, our study analyzed the features of US findings in IMC, as well as their correlation with endoscopic findings, CTCAE grade, and CRP. The US findings showed a correlation between UCEIS and CTCAE. Regarding visualization, the total visualization rates in all segments were significantly higher in US than in CS. In the ascending colon and the right transverse colon, visualization rates also tended to be higher in US. In IMC, patients have a poor general condition, and other concomitant irAEs may also be present. Hence total CS may not be feasible in many cases. Our findings regarding visualization showed the remarkable advantages of US, which is less invasive and does not require pretreatment. Similar to previous reports, the endoscopic findings from our study showed many properties similar to those of UC. Many of the US findings recorded in our study were similar to US findings associated with UC. Meanwhile, in some cases, inflammatory findings such as thickening and loss of stratification were found in the submucosa despite the absence of active inflammatory findings such as thickening in the mucosal layer. This finding is different from those associated with UC, in which inflammation arises from the superficial layer of the mucosa. This may reflect the differences between the underlying mechanisms of IMC and UC. Another finding that differed from UC was that the inflammation was not continuous from the rectum but scattered in a skip-like pattern. The endoscopic or US findings of IMC were analyzed according to the type of ICI, but no significant difference in findings was observed. It has been previously reported that ipilimumab (CTLA-4 antibody) may cause a high incidence of rectal or sigmoid colon inflammation [49], but to our knowledge, no difference in IMC findings between PD-1 and PD-L1 antibodies has been reported. Similarly, in this study, there was no significant difference in US findings between PD-1 and PD-L1 antibodies.
There were several limitations to this study. The first was that since there has been no proposed method for evaluating the US and CS severity in IMC, the scoring method for UC was used alternatively. The second limitation was that US was not performed in a standardized manner in all cases and that the administration of oral laxatives as a pretreatment for CS may have affected US findings. In patients who took bowel cleansing agent before US, the bowel wall may have stretched due to bowel cleansing agent, resulting in an underestimation of BWT. In addition, the study was conducted at a single facility, and the sample size was small. Studies in the future should consider including a larger number of cases (sample size). A scoring method for US findings in IMC should be established.
In conclusion, our study revealed US findings of IMC, and US could be useful method for the evaluation and noninvasive monitoring of IMC based on correlation with CS findings and CTCAE grade.
Footnotes
Funding Source
This work was supported by the Hokkaido University Hospital.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: Sakurai K, Katsurada T, Nishida M, Omotehara S, K Nagashima, Onishi R, Komatsu Y, Sakamoto N. Data curation: Sakurai K, Katsurada T, Nishida M, Omotehara S, Otagiri S. Formal analysis: Sakurai K, Katsurada T, Takagi R. Investigation: Sakurai K, Katsurada T, Nishida M, Fukushima S, Otagiri S. Methodology: Sakurai K, Katsurada T, Nishida M, Nagashima K. Project administration: Sakurai K, Katsurada T, Nishida M, Omotehara S. Resources: Sakurai K, Katsurada T. Supervision: all authors. Validation: Sakurai K, Katsurada T, Nishida M, Komatsu Y. Visualization: Sakurai K, Nishida M, Omotehara S. Writing - original draft: Sakurai K. Writing - review & editing: Sakurai K, Katsurada T, Nishida M, Onishi R, Takagi R, Komatsu Y, Sakamoto N. Approval of final manuscript: all authors.
Others
We thank the staff of Hokkaido University Hospital for their invaluable help with data collection.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1. Ulcerative Colitis Endoscopic Index of Severity
Supplementary Table 2. CTCAE Grade of Colitis Version 5.0
Supplementary Fig. 1. Grading system of color Doppler signal (CDS). The semi-quantitative grading system of the CDSs in the intestinal wall. The amount of blood flow signal within a 10×10 mm square region of interest was evaluated. (A) Grade 0: no CDS. (B) Grade 1: few spotty signals. (C) Grade 2: confluent vessel signals in less than half of the area of the bowel wall. (D) Grade 3: confluent vessel signals in more than half of the area of the bowel wall.
Supplementary Fig. 2. Grading system of transabdominal ultrasonography (US) grades. (A) US grade 1: normal thickness of the colonic wall (arrows). (B) US grade 2: thickened mucosa and submucosa without hypoechoic change of the submucosa (arrows). (C) US grade 3: bowel wall thickness with loss of stratification (arrows). (D) US grade 4: bowel wall thickness with loss of stratification and irregular mucosa or hyperechogenic shallow concavity in the mucosa, which suggests ulceration (arrows).
Supplementary Fig. 3. Correlation between BWT, US grade, CDS grade, and UCEIS at the most severely affected sites in the colon, excluding the rectum. (A) A strong correlation was found between BWT and UCEIS, (B) US grade and UCEIS, and (C) a moderate correlation was found between CDS grade and UCEIS. BWT, bowel wall thickness; US, ultrasonography; CDS, color Doppler signal; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
REFERENCES
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 8.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 11.Nishida T, Iijima H, Adachi S. Immune checkpoint inhibitor-induced diarrhea/colitis: endoscopic and pathologic findings. World J Gastrointest Pathophysiol. 2019;10:17–28. doi: 10.4291/wjgp.v10.i2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056–2067. doi: 10.1136/gutjnl-2018-316948. [DOI] [PubMed] [Google Scholar]
- 13.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 14.Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5:e1128611. doi: 10.1080/2162402X.2015.1128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin C, Rooms I, Fiedler M, et al. Cytomegalovirus reactivation in patients with refractory checkpoint inhibitor-induced colitis. Eur J Cancer. 2017;86:248–256. doi: 10.1016/j.ejca.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Weber JS, Kähler KC, Hauschild A. Management of immunerelated adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda Y, Urata Y, Tohnai R, et al. Immune-related colitis induced by the long-term use of nivolumab in a patient with non-small cell lung cancer. Intern Med. 2018;57:1269–1272. doi: 10.2169/internalmedicine.9230-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubo K, Kato M, Mabe K. Nivolumab-associated colitis mimicking ulcerative colitis. Clin Gastroenterol Hepatol. 2017;15:A35–A36. doi: 10.1016/j.cgh.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi R, Araki T, Mitsuyama K, et al. The characteristics of nivolumab-induced colitis: an evaluation of three cases and a literature review. BMC Gastroenterol. 2018;18:135. doi: 10.1186/s12876-018-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6:95. doi: 10.1186/s40425-018-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geukes Foppen MH, Rozeman EA, van Wilpe S, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3:e000278. doi: 10.1136/esmoopen-2017-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Abu-Sbeih H, Mao E, et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018;24:1695–1705. doi: 10.1093/ibd/izy104. [DOI] [PubMed] [Google Scholar]
- 23.Wright AP, Piper MS, Bishu S, Stidham RW. Systematic review and case series: flexible sigmoidoscopy identifies most cases of checkpoint inhibitor-induced colitis. Aliment Pharmacol Ther. 2019;49:1474–1483. doi: 10.1111/apt.15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haanen JB, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 25.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Sbeih H, Ali FS, Naqash AR, et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol. 2019;37:2738–2745. doi: 10.1200/JCO.19.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the Ulcerative Colitis Endoscopic Index of Severity. Gastroenterology. 2013;145:987–995. doi: 10.1053/j.gastro.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Vuitton L, Peyrin-Biroulet L, Colombel JF, et al. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017;45:801–813. doi: 10.1111/apt.13948. [DOI] [PubMed] [Google Scholar]
- 29.Cheung VT, Gupta T, Olsson-Brown A, et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br J Cancer. 2020;123:207–215. doi: 10.1038/s41416-020-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damore LJ, 2nd, Rantis PC, Vernava AM, 3rd, Longo WE. Colonoscopic perforations: etiology, diagnosis, and management. Dis Colon Rectum. 1996;39:1308–1314. doi: 10.1007/BF02055129. [DOI] [PubMed] [Google Scholar]
- 31.Levin TR, Conell C, Shapiro JA, Chazan SG, Nadel MR, Selby JV. Complications of screening flexible sigmoidoscopy. Gastroenterology. 2002;123:1786–1792. doi: 10.1053/gast.2002.37064. [DOI] [PubMed] [Google Scholar]
- 32.Parente F, Greco S, Molteni M, et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther. 2003;18:1009–1016. doi: 10.1046/j.1365-2036.2003.01796.x. [DOI] [PubMed] [Google Scholar]
- 33.Moreno N, Ripollés T, Paredes JM, et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis. 2014;8:1079–1087. doi: 10.1016/j.crohns.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 35.Migaleddu V, Scanu AM, Quaia E, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology. 2009;137:43–52. doi: 10.1053/j.gastro.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita K, Katsurada T, Nishida M, et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J Gastroenterol. 2019;54:521–529. doi: 10.1007/s00535-018-01534-w. [DOI] [PubMed] [Google Scholar]
- 37.Yamanashi K, Katsurada T, Nishida M, et al. Crohn’s disease activity evaluation by transabdominal ultrasonography: correlation with double-balloon endoscopy. J Ultrasound Med. 2021;40:2595–2605. doi: 10.1002/jum.15645. [DOI] [PubMed] [Google Scholar]
- 38.Nishida M, Shigematsu A, Sato M, et al. Ultrasonographic evaluation of gastrointestinal graft-versus-host disease after hematopoietic stem cell transplantation. Clin Transplant. 2015;129:697–704. doi: 10.1111/ctr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn’s disease: a review with recommendations of an International Panel of Experts. Inflamm Bowel Dis. 2016;22:1168–1183. doi: 10.1097/MIB.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 40.Migaleddu V, Quaia E, Scano D, Virgilio G. Inflammatory activity in Crohn disease: ultrasound findings. Abdom Imaging. 2008;33:589–597. doi: 10.1007/s00261-007-9340-z. [DOI] [PubMed] [Google Scholar]
- 41.Fraquelli M, Sarno A, Girelli C, et al. Reproducibility of bowel ultrasonography in the evaluation of Crohn’s disease. Dig Liver Dis. 2008;40:860–866. doi: 10.1016/j.dld.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Parente F, Maconi G, Bollani S, et al. Bowel ultrasound in assessment of Crohn’s disease and detection of related small bowel strictures: a prospective comparative study versus X ray and intraoperative findings. Gut. 2002;50:490–495. doi: 10.1136/gut.50.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parente F, Greco S, Molteni M, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease: a prospective comparison with conventional ultrasound, X ray studies, and ileocolonoscopy. Gut. 2004;53:1652–1657. doi: 10.1136/gut.2004.041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrich CF. Significance of abdominal ultrasound in inflammatory bowel disease. Dig Dis. 2009;27:482–493. doi: 10.1159/000233287. [DOI] [PubMed] [Google Scholar]
- 45.Martínez MJ, Ripollés T, Paredes JM, Blanc E, Martí-Bonmatí L. Assessment of the extension and the inflammatory activity in Crohn’s disease: comparison of ultrasound and MRI. Abdom Imaging. 2009;34:141–148. doi: 10.1007/s00261-008-9365-y. [DOI] [PubMed] [Google Scholar]
- 46.National Cancer Institute (NCI) NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0 data files. c2019 [cited July 12 2021]. https://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 47.Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:908–921. doi: 10.1016/j.cgh.2020.07.067. [DOI] [PubMed] [Google Scholar]
- 48.Omotehara S, Nishida M, Nagashima K, et al. Immune checkpoint inhibitor-induced colitis successfully followed up by ultrasonography. SN Compr Clin Med. 2020;2:215–221. [Google Scholar]
- 49.Marthey L, Mateus C, Mussini C, et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10:395–401. doi: 10.1093/ecco-jcc/jjv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Ulcerative Colitis Endoscopic Index of Severity
Supplementary Table 2. CTCAE Grade of Colitis Version 5.0
Supplementary Fig. 1. Grading system of color Doppler signal (CDS). The semi-quantitative grading system of the CDSs in the intestinal wall. The amount of blood flow signal within a 10×10 mm square region of interest was evaluated. (A) Grade 0: no CDS. (B) Grade 1: few spotty signals. (C) Grade 2: confluent vessel signals in less than half of the area of the bowel wall. (D) Grade 3: confluent vessel signals in more than half of the area of the bowel wall.
Supplementary Fig. 2. Grading system of transabdominal ultrasonography (US) grades. (A) US grade 1: normal thickness of the colonic wall (arrows). (B) US grade 2: thickened mucosa and submucosa without hypoechoic change of the submucosa (arrows). (C) US grade 3: bowel wall thickness with loss of stratification (arrows). (D) US grade 4: bowel wall thickness with loss of stratification and irregular mucosa or hyperechogenic shallow concavity in the mucosa, which suggests ulceration (arrows).
Supplementary Fig. 3. Correlation between BWT, US grade, CDS grade, and UCEIS at the most severely affected sites in the colon, excluding the rectum. (A) A strong correlation was found between BWT and UCEIS, (B) US grade and UCEIS, and (C) a moderate correlation was found between CDS grade and UCEIS. BWT, bowel wall thickness; US, ultrasonography; CDS, color Doppler signal; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.


